Abstract
Objective
To determine whether indicators of behavioral inhibition and cortisol responses to stressful situations, obtained in infancy, were associated with asthma-related measures (atopy and airway hyper-responsiveness) approximately two years later.
Methods
Measures reflecting inhibited temperament and cortisol response following a 25-hr separation from mother and relocation to a novel room were obtained for 21 rhesus monkeys (mean age 109 days, range 91–122). Inhibited temperament was measured by reduced emotionality and increased vigilance Atopy and airway hyperresponsiveness were assessed after two years (age range 19–35 months) using skin tests to common aeroallergens and inhaled methacholine challenge, respectively.
Results
No associations were found between atopy and either behavioral inhibition or cortisol levels (p > 0.56). Low emotionality was associated with airway hyper-responsiveness (r = 0.47, p = 0.03) and a trend was found for blunted cortisol responsiveness and airway hyper-responsiveness (r = 0.42, p = 0.06).
Conclusions
Inhibited temperament and blunted cortisol responsiveness may be related to the development of airway hyperresponsiveness that is common to both non-atopic and atopic asthma phenotypes, and may indicate risk for non-atopic asthma specifically.
Keywords: asthma, behavioral inhibition, cortisol, temperament, atopy, airway hyper-responsiveness
INTRODUCTION
Asthma has long been recognized as having a significant psychosocial component (1) and a variety of studies have suggested that inhibited temperament in children and anxiety and depression in adults is associated with asthma. These suggestions come from three types of studies. First, comparisons have often been made between individuals with and without asthma. For example, compared to controls, Kim, Ferrara & Chess (2) reported that children with asthma showed a temperamental pattern characteristic of “slow-to-warm-up” children – children that are inhibited (shy or reserved) in unfamiliar situations – and Ortega et al. (3) reported that adolescents with a history of asthma were significantly more likely to have an anxiety disorder. A second set of studies has examined factors associated with severity of disease, such as demonstrating that the relationship between anxiety and asthma was strongest for those with “severe” versus “non-severe” lifetime asthma (4). Third, and perhaps most powerfully, a few studies have employed a prospective methodology. Jonas et al. (5) followed more than 5000 adults for 13 years, and found that, among nonsmokers with no respiratory symptoms at baseline, the relative risks for developing asthma were strong and significant for those with high anxiety or high depression. Similarly, in a three-wave study of 11,000 Finnish adults (6), men that were high in neuroticism had significantly greater odds of developing asthma. Together, these studies point to a possible role of inhibited temperament in asthma. Some of this research has identified a link explicitly (e.g., 2), but other studies described above are consistent with the idea that inhibited temperament early in life, because it is strongly linked to later anxiety, depression, and neuroticism (7–10), may be associated with later development of asthma.
While the retrospective and prospective studies described above are compelling, many relied on self- or parent-reports of asthma, and the definition of asthma used was often very broad. The designs of these studies also do not generally permit understanding of whether inhibition/anxiety precedes asthma, is a consequence of it, or whether both the psychological factors and the asthma phenotype are manifestations of an underlying third cause, such as central and autonomic nervous system organization. Moreover, none of the cited studies distinguished between atopic and non-atopic asthma. The proportion of asthma cases attributable to atopy has been widely debated; however, a recent report (11) using a representative sample from the US determined that about half of all asthma cases were associated with an atopic response (defined as an outcome of a skin-testing procedure). This result has raised questions about the relative lack of attention paid to non-atopic asthma (12). While there are many similarities between atopic and non-atopic asthma, differences have been noted; apart from the obvious difference in aeroallergen involvement, non-atopic asthma tends to appear at relatively older ages, and people with non-atopic asthma show greater bronchial reactivity to challenge with methacholine, a receptor agonist in the parasympathetic nervous system (13, 14). Moreover, a recent Danish study suggests that the increased prevalence of asthma worldwide may be due primarily to non-atopic asthma - between 1986 and 2001, prevalence for non-atopic asthma increased 4.2-fold, but for atopic asthma, the increase was only 1.4-fold (15).
Because behavioral inhibition is associated with autonomic reactivity (16–18), one might expect that inhibited temperament would be related to the feature that is common to both forms of asthma, and that is commonly indexed by measuring responsiveness to autonomic challenge, namely airway hyperresponsiveness (AHR). The relation of inhibition to atopy, however, is unclear. On the one hand, studies that included measures of behavioral inhibition suggest there might be a relationship for measures related to atopy, such as hay fever (19), eczema (20), and dermatitis (21). On the other hand, while Kim et al. (2; described above) reported that children with asthma were more likely to show inhibition, control children that had eczema, allergic rhinitis, or both, did not show this temperamental pattern.
One mechanism by which temperament could influence immune processes relevant to atopy and AHR involves regulation of the hypothalamic-pituitary-adrenal (HPA) axis. Little is known about the characteristics of this altered regulation, however. On the one hand, fearful/inhibited temperament has been linked to blunted cortisol reactivity in boys (22), and several studies have reported HPA dysregulation in people with asthma and other atopic disorders, often taking the form of a blunted cortisol response to stress (23, 24) or reduced basal levels at various points in the circadian cycle (25, 26). Blunted basal and stress levels of cortisol could be achieved through enhanced negative feedback in the HPA system (27), which can be revealed through greater suppression of endogenous cortisol concentrations in response to a dexamethasone challenge. On the other hand, dysregulation might take the form of glucocorticoid desensitization. In fact, Rosenkranz et al. (28) showed that peripheral blood lymphocytes of late-phase responders were less sensitive to suppression by GCs during an antigen challenge. Reduced sensitivity to GCs could result in decreased suppression of inflammatory processes in asthma, and might be revealed by a relative failure to suppress endogenous cortisol levels in response to dexamethasone. Because AHR has an inflammatory component (29), it is unclear to what extent cortisol responsiveness and regulation might be related specifically to the airways response, to the atopic response, or to both.
