Abstract
Echinocandins are a group of antifungal agents that target 1,3-β-glucan synthase, causing disruption of mold growth at cells compromising tips and branch points. In part because echinocandins do not induce clear growth inhibition end points using broth dilution techniques, methods to test susceptibility have not yet been standardized. We developed a novel susceptibility assay that measures growth of Aspergillus species on solid agar media that contain serial dilutions of caspofungin (agar dilution). Results of agar dilution testing of multiple isolates were compared to results obtained by broth microdilution (MIC), microscopic evaluation (minimal effective concentration [MEC]), and a new method to measure fungal burden, quantification of secreted hyphal antigen. MICs obtained by the agar dilution method were within 1 dilution of MECs for 85% of the Aspergillus isolates; the highest agreement was observed for isolates of Aspergillus niger (95%), which were particularly susceptible to caspofungin. Agar dilution MICs were also consistent with those obtained by quantifying antigen secretion. MICs obtained by broth microdilution were different than MICs by any other method. Several Aspergillus isolates with decreased susceptibility to caspofungin were identified. Agar dilution is simple and reproducible, and results were consistent with the results of more technically demanding techniques. This method may be appropriate for use in the clinical laboratory.
Caspofungin (CAS), a member of the echinocandin group of antifungal agents (9), has become a therapeutic option for treatment of severe systemic fungal infections. CAS causes profound changes in Aspergillus hyphal growth, with drug-treated hyphae appearing abnormally swollen and with highly branched hyphal tips. Although CAS is approved for salvage therapy of aspergillosis and has shown to be effective in animal models of infection, in vitro antifungal susceptibility testing methods remain elusive. This is largely because in broth culture methods (National Committee for Clinical Laboratory Standards [NCCLS] standard M38-A) (12), CAS-exposed Aspergillus spp. demonstrate growth in every well, making end points of growth and MICs difficult to determine (1).
Kurtz et al. were the first to propose the term minimal effective concentration (MEC) to denote the lowest echinocandin concentration at which the fungi display microscopic morphological changes (11), and this measure is currently used by investigators to report CAS susceptibilities (1, 17, 18). However, determining MEC by microscopy is labor-intensive and subjective and requires expertise. Other modified versions of CAS susceptibility assays have included E-test and disk diffusion (3, 4, 8), but defining precise MICs by E-test does not appear to be reliable (3, 4).
We hypothesized that drug-induced alterations in filamentous growth may be measured by visual inspection of morphology on solid media and developed an agar dilution method to measure Aspergillus susceptibility to CAS. Because none of the existing testing methods have been standardized, we also sought to develop additional methods to quantify relative fungal growth in the presence of drug. To this end, we developed an additional assay that measures growth according to secretion of a hyphal polysaccharide, galactomannan (GM). Results of multiple experimental comparisons suggest that agar dilution may be an easy, accurate method to assess Aspergillus susceptibility to echinocandin antifungal agents.
MATERIALS AND METHODS
Test isolates.
Fifty-four Aspergillus isolates that were obtained from clinical specimens at the Fred Hutchinson Cancer Research Center were tested. These isolates included Aspergillus fumigatus (n = 34), Aspergillus flavus (n = 1), Aspergillus niger (n = 14), and Aspergillus terreus (n = 2). One Candida albicans isolate and one isolate of Rhizopus sp. (both clinical isolates) were used as susceptible and resistant quality controls, respectively.
Inoculum preparation.
Isolates were stored frozen at −70°C, passaged twice on potato dextrose agar (PDA) at 35°C prior to susceptibility testing, and inocula were prepared as described in the NCCLS M38-A document (12). Briefly, cultures were grown on PDA slants at 35°C for 7 days. To prepare conidial inocula, cultures were flooded with sterile 0.85% saline containing 0.025% Tween 20 (Sigma Chemical Co., St. Louis, Mo.) and gently probed with a pipette tip. The resulting suspension was vortexed, heavy particles were allowed to settle for 3 to 5 min, and the upper layer was adjusted to a transmittance of 80 to 82% by using a spectrophotometer (wavelength, 530 nm). The stock suspensions for Aspergillus and Rhizopus species contained mostly conidia. These nongerminated conidial inoculum suspensions were diluted 1:50 in RPMI 1640 medium (buffered to a pH of 7.0 using 0.165 M morpholinepropanesulfonic acid [MOPS] [both from Sigma Chemical Co.]) for testing by the NCCLS method (Aspergillus and Rhizopus species) and 1:100 in RPMI 1640 medium for testing by the agar dilution method. C. albicans stock inoculum was diluted 1:100 in RPMI 1640 medium for both the agar dilution and the microbroth methods.
