Abstract
Salmonella enterica serotype Newport isolates resistant to at least nine antimicrobials (including extended-spectrum cephalosporins), known as serotype Newport MDR-AmpC isolates, have been rapidly emerging as pathogens in both animals and humans throughout the United States. Resistance to extended-spectrum cephalosporins is associated with clinical failures, including death, in patients with systemic infections. In this study, 87 Salmonella serotype Newport strains were characterized by pulsed-field gel electrophoresis (PFGE) and antimicrobial susceptibility testing and examined for the presence of class 1 integrons and blaCMY genes. Thirty-five PFGE patterns were observed with XbaI, and three of these patterns were indistinguishable among isolates from humans and animals. Fifty-three (60%) Salmonella serotype Newport isolates were identified as serotype Newport MDR-AmpC, including 16 (53%) of 30 human isolates, 27 (93%) of 29 cattle isolates, 7 (70%) of 10 swine isolates, and 3 (30%) of 10 chicken isolates. However, 28 (32%) Salmonella serotype Newport isolates were susceptible to all 16 antimicrobials tested. The blaCMY gene was present in all serotype Newport MDR-AmpC isolates. Furthermore, the plasmid-mediated blaCMY gene was transferable via conjugation to an Escherichia coli strain. The transconjugant showed the MDR-AmpC resistance profile. Thirty-five (40%) of the isolates possessed class 1 integrons. Sequence analyses of the integrons showed that they contained aadA, which confers resistance to streptomycin, or aadA and dhfr, which confer resistance to trimethoprim-sulfamethoxazole. One integron from a swine isolate contained the sat-1 gene, which encodes resistance to streptothricin, an antimicrobial agent that has never been approved for use in the United States. In conclusion, Salmonella serotype Newport MDR-AmpC was commonly identified among Salmonella serotype Newport isolates recovered from humans and food animals. These findings support the possibility of transmission of this organism to humans through the food chain.
Infections with nontyphoid Salmonella enterica serovars represent an important public health problem worldwide. An estimated 1.4 million cases of salmonellosis, leading to 16,000 hospitalizations and nearly 600 deaths, occur each year in the United States (13). Salmonella infections in humans often result from the ingestion of contaminated foods, such as poultry, beef, pork, eggs, milk, seafood, and fresh produce (9). Direct contact with animals also results in transmission of Salmonella to humans (4, 6). Salmonellosis in humans is usually a self-limiting diarrhea that does not warrant antimicrobial therapy. However, these infections can also lead to life-threatening systemic infections that require effective chemotherapy (10, 19). Currently, ceftriaxone is the agent of choice for such chemotherapy.
In the last 20 years, the worldwide emergence of multidrug-resistant phenotypes among Salmonella serotypes, in particular Salmonella serotype Typhimurium (8, 18) and, more recently, Salmonella serotype Newport (2, 4), is of increasing concern. Salmonella serotype Typhimurium DT104, which is resistant to at least five antimicrobials, including ampicillin, chloramphenicol, streptomycin, sulfamethoxazole, and tetracycline, has caused severe infections and deaths in animals and humans worldwide (3, 5, 8). Multidrug-resistant Salmonella serotype Newport has recently been spreading on an epidemic scale in both animals and humans throughout the United States (2, 4). In addition to the resistance to five drugs found in Salmonella serotype Typhimurium DT104, this Salmonella serotype Newport, called serotype Newport MDR-AmpC, is also resistant to amoxicillin-clavulanic acid, cephalothin, cefoxitin, and ceftiofur and exhibits decreased susceptibility to ceftriaxone (MIC ≥ 16 μg/ml). Some serotype Newport MDR-AmpC strains have shown resistance to gentamicin, kanamycin, and trimethoprim-sulfamethoxazole. A multistate outbreak of serotype Newport MDR-AmpC infections was reported by the Centers for Disease Control and Prevention (CDC), and raw or undercooked ground beef was implicated as the vehicle of transmission (2). Serotype Newport MDR-AmpC's resistance to ceftiofur and decreased susceptibility to ceftriaxone has significant clinical implications for both human and veterinary medicine. Ceftriaxone is the drug of choice for treatment of severe salmonellosis in humans, especially in children (11). Ceftiofur is the only cephalosporin approved for systemic use in food animals in the United States. This drug was first approved in 1988 for the treatment of acute bovine respiratory disease and was subsequently approved for use in other food animals, including swine, sheep, chickens, and turkeys (1). Because ceftiofur-resistant organisms also exhibit decreased susceptibility to cephamycins and expanded-spectrum cephalosporins, the use of this antimicrobial in food animals has come under increasing scrutiny as a selective factor responsible for the emergence and dissemination of ceftriaxone-resistant enteric pathogens such as Salmonella (4, 7, 21). Cattle have been identified as the major reservoir for Salmonella serotype Newport MDR-AmpC; however, other animals, such as pigs, horses, dogs, and pigeons, also can be infected with the pathogen, and certain clones of serotype Newport MDR-AmpC have spread among these animals. This spread has been confirmed based on genomic DNA fingerprinting patterns and antimicrobial resistance profiles (16).
