Abstract
Valsartan is a potent antagonist of the type 1 angiotensin receptor (AT1). By blocking the actions of angiotensin II on the AT1, it inhibits vasoconstriction and synthesis of aldosterone thus lowering systemic blood pressure. Valsartan has been approved by the FDA for the treatment of hypertension in children aged 6 years and older. Valsartan can be dosed once a day with a sustained 24-hour effect on blood pressure reduction. The starting dose recommended in children is 1.3 mg/kg once daily (maximum 40 mg) which needs adjustment according to blood pressure response (dose range 1.3–2.7 mg/kg daily; up to 160 mg). A suspension form (4 mg/mL) is available for children who cannot swallow tablets. In patients aged 6 to 16 years, valsartan treatment (from a low dose of 10–20 mg to a high dose of 80–160 mg) resulted in dose-dependent reductions of 7.9–11.5 mmHg in systolic blood pressure and 4.6–7.4 mmHg in diastolic blood pressure. In 1- to 5-year-olds, valsartan (from a low dose of 5–10 mg to a high dose of 40–80 mg) reduced the systolic blood pressure by 8.4–8.6 mmHg and the diastolic blood pressure by 5.5 mmHg. Similar to adults and other antihypertensive medications, the most frequent side effect in children subsequent to valsartan use is headache. Current studies have not shown adverse effects on linear growth, weight gain, head growth, or development in children aged 1 to 5 years subsequent to valsartan use. Based on limited pediatric data, valsartan appears to be well tolerated and efficacious in reducing elevated blood pressure.
Keywords: valsartan, hypertension, angiotensin receptor blockers
Valsartan is an angiotensin receptor blocker (ARB)
The angiotensin II receptor blockers (ARBs) are drugs designed to inhibit the renin-angiotensin system (RAS). ARBs interact with the receptor for angiotensin II thereby selectively blocking its physiological actions. This class of drugs was developed after the angiotensin-converting enzyme (ACE) inhibitors, as an alternative to provide more specific inhibition of the RAS.1 Valsartan (trade name Diovan) was the second orally active ARB to be marketed in Europe and the USA for the treatment of hypertension (HTN) (Table 1).2
Table 1.
Angiotensin receptor blockers
| Generic name | Trade name | Half lifea (metabolite) | Pediatric FDA approval | Dosing | Generic form |
|---|---|---|---|---|---|
| Valsartan | Diovan | 6 hours | Yes | 80–160 mg | No |
| Losartan | Cozaar | 2 (6–9) hours | Yes | 50 mg | Yes |
| Irbesartanb | Avapro | 11–15 hours | No | 150–300 mg | No |
| Telmisartan | Micardis | 24 hours | No | 40–80 mg | No |
| Candesartan cilexetil | Atacand | 9 hours | Yes | 4–32 mg | No |
| Eprosartan | Teveten | 5–9 hours | No | 400–800 mg | No |
| Olmesartan | Benicar | 13 hours | Yes | 10–40 mg | No |
Notes:
From www.medscape.com;
Irbesartan in a study at a dose of up to 4.5 mg/kg/day once daily did not appear to lower blood pressure effectively in pediatric patients aged 6 to 16 years. Avapro has not been studied in pediatric patients less than 6 years old.
The RAS regulates blood pressure through its effect on sodium balance, extracellular fluid volume, and renal and systemic vascular resistance. The final step of the pathway is the activation of the angiotensin II receptors. The actions of angiotensin II are mediated by two types of receptors, type 1 (AT1) and type 2 (AT2). The influence on blood pressure is exerted through activation of AT1. These actions include vasoconstriction of systemic arterioles; activation of the sympathetic nervous system; stimulation of aldosterone secretion which results in sodium and water reabsorption; smooth muscle cell hypertrophy; and stimulation of vascular and myocardial fibrosis. By blocking the action of angiotensin II on the AT1 receptor, valsartan decreases peripheral resistance and causes less sodium and water reabsorption in the kidneys thereby lowering the blood pressure.3 Additionally, blockage of the AT1 receptor leads to a compensatory increase in angiotensin II levels which increases stimulation of the AT2 receptor. In contrast to stimulation of AT1, the stimulation of AT2 leads to the inhibition of cell growth, tissue repair, and possibly vasodilation.1
The increased stimulation of AT2 could possibly promote vasodilation,4,5 and thereby contribute to the blood pressure lowering effect of valsartan. There have been reports that AT2 stimulation also has an antiproliferative effect on cardiac muscle6 by inhibition of cell growth, promotion of cell differentiation, and apoptosis.7 This action could have a beneficial influence on cardiac remodeling in response to elevated blood pressure.
