Abstract
Hepatitis B virus X protein (HBx) expressed in Escherichia coli DH5α by recombinant DNA technology was purified to homogeneity by use of glutathione-Sepharose beads. Immunological characterization of the recombinant HBx protein was performed. Specific binding between the anti-HBx monoclonal antibody and HBx protein showed the specificity of the recombinant HBx protein. The intact HBx protein of the factor Xa-digested glutathione S-transferase-HBx fusion protein was further purified and was used as an antigen for screening the titers of anti-HBx antibodies in sera. Titers of anti-HBx in sera from 20 patients with hepatocellular carcinoma (HCC), 20 patients with chronic hepatitis (CH), and 20 healthy individuals were evaluated by Western blotting and a quantitative enzyme-linked immunosorbent assay. The results indicated that 70% of sera from HCC patients and 5% of sera from CH patients contained antibodies with significant binding to the HBx protein. Western blotting of HBx protein in liver extracts from 20 HCC patients was also performed by using the anti-HBx monoclonal antibody. Results showed that 85% of HCC patients' liver tissues contained a specific HBx protein with the same molecular size as the purified intact HBx. Full correlation was found between anti-HBx antibody positivity in serum and HBx protein positivity in HCC tissues. The data demonstrated that the etiology of HCC is involved with hepatitis B virus (HBV) infection and that HBx in particular plays a role in the development of HBV-related HCC.
Hepatitis B virus (HBV) infection can cause a wide spectrum of sequelae, ranging from asymptomatic chronic infection to chronic active hepatitis. Three hundred million people around the world are chronically infected with HBV (38, 39). Clinically, it is estimated that 5 to 10% of HBV-infected adults will develop chronic hepatitis (CH) and will thus be at a higher (>100 times) risk of developing hepatocellular carcinoma (HCC) (1, 12, 41). Several viral and host factors have been suggested to be involved in the chronicity and diversity of HBV-associated disease (25, 43). The association, at the molecular level, between HBV infection and HBV-related diseases remains unclear.
The small, 3.2-kb DNA genome of HBV contains four known open reading frames, called S, C, P, and X. The hepatitis B virus X (HBx) gene is the smallest, with a length of 465 nucleotides (10). HBx protein is 154 amino acids long, with a molecular weight (MW) of 17,000, and is conserved among all mammalian hepadnaviruses, but the deduced amino acid sequence of the HBx gene product does not correspond to any known viral proteins (26). Although the HBx protein has been shown to stimulate cell cycle progression of quiescent cells (18), it is mainly a pleiotropic transactivator, due to its ability to stimulate not only the HBV promoter and enhancer (28, 33) but also a wide range of other viral promoters (33, 35, 40). The necessity for HBx gene expression during the viral life cycle, in vitro and in vivo, has been suggested (3, 44). The HBx protein acts either through interaction with other cellular transcription factors or via a signal transduction pathway controlled by protein kinase C (15, 30, 31). As a consequence of its activity, the HBx protein appears capable of inducing transformation (32) and liver tumors in a selected strain of mice that express the HBx protein from a transgene (16, 17).
During the natural course of HBV infection, the HBx gene expresses a polypeptide, HBx, that is implicated in HBV-mediated HCC (4, 16). When liver tissue samples from HCC and CH patients were reacted with an anti-HBx antibody and evaluated by immunohistochemistry, reactive antigen was detected in 80% of HCC liver samples and 30% of CH liver samples (4). In another study, the sera of patients with acute hepatitis, CH, and cirrhosis were tested for HBx protein and anti-HBx antibodies by an enzyme-linked immunosorbent assay (ELISA) using a monoclonal antibody and recombinant HBx protein. The results indicated that 23% of patients' sera were HBx positive and 14% of patients' sera were anti-HBx positive (20). In another approach, using HBx oligopeptides as antigens to detect antibodies in the sera of HCC patients, 73% of HCC sera tested positive for anti-HBx antibodies (27). With similar approaches, data showed that 74% of sera from patients with cirrhosis and 54% of sera from patients with HCC were positive for anti-HBx antibodies and HBV surface antigen (HBs) (36). Therefore, the expression of HBx protein in infected patients did not correlate well with the occurrence of HCC (19).