A blaCMY gene encoding a cephalomycinase, an extended-spectrum beta-lactamase, has been shown to be responsible for resistance to ceftiofur and decreased susceptibility to ceftriaxone. We have shown that the blaCMY gene caused resistance to narrow-, expanded-, and broad-spectrum cephalosporins (23). The blaCMY gene has been identified in many gram-negative enteric pathogens, including Salmonella, in many countries (7, 12, 20). The blaCMY gene is transferable through conjugation (23). However, the prevalence of blaCMY in serotype Newport MDR-AmpC isolates and its contribution to multiple-drug resistance has not been fully investigated.
The objectives of the present study were to characterize S. enterica serotype Newport isolates from humans and food animals by using pulsed-field gel electrophoresis (PFGE), to determine their antimicrobial resistance phenotypes, and to screen for the presence of the blaCMY gene and the class 1 integron to determine their contributions to resistance and resistance gene transfer. The results of this study revealed that clonal spread of resistant isolates between animals and humans occurs and that resistant factors exist in previously unreported reservoirs.
MATERIALS AND METHODS
Bacterial strains.
Eighty-seven Salmonella serotype Newport isolates, including 30 from human patients and 57 from sick animals (29 from cattle, 10 from swine, 10 from chickens, and 8 from turkeys), were used in this study. The isolates were obtained from 25 states from 2001 to 2002. Salmonella serotype Newport organisms were grown on Trypticase soy agar (TSA; Difco, Detroit, Mich.) plates supplemented with 5% defibrinated sheep blood (Becton Dickinson Microbiology Systems, Cockeysville, Md.) and stored in Trypticase soy broth (TSB; Difco) containing 15% glycerol at −80°C until they were used.
Antimicrobial susceptibility determination.
Antimicrobial MICs for Salmonella serotype Newport isolates were determined via the Sensititre automated antimicrobial susceptibility system (Trek Diagnostic Systems, Westlake, Ohio) and interpreted according to the National Committee for Clinical Laboratory Standards (NCCLS) MIC interpretive standards (14, 15). Sensititre susceptibility testing was performed according to the manufacturer's instructions. The following antimicrobials were tested: amikacin, amoxicillin-clavulanic acid, ampicillin, cefoxitin, ceftiofur, ceftriaxone, cephalothin, chloramphenicol, ciprofloxacin, gentamicin, kanamycin, nalidixic acid, streptomycin, sulfamethoxazole, tetracycline, and trimethoprim-sulfamethoxazole. E. coli ATCC 25922, E. coli ATCC 35218, Enterococcus faecalis ATCC 29212, Staphylococcus aureus ATCC 29213, and Pseudomonas aeruginosa ATCC 27853 were used as quality control organisms in antimicrobial MIC determinations to ensure that the dilution range for each antimicrobial agent was properly controlled.
PFGE.
