Abstract
A steady increase in the incidence of Guillain-Barré syndrome (GBS) with a seasonal preponderance, almost exclusively related to Campylobacter jejuni, and a rise in the incidence of laboratory-confirmed Campylobacter enteritis have been reported from Curaçao, Netherlands Antilles. We therefore investigated possible risk factors associated with diarrhea due to epidemic C. jejuni. Typing by pulsed-field gel electrophoresis identified four epidemic clones which accounted for almost 60% of the infections. One hundred six cases were included in a case-control study. Infections with epidemic clones were more frequently observed in specific districts in Willemstad, the capital of Curaçao. One of these clones caused infections during the rainy season only and was associated with the presence of a deep well around the house. Two out of three GBS-related C. jejuni isolates belonged to an epidemic clone. The observations presented point toward water as a possible source of Campylobacter infections.
Recently, a steady increase from 1992 to 1999 in the incidence of Guillain-Barré syndrome (GBS) in Curaçao, Netherlands Antilles, was reported (31). A seasonal preponderance and a more-severe clinical course of the disease were remarkable features of GBS on this small Caribbean island located off the northwestern coast of Venezuela. The clinical characteristics, together with serological data, pointed toward an important role for Campylobacter spp. in the pathogenesis of this disease (31). In addition, although there is no consistent surveillance system for reporting clinical cases of intestinal disease, the number of Campylobacter jejuni strains isolated in Curaçao had risen from 45 per 100,000 population in 1988 to 1998 (31) to 120 per 100,000 in 1999 to 2000 (H. P. Endtz, unpublished data). These rates are estimated from the number of isolates obtained from diarrheal stools by the only central microbiology laboratory on the island and from demographic data. During this period, the number of specimens submitted did not rise in proportion to the population and laboratory techniques remained unchanged.
In order to clarify the complex epidemiology of Campylobacter infections in Curaçao, we studied the genetic heterogeneity of Campylobacter isolates from patients with enteritis and investigated possible risk factors associated with infections due to epidemic clones of C. jejuni.
(Part of the information in this report was presented at the 11th International Workshop on Campylobacter, Helicobacter, and Related Organisms in Freiburg, Germany, 1 to 5 September 2001 [abstr. Q05 and N26].)
MATERIALS AND METHODS
Logistics.
Curaçao has only one central microbiology laboratory. Isolation and presumptive identification of campylobacters were performed in Curaçao. All Campylobacter spp. isolated during the study period were freeze-dried and transported to Rotterdam, The Netherlands, for further phenotypic and genetic characterization and to Winnipeg, Canada, for biotyping and serotyping.
Population.
We collected all Campylobacter strains isolated during a 12-month period starting 15 March 1999.
For the case-control study, the source population consisted of inhabitants from Curaçao with diarrhea or abdominal symptoms whose stool samples were submitted to the laboratory between 1 December 1999 and 1 March 2000. A case was defined as a patient with a positive Campylobacter stool culture. For each case, a control patient was selected from a laboratory list of submitted fecal samples. On this list, the nearest sample from a patient of the same sex and in the same (5-year) age category which was negative for Campylobacter, Salmonella, and Shigella spp. was identified. No active effort was made to seek out cases. Also, no collaboration was sought with general practitioners and hospital specialists in order to obtain more samples than usual. For the present study, we used only fecal samples submitted by physicians as part of their routine clinical practice.
Identification and characterization.
Stool samples from patients with gastroenteritis were inoculated on Blaser-Wang Campylobacter selective medium (Oxoid, Basingstoke, United Kingdom) and incubated under a microaerobic atmosphere at 37°C for 48 h. Presumptive identifications were made on the basis of morphology, Gram staining, and catalase, oxidase, and hippurate hydrolysis. The definitive identification was made by a Campylobacter PCR-Line Probe assay (PCR-LiPa; Innogenetics, Ghent, Belgium) (30).