Thus, the usefulness of HBx protein as a prognostic marker for the development of HCC has been questioned (37, 42). Although HBx protein has been observed in sera from HCC patients (14, 24, 27), the significance of the serological data remained to be established. While the study of HBx protein could yield a prognostic marker of HCC, this method requires biopsy of the liver tissues. Whether the titer of anti-HBx in sera or the level of HBx protein in liver tissues could be an alternative choice for molecular detection of HCC has been considered. The specificity of the antibody to the HBx protein was questionable; therefore, predicted levels of anti-HBx antibodies in HCC patients have not yet been established. Discrepancies in measuring the anti-HBx titers of HCC patients have been reported (11, 22, 23, 27, 36). Sera from HCC patients tested 5% positive (8 of 160) for anti-HBx antibodies by use of the recombinant fusion protein as an antigen (23). The clinical significance of this has usually been ignored.
Since the HBx protein plays a role in the development of HCC, the detection of an antibody specific of the HBx protein in hepatoma liver tissues may reveal its possible roles during viral infection. In order to measure the titers of antibody specific to the HBx protein in sera from HCC patients, purified HBx protein and an antibody specific to the HBx protein are required. Due to the difficulties in purifying HBx protein from HBV-infected cells, recombinant DNA technology was used to synthesize HBx protein in Escherichia coli. The expressed HBx protein was purified to homogeneity and used as an immunogen to develop antibodies for further functional identification of the HBx protein. Moreover, immunological characterization of the recombinant HBx protein was performed by using anti-HBx monoclonal antibodies. In this study, anti-HBx antibody titers in sera of HCC patients, CH patients, and healthy individuals were evaluated by using the intact purified recombinant HBx protein. The HBx protein in liver tissues of HCC patients was also detected by use of monoclonal antibody MAb 8419.
MATERIALS AND METHODS
Construction of recombinant plasmids.
DNA copies of the HBx gene were synthesized by using a set of primers containing the sequences 5′-CGGAATTCATGGCTGCTAGGCTGTGC-3′ and 5′-CGGAATTCTTAGGCAGAGGTGAA-3′. This set of primers, anchored with EcoRI restriction enzyme sites, was used to amplify the HBx gene by using the HBV ayw strain (J02203) as the template. The resulting DNA was cloned into EcoRI-digested plasmid pGEX-5X-1 (Pharmacia). The orientation and in-frame location of the insert were confirmed by the presence of an available BamHI restriction enzyme site and by a Dye-terminator cycle sequencing kit (ABI PRISM) (data not shown).
Expression and purification of the HBx protein.
Clones positive for the HBx gene in E. coli DH5α (ATCC 51713) were grown in Terrific broth medium overnight at 37°C. The overnight culture was added to Luria-Bertani medium at a ratio of 1:9. Bacteria were induced with 0.1 mM isopropyl-β-d-thiogalactoside (IPTG) for 5 h at 30°C. Then the cell pellet was collected. The cell lysate was prepared according to published procedures (9, 13). The expressed fusion protein, consisting of glutathione S-transferase (GST) and HBx protein, with a factor Xa-specific protease cutting site, was isolated from the cell lysate by using glutathione Sepharose beads (Sepharose 4B; Pharmacia), according to the manufacturer's protocols.
SDS-PAGE and protein assay.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out according to the procedures suggested by Laemmli except that 12.5% polyacrylamide gels were used (21). The amount of the expressed GST-HBx fusion protein was measured by the Pierce bicinchoninic acid (BCA) protein assay reagent according to the manufacturer's protocols.
Preparation of sera.
Tissues and sera from 20 patients with clinically confirmed HCC (see Table 1) and 20 patients with CH were collected, after informed consent was obtained, from the Department of Surgery, Taichung Veterans General Hospital. These tissues and sera were kept in liquid nitrogen and at −70°C, respectively, until use. The sera tested positive for HBV DNA by a Digene (Beltsville, Md.) Hybrid Capture system assay; they were used for immunodetection by Western blotting and quantitative ELISA. Sera were also obtained from 20 healthy individuals who gave informed consent.
TABLE 1.
