Abstract
Chitosan nanoparticles are good drug carriers because of their good biocompatibility and biodegradability, and can be readily modified. As a new drug delivery system, they have attracted increasing attention for their wide applications in, for example, loading protein drugs, gene drugs, and anticancer chemical drugs, and via various routes of administration including oral, nasal, intravenous, and ocular. This paper reviews published research on chitosan nanoparticles, including its preparation methods, characteristics, modification, in vivo metabolic processes, and applications.
Keywords: chitosan nanoparticle, drug delivery system, preparation, modification, application
Introduction
Many drugs have problems of poor stability, water insolubility, low selectivity, high toxicity, side effects, and so on. Good drug carriers play a significant role in resolving these problems. Chitosan nanoparticles are a drug carrier with wide development potential and have the advantage of slow/controlled drug release, which improves drug solubility and stability, enhances efficacy, and reduces toxicity. Because of their small size, they are capable of passing through biological barriers in vivo (such as the blood–brain barrier) and delivering drugs to the lesion site to enhance efficacy.1 Modified nanoparticles also have other properties such as improved drug targeting. Under the action of enzymes in vivo, biodegradable nanoparticles can produce water and carbon dioxide without adverse effects, and have thus become the focus of increasing research.
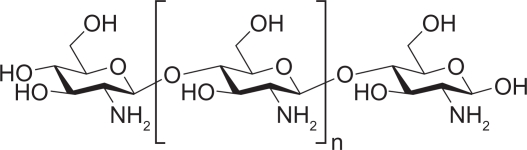
As a copolymer containing β-(1,4)-2-acetamido-D-glucose and β-(1,4)-2-amino-D-glucose unit (Figure 1), chitosan is produced by removing an acetate moiety from chitin through hydration in concentrated alkali. Chitosan is soluble in most diluted acids. Under acidic conditions, chitosan can be dissolved in water after amino protonation to confer positive charges, gelation, and membrane-forming properties. The glycosidic bond of chitosan is hemiacetal, and thus is not stable in acid and will be hydrolyzed under acidic conditions, resulting in decreased viscosity and molecular weight of chitosan. Physical and chemical properties of chitosan depend mainly on its molecular weight and degree of deacetylation.2 As a natural product, chitosan is a renewable pharmaceutic adjuvant with good biocompatibility. Chitosan and its derivatives have strong potential for application as drug carriers.
Figure 1.
Structure of chitosan.
Basic characteristics of chitosan
Adhesivity of chitosan
The amino and carboxyl groups in the chitosan molecule can be combined with glycoprotein in mucus to form a hydrogen bond, leading to an adhesive effect. As mucoprotein in mucus is positively charged, chitosan and mucus are attracted to each other to prolong the retention time of drugs and continuous drug release in vivo as well as improve drug bioavailability.2 Dudhani and Kosaraju,3 who prepared chitosan nanoparticles encapsulating catechin, found that encapsulated catechin had a release percentage of 60% under the action of enzyme. Release testing in vitro showed that 32% of catechin still existed after 24 hours. These results indicated that adhesivity of chitosan enhanced controlled release of catechin and improved its bioavailability.3 Adhesivity of chitosan is strengthened under neutral and acidic conditions. The greater the molecular weight and higher the degree of deacetylation of chitosan, the stronger will be its adhesivity and the amount of adhesion. Some chitosan groups (such as sulfydryl) can be easily modified to strengthen its adhesivity.2
Biodegradability and safety of chitosan
The biodegradability of chitosan is important for its use in drug delivery systems. Chitosan of suitable molecular weight can be cleared by the kidney in vivo, while that of excessive molecular weight can be degraded into fragments suitable for renal clearance.4
Chitosan is degraded mainly by chemical process and enzyme catalysis; the latter is the major in vivo process.4 The higher the degree of deacetylation, the greater the degradation rate. Degradation by enzyme catalysis also depends on the availability of chitosan’s amino group.4 At present, it is generally believed that chitosan is a nontoxic polymer as well as safe drug delivery material. It has been certified as a wound dressings by the US Food and Drug Administration.5 Compared with toxic compounds such as sulfide (LD50 < 20 μg/mL against MCF7 and COS7 cells), chitosan has no obvious toxicity. However, with increasing charge density, toxicity of chitosan will strengthen accordingly.4 Intravenous injection of excessive chitosan may cause death due to blood coagulation.4
Antitumor effect of chitosan
Chitosan can act on tumor cells directly to interfere with cell metabolism, inhibit cell growth, or induce cell apoptosis. It also has an antitumor role through improving the body’s immune function.6 Maeda and Kimura7 showed that low-molecular-weight chitosan and chito-oligosaccharide could inhibit tumor growth in S180-bearing mice. Torzsas et al8 found that a diet containing chitosan could reduce the generation of precancerous lesions in colon cancer induced by azomethane compounds. In vitro antitumor testing of chitosan nanoparticles indicated that inhibition rate of 500 mg/L chitosan nanoparticles was 27% on Hela cells of cervical cancer, 23% on liver SMMC-7721 cells, 29% on gastric cancer BGC-823 cells, and as high as 55% on breast cancer MCF-7 cells.9 These studies suggested that chitosan had antitumor effects in vitro and in vivo, leading to good prospects for their application as a supplementary antitumor drug and drug carrier. Studies have also indicated significant differences in antitumor activity of nanoparticles prepared by chitosan from different producers, and chitosan nanoparticles also had a selectivity for tumor cells.10
Preparation techniques of chitosan nanoparticles
Ionic cross-linking
