Abstract
Macrolide resistance was detected in 64 of 77 (83.1%) Streptococcus pneumoniae isolates from South Korea. Seven (10.9%) isolates contained only mef(A), 32 (50%) contained only erm(B), and 25 (39.1%) contained mef(A) and erm(B). Nineteen isolates containing mef(A) and erm(B) belonged to serotype 19F, and seven isolates were identical to the Taiwan19F-14 clone.
Antimicrobial resistance rates in Streptococcus pneumoniae isolates in South Korea are believed to be the highest in the world (11, 12), but few reports have been published about macrolide-resistant S. pneumoniae (MRSP) isolates in South Korea. The present study was undertaken to evaluate the prevalence, mechanisms, and molecular epidemiology of macrolide resistance in a collection of S. pneumoniae isolates obtained in Seoul from 1996 to 1998.
Forty-five isolates of S. pneumoniae were obtained from throat swabs of healthy children ranging in age from less than 1 year to 5 years old and attending four day care centers. Another 32 isolates were obtained from the respiratory tract, blood, cerebrospinal fluid, and other body sites of children and adults cared for at Ewha Woman's University Tongdaemun Hospital. Organisms were stored frozen at −70°C. Capsular serogroup determinations were performed by agglutination with anticapsular antibodies (Statens Seruminstitut, Copenhagen, Denmark).
The MICs of erythromycin, clindamycin, and penicillin were determined on subcultures prepared from a single colony cloned from stock cultures by the agar gradient diffusion (E-test) technique (AB BIODISK, Piscataway, N.J.) according to the manufacturer's instructions. MRSP isolates were further characterized by PCR assay with primers for erm(B) and mef(A) (16) and by pulsed-field gel electrophoresis (PFGE) (6). Organisms were designated as indistinguishable, closely related, or unrelated according to the criteria described by Tenover et al. (15). PFGE was also performed on 16 international S. pneumoniae clones (6).
Macrolide resistance (erythromycin MIC of >0.25 μg/ml) was documented in 35 of 45 (77.8%) carriage isolates, in 29 of 32 (90.6%) isolates associated with disease, and in 83.1% of isolates overall. Sixty of 64 (93.8%) MRSP isolates were also resistant to penicillin (MIC of >0.06 μg/ml), and 57 (89%) were resistant to clindamycin (MIC of >0.25 μg/ml). A total of 50% of the 64 MRSP isolates contained erm(B) alone, while 39.1% contained both erm(B) and mef(A). The remaining 10.9% contained only mef(A). Isolates containing both erm(B) and mef(A) were obtained from adults and children. Thirteen of these isolates were obtained from surveillance throat cultures, and 12 were obtained from clinical specimens from hospitalized patients. No differences were noted between carriage isolates and those associated with disease with respect to the mechanism of macrolide resistance. Isolates containing both erm(B) and mef(A) were detected in middle-ear discharge, blood, and cerebrospinal fluid specimens from hospitalized patients as well as from the throats of healthy children.
Isolates containing erm(B) alone or erm(B) in combination with mef(A) exhibited the typical macrolide-lincosamide-streptogramin B pattern of resistance in that the MICs of erythromycin and clindamycin were elevated (>256 μg/ml). Isolates containing only mef(A) were fully susceptible to clindamycin (MICs of ≤0.25 μg/ml) and had lower MICs of erythromycin (≤4 μg/ml).
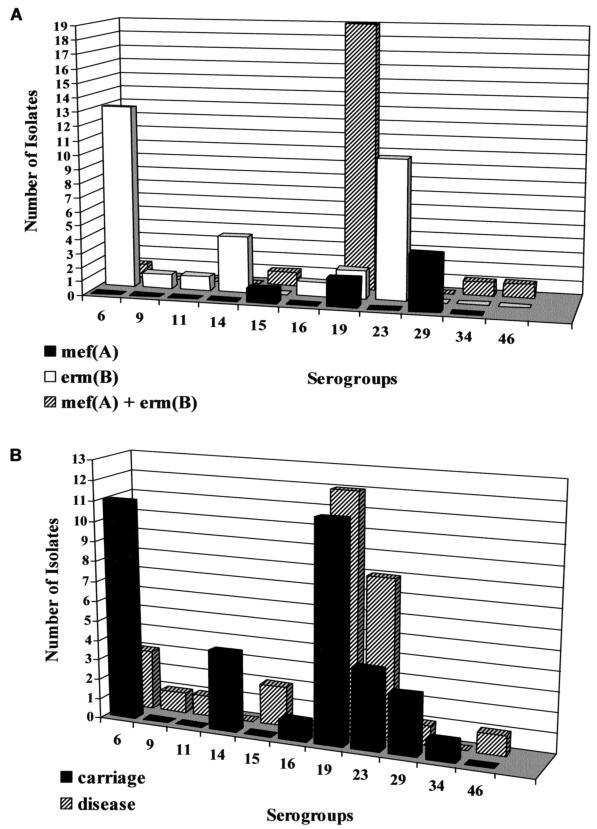
The distribution of serogroups according to the mechanism of resistance and to source is shown in Fig. 1. Serogroups 6, 19, and 23 together accounted for 76.6% of all MRSP isolates. Thirty-two isolates containing erm(B) alone were distributed among seven different serogroups, whereas seven isolates containing mef(A) alone belonged to three serogroups. Among 25 isolates containing both erm(B) and mef(A), serogroup 19 accounted for 76%, with the remaining isolates divided among five other serogroups. Minimal differences in serogroup distribution were noted between carriage isolates (seven serogroups) and those associated with disease (eight serogroups). Four serogroups (6, 19, 23, and 29) were common to both the carriage group and the disease group.
