Abstract
Helicobacter pylori infection is usually acquired in childhood, but precise estimates of the age of acquisition are difficult to obtain in young children. Since serial endoscopic biopsies are not feasible in human infants, we examined acquisition of H. pylori infection that is known to occur in socially housed nonhuman primates. By 12 weeks of age, 8 of 20 newborns (40%) were culture positive for H. pylori, and prevalence reached 90% by 1 year of age. Newborns from infected dams were more commonly infected than those from uninfected dams, particularly during the peripartum period, suggesting that close contact during this time may facilitate transmission. Transient infection was uncommon and occurred only after the first positive culture. These results suggest that in a high-prevalence environment, persistent H. pylori infection may be acquired at an earlier age than was previously thought. Since clean, potable water was readily available, contamination of water supply is not essential for widespread infection at an early age in areas where hygiene is otherwise poor. Furthermore, breastfeeding seems to offer little protection, since newborn macaques breastfeed during the first year of life and typically are fully weaned only when another newborn arrives the following spring.
Helicobacter pylori is a gram-negative bacterium that infects the stomachs of approximately half of all adults in developed countries and up to 90% of the population in developing countries. Infection causes chronic histologic gastritis that is usually asymptomatic. However, approximately 15% of infected individuals will at some time develop peptic ulcer disease or gastric adenocarcinoma, which is the second-most-common cause of cancer death worldwide (13).
Better understanding of natural acquisition of H. pylori infection is important because it may permit a more rational focus of efforts to prevent transmission. The incidence of H. pylori infection is greatest in childhood, particularly in developing countries where prevalence of seropositivity can be as high as 50% by age 5 years (3). However, precise estimates of the age of acquisition are difficult to obtain. Since acute H. pylori infection rarely comes to clinical attention, most estimates of the age of acquisition come from retrospective cross-sectional studies or birth cohort serosurveys (3, 14, 27, 28, 30). These analyses, as well as some more recent prospective studies (15, 32), have by necessity relied on noninvasive methods for H. pylori detection, which in many cases have not been well validated in young children. Detection of anti-H. pylori immunoglobulin G (IgG) antibodies in serum has been most commonly used to document acquisition (and loss) of infection. However, the sensitivity of serodiagnosis of H. pylori in children is unacceptably low, particularly among very young children and infants (23, 29, 31), which is the very group that is of greatest interest. The [13C]-urea breath test and stool immunoassay are more accurate than serology for detection of H. pylori in children (5, 7, 23, 24, 33, 42). However, neither has been thoroughly validated for infants, and some studies suggest that both methods may be less accurate in children less than 6 years of age (4, 21, 23).
The rhesus monkey model provides an opportunity to examine natural acquisition of H. pylori using an experimental system that closely resembles human infection. Captive rhesus monkeys are commonly infected with H. pylori that is indistinguishable from that which infects humans (8). Seroprevalence studies suggest that, as in some human populations, infection is acquired at an early age and is nearly universal in adult animals (11, 12, 35). Once acquired, infection is associated with chronic gastritis that resembles that seen in humans. Neither the humoral nor the cellular immune response is sufficient to clear the infection. Some animals may go on to develop atrophic gastritis, the histologic precursor to gastric adenocarcinoma (10). The similarities between H. pylori infection in rhesus monkeys and humans and the opportunity to perform repeat endoscopic biopsies beginning soon after birth provide a unique opportunity to study early events in the natural acquisition of H. pylori. We therefore sought to use this model to examine natural acquisition and loss of H. pylori infection in newborn rhesus monkeys by using serial cultures of gastric biopsies. Identification of H. pylori by culture, rather than serology or urea breath test, also afforded us the opportunity to examine the dynamics of H. pylori strain diversity early in natural infection.
MATERIALS AND METHODS
Animals.
Male and female rhesus macaques were located at the California National Primate Research Center, which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Newborn monkeys (n = 20) and their dams (n = 16; four dams produced two infants each in consecutive years) were housed in one of five outdoor field cages. Each cage measured 100 × 200 ft and contained 60 to 120 male (∼30%) and female (∼70%) rhesus monkeys ranging in age from newborn to over 20 years. During the study period, newborns and dams ranged in age from 12 to 64 weeks and 3 to 16 years, respectively. Animals were fed twice daily with commercial primate chow (Purina) that was supplemented with enrichment (e.g., sunflower seeds, grain) and occasional fresh fruit. Water was available ad libitum. All procedures were approved by the California National Primate Research Center Research Advisory Committee and the University of California—Davis Chancellor's Animal Use and Care Administrative Advisory Committee.
