Bacterial pathogens have evolved sophisticated mechanisms to evade host defenses and cause disease.[1] The emergence of new and antibiotic-resistant bacterial pathogens demands a better understanding of virulence mechanisms for antibacterial drug discovery. While the discovery of bacterial toxins, quorum sensing and protein secretion pathways has revealed some key virulence mechanisms, the precise mechanisms by which intracellular bacterial pathogens subvert host immune responses are still unclear.[1] The analysis of individual virulence factors has demonstrated that bacterial pathogens alter their protein expression to infect and replicate in host tissues.[2] However, the system-wide identification and analysis of bacterial proteins that are uniquely expressed or secreted during infection is paramount for understanding mechanisms of bacterial pathogenesis.[2, 3] Comparative genomics and mutagenesis studies have revealed bacterial genes that are important for infection, but their precise biochemical mechanisms and temporal expression pattern can be elusive due to post-transcriptional regulation.[2, 3] Direct biochemical analysis of bacterial proteomes during infection is needed.[3] The large excess of host proteins in mixed pathogen-host lysates presents a significant challenge for proteomic analysis of bacterial proteins during infections[3] and even after physical isolation of intact bacteria significant amounts of host proteins still remain.[4, 5] This is particularly important since many bacterial virulence factors are often expressed at low levels.[2] New strategies are therefore required to selectively enrich bacterial proteins from host proteomes for their analysis during infection.
The incorporation of unnatural amino acids in bacteria has provided new methods to differentiate bacterial proteins from host proteomes. For example, the incorporation of phenylalanine analogs in mycobacteria by amber stop codon suppression technology has enabled the selective labeling of green fluorescent protein expressed in M. tuberculosis during intracellular infection of macrophages.[6] Alternatively, alkyne- or azide-functionalized methionine (Met) surrogates can be incorporated by the endogenous methionyl-tRNA synthetase (MetRS) into bacterial proteomes.[7, 8] These amino acid chemical reporters allow the metabolic labeling of newly synthesized proteins, which in combination with bioorthogonal ligation methods, such as Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC)[9] can be used to detect and identify Met-containing proteins.[10] Additionally, MetRS mutants have been identified that can incorporate azidonorleucine (ANL, Figure 1a),[11, 12] a Met surrogate that is not efficiently activated by the wild-type MetRS or other endogenous aminoacyl-tRNA synthetases. ANL can therefore be used as an orthogonal amino acid reporter to selectively label proteins in non-pathogenic E. coli by bacterial expression of MetRS mutants in the presence of mammalian cells.[13] While these studies have demonstrated selective targeting of bacterial proteomes in the presence of host cells,[6, 13] the analysis of endogenously expressed bacterial proteins during infection has not been achieved using unnatural amino acid reporters. Herein, we report a new orthogonal alkynyl-amino acid reporter for specific imaging and enrichment of bacterial proteomes during infection of mammalian cells with the gram-negative intracellular bacterial pathogen Salmonella typhimurium (Figure 1b).