One strategy for understanding whether temperament and HPA function could be risk factors for atopy and AHR would be to use an animal model. Rhesus monkeys provide an excellent model for the study of asthma and related outcomes (30, 31), and have been useful in studies of airways remodeling and asthma pathogenesis (32, 33). Moreover, nearly three decades of research suggest great similarity in temperament between human and nonhuman primates. For example, behavioral manifestations of various temperament dimensions are similar between the two species. Inhibited (sometimes referred to as anxious or fearful) temperament in rhesus monkeys is often indicated by vigilant behavior and reduction in vocalization in unfamiliar and novel situations (34), while in humans inhibited temperament is typically manifested as responses that are characterized as shy, emotionally reserved, withdrawn, and timid in the presence of strangers (35). These behavioral similarities are paralleled by similarities in measures of brain activity in both species (34). In addition, we have demonstrated with adult rhesus monkeys that reduced basal plasma cortisol levels are associated with natural variation in a personality dimension that is associated with anxious responding during challenging situations (36). A monkey model could also help identify causal pathways – whether inhibition precedes or follows asthma, or if both are related to a common underlying third variable.
The present study examines the relationship, in rhesus monkeys, between temperament and plasma cortisol concentrations assessed in infancy, and airway responsiveness and atopy measured approximately two years later. An atopic response to skin testing and measures of AHR have been used to diagnose and characterize asthma in both human (37, 38) and nonhuman (31) studies. Based on the literature, we expected that measures reflecting inhibited temperament (reduced emotionality and increased vigilance) in a novel, stressful situation would be related to AHR. We also expected that a blunted cortisol response to a stressor would be predictive of atopy, and possibly, AHR. In addition, we examined cortisol levels in response to dexamethasone administration to identify possible mechanisms by which HPA regulation might influence atopy and/or AHR.
METHODS
Subjects
Subjects for the current report were 21 (14 females) juvenile rhesus monkeys (Macaca mulatta), ranging in age from 19 – 35 months. These animals were drawn from a larger pool of monkeys (n=79) that were screened for a study of asthma, and the final sample includes every animal that a) was skin-tested, b) was given a pulmonary function test, and c) participated in the infant BioBehavioral Assessment (BBA) program (see below). At the time of screening, investigators were unaware that any animals had participated in the BBA program. All subjects were born and reared with their mothers at the California National Primate Research Center (CNPRC) in either indoor or outdoor cages. Preliminary analyses revealed no effects of sex or rearing history on the outcome measures; consequently data from all animals were combined for analysis. All procedures were approved by the UC Davis Institutional Animal Care and Use Committee, and followed the Guide to the Care and Use of Laboratory Animals (39). UC Davis and the CNPRC are accredited by AAALAC.
BBA program
At a mean age of 108.6 days (range: 92–122 days), animals participated in CNPRC’s BBA program (40, 41). Briefly, infants were removed from their home cages, separated from their mothers, and housed individually in a novel, indoor location for a 25-hour period, during which each infant experienced several situations that were designed to assess infants' behavioral and physiological reactivity. At the end of the 25-hr testing period, animals were returned to their mothers and to their original home cages.
Behavioral inhibition in humans is typically assessed as responses to unfamiliar environments or people, and in such situations, inhibited children are usually described as being vigilant, showing subdued affect, and withdrawing to the proximity of their caregivers (16, 42). For the present analysis, we selected relevant measures from two assessments in the BBA program: Emotionality from the focal behavioral observations, and Vigilance from the temperament assessments.
Focal behavioral observations
After the animals had been in their temporary individual cages for at least 15 minutes, behavioral data were collected for five minutes from each animal in a predetermined random order. Inter-observer reliability for recording of behaviors (which are described in 41) was established at better than 85% agreement between two independent observers. Data reduction for measures of behavioral responsiveness and temperament (see next section) was accomplished via exploratory and confirmatory factor analyses using MPlus statistical software (43) in order to derive reliable scales that could be used as outcome measures. Factor analytic procedures (which utilized data from more than 1,400 infants collected over a 5-year period), and all indices of factor model fit, are described in detail in (41). Briefly, an exploratory factor analysis, using weighted least squares with robust standard errors, identified a two-factor solution, which was given a promax rotation. Separate confirmatory factor analyses were performed on separate data sets, and fit was excellent based on traditional fit statistics (see 41). Scales were constructed by summing z-scores for the items that loaded on a given factor. Cronbach’s alphas ranged from 0.6 to 0.8. The final scales were then z-scored and labeled ‘‘Activity’’ (comprising the proportion of time animals were locomoting; proportion of time animals were not in a hang position from the side or top of the cage; rate of environmental exploration; and whether animals ate food; drank water; and were in a crouched posture) and ‘‘Emotionality’’ (rate of vocalizing [coos and barks]; and whether the animal displayed threats, lipsmacks, and self-scratch).