Advantages of ARBs over the more commonly used ACE inhibitors
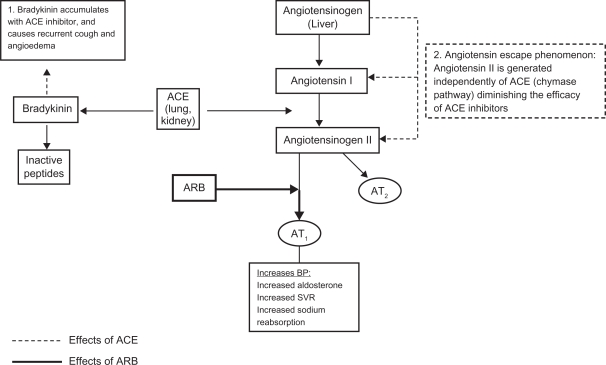
Due to the direct blockage of angiotensin II, the ARBs do not have the same limitations as ACE inhibitors. Bradykinin, which is also a substrate for ACE, accumulates with the use of ACE inhibitors. This accounts for the two most common side effects of ACE inhibitors, cough, and angioedema. When there is competitive inhibition of ACE, there can be a reactive increase in renin and angiotensin I levels which may overcome the blockade effect.8 There are also alternative pathways through which angiotensin II is formed independently of ACE. In patients on chronic ACE inhibitor treatment, an “angiotensin escape phenomenon” is described, whereby the reduced stimulation of AT1 and AT2 receptors is counteracted by an increase in AT1 receptor stimulation of angiotensin II formed by an alternative pathway.4 The use of ARBs circumvents this problem by acting directly on the angiotensin II receptor and therefore resulting in complete angiotensin II inhibition (Figure 1).
Figure 1.
Effects of ACE and ARB on the renin angiotensin system.
Abbreviations: ACE, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker; SVR, systemic vascular resistance.
Hypertension in children and adolescents
HTN is the most commonly diagnosed disease in the United States.9 The recent increase in obesity rates in the US and higher incidence and risk for HTN in obese individuals has increased the focus on the diagnosis and management of high blood pressure. HTN in children can be either secondary, in which an underlying cause is identified, or primary which is a diagnosis of exclusion that is reached after a systematic work-up is done looking for secondary causes. Pharmacologic treatment of HTN is indicated in the presence of secondary HTN, symptomatic HTN, when blood pressure remains elevated in spite of lifestyle modifications, and when there is evidence of end organ damage.10 The goal of antihypertensive treatment should be a reduction of blood pressure to below the 95th percentile for gender, age, and height for children without comorbidities. For children with chronic kidney disease, diabetes, and target-organ damage the goal blood pressure should be less than the 90th percentile.10 Since there is a lack of studies comparing long-term clinical endpoint data of multiple antihypertensive medications in children, the initial drug is chosen based on the prescribing physicians’ own preference. The Fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents recommends ACE inhibitors, ARBs, beta blockers, calcium channel blockers, and diuretics as acceptable drug classes for use in children.10
Role of RAS activation in pediatric hypertension
The association between obesity and HTN has been known for several decades.11–16 Activation of the RAS in these patients may represent an important link between obesity and HTN.17 Since adipocytes can produce angiotensinogen, increased adiposity in obese patients can lead to inappropriately normal or elevated plasma renin activity (PRA).18 Despite increased sodium intake, sodium/water retention, and blood pressure, increased levels of angiotensinogen, renin, aldosterone, ACE, and