Detection of HBx protein in liver tissues and anti-HBx antibody in sera of HCC patients
| Patient no. | Sex | Age (yr) | Operation date (mo/day/yr) | Detectiona of:
|
|
|---|---|---|---|---|---|
| HBxb | Anti-HBxc | ||||
| 1 | M | 54 | 5/24/1994 | + | + |
| 2 | M | 38 | 5/15/1995 | + | + |
| 3 | F | 58 | 6/8/1995 | + | + |
| 4 | M | 50 | 7/31/1995 | + | + |
| 5 | M | 66 | 8/2/1995 | + | + |
| 6 | M | 58 | 8/26/1995 | + | + |
| 7 | M | 69 | 12/18/1995 | + | + |
| 8 | M | 69 | 4/4/1996 | + | + |
| 9 | M | 41 | 2/15/1996 | + | + |
| 10 | M | 53 | 2/15/1996 | + | + |
| 11 | M | 64 | 2/26/1996 | − | − |
| 12 | M | 40 | 3/4/1996 | + | + |
| 13 | M | 44 | 3/21/1996 | + | + |
| 14 | F | 45 | 3/25/1996 | + | + |
| 15 | M | 68 | 5/6/1996 | + | + |
| 16 | M | 73 | 5/13/1996 | − | − |
| 17 | F | 65 | 8/6/1996 | + | − |
| 18 | M | 29 | 8/7/1996 | − | − |
| 19 | M | 36 | 8/26/1996 | + | − |
| 20 | M | 60 | 9/2/1996 | + | − |
+, positive response; −, negative response.
HBx protein in HCC liver tissues was detected by Western blotting with an anti-HBx monoclonal antibody.
Antibodies in patients' sera were detected by Western blotting with the E. coli-expressing HBx recombinant protein as an antigen.
Western blotting of anti-HBx antibodies using recombinant HBx protein.
The specificities of antibodies and sera of HCC patients against HBx protein were defined by Western blotting. Proteins (30 μg/well) were separated by SDS-PAGE using 12.5% polyacrylamide gels. After electrophoresis, the proteins on the gels were transferred to a nitrocellulose membrane. Duplicate blots were generated by diffusion for 24 h at room temperature. Western blotting of the recombinant HBx and protease-digested fusion protein on nitrocellulose membranes was carried out by using a mouse anti-HBx monoclonal antibody (MAb 8419; Chemicon, Temecula, Calif.) and patients' sera. The reactivities of sera were determined by using alkaline phosphatase-conjugated goat anti-human immunoglobulin G (IgG; dilution, 1:2,000). The dilution of each patient's serum sample was 1:1,000.
ELISA.
ELISA was performed using sera from HCC patients, CH patients, and healthy individuals. A direct ELISA method was used to determine the extent of binding of antibodies to the purified GST and the GST-HBx fusion protein. Purified GST and the GST-HBx fusion protein were immobilized overnight at 4°C on the bottoms of wells of a 96-well plate in triplicate (10 μg/well). After wells were blocked with 3% bovine serum albumin and washed, the antigen-antibody complex was detected by a peroxidase-conjugated goat anti-human IgG (1:2,000) and the oxidation of ortho-phenyldiamine (Zymed, South San Francisco, Calif.). The degree of oxidation was measured at 490 nm. The dilution of each of the patient's serum was 1:100.
Tukey's statistical test.
Multiple comparisons for statistically significant differences in anti-HBx antibody titers (measured in optical density [OD]) in sera among HCC patients, CH patients, and healthy individuals were performed by a Tukey test (34). The test result was considered significant at an α value of 0.05.
Western blotting of HBx protein in liver tissues of HCC patients by use of a monoclonal antibody.
Liver tissues obtained by surgical resection (1 mm3) from the 20 HCC patients, with their corresponding sera, for the anti-HBx antibody studies were washed in phosphate-buffered saline and suspended in a buffer (100 mM NaCl, 10 mM Tris-HCl [pH 7.6], 1 mM EDTA [pH 8.0], 1 μg of aprotinin/ml, and 100 μg of phenylmethylsulfonyl fluoride/ml) equivalent to 5 times the tissue volume. A 2× SDS-gel loading buffer was added, and the mixture was boiled for 10 min and sonicated about 1 min. After centrifugation, the resulting total-protein solution was obtained, and protein concentrations from each sample were determined by the BCA method. Protein lysates (100 μg/well) were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and detected with a monoclonal antibody (MAb 8419; Chemicon) by an enhanced chemiluminescence (ECL) plus immunodetection system (NEN, Boston, Mass.). The reactivity of the monoclonal antibody was determined by using horseradish peroxidase-conjugated goat anti-mouse IgG (1:5,000). The dilution of the monoclonal antibody was 1:3,000.
RESULTS
Expression of recombinant HBx protein.