As a widely used method for preparing chitosan nanoparticles, ionic cross-linking is generated by auto-aggregation between chitosan or its derivatives and macromolecules of opposite charge, or when ionic cross-linking agent exists. The most commonly used cross-linking agent is sodium tripolyphosphate. When it is added continuously to a water solution of chitosan with constant stirring under a moderate temperature, two components with opposite charges will combine to form nanoparticles.11 In the chitosan–Arabic gelatin nanoparticles of insulin prepared by Avadi et al12 minimum particle size and maximum combining rate with insulin were reached at a chitosan concentration of 10 mg/mL, Arabic gelatin concentration of 5 mg/mL, and insulin concentration of 5 mg/mL.12 As ionic cross-linking can be performed at room temperature to avoid the use of organic solvent, and uniform nanoparticles with an adjustable size can be obtained easily, cross-linking has been widely used in encapsulation of protein and gene drugs. Using ionic cross-linking, chitosan nanoparticles were prepared by Du et al13 and then loaded with Ag+, Cu2+, Zn2+, Mn2+, and Fe2+. The zeta potential of chitosan nanoparticles is intensified significantly by positive charge of ions thus improving the stability of nanoparticles and greatly enhancing the antibacterial potential of nanoparticles.13
Covalent cross-linking
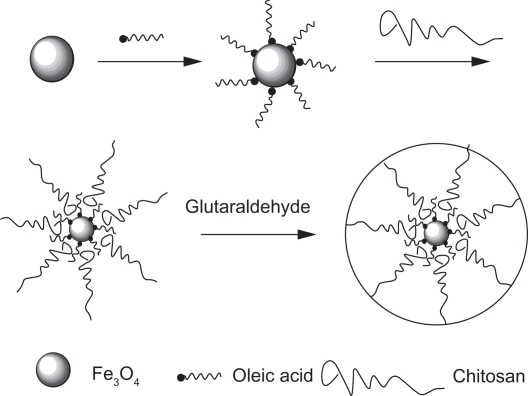
Chitosan and its derivatives can be prepared as nanoparticle drug carriers by using covalent cross-linking,14 which involves mainly the formation of covalent bonds between the chitosan chain and a functional cross-linking agent. This method was first used to prepare chitosan nanoparticles by encapsulating 5-fluorouracil by cross-linking glutaraldehyde with amino groups in the molecular chain of chitosan.15 Commonly applied agents also include polyethylene glycol (PEG) dicarboxylic acid, glutaraldehyde, or monofunctional agents such as epichlorohydrin.16,17 Fe3O4–chitosan nanoparticles were prepared by Qu et al18 by covalent cross-linking (Figure 2). Oleic acid-coated Fe3O4 nanoparticles are absorbed by chitosan and cross-linked with glutaraldehyde, resulting in Fe3O4–chitosan nanoparticles of average size of 10.5 nm and a narrow size distribution. These nanoparticles have been shown to have a highly saturated magnetization effect, superparamagnetic properties, and a sufficiently high temperature to induce hyperthermia.18
Figure 2.
A schematic representation of Fe3O4–chitosan nanoparticles.18
Precipitation
There are two kinds of approaches for nanoparticle precipitation. One is desolvation, in which flocculant (commonly sodium sulfate) is added to a water solution of chitosan and solubility of chitosan is decreased by the combination of water and sodium sulfate, leading to the precipitation of nanoparticles due to hydrogen bonding between molecules. This method was first applied by Berthold et al19 to prepare chitosan microspheres. Technical improvements then enabled Tian and Groves20 to prepare 600- to 800-nm chitosan nanoparticles. The other type is based on diffusion of emulsified solvent. Under the action of emulsified solvent, the water phase containing chitosan is dispersed in the organic phase encapsulating the drug, where turbulence appears between the interfaces of the two phases and chitosan is precipitated, resulting in the generation of nanoparticles.21 In this method, organic solvent is used and the large nanoparticles obtained restricting their application.
Polymerization
Radical polymerization was initially developed by Chauvierre et al.22–24 It was later used to prepare chitosan and thiolated chitosan-poly (isobutyl cyanoacrylate) core-shell nanoparticles by Bravo-Osuna et al.25 Diluted nitric acid solution of chitosan was stirred continuously together with a mixed solution of ceric ammonium nitrate, nitric acid, and isobutyl cyanoacrylate at 40°C for 40 minutes in an argon environment. After cooling to room temperature, sodium hydroxide was added to adjust pH to 4.5 and a nanoparticle suspension was then obtained.25 Radical polymerization, by combining different monomers, can regulate both nanoparticle shell and core properties. de Moura et al26 prepared chitosan–methyl acrylic acid (CS–PMMA) nanoparticles by polymerization through ionic interaction between COO− produced by carboxyl dissociation from methyl acrylic acid and the amino group of chitosan. It was shown in a transmission electron microscope micrograph that these nanoparticles were uniformly spherical and quite consistent in size distribution.
Self-assembly
Amphiphilic compounds dispersed in water can form nanoparticles with a core-shell structure by self-assembly. With a hydrophobic core and a hydrophilic shell, amphiphilic nanoparticles can be used as carriers for hydrophobic and hydrophilic drugs simultaneously. The hydrophilic shell greatly reduces macrophage phagocytosis. Therefore, amphiphilic nanoparticles have attracted increasing attention. Kim et al27 used hydrophobic cholanic acid to modify glycol chitosan and then form nanoparticles by self-assembly in water solution. The antitumor drug cisplatin was encapsulated into the hydrophobic core with a drug loading of 80%.28 The amphiphilic derivatives of N-octyl-O, N-carboxymethyl chitosan (OCC) prepared by Huo et al28 enhanced the solubility of the insoluble antitumor drug paclitaxel. Solubility of paclitaxel was improved nearly 500-fold with a drug loading of 34.6% and encapsulation rate of 89.9%, indicating that OCC is a potentially excellent solubilization carrier for insoluble drugs.
Chitosan–drug complexes
Because of the cationic properties of chitosan, ion complexes can be formed by electrostatic interaction between chitosan and anionic drugs. Cheng et al29 prepared chitosan nanoparticles linked with antisense oligonucleotide (asON). By the mixing method, the nanoparticles could be made easily under the optimal condition of 2:1 M proportion of chitosan phosphate and asON. The size of the nanoparticles was 102.6 ± 12.0 nm, their zeta potential was 1.45 ± 1.75, and the encapsulated ratio of chitosan cross-linked asON was 87.6 ± 3.5%. Kim et al30 encapsulated retinol into chitosan nanoparticles through the electrostatic interaction between the amine group of chitosan and the hydroxyl group of retinol. Encapsulation into chitosan nanoparticles was shown to increase solubility of retinol by more than 1600-fold.