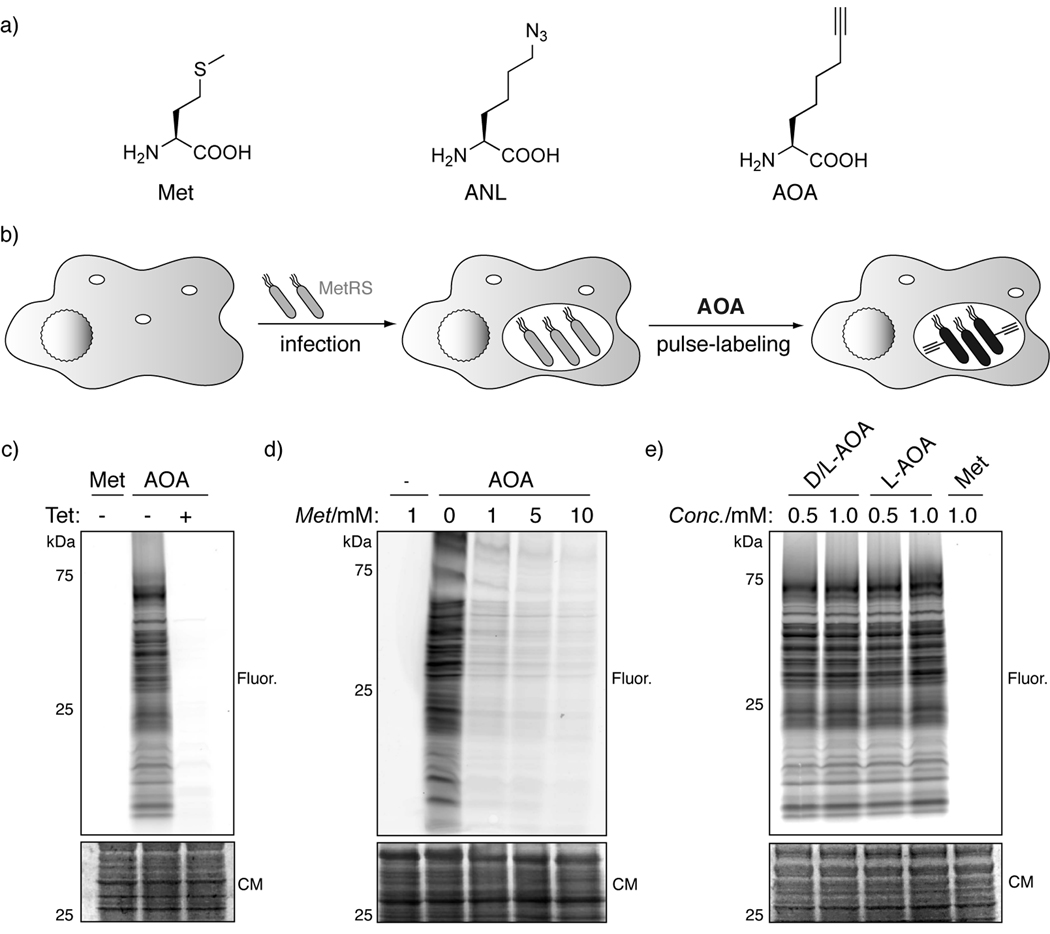
Figure 1.
Selective labeling of bacterial proteomes during infection with orthogonal amino acid reporters. a) Methionine (Met), azido-norleucine (ANL), and 2-aminooctynoic acid (AOA). b) Infection of host cells with bacterial pathogens expressing mutant methionyl-tRNA synthetase (MetRS) followed by pulse-labeling with orthogonal amino acid reporters (i.e. AOA) enables specific imaging and proteomic analysis of bacterial proteomes using CuAAC or “click chemistry“. c) MetRS-NLL S. typhimurium were labeled with 1 mM Met or 2 mM AOA ± tetracycline for 30 min. Cell lysates were reacted with az-Rho by CuAAC and analyzed by in-gel fluorescence. d) Met competition for 2mM AOA labeling. e) Comparison of labeling intensity of d/l-AOA and l-AOA. (CM-coomassie; Fluor. - in-gel fluorescence scanning)
Based on the reported superior selectivity of azide- compared to alkyne-functionalized secondary CuAAC reagents[14] and our own experience with fatty acid chemical reporters,[15] we evaluated whether an alkynyl-isostere of ANL, 2-aminooctynoic acid (AOA, Figure 1a), could be accepted by previously reported MetRS mutants in S. typhimurium.[13, 16, 17] MetRS mutants were generated from the E. coli metG gene (the S. typhimurium metG gene is 95% identical to E. coli metG) by site-directed mutagenesis and ligated into the low copy number plasmid pWSK29 under the expression control of the lac promoter, which provides constitutive expression in S. typhimurium. All plasmids were transformed into the S. typhimurium strain IR715. AOA was synthesized by alkylation of diethyl acetamidomalonate with hex-5-ynyl-4-methylbenzenesulfonate and sequentially deprotected to yield the racemic product (Supporting Scheme 1). For the in vitro analysis of the S. typhimurium strains expressing MetRS mutants, bacteria were grown in full Luria-Bertani (LB) medium to stationary phase and diluted into minimal medium containing Met or AOA. Protein lysates were reacted with the azido-rhodamine (az-Rho)[15] detection tag by CuAAC and analyzed by SDS-PAGE and in-gel fluorescence scanning. AOA selectively and efficiently labeled proteins in S. typhimurium expressing the MetRS-NLL mutant (L13N-Y260L-H301L) (Figure 1c). To confirm selectivity of AOA for newly synthesized Met-containing proteins, we conducted