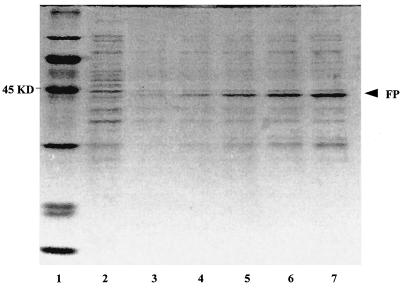
To clone the HBx gene into plasmid pGEX-5X-1, one set of primers anchored with an EcoRI restriction enzyme site was used for amplification. Genomic DNA from the HBV ayw strain was used as the template. A bacterial clone that contained pGEX-5X-1-HBx was screened, confirmed by sequencing, and induced with 1 mM IPTG for maximal expression of the GST-HBx fusion protein. The purified fusion protein could also be specifically cleaved by the protease factor Xa. As shown in Fig. 1, the cloned E. coli was induced by IPTG for 5 h at 30°C for expression of the fusion protein. By use of the affinity of GST and glutathione Sepharose 4B, the fusion protein was purified from the cell lysate. After elution and factor Xa digestion, proteins were analyzed by SDS-12.5% PAGE and immunodetected by MAb 8419. As shown in Fig. 2, the purified GST-HBx fusion protein has a molecular size of about 43 kDa and is composed of a 26-kDa GST protein and a 17-kDa intact HBx protein moiety.
FIG. 1.
SDS-PAGE analyses of the GST-HBx fusion protein (FP) expressed in IPTG-induced E. coli cultures grown for different times. Lane 1, protein marker; lane 2, noninduced cell lysates of E. coli cultures; lanes 3 to 7, cell lysates of E. coli cultures induced with IPTG at 1, 2, 3, 4, and 5 h, respectively. The molecular size of the GST-HBx fusion protein (in kilodaltons) is given on the left.
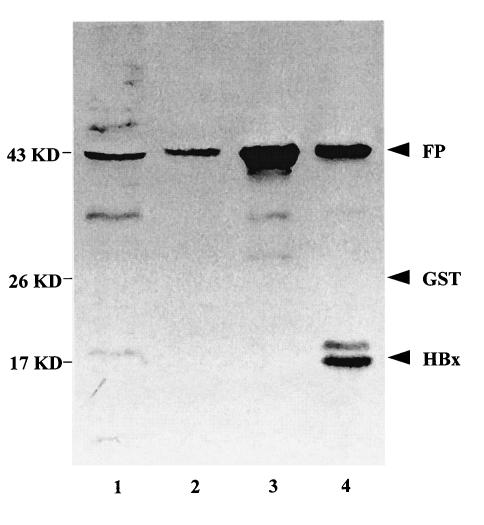
FIG. 2.
Western blot analysis of recombinant HBx protein. Lane 1, cell lysate of the GST-HBx recombinant clone; lanes 2 and 3, the purified GST-HBx fusion protein (FP); lane 4, the factor Xa-digested fusion protein.
Characterization of the HBx protein.
As shown in Fig. 2, both forms of the recombinant HBx protein—the GST-HBx fusion protein and the purified (factor Xa-digested) form—reacted well with the monoclonal antibody while the GST protein alone did not react with the antibody. Thus, the specificity of the recombinant HBx protein was confirmed. The purified HBx protein was obtained and stored at −20°C for screening of titers in sera.
Screening the sera of HCC patients.
Nitrocellulose membrane blots containing intact HBx protein were cut into strips with about 30 μg of protein/strip. Anti-HBx antibodies in the sera were used to detect the HBx protein by Western blotting. As shown in Fig. 3A, almost all serum samples from 20 HCC patients showed the anti-HBx antibody response, and about 70%, for which a visible band was detected (14 out of 20; exceptions were patients 11, 16, 17, 18, 19, and 20), showed significant binding with the recombinant HBx protein. When the same serum dilution (1:1,000) was used for the 20 CH cases (Fig. 3B), only the serum sample of patient 9 appeared to exhibit the anti-HBx antibody response. HBV DNA detection indicated that the serum sample of CH patient 9 contained a relatively large amount of HBV DNA (4,773 pg/ml). In contrast, all the serum samples from healthy individuals (Fig. 3C) exhibited a negative response. Therefore, it was evident that the sera of HCC patients had higher anti-HBx antibody titers.
FIG. 3.
Western blot analysis of recombinant HBx protein with serum samples from patients with clinically confirmed HCC (A) or CH (B) or from healthy individuals (C). Lanes P, positive control detected by MAb 8419.
ELISA.