Genetic characterization was performed by pulsed-field gel electrophoresis (PFGE) as previously described (8). Briefly, samples of genomic DNA extracted from overnight cultures of the isolates were digested with SmaI (Boehringer Mannheim, Mannheim, Germany). Electrophoresis was performed in 1% SeaKem agarose in 0.5× Tris-borate-EDTA by using a Bio-Rad CHEF mapper, programmed in the autoalgorithm mode (run time, 19 h; switch time, 6.75 to 25 s). Gels were stained with ethidium bromide for 15 min, destained in distilled water for 1 h, and photographed using UV irradiation. The gels were inspected visually by two independent investigators. The patterns were interpreted according to the criteria described by Tenover et al. (28). Isolates that differed by one to three bands, consistent with one single differentiating genetic event, were assigned the same capital letter, with a number designating the subtype. Four or more band differences between two isolates indicated distinct genotypes, which were designated by different capital letters.
Serotyping was performed at the National Laboratory for Enteric Pathogens, Winnipeg, Canada, by using the heat-stable (HS or O) and heat-labile (HL) serotyping schemes of Penner et al. (23) and Lior et al. (14), respectively.
Case-control study.
All physicians who had submitted fecal specimens for the cases and controls were contacted, and consent was obtained to contact the patients. Subsequently, we sent out invitations to participate in the study. Patients were called at home after a few days' interval and asked if they agreed to participate and to be interviewed. If the case patient was <12 years old, the interview was conducted with an adult living in the household of the child. Case patients between the ages of 13 and 18 years were interviewed in the presence of the parent or guardian. The language used was either Dutch, the official language, or Papiamento, the language most commonly spoken. Respondents who could not communicate in Dutch or Papiamento were excluded from the study. Those who agreed to participate were visited by the interviewers (H.V.W. and L.D.H.). All case and control patients were interviewed by using a standardized questionnaire.
The Department of Spatial and Urban Planning of Curaçao has divided the island into 65 geographical zones, which have been used previously in epidemiological studies (2). The domiciles of all case and control patients were thus classified according to these zones. The interview included questions about water and food consumption and handling, exposure to animals, lifestyle habits, and socioeconomic status (SES). The SES score was composed of educational level and net household income. The educational level was encoded according to the International Standard Classification of Education, developed by the United Nations Educational, Scientific, and Cultural Organization. Participants classified their net household monthly incomes in 1 of 11 categories ranging from less than NAF 500 to more than NAF 5,000 (NAF 1 = U.S. $0.57) (2).
Subgroup analysis.
Four subgroups were identified a priori as deserving separate analysis. Because the epidemiology of campylobacter infections in young children may differ from that for adults, we conducted analyses for two age strata: children <5 years old (n = 18) and persons ≥5 years old (n = 88). Furthermore, the observed clonality of specific PFGE types warranted subgroup analysis, since particular clonal types could be associated with common sources and transmission routes. Thus, two more subgroups were identified: one group of cases belonging to all epidemic clones (PFGE types A, D, G and M) (n = 53) and one group of cases with the seasonal and highly prevalent C. jejuni PFGE type M (n = 22). For the latter analyses, subgroups of case patients were compared with all control patients.
Statistical analysis.
Pearson's chi-square test was used to analyze differences in percentages. Differences between means were tested by the Student t test. All tests were two-tailed and were considered significant at a P value of ≤0.05.
RESULTS
Microbial identification and serological and molecular characterization.
A total of 240 Campylobacter isolates were recovered from 240 patients, including 2 from patients with GBS. Five isolates were not viable upon arrival in Rotterdam; thus, 235 of 240 isolates were available for the study. One additional C. jejuni isolate, recovered in May 2000 from a GBS patient, was also characterized. Out of 236 isolates obtained during the study period, 235 were identified as C. jejuni and 1 was identified as Campylobacter coli.