increased expression of the angiotensinogen gene in visceral adipose tissue are found in obese patients. The levels of angiotensinogen, renin, aldosterone, and ACE decrease after 5% in weight loss and the reduction in angiotensinogen expression and levels correlates with reduction in the systolic blood pressure.19 Recently, a treatment approach for hypertension based on PRA has been proposed. Hypertensive patients with low levels of PRA have an excess of sodium and volume, and would benefit more from diuretic therapy, whereas patients with high PRA levels would respond better to RAS inhibitors.20,21
The effects of RAS that are important in the progression of chronic kidney disease (CKD) include intraglomerular hypertension and increased filtration of proteins, cell growth, inflammation, and fibrosis.22 In view of this; ACE/ARB are considered the preferred antihypertensive medications in children with kidney disease as inhibition of the RAS can additionally decrease the rate of progression of CKD.23,24
Valsartan: pharmacokinetics and palatability
Adult studies have shown that the bioavailability of valsartan is 23% for the capsule and 39% for the solution.25 Valsartan has a higher affinity for the AT1 receptor than losartan, but a lower affinity than candesartan, telmisartan, and olmesartan.26 The half life of the drug is around 7 hours which is shorter than the half life of telmisartan and olmesartan, however it can be given once a day with a sustained 24-hour effect on blood pressure reduction.26 Valsartan undergoes little hepatic metabolism and is excreted primarily as the unchanged drug via biliary elimination. Most of the drug is excreted in the feces, with the rest being renally excreted (7%–13% of the drug).26
The pharmacokinetics of valsartan in a pediatric population has been studied in a group of children aged 1 to 16 years. These patients received a dose of 2 mg/kg of valsartan suspension up to a maximum dose of 80 mg. Initially age and body size were found to have similar influence in the clearance of the drug. However, as valsartan is expected to have minimal distribution in fatty tissues, adjustment was done for body size using fat free mass. This subsequent analysis accounting for body size showed that the effect of increasing age was only 2% per year on valsartan clearance, which was considered to be not significant. Therefore, this study shows that body weight dosing up to 80 mg provides comparable exposure in children as in adults.3
In pediatric populations, the taste of a medication is an important factor to consider in the adherence to a treatment regimen. The taste and smell acceptability of several ARBs has been compared in young children (4 to 11 years of age) and found valsartan to have low ratings of palatability compared to candesartan and telmisartan, which were rated by the patients to have better taste.27 However this study gave the medications in the form of crushed and pulverized tablets. Pediatric patients who are not able to swallow a tablet take the suspension form of the medication. A suspension form is available for preparation from the tablets for valsartan and olmesartan. The taste of valsartan suspension has not been compared to other ARBs.