Spray-drying
Spray-drying method can be used as a one-step preparation of nanoparticle powder. Grenha et al31 prepared mannitol microspheres containing chisosan nanoparticles-loaded protein by this method. Huang et al32 prepared chitosan–iron oxide nanoparticles with various chitosan:iron oxide ratios by spray-drying. Atomic absorption spectrometry results implied that chitosan had strong chelation with iron. Meanwhile, Fe3O4 was crystallized and distributed in the chitosan matrix. These chitosan–iron oxide nanoparticles were stable in water with strong superparamagnetism.
Characteristics of chitosan nanoparticles
General characteristics
Nanoparticles are solid colloid particles of 1 to 1000 nm. Compared with micron-grade particles, nanoparticles have strong mobility because of their small size and can enter cells easily to accumulate at the lesion site. Therefore, cell uptake rate is high.33
Characteristics of sustained/controlled release
Drugs carried by chitosan nanoparticles can be released through degradation and corrosion of chitosan, leading to a clear sustained-release effect. Because of varied degradation rate and time of chitosan of different molecular weight and degree of deacetylation degree, different types of nanoparticles can be used to regulate drug-release rate. Meanwhile, chitosan can also be modified to achieve the sustained/controlled release. Chitosan–alumino-silicate nanoparticles prepared by Yuan et al34 had significant sustained/controlled-release effects. pH of the environment and chitosan:alumino-silicate ratio also influence drug release.
Targeting of chitosan nanoparticles
Positive charges of chitosan have selective adsorption and neutralizing effects on the tumor cell surface. As a drug carrier, it has a targeting function to liver, spleen, lung, and colon.35 Ethylene chitosan nanoparticles had a prolonged circulating time in the blood with a high tumor-cell selectivity.36 Moreover, this kind of effect was more obvious in ethylene chitosan nanoparticles of large molecular weight. Doxorubicin–chitosan polymeric micelles had excellent drug-loading properties, were suitable for targeting the liver and spleen, and significantly reduced drug toxicity to the heart and kidney.37
Modification of chitosan nanoparticles
In order to improve targeting and bioavailability of chitosan nanoparticles, an increasing number of studies are focusing on modification of chitosan. Modified chitosan nanoparticles are characterized by pH sensitivity, thermosensitivity, and targeting accuracy.
Modification of pH sensitivity
A pH-sensitive nanocarrier is a drug delivery system that increases drug release by changing carrier properties under a certain acid-base environment in vivo, and targets the lesion tissue. Poly(propyl acrylic acid) (PPAA) is a polymer that is highly sensitive to pH. At a pH lower than 6.0, its high membrane fragmentation ability was shown to cause rupture of endosomal membrane and release vesicular materials into cytochylema.38 Therefore, Kiang et al39 added PPAA to chitosan–DNA complex to improve gene transfection efficiency. The results showed that adding PPAA to chitosan–DNA complexes enhanced gene expression in both HEK293 and HeLa cells compared with chitosan nanoparticles alone. Camptothecine-loaded N-isopropylacrylamide–chitosan nanoparticles were sensitive to pH: the nanoparticles were most sensitivite to tumor pH when the charge ratio of N-isopropylacrylamide:chitosan was 4:1.40 At a pH of 6.8, cumulative release rate of camptothecine was highest and cell toxicity was significantly stronger. Cell toxicity was minimum at a pH of 7.4. Thus, N-isopropylacrylamide–chitosan nanoparticles have a good potential for use as a targeted anticancer drug carrier.40 Chitosan nanoparticles prepared with sodium tripolyphosphate and glycidoxypropyltrimethoxysilane cross-linking were pH sensitive.41 With antihuman IgG antibody as a model protein drug, the release of antibody was increased from 5.6% to 50% when solution pH was adjusted from 7.4 to 6.0. Therefore, chitosan nanoparticles prepared by two-step cross-linking are a potential drug carrier sensitive to pH.41
Modification of thermosensitivity
Drug release is regulated by structural change of thermosensitive drug carriers at different temperatures. Poly(N-isopropylacrylamide) is a well-known thermosensitive polymer widely used in drug carriers.42,43 Chitosan–polyvinylcaprolactan graft copolymer nanoparticles were sensitive to temperature, with a critical solution temperature at 38°C.44 With 5-fluorouracil as a model drug, drug release mainly occurred above 38°C with high toxicity to tumor cells but low toxicity to normal cells.
Modification of targeting
Active targeting can be obtained in chitosan nanoparticles through chemical modification, so as to make the drug identify the target accurately. With resveratrol as a model drug, Yao45 prepared chitosan nanoparticles using ligands of both avidin and biotin to modify the nanoparticles. The resulting delivery system passively targeted the liver and positively targeted hepatoma cells. Two kinds of targeting mechanisms were thus combined in the new drug delivery system to achieve targeting to specific cells in specific tissues, further improving therapeutic effects and reducing toxic and side effects.45 Chitosan nanoparticles modified by glycyrrhizic acid, strengthened the active liver-targeting delivery of drug-loaded carriers through the mediation of glycyrrhizic acid because there were binding sites of glycyrrhizic acid on the surface of liver parenchyma.46 Kim et al27 used hydrophobic cholanic acid to modify glycol chitosan and prepare nanoparticles through self-assembly. The antitumor drug cisplatin could be encapsulated easily in a hydrophobic core of nanoparticles. It was proven that due to prolonged circulating time in vivo and strengthened cell permeability and drug effect, drug-loaded nanoparticles were concentrated in tumor tissues of mice successfully with better antitumor effect and lower toxicity.27 Min et al47 used chemical binding to bind hydrophobic 5β-cholanic acid with the skeleton of hydrophilic glycol chitosan (HGC) to prepare nanoparticles. Using a dialysis method, camptothecine could be encapsulated into the nanoparticles easily, with drug-loading of more than 80%. Camptothecine (CPT) was effectively protected from hydrolyzation by the hydrophobic core of HGC nanoparticles under a physiological environment, producing CPT-HGC nanoparticles with a significant antitumor effect and high targeting to MDA-MB231 human breast cancer cells.47 Hydrophobic glycol chitosan nanoparticles were shown to be excellent carriers for insoluble antitumor drugs.