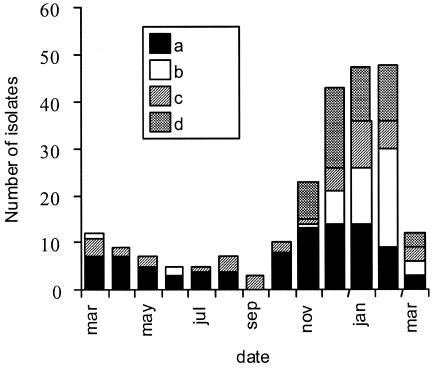
PFGE analysis was performed on all isolates. The PFGE patterns comprised six to nine bands. Eleven PFGE patterns were observed more than once and accounted for 193 (85%) of the 236 isolates. PFGE patterns A (46 of 236; 19%) and M (45 of 236; 19%) were observed most frequently, followed by patterns G (29 of 236; 12%) and D (17 of 236; 7%). Thus, only four PFGE patterns (A, D, G, and M) accounted for 137 (58%) of the 236 C. jejuni isolates; these were designated “epidemic clones.” The three clonal types A, D, and G (92 of 236; 39%) were present throughout the year (Fig. 1). In contrast, PFGE types L, M, P, T, V, X, Y, and Z (102 of 236; 43%) were found almost exclusively during the rainy season (November through February; 91 of 102 isolates [90%]). Two out of three GBS-related isolates had PFGE pattern A, while the third had a unique PFGE pattern not observed in any other strain.
FIG. 1.
Number and seasonal distribution of C. jejuni isolates of different PFGE types isolated in Curaçao between 15 March 1999 and 15 March 2000. Designations: a, year-round epidemic strains (PFGE types A, G, and D); b, seasonal epidemic strain (PFGE type M); c, all unique strains; d, other strains.
A selection of 20 isolates, representing 4 to 6 C. jejuni isolates of each of the major clonal PFGE types, were serotyped. The congruent results of PFGE, HS serotyping, and HL serotyping are shown in Table 1. The Penner O:4 complex (O:4, O:13, O:50, and O:64) was distributed into two PFGE groups: PFGE type A contained the O:13 and O:50 isolates, and PFGE type G contained the O:4 and O:64 isolates. Moreover, PFGE type D consisted almost exclusively of O:41 isolates.
TABLE 1.
HL and HS serotyping and biotyping of 20 clonally related C. jejuni isolates from Curaçao, Netherlands Antilles
| Strain no. | PFGE group | Biotype | HL serotype | HS serotype |
|---|---|---|---|---|
| CURA 7a | A | III | 7 | 0:4, 0:13 |
| CURA 27 | A | III | 51 | 0:13, 0:50 |
| CURA 29 | A | III | 51 | 0:13, 0:65 |
| CURA 30 | A | III | 7 | 0:13, 0:43 |
| CURA 40 | A | III | 51 | 0:13, 0:50 |
| CURA 276a | A | III | 7 | 0:13, 0:50 |
| CURA 14 | D | III | 27 | 0:41 |
| CURA 16 | D | III | 27 | 0:41 |
| CURA 23 | D | III | 27 | 0:41 |
| CURA 32 | D | III | 27 | 0:41 |
| CURA 52 | D | I | 76 | 0:12 |
| CURA 24 | G | I | 1 | 0:4 |
| CURA 26 | G | I | 1 | 0:4, 0:64 |
| CURA 36 | G | I | 1 | 0:4 |
| CURA 37 | G | I | 125 | 0:4, 0:64 |
| CURA 15 | G | I | 1 | 0:4 |
| CURA 119 | M | I | 91 | UT |
| CURA 121 | M | I | 91 | 0:17 |
| CURA 129 | M | I | 91 | 0:8 |
| CURA 130 | M | I | 51 | 0:17 |
GBS-related strain.
Case-control study.
A total of 142 patients with culture-confirmed C. jejuni infections were contacted. Of these, 106 (75%) enrolled in the case-control study. The most frequently reported reasons for exclusion were as follows: no telephone available or no one answering the call, an incorrect or untraceable address, or a household contact already included in the study. Within the control group, 108 of 142 selected patients could be included. There were no significant differences between cases and controls with respect to mean age, sex, number of children in the household, educational level, and net household income (Table 2). However, the “under-5-year” category tended to contain fewer cases than controls. Exposure to food of various origins, poor hygiene during preparation of meals, and contact with various animals did not differ significantly between cases and controls. However, case patients more often reported diarrhea (P < 0.001). In the interview, special attention was given to the consumption and sources of water, milk, milk products, and eggs. No significant differences between the two groups were observed. Contact with or the presence of dogs, cats, goats, birds, and reptiles (iguana), foreign travel, and beach exposure were not related to Campylobacter infections; neither was the presence of a deep well around the house.