Temperament ratings
At the end of the 25-hr. period, observers rated each animal using a 1–7 scale for each of 16 trait adjectives. These ratings provide overall summaries of the observers’ experiences with the animals throughout the testing period, during the behavioral observations, the handling of the animals for blood sampling, the transport of the animals to testing cages, etc. For each trait, agreement between independent raters was significantly greater than chance using chi-square (p<0.001) when different observers' ratings were allowed to vary from each other by one point. Exploratory and confirmatory factor analyses were performed as described above (details in 41, 44) and utilized maximum likelihood estimation and promax rotation. Fit indices revealed a close fit (41). Factor scores were calculated by summing the z-scores for all adjective items loading on a given factor, and then z-scoring each scale. Cronbach’s alpha values for the scales ranged from 0.6 to 0.9. The four scales, named for the adjective with the highest factor loading, were: Confident (active, bold, confident, curious, playful), Gentle (calm, curious, flexible, gentle), Vigilant (vigilant, not depressed, not tense, not timid), Nervous (fearful, nervous, timid, not calm, not confident). The principal measure of interest was Vigilance. While behavioral inhibition may also appear to be related to other temperament factors such as nervousness, and while, in fact some of these factors are correlated with each other (e.g., the correlation between vigilant and nervous in this sample is r = −0.721), correlational analyses, multiple regressions, and logistic regressions (not shown) confirmed that in nearly all cases, the strongest relationships to all of the outcome measures were indeed with Vigilance.
Plasma cortisol concentrations
Blood was sampled via femoral venipuncture following manual restraint on four occasions during the 25-hr. assessment period: two hours after maternal separation (1100 hrs: Sample 1); five hours later (1600 hrs: Sample 2); at 0830 on Day 2 (Sample 3); and at 0900 on Day 2 (Sample 4). Immediately following Sample 2, animals were injected with 500ug/kg dexamethasone i.m., and immediately following Sample 3, animals received 2.5 I.U. ACTH, i.m.; thus, Samples 3 and 4 reflect pharmacologic treatments designed to assess regulation of the hypothalamic-pituitary-adrenal axis. 0.5 ml of whole blood from each sample was centrifuged for 10 minutes at 3000 RPM at 4° C. Plasma was removed and decanted into tubes for storage at −80° C, and was later assayed in duplicate using commercially available kits (Diagnostics Products Corporation, Los Angeles, CA). Inter- and intra-assay coefficients of variation were 5.8% and 7.9 %, respectively. Because we were interested in testing hypotheses specifically about blunted cortisol responses to stress (after 23), we averaged the cortisol measures from the first two blood samples, and used this value in the analyses (preliminary analyses revealed that these samples did not differ significantly from each other). Sample 3 values were used to determine whether cortisol’s influence may be due to enhanced negative feedback or to glucocorticoid desensitization. Values from Sample 4 were not used, inasmuch as we had no specific hypotheses about adrenal responsiveness to ACTH challenge.
Skin testing
Intradermal skin testing was done according to published procedures (31). Briefly, hair was clipped from an area of the thorax, and 8 separate intradermal injections (0.1 ml each) were made. The 8 injections consisted of PBS alone (negative control), PBS with histamine (positive control), and six test antigens: house dust mite, cockroach, mold, trees, weeds, or grasses. Wheal diameters were measured after 20 minutes. A skin-test positive response was defined as a wheal diameter for any test antigen that was greater than or equal to that halfway between the diameter of the wheals produced by the positive- and negative-controls (31). By this definition, 8 animals were atopic, and 13 were not.
Airway responsiveness testing
Airway responsiveness to doubling concentrations of inhaled methacholine aerosols was quantified using previously published procedures (31). Briefly, each animal was anesthetized with propofol (0.1 mg/kg/min), intubated, and placed in a head-out body plethysmograph with the intubation tube attached to a pneumotachograph assembly. Airways resistance was assessed using a forced oscillatory technique (31, 45). Methacholine was administered as an aerosol at a set tidal volume and breathing frequency (15.0 ml/kg and 20.0 bpm) using a compressed air nebulizer in series with a positive pressure ventilator. We used repeated 30-sec challenge periods separated by 240-sec data collection periods, beginning with saline followed by doubling concentrations of methacholine starting at 0.0625 mg/ml and ending at the concentration that gives a 150 percent increase over baseline airways resistance (EC150). Responses by the 21 animals ranged from 0.39 to 32.0 mg/ml.
Statistical analysis
The principal behavioral measures were Emotionality and Vigilance, and the hormonal measure was the mean concentration of cortisol from the first two blood samples. Because of its non-normal distribution, the measure of AHR was subjected to a log10 transformation. Bivariate correlation coefficients were calculated between predictors and both outcome measures (atopy, AHR), followed by multiple linear regression for the AHR measure, and logistic regression for the measure of atopy. We conducted an additional analysis, using logistic regression, of AHR by dichotomizing this measure, inasmuch as experience with more than 70 animals has suggested that hyper-responsive individuals as a group have EC150 values of about 3.0 or lower, and show little within-group variation in response during asthma induction. Examination of the distribution of EC150 responses for this cohort revealed that 10 animals (designated hyper-responders) had EC150 of 0.39 to 3.21 mg/ml, and 11 animals (normals) had EC150 of 4.98 to 32.0 mg/ml. In the logistic regression analyses, we emphasized classification of the cases based upon the three predictors. By chance, 61.9% of cases (13 of 21) would be classified for the atopy measure, and 52.4% (11 of 21) for the AHR measure. Finally, to identify possible mechanisms by which glucocorticoids could affect AHR and/or atopy, we conducted analyses of variance on the dex-suppressed levels of cortisol in relation to the measure of atopy and the dichotomized measure of AHR.
