Abstract
Background:
The purpose of this study was to investigate the short-term effect of topical antiglaucoma medication on tear-film stability, tear secretion, and corneal sensitivity in healthy subjects.
Methods:
In this prospective, double-blind crossover trial, break-up time and basal secretion (Jones test) were measured 60 minutes before, and 30, 60, and 90 minutes after topical antiglaucoma drop application in 30 healthy subjects. Corneal sensitivity was measured 60 minutes before, and five, 10, and 15 minutes after drop application using a Cochet–Bonnet esthesiometer.
Results:
Reduction of break-up time in the latanoprost group was −23.8% after 30 minutes (P = 0.21), −26.7% after 60 minutes (P = 0.03) and −51.4% after 90 minutes (P ≤ 0.003), which was statistically significant. Reduction of break-up time in all other treatment groups was not statistically significant. The Jones test revealed a significant reduction of basal secretion after application of brimonidine (−17.8%, P = 0.002; −22.5%, P < 0.001; −30.5%, P < 0.001), followed by apraclonidine (−10%, P = 0.06; −20.1%, P = 0.02; −22.1%, P = 0.002), latanoprost (−2.4%, P = 0.64; −18.6%, P = 0.001; −20.1%, P = 0.001) and dorzolamide (−0.5%, P = 0.9; 14.3%, P = 0.018; −17.3%, P = 0.004) at 30, 60, and 90 minutes after drop application. Reduction of basal secretion in all other treatment groups was not statistically significant.
Conclusion:
Latanoprost showed the most statistically significant reduction in break-up time, and brimonidine showed the most significant reduction in basal secretion of all the glaucoma medications used in this study. In conclusion, our data may be helpful for treatment decisions in glaucoma patients who also suffer from ocular surface problems.
Keywords: tear-film, tear secretion, corneal sensitivity, antiglaucoma medication
Introduction
Antiglaucoma medication is the major treatment modality for glaucoma. There is increasing evidence that topically administered medication affects the structure and the integrity of the ocular surface.1–4 Besides its intraocular pressure-lowering effect, local adverse effects, such as conjunctival hyperemia, foreign body sensation, or other symptoms of dry eye disease, can accompany its use. These symptoms can be related to the negative impact of topical antiglaucoma medication and its preservatives on tear-film stability. Because glaucoma therapy typically consists of long-term treatment, these symptoms usually become worse over the long term, eventually leading to a drop in compliance or to complete break-up of drop application in glaucoma patients. At this point, patient care and guidance suffer.
In contrast with studies5,6 investigating their long-term effects on the ocular surface, quantitative investigations to evaluate the short-term effects of relatively “new” topical antiglaucoma medications on tear-film stability and corneal sensitivity are comparatively limited at the present time.
Therefore, the aim of this study was to investigate the short-term effect of the commonly used antiglaucoma medications, ie, brimonidine, dorzolamide, latanoprost, timolol, and apraclonidine, as well as a fixed combination of dorzolamide and timolol, on tear-film stability, tear secretion, and corneal sensitivity in healthy subjects.
Methods and materials
Thirty healthy subjects were included in this prospective, double-blind, crossover study. The study protocol was approved by the ethics committee of Dresden in accordance with the Declaration of Helsinki. All subjects signed an informed consent form before participating in the study. Break-up time and a Jones test were performed 60 minutes before, and 30, 60, and 90 minutes after drop application. Corneal sensitivity was determined 60 minutes before, and 5, 10, and 15 minutes after topical drop application. In accordance with the crossover design, all subjects received one topical medication, which was applied once into the lower fornix of the conjunctiva of one randomly chosen eye. Additionally, benzalkonium chloride (BAK) 0.02% and 0.005% and sodium chloride solution 0.9% as control substances were applied once in one randomly chosen eye.
Break-up time
For determination of break-up time, 10 μL of 0.2% fluorescein solution was applied to the inferior fornix, and the participant was asked to close his/her eyes. Using the blue light of the slit lamp, the time in seconds between eyelid opening and the appearance of initial defects in the tear film was measured. Values longer than 10 seconds were accepted as reference with regard to high inter- and intraindividual variability.7
Corneal sensitivity
Corneal sensitivity was measured using a Cochet–Bonnet esthesiometer according to standard protocols.8 Briefly, the corneal surface was touched orthogonally with a defined nylon fiber. Eyelid closure was considered to be a positive response to the stimulus. The intensity of response was defined by the length and the stiffness of the fiber, which was then converted into pressure values.