FIG. 1.
Serogroup distribution of S. pneumoniae isolates according to mechanism of resistance (A) and source (B).
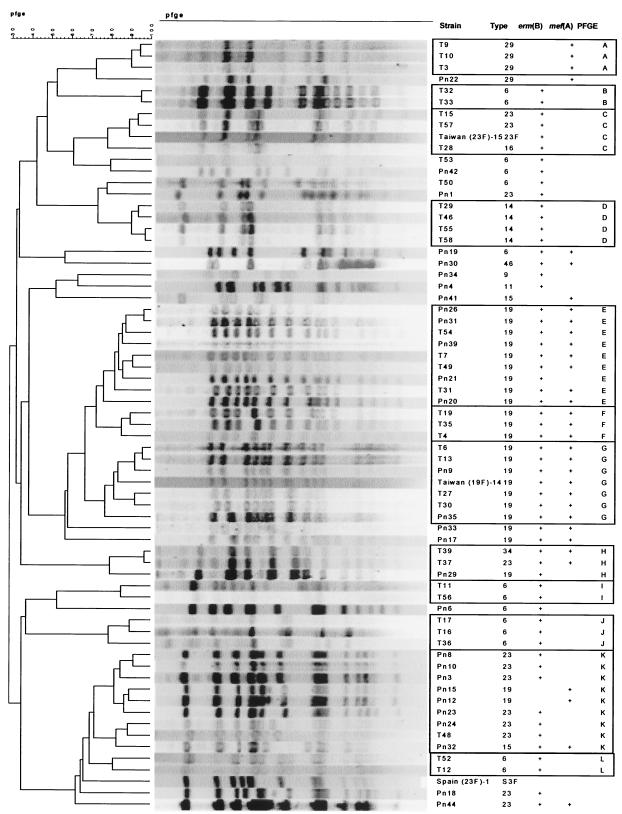
PFGE analysis (Fig. 2) showed 12 groupings covering 49 MRSP isolates. Most MRSP isolates that were grouped together by PFGE belonged to a single serogroup. Seven groups (A, B, C, D, I, J, and L) consisted solely of 19 isolates from surveillance throat cultures. Five groups (E, F, G, H, and K) were comprised of 30 isolates obtained from surveillance throat cultures as well as from patients with disease. The remaining 15 isolates were not closely related to one another and represented unique PFGE profiles. Groups E, F, and G, comprising 17 of 25 (68%) isolates containing both erm(B) and mef(A), were identical or very closely related, with only two or three band differences. All of these isolates belonged to serogroup 19. Seven isolates in group G, including both carriage and invasive organisms, were identical to international clone Taiwan19F-14. A second international clone, Taiwan23F-15, was identical to carriage isolates T15 and T57 in group C. The Spain23F-1 clone, known to be common in drug-resistant pneumococci in South Korea, was surprisingly not related to any of the MRSP isolates. We were also unable to demonstrate relationships between isolates in this collection and other international pneumococcal clones (6) (PFGE data not shown). McGee et al. (9) reported the dissemination of a serotype 19F multidrug-resistant pneumococcal clone containing erm(B) and mef(A) in South Africa that was later shown to be identical to Taiwan19F-14 (L. McGee, personal communication). Our PFGE analysis indicated that this international clone is present in Korea and that it has also acquired dual resistance mechanisms, just as in South Africa.
FIG. 2.
PFGE analysis of 25 macrolide-resistant S. pneumoniae isolates showing 12 groupings of 49 isolates. The remaining 15 isolates are not closely related to one another and represent unique PFGE profiles. The seven isolates in group G are closely related or identical to international clone Taiwan19F-14. T, isolate obtained from surveillance throat culture; Pn, isolate obtained from patient with disease.