RESULTS
Vigilance and Emotionality were uncorrelated in the present sample (r=0.067, p=.77), which precluded construction of a single scale of behavioral inhibition. Cortisol levels were also not correlated with Vigilance (r=0.078, p=.74) or Emotionality (r=0.309, p=.17).
Atopy
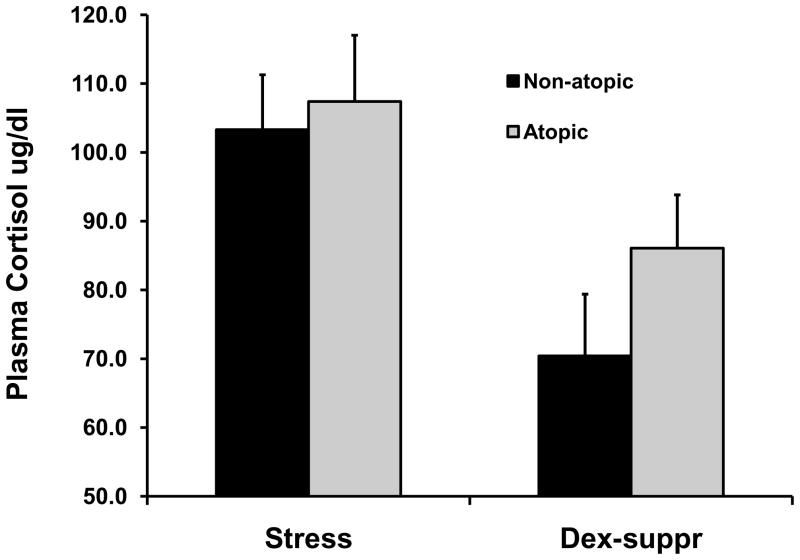
Bivariate correlation coefficients were all non-significant between the three principal BBA predictors and the measure of atopy (all p > 0.56; Table 1, row 1). Because we originally defined atopy somewhat conservatively, however, we redefined atopy similar to Sears et al. (46), as a wheal diameter at least 2mm larger than the negative control for the skin test. By this definition, 13 animals (compared to 8 for the original definition) were atopic. Bivariate analysis again revealed that no measure was correlated with BBA predictors (all p > 0.31; Table 1, row 2). Logistic regression revealed that the three predictors were unrelated to either the original measure of atopy (Chisquare(3)=1.13, p=0.77) or the revised measure (Chisquare(3)=1.18, p=0.76). Classification analysis revealed that the three predictors resulted in 66.7% correct classification of the cases for either model; as described above, chance classification was 61.9%. To identify whether measures of HPA regulation were related to the original measure of atopy, we conducted an analysis of variance on the two cortisol values. Although the dex-suppressed values were higher for atopic animals, neither the main effect nor interaction effect achieved statistical significance (both p>.38). Both the stressed and dex-suppressed values of cortisol are shown in Figure 1A.
Table 1.
Pearson product-moment correlation coefficients between biobehavioral data and measures of atopy and airway hyper-responsiveness (AHR).
| Emotionality | Vigilance | Cortisol | |
|---|---|---|---|
| Atopy (original) | −0.134 | 0.127 | 0.074 |
| Atopy (revised) | 0.061 | 0.231 | 0.014 |
| AHR (continuous) | 0.470, p=.03 | −0.235 | 0.423, p=.06 |
| AHR (hyperresponders) n=21 for all correlations | −0.420, p=.06 | 0.347 | −0.502, p=.02 |
Revised measure of atopy follows Sears et al. 2003; for hyperresponsiveness measure of AHR, 0 = normal responder, 1 = hyperresponder -- see text for details. Note that this classification is the reverse of the continuous measure, which accounts for the differences in signs.
Figure 1.
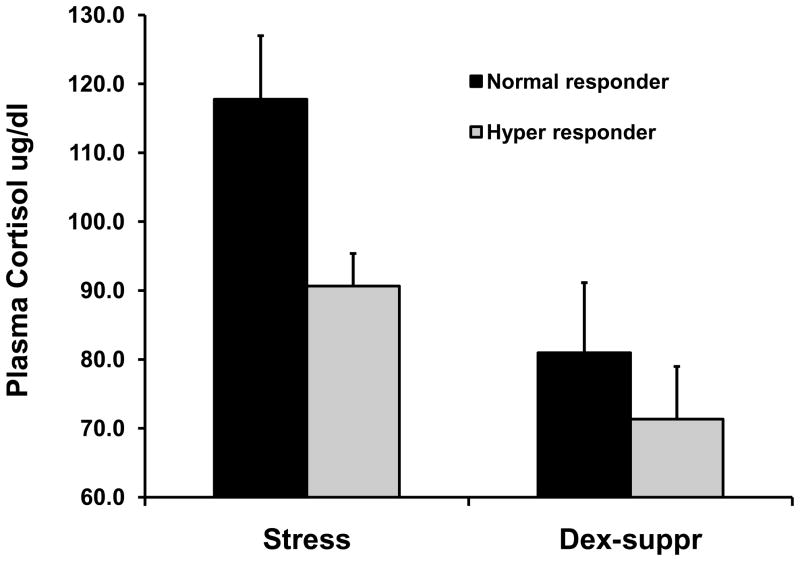
Mean (+/− SEM) plasma cortisol concentrations for stressed samples and samples obtained following overnight dexamethasone suppression. A) Cortisol concentrations based upon original definition of atopy (see text); all results are non-significant. B) Cortisol concentrations based upon dichotomized measure of airways hyper-responsiveness (see text). Left panel of B shows significant effect for stress samples; group differences for dexamethasone-suppressed samples are non-significant.