In vivo metabolic process of chitosan nanoparticles
Nanoparticles are recognized as foreign matter in vivo and are absorbed by antibodies generated in the human body. Plasma protein, lipoprotein, immune protein, and complement C protein in plasma are also adsorbed on nanoparticles, accelerating the reorganization of the reticuloendothelial system. Nanoparticles are engulfed by macrophage and cleared from the body’s circulation.48 The bridge between nanoparticles and macrophage is formed because of plasma protein adsorbed on the nanoparticle surface. The ability of nanoparticles to adsorb plasma protein is determined by surface charge of nanoparticles, thereby influencing the transfer intensity of nanoparticles by macrophage.49 Nanoparticles with polarity and high surface potential as well as amphipathic or hydrophilic nanoparticles are engulfed less and have a longer circulating time in vivo. Nam et al50 found that glycol–chitosan nanoparticles after hydrophobic modification are more distributed in all cells compared with unmodified nanoparticles.
Drug-loaded chitosan nanoparticles are decomposed into free chitosan and drug in vivo. Drugs enter cell and targeted tissues to generate therapeutic effects. Chitosan is mainly degraded under catalysis of lysozyme and bacterial enzyme in the colon. The chitosan absorbed into blood is cleared by the kidney and the rest is discharged through excrement. Degree of deacetylation as well as molecular weight also influence degradation rate and degree of chitosan in vivo.51,52
Application of chitosan nanoparticles
Studies have shown that chitosan nanoparticles can carry many drugs including gene drugs, protein drugs, anticancer chemical drugs, and antibiotics, and via various routes of administration including oral, nasal, intravenous, and ocular.
Carrier for various drugs
Carrier of gene drugs
As a gene carrier, conventional virus has the disadvantages of low transfection rate and cell toxicity,53 and even causes serious immune response. As a nonvirus carrier, chitosan has excellent biocompatibility and biodegradation, which has led to increasing application of chitosan nanoparticles in gene drug delivery.54,55 Gene silencing mediated by double-stranded small interfering RNA (siRNA) has been widely investigated as a potential therapeutic approach for diseases caused by genetic defects.54 However, its application is restricted by rapid degradation and poor cell absorption. Drug loading of chitosan nanoparticles prepared by ionic gelation by Katas and Alpar54 reached 100%, protecting well siRNA from nuclease degradation. With natural positive ion chitosan as a carrier material and using electrostatic interaction of polyelectrolyte, siRNA of silencing green fluorescent protein was compounded directly by Liu55 to prepare stable siRNA nanoparticles with a complex rate of 83% to 94%. It was also found that more stable nanoparticles with positive surface charges could be generated by siRNA and chitosan with high molecular weight and degree of deacetylation. The product was not only easily adsorbed onto the cell surface to increase chance of cellular endocytosis, but also could protect siRNA activity effectively during the transfection in cells to improve gene silencing efficiency. However, gene transfection efficiency of chitosan nanoparticles is low generally and also influenced by molecular weight, degree of deacetylation, the chitosan:DNA ratio, environmental pH, and nanoparticle preparation method.56 Improving transfection efficiency is a challenge for using chitosan nanoparticles as a gene carrier. Transfection efficiency of chitosan with different degrees of deacetylation and molecular weights was studied by Lavertu et al,57 who found that maximum transgene expression occurred when the ratio of the degree of deacetylation (DDA) to the molecular weight (MW) moves from high DDA/low MW to low DDA/high MW. Moreover, several chitosan–pDNA (plasmid DNA) complex formulations achieved levels of transgene expression approaching those of the positive controls (Lipofectamine™, Life Technologies, Carlsbad, CA; and FuGENE® 6, Roche Diagnostics, Basel, Switzerland), while two optimal conditions (92-10-5:1 and 80-10-10:1 [DDA-MW-N:P ratio] both at pH 6.5) were particularly effective, showing equivalent transfection efficiency compared with the best positive control.57 This provided a good example for the application of chitosan nanoparticles in gene transfection. Moreover, chitosan could also be modified by folic acid to improve transfection efficiency. Folic acid could be easily absorbed by cells, promoting the targeting and internalization of drug. Mansouri et al58 used folic acid to modify chitosan for improving gene transfection efficiency. They studied systematically the characteristics of folic acid for gene treatment, finding that folic acid-modified chitosan nanoparticles had low cell toxicity and could condense DNA effectively with ideal size and zeta potential. The results showed that folic acid-modified chitosan nanoparticles were a nonvirus gene carrier with a good application potential.