Valsartan: dosing, efficacy, and safety
Valsartan was initially approved in Europe in 1996 and in the USA in 1997 for the treatment of HTN in adults. This drug has shown efficacy in reducing blood pressure in adult populations. Additionally, it has been shown to have cardioprotective effects, with reduced morbidity and mortality in adults with heart failure and following myocardial infarction.26 These effects are comparable to those achieved with ACE inhibitors, with the additional benefit of a lower incidence of side effects such as cough and angioedema.26
In December of 2007 the FDA approved valsartan for the treatment of HTN in children aged 6 to 16 years.28 The starting dose recommended in children is 1.3 mg/kg once daily (maximum dose 40 mg) which should be adjusted according to blood pressure response. The dose range is 1.3–2.7 mg/kg once daily (up to 40–160 mg). A suspension form of the drug (4 mg/mL) can be prepared for children who cannot swallow tablets or for children for whom the dose does not correspond to the available tablet strengths. The recipe for preparation of the suspension is available in the valsartan package insert.28 An oral suspending vehicle (Ora-Plus®) and an oral sweetening vehicle (Ora-Sweet SF®), both registered trademarks of Paddock Laboratories, Inc are recommended for making the oral suspension. As per the recommendations, 8 tablets of valsartan (80 mg each) are mixed with 80 mL of Ora-Plus. After shaking this mix and allowing it to stand for 1 hour, 80 mL of the Ora-Sweet SF are added. The suspension obtained has a concentration of valsartan of 4 mg/mL. This suspension can be stored for 30 days at room temperature or up to 75 days refrigerated. The exposure to valsartan with the suspension is 1.6 times greater than with the tablet, therefore when the suspension is replaced by a tablet the dose may have to be increased.28
Two studies with the same design except for the ages of the study cohort were done to assess the efficacy and safety of valsartan in pediatric populations. The studies were conducted in two phases. In Phase I the patients were randomly assigned to receive low, medium, and high doses of valsartan for two weeks. Children who completed Phase I were then re-randomized to continue the same dose of valsartan or to receive placebo for two additional weeks. The study by Wells et al29 which included children aged 6 to 16 years (n = 261) showed a reduction in systolic and diastolic BP in all three study groups (Table 2). Flynn et al’s study30 with a similar design including patients aged 1 to 5 years (n = 90) also showed reduction in both systolic and diastolic blood pressure (Table 2). However, it is important to note that the reduction in the systolic and diastolic BP is dose dependent in older children (6 to 16 years)29 while this effect was not reported in the younger age group (1 to 5 years).30 It should be noted that both studies do not comment on the statistical significance of reduction in the systolic and diastolic BP noted in study Phase I.
Table 2.
Results of randomized, double-blind clinical studies on the safety and effectiveness of valsartan in children
| Valsartan dose group | Dose according to patients’ weight (mg) | Blood pressure reduction (mmHg) | ||
|---|---|---|---|---|
| Wells et al29 | ||||
| <35 kg | ≥35 kg | SBP | DBP | |
| Low dose | 10 | 20 | 7.9 | 4.6 |
| Medium dose | 40 | 80 | 9.6 | 5.8 |
| High dose | 80 | 160 | 11.5 | 7.4 |
| Flynn et al | ||||
| <18 kg | ≥18 kg | SBP | DBP | |
| Low dose | 5 | 10 | 8.4 | 5.5 |
| Medium dose | 20 | 40 | 8.3 | 6.4 |
| High dose | 40 | 80 | 8.6 | 5.5 |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
In Phase II, in 6- to 16-year-olds, the systolic blood pressure of patients who continued to receive valsartan at medium (40–80 mg) and high doses (80–160 mg) was 4 and 7 mmHg lower than patients who received placebo.29 The statistical significance of this reduction was not mentioned. In 1- to 5-year-olds the mean difference in the change of systolic (3.9 mmHg, P = 0.02) and diastolic blood pressures (3.7 mmHg, P < 0.01) between the pooled valsartan and placebo groups was significant.30
Prescription patterns for ARB
The therapeutic efficacy of ARB when compared to ACE inhibitors is a critical issue and one that has important implications for ARB prescriptions. Currently there is no consensus as to whether pharmacological differences between ACE inhibitors and ARB translate into significant differences in therapeutic outcomes. Existing literature suggests that the antihypertensive effects of ARB are similar to ACE inhibitors and that they are an acceptable alternative as first choice drugs for the management of hypertension especially in patients intolerant to ACE inhibitors.31 In adults, ACE inhibitors are the drug of choice in heart failure, cardiac dysfunction after MI, and diabetic nephropathy, and ARBs offer an acceptable alternative. A retrospective study evaluating the use of ARBs as antihypertensives showed that 53.4% of the patients were new to all antihypertensive therapies and more than 40% of these had no other antihypertensive. The study also reported increased use of fixed drug combinations with diuretics when losartan and valsartan were used initially to control BP in comparison to the newer ARB such as telmisartan, irbesartan, or candesartan.32