Enzyme-linked immunosorbent assay.
Blood was collected by femoral venipuncture, and sera were separated by centrifugation and stored at −70°C. Serum samples were assayed for IgG antibodies to H. pylori using an enzyme-linked immunosorbent assay that is 96% sensitive and 88% specific for H. pylori infection (35). All samples were run in triplicate.
Endoscopy and quantitative bacterial cultures.
Infants and their dams were hand caught by primate center staff and housed together indoors for approximately 24 h. The first biopsy in most infants was performed at 12 weeks of age, the age at which it was first technically feasible to perform endoscopy with a 3.5-mm (diameter) pediatric bronchoscope (Olympus BF 3C20) fitted with a 1-mm biopsy forceps. Subsequent biopsies were performed when possible at 4-week intervals to a maximum of 64 weeks of age. Not all time points were available for all newborns. For example, all newborns were born in the spring and no biopsies were performed from approximately January to April because of the stress induced by hand catching during the rainy winter months. All dams were biopsied twice with a pediatric gastroscope (Pentax FG-16X) with a 1.8-mm biopsy forceps.
Endoscopy was performed under ketamine anesthesia (10 mg/kg of body weight intramuscularly) after an overnight fast. In most cases, three antral and three corpus biopsies were obtained at each endoscopy and processed separately, though in some cases (predominantly at early time points) only antral biopsies were obtained. The biopsies were placed in vials containing 250 μl of brucella broth and transported immediately to the laboratory. The tissue was homogenized with a sterile ground-glass pestle, serially diluted, and inoculated onto brucella agar containing 5% bovine calf serum (GIBCO-BRL) and antibiotics (5 mg of trimethoprim/liter, 10 mg of vancomycin/liter, 2.5 IU of polymyxin B/liter, and 4 mg of amphotericin B/liter; all from Sigma). The number of CFU per three biopsies was calculated by enumerating colonies and adjusting for the dilution. All plates were incubated in an atmosphere of 5% CO2 for up to 10 days. H. pylori was identified in the conventional manner by colony morphology (pinhead-sized translucent colonies), microscopy (gram-negative curved organisms), and biochemistry (oxidase-, catalase-, and urease-positive).
DNA fingerprinting.
Repetitive extragenic palindromic PCR (Rep-PCR) was used in order to type strains isolated from each infected infant monkey and from each infected dam by using methods previously described (16, 40, 41). Briefly, chromosomal DNA was prepared from plate-grown bacteria by using the cetyltrimethylammonium bromide method (2). The degenerate oligonucleotide primers (50 pmol each) REP1R-Dt (5′IIINCGNCGNCATCNGGC3′) and REP2-Dt (5′NCGNCTTATCNGGCCTAC3′) were added to 25-μl PCRs that contained 100 ng of template DNA, 6 mM MgCl2, 0.6 mM (each) dNTP, and 2 units of Amplitaq DNA polymerase (Perkin-Elmer). Amplification was carried out as follows: initial denaturation at 94°C for 2 min; 30 cycles of 94°C for 30 s, 45°C for 1 min, and 72°C for 3 min; and one final extension of 72°C for 5 min. Aliquots of PCR products were run on 1.5% agarose gels, and fragments were visualized by ethidium bromide staining.
RESULTS
Culture and serology.