PFGE was performed to determine genomic DNA fingerprinting profiles of Salmonella serotype Newport isolates according to the procedures developed by the CDC. Briefly, bacteria were grown on plates containing TSA supplemented with 5% defibrinated sheep blood (Becton Dickinson) at 37°C for 18 h. Bacterial colonies were suspended in cell suspension buffer (100 mM Tris HCl, 100 mM EDTA [pH 8.0]) and adjusted to an optical density of 0.52 to 0.56 by using a MicroScan turbidity meter (Dade Behring, Inc., West Sacramento, Calif.). The cell suspension (200 μl) was mixed with 10 μl of proteinase K (10 mg/ml) and an equal volume of melted 1% SeaKem Gold agarose (FMC BioProducts, Rockville, Maine) containing 1% sodium dodecyl sulfate. The mixture was dispensed into a sample mold (Bio-Rad, Hercules, Calif.). After solidification, the plugs were transferred to a tube containing 5 ml of lysis buffer (50 mM Tris HCl, 50 mM EDTA [pH 8.0], 1% sarcosyl) and 0.1 mg of proteinase K per ml. Cells were lysed overnight in a water bath at 54°C with agitation at 180 rpm. After lysis, the plugs were washed twice with deionized distilled water and four times with TE buffer (10 mM Tris HCl, 1 mM EDTA [pH 8.0]) for 15 min per wash at 50°C with agitation at 180 rpm. Agarose-embedded DNA was digested with 50 U of XbaI (Boehringer Mannheim, Indianapolis, Ind.) overnight in a water bath at 37°C. The plugs were placed in a 1% SeaKem Gold agarose gel. Restriction fragments were separated by electrophoresis in 0.5× Tris-borate-EDTA buffer at 14°C for 18 h by using a Chef Mapper (Bio-Rad) with pulse times of 2.16 to 63.8 s. The gel was stained with ethidium bromide, and DNA bands were visualized with a UV transilluminator. The interpretation of the PFGE patterns was aided by the use of Molecular Analyst Fingerprinting Plus software, version 1.6 (Bio-Rad).
Bacterial DNA preparation, PCR, and DNA sequencing.
The presence of blaCMY and the class 1 integron was detected by PCR assays with primers cmy-F (5′-GACAGCCTCTTTCTCCACA-3′) and cmy-R (5′-TGGAACGAAGGCTACGTA-3′) and primers 5′-CS (5′-GGCATCCAAGCACAAGC-3′) and 3′-CS (5′-AAGCAGACTTGACTGAT-3′), respectively. DNA template was prepared by boiling isolates for 10 min and then centrifuging them for 30 s. Amplifications were carried out with 10 μl of boiled bacterial suspensions (ca. 159 ng of DNA), 250 μM (each) deoxynucleoside triphosphate, 2.5 mM MgCl2, 50 pmol of primers, and 1 U of Gold Taq polymerase (Perkin-Elmer, Foster City, Calif.) in 50-μl reaction mixtures. PCR cycling conditions were 30 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min and a final extension at 72°C for 10 min. The annealing temperature of 54°C was used for the amplification of the class 1 integron. The amplified products were separated by gel electrophoresis in a 1.0% agarose gel stained with ethidium bromide, visualized under UV light, and recorded with a gel documentation system (Bio-Rad). For each set of blaCMY PCRs, cephalosporin-resistant S. enterica serotype Typhimurium CVM1290 and susceptible S. enterica serotype Typhimurium CVM785 were included as positive and negative controls. S. enterica serotype Typhimurium DT 104 CVM4011 and E. coli CVM996 were included as positive and negative controls, respectively, for class 1 integron PCRs. The PCR-generated DNA fragments were then purified with a PCR purification kit (Boehringer Mannheim) and sequenced in an ABI automatic DNA sequencer (model 3700; Perkin-Elmer). DNA sequences were analyzed by searching the GenBank database of the National Center for Biotechnology Information via the BLAST network service.
Conjugation experiments.
For conjugation studies, Salmonella serotype Newport strains CVM20776 (from cattle) and CVM21547 (from swine) were used as donor strains, and E. coli CVM19752 (from food) was used as the recipient strain. Streptomycin and nalidixic acid were used as selective agents for the donor and recipient strains, respectively. Conjugation was performed by the filter mating method (17). Briefly, the donor and recipient strains were grown overnight at 37°C in TSB with the appropriate antibiotics. The recipient and donor cells were mixed at a 1:10 ratio and placed on a filter paper, which was placed on a plate containing antibiotic-free TSA supplemented with 5% defibrinated sheep blood and incubated overnight at 37°C. Transconjugants from the filter paper were resuspended in TSB and plated on TSA containing 64 μg of streptomycin/ml and 32 μg of nalidixic acid/ml. Following incubation at 37°C for 24 h, transconjugants from each mating were selected and subjected to antimicrobial susceptibility testing and Vitek identification. The transfer of blaCMY and the class 1 integron was confirmed by PCR followed by DNA sequence analysis of the PCR products.
RESULTS
Antimicrobial susceptibility profiles.