protein synthesis inhibitor and Met competition experiments. Pre- and co-incubation of MetRS-NLL S. typhimurium with the protein synthesis inhibitor tetracycline (Tet) effectively abrogated AOA-labeling (Figure 1c). Met efficiently competed away AOA-incorporation in a dose-dependent manner (Figure 1d). Since the synthetic route of AOA results in a racemic mixture, we determined whether the presence of the d-AOA isomer in the racemate impairs S. typhimurium labeling. Pig acylase I was used to kinetically resolve the racemic mixture of AOA,[18] which afforded enantiomerically pure l-AOA (Supporting Figure 1). Racemic AOA was used at 2 mM and compared to 1 mM l-AOA. Since both preparations showed identical labeling efficiencies at comparable effective concentrations (Figure 1e), we decided to use the racemic AOA preparation for subsequent experiments. These data demonstrate that AOA can selectively label newly synthesized Met-containing proteins in gram-negative bacterial pathogens such as MetRS-NLL S. typhimurium.
To determine the most efficient MetRS mutant and orthogonal amino acid reporter combination for selective labeling of bacterial proteomes during infection of host cells, we evaluated other reported MetRS mutants with ANL and AOA in Salmonella. We generated three previously reported MetRS mutants with high activation efficiency for ANL to evaluate their utility for metabolic labeling of S. typhimurium proteins with orthogonal amino acid reporters: L13G (MetRS-L13G), L13N-Y260L-H301L (MetRS-NLL), and L13P-Y260L-H301L (MetRS-PLL).[11] All three MetRS mutants permit incorporation of ANL and AOA into Salmonella proteomes (Figure 2a). Expression level of all MetRS mutants were comparable as judged by S-tag Western blot analysis of the epitope tag fused to all MetRS constructs (Supporting Figure 2). Both triple mutants (NLL, PLL) conveyed more efficient incorporation of ANL and AOA over the single mutant (L13G). In addition, AOA showed superior signal-to-noise ratios compared to labeling with ANL for all three MetRS mutants (Supporting Figure 3a). These data demonstrate that both orthogonal amino acid reporters function in Salmonella expressing MetRS mutants, but MetRS-NLL in combination with AOA affords the optimal orthogonal enzyme-substrate pair for metabolic labeling of Salmonella proteins.
