Abstract
Objective
This study examined whether a polymorphism (5-HTTLPR) in the promoter of the serotonin transporter gene (SLC6A4) moderates cardiovascular reactivity to social threat.
Methods
Psychologically healthy young adults delivered a speech and performed mental arithmetic in one of three conditions: a) an evaluative audience condition that gave disapproving and negative nonverbal social signals (n=59); b) an evaluative audience condition that provided supportive social signals (n=60); or c) a no audience condition (n=65). Heart rate (HR) and systolic and diastolic blood pressure (SBP, DBP) were measured before, during, and after the stress tasks to assess cardiovascular reactivity and recovery.
Results
In the negative audience condition, there was a significant association between the 5-HTTLPR and SBP, DBP, and HR reactivity. Individuals with the short/short genotype showed the greatest reactivity. The DBP and HR reactivity of short/short individuals in the negative audience condition was also greater than that of individuals with the short/short genotype in the no audience condition. These associations of the 5-HTLPR with HR reactivity were moderated by gender, being limited to females. With respect to cardiovascular recovery, short/short individuals in the negative audience condition exhibited impaired DBP recovery relative to other genotypes in the same condition as well as short/short individuals in the no audience condition.
Conclusions
The 5-HTTLPR moderates cardiovascular reactivity to stress in a threatening evaluative social context, which suggests that the serotonin system may be involved in the processes by which stressful, conflict-ridden social environments affect risk for cardiovascular-related health outcomes.
Keywords: Serotonin, Social, Stress, Trier, Blood Pressure, Genetic variation
Introduction
Over the last several decades, an accumulating body of evidence has established strong links between social factors and cardiovascular disease. For example, impoverished social connections are a predictor of coronary heart disease (1, 2), and a lack of social support is associated with an increased likelihood of hypertension (3). The presence of conflict-filled relationships can also worsen prognosis for heart disease, as evidenced by studies of strained marriages (e.g., (4)). Similarly, being raised in a threat-filled family environment ridden with frequent strife increases the probability of heart disease (5).
One possible means by which socially threatening environments may influence cardiovascular-related health outcomes is by influencing the extent to which the cardiovascular system, particularly blood pressure, is activated by psychological stress (6). In prospective studies, greater cardiovascular reactivity (CVR) to stress has been linked with preclinical markers of coronary heart disease, such as coronary artery calcification (7) and carotid intima-media thickness (8). Furthermore, a recent meta-analysis of prospective studies (9) indicates that CVR is a significant predictor of higher resting systolic (SBP) and diastolic blood (DBP) pressure.
In line with such a model, growing up in a childhood environment characterized by frequent social conflict increases CVR to laboratory-based psychological stressors among adolescents (10, 11) and young adults (12), although findings are not always consistent across genders. An environment of social conflict in adulthood has similar effects, as marital strife also increases cardiovascular reactivity to psychological stressors in the laboratory (13). In so far as CVR to adverse social environments is a risk factor for cardiovascular disease, it is important to better understand the biological processes affecting reactivity to social threats.
One factor that may be involved in this process is the neurotransmitter serotonin because pharmacological alteration of the serotonin system affects CVR to psychological stressors. In double-blind crossover studies with psychiatrically healthy subjects, chronic selective serotonin reuptake inhibitor (SSRI) treatment reduced CVR to psychological stress (14, 15) compared to placebo. Similar results have been seen in studies of clinical samples that did not use a crossover design (16-18). Conversely, reductions in central serotonin levels via tryptophan depletion increases CVR to psychological stress in post-traumatic stress disorder patients successfully treated with an SSRI (19). Thus, it appears that augmenting central serotoninergic neurotransmission decreases CVR, while reducing central serotoninergic signaling increases CVR. This suggests that genetic variation in the serotonin system is likely to modulate CVR.
Within the promoter region of the serotonin transporter gene (SLC6A4), there is a polymorphism (5-HTTLPR) that gives rise to two principal alleles: long and short. The long allele is associated with greater transcription in lymphocytes (20) and consistent with the functional role of the serotonin transporter in serotonin reuptake leads to greater serotonin uptake into platelets in some (21-23) but not all studies (24). In terms of central effects, the short allele is associated with reduced serotoninergic neurotransmission (25-27), as assayed using a pharmacological challenge (prolactin release following alteration of serotonin transporter function). Because low levels of central serotoninergic transmission are associated with risk factors for cardiovascular disease such as elevated systolic and diastolic blood pressure (28), the metabolic syndrome (29), and carotid artery intima-media thickness (30), the short allele would be expected to be associated with greater CVR to stressors involving social threat.