TABLE 2.
Baseline characteristics of case and control patients
| Characteristic | Value for group
|
|
|---|---|---|
| Case (n = 106) | Control (n = 108) | |
| Age (mean [SD]) | 26.7 (21.9) | 22.6 (19.9) |
| Sex (% female) | 43 | 51 |
| Education (on a scale of 1 to 8 [SD]) | 3.4 (1.9) | 3.3 (1.8) |
| Net household income (on a scale of 1 to 11 [SD]) | 6.2 (3.3) | 6.3 (3.3) |
| Civil state (% single) | 61.3 | 64.8 |
| No. in household (median) | 4 | 4 |
Subgroup analysis.
No specific risk factor was associated with Campylobacter infection in the age group below 5 years. It should be noted that the small number of cases in this subgroup resulted in limited power to detect differences between cases and controls.
Case patients with an epidemic clone of C. jejuni (PFGE type A, D, G, or M) reported diarrhea more often than controls (P < 0.001). In contrast to the results of analyses including cases with nonepidemic PFGE types, there appeared to be geographic clustering of the epidemic clones in districts 31 to 37 or 53 to 59, all located in the center of Willemstad, the capital of Curaçao (P = 0.8 versus P < 0.001). Almost half (48%) of case patients with PFGE type A, D, G, or M isolates were living in these districts versus 26% of those with other PFGE types. Also, 66% of case patients with PFGE type M were living in this central part of Willemstad (P < 0.001). In addition, more PFGE type M case patients than controls had a deep well in or around the house (P < 0.05).
DISCUSSION
This is the first report of an epidemiological study designed to investigate the genetic epidemiology of Campylobacter and to identify risk factors associated with Campylobacter infection in the Caribbean. Although Campylobacter is one of the leading causes of bacterial diarrhea worldwide, data on the incidence of Campylobacter infections in the Caribbean are lacking. A few small-scale studies, however, have examined the etiology of diarrhea in this region (6, 15, 22). In a group of young children from Guadeloupe with acute diarrhea, no Campylobacter was isolated (6). A study performed in Puerto Rico revealed an incidence of only 3% in a selected group of patients with gastroenteritis (15). Paredes et al. examined the stools of 332 travelers with diarrhea in Jamaica. C. jejuni was isolated from only 4 of 322 (1.2%) specimens (22). The low incidences reported in these studies contrast with concurrent data from Curaçao, where in the year 2000 C. jejuni was detected in 2.6% of all fecal samples submitted (E. Leyde, personal communication).
In contrast to other islands in the region, Curaçao has faced a considerable burden of GBS. It is tempting to speculate that the low incidence of Campylobacter infections in the rest of the Caribbean may also account for the low incidence of GBS in this region, as opposed to the high incidence of Campylobacter-associated GBS in Curaçao (31).
Although we depended on physicians to submit fecal specimens from patients with diarrhea to the microbiology laboratory, we have little doubt that these specimens were representative for all patients with relatively severe enteritis or abdominal complaints, because the entire Curaçao population has access to health care. However, one cannot exclude variation in health care-seeking behavior among different subgroups of the population.
PFGE has recently been proven to be a very useful epidemiological tool for the characterization of Campylobacter (7-9, 12, 19, 20, 24, 33). In our study, only two epidemic clones accounted for almost 40% of Campylobacter infections. The majority of infections were caused by four epidemic clones (PFGE types A, G, D, and M). One of these, PFGE type M, was isolated only during the rainy season. However, because analyses of isolates ended in March 2000, we could not detect the disappearance of type M at the end of the rainy season. The seasonal occurrence of a specific C. jejuni subtype has not been reported earlier in this area. van Koningsveld et al. described a seasonal occurrence of GBS in Curaçao (31). Two out of three patients with C. jejuni-related GBS had isolates of PFGE type A. It is not clear whether this C. jejuni PFGE type A represents a more pathogenic strain, more likely to induce GBS. Recent studies have shown that the lipo-oligosaccharide gene cluster of the GBS-associated isolates harbors genes that may have a role in the pathogenesis of polyneuropathy (29). Detection of these genes among the epidemic isolates from Curaçao may further highlight their putative involvement in neuropathogenicity.