In children, the FDA has approved the use of valsartan for elevated blood pressure for those who are older than 6 years of age. In addition to the reduction of blood pressure, valsartan’s antiproteinuric effects contribute towards additional benefit in children with CKD.33,34 Although ARBs have shown to be effective in the reduction of proteinuria and the rate of progression of renal failure,35,36 there is limited information regarding the renoprotective effects of ARBs in children with CKD. A study in children (n = 10) with CKD showed that adding an ARB (losartan) to ACE inhibitor treatment further reduced proteinuria.37 Valsartan may have an important role in this population and more studies are needed in children with CKD to further determine the benefits of valsartan in slowing the progression of renal disease. There is a clinical trial being conducted to compare the long-term safety and effectiveness of valsartan in combination with enalapril versus enalapril alone in children with CKD. This trial will also evaluate proteinuria reduction, renopreservation and tolerability of valsartan and enalapril in combination versus enalapril monotherapy in patients with CKD.38
Valsartan versus other ARBs
A meta-analysis of published randomized controlled trials involving ARB (valsartan, losartan, irbesartan, and candesartan) has shown a similar blood pressure lowering efficacy within the ARB class, a flat ARB-dose response when titrating from lower to maximum recommended doses, and potentiation of the antihypertensive effect with the addition of a diuretic (hydrochlorthiazide).39 In children, studies are needed to compare the efficacy of the different ARBs. It is possible that olmesartan may offer a better alternative in view of its longer half life (Table 1) and therefore better control with daily dosing.
Valsartan in combination with other antihypertensives is widely used in adults. These combinations have been shown to have better efficacy than valsartan monotherapy in the reduction of blood pressure.40 The commercially available combinations include: 1) Valturna (valsartan with aliskiren, a direct renin inhibitor); 2) Exforge (valsartan and amlodipine, calcium channel blocker); 3) Diovan HCT (valsartan and hydrochlorothiazide, diuretic); and 4) Exforge HCT (valsartan with both amlodipine and hydrochlorothiazide). These combinations have not yet been approved for use in children.
Valsartan: side effects
Adverse reactions to valsartan are generally mild and transient. The discontinuation rate due to adverse effects is only 2.3%. The most common reasons for discontinuation of this drug are headache and dizziness.28 Cardiovascular side effects of ARBs are similar to other antihypertensives and include hypotension, postural hypotension, and syncope. Central nervous system side effects include dizziness, fatigue, postural dizziness, headache, blurred vision, and vertigo. Gastrointestinal side effects include diarrhea, abdominal pain, and nausea; rarely do ARBs cause elevated liver enzymes. Hematologic side effects include neutropenia and decrease in hemoglobin. The incidence of cough is 2.6%, which is less than when taking ACE inhibitors.28 Renal side effects include hyperkalemia and renal dysfunction with elevated creatinine. Musculoskeletal side effects include arthralgia and back pain. Rash can also be caused by ARBs and angioedema has also been reported although it is considered to be a rare side effect.
Drugs that inhibit the renin-angiotensin system can cause fetal defects if used during pregnancy. These defects include neonatal skull hypoplasia, anuria, oligohydramnios associated with craniofacial deformities and hypoplastic lungs, prematurity, renal failure, intrauterine growth retardation, patent ductus arteriosus, and death. In pediatrics, if used in female adolescents they should be counseled regarding these risks and if sexually active should take contraceptives or be prescribed alternative antihypertensives.
Although the risk of derangement of liver function tests, potassium, and creatinine is low, the prescriber may want to consider monitoring these tests when valsartan therapy is started.
A recent warning has been issued by the FDA regarding the possible association between the use of ARB and cancer. This warning is based on a recent meta-analysis that reported the frequencies of new cancer occurrence to be 7.2% for patients receiving ARBs compared to 6.0% for patients not taking ARBs. The risk ratio was 1.08 with a 95% confidence interval of 1.01–1.15.41 However, this study has several limitations and the FDA has not concluded that ARBs increase the risk of cancer and is still evaluating this safety concern. In children, the duration of ARB therapy could be longer given their young age; therefore the association between ARB and cancer could possibly influence the prescription patterns for this medicine class.