Broth microdilution methods.
CAS, provided as powder by Merck Research Laboratories (Rahway, N.J.), was diluted in RPMI 1640 medium (with l-glutamine, without bicarbonate, buffered to pH 7.0 with 0.165 M MOPS [Sigma Chemical Co.]). Doubling dilutions of the drug were prepared in microdilution wells, and 100 μl of diluted conidial inoculum suspension was added to 100 μl of drug solution, yielding final CAS concentrations ranging from 0.03125 to 32 μg/ml. Growth and sterility controls were included for each isolate. A C. albicans strain with known CAS susceptibility was tested in parallel whenever a set of isolates was evaluated. Microdilution trays were incubated at 35°C and examined after 48 h for MIC determination. MICs were interpreted as the drug concentration that demonstrated 50% growth inhibition, as described previously (1). MECs were determined microscopically as the lowest concentration of CAS causing abnormal hyphal growth with short abundant branchings (1).
Agar dilution method.
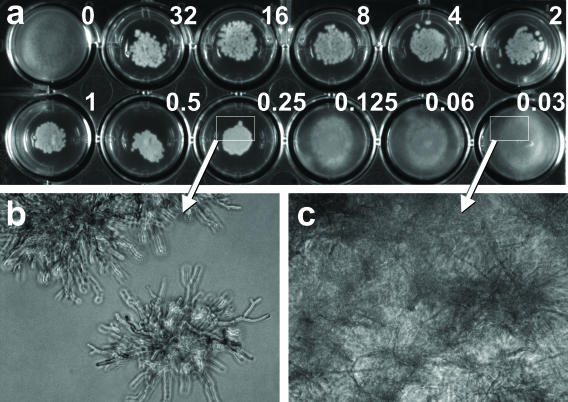
CAS was serially diluted in molten medium equilibrated at 50°C (RPMI 1640 medium with 2% glucose [Sigma Chemical Co.] with 1.5% Bacto Agar) to achieve final drug concentrations ranging from 0.03 to 32 μg/ml. One milliliter was added to each well in a 24-well plate with a flat bottom (catalog no. 353847; Becton Dickinson, Franklin Lakes, N.J.) and allowed to solidify. The center of each well was inoculated with 10 μl of the conidial suspension. Drug-free growth controls were included for each isolate. MICs for filamentous fungi were determined after 48 h at 35°C. MICs were defined as the lowest drug concentrations that had granular appearing microcolonies of growth instead of filamentous radiating colonies on solid agar (Fig. 1a). For C. albicans, the MIC was the lowest drug concentration associated with no growth on agar.
FIG. 1.
Agar dilution method. (a) Well 1 is a positive control (without CAS), and wells 2 to 12 contain drug concentrations ranging from 32 to 0.03 μg/ml (as shown). The MIC is 0.25 μg/ml, interpreted as the lowest drug concentration showing compact granular microcolonies compared to the radial filamentous colonies of the growth control and wells containing lower drug concentrations (0.125 to 0.03 μg/ml). The corresponding microscopic appearance of colonies at effective concentrations and subinhibitory concentrations of drug are shown in panels b and c, respectively.
GM antigen release method.
To measure relative fungal growth independent of colony morphology or culture turbidity, we developed a GM antigen release method. This method was chosen, because previous studies had demonstrated that GM is secreted into growth medium at concentrations that correlate with fungal burden (R. M. Winn, A. Warris, T. G. Abrahmsen, and P. Gaustad, Abstr. 6th Congr. Eur. Confed. Med. Mycol. Soc., abstr. P3-016, 2000). Microdilution trays were prepared with antifungal agents and fungal inocula as described above for the NCCLS M38-A method (12). After 24 h of incubation at 35°C, 5 μl of supernatant from each well was diluted in 5 ml of saline (1:1,000), and secreted GM was measured by enzyme immunoassay (Platelia Aspergillus; Bio-Rad Laboratories, Redmond, Wash.) according to the manufacturer's instructions. Briefly, 50 μl of the dilution was added to each well of a microtitration plate coated with a rat monoclonal antibody EB-A2 (directed against Aspergillus GM) and incubated at 37°C. After 90 min, the plates were washed, and 100 μl of buffer containing orthophenylenediamine dihydrochloride solution was added. The plates were incubated for another 30 min in the dark at room temperature, before 100 μl of 1.5 M sulfuric acid was added to stop the reaction. The optical density (OD) was read at 450 nm. The OD index for treated samples was calculated by dividing the OD of each sample by the OD of a control containing 1 ng of GM per ml. The ratio of GM indices in samples compared to growth controls was calculated, and the drug concentration at which the ratio approximated 0.5 was interpreted as the MIC50.