The results we obtained by PFGE typing were corroborated by serotyping. A remarkable observation is the frequent isolation of C. jejuni O:41. All C. jejuni O:41 isolates were characterized as PFGE type D. In other parts of the world, this serotype is rarely isolated from diarrheal patients. However, C. jejuni O:41 isolates were found in association with GBS in South Africa, and these isolates appear to be genetically indistinguishable by amplified fragment length polymorphism (AFLP) analysis and flagellin typing, indicating that they are representative of a genetically stable clone (13, 32). AFLP analysis also will be performed on the isolates from Curaçao to investigate the genetic homogeneity of C. jejuni O:41 in two distant geographic areas; this work is in progress.
The case-control part of the study was designed to detect differences in risk factor profile between Campylobacter infections and culture-negative enteritis. Intake of contaminated food and water has been found in previous studies to be a major risk factor for acquisition of a Campylobacter infection (10). Among food-borne outbreaks, consumption of unpasteurized milk and cross-contamination events in the kitchen from raw poultry were most commonly implicated. Although the questionnaire in our study was very detailed with respect to the intake and sources of food and water, no association with any of these risk factors could be established. However, there did appear to be geographical clustering of the cases. Patients, especially those who were infected with one of the so-called “epidemic clones,” appeared to be concentrated in a specific area of Willemstad. Moreover, patients who had a deep well in or around their house were more often infected with C. jejuni PFGE type M. This association is interesting because it suggests that these wells are contaminated with a specific Campylobacter subtype and may play a role in the transmission of these Campylobacter subtypes to humans. The wells are sometimes connected to the tap water system, although more frequently the well water is used for gardening and may be the source of colonization of animals that live in or around the houses. Although our analyses did not reveal this association, close contacts with animals may still be a risk factor for the transmission of Campylobacter to humans. This has been shown to be the typical transmission route of Campylobacter in developing countries and for infants and young children (18, 27).
Several water sources have been identified as risk factors in the literature (1, 5, 16, 17, 25, 26). Surface waters and municipal water systems, but also seawater and sea sand, may be contaminated with Campylobacter species and are thus possible sources for Campylobacter enteritis (4, 11). Although we did not observe an association between beach attendance or the frequenting of specific beaches and Campylobacter-associated diarrhea, one cannot exclude a role of contaminated surface waters in this epidemic. Although a country health profile by the Pan American Health Organization (PAHO) reported on the excellent quality of the water from desalinization plants in 1998 (21), recurrent articles in the local press drew attention to the poor quality of the water supply in 2000, following reports of local outbreaks of diarrheal disease in Curaçao (3). Advice from the PAHO included permanent chlorination of the water. However, this measure was discontinued after several weeks.
We are aware of some limitations of the case-control study. First, enrollment in this study was 77%. Although the mean age and the district code did not appear to differ between those who enrolled and those who did not, bias may have been introduced at this point. Second, there is the possibility that the study is biased toward the null for risk factors common to both groups. Third, some controls may have been misclassified if antibiotics were used before specimen collection; such misclassification would also result in a decreased likelihood of finding an association. Fourth, this investigation was retrospective. In conclusion, the relationship between seasonal occurrence of epidemic Campylobacter isolates, the geographical clustering of cases with epidemic PFGE types, the presence of a well in or around the house, and recurrent contamination of the drinking water on the island is not clear. However, though the present evidence is circumstantial, the results of the case-control study and local observations in Curaçao do point toward the likelihood of water as a source of Campylobacter infections. Therefore, microbiological studies of tap and well water could disclose a source of C. jejuni. A larger, prospective epidemiological study with active case finding might circumvent the limitations of the present study design. Great effort should be directed to achieving optimal recovery of C. jejuni from the stools of GBS patients.
Acknowledgments
We thank Marian Humphrey for revision of the English text.