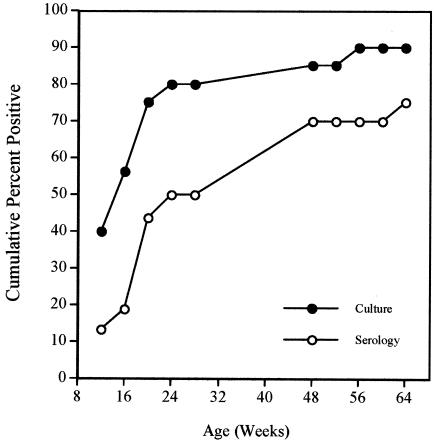
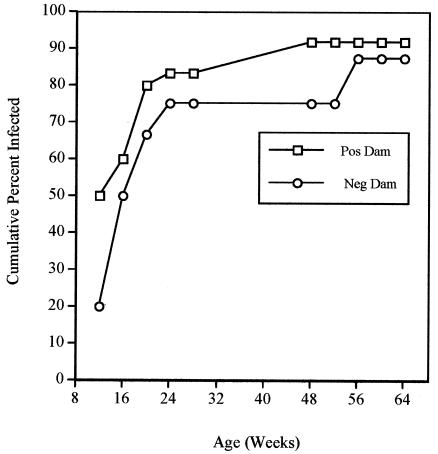
Twenty newborn monkeys underwent a mean of 5.4 (standard deviation [SD], 2.6) endoscopies between 12 and 64 weeks of age. Eighteen monkeys (90%) had a culture positive for H. pylori on one or more occasions. By the time of the first endoscopy performed at 12 weeks of age, 6 of 15 animals (40%) were already culture positive. The cumulative percentage of monkeys that was culture positive on one or more occasions increased rapidly from 40% at 12 weeks to 75% by 24 weeks and subsequently increased more slowly to 90% by 56 weeks of age (Fig. 1). The cumulative percentage of animals seropositive for H. pylori lagged behind the results for culture, reaching 70% at 1 year of age (Fig. 1). This finding is essentially identical to the seroprevalence at 1 year that was reported previously in a cross-sectional study (35). Of the 16 dams, 10 (62.5%) were culture positive for H. pylori on one or both endoscopies. At each time point, H. pylori infection was more prevalent among babies of infected dams than among those of uninfected dams. This difference was particularly apparent at the earliest time point (12 weeks), when positive cultures were obtained from 5 of 10 (50%) newborns from infected dams but from only 1 of 5 (20%) newborns from uninfected dams (Fig. 2). By 64 weeks of age, the cumulative prevalence of infection among babies from infected dams (91.7%) was similar to that from uninfected dams (87.5%).
FIG. 1.
Cumulative percentage of babies positive for H. pylori by culture (filled circles) and serology (open circles) as a function of age (weeks).
FIG. 2.
Cumulative percentage of babies from infected (open squares) or uninfected (open circles) dams that were culture positive for H. pylori as a function of age.
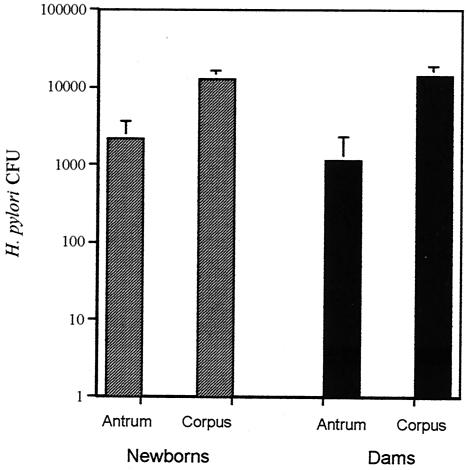
Evidence from longitudinal studies using both anti-H. pylori IgG antibodies (26, 28) and the [13C]-urea breath test (22) has suggested that H. pylori infection may sometimes be acquired and spontaneously cleared during childhood. We therefore examined whether infection in newborn rhesus monkeys was persistent over serial endoscopic cultures. The 18 monkeys that had at least one positive culture were examined in a total of 60 subsequent endoscopies (mean, 3.4 per animal; SD, 2.2). Of those endoscopies, four (6.7%) from two animals (11% of those infected) were negative for H. pylori. In one of these monkeys, no corpus biopsies were obtained at one of the negative endoscopies. Since the bacterial load in the corpus was typically greater than that in the antrum (Fig. 3), this negative endoscopy could have been falsely negative. In the other monkey, initial biopsies from the antrum and corpus yielded a total of 2 CFU, suggesting that the bacterial load was very low.
FIG. 3.
Mean (SD) H. pylori CFU in the gastric antrum and corpus of newborn babies and their dams.
Since the prevalence of H. pylori infection among newborns reached 90% at approximately 1 year of age, it was surprising that the prevalence among dams was only 62.5%. Interestingly, the seroprevalence in the dams was 100%, which is consistent with our earlier cross-sectional findings (35). In humans, a similar discordance has been observed between serology and the presence of H. pylori by Giemsa staining of gastric tissue (20). It has been suggested that H. pylori may be cleared later in life, which is thought to result from hypochlorhydria due to atrophic gastritis (20). We therefore examined whether there was a relationship between H. pylori infection and the age of the dams. The mean age (calculated at the time of the first biopsy) for H. pylori-infected and uninfected dams was 7.3 and 10.4 years, respectively (two-tailed student's t test, P < 0.05). Although we have not systematically examined H. pylori seroprevalence in captive macaques after the age of 4 years, these results suggest that, as in humans, the prevalence of H. pylori infection may decline in older age.