Carrier of protein drugs
Protein drugs can be degraded easily by enzymes in vivo and have poor permeability and stability as well as a short half-life. However, chitosan can protect protein well and promote the contact between drug and biomembrane, thereby improving bioavailability.11 Gan and Wang59 showed that changing the size and surface charge of chitosan–bovine serum albumin nanoparticles could regulate the encapsulation efficiency and release kinetics of bovine serum albumin, but it was difficult to control the burst release of protein of high molecular weight. Zhang et al60 used insulin and cationic β-cyclodextrin to form a complex encapsulated into alginate–chitosan nanoparticles. Binding rate and drug-loading amount were 87% and 9.5%, respectively, and cumulative release of insulin in simulated intestinal fluid reached 40%. Insulin was protected well in the nanoparticle core, avoiding the degradation in simulated gastric fluid, as well as the structure of insulin during release. Glycol chitosan nanoparticles modified by 5β-cholanic acid (HGC) and RGD (Arg-Gly-Asp) polypeptide were easily encapsulated into nanoparticles with a drug-loading amount greater than 85%.61 RGD–HGC nanoparticles showed a one-week sustained-release effect. RGD–HGC displayed antiangiogenic efficacy by inhibiting human umbilical venous cord endothelial cell adhesion to a βig-h3 protein-coated surface, markedly suppressing bFGF-induced angiogenesis as well as decreasing hemoglobin content in Matrigel plugs in vivo. Therefore, RGD-HGC nanoparticles can inhibit tumor cell growth and reduce microvessel density significantly.61
Carrier of anticancer chemical drugs
Chitosan itself has a certain antitumor activity and its positive charge can neutralize the negative charge on the tumor cell surface, resulting in selective absorption. Thus, chitosan nanoparticles can increase drug concentration in the tumor site and improve therapeutic effects. Doxorubicin/methoxy PEG grafted carboxymethyl chitosan nanoparticles with higher cell toxicity could enter cell and inhibit tumor-cell proliferation effectively.62 Paclitaxel chitosan nanoparticles had a high encapsulation rate of 94.0% ± 16.73% with sustained-release effect.63 Cell toxicity testing showed that paclitaxel–chitosan nanoparticles had a higher toxicity than that of paclitaxel alone, and with a higher cell uptake rate. Using an evaporation method of composite microemulsion solvent, Trickler et al64 combined glyceryl monooleate (GMO) and chitosan to prepare nanoparticles with a hydrophobic core and a hydrophilic shell. When encapsulated in the nanoparticle, paclitaxel had clear sustained-release characteristics; cell uptake increased four-fold and median lethal dose (IC50) of paclitaxel decreased 1000-fold, leading to a maximum reduction of the side effects of paclitaxel. Kim et al65 linked 5β-cholanic acid to the main chain of glycol chitosan for the preparation of amphiphilic HGC nanoparticles. Encapsulated into nanoparticles by the dialysis method, paclitaxel had a drug-loading amount greater than 80%. With significant sustained-release effect, paclitaxel–HGC nanoparticles had low toxicity to B16F10 melanoma cells but a clear anticancer cell effect.
Carrier of other drugs
Chitosan nanoparticles also can load other drugs including antivirus drugs, antiallergic drugs, and hormone drug. Hao and Deng66 prepared acyclovir-loaded chitosan nanoparticles with a drug loading of 17.8% and an encapsulation rate of 87.5% by an ionic cross-linking method. Li and Luan67 prepared tranilast-loaded chitosan nanoparticles for allergic diseases with a particle size of 285.5 nm and an encapsulation rate of 82.4%.
Routes of administration
Routes of administration of chitosan nanoparticles have been developed. Oral nanoparticles can protect drugs from degradation in the gastrointestinal tract and improve drug absorption.68 Yin et al69 developed a promising vehicle for oral delivery. Trimethyl chitosan–cysteine conjugate (TMC–Cys) was synthesized in an attempt to combine the mucoadhesion- and the permeation-enhancing effects of TMC and thiolated polymers related to different mechanisms for oral absorption. The TMC–Cys nanoparticles, obtained via self-assembly, possessed spherical morphology, uniform size, positive zeta potentials, and high insulin encapsulation efficiency. Mucoadhesion and permeation enhancing effects of TMC–Cys nanoparticles were significantly higher than those of TMC nanoparticles. Biocompatibility assessment revealed lack of toxicity of TMC–Cys nanoparticles.69 Because of poor stability and intestinal absorption of catechins, Dube et al70 encapsulated (+)-catechin (C) and (−)-epigallocatechin gallate (EGCg) in chitosan nanoparticles. The encapsulation significantly enhanced intestinal absorption and the cumulative amounts transported after encapsulation were significantly higher.70 An insulin-loaded, pH-sensitive chitosan nanoparticle was formulated by ionic cross-linking with hydroxypropyl methylcellulose phthalate (HPMCP) as a pH-sensitive polymer.71 In vitro results revealed a superior acid stability of CS–HPMCP nanoparticles with a significant control over insulin release and degradation in simulated acidic conditions with or without pepsin. Moreover, fluorescently labeled CS–HPMCP nanoparticles showed a 2- to 4-fold improvement in the intestinal mucoadhesion and penetration compared with CS–TPP nanoparticles.71 Amidi et al72 investigated the potential of N-trimethyl chitosan (TMC) nanoparticles as a carrier system for the nasal delivery of proteins. TMC nanoparticles have an excellent loading capacity for proteins, and a positive surface charge, suitable for attaching to nasal mucosa. In vivo experiments showed that TMC nanoparticles loaded with fluorescein isothiocyanate–albumin, when administered in the nasal cavity, were able to cross the mucosal layer, be taken up by rat nasal epithelia and NALT cells, and transported to submucosal layers. TMC nanoparticles are a potential new delivery system for protein transport through the nasal mucosa.72 Wang et al,73 who prepared estradiol-loaded chitosan nanoparticles and investigated the levels of estradiol in blood and cerebrospinal fluid in rats after intranasal administration, showed that estradiol levels in the cerebrospinal fluid after intranasal administration were significantly higher than after intravenous administration. The drug targeting index (DTI) of the nasal route was 3.2 and drug targeting percent (DTP%) was 68.4%.73 The combination of bioadhesion and paracellular transport effects has led to chitosan to be considered for the delivery of estradiol via the nasal cavity. Huo et al,74 who used N-octyl-O-glycol chitosan (OGC) as the carrier of paclitaxel for intravenous administration, found that OGC for intravenous administration had good biocompatibility and no toxicity. Moreover, paclitaxel-loaded OGC micelles had low toxicity and a higher tolerated dose. In vivo studies of chitosan-fluorescent nanoparticles (CS-fl) prepared for ocular administration showed that the amounts of CS-fl in cornea and conjunctiva were significantly higher for CS-fl nanoparticles than for a control CS-fl solution, these amounts being fairly constant for up to 24 hours.75
Future prospects
As a drug delivery system, chitosan nanoparticles have attracted increasing attention because of their good bio-compatibility, degradability, and nontoxicity. Absorption and bioavailability of drug encapsulated into chitosan nanoparticles can be improved, so they can be used to deliver protein drugs, gene drugs, and other drugs and can protect them effectively from enzyme degradation in vivo. Chitosan nanoparticles are now being modified for sustained/controlled release and targeting. As the active antitumor components of plant drugs are being constantly discovered and developed, developing targeted chitosan carriers for sustained/controlled release plant drugs is also an area of future development. While great progress has been achieved in the application of chitosan nanoparticles as drug carriers, some problems remain to be resolved urgently. For example, chitosan has poor solubility and unmodified chitosan nanoparticles can encapsulate only some hydrophilic drugs. Although chitosan can be modified easily to encapsulate hydrophobic drugs, further investigation is required on the biocompatibility of modified chitosan and its derivatives. In conclusion, chitosan and its derivatives as drug carriers have potential for a wider application.