AHR
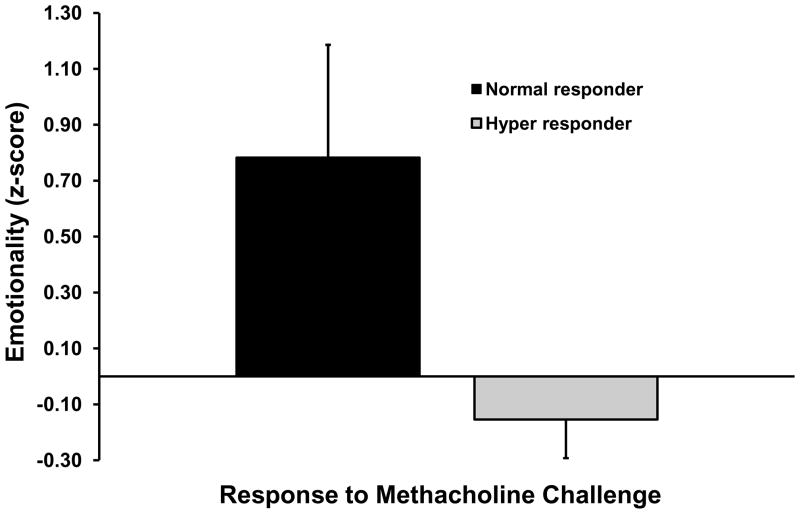
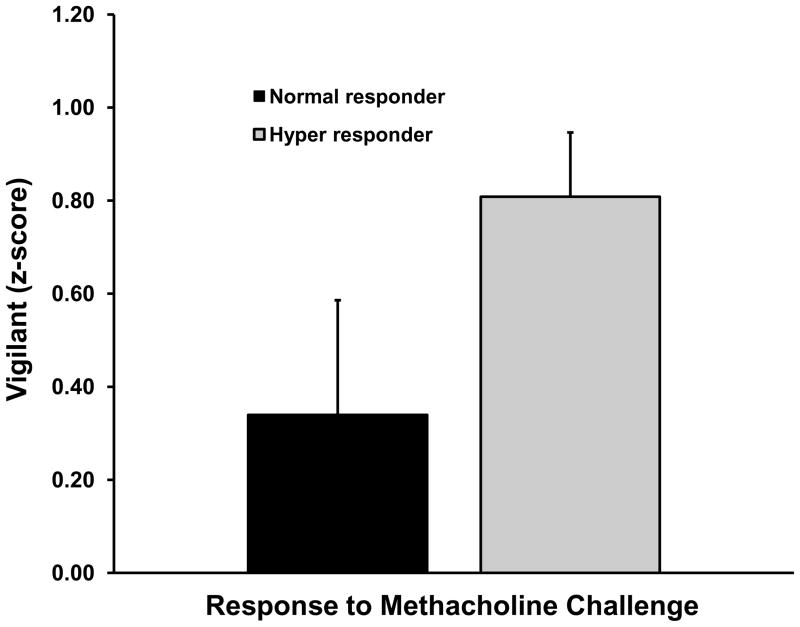
In contrast to the results for atopy, animals that showed hyperresponsive airways (lower values for EC150) displayed less Emotionality (p=.03), and blunted cortisol responsiveness (p=.06; Table 1, row 3). The correlation between the airways response and Vigilance was in the expected direction but was not significant (r=−0.235, p=.31). Inclusion of the three predictors in a multiple regression analysis resulted in a significant model fit (F(3,17)=3.59, p=.04), with an adjusted R2=0.28. A similar pattern of results was found for the dichotomized measure of hyperresponsiveness (Table 1, row 4; note that the correlation for cortisol is now statistically significant). Finally, the logistic regression involving the dichotomized measure of AHR revealed a significant model fit (Chisquare(3)=15.99, p=.001) and correct classification of 95.2% (i.e., 20 of 21) of the cases (chance classification was 52.4%). Maximal classification was obtained only when all three predictors were in the logistic model; for example, although Vigilance was not significant in any bivariate analysis, removal of this measure decreased classification from 95.2% to 66.7%, suggesting that it was the combination of low Emotionality and Vigilance, which together are hallmarks of behavioral inhibition, that was the important construct. Figure 2 shows mean values for the Emotionality and Vigilance measures for hyper-responders and normal-responders. Analysis of variance for the two cortisol values using the dichotomized measure of AHR revealed a trend for AHR (F(1,19)=3.26, p=.09), with hyper-responders showing lower cortisol concentrations. The interaction of sample and AHR response type was non-significant (p=.17). Means for both cortisol measures are shown in Fig. 1B.
Figure 2.
Mean (+/− SEM) values of A) Emotionality (p=.06) and B) Vigilance (not significant) for monkeys that displayed a hyper-responsive or normal response to methacholine challenge (see text for definition). Cortisol concentrations are displayed in left panel of Figure 1B.
DISCUSSION
Indicators of behavioral inhibition (reduced emotionality, and to a lesser extent, greater vigilance) and blunted cortisol responsiveness were associated with the airways response that is common to both atopic and non-atopic asthma, but not with the atopic response in the animals. Our inability to find relationships between inhibition and atopy suggests that behavioral inhibition may not be related to the allergic component of asthma, a suggestion that is at variance with other reports described above that did find relationships between inhibition-related measures and atopy (19–21). The results are, however, consistent with the initial study by Kim et al. (2). While we acknowledge that further research is needed, we propose that behavioral inhibition may be a marker for non-atopic asthma.