The antimicrobial susceptibilities of 87 Salmonella serotype Newport isolates were determined by a microdilution broth method. Fifty-three (60%) Salmonella serotype Newport isolates were identified as serotype Newport MDR-AmpC; these isolates were resistant to ampicillin, chloramphenicol, streptomycin, sulfamethoxazole, tetracycline, amoxicillin-clavulanic acid, cephalothin, cefoxitin, and ceftiofur and exhibited decreased susceptibility to ceftriaxone (MIC ≥ 16 μg/ml). The isolates identified as serotype Newport MDR-AmpC included 27 (93%) of 29 isolates from cattle, 7 (70%) of 10 isolates from swine, 3 (30%) of 10 isolates from chickens, and 16 (53%) of 30 isolates from humans. None of the turkey isolates were identified as serotype Newport MDR-AmpC. For one serotype Newport MDR-AmpC strain isolated from a human, the ceftriaxone MIC was ≥8 μg/ml, and for two strains, one from a cow and one from a human, the ceftriaxone MICs were ≥64 μg/ml, which is the MIC resistance breakpoint according to NCCLS interpretive standards. Three cattle isolates, two swine isolates, and one chicken isolate showed resistance to gentamicin, and nine human isolates, two cattle isolates, and three swine isolates showed resistance to trimethoprim-sulfamethoxazole. All 87 serotype Newport isolates were susceptible to amikacin, ciprofloxacin, and nalidixic acid. However, 28 (32%) of the Salmonella serotype Newport isolates—14 from humans, 1 from a cow, 5 from chickens, 1 from swine, and 7 from turkeys—were susceptible to all 16 antimicrobials tested.
PFGE profiles.
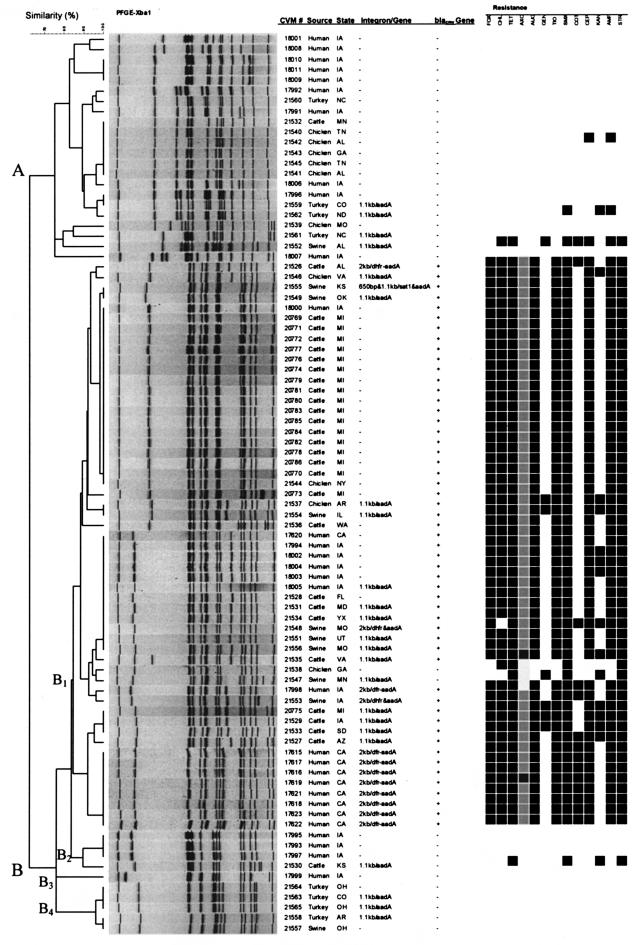
PFGE was used to assess genetic relatedness among the S. enterica serotype Newport isolates recovered from humans and sick food animals. PFGE revealed 35 banding patterns among the 87 serotype Newport isolates, which grouped into two clusters with 60% pattern similarity (Fig. 1). The PFGE clusters showed good correlation with the antimicrobial susceptibility profiles. Cluster A contained 22 isolates with 14 PFGE patterns. The 22 isolates in this cluster were isolated from different states and were classified as non-serotype Newport MDR-AmpC. The majority of these isolates were susceptible to all 16 antimicrobials tested, with the exception of one isolate from a chicken (CVM21542), one isolate from a turkey (CVM21562), and one isolate from a pig (CVM21552). More importantly, the PFGE patterns for one human isolate (CVM17996) and one turkey isolate (CVM21559) from different states were indistinguishable. Cluster B contained the remaining 65 isolates, with four subclusters and 21 PFGE patterns. All isolates in subcluster B1 except two were identified as serotype Newport MDR-AmpC. The two isolates in this subcluster that were not identified as serotype Newport MDR-AmpC were from a chicken (CVM21538) and a pig (CVM21547) and showed similar PFGE patterns (with a difference of one band). There were two major PFGE patterns (PulseNet patterns JJPX01.0014 and JJPX01.0042) in subcluster B1 that were shared among isolates from humans and animals from different states. The PFGE pattern for 1 human isolate (CVM18000) from Iowa (PulseNet pattern JJPX01.0042) was indistinguishable from those for 16 cattle isolates from Michigan and 2 chicken isolates from Iowa and New York, whereas 5 human isolates that were also from Iowa shared a PFGE pattern (JJPX01.0014) with 3 cattle and 3 swine isolates obtained from five different states. None of the 10 isolates in subclusters B2, B3, and B4 were of serotype Newport MDR-AmpC. All of them except one cattle isolate were susceptible to 16 antimicrobials.