Indeed, prior work has found that in a sample of healthy young European-American adults (31), the short/short genotype was associated with greater heart rate (HR) reactivity to a psychological performance stressor (Stroop task and mental arithmetic), although the effect was limited to females. In contrast, in an older sample of healthy African-American and white participants (32), the long/long genotype was associated with greater heart rate and BP reactivity to the recall of an emotionally-charged event in front of a small audience. These discrepant results suggest the need for further studies to clarify the nature of the relationship between the 5-HTTLPR and CVR as well as identify potential variables that may moderate these effects. One such variable may be the degree to which a stressor invokes socially evaluative threat, as the presence of an evaluative audience can alter CVR (33). Recent work indicates that the serotonin system affects sensitivity to both positive and negative social experiences (34, 35) and therefore may be particularly involved in responding to signals of social support and threat.
In the present study, this hypothesis was tested by having participants perform a speech and do mental arithmetic in front of a disapproving evaluative audience, a supportive evaluative audience, or a videocamera without an audience present. It was hypothesized that individuals with the short/short genotype would be most responsive to the negative, threatening social context and exhibit the highest CVR in this condition. It was expected that the positive social evaluation condition would elicit reduced reactivity relative to the negative audience condition, and the short/short genotype would be the most responsive to these differences in social context. Finally, it was anticipated that there would be the least CVR in the no audience condition without genotype dependent differences in CVR. As impaired cardiovascular recovery from psychological stressors is associated with adverse cardiovascular related health outcomes (9), the relationship between the 5-HTTLPR and cardiovascular recovery was also assessed.
Methods
Participants
Participants responded to a poster offering $120 compensation for participation in the study. Prospective participants were screened during a telephone interview and were excluded from the study if: they were currently being treated by a mental health professional; had mental or physical health problems (including post-traumatic stress disorder); or were using mental-health related (e.g. SSRI's) or other medications that affect cardiovascular or endocrine function. In addition, pregnant or lactating women were excluded. All procedures were approved by the Institutional Review Board from the University of California, Los Angeles. Data were collected between September 2006 and August 2008. The final sample consisted of 185 participants (39% male; 61% female; age range:18 to 35). As participants were affiliated with the university as students, employees, or both, the sample reflects these demographics and was 37% Asian-American, 22% European-American, 16% Latino, 23% of “mixed” ethnicity and 2% African American (These last three groups are designated “other” in the analyses to be reported).
Procedure
Participants reported to the University's Clinical Research Center in the mid to late afternoon. After arrival, participants were further screened to ensure that they had: 1) blood pressure less than 140/90 mmHg; 2) a resting pulse between 60 and 100; 3) normal lung auscultation; and 4) a normal cardiac exam (no evidence of congestive heart failure or arrythmia). The nurse then inserted an indwelling catheter and performed a blood draw to assay gonadal hormone and peptide levels (data not reported here). Blood pressure and heart rate were assessed every three minutes via a Critikon Dinamap Vital Signs Monitor Model 1846SX.
Each participant then took part in the Trier Social Stress Task (TSST), a widely-used laboratory stress challenge known to elicit autonomic and HPA axis stress responses (36). Participants were given five minutes to prepare a five-minute speech on why they would be a good administrative assistant, a popular campus job for students and employees. They were then randomly assigned to deliver the speech in one of three audience conditions. In the no audience condition, participants delivered the speech only in front of the videocamera, which they were told was so that experts could later rate their performance. In both of the audience conditions, participants were told that they would not only be presenting in front of the videocamera for later evaluation, but also in front of a live audience of trained evaluators who would be making ratings of their performance.
In the negative audience condition, the participant delivered his/her speech to two members of an evaluative panel who gave non-verbal indications of frustration over the quality of the speech. They displayed non-verbal signs of boredom and exchanged glances with each other that communicated mutual negative assessments. This manipulation represents a somewhat stronger version of the standardized audience condition for the TSST. In the positive audience condition, the two audience members leaned forward, smiled, and gave non-verbal indications of approval. They occasionally exchanged glances with each other that communicated mutual positive assessments and when not explicitly communicating positive assessments, their demeanor communicated interest in what the participant was saying. The two audience conditions mirrored each other precisely in terms of the timing and type of feedback, with the exception that the nonverbal feedback was positive in one condition and negative in the other (37). All panels included one man and one woman, and measures were taken to ensure the participant and audience members were unacquainted. The experimenter sat off to the side and out of direct view of the participant and did not give any verbal or nonverbal indications of positive or negative reactions to the participant's speech performance.
Immediately after the speech, participants performed challenging mental arithmetic tasks for 5 minutes that required counting backwards by 7s and by 13s from 2,935 out loud, during which time they were urged by the experimenter to try to go faster. For the participants in the two audience conditions, these math problems were completed in front of the audience as well. Following the mental arithmetic task, participants completed questionnaires. To assess the degree of recovery in the cardiovascular measures, blood pressure and heart rate was measured five minutes after conclusion of the mental arithmetic task. At the conclusion of the experiment, the participant was debriefed and then dismissed.