Overall, in the clinical studies that looked at the safety of valsartan in children,29,30 this drug was well tolerated. In the 6- to 16-year-old group there was a similar adverse event profile to that seen in adults, with headache as the most frequent adverse event noted.29 The 1- to 5-year-old group had a low incidence of adverse events, with the most frequent being cough, fever, upper respiratory infection, and diarrhea which are common illnesses in children in that age group. Four patients had increases in liver enzymes; however, no relationship to the study drug could be determined. The effects of treatment with valsartan on growth and development were studied in this age group. Valsartan did not appear to have adverse effects on linear growth, weight gain, head growth, or development over the 13 months of the study.30
Summary
Valsartan is one of the ARBs that are FDA approved for use in hypertensive children. This drug has a high affinity for the AT1 receptor and lowers the blood pressure by inhibiting vasoconstriction and synthesis of aldosterone. In comparison to ACE inhibitors, valsartan blocks the actions of angiotensin II by inhibiting its interaction with AT1. Thus, valsartan and other ARBs have fewer side effects of recurrent cough and angioedema in comparison to ACE inhibitors. Valsartan is a good candidate for the treatment of hypertensive children as it has been shown to be effective in reducing systolic and diastolic blood pressure and is well tolerated.29,30 The activation of the RAS is a contributing factor in progressive CKD; therefore valsartan is a good choice for managing hypertension and/or proteinuria in children with CKD.
Footnotes
Disclosure
No conflicts of interest were declared in relation to this paper.
References
- 1.Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet. 2000;355:637–645. doi: 10.1016/s0140-6736(99)10365-9. [DOI] [PubMed] [Google Scholar]
- 2.Chiolero A, Burnier M. Pharmacology of valsartan, an angiotensin II receptor antagonist. Expert Opin Investig Drug. 1998;7:1915–1925. doi: 10.1517/13543784.7.11.1915. [DOI] [PubMed] [Google Scholar]
- 3.Habtemariam B, Sallas W, Sunkara G, Kern S, Jarugula V, Pillai G. Population pharmacokinetics of valsartan in pediatrics. Drug Metab Pharmacokinet. 2009;24:145–152. doi: 10.2133/dmpk.24.145. [DOI] [PubMed] [Google Scholar]
- 4.Werner C, Baumhakel M, Teo KK, et al. RAS blockade with ARB and ACE inhibitors: current perspective on rationale and patient selection. Clin Res Cardiol. 2008;97:418–431. doi: 10.1007/s00392-008-0668-3. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez-Hernandez R, Sosa-Canache B, Velasco M, Armas-Hernandez MJ, Armas-Padilla MC, Cammarata R. Angiotensin II receptor antagonists role in arterial hypertension. J Hum Hypertens. 2002;16:S93–S99. doi: 10.1038/sj.jhh.1001352. [DOI] [PubMed] [Google Scholar]
- 6.Booz GW, Baker KM. Role of type 1 and type 2 angiotensin receptors in angiotensin II-induced cardiomyocyte hypertrophy. Hypertension. 1996;28:635–640. doi: 10.1161/01.hyp.28.4.635. [DOI] [PubMed] [Google Scholar]
- 7.Stoll M, Steckelings M, Paul M, Bottari SP, Metger R, Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 1995;95:651–657. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barreras A, Gurk-Turner C. Angiotensin II receptor blockers. BUMC Proc. 2003;16:123–126. doi: 10.1080/08998280.2003.11927893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2000 summary. Advance Data. 2002;328:1–32. [PubMed] [Google Scholar]