FIG.1.
PFGE profiles, presence of blaCMY, class 1 integron, and associated resistance genes, and antimicrobial resistance profiles of S. enterica serotype Newport isolates from humans and food animals from 25 states. AUG, amoxicillin-clavulanic acid; AMP, ampicillin; TIO, ceftiofur; AXO, ceftriaxone; CEP, cephalothin; FOX, cefoxitin; CHL, chloramphenicol; KAN, kanamycin; STR, streptomycin; SMX, sulfamethoxazole; TET, tetracycline; GEN, gentamicin; COT, trimethoprim-sulfamethoxazole. Dark blocks indicate resistance, and light blocks indicate decreased susceptibility to AXO (MIC ≥ 16 μg/ml).
Presence of blaCMY gene, class I integron, and integron-associated genes.
All 53 serotype Newport MDR-AmpC isolates contained the blaCMY gene. DNA sequence analysis of 1.0-kb PCR amplicons of the blaCMY gene demonstrated 95 to 99% homology to a previously reported blaCMY-2 gene from Klebsiella pneumoniae and S. enterica serotype Senftenberg.
The class 1 integron was identified in 36 isolates. The integron was present in both serotype Newport MDR-AmpC isolates and non-serotype Newport MDR-AmpC isolates, even in some of the antimicrobial-susceptible isolates. Twenty-three isolates contained a 1.1-kb amplicon, 12 contained a 2.0-kb amplicon, and 1 contained two amplicons with sizes of 0.65 and 1.1 kb. DNA sequencing revealed that all of the 1.1-kb integrons contained aadA, which encodes resistance to streptomycin; that the 2.0-kb integron aadA and dfrh, which confer resistance to trimethoprim; and that the 0.65-kb integron contained the sat-1 gene, which encodes resistance to streptothricin. Streptothricin, an early aminoglycoside, has never been approved for therapeutic use in the United States due to its toxicity.
Conjugal transfer of plasmids harboring the blaCMY gene and the class 1 integron.
Conjugation studies were conducted to determine whether the blaCMY gene and the class 1 integron were transferable to E. coli. Results obtained by a filter mating method indicated that Salmonella serotype Newport MDR-AmpC strain CVM20766 was able to transfer the blaCMY gene to E. coli strain CVM19752. Transconjugant 20776/19752 acquired the serotype Newport MDR-AmpC resistance profile, exhibiting resistance to ampicillin, amoxicillin-clavulanic acid, cephalothin, cefoxitin, ceftiofur, chloramphenicol, streptomycin, sulfamethoxazole, and tetracycline and decreased susceptibility to ceftriaxone (MIC ≥ 32 μg/ml). Salmonella serotype Newport strain CVM21547 was able to transfer a class 1 integron to the E. coli strain. Transconjugant 21547/19752 acquired resistance not only to streptomycin, which was the integron-encoded resistance profile, but also to gentamicin, kanamycin, sulfamethoxazole, and tetracycline (Table 1). The transconjugants were confirmed to be E. coli by Vitek identification, and transfer of the blaCMY gene and the class 1 integron were confirmed by PCR and DNA sequencing analysis.
TABLE 1.