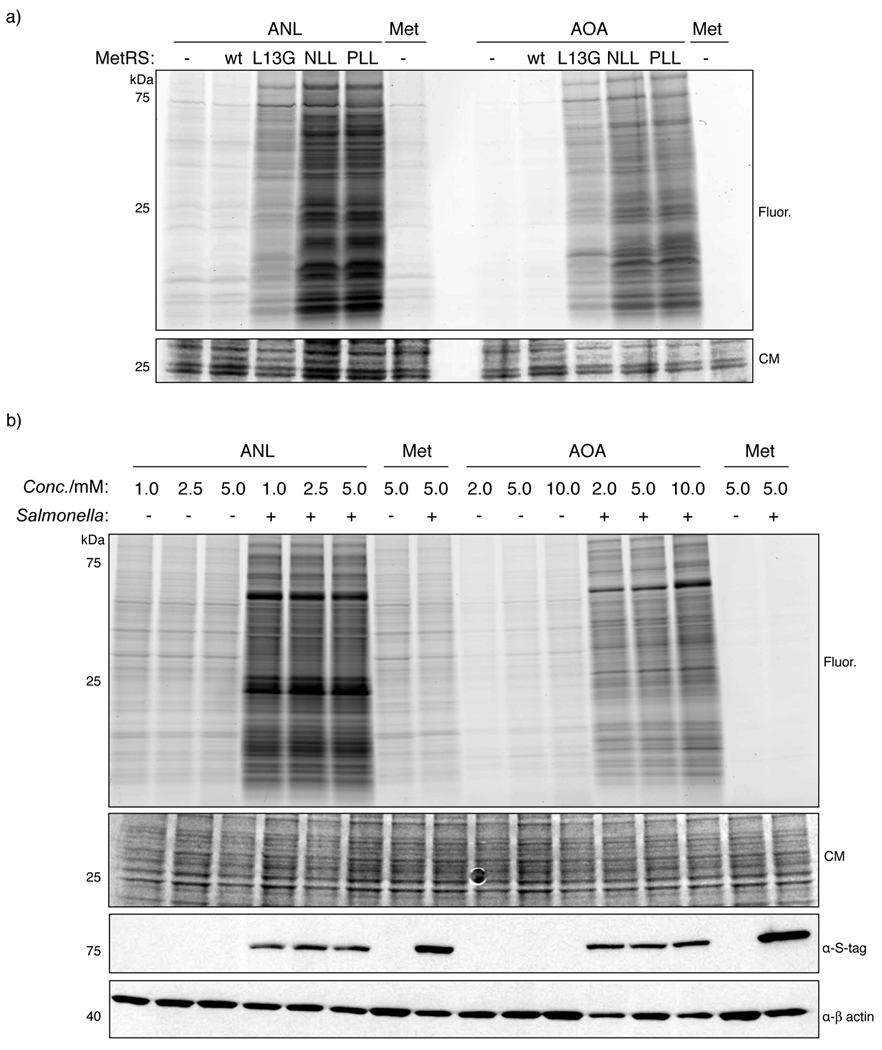
Figure 2.
Comparative analysis of orthogonal amino acid reporters and MetRS mutants in S. typhimurium. a) S. typhimurium expressing different MetRS mutants was labeled with 2 mM AOA or 1 mM ANL in minimal medium and cell lysates were analyzed by CuAAC with az- or alk-Rho and in-gel fluorescence scanning. b) Quantification of fluorescence intensity for (a). c) Infected and non-infected Raw264.7 cells with MetRS-NLL S. typhimurium were labeled with different concentrations of ANL or AOA. Total cell lysates were analyzed by CuAAC with az- or alk-Rho. Western blot analysis was performed for mutant MetRS expression and host protein β-actin levels. d) Quantification fluorescence intensity for (c). (CM - coomassie; F.I. - normalized fluorescence intensity; Fluor. - in-gel fluorescence scanning)
We then evaluated the efficiency of ANL- and AOA-labeling of S. typhimurium proteomes during intracellular infection of mammalian cells. To analyze selective incorporation of orthogonal amino acid reporters in intracellular bacterial pathogens, Raw264.7 murine macrophages were infected with MetRS-NLL S. typhimurium at a multiplicity of infection (MOI) of 100 for 30 minutes. Following infection, all extracellular bacteria were killed by addition of gentamicin, a cell-impermeable antibiotic, to the cell culture medium. After two hours, MetRS-NLL S. typhimurium infected-macrophages were labeled with different concentrations of Met, ANL or AOA in the growth medium for 3 hours. Cell pellets containing bacteria and mammalian cells were lysed with 4% SDS lysis buffer, reacted with alk-Rho (alkyne-rhodamine)[17] or az-Rho and analyzed by in-gel fluorescence. Samples infected with MetRS-NLL S. typhimurium, validated by S-tag Western blot analysis, showed robust labeling with ANL and AOA compared to Met (Figure 2c). Uninfected mammalian cells treated with Met showed no difference in fluorescent labeling compared to mammalian cells infected with S. typhimurium not expressing MetRS-NLL (Figure 2c), emphasizing the necessity for MetRS-NLL expression to allow incorporation of orthogonal amino acid reporters into the bacterial proteome. The comparison of the respective Met controls for AOA- or ANL-treated samples demonstrates the considerably higher non-specific background signal of the alkyne-functionalized detection tag (Figure 2c), as observed earlier in vitro (Figure 2a). Close inspection of AOA-treated uninfected macrophages revealed marginal concentration dependent labeling of mammalian proteomes that was not observed with ANL (Figure 2c). Given the higher non-specific reactivity of alkyne detection reagents (Figure 2a,c), low levels of ANL labeling in mammalian cells are likely undetectable above background. Quantification of relative fluorescence intensities of ANL and AOA suggests that 2 mM of AOA affords the most sensitive labeling of Salmonella proteins during infection (Supporting Figure 3b). These data show that ANL and AOA can be selectively incorporated into S. typhimurium during intracellular infection by bacterial expression of MetRS-NLL. Furthermore, we show that AOA, together with azide detection reagents, displays superior labeling sensitivity in all tested concentrations (Supporting Figure 3b).