- 10.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 11.Robinson RF, Batisky DL, Hayes JR, Nahata MC, Mahan JD. Body mass index in primary and secondary pediatric hypertension. Pediatr Nephrol. 2004;19:1379–1384. doi: 10.1007/s00467-004-1588-8. [DOI] [PubMed] [Google Scholar]
- 12.Pludowski P, Litwin M, Sladowska J, et al. Bone mass and body composition in children and adolescents with primary hypertension. Hypertension. 2008;51:77–83. doi: 10.1161/HYPERTENSIONAHA.107.100602. [DOI] [PubMed] [Google Scholar]
- 13.McGavock JM, Torrance B, McGuire KA, Wozny P, Lewanczuk RZ. The relationship between weight gain and blood pressure in children and adolescents. Am J Hypertens. 2007;20:1038–1044. doi: 10.1016/j.amjhyper.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Urrutia-Rojas X, Egbuchunam CU, Bae S, et al. High blood pressure in school children: prevalence and risk factors. BMC Pediatrics. 2006;6:32–38. doi: 10.1186/1471-2431-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiramatsu K, Yamada T, Ichikawak T, Izumiyama T, Nagata H. Changes in endocrine activity to obesity in patients with essential hypertension. J Am Geriatr Soc. 1981;29:25–30. doi: 10.1111/j.1532-5415.1981.tb02389.x. [DOI] [PubMed] [Google Scholar]
- 16.Tuck MI, Sowers J, Dornfield L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity and plasma aldosterone level in obese patients. N Engl J Med. 1981;304:930–933. doi: 10.1056/NEJM198104163041602. [DOI] [PubMed] [Google Scholar]
- 17.Rocchini AP. Childhood obesity and blood pressure regulation. In: Portman RJ, Sorof JM, Ingelfinger JR, editors. Pediatric Hypertension. Totowa, NJ: Humana Press; 2004. pp. 307–334. [Google Scholar]
- 18.Sarzani R, Salvi F, Dessi-Fulgheri P, Rappeli A. Renin-angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: an integrated view in humans. J Hypertens. 2008;26:831–843. doi: 10.1097/HJH.0b013e3282f624a0. [DOI] [PubMed] [Google Scholar]
- 19.Engeli S, Bohnke J, Gorzelniak K, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 20.Laragh J. Laragh’s lessons in pathophysiology and clinical pearls for treating hypertension. Am J Hypertens. 2001;14:186–194. doi: 10.1016/s0895-7061(00)01317-0. [DOI] [PubMed] [Google Scholar]
- 21.Egan BM, Basile JN, Rehman SU, et al. Plasma renin test-guided drug treatment algorithm for correcting patients with treated but uncontrolled hypertension: a randomized controlled trial. Am J Hypertens. 2009;22:792–801. doi: 10.1038/ajh.2009.63. [DOI] [PubMed] [Google Scholar]
- 22.Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int. 2005;68:S57–S65. doi: 10.1111/j.1523-1755.2005.09911.x. [DOI] [PubMed] [Google Scholar]
- 23.Flynn JT, Mitsnefes M, Pierce C, et al. for the Chronic Kidney Disease in Children Study Group Blood pressure in children with chronic kidney disease. A report from the Chronic Kidney Disease in Children Study. Hypertension. 2008;52:1–7. doi: 10.1161/HYPERTENSIONAHA.108.110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wuhl E, Trivelli A, Picca S, et al. Strict blood pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 25.Flesch G, Muller P, Lloyd P. Absolute bioavailability and pharmacokinetics of valsartan, an angiotensin II receptor antagonist, in man. Eur J Clin Pharmacol. 1997;52:115–120. doi: 10.1007/s002280050259. [DOI] [PubMed] [Google Scholar]