REFERENCES
- 1.Aho, M., M. Kurki, H. Rautelin, and T. U. Kosunen. 1989. Waterborne outbreak of Campylobacter enteritis after outdoors infantry drill in Utti, Finland. Epidemiol. Infect. 103:133-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberts, J. F., R. Sanderman, J. M. Eimers, and W. J. van den Heuvel. 1997. Socioeconomic inequity in health care: a study of services utilization in Curaçao. Soc. Sci. Med. 45:213-220. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 28 December 2000. Geen garantie voor kwaliteit drinkwater. Amigoe, Curaçao, Netherlands Antilles.
- 4.Bolton, F. J., S. B. Surman, K. Martin, D. R. Wareing, and T. J. Humphrey. 1999. Presence of Campylobacter and Salmonella in sand from bathing beaches. Epidemiol. Infect. 122:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brieseman, M. A. 1987. Town water supply as the cause of an outbreak of Campylobacter infection. N. Z. Med. J. 100:212-213. [PubMed] [Google Scholar]
- 6.Courouble, G., D. Dufillot, A. Sans, E. Malpote, C. Berchel, and M. Nicolas. 2000. Acute childhood gastroenteritis study at Central University Hospital of Pointe-a-Pitre/Abymes, Guadeloupe, from November 1997 to March 1998. Bull. Soc. Pathol. Exot. 93:58-61. (In French.) [PubMed] [Google Scholar]
- 7.de Boer, P., B. Duim, A. Rigter, J. van Der Plas, W. F. Jacobs-Reitsma, and J. A. Wagenaar. 2000. Computer-assisted analysis and epidemiological value of genotyping methods for Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 38:1940-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endtz, H. P., C. W. Ang, N. van Den Braak, B. Duim, A. Rigter, L. J. Price, D. L. Woodward, F. G. Rodgers, W. M. Johnson, J. A. Wagenaar, B. C. Jacobs, H. A. Verbrugh, and A. van Belkum. 2000. Molecular characterization of Campylobacter jejuni from patients with Guillain-Barré and Miller Fisher syndromes. J. Clin. Microbiol. 38:2297-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald, C., L. O. Helsel, M. A. Nicholson, S. J. Olsen, D. L. Swerdlow, R. Flahart, J. Sexton, and P. I. Fields. 2001. Evaluation of methods for subtyping Campylobacter jejuni during an outbreak involving a food handler. J. Clin. Microbiol. 39:2386-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, D.C.
- 11.Ghinsberg, R. C., L. Bar Dov, M. Rogol, Y. Sheinberg, and Y. Nitzan. 1994. Monitoring of selected bacteria and fungi in sand and sea water along the Tel Aviv coast. Microbios 77:29-40. [PubMed] [Google Scholar]
- 12.Hanninen, M. L., S. Pajarre, M. L. Klossner, and H. Rautelin. 1998. Typing of human Campylobacter jejuni isolates in Finland by pulsed-field gel electrophoresis. J. Clin. Microbiol. 36:1787-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lastovica, A. J., E. A. Goddard, and A. C. Argent. 1997. Guillain-Barré syndrome in South Africa associated with Campylobacter jejuni O:41 strains. J. Infect. Dis. 176(Suppl. 2):S139-S143. [DOI] [PubMed] [Google Scholar]
- 14.Lior, H., D. L. Woodward, J. A. Edgar, and L. J. LaRoche. 1981. Serotyping by slide agglutination of Campylobacter jejuni and epidemiology. Lancet ii:1103-1104. [DOI] [PubMed]
- 15.Lopez Ortiz, W., and R. A. Solivan. 1999. Campylobacter jejuni among patients with gastroenteritis: incidence at a reference microbiology laboratory in San Juan, Puerto Rico. P. R. Health Sci. J. 18:273-276. [PubMed] [Google Scholar]
- 16.Melby, K. K., J. G. Svendby, T. Eggebo, L. A. Holmen, B. M. Andersen, L. Lind, E, Sjogren, and B. Kaijser. 2000. Outbreak of Campylobacter infection in a subarctic community. Eur. J. Clin. Microbiol. Infect. Dis. 19:542-544. [DOI] [PubMed] [Google Scholar]
- 17.Millson, M., M. Bokhout, J. Carlson, L. Spielberg, R. Aldis, A. Borczyk, and H. Lior. 1991. An outbreak of Campylobacter jejuni gastroenteritis linked to meltwater contamination of a municipal well. Can. J. Public Health 82:27-31. [PubMed] [Google Scholar]
- 18.Oberhelman, R. A., and D. N. Taylor. 2000. Campylobacter infections in developing countries, p. 139-153. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, D.C.