Topography of infection.
Quantitation of H. pylori in both the gastric antrum and corpus was performed during 36 endoscopies performed on one or more occasions on 17 of 18 infected infants and all 10 infected dams. Since the small size of biopsies obtained from infants precluded accurate measurement of tissue weight, results were expressed as CFU/three biopsies. The bacterial load (Fig. 3) was significantly greater in the corpus than in the antrum for both newborns (two-tailed student's t test, P < 0.0004) and dams (two-tailed student's t test, P < 0.008). Since the corpus was sampled less frequently during early time points yet contained larger numbers of bacteria, it is likely that the prevalence of H. pylori at 12 and perhaps also at 16 weeks of age is in fact even greater than we have estimated (Figs. 1 and 2).
Strain typing.
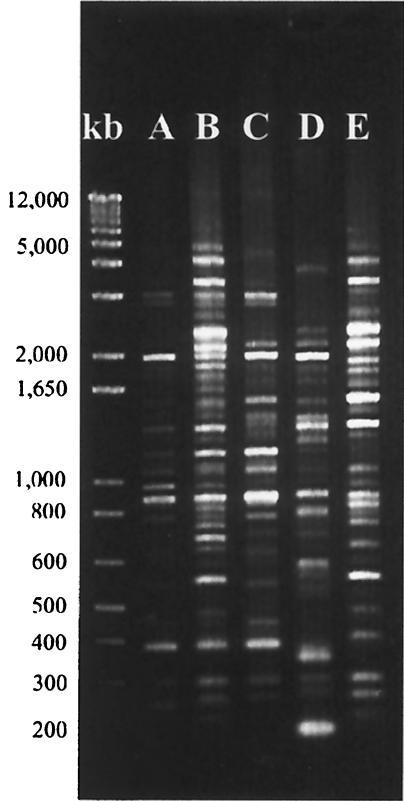
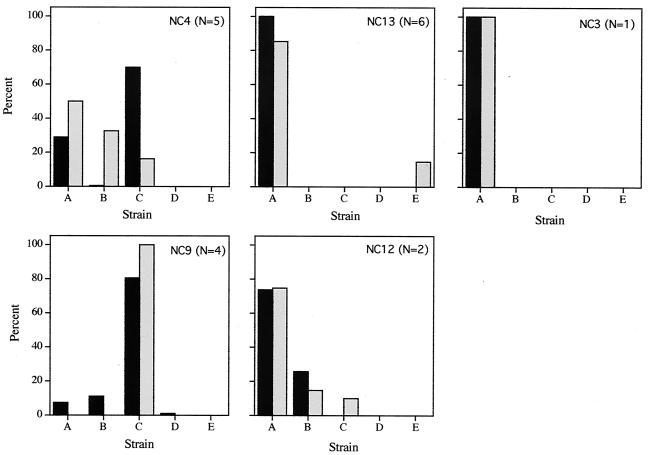
Rep-PCR typing of 370 colonies from newborns (mean and SD, 21 and 11 colonies per animal, respectively) and 109 colonies from dams (mean and SD, 11 and 7 colonies per animal, respectively) identified five strains of H. pylori, which we designated A through E (Fig. 4). Strains A, B, and C accounted for all but 7 of the 479 colonies studied. Two or more strains were identified in 7 of 18 babies (38.9%) and only 2 of 10 (20%) dams, though this result may simply reflect the larger number of colonies studied in the newborns. The strain types A through E were not equally distributed in each of the five field cages from which the animals were drawn (Fig. 5). The animals from cages NC13 and NC3 were almost exclusively strain A, those from cage NC9 were predominantly strain C, and those from the other two cages contained mixtures of A, B, and C. In each field cage, the strains recovered from the babies matched those recovered from the dams (Fig. 5).
FIG. 4.
Rep-PCR products electrophoresed in a 1.5% agarose gel stained with ethidium bromide. Representative fingerprints from each of the five strains (A through E) are shown. A DNA kilobase (kb) ladder is at left.
FIG. 5.
Distribution of the five H. pylori strain types (A through E) among babies (solid bars) and their dams (shaded bars) from each of the five field cages. The field cage designation and the number of baby-dam pairs is shown in the upper right of each panel.