Agreement between different susceptibility testing methods.
Each of the isolates was tested at least three times by the agar dilution and NCCLS methods (MECs and MICs). Fifteen isolates of A. fumigatus and A. niger were also tested at least twice using the antigen release method. For each strain, the percentage of agreement between the methods was defined as the proportion of MICs that fell within 1 dilution of the MIC determined by the NCCLS method, the antigen method, and the agar dilution method, respectively. For comparative evaluation of the broth microdilution and agar dilution methods, the geometric mean and range of the MICs and MECs were calculated for each genus-species combination. High and low off-scale MICs were included in the analysis by converting to the next higher or lower drug concentration, respectively.
RESULTS
The MICs obtained by agar dilution were compared to the MICs and MECs obtained by broth microdilution for multiple clinical isolates of A. fumigatus (Table 1) and non-A. fumigatus species (Table 2). As shown in Table 1, agar dilution MICs and NCCLS MECs of different A. fumigatus isolates ranged from very low (≤0.25 μg/ml) to high (≥16 μg/ml). In contrast, MICs determined by serial broth microdilution tended to be higher for all A. fumigatus isolates tested. The geometric means of agar dilution MICs and MECs and broth microdilution MICs for A. fumigatus isolates were 0.4, 1.2, and 6.5 μg/ml, respectively. CAS susceptibility was high for seven A. fumigatus isolates (Af28 to Af34), using all three methods. Agar dilution MICs and MECs fell within 1 dilution in 75.5% of isolates, and 84.8% of isolates tested had results within 2 dilutions. Only four isolates had agar dilution MICs and MECs that differed by more than 4 dilutions of drug.
TABLE 1.
Agar dilution and NCCLS broth microdilution results obtained for multiple A. fumigatus isolatesa
| A. fumigatus isolate | Agar dilution MIC (μg/ml)
|
NCCLS
|
||||
|---|---|---|---|---|---|---|
| Mean | Range | MEC (μg/ml)
|
MIC (μg/ml)
|
|||
| Mean | Range | Mean | Range | |||
| Af1 | 0.10 | 0.0625-0.125 | 0.35 | 0.25-0.5 | 5.66 | 1->32 |
| Af2 | 0.13 | 0.0625-25 | 0.35 | 0.25-0.5 | 8 | 2->32 |
| Af3 | 0.16 | 0.125-0.25 | 0.25 | 0.125-0.5 | 4 | 0.5->32 |
| Af4 | 0.16 | 0.125-0.25 | 0.25 | 0.125-0.5 | 5.66 | 1->32 |
| Af5 | 0.16 | 0.125-0.25 | 0.5 | 0.25-1 | 1 | 0.125->32 |
| Af6 | 0.20 | 0.125-0.25 | 0.5 | NR | 0.71 | 0.5-1 |
| Af7 | 0.20 | 0.125-0.25 | 0.5 | 0.25-1 | >32 | NR |
| Af8 | 0.20 | 0.125-0.25 | 0.5 | 0.25-1 | 2.52 | 0.5->32 |
| Af9 | 0.20 | 0.125-0.25 | 0.18 | 0.125-0.25 | 0.5 | 0.5->32 |
| Af10 | 0.20 | 0.125-0.25 | 0.63 | 0.5-1 | 3.17 | 1->32 |
| Af11 | 0.20 | 0.125-0.25 | 3.17 | 0.125-16 | 6.35 | 0.25->32 |
| Af13 | 0.25 | 0.125-0.5 | 0.5 | 0.25-1 | 22.63 | 16->32 |