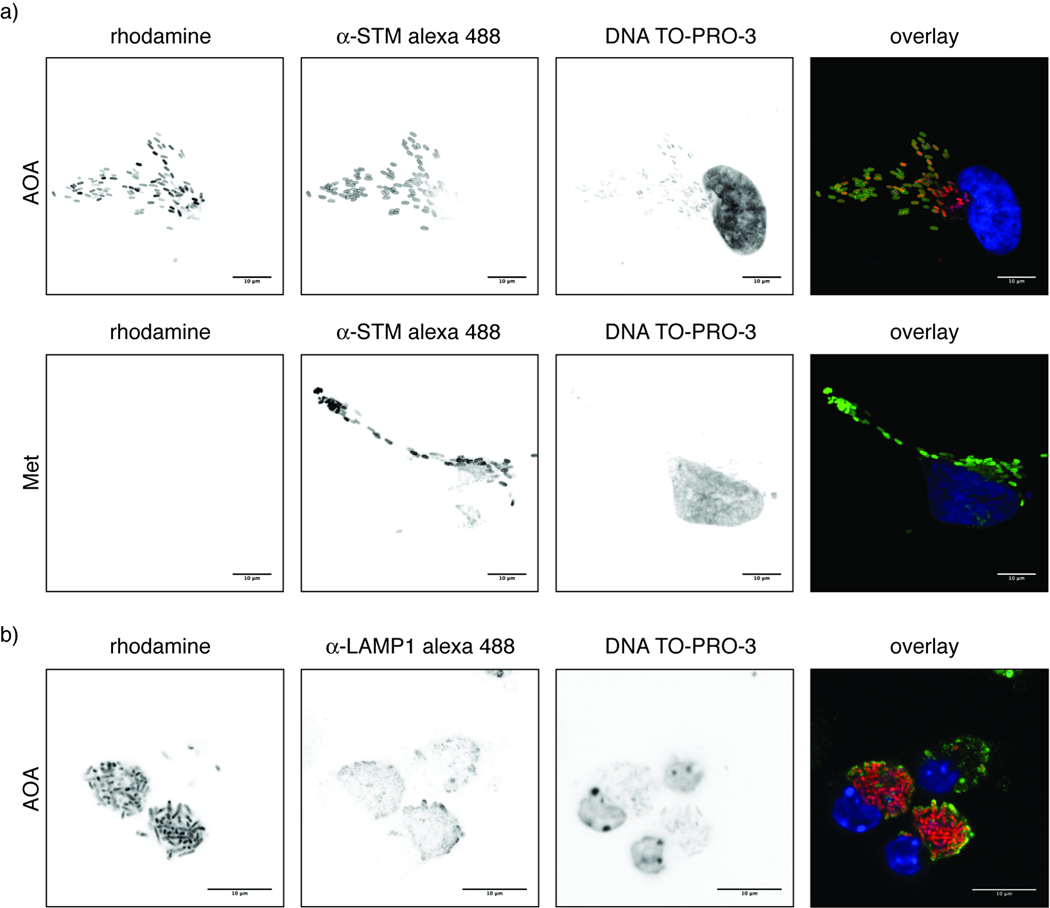
To confirm selective labeling of Salmonella with AOA inside mammalian cells, we performed fluorescence imaging studies of infected cells. HeLa cells were infected with MetRS-NLL S. typhimurium (MOI = 100) for 16 hours, pulse-labeled with AOA or Met for 1 hour, fixed, permeabilized, reacted with az-Rho and stained with anti-Salmonella serum. Imaging of rhodamine fluorescence demonstrated robust and selective labeling of intracellular bacteria with AOA (Figure 3a). No rhodamine fluorescence was observed in host cells or MetRS-NLL S. typhimurium-infected cells treated with Met under these conditions (Figure 3a). AOA-labeled S. typhimurium co-localized with anti-Salmonella serum (Figure 3a), validating selective incorporation of AOA into the bacterial proteome and exclusion from host proteins. In addition, we analyzed S. typhimurium-infected Raw264.7 macrophages along with LAMP-1 antibody staining, a marker for the Salmonella containing vacuole (SCV) of infected host cells[2]. AOA-labeled MetRS-NLL S. typhimurium inside Raw264.7 macrophages were enclosed in LAMP-1 positive compartments (Figure 3b), suggesting that AOA labeling does not significantly disturb intracellular trafficking of Salmonella in host cells. These data further validates the utility of AOA for selective metabolic labeling of Salmonella proteins inside mammalian cells.
Figure 3.
Fluorescence microscopy of AOA-labeled MetRS-NLL S. typhimurium-infected mammalian cells. a) HeLa cells were infected with MetRS-NLL S. typhimurium and were labeled 16 h post infection with 2 mM AOA or 1 mM Met. Fixed cells were stained for S. typhimurium (α-STM alexa 488, green) and DNA (TO-PRO-3, blue) after CuAAC with az-Rho (rhodamine, red). b) Raw264.7 cells were infected with MetRS-NLL S. typhimurium and were labeled 16 h post infection with 2 mM AOA. Fixed cells were stained for LAMP-1 (α-LAMP-1 alexa 488, green) and DNA (TO-PRO-3, blue) after CuAAC with az-Rho (rhodamine, red). (Scale bar represents 10 µm)
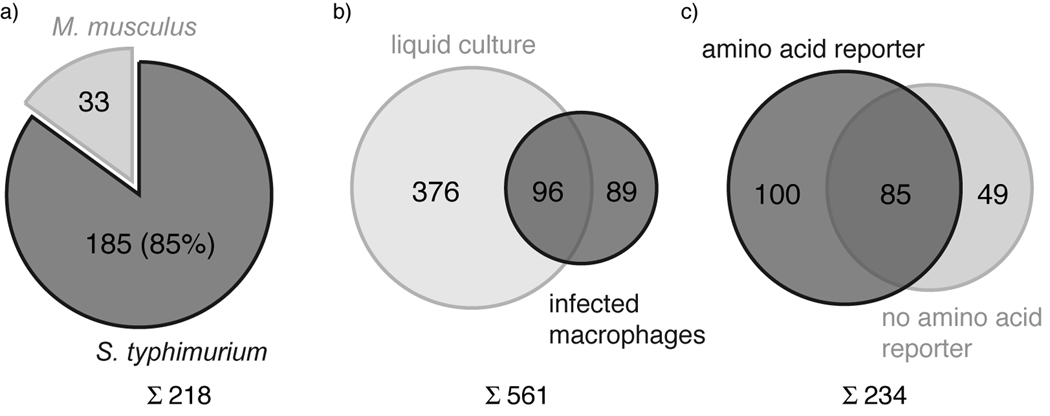