DISCUSSION
This study examined for the first time the natural acquisition of H. pylori in newborns by using bacterial culture. This method avoids problems that are inherent in noninvasive techniques such as serology and the urea breath test, neither of which has been well validated in newborns. Since serial endoscopic biopsies are not feasible in human infants, we took advantage of natural H. pylori infection that is known to occur in socially housed nonhuman primates (11, 12, 35), the best studied of which are rhesus macaques. The results from cultures showed that H. pylori infection is common within the first few months of life and is nearly universal by the age of 1 year. Seropositivity lagged behind positive cultures by about 2 months (Fig. 1), a result that is similar to what has been seen in experimental infection of rhesus monkeys (9). Maternal IgG in macaques has a half-life of only 15 days, so the serologic response we observed likely reflects newborn antibody production. In some cases, infection may be acquired as early as the first day of life, since newborns isolated from their dams on the day of birth and raised in the nursery are occasionally infected with H. pylori (35). Therefore, colonization of the stomach with H. pylori in a high-prevalence environment almost resembles colonization of the gut with facultative microorganisms, which fully colonize the gastrointestinal tract of newborn infants within the first 2 weeks of life (34).
The incidence of H. pylori in newborn macaques was greater than is generally reported in humans, even in developing countries where the prevalence is highest (3). This may reflect in part the well-described limitations of serology and the urea breath test for detection of H. pylori in young children (4, 21, 23, 29, 31). The use of serology in newborn children is complicated further by the fact that maternal IgG confounds serodetection of H. pylori in children during the first year of life. IgM, which does not cross the placenta, is not well validated and is apparently less sensitive than IgG for detection of new infection (32). The increased incidence of H. pylori in rhesus monkeys compared to that in children may also be due to poor sanitation and hygiene in communities of captive nonhuman primates. It is notable, however, that infection rates were high despite readily available clean, potable water, suggesting that contamination of water supply is not essential for widespread infection at an early age in areas where hygiene is otherwise poor. Furthermore, breastfeeding seems to offer little protection, since newborn macaques breastfeed during the first year of life and typically are fully weaned only when another newborn arrives the following spring.
Although spontaneous clearance of H. pylori infection is thought to be uncommon in adults, many studies have described seroreversion of H. pylori in children at an annual rate of 1 to 2% (18, 26 to 28, 39). In some studies, however, spontaneous elimination of H. pylori in children appears much more common. For example, in a cohort of Swedish children monitored from age 6 months to 11 years, 25 of 40 children (62.5%) who were positive at some time during follow-up were negative by 11 years of age (18). Similarly, in a study of Peruvian children between 6 and 30 months of age using the urea breath test, 36 of 56 children (64.3%) had one or more negative tests after a positive test (22). We found that of 18 infected monkeys, only 2 had a negative culture following a positive one, which in both cases occurred after the first positive culture. Our results suggest that at least in a high-prevalence environment spontaneous clearance of H. pylori is uncommon but may occasionally occur after initial exposure. This finding is consistent with the results of some human studies that have found an inverse relationship between age and frequency of seroreversion (32). However, since even a very specific and sensitive test may have false-negative results, it is difficult to estimate the true frequency of spontaneous elimination of H. pylori infection.
H. pylori strain heterogeneity in isolates from socially housed macaques was substantially less than is found among isolates from unrelated humans. This finding is not surprising, since each field cage functions as a breeding colony, and once a colony is formed, no additional animals are introduced. Interestingly, the colonies from cages NC9, NC4, and NC12 (Fig. 5), which showed different profiles of strain diversity, were formed independently. On the other hand, the colony from cage NC13 was formed from that of cage NC3, and nearly all animals in these two cages have essentially the same strain profile (Fig. 5). Although molecular fingerprinting of human isolates suggests in some cases that children acquire infection from their parents (37, 43), the diversity among isolates from macaques was insufficient to implicate a route of infection. However, the markedly higher prevalence of infection among newborns of infected dams at 12 weeks of age suggests that infection may frequently be acquired from the dam during the peripartum period, during which physical contact between dam and newborn is greatest and that with other animals is least.
We were surprised to find in both newborns and dams that H. pylori colonization of the corpus was approximately 10-fold higher than that in the antrum (Fig. 3). This result stands in contrast to findings from most studies of naturally infected humans (17, 19, 25) and nonhuman primates (11) as well as to results for macaques experimentally infected with human-derived H. pylori (36). However, recent evidence suggests that strains of H. pylori differ in how effectively they colonize the gastric antrum and corpus (1). The hypothesis that local acid production may be important in the topography of H. pylori infection (6, 38) is a subject of ongoing studies.