| Af14 | 0.25 | 0.125-0.5 | 0.35 | 0.25-0.5 | 2.52 | 0.5->32 |
| Af15 | 0.25 | 0.125-0.5 | 0.5 | 0.25-1 | 12.70 | 2->32 |
| Af16 | 0.25 | NR | 0.31 | 0.25-0.5 | 3.17 | 1->32 |
| Af17 | 0.25 | NR | 0.31 | 0.25-0.5 | 2.52 | 0.5->32 |
| Af18 | 0.25 | NR | 0.79 | 0.5-2 | 4 | 1->32 |
| Af19 | 0.25 | NR | 0.5 | 0.25-1 | 6.35 | 0.5->32 |
| Af20 | 0.25 | NR | 0.40 | 0.25-0.5 | 3.17 | 1->32 |
| Af21 | 0.25 | NR | 0.63 | 0.5-1 | 3.17 | 0.5->32 |
| Af22 | 0.25 | NR | 0.71 | 0.5-1 | 32 | NR |
| Af23 | 0.31 | 0.25-0.5 | 0.35 | 0.25-0.5 | 4 | 0.5->32 |
| Af24 | 0.31 | 0.25-0.5 | 4 | 1-16 | 22.63 | 16->32 |
| Af25 | 0.40 | 0.25-1 | 2 | 0.5-8 | 10.08 | 1->32 |
| Af26 | 0.40 | 0.25-0.5 | 0.63 | 0.5-1 | 1 | 0.5-2 |
| Af27 | 0.40 | 0.25-0.5 | 0.40 | 0.25-0.5 | 0.63 | 0.25-1 |
| Af28 | 2.52 | 2-4 | 16 | NR | >32 | NR |
| Af29 | 2.52 | 2-4 | 4 | 2-8 | >32 | NR |
| Af30 | 5.04 | 4-8 | 11.31 | 8-16 | 16 | NR |
| Af31 | 6.35 | 4-8 | >32 | NR | >32 | NR |
| Af32 | 8.00 | 4-16 | 16 | 16 | >32 | NR |
| Af33 | 16 | NR | 25.40 | 16->32 | >32 | NR |
| Af34 | 16 | NR | 32 | NR | >32 | NR |
Geometric mean MICs and MECs, calculated from three individual experiments, and ranges are shown for each isolate. NR, no range was generated, as all experiments yielded the same results.
TABLE 2.
Agar dilution and NCCLS broth microdilution results obtained for multiple isolates of three Aspergillus speciesa
| Aspergillus isolate | Agar dilution MIC (μg/ml)
|
NCCLS
|
||||
|---|---|---|---|---|---|---|
| Mean | Range | MEC (μg/ml)
|
MIC (μg/ml)
|
|||
| Mean | Range | Mean | Range | |||
| An1 | 0.31 | 0.25-0.5 | 0.25 | 0.125-0.5 | 0.31 | 0.25-0.5 |
| An2 | 0.16 | 0.125-0.25 | 0.20 | 0.125-0.25 | 0.31 | 0.25-0.5 |
| An3 | 0.125 | 0.125 | 0.31 | 0.25-0.5 | 0.25 | 0.125-0.5 |
| An4 | 0.18 | 0.125-0.25 | 0.16 | 0.125-0.25 | 0.25 | 0.125-0.5 |
| An5 | 0.16 | 0.125-0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| An6 | 0.16 | 0.125-0.25 | 0.20 | 0.125-0.5 | 0.20 | 0.125-0.5 |
| An7 | 0.20 | 0.125-0.25 | 0.16 | 0.125-0.25 | 0.20 | 0.125-0.5 |
| An8 | 0.31 | 0.25-0.5 | 0.20 | 0.125-0.5 | 0.40 | 0.25-0.5 |
| An9 | 0.31 | 0.25-0.5 | 0.40 | 0.25-0.5 | 0.40 | 0.25-0.5 |
| An10 | 0.31 | 0.25-0.5 | 0.31 | 0.25-0.5 | 0.31 | 0.25-0.5 |
| An11 | 0.25 | 0.25 | 0.40 | 0.25-0.5 | 0.31 | 0.25-0.5 |
| An12 | 0.16 | 0.125-0.25 | 0.20 | 0.125-0.25 | 0.31 | 0.25-0.5 |
| An13 | 0.20 | 0.125-0.25 | 0.125 | 0.125 | 0.20 | 0.125-0.5 |
| An14 | 0.20 | 0.125-0.5 | 0.25 | 0.125-0.5 | 0.40 | 0.5-1 |
| Afl1 | 0.11 | 0.0625-0.125 | 0.25 | 0.25-0.5 | 0.63 | 0.25-0.5 |
| At1 | 0.20 | 0.125-0.5 | 0.31 | 0.125-0.5 | 0.50 | 0.125-0.5 |
| At2 | 0.16 | 0.125-0.25 | 0.63 | 0.5-1 | 0.31 | 0.5-1 |
Geometric mean MICs and MECs calculated from three individual experiments and ranges are shown for multiple A. niger (An1 to An14), A. flavus (Af1), and A. terreus (At1 and At2) isolates.