The horizontal transfer of capsular biosynthetic genes has also been documented in South Korea, resulting in the capsular transformation of multidrug-resistant serotype 23F to serotypes 19F and 14, as evidenced by homogeneous PFGE patterns (8, 14). In our study, in the cases of groups C, H, and K, the placement of organisms of different serogroups within a single group suggests that capsular transformation may have occurred in this group of organisms.
Erythromycin resistance has now exceeded penicillin resistance in South Korea (5, 7, 12). The highest erythromycin resistance rate reported from South Korea previously was 87.6%, as recorded in the PROTEKT surveillance study for 137 isolates collected from 1999 to 2000 (5). Erythromycin resistance rates for carriage and invasive S. pneumoniae isolates collected in South Korea in the late 1990s as reported by the Asian Network for surveillance of Resistant Pathogens (ANSORP) were 74.6% and 77.8%, respectively. Our findings are consistent with these reports.
These high levels of pneumococcal antimicrobial resistance are undoubtedly related to antibiotic pressure due to the widespread prescribing of ampicillin, erythromycin, and oral cephalosporins for upper respiratory infections and to the sale of many of these products without physician oversight (11). Our most striking finding was the presence of dual mechanisms of resistance involving both the mef(A) and erm(B) genes in 39% of the MRSP isolates. The few reports of dual mechanisms of macrolide resistance in S. pneumoniae isolates have most often involved 4% or fewer of the isolates studied. However, a report from Japan found that 16.1% of MRSP isolates contained both mef(A) and erm(B) (10), and a serotype 19F pneumococcal clone containing both genes and accounting for 30.5% of 118 MRSP isolates was documented in South Africa (9). Farrell et al. (4) analyzed 120 MRSP isolates from South Korea and found results similar to those from our study in that erm(B) alone was present in 43.3% of the isolates and mef(A) alone was present in 18.4%, while both genes were present in 38.3%.
All 64 MRSP isolates could be categorized as having erm(B), mef(A), or both genes. Thus, nucleotide substitutions in the 23S rRNA or in genes that encode ribosomal proteins L4 and L2 that are sometimes involved with macrolide resistance in S. pneumoniae were not present independently (2, 13). Whether these mechanisms of resistance might have been present in addition to the other genes was not assessed. A recent study of pneumococcal isolates from several different countries indicated a rate of only 1.5% for MRSP isolates not mediated by erm(B) and/or mef(A) (3), but no large-scale studies have been performed to determine how commonly such alternative mechanisms might be present in organisms that already contain mef(A) and/or erm(B).
The high prevalence of serotypes 19F and 23F is one of several explanations for the high frequencies of drug resistance in South Korea since these serotypes are particularly likely to be drug resistant (1, 11). Song (11) suggested that the emergence of drug resistance in S. pneumoniae in Korea has resulted from the introduction of foreign genes, the spread of resistant clones, and the redistribution of genes from resistant clones into other clones, aided by overcrowded living conditions that facilitate person-to-person transmission of resistant organisms. These logical conclusions are supported by our PFGE analysis that demonstrated several clonally related groups.
In conclusion, this study is the first to characterize macrolide resistance in S. pneumoniae in carriage as well as invasive isolates from South Korea, to determine mechanisms of resistance and capsular serotypes, and to assess the genetic relatedness of resistant organisms by PFGE. This investigation has determined that a clone of S. pneumoniae serogroup 19 that is closely related or identical to the international clone Taiwan19F-14 and that carries dual macrolide resistance mechanisms has been disseminated during the 1990s through asymptomatic carriage among children in Seoul, South Korea, and has been involved in invasive infections involving adults and children.