In summary, we report for the first time a study of the natural acquisition of H. pylori in newborns using serial cultures of gastric biopsies from rhesus monkeys. H. pylori infection is common within the first few months of life and is nearly universal by 1 year of age. Infection is likely acquired from the dam during the early peripartum period. Although transient infections may occasionally occur, in a high-prevalence environment this occurrence is unusual and is probably restricted to the initial episode of colonization.
Acknowledgments
This work was supported by National Institutes of Health (NCRR) grant R01 RR14298.
REFERENCES
- 1.Akada, J. K., K. Ogura, D. Dailidiene, G. Dailide, J. M. Cheverud, and D. E. Berg. 2003. Helicobacter pylori tissue tropism: mouse-colonizing strains can target different gastric niches. Microbiology 149:1901-1909. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1990. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Bardhan, P. K. 1997. Epidemiological features of Helicobacter pylori in developing countries. Clin. Infect. Dis. 25:973-978. [DOI] [PubMed] [Google Scholar]
- 4.Braden, B., H. G. Posselt, P. Ahrens, R. Kitz, C. F. Dietrich, and W. F. Caspary. 2000. New immunoassay in stool provides an accurate noninvasive diagnostic method for Helicobacter pylori screening in children. Pediatrics 106:115-117. [DOI] [PubMed] [Google Scholar]
- 5.Corvaglia, L., P. Bontems, J. M. Devaster, P. Heimann, Y. Glupczynski, E. Keppens, and S. Cadranel. 1999. Accuracy of serology and 13C-urea breath test for detection of Helicobacter pylori in children. Pediatr. Infect. Dis. J. 18:976-979. [DOI] [PubMed] [Google Scholar]
- 6.Danon, S. J., J. L. O'Rourke, N. D. Moss, and A. Lee. 1995. The importance of local acid production in the distribution of Helicobacter felis in the mouse stomach. Gastroenterology 108:1386-1395. [DOI] [PubMed] [Google Scholar]
- 7.Delvin, E. E., J. L. Brazier, C. Deslandres, F. Alvarez, P. Russo, and E. Seidman. 1999. Accuracy of the [13C]-urea breath test in diagnosing Helicobacter pylori gastritis in pediatric patients. J. Pediatr. Gastroenterol. Nutr. 28:59-62. [DOI] [PubMed] [Google Scholar]
- 8.Drazek, E. S., A. Dubois, and R. K. Holmes. 1994. Characterization and presumptive identification of Helicobacter pylori isolates from rhesus monkeys. J. Clin. Microbiol. 32:1799-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois, A., D. E. Berg, E. T. Incecik, N. Fiala, L. M. Heman-Ackah, J. Del Valle, M. Yang, H. P. Wirth, G. I. Perez-Perez, and M. J. Blaser. 1999. Host specificity of Helicobacter pylori strains and host responses in experimentally challenged nonhuman primates. Gastroenterology 116:90-96. [DOI] [PubMed] [Google Scholar]
- 10.Dubois, A., D. E. Berg, E. T. Incecik, N. Fiala, L. M. Heman-Ackah, G. I. Perez-Perez, and M. J. Blaser. 1996. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect. Immun. 64:2885-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubois, A., N. Fiala, L. M. Heman-Ackah, E. S. Drazek, A. Tarnawski, W. N. Fishbein, G. I. Perez-Perez, and M. J. Blaser. 1994. Natural gastric infection with Helicobacter pylori in monkeys: a model for spiral bacteria infection in humans. Gastroenterology 106:1405-1417. [DOI] [PubMed] [Google Scholar]
- 12.Dubois, A., N. Fiala, R. H. Weichbrod, G. S. Ward, M. Nix, P. Mehlman, D. M. Taub, G. I. Perez-Perez, and M. J. Blaser. 1995. Seroepizootiology of Helicobacter pylori gastric infection in nonhuman primates housed in social environments. J. Clin. Microbiol. 33:1492-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997. Helicobacter pylori. Clin. Microbiol. Rev. 10:720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figueroa, G., R. Acuna, M. Troncoso, D. P. Portell, M. S. Toledo, and J. Valenzuela. 1997. Helicobacter pylori infection in Chile. Clin. Infect. Dis. 25:983-989. [DOI] [PubMed] [Google Scholar]