The mechanisms by which inhibited temperament may influence AHR cannot be determined by the present analysis. Airway hyperresponsiveness is a complex condition, with evidence implicating several underlying factors, including abnormalities in smooth muscle, inflammation, and neural control (29). Studies of behavioral inhibition in humans and monkeys have revealed that this temperamental pattern is associated with autonomic reactivity during stressful situations (16–18) and with reduced cellular immune function measured in vitro (47). Either of these mechanisms might link temperament with airway responses, and both bear prospective investigation.
In the present study, measures of inhibition and cortisol were obtained in infancy, while the asthma-related measures were obtained approximately two years later. While there is generally good continuity in inhibited temperament across time (16), the behavior of some individuals does normalize. Unfortunately, we did not have contemporary measures of behavior to assess the animals’ functioning at the time of the skin-testing and methacholine challenge. It is possible, for example, that stronger relationships between behavioral function and AHR (or even the skin test data) may have been found among a subset of animals whose current (as well as infant) functioning reflected inhibition.
We examined the cortisol response to stress and to an overnight dexamethasone suppression test to determine whether an HPA-related mechanism may be involved in either the atopic response or AHR. All analyses for atopy were non-significant, but there was a significant blunting of stress-related cortisol in hyper-responders compared to normal responders. This may be due to reduced activation of the HPA system in response to stress, or to enhanced negative feedback associated with a greater number or efficiency of glucocorticoid receptors. Although non-significant, dex-suppressed cortisol concentrations were also lower for hyper-responders, a result consistent with the negative feedback interpretation. If AHR is associated with enhanced negative feedback, however, then this finding is counterintuitive to glucocorticoids’ anti-inflammatory effects – one would expect a greater anti-inflammatory effect, not a reduction. It seems likely, therefore, that the altered HPA regulation suggested for the hyper-responders extends beyond simple enhancement of negative feedback, and involves other mechanisms not examined in this study.
We recognize the limitations of the present analysis, and urge caution in interpretation. Sample size was relatively small for an “individual differences” analysis, and may have been underpowered to find an effect on atopy. Moreover, retrospective analysis such as this runs the risk of generating spurious results owing to capitalization on chance associations in the data. Nevertheless, the fact that the results for AHR are consistent with human data that were obtained using a variety of approaches, both prospective and retrospective, provides more confidence in the results. We also note that, inasmuch as this was a retrospective study, we did not have measures of AHR at the time of the BBA assessments. Consequently, we do not know the respiratory status of the behaviorally inhibited animals at 3–4 months of age. Unfortunately, the animals are too small at that age to perform the methacholine challenge test as described. We do know, however, that the animals showed no obvious wheezing or respiratory difficulties that required veterinary intervention at the time of biobehavioral assessment. Regardless of whether the present measures of behavioral inhibition reflect existing pulmonary function or predict later-developing hyperresponsiveness, the present data do suggest that biobehavioral measures that are relatively easy to obtain can identify animals at-risk for hyperresponsive airways.
The rhesus monkey provides an excellent model system to understand the pathophysiologic mechanisms associated with asthma (30), and we believe that the present analysis extends the usefulness of this model into examination of the role of psychosocial factors in the development of asthma. While the present study does not elucidate whether inhibition precedes asthma, follows it, or whether both are manifestations of a third underlying variable, the monkey model could be extremely valuable in addressing this issue, as well as enabling prospective studies to test hypotheses about specific developmental mechanisms, and to attempt interventions that might forestall the development of asthma. In addition, this model is valuable because it permits one to create animals with specific temperamental characteristics. For example, prenatal stress results in offspring that display an inhibited temperament (48) and impaired cellular immunity, as well as evidence of glucocorticoid resistance in immune cells (49). Having a ready source of animals with a risky temperamental phenotype for prospective investigation could be extremely useful in understanding the immune and neural mechanisms associated with AHR and atopy.
Acknowledgments
The authors thank L. Del Rosso and L. Calonder for assistance with the biobehavioral assessments, and the veterinary and animal care staffs for technical assistance. This research was supported by grants from NIH (R24RR019970 [JPC, WAM, SPM], P51RR000169 [all authors], P01ES00628 (DMH, LAM, ESS), P01ES11617 (DMH, LAM, ESS), R01HL081286 (LAM)), R21HL089148 (JPC, DMH, LAM, ESS) and a contract with Genentech (DMH, LAM, ESS).
Acronyms
- AHR
airway hyperresponsiveness
- HPA
hypothalamic-pituitary-adrenal
- GC
glucocorticoid
- BBA
BioBehavioral Assessment
- ACTH
adrenocorticotrophic hormone
- EC150
concentration of methacholine that gives a 150% increase over baseline airways resistance
Footnotes
The authors declare that they have no conflicts of interest.