Antimicrobial susceptibility profiles of Salmonella and E. coli strains used in conjugation experimentsa
| Strain | Designation | MIC (μg/ml) of antimicrobial agent
|
Presence or absence of:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUG | AMP | TIO | AXO | CEP | FOX | CHL | KAN | NAL | STR | SMX | TET | CIP | GEN | blaCMY | Integron | ||
| CVM20776 | Donor (serotype Newport) | >32 | >32 | >8 | 16 | >32 | >16 | >32 | <8 | ≤4 | >64 | >512 | >32 | 0.015 | 0.25 | + | − |
| CVM21547 | Donor (serotype Newport) | 2 | 2 | ≤0.25 | ≤0.25 | 4 | ≤4 | ≤4 | >64 | ≤4 | >64 | >512 | >32 | 0.015 | 16 | − | + |
| CVM19752 | Recipient (E. coli) | 2 | 4 | ≤0.25 | ≤0.25 | 16 | 4 | 8 | <8 | >32 | <32 | <16 | 4 | >4 | 1 | − | − |
| 20776/19752 | Transconjugant (E. coli) | >32 | >32 | >8 | 32 | >32 | >16 | >32 | <8 | >32 | >64 | >512 | >32 | >4 | 0.5 | + | − |
| 21547/19752 | Transconjugant (E. coli) | 2 | 4 | ≤0.25 | ≤0.25 | 8 | 4 | 4 | >64 | >32 | >64 | >512 | >32 | >4 | >16 | − | + |
AUG, amoxicillin-clavulanic acid; AMP, ampicillin; TIO, ceftiofur; AXO, ceftriaxone; CEP, cephalothin; FOX, cefoxitin; CHL, chloramphenicol; KAN, kanamycin; NAL, nalidixic acid; STR, streptomycin; SMX, sulfamethoxazole; TET, tetracycline; CIP, ciprofloxacin; GEN, gentamicin. Numbers in bold are resistance breakpoints.
DISCUSSION
Several reports from the United States have documented the association of exposure to dairy farms, ill cattle, and cheese made from unpasteurized milk with increased human Salmonella serotype Newport MDR-AmpC infections (4, 16). In a recent outbreak, most patients had eaten lean or extra-lean ground beef; in the United States, dairy cattle are an important source of lean or extra-lean ground beef (2). Recent U.S. surveys indicate that 11 to 28% of persons report eating raw or undercooked ground beef and that approximately one-third of those surveyed do not use safe food-handling practices to prevent cross-contamination in the kitchen (2). These data suggest that cattle, particularly dairy cattle, might be a source of Salmonella serotype Newport MDR-AmpC infections in humans. Rankin et al. reported that cattle are the major reservoir of serotype Newport MDR-AmpC but that horses, dogs, and pigeons also can carry the pathogen (16). In the present study, Newport MDR-AmpC isolates were identified in swine and chickens in addition to cattle and other animals.
All 53 serotype Newport MDR-AmpC isolates in our study contained the blaCMY gene, which is responsible for resistance to ceftiofur and decreased susceptibility to ceftriaxone. Our previous study indicated that the blaCMY gene was associated with resistance to narrow-, expanded-, and broad-spectrum cephalosporins and was widespread in many other gram-negative enteric pathogens as well (23). The conjugation study showed that the blaCMY gene was located on a conjugative plasmid that also contained genes conferring resistance to chloramphenicol, streptomycin, sulfamethoxazole, and tetracycline. Many serotype Newport MDR-AmpC isolates contained a class 1 integron carrying aadA and dhfr gene cassettes. Five isolates contained silent aadA cassettes in the integron. Our previous study indicated that such silent integron-borne aadA genes can be fully expressed when transferred to a new host (22). The conjugation experiment also showed that other resistance genes in addition to those associated with the integron reside in this transferable plasmid. Both DNA sequence and conjugation studies showed that the blaCMY gene does not locate within the integron cassette. In fact, the blaCMY gene and the integron reside in different conjugative plasmids. Serotype Newport MDR-AmpC possesses resistance to nine or more antimicrobials. The presence of any of these antimicrobials may contribute to the overall selective pressures involved in maintaining these resistance genes and also may play a significant role in the dissemination of multidrug resistance genes to other susceptible bacteria in the environment.