Basal secretion
Measurement of basal secretion was performed according to the Jones test, but without mechanical irritation of the nasal mucosa.9 Sixty seconds after topical anesthesia with proxymetacain 0.5%, the tear film was sponged with a cotton swab, a standard test strip was placed in the outer third of the lower eyelid and then removed after five minutes, and the length of the moistened strip was measured. Despite considerable variability, results >20 mm were considered normal.10
Statistical analysis
Statistical analysis was performed using SPSS software (version 11.0, SPSS Inc, Chicago, IL). Data analysis of follow-up examinations after five, 10, and 15 minutes (corneal sensitivity) and after 30, 60, and 90 minutes (break-up time and basal secretion) were performed using analysis of variance for repeated measurements. In the event of a statistically significant difference, a t-test was performed to determine the time point at which a significant difference from baseline examination was observed. Based on multiple testing for significant differences, the level of significance was adapted according to the Bonferroni method as follows: P = 0.05/3 = 0.017. P = 0.017 was therefore considered statistically significant. Statistical analyses of results between the medications at different time points were performed using analysis of variance. Statistical significance was tested with post hoc analysis using the Bonferroni method to assess significant differences among the medications. In cases of significant differences in the test of homogeneity (Levene’s test), analysis of variance could not be performed, so the Kruskal–Wallis test was used for further statistical analysis.
Results
Thirty healthy subjects (16 women and 14 men) of mean age 32.1 ± 8.8 (range 19–59) years were included in this study.
Break-up time
Due to interindividual variability and for a better comparability, measurement results were normalized to 100% and the relative changes in break-up time were determined. Results of break-up time changes are shown in Figures 1 and 2. Statistical analysis revealed no statistically significant differences at 30, 60, or 90 minutes after drop application among the different medications (P = 0.83, P = 0.87, P = 0.12, data not shown).
Figure 1.
Relative changes in break-up time of brimonidine, latanoprost, apraclonidine, dorzolamide, the fixed dorzolamide/timolol combination, and timolol 60 minutes before (T−60) and 30 (T+30), 60 (T+60), and 90 (T+90) minutes after drop application.
Notes: *P < 0.05; **P < 0.01.
Abbreviation: BUT, break-up time.
Figure 2.
Relative changes in break-up time of BAK 0.02%, BAK 0.005%, and sodium chloride 0.9% 60 minutes before (T−60) and 30 (T+30), 60 (T+60), and 90 (T+90) minutes after drop application.
Note: *P < 0.05.
Abbreviations: BUT, break-up time; BAK, benzalkonium chloride.
Analysis of significant differences of one medication during the time course revealed statistically significant changes for latanoprost (P = 0.008), BAK 0.02% (P = 0.02), BAK 0.005% (P = 0.039), and the fixed combination of dorzolamide and timolol (P = 0.015). The results for brimonidine (P = 0.24), sodium chloride 0.9% (P = 0.10), apraclonidine (P = 0.19), dorzolamide (P = 0.21), and timolol (P = 0.67) showed no statistically significant changes.
The strongest reduction of break-up time was observed after latanoprost application, with statistical significance after 90 minutes (−23.8% after 30 minutes, −26.7% after 60 minutes, −51.4% after 90 minutes; P < 0.003). The fixed combination of dorzolamide and timolol reduced break-up time at all time points, with statistical significance after 90 minutes (−17.3%, P = 0.20; −18.3%, P = 0.10; −34.6%; P < 0.017). The change in break-up time in the BAK 0.02% group was −4.3% (P = 0.74) after 30 minutes, −6.5% (P = 0.67) after 60 minutes, and −39.1% (P < 0.003) at 90 minutes. In the BAK 0.005% group, the reductions were −3.6% (P = 0.71) after 30 minutes, −10.8% (P = 0.20) after 60 minutes, and −28.9% (P < 0.017) after 90 minutes. Break-up time after apraclonidine (−24.7%, −19.3%, −35.5%), brimonidine (−17.5%, −15.0%, −20.0%), dorzolamide (±0%, −8.2%, −21.2%), timolol (−2.2%, −16.3%, −7.6%), and sodium chloride 0.9% (−7.3%, 0%, 21.9%) showed trends towards reduction but did not reach statistical significance.