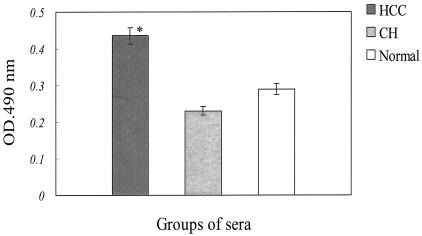
As seen in the ELISA data shown in Fig. 4, the quantities of anti-HBx antibody in the sera of 20 HCC patients, 20 CH patients, and 20 healthy individuals indicated that HCC patients had higher anti-HBx antibody titers than the other two groups. After multiple comparison tests for the significance of differences in anti-HBx antibody titers among the three groups, anti-HBx antibody titers in the sera of HCC patients were found to be significantly higher than those in the sera of healthy individuals or CH patients.
FIG. 4.
ELISA results for recombinant HBx protein in serum samples from patients with clinically confirmed HCC, patients with CH, and healthy individuals. The same amount (10 μg/well) of intact HBx protein, obtained by digestion of the GST-HBx fusion protein with factor Xa, was applied to 96-well plates in triplicate. The asterisk indicates a statistically significant comparison between two groups. Error bars, standard errors (0.0176 OD unit).
Western blotting of the HBx protein in liver tissues of HCC patients.
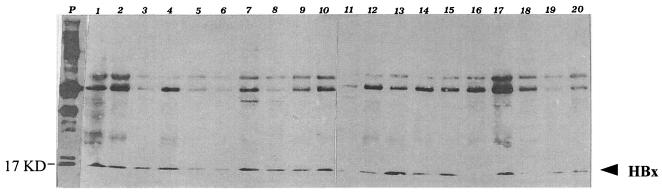
Protein lysates containing various amounts of HBx protein were detected by a monoclonal antibody. The same amount of protein lysates (100 μg) was loaded in each well. As shown in Fig. 5, HBx protein with a molecular size of about 17 kDa was detected among these 20 samples. Lysates from 85% (17 out of 20) of patients tested HBx positive, and only lysates from 3 patients (patients 11, 16, and 18) tested negative. The MW of the HBx protein detected in liver tissues of HCC patients is the same as that of the prokaryotic recombinant protein, as shown by migration on SDS-PAGE gels.
FIG. 5.
Western blotting of HBx protein in cell lysates of 20 HCC tumor tissues with a monoclonal antibody (MAb 8419). Lane P, positive control detected by MAb 8419.
DISCUSSION
Data from previous studies have shown that anti-HBx antibody titers in the sera of CH and HCC patients varied considerably according to the fusion protein or HBx oligopeptides used (5, 20, 22, 27, 36). A study using the oligopeptides lacked sufficient specificity, and a study using the fusion protein may have exhibited interference (2, 29). Therefore, it would be appropriate to use full-length, intact, purified HBx as an antigen for accurate titering of the sera from various sources. Western blotting was performed, and the HBx protein was detected by the anti-HBx monoclonal antibody (MAb 8419). In this study, the HBx recombinant protein was successfully induced and purified. Both the recombinant proteins, that expressed as a GST fusion protein and the purified (factor Xa-digested) form, reacted well with the monoclonal antibody, while the GST protein did not react with the antibody. Thus, the specificity of the recombinant HBx protein was confirmed. The purified HBx protein was obtained and stored at −20°C for screening of titers in sera.
Studies using the same strategy with oligopeptides of HBx or other forms of recombinants to detect anti-HBx in sera of HCC patients showed inconclusive results. The specificities of antibodies and sera of HCC patients, CH patients, and healthy individuals against HBx protein were defined by Western blotting. In this study, higher anti-HBx antibody titers in the sera of HCC patients were evident. The significant result of our study is due to the availability of the highly purified HBx protein. Among the HCC sera detected, the serum sample from HCC patient 8 showed the strongest response, with the same binding level as the monoclonal antibody (MAb 8419; Chemicon). This antibody would be very useful in the detection and functional identification of the HBx protein in HCC. We also used ELISA to confirm these results. After multiple comparison tests for the significance of the anti-HBx antibody titers in the sera of HCC patients, CH patients, and healthy individuals, the anti-HBx antibody titers in the sera of HCC patients were found to be significantly higher than those for healthy individuals or CH patients. According to both Western blotting and ELISA, anti-HBx antibody levels in the sera of HCC patients were evidently higher than those in the sera of CH patients and healthy individuals. Anti-HBx antibody in the serum could be a prognostic marker of HBV infection, especially in the detection of HCC. Furthermore, expression of the anti-HBx antibody in sera correlated well with the markers of HBV replication, such as HBsAg and HBcAg proteins (6, 22). The role of the anti-HBx antibody in viral replication remains unclear, but our data support the notion that sera of HCC patients do have higher levels of anti-HBx antibodies.