The susceptibilities of several A. niger, A. flavus, and A. terreus isolates are shown in Table 2. Compared to A. fumigatus, A. niger isolates had lower geometric mean MICs and MECs (agar dilution MIC, 0.2 μg/ml; agar dilution MEC, 0.23 μg/ml; broth microdilution MIC, 0.28 μg/ml). No non-A. fumigatus isolates with high MICs or MECs were identified.
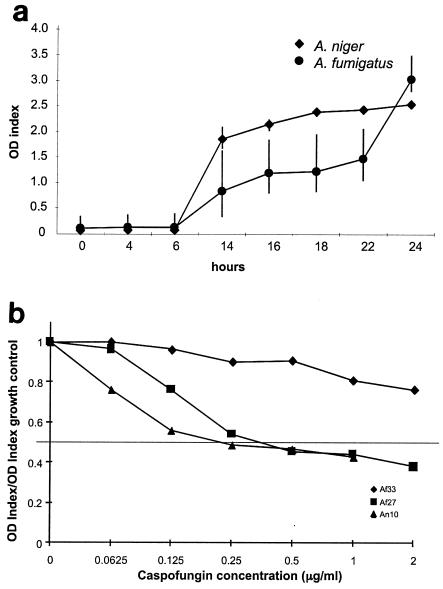
Because of the lack of agreement between broth microdilution MICs and other methods, we compared both results with results of the GM release assay. Initial experiments demonstrated that GM secreted in culture medium increased over time in the absence of drug for all species tested; results for A. fumigatus and A. niger cultures are shown in Fig. 2a. In the presence of increased concentrations of CAS, secreted GM decreased predictably. Relative growth curves for one A. fumigatus isolate that had high MICs and MECs and two isolates with low MICs and MECs are shown in Fig. 2b.
FIG. 2.
Antigen release assay. (a) OD index of secreted GM in A. fumigatus and A. niger after 24 h of growth (means ± standard deviations [error bars] from three experiments). (b) Ratio of secreted GM relative to growth control is shown for one A. fumigatus isolate with a high MIC (Af33), one A. fumigatus isolate with a low MIC (Af27), and one A. niger isolate (An10). The drug concentration at which the ratio approximated 0.5 was interpreted as the MIC50.
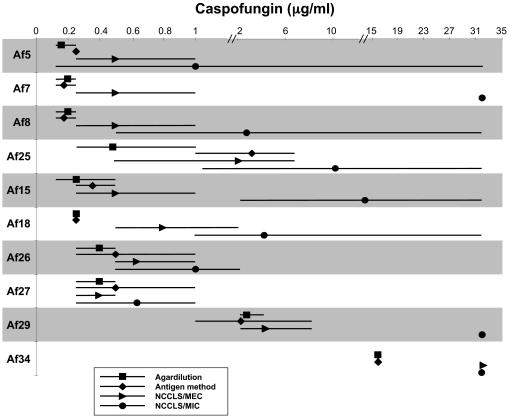
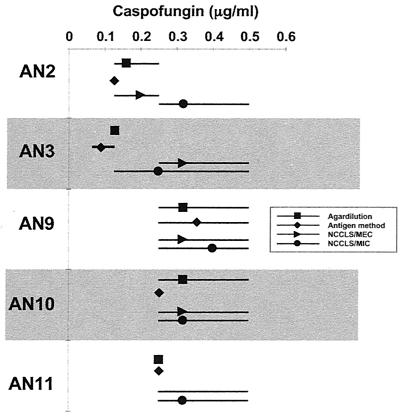
Fifteen isolates that had disparate high and low MICs and MECs by previous testing were selected to undergo further tests by GM secretion. Comparative results of all methods, with interexperimental agreement, are shown in Fig. 3 and 4. In general, agar dilution and antigen secretion results had a high degree of agreement with MECs and very little interexperimental variability. High and variable MICs were obtained by the broth microdilution assay, especially for A. fumigatus. The antigen secretion method confirmed that there was very little growth of all A. niger isolates in the presence of low concentrations of CAS (Fig. 4).