Acknowledgments
This work was supported by grants from the Foundation of Zhejiang Science and Technology Department (2009C33005), National Natural Science Foundation of China (81001647), China Postdoctoral Science Foundation (20100471757), and National Natural Science Foundation of China (20906016).
Footnotes
Disclosure
The authors report no conflicts of interest. The authors are solely responsible for the content and writing of the article.
References
- 1.Shi XY, Fan XG. Advances in nanoparticle system for deliverying drugs across the biological barriers. J China Pharm Univ. 2002;33(3):169–172. [Google Scholar]
- 2.Jin MX, Hu QH. Characterization and application in bioadhesive drug delivery system of chitosan. Centr South Pharm. 2008;6(003):324–327. [Google Scholar]
- 3.Dudhani AR, Kosaraju SL. Bioadhesive chitosan nanoparticles: preparation and characterization. Carbohydr Polym. 2010;81(2):243–251. [Google Scholar]
- 4.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62(1):3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Wedmore I, McManus J, Pusateri A, Holcomb J. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60(3):655–658. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 6.Cao J, Zhou NJ. Progress in antitumor studies of chitosan. Chin J Biochem Pharm. 2005;26(2):127–127. [Google Scholar]
- 7.Maeda Y, Kimura Y. Antitumor effects of various low-molecular-weight chitosans are due to increased natural killer activity of intestinal intra-epithelial lymphocytes in sarcoma 180-bearing mice. J Nutr. 2004;134(4):945–950. doi: 10.1093/jn/134.4.945. [DOI] [PubMed] [Google Scholar]
- 8.Torzsas T, Kendall C, Sugano M, Iwamoto Y, Rao A. The influence of high and low molecular weight chitosan on colonic cell proliferation and aberrant crypt foci development in CF1 mice. Food Chem Toxicol. 1996;34(1):73–77. doi: 10.1016/0278-6915(95)00083-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhou SH, Hong Y, Fang GJ. Preparation, characterization and anticancer effect of chitosan nanoparticles. J Clin Rehab Tiss Engin Res. 2007;11(48):9688–9691. [Google Scholar]
- 10.Fang GJ, Hong Y, Jiang YY. Comparison of antitumor effects of chitosan nanoparticles from different sources in vitro. J Clin Rehab Tiss Engin Res. 2007;11(48):9696–9699. [Google Scholar]
- 11.Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62(1):59–82. doi: 10.1016/j.addr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Avadi MR, Sadeghi AMM, Mohammadpour N, et al. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomed Nanotechnol Biol Med. 2010;6(1):58–63. doi: 10.1016/j.nano.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Du W, Niu S, Xu Y, Xu Z, Fan C. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr Polym. 2009;75(3):385–389. [Google Scholar]
- 14.Prabaharan M, Mano J. Chitosan-based particles as controlled drug delivery systems. Drug Deliv. 2004;12(1):41–57. doi: 10.1080/10717540590889781. [DOI] [PubMed] [Google Scholar]
- 15.Ohya Y, Shiratani M, Kobayashi H, Ouchi T. Release behavior of 5-fluorouracil from chitosan-gel nanospheres immobilizing 5-fluorouracil coated with polysaccharides and their cell specific cytotoxicity. J Macromol Sci Part A. 1994;31(5):629–642. [Google Scholar]
- 16.Goldberg M, Langer R, Jia X. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Ed. 2007;18(3):241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodnar M, Hartmann J, Borbely J. Preparation and characterization of chitosan-based nanoparticles. Biomacromolecules. 2005;6(5):2521–2527. doi: 10.1021/bm0502258. [DOI] [PubMed] [Google Scholar]
- 18.Qu J, Liu G, Wang Y, Hong R. Preparation of Fe3O4-chitosan nanoparticles used for hyperthermia. Adv Powder Technol. 2010;21(4):461–467. [Google Scholar]
- 19.Berthold A, Cremer K, Kreuter J. Preparation and characterization of chitosan microspheres as drug carrier for prednisolone sodium phosphate as model for anti-inflammatory drugs. J Control Release. 1996;39(1):17–25. [Google Scholar]
- 20.Tian X, Groves M. Formulation and biological activity of antineoplastic proteoglycans derived from Mycobacterium vaccae in chitosan nanoparticles. J Pharm Pharmacol. 1999;51(2):151–157. doi: 10.1211/0022357991772268. [DOI] [PubMed] [Google Scholar]
- 21.El-Shabouri M. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int J Pharm. 2002;249(1–2):101–108. doi: 10.1016/s0378-5173(02)00461-1. [DOI] [PubMed] [Google Scholar]
- 22.Chauvierre C, Labarre D, Couvreur P, Vauthier C. Radical emulsion polymerization of alkylcyanoacrylates initiated by the redox system dextran-cerium (IV) under acidic aqueous conditions. Macromolecules. 2003;36(16):6018–6027. [Google Scholar]
- 23.Chauvierre C, Labarre D, Couvreur P, Vauthier C. Plug-in spectrometry with optical fibers as a novel analytical tool for nanoparticles technology: application to the investigation of the emulsion polymerization of the alkylcyanoacrylate. J Nanopart Res. 2003;5(3):365–371. [Google Scholar]
- 24.Chauvierre C, Labarre D, Couvreur P, Vauthier C. Novel polysaccharide-decorated poly (isobutyl cyanoacrylate) nanoparticles. Pharm Res. 2003;20(11):1786–1793. doi: 10.1023/b:pham.0000003376.57954.2a. [DOI] [PubMed] [Google Scholar]