After selectivity and efficiency of AOA labeling was established, we compared Salmonella proteins that are metabolically labeled by AOA during growth in liquid culture to Salmonella proteins that are labeled during infection of macrophages. For this comparative analysis, MetRS-NLL S. typhimurium grown in liquid culture (in vitro) and Raw264.7 macrophages infected with MetRS-NLL S. typhimurium (16 h post infection) were pulse-labeled with AOA or Met for 1 hour. Cell lysates were then reacted with azido-diazo-biotin,[19] affinity purified with streptavidin and eluted from beads for protein identification by gel-based proteomics (Supporting Figure 4). The generated raw data was searched against a concatenated mouse-Salmonella database for protein identification (Supporting Information). Only protein identifications with 2 unique peptides and no detection in the Met control were considered for this study. The analysis of infected Raw264.7 macrophages identified a total of 218 proteins that sufficed our filter criteria (Supporting Table 1). Amongst these proteins, 185 were assigned to the Salmonella proteome (85%), the remainder of which were mouse proteins (Figure 4a). Additionally, we identified 472 proteins from MetRS-NLL S. typhimurium pulse-labeled with AOA in minimal medium (Supporting Table 2). Between the in vitro Salmonella and the intracellular Salmonella dataset we could identify 96 common proteins, while 89 proteins (48%) were exclusively observed in the sample derived from infected macrophages (Figure 4b). Interestingly, at least 5 Salmonella proteins (SodM, SsrB, SseA, PipB2 and PhoP) from infected Raw264.7 cells were previously described as virulence factors.[20] Compared to other proteomic datasets generated from macrophages infected with S. typhimurium.[4] our results demonstrate that AOA-pulse-labeling in conjunction with bioorthogonal ligation methods allows better enrichment (85 % Salmonella proteins with AOA-labeling compared to ~45 % under comparable conditions without AOA)[4] of bacterial proteins and reveals additional bacterial proteins that may be preferentially expressed during infection (Figure 4c). While the use of AOA and MetRS-NLL only targets Met-containing proteins, 96% of the S. typhimurium LT2 proteome contains one or more Met residues in addition to the N-terminal initiator Met residue. Hence, the majority of the Salmonella proteome is amenable to labeling with AOA (Supporting Figure 5). Our preliminary proteomic studies demonstrate that AOA-pulse-labeling in combination with CuAAC allows efficient enrichment and identification of endogenously expressed bacterial proteins that are differentially synthesized by Salmonella during infection of host cells.