Basal secretion
Results of the basal secretion test are shown in Figures 3 and 4. Similar to the case with break-up time, measurement results were normalized to 100%, and the relative changes in basal secretion were determined. Neither the baseline results (P = 0.99) nor the results after 30 minutes (P = 0.96), 60 minutes (P = 0.91), or 90 minutes revealed any statistically significant differences between the medications (data not shown).
Figure 3.
Relative changes of basal secretion of brimonidine, latanoprost, apraclonidine, dorzolamide, the fixed combination dorzolamide/timolol and timolol 60 minutes before (T−60) and 30 (T+30), 60 (T+60) and 90 (T+90) minutes after drop application.
Notes: *P < 0.05; **P < 0.01; ***P < 0.001.
Abbreviation: BS, basal secretion.
Figure 4.
Relative changes of basal secretion of BAK 0.02%, BAK 0.005% and sodium chloride 0.9% 60 minutes before (T−60) and 30 (T+30), 60 (T+60), and 90 (T+90) minutes after drop application.
Note: **P < 0.01.
Abbreviations: BS, basal secretion; BAK, benzalkonium chloride.
Analysis of statistically significant differences associated with individual medications during the time course showed significant changes after application of brimonidine (P < 0.001), latanoprost (P < 0.001), BAK 0.02% (P = 0.02), BAK 0.005% (P = 0.02), sodium chloride 0.9% (P = 0.02), apraclonidine (P < 0.001), and dorzolamide (P < 0.001). Basal-secretion changes after timolol (P = 0.09) and after the fixed combination of dorzolamide and timolol (P = 0.27) were not statistically significant. The strongest reduction in basal secretion was observed after brimonidine (−17.8%, P = 0.002 after 30 minutes; −22.5%, P < 0.001 after 60 minutes; −30.5%, P < 0.001 after 90 minutes), followed by apraclonidine (−10%, P = 0.06; −20.1%, P = 0.002; −22.1%, P = 0.002), latanoprost (−2.4%, P = 0.64; −18.6%, P = 0.0006; −20.1%, P = 0.001) and BAK 0.02% (−1.6%, P = 0.67; −13.1%, P = 0.03; −11.6%, P = 0.002), with a significant reduction after 90 minutes only.
Timolol (−4.7%; −8.6%; −14.3%) and the f ixed combination of dorzolamide and timolol showed a trend towards reduction of basal secretion but did not reach statistical significance. After adjustment of the P value, neither sodium chloride 0.9% (−6.7%, P = 0.24; −13.4%, P = 0.03; −14.4%, P = 0.03) nor BAK 0.005% (0%, P = 0.1; −10.1%, P = 0.13; −16.7%, P = 0.03) reached statistical significance. BAK 0.02% showed a significant reduction in basal secretion after 90 minutes (−1.6%, P = 0.67; −13.1%, P = 0.03; −11.6%, P = 0.002).
Corneal sensitivity
Similar to break-up time and basal secretion, measurement results were normalized to 100% and the relative changes of corneal sensitivity were determined. Figures 5 and 6 indicate the relative changes in corneal sensitivity five, 10, and 15 minutes after drop application in relation to baseline examination. Data analysis revealed no significant differences in corneal sensitivity between the different drops after five minutes (P = 0.59), 10 minutes (P = 0.69), or 15 minutes (P = 0.69, data not shown).
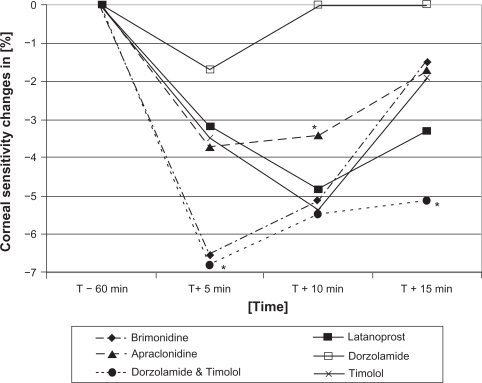
Figure 5.
Relative changes of corneal sensitivity of brimonidine, latanoprost, apraclonidine, dorzolamide, the fixed combination dorzolamide/timolol and timolol 60 minutes before (T−60) and 5 (T+5), 10 (T+10) and 15 (T+15) minutes after drop application.
Note: *P < 0.05.
Figure 6.