In order to study the correlation between HBV infection and the development of HBV-related HCC, the molecular aspects of the titer of anti-HBx antibody in serum and the viral HBx protein in HCC were approached. Therefore, we also studied the expression of HBx protein in 20 clinically confirmed HCC tissues. Protein lysates containing various amounts of HBx protein were detected by a monoclonal antibody (MAb 8419). The results indicated that 17 out of 20 patients' tissues (85%) were HBx positive, with only the lysates from patients 11, 16, and 18 testing HBx negative. Since the MW of the HBx protein detected in liver tissues of HCC patients was the same as that of the prokaryotic recombinant protein, as revealed by migration on SDS-PAGE gels, these results also suggested that no substantial protein modification was found in the HBx protein of HCC. Among the 17 HCC patients whose liver tissues tested HBx positive, patients 17, 19, and 20 failed to produce detectable antibody. Therefore, patients whose sera were positive for the antibody (70%) all had HBx-positive HCC tissues. Immunologically, induction of an antigen is required for the production of an antibody. Patients with viral antigens do not necessarily have antibody-positive sera. Those three patients whose HCC tissue samples contained no HBx protein (patients 11, 16, and 18) were also in the anti-HBx antibody-negative group (consisting of patients 11, 16, 17, 18, 19, and 20) (Table 1), showing full correlation between the presence of HBV anti-HBx antibodies in sera and HBx protein in HCC tissues. This would certainly justify the specificity of the HBx protein and the antibodies used in this study.
Previous studies have indicated that anti-HBx antibodies in sera could be an early marker of HBV infection and viral replication (7, 8). In this study, we clearly demonstrated the clinical significance of the anti-HBx antibody in the sera of HCC patients (70%) compared with the sera of HBV DNA-positive CH patients (5%). Detection of the HBx protein in cell lysates from liver tissues of the same group of HCC patients with higher anti-HBx antibody titers showed that 85% were HBx positive. The HBx-negative patients (patients 11, 16, and 18), for whom no visible band was detected, showed no anti-HBx antibodies in sera. HBx antigen positivity in HCC tissues is required for anti-HBx antibody positivity in serum. However, whether the anti-HBx antibody in serum could be used as a prognostic marker will require further systematic studies.
Acknowledgments
We appreciate the support of V. C. Yang, the director of the Life Science Research Center, and C. S. Chen, the Dean of Science at Tunghai University. This work was supported by the National Science Council, R.O.C. (grants 87-2311-B-029-004 and 86-2316-B-029-001).
REFERENCES
- 1.Beasley, R. P. 1988. Hepatitis B virus: the major etiology of hepatocellular carcinoma. Cancer 61:1942-1956. [DOI] [PubMed] [Google Scholar]
- 2.Cartwright, G. A., J. S. Rothel, and M. W. Lightowlers. 1995. Conventional immunoassays underestimate anti-GST antibody titre. J. Immunol. Methods 179:31-35. [DOI] [PubMed] [Google Scholar]
- 3.Chen, H. S., S. Kaneko, R. Girones, R. W. Anderson, W. E. Hornbuckle, B. C. Tennant, P. J. Cote, J. L. Gerin, R. H. Purcell, and R. H. Miller. 1993. The woodchuck hepatitis virus X gene is important for establishment of virus infection in woodchucks. J. Virol. 67:1218-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamantis, I. D., C. E. McGandy, T. J. Chen, Y. F. Liaw, F. Gudat, and L. Bianchi. 1992. Hepatitis B X gene expression in hepatocellular carcinoma. J. Hepatol. 15:400-403. [DOI] [PubMed] [Google Scholar]
- 5.Elfassi, E., W. A. Haseltine, and J. L. Dienstag. 1986. Detection of hepatitis B virus X product using an open reading frame Escherichia coli expression vector. Proc. Natl. Acad. Sci. USA 83:2219-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feitelson, M. A., and M. M. Clayton. 1990. X antigen polypeptides in the sera of hepatitis B virus-infected patients. Virology 177:367-371. [DOI] [PubMed] [Google Scholar]
- 7.Feitelson, M. A., M. M. Clayton, and B. S. Blumberg. 1990. X antigen/antibody markers in hepadnavirus infections. Gastroenterology 98:1071-1078. [DOI] [PubMed] [Google Scholar]
- 8.Feitelson, M. A., and L. X. Duan. 1997. Hepatitis B virus X antigen in the pathogenesis of chronic infections and the development of hepatocellular carcinoma. Am. J. Pathol. 150:1141-1157. [PMC free article] [PubMed] [Google Scholar]
- 9.Frangioni, J. V., and B. G. Neel. 1993. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion protein. Anal. Biochem. 210:179-187. [DOI] [PubMed] [Google Scholar]
- 10.Galibert, F., E. Mandart, F. Fitoussi, P. Tiollais, and P. Charnay. 1979. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature 281:646-650. [DOI] [PubMed] [Google Scholar]
- 11.Hess, J., M. Stemler, H. Will, C. H. Schröder, J. Kühn, and R. Braun. 1988. Frequent detection of antibodies to hepatitis B virus X-protein in acute, chronic and resolved infection. Med. Microbiol. Immunol. 177:195-205. [DOI] [PubMed] [Google Scholar]
- 12.Hollinger, F. B. 1990. Hepatitis B virus, p. 2171-2236. In B. N. Fields (ed.), Virology. Raven Press, Ltd., New York, N.Y.