- 15.Glynn, M. K., C. R. Friedman, B. D. Gold, B. Khanna, L. Hutwagner, N. Iihoshi, C. Revollo, and R. Quick. 2002. Seroincidence of Helicobacter pylori infection in a cohort of rural Bolivian children: acquisition and analysis of possible risk factors. Clin. Infect. Dis. 35:1059-1065. [DOI] [PubMed] [Google Scholar]
- 16.Go, M. F., K. Y. Chan, J. Versalovic, T. Koeuth, D. Y. Graham, and J. R. Lupski. 1995. Cluster analysis of Helicobacter pylori genomic DNA fingerprints suggests gastroduodenal disease-specific associations. Scand. J. Gastroenterol. 30:640-646. [DOI] [PubMed] [Google Scholar]
- 17.Graham, D. Y., R. Genta, D. G. Evans, R. Reddy, J. E. Clarridge, C. A. Olson, A. L. Edmonds, and N. Siepman. 1996. Helicobacter pylori does not migrate from the antrum to the corpus in response to omeprazole. Am. J. Gastroenterol. 91:2120-2124. [PubMed] [Google Scholar]
- 18.Granstrom, M., Y. Tindberg, and M. Blennow. 1997. Seroepidemiology of Helicobacter pylori infection in a cohort of children monitored from 6 months to 11 years of age. J. Clin. Microbiol. 35:468-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackelsberger, A., T. Günther, V. Schultze, J. Labenz, A. Roessner, and P. Malfertheiner. 1997. Prevalence and pattern of Helicobacter pylori gastritis in the gastric cardia. Am. J. Gastroenterol. 92:2220-2224. [PubMed] [Google Scholar]
- 20.Karnes, W. E., Jr., I. M. Samloff, M. Siurala, M. Kekki, P. Sipponen, S. W. Kim, and J. H. Walsh. 1991. Positive serum antibody and negative tissue staining for Helicobacter pylori in subjects with atrophic body gastritis. Gastroenterology 101:167-174. [DOI] [PubMed] [Google Scholar]
- 21.Kindermann, A., H. Demmelmair, B. Koletzko, S. Krauss-Etschmann, B. Wiebecke, and S. Koletzko. 2000. Influence of age on 13C-urea breath test results in children. J. Pediatr. Gastroenterol. Nutr. 30:85-91. [DOI] [PubMed] [Google Scholar]
- 22.Klein, P. D., R. H. Gilman, R. Leon-Barua, F. Diaz, E. O. Smith, and D. Y. Graham. 1994. The epidemiology of Helicobacter pylori in Peruvian children between 6 and 30 months of age. Am. J. Gastroenterol. 89:2196-2200. [PubMed] [Google Scholar]
- 23.Koletzko, S., and A. Feydt-Schmidt. 2001. Infants differ from teenagers: use of non-invasive tests for detection of Helicobacter pylori infection in children. Eur. J. Gastroenterol. Hepatol. 13:1047-1052. [DOI] [PubMed] [Google Scholar]
- 24.Konstantopoulos, N., H. Russmann, C. Tasch, T. Sauerwald, H. Demmelmair, I. Autenrieth, and S. Koletzko. 2001. Evaluation of the Helicobacter pylori stool antigen test (HpSA) for detection of Helicobacter pylori infection in children. Am. J. Gastroenterol. 96:677-683. [DOI] [PubMed] [Google Scholar]
- 25.Kuipers, E. J., A. M. Uyterlinde, A. S. Peña, H. J. Hazenberg, E. Bloemena, J. Lindeman, E. C. Klinkenberg-Knol, and S. G. Meuwissen. 1995. Increase of Helicobacter pylori-associated corpus gastritis during acid suppressive therapy: implications for long-term safety. Am. J. Gastroenterol. 90:1401-1406. [PubMed] [Google Scholar]
- 26.Kumagai, T., H. M. Malaty, D. Y. Graham, S. Hosogaya, K. Misawa, K. Furihata, H. Ota, C. Sei, E. Tanaka, T. Akamatsu, T. Shimizu, K. Kiyosawa, and T. Katsuyama. 1998. Acquisition versus loss of Helicobacter pylori infection in Japan: results from an 8-year birth cohort study. J. Infect. Dis. 178:717-721. [DOI] [PubMed] [Google Scholar]
- 27.Malaty, H. M., A. El-Kasabany, D. Y. Graham, C. C. Miller, S. G. Reddy, S. R. Srinivasan, Y. Yamaoka, and G. S. Berenson. 2002. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet 359:931-935. [DOI] [PubMed] [Google Scholar]
- 28.Malaty, H. M., D. Y. Graham, W. A. Wattigney, S. R. Srinivasan, M. Osato, and G. S. Berenson. 1999. Natural history of Helicobacter pylori infection in childhood: 12-year follow-up cohort study in a biracial community. Clin. Infect. Dis. 28:279-282. [DOI] [PubMed] [Google Scholar]