Figure 4.
Proteomic analysis of AOA-labeled MetRS-NLL S. typhimurium-infected mammalian cells. a) A total of 218 S. typhimurium proteins were identified from infected Raw264.7 cells, of which 185 (85%) were Salmonella proteins and 33 were mouse proteins. b) Comparison of Salmonella proteins identified from infected macrophages and Salmonella grown in minimal liquid culture. 376 proteins were identified in liquid culture only, 89 proteins were identified in infected macrophages only and 96 were identified in both samples. c) Comparison of proteins identified from AOA-pulse-labeled infected Raw264.7 cells (amino acid reporter) and a previously published dataset of Salmonella proteins identified from infected Raw246.7 cells under comparable conditions without AOA labeling (no amino acid reporter).[4] 100 proteins were exclusively identified in our data set, 85 proteins were identified in both datasets and 49 proteins were only identified in the previously published dataset.[4]
In summary, we are presenting a new and efficient orthogonal amino acid reporter for selective labeling of bacterial proteomes during infection. The combination of CuAAC with AOA-pulse-labeling allows imaging of bacteria within mammalian cells as well as enrichment and proteomic analysis of endogenously expressed Salmonella proteins from infected mammalian cells. The application of orthogonal amino acid reporters should facilitate new and exciting opportunities for imaging and proteomic investigation of different bacterial pathogens during infection in the future.
Supplementary Material
Acknowledgments
We thank The Rockefeller University Proteomics Resource Center for mass spectrometry analysis, James Flexner for help with the ANL synthesis and members of the Hang Laboratory for providing secondary detection reagents. M.M.Z. is supported by A*STAR, Singapore. H.C.H. acknowledges support from The Rockefeller University, Irma T. Hirschl/Monique Weill-Caulier Trust, Lerner Family and Northeastern Biodefense Center NIH/NIAID (2 U54 AI057158-06).