- 26.Black HR, Bailey J, Zappe D, Samuel R. Valsartan: more than a decade of experience. Drugs. 2009;69:2393–2414. doi: 10.2165/11319460-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Meier CM, Simonetti GD, Ghiglia S, et al. Palatability of angiotensin II antagonists among nephropathic children. Br J Clin Pharmacol. 2007;63:628–631. doi: 10.1111/j.1365-2125.2006.02814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.East Hanover, New Jersey: Novartis Pharmaceuticals Corporation; Diovan (valsartan) tablets [prescribing information] [cited 2008 Dec]. Available from: http://www.pharma.us.novartis.com/product/pi/pdf/diovan.pdf. Accessed February 16, 2011. [Google Scholar]
- 29.Wells T, Blumer J, Litwin M, et al. Safety and effectiveness of valsartan in hypertensive children ages 6–16 years. J Clin Hypertens (Greenwich) 2007;9(5 Suppl A):A79–A80. [Google Scholar]
- 30.Flynn JT, Meyers KEC, Pacheco Neto J, et al. for the Pediatric Valsartan Study Group Efficacy and safety of the angiotensin receptor blocker valsartan in children with hypertension aged 1 to 5 years. Hypertension. 2008;52:222–228. doi: 10.1161/HYPERTENSIONAHA.108.111054. [DOI] [PubMed] [Google Scholar]
- 31.Rodgers JE, Patterson JH. Angiotensin II-receptor blockers: clinical relevance and therapeutic role. Am J Health Syst Pharm. 2001;58:671–683. doi: 10.1093/ajhp/58.8.671. [DOI] [PubMed] [Google Scholar]
- 32.Young CH, Zhang K, Poret AW. Patterns of antihypertensive therapy in new users of angiotensin II-receptor blockers. Am J Health Syst Pharm. 2005;62:2381–2385. doi: 10.2146/ajhp040583. [DOI] [PubMed] [Google Scholar]
- 33.Wuhl E, Schaefer F. Therapeutic strategies to slow chronic kidney disease progression. Pediatr Nephrol. 2008;23:705–716. doi: 10.1007/s00467-008-0789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaefer F, Mehls O. Hypertension in chronic kidney disease. In: Portman RJ, Sorof JM, Ingelfinger JR, editors. Pediatric Hypertension. Totowa, NJ: Humana Press; 2004. pp. 371–387. [Google Scholar]
- 35.Jafar TH, Schmid CH, Landa M, et al. ACE inhibition in progressive renal disease study group Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A metaanalysis of patient-level data. Ann Intern Med. 2001;135:73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- 36.Izuhara Y, Nangaku M, Inagi R, et al. Renoprotective properties of angiotensin receptor blockers beyond blood pressure lowering. J Am Soc Nephrol. 2005;16:3631–3641. doi: 10.1681/ASN.2005050522. [DOI] [PubMed] [Google Scholar]
- 37.Seeman T, Pohl M, Misselwitz J, John U. Angiotensin receptor blocker reduces proteinuria independently of blood pressure in children already treated with angiotensin-converting enzyme inhibitors. Kidney Blood Press Res. 2009;32:440–444. doi: 10.1159/000266478. [DOI] [PubMed] [Google Scholar]
- 38.Clinical Trials Search. Worldwide Clinical Trials Listings. Hypertension. Available from: http://www.clinicaltrialssearch.org/extension-study-to-assess-long-term-safety-tolerability-and-efficacy-of-valsartan-and-enal-april-combined-and-alone-in-children-with-hypertension-nct00446511.html Accessed February 16, 2011
- 39.Conlin PR, Spence JD, Williams B, et al. Angiotensin II antagonists for hypertension: are there differences in efficacy? Am J Hypertens. 2000;13(4 Pt 1):418–426. doi: 10.1016/s0895-7061(99)00237-x. [DOI] [PubMed] [Google Scholar]
- 40.Weinberger MH, Glazer RD, Crikelair NA, Chiang YT. Achieving blood pressure goal: initial therapy with valsartan/hydrochlorothiazide combination compared with monotherapy. J Hum Hypertens. 2010;24:823–830. doi: 10.1038/jhh.2010.17. [DOI] [PubMed] [Google Scholar]
- 41.Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomized controlled trials. Lancet Oncol. 2010;11:627–636. doi: 10.1016/S1470-2045(10)70106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]