Salmonella serotype Newport has emerged as the third most common Salmonella serotype causing human salmonellosis in the United States (2). According to the CDC, the number of laboratory-confirmed Salmonella serotype Newport infections increased from 1,584 (5%) of 34,608 reported Salmonella infections in 1997 to 3,152 (10%) of 31,607 reported infections in 2001 (2). The increasing number of Salmonella serotype Newport infections appears to be associated with the emergence and rapid dissemination of multidrug-resistant strains of Salmonella serotype Newport. The National Antimicrobial Resistance Monitoring System study showed that 33 (26%) of 128 Salmonella serotype Newport isolates tested were of serotype Newport MDR-AmpC in 2001, compared with 1 (1%) of 78 isolates in 1998. A Danish study reported significantly increased risks of hospitalization, morbidity, and mortality for patients infected with multidrug-resistant Salmonella serotype Typhimurium (10). The highest risks were associated with fluoroquinolone-resistant strains, and fluoroquinolones were the empirical drug of choice during the study period. That study evaluated strains collected from 1995 until October 1999, of which only 1.4% were resistant to ceftriaxone, the drug of choice for treating serious Salmonella infections. It is reasonable to expect that in the United States, especially among children, for whom ceftriaxone is the only recommended drug, an increase in ceftriaxone resistance such as that shown in our study will be followed by related increases in morbidity and mortality. The present study showed clearly that previously unsuspected numbers of strains carry the capability, via conjugative plasmids and class 1 integrons, to transfer additional resistance genes broadly among enteric pathogens and other gut bacteria. Importantly, this study showed the presence of these genetic elements in Salmonella isolates from food animals other than cattle, including swine and chickens. The presence of serotype Newport MDR-AmpC resistance in dairy cattle and the findings of identical serotype Newport MDR-AmpC clones in animals and humans further demonstrate that food animals can be a source of the pathogen, and these findings emphasize the need to modify both antibiotic dosing practices and feed supplementation in animals. The overuse and misuse of antimicrobials may provide selective pressure for the spread of serotype Newport MDR-AmpC. Therefore, efforts to promote the appropriate use of antimicrobials in both humans and animals and to enhance surveillance are essential for the control of multidrug resistance of bacterial pathogens. In addition, should increased morbidity be recognized among Salmonella-infected patients, recommendations for empirical therapy for humans with serious Salmonella disease may need revision.
REFERENCES
- 1.Bradford, P. A., P. J. Petersen, I. M. Fingerman, and D. G. White. 1999. Characterization of expanded-spectrum cephalosporin resistance in E. coli isolates associated with bovine calf diarrhoeal disease. J. Antimicrob. Chemother. 44:607-610. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2002. Outbreak of multidrug-resistant Salmonella Newport—United States, January-April 2002. Morb. Mortal. Wkly. Rep. 51:545-548. [PubMed] [Google Scholar]
- 3.Cody, S. H., S. L. Abbott, A. A. Marfin, B. Schulz, P. Wagner, K. Robbins, J. C. Mohle-Boetani, and D. J. Vugia. 1999. Two outbreaks of multidrug-resistant Salmonella serotype Typhimurium DT104 infections linked to raw-milk cheese in northern California. JAMA 281:1805-1810. [DOI] [PubMed] [Google Scholar]
- 4.Dunne, E. F., P. D. Fey, P. Kludt, R. Reporter, F. Mostashari, P. Shillam, J. Wicklund, C. Miller, B. Holland, K. Stamey, T. J. Barrett, J. K. Rasheed, F. C. Tenover, E. M. Ribot, and F. J. Angulo. 2000. Emergence of domestically acquired ceftriaxone-resistant Salmonella infections associated with ampC beta-lactamase. JAMA 284:3151-3156. [DOI] [PubMed] [Google Scholar]
- 5.Evans, S., and R. Davies. 1996. Case control study of multiple-resistant Salmonella Typhimurium DT104 infection of cattle in Great Britain. Vet. Rec. 139:557-558. [PubMed] [Google Scholar]