- 13.Hwang, G. Y., J. C. Wang, and C. C. Wu. 1999. Immunological characterization of two major secreted forms of recombinant hepatitis B virus e antigen. Virus Res. 59:203-210. [DOI] [PubMed] [Google Scholar]
- 14.Kay, A., E. Mandart, C. Trepo, and F. Galibert. 1985. The HBV HBx gene expressed in E. coli is recognized by sera from hepatitis patients. EMBO J. 4:1287-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kekule, A. S., U. Lauer, L. Weiss, B. Luber, and P. H. Hofschneider. 1993. Hepatitis B virus transactivator Hbx uses a tumor promoter signalling pathway. Nature 361:742-745. [DOI] [PubMed] [Google Scholar]
- 16.Kim, C. M., K. Koike, I. Saito, T. Miyamura, and J. Gilbert. 1991. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature (London) 351:317-320. [DOI] [PubMed] [Google Scholar]
- 17.Koike, K., K. Moriya, S. Iino, H. Yotsuyanagi, Y. Endo, T. Miyamura, and K. Kurokawa. 1994. High-level expression of hepatitis B virus HBx gene and hepatocarcinogenesis in transgenic mice. Hepatology 19:810-819. [PubMed] [Google Scholar]
- 18.Koike, K., K. Moriya, H. Yotsuyanagi, S. Iino, and K. Kurokawa. 1994. Induction of cell cycle progression by hepatitis B virus HBx gene expression in quiescent mouse fibroblasts. J. Clin. Investig. 94:44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koike, K. 1999. Transgenic mouse models of viral hepatitis: insight into viral hepatocarcinogenesis. Viral Hepatitis 5:177-203. [Google Scholar]
- 20.Kumar, V., N. Jayasuryan, H. Reddi, D. Sahal, and S. K. Panada. 1998. A monoclonal antibody against the X protein of hepatitis B virus: fine mapping of its epitope and application in a quantitative ELISA of the X protein in sera of hepatitis B patients. Hybridoma 17:157-164. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Levrero, M., M. Stemler, C. Pasquinelli, A. Alberti, O. Jean-Jean, A. Franco, C. Balsano, D. Diop, C. Brechot, M. Melegari, E. Villa, V. Barnaba, M. Perricaudet, and H. Will. 1991. Significance of anti-HBx antibodies in hepatitis B virus infection. Hepatology 13:143-149. [PubMed] [Google Scholar]
- 23.Levrero, M., O. Jean-Jean, C. Balsano, H. Will, and M. Perricaudet. 1990. Hepatitis B virus (HBV) X gene expression in human cells and anti-HBx antibodies detection in chronic HBV infection. Virology 174:299-304. [DOI] [PubMed] [Google Scholar]
- 24.Meyers, M. L., L. V. Trepo, N. Nath, and J. J. Sninsky. 1986. Hepatitis B virus polypeptide X: expression in Escherichia coli and identification of specific antibodies in sera from hepatitis B virus-infected humans. J. Virol. 57:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milich, D. R., J. E. Jones, J. L. Hughes, J. Price, A. K. Raney, and A. McLanchlan. 1990. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc. Natl. Acad. Sci. USA 87:6599-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, R. H., and W. S. Robinson. 1986. Common evolutionary origin of hepatitis B virus and retroviruses. Proc. Natl. Acad. Sci. USA 83:2531-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriarty, A. M., H. Alexander, R. A. Lerner, and G. B. Thornton. 1985. Antibodies to peptides detect new hepatitis B antigen: serological correlation with hepatocellular carcinoma. Science 227:429-433. [DOI] [PubMed] [Google Scholar]
- 28.Nakatake, H., O. Chisaka, S. Yamamoto, K. Matsubara, and R. Koshy. 1993. Effect of X protein on transactivation of hepatitis B virus promoters and on viral replication. Virology 195:305-314. [DOI] [PubMed] [Google Scholar]