REFERENCES
- 1.Chong, Y., K. Lee, O. H. Kwon, and J. Henrichsen. 1995. Capsular types and antimicrobial resistance of Streptococcus pneumoniae isolated in Korea. Eur. J. Clin. Microbiol. Infect. Dis. 14:528-531. [DOI] [PubMed] [Google Scholar]
- 2.Depardieu, F., and P. Courvalin. 2001. Mutation in 23S rRNA responsible for resistance to 16-membered macrolides and streptogramins in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:319-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrell, D. J., S. Douthwaite, I. Morrissey, S. Bakker, J. Poehlsgaard, L. Jakobsen, and D. Felmingham. 2003. Macrolide resistance by ribosomal mutation in clinical isolates of Streptococcus pneumoniae from the PROTEKT 1999-2000 study. Antimicrob. Agents Chemother. 47:1777-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrell, D. J., I. Morrissey, S. Bakker, and D. Felmingham. 2002. Molecular characterization of macrolide resistance mechanisms among Streptococcus pneumoniae and Streptococcus pyogenes isolated from the PROTEKT 1999-2000 study. J. Antimicrob. Chemother. 50(Suppl. S1):39-47. [DOI] [PubMed] [Google Scholar]
- 5.Felmingham, D., R. R. Reinert, Y. Hirakata, and A. Rodloff. 2002. Increasing prevalence of antimicrobial resistance among isolates of Streptococcus pneumoniae from the PROTEKT surveillance study, and compatative in vitro activity of the ketolide, telithromycin. J. Antimicrob. Chemother. 50(Suppl. S1):25-37. [DOI] [PubMed] [Google Scholar]
- 6.Johnson, C. N., W. H. Benjamin, Jr., S. A. Moser, S. K. Hollingshead, X. Zheng, M. J. Crain, M. H. Nahm, and K. B. Waites. 2003. Genetic relatedness of levofloxacin-nonsusceptible Streptococcus pneumoniae isolates from North America. J. Clin. Microbiol. 41:2458-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, N. Y., J. H. Song, S. Kim, K. R. Peck, K. M. Ahn, S. I. Lee, Y. Yang, J. Li, A. Chongthaleong, S. Tiengrim, N. Aswapokee, T. Y. Lin, J. L. Wu, C. H. Chiu, M. K. Lalitha, K. Thomas, T. Cherian, J. Perera, T. T. Yee, F. Jamal, U. C. Warsa, P. H. Van, C. C. Carlos, A. M. Shibl, M. R. Jacobs, and P. C. Appelbaum. 2001. Carriage of antibiotic-resistant pneumococci among Asian children: a multinational surveillance by the Asian Network for Surveillance of Resistant Pathogens (ANSORP). Clin. Infect. Dis. 32:1463-1469. [DOI] [PubMed] [Google Scholar]
- 8.McGee, L., K. P. Klugman, D. Friedland, and H. J. Lee. 1997. Spread of the Spanish multi-resistant serotype 23F clone of Streptococcus pneumoniae to Seoul, Korea. Microb. Drug Resist. 3:253-257. [DOI] [PubMed] [Google Scholar]
- 9.McGee, L., K. P. Klugman, A. Wasas, T. Capper, A. Brink, and The Antibiotics Surveillance Forum of South Africa. 2001. Serotype 19f multiresistant pneumococcal clone harboring two erythromycin resistance determinants [erm(B) and mef(A)] in South Africa. Antimicrob. Agents Chemother. 45:1595-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishijima, T., Y. Saito, A. Aoki, M. Toriya, Y. Toyonaga, and R. Fujii. 1999. Distribution of mefE and ermB genes in macrolide-resistant strains of Streptococcus pneumoniae and their variable susceptibility to various antibiotics. J. Antimicrob. Chemother. 43:637-643. [DOI] [PubMed] [Google Scholar]
- 11.Song, J. H. 1998. Emergence and spread of antimicrobial resistance of Streptococcus pneumoniae in Korea. Yonsei Med. J. 39:546-553. [DOI] [PubMed] [Google Scholar]
- 12.Song, J. H., N. Y. Lee, S. Ichiyama, R. Yoshida, Y. Hirakata, W. Fu, A. Chongthaleong, N. Aswapokee, C. H. Chiu, M. K. Lalitha, K. Thomas, J. Perera, T. T. Yee, F. Jamal, U. C. Warsa, B. X. Vinh, M. R. Jacobs, P. C. Appelbaum, and C. H. Pai. 1999. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study. Clin. Infect. Dis. 28:1206-1211. [DOI] [PubMed] [Google Scholar]
- 13.Tait-Kamradt, A., T. Davies, M. Cronan, M. R. Jacobs, P. C. Appelbaum, and J. Sutcliffe. 2000. Mutations in 23S rRNA and ribosomal protein L4 account for resistance in pneumococcal strains selected in vitro by macrolide passage. Antimicrob. Agents Chemother. 44:2118-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarasi, A., Y. Chong, K. Lee, and A. Tomasz. 1997. Spread of the serotype 23F multidrug-resistant Streptococcus pneumoniae clone to South Korea. Microb. Drug Resist. 3:105-109. [DOI] [PubMed] [Google Scholar]
- 15.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swamminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 32:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waites, K., C. Johnson, B. Gray, K. Edwards, M. Crain, and W. Benjamin, Jr. 2000. Use of clindamycin disks to detect macrolide resistance mediated by ermB and mefE in Streptococcus pneumoniae isolates from adults and children. J. Clin. Microbiol. 38:1731-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]