- 25.Bravo-Osuna I, Vauthier C, Farabollini A, Palmieri GF, Ponchel G. Mucoadhesion mechanism of chitosan and thiolated chitosan-poly(isobutyl cyanoacrylate) core-shell nanoparticles. Biomaterials. 2007;28(13):2233–2243. doi: 10.1016/j.biomaterials.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 26.de Moura MR, Aouada FA, Mattoso LHC. Preparation of chitosan nanoparticles using methacrylic acid. J Colloid Interface Sci. 2008;321(2):477–483. doi: 10.1016/j.jcis.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Kim YS, Park K, et al. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J Control Release. 2008;127(1):41–49. doi: 10.1016/j.jconrel.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Huo MR, Zhou JP, Zhang Y. Synthesis and characterization of novel amphiphilic chitosan derivatives and its solubilizing abilities for water-insoluble drugs. Chem J Chinese U. 2007;28(10):1995–1999. [Google Scholar]
- 29.Cheng MH, Huang YX, Zhou HJ, Liu Z, Li JF. Rapid preparation and characterization of chitosan nanoparticles for oligonucleotide. Curr Appl Phys. 2010;10(3):797–800. [Google Scholar]
- 30.Kim D, Jeong Y, Choi C, et al. Retinol-encapsulated low molecular water-soluble chitosan nanoparticles. Int J Pharm. 2006;319(1–2):130–138. doi: 10.1016/j.ijpharm.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 31.Grenha A, Seijo B, Serra C, Remu án-López C. Chitosan nanoparticle-loaded mannitol microspheres: structure and surface characterization. Biomacromolecules. 2007;8(7):2072–2079. doi: 10.1021/bm061131g. [DOI] [PubMed] [Google Scholar]
- 32.Huang HY, Shieh YT, Shih CM, Twu YK. Magnetic chitosan/iron (II, III) oxide nanoparticles prepared by spray-drying. Carbohydr Polym. 2010;81(4):906–910. [Google Scholar]
- 33.Singh R, Lillard J., Jr Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan Q, Shah J, Hein S, Misra RDK. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 2010;6(3):1140–1148. doi: 10.1016/j.actbio.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Park JH, Saravanakumar G, Kim K, Kwon IC. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Deliv Rev. 2010;62(1):28–41. doi: 10.1016/j.addr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Park K, Kim J, Nam Y, et al. Effect of polymer molecular weight on the tumor targeting characteristics of self-assembled glycol chitosan nanoparticles. J Control Release. 2007;122(3):305–314. doi: 10.1016/j.jconrel.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Xu XY, Zhou JP, Li L. Preparation of doxorubicin-loaded chitosan polymeric micelle and study on its tissue biodistribution in mice. Acta Pharm Sin. 2008;43(7):743–748. [PubMed] [Google Scholar]
- 38.Jones R, Cheung C, Black F, et al. Poly (2-alkylacrylic acid) polymers deliver molecules to the cytosol by pH-sensitive disruption of endosomal vesicles. Biochem J. 2003;372(Pt 1):65–75. doi: 10.1042/BJ20021945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiang T, Bright C, Cheung C, Stayton P, Hoffman A. Formulation of chitosan-DNA nanoparticles with poly (propyl acrylic acid) enhances gene expression. J Biomater Sci, Polym Ed. 2004;15(11):1405–1421. doi: 10.1163/1568562042368112. [DOI] [PubMed] [Google Scholar]
- 40.Fan L, Wu H, Zhang H, et al. Novel super pH-sensitive nanoparticles responsive to tumor extracellular pH. Carbohydr Polym. 2008;73(3):390–400. [Google Scholar]
- 41.Pan A, Wu B, Wu J. Chitosan nanoparticles crosslinked by glycidoxypropyltrimethoxysilane for pH triggered release of protein. Chin Chem Lett. 2009;20(1):79–83. [Google Scholar]
- 42.Chung J, Yokoyama M, Aoyagi T, Sakurai Y, Okano T. Effect of molecular architecture of hydrophobically modified poly (N-isopropylacrylamide) on the formation of thermoresponsive core-shell micellar drug carriers. J Control Release. 1998;53(1–3):119–130. doi: 10.1016/s0168-3659(97)00244-7. [DOI] [PubMed] [Google Scholar]
- 43.Chung J, Yokoyama M, Yamato M, Aoyagi T, Sakurai Y, Okano T. Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly (N-isopropylacrylamide) and poly (butylmethacrylate) J Control Release. 1999;62(1–2):115–127. doi: 10.1016/s0168-3659(99)00029-2. [DOI] [PubMed] [Google Scholar]
- 44.Rejinold NS, Chennazhi KP, Nair SV, Tamura H, Jayakumar R. Biodegradable and thermo-sensitive chitosan-g-poly(N-vinylcaprolactam) nanoparticles as a 5-fluorouracil carrier. Carbohydr Polym. 2011;83(2):776–786. [Google Scholar]
- 45.Yao Q. Study on the two-ligand modified chitosan nanoparticles actively targeting to malignant liver cells. Doctoral Paper of Sichuan University. 2006 [Google Scholar]
- 46.Huang Y, Lin AH, Zhang X. Targeting binding of chitosan nanoparticles with glycyrrhizin surface modification to hepatic parenchymal cells in vitro. Tradit Chin Drug Res Pharmacol. 2008;19(6):495–498. [Google Scholar]
- 47.Min K, Park K, Kim Y, et al. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J Control Release. 2008;127(3):208–218. doi: 10.1016/j.jconrel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Mei ZN, Yang XL, Xu HB. Biodegradable polymer long-circulating nanoparticle. Chin J Hosp Pharm. 2002;22(7):433–435. [Google Scholar]
- 49.Redhead H, Davis S, Illum L. Drug delivery in poly (lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: in vitro characterisation and in vivo evaluation. J Control Release. 2001;70(3):353–363. doi: 10.1016/s0168-3659(00)00367-9. [DOI] [PubMed] [Google Scholar]
- 50.Nam HY, Kwon SM, Chung H, et al. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. J Control Release. 2009;135(3):259–267. doi: 10.1016/j.jconrel.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 51.Yang YM, Hu W, Wang XD, et al. The controlling biodegradation of chitosan fibers by N-acetylation in vitro and in vivo. J Mater Sci: Mater Med. 2007;18(11):2117–2121. doi: 10.1007/s10856-007-3013-x. [DOI] [PubMed] [Google Scholar]