- 29.Park, O. Y., Y. H. Jin, M. Lee, H. J. Shin, H. I. Kim, H. Cho, C. W. Yun, J. K. Youn, and S. Park. 2000. Characterization and gene cloning of monoclonal antibody specific for the hepatitis B virus X protein. Hybridoma 19:73-80. [DOI] [PubMed] [Google Scholar]
- 30.Rossner, M. T. 1992. Hepatitis B virus X gene product: a promiscuous transcriptional activator. J. Med. Virol. 36:101-117. [DOI] [PubMed] [Google Scholar]
- 31.Seto, E., P. J. Mitchell, and T. S. Yen. 1989. Transactivation by the hepatitis B virus X protein depends on AP-2 and other transcription factors. Nature (London) 344:72-74. [DOI] [PubMed] [Google Scholar]
- 32.Shirakata, Y., M. Kawada, Y. Fujiki, H. Sano, M. Oda, K. Yaginuma, M. Kobayashi, and K. Koike. 1989. The X gene of hepatitis B virus induced growth stimulation and tumorigenic transformation of mouse NIH3T3 cells. Jpn. J. Cancer Res. 80:617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spandau, D. F., and C. H. Lee. 1988. trans-activation of viral enhancers by the hepatitis B virus X protein. J. Virol. 62:427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steel, R. G. D., J. H. Torrie, and D. A. Dickey. 1997. Principles and procedures of statistics, 3rd ed. McGraw-Hill, Inc., New York, N.Y.
- 35.Twu, J. S., K. Chu, and W. S. Robinson. 1989. Hepatitis B virus X gene activates κB-like enhancer sequences in the long terminal repeat of human immunodeficiency virus 1. Proc. Natl. Acad. Sci. USA 86:5168-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vitvitski-Trépo, L., A. Kay, C. Pichoud, P. Chevallier, S. de Dinechin, B.-M. Shamoon, E. Mandart, C. Trépo, and F. Galibert. 1990. Early and frequent detection of HBxAg and/or anti-HBx in hepatitis B virus infection. Hepatology 12:1278-1283. [DOI] [PubMed] [Google Scholar]
- 37.Wang, W., W. T. London, L. Lega, and M. A. Feitelson. 1991. HBxAg in the liver from carrier patients with chronic hepatitis and cirrhosis. Hepatology 14:29-37. [DOI] [PubMed] [Google Scholar]
- 38.Yeh, C. T. 2000. Hepatitis B virus X protein: searching for a role in hepatocarcinogenesis. J. Gastroenterol. Hepatol. 15:339-341. [DOI] [PubMed] [Google Scholar]
- 39.Yen, T. S. B. 1996. Hepadnaviral X protein: review of recent progress. J. Biomed. Sci. 3:20-30. [DOI] [PubMed] [Google Scholar]
- 40.Zahm, P., P. H. Hofschneider, and R. Koshy. 1988. The HBV X-ORF encodes a transactivator: a potential factor in viral hepatocarcinogenesis. Oncogene 3:169-177. [PubMed] [Google Scholar]
- 41.Zentgraf, H., G. Herrmann, R. Klein, P. Schranz, I. Loncarevic, D. Herrmann, K. Hubner, and C. H. Schroder. 1990. Mouse monoclonal antibody directed against hepatitis B virus X protein synthesized in Escherichia coli: detection of reactive antigen in liver cell carcinoma and chronic hepatitis. Oncology 47:143-148. [DOI] [PubMed] [Google Scholar]
- 42.Zhu, M., W. T. London, L. X. Duan, and M. A. Feitelson. 1993. The value of hepatitis B X antigen as a prognostic marker in the development of hepatocellular carcinoma. Int. J. Cancer 55:571-576. [DOI] [PubMed] [Google Scholar]
- 43.Zinkernagel, R. M., and P. C. Doherty. 1974. Restriction of in vitro T cell-mediated cytotoxicity in lympholytic choriomeningitis within a syngeneic or semiallogeneic system. Nature 248:701-702. [DOI] [PubMed] [Google Scholar]
- 44.Zoulim, F., J. Saputelli, and C. Seeger. 1994. Woodchuck hepatitis virus X protein is required for viral infection in vivo. J. Virol. 68:2026-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]