- 19.Olsen, S. J., G. R. Hansen, L. Bartlett, C. Fitzgerald, A. Sonder, R. Manjrekar, T. Riggs, J. Kim, R. Flahart, G. Pezzino, and D. L. Swerdlow. 2001. An outbreak of Campylobacter jejuni infections associated with food handler contamination: the use of pulsed-field gel electrophoresis. J. Infect. Dis. 183:164-167. [DOI] [PubMed] [Google Scholar]
- 20.Owen, R. J., K. Sutherland, C. Fitzgerald, J. Gibson, P. Borman, and J. Stanley. 1995. Molecular subtyping scheme for serotypes HS1 and HS4 of Campylobacter jejuni. J. Clin. Microbiol. 33:872-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan American Health Organization. 1998. Health in the Americas, p. 375-381. Pan American Health Organization, Washington, D.C.
- 22.Paredes, P., S. Campbell-Forrester, J. J. Mathewson, D. Ashley, S. Thompson, R. Steffen, Z. D. Jiang, A. M. Svennerholm, and H. L. DuPont. 2000. Etiology of travelers' diarrhea on a Caribbean island. J. Travel Med. 7:15-18. [DOI] [PubMed] [Google Scholar]
- 23.Penner, J. L., J. N. Hennessy, and R. V. Congi. 1983. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur. J. Clin. Microbiol. 2:378-383. [DOI] [PubMed] [Google Scholar]
- 24.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosef, O., G. Rettedal, and L. Lageide. 2001. Thermophilic campylobacters in surface water: a potential risk of campylobacteriosis. Int. J. Environ. Health Res. 11:321-327. [DOI] [PubMed] [Google Scholar]
- 26.Stehr-Green, J. K., C. Nicholls, S. McEwan, A. Payne, and P. Mitchell. 1991. Waterborne outbreak of Campylobacter jejuni in Christchurch: the importance of a combined epidemiologic and microbiologic investigation. N. Z. Med. J. 104:356-358. [PubMed] [Google Scholar]
- 27.Tenkate, T. D., and R. J. Stafford. 2001. Risk factors for campylobacter infection in infants and young children: a matched case-control study. Epidemiol. Infect. 127:399-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Belkum, A., N. van Den Braak, P. Godschalk, W. Ang, B. Jacobs, M. Gilbert, W. Wakarchuk, H. Verbrugh, and H. Endtz. 2001. A Campylobacter jejuni gene associated with immune-mediated neuropathy. Nat. Med. 7:752-753. [DOI] [PubMed] [Google Scholar]
- 30.van Doorn, L. J., A. Verschuuren-van Haperen, A. Burnens, M. Huysmans, P. Vandamme, B. A. Giesendorf, M. J. Blaser, and W. G. Quint. 1999. Rapid identification of thermotolerant Campylobacter jejuni, Campylobacter coli, Campylobacter lari, and Campylobacter upsaliensis from various geographic locations by a GTPase-based PCR-reverse hybridization assay. J. Clin. Microbiol. 37:1790-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Koningsveld, R., R. Rico, I. Gerstenbluth, P. I. Schmitz, C. W. Ang, I. S. Merkies, B. C. Jacobs, Y. Halabi, H. P. Endtz, F. G. van Der Meche, and P. A. van Doorn. 2001. Gastroenteritis-associated Guillain-Barré syndrome on the Caribbean island Curaçao. Neurology 56:1467-1472. [DOI] [PubMed] [Google Scholar]
- 32.Wassenaar, T. M., B. N. Fry, A. J. Lastovica, J. A. Wagenaar, P. J. Coloe, and B. Duim. 2000. Genetic characterization of Campylobacter jejuni O:41 isolates in relation with Guillain-Barré syndrome. J. Clin. Microbiol. 38:874-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wassenaar, T. M., and D. G. Newell. 2000. Genotyping of Campylobacter spp. Appl. Environ. Microbiol. 66:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]