- 29.Malaty, H. M., T. Haveman, D. Y. Graham, and J. K. Fraley. 2002. Helicobacter pylori infection in asymptomatic children: impact of epidemiologic factors on accuracy of diagnostic tests. J. Pediatr. Gastroenterol. Nutr. 35:59-63. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell, H. M., Y. Y. Li, P. J. Hu, Q. Liu, M. Chen, G. G. Du, Z. J. Wang, A. Lee, and S. L. Hazell. 1992. Epidemiology of Helicobacter pylori in southern China: identification of early childhood as the critical period for acquisition. J. Infect. Dis. 166:149-153. [DOI] [PubMed] [Google Scholar]
- 31.Okuda, M., E. Miyashiro, M. Koike, T. Tanaka, M. Bouoka, S. Okuda, and N. Yoshikawa. 2002. Serodiagnosis of Helicobacter pylori infection is not accurate for children aged below 10. Pediatr. Int. 44:387-390. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Perez, G. I., R. B. Sack, R. Reid, M. Santosham, J. Croll, and M. J. Blaser. 2003. Transient and persistent Helicobacter pylori colonization in native American children. J. Clin. Microbiol. 41:2401-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shepherd, A. J., C. L. Williams, C. P. Doherty, M. Hossack, T. Preston, K. E. McColl, and L. T. Weaver. 2000. Comparison of an enzyme immunoassay for the detection of Helicobacter pylori antigens in the faeces with the urea breath test. Arch. Dis. Child. 83:268-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon, G. L., and S. L. Gorbach. 1995. Normal alimentary tract microflora, p. 53-69. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract. Raven Press, New York, N.Y.
- 35.Solnick, J. V., D. R. Canfield, S. Yang, and J. Parsonnet. 1999. The rhesus monkey (Macaca mulatta) model of Helicobacter pylori: noninvasive detection and derivation of specific pathogen free monkeys. Lab. Anim. Sci. 49:197-201. [PubMed] [Google Scholar]
- 36.Solnick, J. V., L. M. Hansen, D. R. Canfield, and J. Parsonnet. 2001. Determination of the infectious dose of Helicobacter pylori during primary and secondary infection in rhesus monkeys (Macaca mulatta). Infect. Immun. 69:6887-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taneike, I., Y. Tamura, T. Shimizu, Y. Yamashiro, and T. Yamamoto. 2001. Helicobacter pylori intrafamilial infections: change in source of infection of a child from father to mother after eradication therapy. Clin. Diagn. Lab. Immunol. 8:731-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Zanten, S. J., M. F. Dixon, and A. Lee. 1999. The gastric transitional zones: neglected links between gastroduodenal pathology and helicobacter ecology. Gastroenterology 116:1217-1229. [DOI] [PubMed] [Google Scholar]
- 39.Veldhuyzen van Zanten, S. J., P. T. Pollak, L. M. Best, G. S. Bezanson, and T. Marrie. 1994. Increasing prevalence of Helicobacter pylori infection with age: continuous risk of infection in adults rather than cohort effect. J. Infect. Dis. 169:434-437. [DOI] [PubMed] [Google Scholar]
- 40.Versalovic, J., V. Kapur, E. O. Mason, Jr., U. Shah, T. Koeuth, J. R. Lupski, and J. M. Musser. 1993. Penicillin-resistant Streptococcus pneumoniae strains recovered in Houston: identification and molecular characterization of multiple clones. J. Infect. Dis. 167:850-856. [DOI] [PubMed] [Google Scholar]
- 41.Versalovic, J., T. Koeuth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vincent, P., L. Michaud, E. Martin de Lasalle, B. Benon, D. Turck, and F. Gottrand. 1999. 13C-urea breath test and gastric mucosal colonization by Helicobacter pylori in children: quantitative relation and usefulness for diagnosis of infection. Helicobacter 4:233-237. [DOI] [PubMed] [Google Scholar]
- 43.Wang, J. T., J. C. Sheu, J. T. Lin, T. H. Wang, and M. S. Wu. 1993. Direct DNA amplification and restriction pattern analysis of Helicobacter pylori in patients with duodenal ulcer and their families. J. Infect. Dis. 168:1544-1548. [DOI] [PubMed] [Google Scholar]