Relative changes of corneal sensitivity of BAK 0.02%, BAK 0.005% and sodium chloride 0.9% 60 minutes before (T−60) and 5 (T+5), 10 (T+10) and 15 (T+15) minutes after drop application.
After five minutes, the strongest reduction in corneal sensitivity was observed after brimonidine and the fixed combination of timolol and dorzolamide (both −6.8%), but only after brimonidine was the reduction of corneal sensitivity statistically significant (P = 0.005). Latanoprost (−3.3%), dorzolamide (−1.7%), apraclonidine (−3.4%), and timolol (−3.4%) showed tendencies towards reduction of corneal sensitivity, but these were not statistically significant.
After 10 minutes, apraclonidine showed a significant reduction of −3.4% (P = 0.007). Latanoprost (−5.0%), dorzolamide (±0.0%), timolol (−5.2%), and the fixed combination of timolol and dorzolamide (−5.1%) showed no significant reduction in corneal sensitivity.
After 15 minutes, only the fixed combination of dorzolamide and timolol (−5.1%) showed a significant reduction of corneal sensitivity (P = 0.01). Latanoprost (−3.3%), apraclonidine (−1.7%), brimonidine (−1.7%), and timolol (−1.7%) showed no significant reduction. Dorzolamide showed no change of corneal sensitivity after 15 minutes (±0%).
Reductions of corneal sensitivity after BAK 0.02% (−1.7% after five minutes; ±0% after 10 minutes; −1.7% after 15 minutes), BAK 0.005% (−1.7%; 0%; −1.7%) and sodium chloride 0.9% (−1.7%; 0%; 0%) were not statistically significant.
No statistically significant results were found between men and women for break-up time, Jones test, or corneal sensitivity.
Discussion
Because glaucoma therapy represents a long-term treatment, symptoms usually become worse during the course of treatment, eventually leading to a reduction in compliance or to break-up of drop application. This results from the negative effects of antiglaucoma medication and its preservatives on the corneal tear film,5,6 and these become worse with increasing amounts of drops being applied. Recent studies investigating the effect of topical antiglaucoma medication on tear secretion,11,12 tear-film stability,13,14 and corneal sensitivity15,16 have mainly focused on the side effects of topical β-blockers. Quantitative investigations of relatively new antiglaucoma medications are limited at the present time. Therefore, this study was conducted to address this issue.
Latanoprost induced the highest reduction in break-up time, ie, to 50%. In contrast, Thygesen et al17 observed no changes in break-up time at 30 minutes and 28 days after latanoprost therapy. There are some methodological differences between our study and the one by Thygesen et al in that in our study, baseline measurement was performed one day before the first drop application, and the amount and concentration of fluorescein used was different in both studies (2.5 μL versus 10 μL and 1% versus 0.2%). Although latanoprost contains the highest amount of BAK (0.02%), the application of BAK alone led to a significant reduction of break-up time (40%), but not until 90 minutes had passed. After 30 and 60 minutes, no significant changes could be observed. As a consequence, it is conceivable that the high concentration of BAK is not only responsible for the reduction in break-up time but also of latanoprost itself. This finding is supported by the result for apraclonidine, containing BAK 0.01%, leading to a reduction of break-up time, although this was not statistically significant.
The short-term effect of preservative-containing timolol on break-up time showed a reduction of up to 16%, but this was not statistically significant. Strempel18 reported a reduction of 33% with both timolol and sodium chloride. The differences in percentage may be related to the time interval between drop application and the first break-up time measurement (20 minutes versus 30 minutes). Additionally, the abovementioned study was not a randomized, double-blind crossover study, but was an investigation of different groups of healthy subjects.
Break-up time was clearly higher after application of the fixed combination of dorzolamide and timolol than it was after application of dorzolamide and timolol separately. Even after taking the concentrations of BAK into account, and accounting for the approximately one-quarter higher BAK concentration than in the fixed combination, there is no reasonable explanation for our observation. It can only be assumed that the active agents themselves or other contributing factors which are included in the original substances are responsible for this effect on tear-film stability, which is, of course, rather hypothetical and cannot be derived from our data.
The reduction of basal secretion was highest in the apra-clonidine and brimonidine groups, which can be explained by vasoconstriction and the consequent water and electrolyte secretion by α-agonists.19 Moreover, α1-adrenergic receptors exist in lacrimal gland acinar cells, stimulating the secretion of proteins.20 However, it is not known whether activation of these receptors promotes an increase in tear secretion.