- 6.Fey, P. D., T. J. Safranek, M. E. Rupp, E. F. Dunne, E. Ribot, P. C. Iwen, P. A. Bradford, F. J. Angulo, and S. H. Hinrichs. 2000. Ceftriaxone-resistant Salmonella infection acquired by a child from cattle. N. Engl. J. Med. 342:1242-1249. [DOI] [PubMed] [Google Scholar]
- 7.Gazouli, M., S. V. Sidorenko, E. Tzelepi, N. S. Kozlova, D. P. Gladin, and L. S. Tzouvelekis. 1998. A plasmid-mediated beta-lactamase conferring resistance to cefotaxime in a Salmonella Typhimurium clone found in St. Petersburg, Russia. J. Antimicrob. Chemother. 41:119-121. [DOI] [PubMed] [Google Scholar]
- 8.Glynn, M. K., C. Bopp, W. Dewitt, P. Dabney, M. Mokhtar, and F. J. Angulo. 1998. Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. N. Engl. J. Med. 338:1333-1338. [DOI] [PubMed] [Google Scholar]
- 9.Gomez, T. M., Y. Motarjemi, S. Miyagawa, F. K. Kaferstein, and K. Stohr. 1997. Foodborne salmonellosis. World Health Stat. Q. 50:81-89. [PubMed] [Google Scholar]
- 10.Helms, M., P. Vastrup, P. Gerner-Smidt, and K. Molbak. 2002. Excess mortality associated with antimicrobial drug-resistant Salmonella Typhimurium. Emerg. Infect. Dis. 8:490-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hohmann, E. L. 2001. Nontyphoidal salmonellosis. Clin. Infect. Dis. 32:263-269. [DOI] [PubMed] [Google Scholar]
- 12.Kesah, C. N., A. O. Coker, S. A. Alabi, and D. K. Olukoya. 1996. Prevalence, antimicrobial properties and beta-lactamase production of haemolytic enterobacteria in patients with diarrhoea and urinary tract infections in Lagos, Nigeria. Cent. Afr. J. Med. 42:147-150. [PubMed] [Google Scholar]
- 13.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals, 5th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 16.Rankin, S. C., H. Aceto, J. Cassidy, J. Holt, S. Young, B. Love, D. Tewari, D. S. Munro, and C. E. Benson. 2002. Molecular characterization of cephalosporin-resistant Salmonella enterica serotype Newport isolates from animals in Pennsylvania. J. Clin. Microbiol. 40:4679-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simjee, S., A. P. Fraise, and M. J. Gill. 1999. Plasmid heterogeneity and identification of a Tn5281-like element in clinical isolates of high-level gentamicin-resistant Enterococcus faecium isolated in the UK. J. Antimicrob. Chemother. 43:625-635. [DOI] [PubMed] [Google Scholar]
- 18.Threlfall, E. J., L. R. Ward, J. A. Frost, and G. A. Willshaw. 2000. The emergence and spread of antibiotic resistance in food-borne bacteria. Int. J. Food Microbiol. 62:1-5. [DOI] [PubMed] [Google Scholar]
- 19.Travers, K., and M. Barza. 2002. Morbidity of infections caused by antimicrobial-resistant bacteria. Clin. Infect. Dis. 34(Suppl. 3):S131-S134. [DOI] [PubMed] [Google Scholar]
- 20.Verdet, C., G. Arlet, S. Ben Redjeb, A. Ben Hassen, P. H. Lagrange, and A. Philippon. 1998. Characterisation of CMY-4, an AmpC-type plasmid-mediated beta-lactamase in a Tunisian clinical isolate of Proteus mirabilis. FEMS Microbiol Lett. 169:235-240. [DOI] [PubMed] [Google Scholar]
- 21.Winokur, P. L., A. Brueggemann, D. L. DeSalvo, L. Hoffmann, M. D. Apley, E. K. Uhlenhopp, M. A. Pfaller, and G. V. Doern. 2000. Animal and human multidrug-resistant, cephalosporin-resistant Salmonella isolates expressing a plasmid-mediated CMY-2 AmpC β-lactamase. Antimicrob. Agents Chemother. 44:2777-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao, S., D. G. White, B. Ge, S. Ayers, S. Friedman, L. English, D. Wagner, S. Gaines, and J. Meng. 2001. Identification and characterization of integron-mediated antibiotic resistance among Shiga toxin-producing Escherichia coli isolates. Appl. Environ. Microbiol. 67:1558-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao, S., D. G. White, P. F. McDermott, S. Friedman, L. English, S. Ayers, J. Meng, J. J. Maurer, R. Holland, and R. D. Walker. 2001. Identification and expression of cephamycinase blaCMY genes in Escherichia coli and Salmonella isolates from food animals and ground meat. Antimicrob. Agents Chemother. 45:3647-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]