FIG. 3.
Comparison of agar dilution MICs, broth microdilution MICs and MECs, and antigen secretion MICs for selected A. fumigatus isolates. The geometric means (symbols) and ranges (lines) from at least three experiments per isolate are shown.
FIG. 4.
Comparison of agar dilution MICs, broth microdilution MICs and MECs, and antigen secretion MICs for selected A. niger isolates. The geometric means (symbols) and ranges (lines) from at least three experiments per isolate are shown.
DISCUSSION
The development of glucan synthase inhibitors presents an important advance in therapy of aspergillosis and candidiasis but a challenge with regards to in vitro susceptibility testing. While the fungicidal activity of these drugs against Candida species provides clear growth end points in vitro, the mechanism by which these drugs inhibit growth of Aspergillus species has presented difficulties in measuring relative activity in the laboratory. We have developed a simple method to assess CAS activity by visual inspection of colony morphology on drug-containing agar. Screening of a large number of Aspergillus isolates, using multiple methodologies, demonstrated differential activity of CAS against A. fumigatus isolates and between species.
Multiple laboratories have reported difficulties in testing and inconsistency in MIC results for CAS using traditional broth microdilution methods (2, 4) (A. M. Flattery, P. S. Hicks, A. Wilcox, and H. Rosen, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 936, 2000). The results of our studies are consistent with those of prior reports. In the data presented here, broth microdilution MICs were consistently higher than all other measures of fungal growth, and interpretation was associated with a great deal of interexperimental variability. While microscopic MECs were consistently lower, they too were associated with a large amount of measurement variability between experiments. It is not surprising that the results of the agar dilution assay closely reflected MECs, as colony morphology and microscopic appearance are closely linked (Fig. 1). However, the agar dilution MICs were relatively more reproducible between experiments. Susceptibility measured by broth microdilution was notably lower for all isolates. Which microdilution measure (MIC versus MEC) is the clinically relevant observation still needs to be resolved with additional studies; however, we believe that the large degree of experimental variability makes broth microdilution MICs both difficult to interpret and of questionable value.
Additional support for employing the microscopic or agar dilution method to assess relative growth was provided by the results of the antigen release assay. For all discrepant isolates tested, the antigen release MICs were more consistent with agar dilution MICs and MECs than the results generated by broth turbidity.
Although the laborious nature of the antigen release assay limits its utility as a susceptibility test, our data are in agreement with prior findings that GM release correlates with hyphal growth (Winn et al., Abstr. 6th Congr. Eur. Confed. Med. Mycol. Soc.). These in vitro results are, however, inconsistent with in vivo observations that GM antigen values increased in echinocandin-treated animals (14, 15). Specifically, prior studies showed that GM indices continued to rise despite improved clinical outcomes (survival) in CAS- and micafungin-treated rabbits (14, 15). It was proposed that this effect might be caused by drug-induced hyphal fragmentation. This apparent discrepancy in the kinetics of GM antigen values in in vitro versus animal studies may be explained by numerous laboratory and/or biological variables. For instance, it is possible that the impact of echinocandins on Aspergillus GM secretion may be dependent on the cellular state of the organism (conidia versus hyphae) at the time of drug exposure. Detailed studies will be necessary to define the kinetics of GM release using multiple models.
Although most of the Aspergillus isolates appeared very susceptible to CAS, 5 of 50 isolates examined demonstrated a relative resistance to the drug in all assays. Caspofungin-resistant mutants of Saccharomyces and Candida species have previously been generated (5, 6, 7, 10, 13, 16), and different susceptibilities of A. fumigatus isolates have only recently been observed (8). It is also noteworthy that all A. niger isolates appeared unusually susceptible in vitro; the clinical significance of these findings requires confirmation in animal models. It is possible that this species may be particularly susceptible because of a difference in cell wall composition.