Basal secretion after latanoprost application was reduced significantly after 60 and 90 minutes (−18.6% and −20.1%, respectively). In contrast, in an animal model, prostaglandin E1 showed a stimulating effect on tear secretion of the lacrimal gland under the additional effect of β-receptors.21 No change in tear secretion was observed by Thygesen et al17 two hours after topical prostaglandin application, which is in contrast with our study results. However, there are three major factors which make a direct comparison of these two studies quite difficult. First, Thygesen et al performed Schirmer’s I test instead of the Jones test. Second, baseline measurement of basal secretion was conducted one day (versus 60 minutes) before drop application. Third, patients with primary open-angle glaucoma and ocular hypertension, a mean age of 69 years, and a drop abstention period of 3–4 weeks (versus healthy subjects with a mean age of 32 years) were included. Basal secretion was not significantly affected by the fixed combination of dorzolamide and timolol, which is surprising, because neither dorzolamide nor timolol alone induced a reduced basal secretion. There is no reasonable explanation for our observation, because sodium chloride and BAK in both concentrations affected basal secretion negatively.
Timolol induced a reduction in basal secretion (−14.3%) which was statistically significant after 90 minutes. Other studies have focused on the long-term effect of timolol on basal secretion. Merte and Merkle11 reported a reduction of 10.2% in basal secretion in 25 patients applying topical timolol in concentrations of 0.1% and 0.5% over two years. Other research reported reductions of 10%–25% in basal secretion.22 In contrast, Kitazawa and Tsuchisaka did not find any reduction in basal secretion after 13 months of topical timolol application.23 However, a temporary reduction in basal secretion after one week of treatment, as measured by fluorometry, was observed by Gobbels et al which resolved after 4–7 months.24 Kuppens et al also observed a significant reduction of basal secretion in glaucoma patients after six months of timolol therapy, in contrast with basal secretion in healthy subjects.25
In our study, corneal sensitivity decreased by approximately 5% from baseline after 10 minutes. Höh observed a significant reduction in corneal sensitivity at one minute after a single application of timolol 0.5%. After minutes 3, 6, 10, 15 and 30, no statistically significant changes were detected.26 Unfortunately, additional data about the short-term effect of β-blockers are only available for pindolol, levobunolol, befunolol, metripranolol, carteolol, bupranolol, and betaxolol.27–31
Latanoprost showed a decrease of 5% in corneal sensitivity after 10 minutes. In contrast, Thygesen et al did not detect any decrease in corneal sensitivity after 30 minutes or after 28 days.17
Consistent with the findings of Kohlhaas et al32 in our study we found that dorzolamide caused a reduction in corneal sensitivity after five, 10, and 15 minutes, but this was not statistically significant. In contrast with our study, the study by Kohlhaas et al32 determined measurements of corneal sensitivity using an electronic-optical esthesiometer.
To the best of our knowledge, the present study is the first in which the effect of apraclonidine, brimonidine, and the fixed combination of dorzolamide and timolol on corneal sensitivity has been investigated. Brimonidine and the fixed combination of dorzolamide and timolol showed the strongest reduction in sensitivity, but only the changes after five minutes (brimonidine) and after 15 minutes (fixed combination) were statistically significant. The statistical significance of the fixed combination is surprising, because neither timolol nor dorzolamide showed a trend towards a reduction of sensitivity.
There are three major limitations in our study. First, the short-term effects of antiglaucoma medication on break-up time, basal secretion, and corneal sensitivity were investigated only in healthy patients. An extrapolation of our results to patients with glaucoma is limited, because glaucoma therapy is mainly a long-term treatment and, therefore, alterations of the ocular surface with disturbances of tear film-stability are also the result of long-term drop application. Nevertheless, in light of a negative additive effect on tear-film stability and a higher prevalence of dry eye disease in glaucoma patients,33 it is conceivable that our results, observed in young and healthy participants, are also relevant for glaucoma patients.
Second, the measurement techniques that we used in our study resulted in considerable inter- and intraindividual variability, which could have influenced the data analysis. However, we chose a double-blind, crossover design, and all antiglaucoma medications in our study were subject to the same measurement procedures and were used in an identical study population. The double-blindness of the study design reduced the subjective influence of both the investigator and the participant on measurement results.