References
- 1.Wright RJ, Rodriguez M, Cohen S. Review of psychosocial stress and asthma: An integrated biopsychosocial approach. Thorax. 1998;53:1066–1074. doi: 10.1136/thx.53.12.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SP, Ferrara A, Chess S. Temperament of asthmatic children: A preliminary study. J Ped. 1980;97:483–486. doi: 10.1016/s0022-3476(80)80214-9. [DOI] [PubMed] [Google Scholar]
- 3.Ortega AN, Huertas SE, Canino G, Ramirez R, Rubio-Stipec M. Childhood asthma, chronic illness, and psychiatric disorders. J Nerv Ment Dis. 2002;190:275–281. doi: 10.1097/00005053-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin RD, Jacobi F, Thefeld W. Mental disorders and asthma in the community. Arch Gen Psychiatry. 2003;60:1125–1130. doi: 10.1001/archpsyc.60.11.1125. [DOI] [PubMed] [Google Scholar]
- 5.Jonas BS, Wagener DK, Lando JF, Feldman JJ. Symptoms of anxiety and depression as risk factors for development of asthma. J Appl Biobehav Res. 1999;4:91–110. [Google Scholar]
- 6.Huovinen E, Kaprio J, Koskenvuo M. Asthma in relation to personality traits, life satisfaction, and stress: A prospective study among 11,000 adults. Allergy. 2001;56:971–977. doi: 10.1034/j.1398-9995.2001.00112.x. [DOI] [PubMed] [Google Scholar]
- 7.Rapee RM. The development and modification of temperamental risk for anxiety disorders: Prevention of a lifetime of anxiety? Biol Psychiatry. 2002;52:947–957. doi: 10.1016/s0006-3223(02)01572-x. [DOI] [PubMed] [Google Scholar]
- 8.Gladstone GL, Parker GB. Is behavioral inhibition a risk factor for depression? J Affective Dis. 2006;95:85–94. doi: 10.1016/j.jad.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Moffitt TE, Caspi A, Harrington H, Milne BJ, Melchior M, Goldberg D, Poulton R. Generalized anxiety disorder and depression: childhood risk factors in a birth cohort followed to age 32. Psychol Med. 2007;37:441–452. doi: 10.1017/S0033291706009640. [DOI] [PubMed] [Google Scholar]
- 10.Muris P, Dietvorst R. Underlying personality characteristics of behavioral inhibition in children. Child Psychiatry Hum Dev. 2006;36:437–445. doi: 10.1007/s10578-006-0014-9. [DOI] [PubMed] [Google Scholar]
- 11.Arbes SJ, Gergen PJ, Vaughn B, Zeldin DC. Asthma cases attributable to atopy: Results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2007;120:1139–1145. doi: 10.1016/j.jaci.2007.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ownby DR, Joseph CLM. Should nonatopic asthma get equal attention? J Allergy Clin Immunol. 2007;120:1018–1020. doi: 10.1016/j.jaci.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Kelley CF, Mannino DM, Homa DM, Savage-Brown A, Holguin F. Asthma phenotypes, risk factors, and measures of severity in a national sample of US children. Pediatrics. 2005;115:726–731. doi: 10.1542/peds.2004-0529. [DOI] [PubMed] [Google Scholar]
- 14.Mochizuki H, Shigeta M, Tokuyama K, Morikawa A. Difference in airway reactivity in children with atopic vs nonatopic asthma. Chest. 1999;116(3):619–24. doi: 10.1378/chest.116.3.619. [DOI] [PubMed] [Google Scholar]
- 15.Thomsen SF, Ulrik CS, Larsen K, Backer V. Change in prevalence of asthma in Danish children and adolescents. Ann Allergy, Asthma, Immunol. 2004;92:506–511. doi: 10.1016/S1081-1206(10)61757-7. [DOI] [PubMed] [Google Scholar]
- 16.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 17.Suomi SJ. Social development in rhesus monkeys: Consideration of individual differences. In: Oliverio A, Zapella M, editors. The behavior of human infants. New York: Plenum; 1983. pp. 71–91. [Google Scholar]
- 18.Keltikangas-Jarvinen L, Kettunen J, Ravaja N, Naatanen P. Inhibited and disinhibited temperament and autonomic stress reactivity. Int J Psychophysio. 1999;33:185–196. doi: 10.1016/s0167-8760(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 19.Bell IR, Jasnoski ML, Kagan J, King DS. Is allergic rhinitis more frequent in young adults with extreme shyness? A preliminary survey. Psychosom Med. 1990;52:517–525. doi: 10.1097/00006842-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Kagan J, Snidman N, Julia-Seller M, Johnson MO. Temperament and allergic symptoms. Psychosom Med. 1991;53:332–340. doi: 10.1097/00006842-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Kim T-S, Pae C-U, Jeong J-T, Kim S-D, Chung K-I, Lee C. Temperament and character dimensions in patients with atopic dermatitis. J Dermatol. 2006;1:10–15. doi: 10.1111/j.1346-8138.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 22.Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Dev Psychopath. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buske-Kirschbaum A, von Auer K, Krieger S, Weis S, Rauh W, Hellhammer D. Blunted cortisol responses to psychosocial stress in asthmatic children: A general feature of atopic disease? Psychosom Med. 2003;65:806–810. doi: 10.1097/01.psy.0000095916.25975.4f. [DOI] [PubMed] [Google Scholar]
- 24.Chida Y, Sudo N, Sonoda J, Hiramoto T, Kubo C. Early life stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med. 2007;175:316–322. doi: 10.1164/rccm.200607-898OC. [DOI] [PubMed] [Google Scholar]
- 25.Kauffmann F, Guiochon-Mantel A, Neukirch F. Is low endogenous cortisol a risk factor for asthma? Am J Resp Crit Care Med. 1999;160:1428. doi: 10.1164/ajrccm.160.4.16040_1. [DOI] [PubMed] [Google Scholar]