- 52.Xu J, McCarthy S, Gross R, Kaplan D. Chitosan film acylation and effects on biodegradability. Macromolecules. 1996;29(10):3436–3440. [Google Scholar]
- 53.Jayakumar R, Chennazhi KP, Muzzarelli RAA, Tamura H, Nair SV, Selvamurugan N. Chitosan conjugated DNA nanoparticles in gene therapy. Carbohydr Polym. 2010;79(1):1–8. [Google Scholar]
- 54.Katas H, Alpar H. Development and characterization of chitosan nanoparticles for siRNA delivery. J Control Release. 2006;115(2):216–225. doi: 10.1016/j.jconrel.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 55.Liu XD. Chitosan-siRNA complex nanoparticles for gene silencing. J Biomed Engin. 2010;27(1):97–101. [PubMed] [Google Scholar]
- 56.Mao S, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62(1):12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Lavertu M, Methot S, Trankhanh N, Buschmann M. High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials. 2006;27(27):4815–4824. doi: 10.1016/j.biomaterials.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 58.Mansouri S, Cuie Y, Winnik F, et al. Characterization of folate-chitosan-DNA nanoparticles for gene therapy. Biomaterials. 2006;27(9):2060–2065. doi: 10.1016/j.biomaterials.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 59.Gan Q, Wang T. Chitosan nanoparticle as protein delivery carrier-systematic examination of fabrication conditions for efficient loading and release. Colloids Surf B. 2007;59(1):24–34. doi: 10.1016/j.colsurfb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Zhang N, Li J, Jiang W, et al. Effective protection and controlled release of insulin by cationic β-cyclodextrin polymers from alginate/chitosan nanoparticles. Int J Pharm. 2010;393(1–2):213–219. doi: 10.1016/j.ijpharm.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Kim JH, Kim YS, Park K, et al. Self-assembled glycol chitosan nanoparticles for the sustained and prolonged delivery of antiangiogenic small peptide drugs in cancer therapy. Biomaterials. 2008;29(12):1920–1930. doi: 10.1016/j.biomaterials.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 62.Jeong YI, Jin SG, Kim IY, et al. Doxorubicin-incorporated nanoparticles composed of poly(ethylene glycol)-grafted carboxymethyl chitosan and antitumor activity against glioma cells in vitro. Colloids Surf B. 2010;79(1):149–155. doi: 10.1016/j.colsurfb.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 63.Li F, Li J, Wen X, et al. Anti-tumor activity of paclitaxel-loaded chitosan nanoparticles: An in vitro study. Mater Sci Eng, C. 2009;29(8):2392–2397. [Google Scholar]
- 64.Trickler WJ, Nagvekar AA, Dash AK. A novel nanoparticle formulation for sustained paclitaxel delivery. AAPS Pharm Sci Tech. 2008;9(2):486–493. doi: 10.1208/s12249-008-9063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JH, Kim YS, Kim S, et al. Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J Control Release. 2006;111(1–2):228–234. doi: 10.1016/j.jconrel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 66.Hao PP, Deng SH. Preparation and detection of acyclovir loaded chitosan nanoparticles. Chin Med Herald. 2008;5(1):28–29. [Google Scholar]
- 67.Li FB, Luan LB. Preparation and in vitro release of tranilast-loaded chitosan nanoparticles. Pharm Clin Res. 2008;16(4):282–284. [Google Scholar]
- 68.Samstein R, Perica K, Balderrama F, Look M, Fahmy T. The use of deoxycholic acid to enhance the oral bioavailability of biodegradable nanoparticles. Biomaterials. 2008;29(6):703–708. doi: 10.1016/j.biomaterials.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 69.Yin L, Ding J, He C, Cui L, Tang C, Yin C. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials. 2009;30(29):5691–5700. doi: 10.1016/j.biomaterials.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 70.Dube A, Nicolazzo JA, Larson I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur J Pharm Sci. 2010;41(2):219–225. doi: 10.1016/j.ejps.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 71.Makhlof A, Tozuka Y, Takeuchi H. Design and evaluation of novel pH-sensitive chitosan nanoparticles for oral insulin delivery. Eur J Pharm Sci. 2010 doi: 10.1016/j.ejps.2010.12.007. In press. [DOI] [PubMed] [Google Scholar]
- 72.Amidi M, Romeijn S, Borchard G, Junginger H, Hennink W, Jiskoot W. Preparation and characterization of protein-loaded N-trimethyl chitosan nanoparticles as nasal delivery system. J Control Release. 2006;111(1–2):107–116. doi: 10.1016/j.jconrel.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 73.Wang X, Chi N, Tang X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur J Pharm Biopharm. 2008;70(3):735–740. doi: 10.1016/j.ejpb.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Huo M, Zhang Y, Zhou J, et al. Synthesis and characterization of low-toxic amphiphilic chitosan derivatives and their application as micelle carrier for antitumor drug. Int J Pharm. 2010;394(1–2):162–173. doi: 10.1016/j.ijpharm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 75.De Campos A, Diebold Y, Carvalho E, Sanchez A, Jose Alonso M. Chitosan nanoparticles as new ocular drug delivery systems: in vitro stability, in vivo fate, and cellular toxicity. Pharm Res. 2004;21(5):803–810. doi: 10.1023/b:pham.0000026432.75781.cb. [DOI] [PubMed] [Google Scholar]