Third, due to methodological differences compared with other studies and limited availability of reliable data, a comparison of study results was difficult. Nevertheless, despite a certain heterogeneity in our study results, our data might be helpful for making treatment decisions for glaucoma patients who also suffer from ocular surface problems.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Broadway DC, Grierson I, O′ Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication I. The conjunctival cell profile. Arch Ophthalmol. 1994;112:1437–1445. doi: 10.1001/archopht.1994.01090230051020. [DOI] [PubMed] [Google Scholar]
- 2.Steuhl KP, Knorr M, Frohn A, Thiel HJ. Effect of anti-glaucoma eye drops on cell differentiation of the conjunctiva. Fortschr Ophthalmol. 1991;88:865–9. German. [PubMed] [Google Scholar]
- 3.Schwab IR, Linberg JV, Gioia VM, Benson WH, Chao GM. Foreshortening of the inferior conjunctival fornix associated with chronic glaucoma medications. Ophthalmology. 1992;99:197–202. doi: 10.1016/s0161-6420(92)32001-9. [DOI] [PubMed] [Google Scholar]
- 4.Nuzzi R, Vercelli A, Finazzo C, Cracco C. Conjunctiva and subcon-junctival tissue in primary open-angle glaucoma after long-term topical treatment: An immunohistochemical and ultrastructural study. Graefes Arch Clin Exp Ophthalmol. 1995;233:154–162. doi: 10.1007/BF00166608. [DOI] [PubMed] [Google Scholar]
- 5.Liesegang TJ. Conjunctival changes associated with glaucoma therapy: Implications for the external disease consultant and the treatment of glaucoma. Cornea. 1998;17:574–583. doi: 10.1097/00003226-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Sherwood MB, Grierson I, Millar L, Hitchings RA. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon’s capsule in glaucomatous patients. Ophthalmology. 1989;96:327–335. doi: 10.1016/s0161-6420(89)32888-0. [DOI] [PubMed] [Google Scholar]
- 7.Zirm M. Schirmer test and break-up time. In: Marquardt R, editor. Chronic Conjunctivitis – Dry Eye. Wien, NY: Springer Verlag; 1982. [Google Scholar]
- 8.Cochet P, Bonnet R. Lèstesie corneenne. Clin Ophthal. 1960;4:2–27. [Google Scholar]
- 9.Jones LT. The lacrimal secretory system and its treatment. Am J Ophthalmol. 1966;62:47–60. doi: 10.1016/0002-9394(66)91676-x. [DOI] [PubMed] [Google Scholar]
- 10.Feldmann F, Wood MM. Evaluation of the Schirmer tear test. Can J Ophthalmol. 1979;14:257–259. [PubMed] [Google Scholar]
- 11.Merte HJ, Merkle W. Results of long-term treatment of glaucoma with timolol ophthalmic solution. Klin Monatsbl Augenheilkd. 1980;177:562–571. German. [PubMed] [Google Scholar]
- 12.Petounis AD, Akritopoulos P. Influence of topical and systemic beta-blockers on tear production. Int Ophthalmol. 1989;13:75–80. doi: 10.1007/BF02028642. [DOI] [PubMed] [Google Scholar]
- 13.Marquardt R, Schubert T. Modification of tear film break-up time by beta blocker eyedrops without preservatives. Klin Monatsbl Augenheilkd. 1991;99:75–78. doi: 10.1055/s-2008-1046051. German. [DOI] [PubMed] [Google Scholar]
- 14.Baudouin C, de Lunardo C. Short-term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br J Ophthalmol. 1998;82:39–42. doi: 10.1136/bjo.82.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Buskirk EM. Adverse reactions from timolol administration. Ophthalmology. 1980;87:447–450. doi: 10.1016/s0161-6420(80)35215-9. [DOI] [PubMed] [Google Scholar]
- 16.Höh H. The local anesthetic effect and subjective tolerance of 0.5% befunolol and 1% pindolol in healthy eyes. Klin Monatsbl Augenheilkd. 1990;196:219–224. German. [PubMed] [Google Scholar]