References
- 1.Bhavsar AP, Guttman JA, Finlay BB. Nature. 2007;449:827. doi: 10.1038/nature06247. [DOI] [PubMed] [Google Scholar]
- 2.Haraga A, Ohlson MB, Miller SI. Nat. Rev. Microbiol. 2008;6:53. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 3.Rodland KD, Adkins JN, Ansong C, Chowdhury S, Manes NP, Shi L, Yoon H, Smith RD, Heffron F. Future Microbiol. 2008;3:625. doi: 10.2217/17460913.3.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi L, Adkins JN, Coleman JR, Schepmoes AA, Dohnkova A, Mottaz HM, Norbeck AD, Purvine SO, Manes NP, Smallwood HS, Wang H, Forbes J, Gros P, Uzzau S, Rodland KD, Heffron F, Smith RD, Squier TC. J. Biol. Chem. 2006;281:29131. doi: 10.1074/jbc.M604640200. [DOI] [PubMed] [Google Scholar]
- 5.Becker D, Selbach M, Rollenhagen C, Ballmaier M, Meyer TF, Mann M, Bumann D. Nature. 2006;440:303. doi: 10.1038/nature04616. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Robbins S, Guo J, Shen W, Schultz PG. PLoS ONE. 2010;5:e9354. doi: 10.1371/journal.pone.0009354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beatty KE, Liu JC, Xie F, Dieterich DC, Schuman EM, Wang Q, Tirrell DA. Angew. Chem. Int. Ed. Engl. 2006;45:7364. doi: 10.1002/anie.200602114. [DOI] [PubMed] [Google Scholar]
- 8.Kiick KL, Saxon E, Tirrell DA, Bertozzi CR. Proc. Natl. Acad. Sci. USA. 2002;99:19. doi: 10.1073/pnas.012583299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meldal M, Tornøe CW. Chem. Rev. 2008;108:2952. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 10.Dieterich DC, Link AJ, Graumann J, Tirrell DA, Schuman EM. Proc. Natl. Acad. Sci. U S A. 2006;103:9482. doi: 10.1073/pnas.0601637103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanrikulu IC, Schmitt E, Mechulam Y, Goddard WA, Tirrell DA. Proc. Natl. Acad. Sci. USA. 2009 doi: 10.1073/pnas.0905735106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Link AJ, Vink MK, Agard NJ, Prescher JA, Bertozzi CR, Tirrell DA. Proc Natl Acad Sci USA. 2006;103:10180. doi: 10.1073/pnas.0601167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngo JT, Champion JA, Mahdavi A, Tanrikulu IC, Beatty KE, Connor RE, Yoo TH, Dieterich DC, Schuman EM, Tirrell DA. Nat. Chem. Biol. 2009;5:715. doi: 10.1038/nchembio.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speers AE, Cravatt BF. Chem. Biol. 2004;11:535. doi: 10.1016/j.chembiol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Charron G, Zhang MM, Yount JS, Wilson J, Raghavan AS, Shamir E, Hang HC. J. Am. Chem. Soc. 2009;131:4967. doi: 10.1021/ja810122f. [DOI] [PubMed] [Google Scholar]
- 16.Beatty KE, Tirrell DA. Bioorg. Med. Chem. Lett. 2008 doi: 10.1016/j.bmcl.2008.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charron G, Zhang MM, Yount JS, Wilson J, Raghavan AS, Shamir E, Hang HC. J. Am. Chem. Soc. 2009 doi: 10.1021/ja810122f. [DOI] [PubMed] [Google Scholar]
- 18.Chenault HK, Dahmer J, Whitesides GM. J. Am. Chem. Soc. 1989;111:6354. [Google Scholar]
- 19.Yang YY, Ascano JM, Hang HC. J. Am. Chem. Soc. 2010;132:3640. doi: 10.1021/ja908871t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Chen L, Sun L, Yu J, Jin Q. Nucleic Acids Res. 2008;36:D539. doi: 10.1093/nar/gkm951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.