In conclusion, the agar dilution method appears to be a valuable test for in vitro determination of the susceptibilities of Aspergillus isolates against echinocandins. Its simplicity and low cost may allow for application in a clinical microbiology laboratory. Future studies are necessary to determine interlaboratory reproducibility and to evaluate the clinical significance of the apparently different CAS susceptibilities of different Aspergillus isolates.
Acknowledgments
This research was funded in part by research grants from Merck Research Laboratories, NIH grants K08 AI01571 (K.A.M.) and R21 AI055928 (K.A.M.), and Swiss National Science Foundation grant PBBEA-102316 (A.I.).
REFERENCES
- 1.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2002. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob. Agents Chemother. 46:245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arikan, S., V. Paetznick, and J. H. Rex. 2002. Comparative evaluation of disk diffusion with microdilution assay in susceptibility testing of caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 46:3084-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartizal, K., C. J. Gill, G. K. Abruzzo, A. M. Flattery, L. Kong, P. M. Scott, J. G. Smith, C. E. Leighton, A. Bouffard, J. F. Dropinski, and J. Balkovec. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal agent MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2326-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas, C. M., J. A. D'Ippolito, G. J. Shei, M. Meinz, J. Onishi, J. A. Marrinan, W. Li, G. K. Abruzzo, A. Flattery, K. Bartizal, A. Mitchell, and M. B. Kurtz. 1997. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41:2471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas, C. M., F. Foor, J. A. Marrinan, N. Morin, J. B. Nielsen, A. M. Dahl, P. Mazur, W. Baginsky, W. Li, M. el-Sherbeini, et al. 1994. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-beta-D-glucan synthase. Proc. Natl. Acad. Sci. USA 91:12907-12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas, C. M., J. A. Marrinan, W. Li, and M. B. Kurtz. 1994. A Saccharomyces cerevisiae mutant with echinocandin-resistant 1,3-β-d-glucan synthase. J. Bacteriol. 176:5686-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A. 2003. Evaluation of broth microdilution testing parameters and agar diffusion Etest procedure for testing susceptibilities of Aspergillus spp. to caspofungin acetate (MK-0991). J. Clin. Microbiol. 41:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groll, A. H., and T. J. Walsh. 2002. Antifungal chemotherapy: advances and perspectives. Swiss Med. Wkly. 132:303-311. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz, M. B., G. Abruzzo, A. Flattery, K. Bartizal, J. A. Marrinan, W. Li, J. Milligan, K. Nollstadt, and C. M. Douglas. 1996. Characterization of echinocandin-resistant mutants of Candida albicans: genetic, biochemical, and virulence studies. Infect. Immun. 64:3244-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against 1,3-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Osherov, N., G. S. May, N. D. Albert, and D. P. Kontoyiannis. 2002. Overexpression of Sbe2p, a Golgi protein, results in resistance to caspofungin in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 46:2462-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petraitiene, R., V. Petraitis, A. H. Groll, T. Sein, R. L. Schaufele, A. Francesconi, J. Bacher, N. A. Avila, and T. J. Walsh. 2002. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrob. Agents Chemother. 46:12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petraitis, V., R. Petraitiene, A. H. Groll, K. Roussillon, M. Hemmings, C. A. Lyman, T. Sein, J. Bacher, I. Bekersky, and T. J. Walsh. 2002. Comparative antifungal activities and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 46:1857-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuetzer-Muehlbauer, M., B. Willinger, G. Krapf, S. Enzinger, E. Presterl, and K. Kuchler. 2003. The Candida albicans Cdr2p ATP-binding cassette (ABC) transporter confers resistance to caspofungin. Mol. Microbiol. 48:225-235. [DOI] [PubMed] [Google Scholar]
- 17.Serrano Mdel, C., A. Valverde-Conde, M. M. Chavez, S. Bernal, R. M. Claro, J. Peman, M. Ramirez, and E. Martin-Mazuelos. 2003. In vitro activity of voriconazole, itraconazole, caspofungin, anidulafungin (VER002, LY303366) and amphotericin B against Aspergillus spp. Diagn. Microbiol. Infect. Dis. 45:131-135. [DOI] [PubMed] [Google Scholar]
- 18.Shalit, I., Y. Shadkchan, Z. Samra, and N. Osherov. 2003. In vitro synergy of caspofungin and itraconazole against Aspergillus spp.: MIC versus minimal effective concentration end points. Antimicrob. Agents Chemother. 47:1416-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]