- 17.Thygesen J, Aaen K, Theodorsen F, Kessing SV, Prause JU. Short-term effect of latanoprost and timolol eye drops on tear fluid and the ocular surface in patients with primary open-angle glaucoma and ocular hypertension. Acta Ophthalmol. 2000;78:37–44. doi: 10.1034/j.1600-0420.2000.078001037.x. [DOI] [PubMed] [Google Scholar]
- 18.Strempel I. Effect of commercial beta blockers and their combination with artificial tears on the duration of tear film stability. Ophthalmologica. 1987;195:61–68. doi: 10.1159/000309788. [DOI] [PubMed] [Google Scholar]
- 19.Dartt DA. Signal transduction and control of lacrimal gland protein secretion: A review. Curr Eye Res. 1989;8:619–636. doi: 10.3109/02713688908995762. [DOI] [PubMed] [Google Scholar]
- 20.Botelho SY, Martinez EV, Pholpramool C, Prooyen HC, Janssen JT, De Palau A. Modification of stimulated lacrimal gland flow by sympathetic nerve impulses in rabbit. Am J Physiol. 1976;230:80–84. doi: 10.1152/ajplegacy.1976.230.1.80. [DOI] [PubMed] [Google Scholar]
- 21.Pholpramool C. Secretory effect of prostaglandins on the rabbit lacrimal gland in vivo. Prostaglandins Med. 1979;2:185–192. doi: 10.1016/0161-4630(79)90102-2. [DOI] [PubMed] [Google Scholar]
- 22.De Popa DP, Andreescu G, Albu C. Tearing in a patient with glaucoma. Ophthalmologica. 1998;42:41–44. [PubMed] [Google Scholar]
- 23.Kitazawa Y, Tsuchisaka H. Effects of timolol on corneal sensitivity and tear production. Int Ophthalmol. 1980;3:25–29. doi: 10.1007/BF00136210. [DOI] [PubMed] [Google Scholar]
- 24.Gobbels M, Monks T, Spitznas M. Effect of topical 0.5% timolol on tear flow in patients with primary open-angle glaucoma as assessed by fluorophotometry. Ger J Ophthalmol. 1993;2:241–245. [PubMed] [Google Scholar]
- 25.Kuppens EV, Stolwijk TR, de Keizer RJ, van Best JA. Basal tear turnover and topical timolol in glaucoma patients and healthy controls by fluorophotometry. Invest Ophthalmol Vis Sci. 1992;33:3442–3448. [PubMed] [Google Scholar]
- 26.Höh H. Corneal sensitivity after single doses of timolol, betaxolol or placebo in persons with healthy eyes – a randomized, prospective double-blind study. Fortschr Ophthalmol. 1988;85:132–138. German. [PubMed] [Google Scholar]
- 27.Höh H. The local anesthetic effect and subjective tolerance of 0.5% befunolol and 1% pindolol in healthy eyes. Klin Monatsbl Augenheilkd. 1990;196:219–224. German. [PubMed] [Google Scholar]
- 28.Höh H. Local anesthetic effect and subjective tolerance of 0.5% levobunolol in normal eyes. Klin Monatsbl Augenheilkd. 1990;197:20–26. German. [PubMed] [Google Scholar]
- 29.Höh H. Local anesthetic effect and subjective tolerance of 2% carteolol and 0.6% metripranolol on healthy eyes. Klin Monatsbl Augenheilkd. 1989;194:241–248. German. [PubMed] [Google Scholar]
- 30.Bastian B, Höh H. Local anesthetic effect and subjective tolerance of a single dose of 0.25% bupranolol in probands with healthy eyes. A randomized parallel double-blind study. Klin Monatsbl Augenheilkd. 1991;198:25–27. doi: 10.1055/s-2008-1045923. German. [DOI] [PubMed] [Google Scholar]
- 31.Höh H. Corneal sensitivity after single doses of timolol, betaxolol or placebo in persons with healthy eyes – a randomized, prospective double-blind study. Fortschr Ophthalmol. 1988;85:132–138. German. [PubMed] [Google Scholar]
- 32.Kohlhaas M, Mammen A, Richard G. Change in corneal sensitivity after topical dorzolamide adminutesistration. A comparative study. Ophthalmologe. 1997;94:424–427. doi: 10.1007/s003470050137. German. [DOI] [PubMed] [Google Scholar]
- 33.Rossi GC, Tinelli C, Pasinetti GM, et al. Dry eye syndrome-related quality of life in glaucoma patients. Eur J Ophthalmol. 2009;19:572–579. doi: 10.1177/112067210901900409. [DOI] [PubMed] [Google Scholar]