- 26.Landstra AM, Postma DS, Boezen HM, van AAlderen WMC. Role of serum cortisol levels in children with asthma. Am J Respir Crit Care Med. 2002;165:708–712. doi: 10.1164/ajrccm.165.5.2102115. [DOI] [PubMed] [Google Scholar]
- 27.Capitanio JP, Mendoza SP, Lerche NW, Mason WA. Social stress results in altered glucocorticoid regulation and shorter survival in simian acquired immune deficiency syndrome. Proc Natl Acad Sci. 1998;95:4714–4719. doi: 10.1073/pnas.95.8.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenkranz MA, Busse WW, Johnstone T, Swenson CA, Crisafi GM, Jackson MM, Bosch JA, Sheridan JF, Davidson RJ. Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. PNAS. 2005;102:13319–13324. doi: 10.1073/pnas.0504365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berend N, Salome CM, King GG. Mechanisms of airway hyperresponsiveness in asthma. Respirology. 2008;13:624–631. doi: 10.1111/j.1440-1843.2008.01330.x. [DOI] [PubMed] [Google Scholar]
- 30.Coffman RL, Hessel EM. Nonhuman primate models of asthma. J Exp Med. 2005;201:1875–1879. doi: 10.1084/jem.20050901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schelegle ES, Gershwin LJ, Miller LA, Fanucchi MV, Van Winkle LS, Gerriets JP, Walby WF, Omlor AM, Buckpitt AR, Tarkington BK, Wong VJ, Joad JP, Pinkerton KB, Wu R, Evans MJ, Hyde DM, Plopper CG. Allergic asthma induced in rhesus monkeys by house dust mite (Dermatophagoides farinae) Am J Pathol. 2001;158:333–41. doi: 10.1016/S0002-9440(10)63973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller LA, Hurst SD, Coffman RL, Tyler NK, Stovall MY, Chou DL, Putney LF, Gershwin LJ, Schelegle ES, Plopper CG, Hyde DM. Airway generation-specific differences in the spatial distribution of immune cells and cytokines in allergen-challenged rhesus monkeys. Clin Exp Allergy. 2005;35:894–906. doi: 10.1111/j.1365-2222.2005.02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plopper CG, Smiley-Jewell SM, Miller LA, Fanucchi MV, Evans MJ, Buckpitt AR, Avdalovic M, Gershwin LJ, Joad JP, Kajekar R, Larson S, Pinkerton KE, Van Winkle LS, Schelegle ES, Pieczarka EM, Wu R, Hyde DM. Asthma/allergic airways disease: does postnatal exposure to environmental toxicants promote airway pathobiology? Toxicol Pathol. 2007;35:97–110. doi: 10.1080/01926230601132030. [DOI] [PubMed] [Google Scholar]
- 34.Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann NY Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J Am Acad Child Adolesc Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Capitanio JP, Mendoza SP, Bentson K. Personality characteristics and basal cortisol concentrations in adult male rhesus macaques (Macaca mulatta) Psychoneuroendo. 2004;29:1300–1308. doi: 10.1016/j.psyneuen.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Frank PI, Morris JA, Hazell ML, Linehan MF, Frank TL. Long term prognosis in preschool children with wheeze: Longitudinal postal questionnaire study 1993–2004. BMJ. 2008;336:1423–1426. doi: 10.1136/bmj.39568.623750.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner SW, Young S, Goldblatt J, Landau LI, Le Souef PN. Childhood asthma and increased airway responsiveness: A relationship that begins in infancy. Am J Respir Crit Care Med. 2009;179:98–104. doi: 10.1164/rccm.200805-804OC. [DOI] [PubMed] [Google Scholar]
- 39.Guide to the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 40.Capitanio JP, Mason WA, Mendoza SP, Del Rosso LA, Roberts JA. Nursery rearing and biobehavioral organization. In: Sackett GP, Ruppenthal G, Elias K, editors. Nursery Rearing of Nonhuman Primates in the 21st Century. New York: Springer; 2006. pp. 191–213. [Google Scholar]
- 41.Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobiol. 2009;51(1):47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kagan J, Saudino KJ. Behavioral inhibition and related temperaments. In: Emde RN, Hewitt JK, editors. Infancy to early childhood: Genetic and environmental influences on developmental change. New York: Oxford University Press; 2001. pp. 111–119. [Google Scholar]
- 43.Muthen LK, Muthen BO. Mplus user’s guide. 2. Los Angeles, CA: Muthen & Muthen; 1998–2001. [Google Scholar]
- 44.Hinde K, Capitanio JP. Lactational programming? Mother’s milk energy predicts infant behavior and temperament in rhesus macaques (Macaca mulatta) Am J Primatol. doi: 10.1002/ajp.20806. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madwed JB, Jackson AC. Determination of airway and tissue resistances after antigen and methacholine in nonhuman primates. J Appl Physiol. 1997;83:1690–6. doi: 10.1152/jappl.1997.83.5.1690. [DOI] [PubMed] [Google Scholar]
- 46.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva A, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed into adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 47.Capitanio JP. Nonhuman primate personality and immunity: Mechanisms of health and disease. In: Weiss A, King JE, Murray L, editors. Personality, temperament, and behavioral syndromes in nonhuman primates. New York: Springer; in press. [Google Scholar]
- 48.Clarke AS, Schneider ML. Effects of prenatal stress on behavior in adolescent rhesus monkeys. Ann NY Acad Sci. 1997;807:490–491. doi: 10.1111/j.1749-6632.1997.tb51947.x. [DOI] [PubMed] [Google Scholar]
- 49.Coe CL, Lubach GR, Karaszewski JW, Ershler WB. Prenatal endocrine activation alters postnatal cellular immunity in infant monkeys. Br Beh Immun. 1996;10:221–234. doi: 10.1006/brbi.1996.0020. [DOI] [PubMed] [Google Scholar]