Genotyping
DNA was collected from saliva using Oragene kits (DNA Genotek, Ottawa, Ontario, Canada) and extracted according to the manufacturer's recommendations. The 5-HTTLPR was genoptyed as described previously (38) using a protocol modified from (20). Briefly, the forward primer was 5’-GGC GTT GCC GCT CTG AAT GC-3’ (labeled with 6-carboxyfluorescein fluorophore), and the reverse primer was 5’- GAG GGA CTG AGC TGG ACA ACC AC-3’, which yielded 486-bp (short) and 529-bp (long) fragments. Polymerase chain reaction (PCR) was performed in a total volume of 25μl, containing 100 ng of DNA, 160 nM of each primer, 1mM Tris-HCL (pH 8.3), 5mM KCl, 1.5 mM MgCl2, 2% DMSO (v/v), 2.5 U Amplitaq Gold DNA polymerase (Applied Biosystems, Foster City, CA), 200 μM of dATP, dCTP, dTTP, and 100 μM of dGTP, and 7-deaza-2'-dGTP. After an initial denaturation at 94°C for 5 min, 35 cycles of denaturation (94°C for 30 sec), annealing (63°C for 30 sec), and extension (72°C for 1 min) was performed followed by a final extension at 72°C for 20 min. The PCR products were electrophoresed on an ABI 3730 DNA analyzer (Applied Biosystems) with a Mapmaker size standard (Bioventures, Murfreesboro, TN). Data collection and analysis used GeneScan and Genotyper software (Applied Biosystems).
Analyses
SBP, DBP, and HR reactivity were assessed by subtracting the baseline level of the respective measure from the average of the peak level during the speech stressor and mental arithmetic stressor (39). Recovery was assessed by subtracting the baseline level of each cardiovascular measure from the level of the measure five minutes after completion of the stress tasks. Tests of Hardy–Weinberg equilibrium for the entire sample and each ethnic grouping were conducted using the software program Haploview v3.32 (http://www.broad.mit.edu/mpg/haploview/) (40). The relationship between the 5-HTTLPR and each cardiovascular dependent measure was assessed using analyses of covariance. SPSS 17.0 (Chicago, IL) was used for all analyses except for the three-way ANOVA which used Stata 11.0 (College Station, TX) with the “anovalator” and “smecriticalvalue” programs downloaded from http://www.ats.ucla.edu/stat/stata/ado/analysis/. Baseline levels of each dependent measure were entered as a covariate in each analysis. For the assessment of genetic effects, two additional covariates for self-reported ethnicity (using two dummy variables: East Asians = 1, all others = 0; and European-American's = 1, all others = 0) were used to control for population stratification concerns. All statistical tests were two-tailed with alpha set to p < 0.05.1
Results
Descriptive Analyses
One participant could not be genotyped, leaving a sample of 184 participants. Across the three experimental conditions, there were no differences in the distribution of gender (χ2(2,N=184)= .08, p =.96), ethnicity (χ2(4,N=184)= 1.7, p =.76), or 5-HTTLPR genotype (χ2(4,N=184) =2.11, p =.72). Similarly, there were no differences between the groups in baseline SBP (F(2,181) = .097, p =.91), DBP (F(2,181) =.002, p =.99), or HR (F(2,181) =.41, p =.67). Hardy-Weinberg equilibrium calculations showed no significant deviation from equilibrium for each ethnic grouping (all p's > 0.4), as was the case for the calculation using the entire sample (p =0.19).
Audience Effects on Cardiovascular Reactivity
First, the effects of audience condition upon CVR were assessed irrespective of genotype. There were significant effects of audience condition upon SBP reactivity (F(2,180) =8.31, p < .001, η2=.085), DBP Reactivity (F(2,180) =6.57, p =.002, η2=.068), and HR reactivity (F(2,181) =9.16, p < .001, η2 = .09). For each dependent measure, there were significant differences between the no audience condition and both the positive audience condition (SBP: F(1,180)= 12.83, p < .001; DBP: F(1,180)= 11.72, p = .001; HR: F(1,180)=5.61, p =.019) and the negative audience condition (SBP: F(1,180)=11.72, p < .001; DBP: F(1,180)=7.67, p =.006; HR: F(1,180)=18.05 p < .001), with reactivity higher in both audience conditions than in the no audience control. There were no significant differences in SBP or DBP reactivity between the two audience conditions (p's > .53), while the HR reactivity differences approached significance HR: F(1,180)= 3.46, p =.065). To determine if there was significant CVR in the control condition, a repeated measures ANOVA was used with baseline and peak reactivity as the within-subjects factor. Indeed, there were robust increases in SBP (F(1,64) =266.54, p < .001); DBP (F(1,64) =457.07, p < .001), and HR (F(1,64) =124.73, p < .001) in the absence of an audience.
The 5-HTTLPR and Cardiovascular Reactivity to Psychological Stress
In the entire sample, there were no relationships between the 5-HTTLPR and baseline measures of SBP (F(2,179) =.05, p =.95), DBP (F(2,179) =1.57, p =.21) or HR (F(2,179) = .004, p = .996). Because prior work has examined the relationship between the 5-HTTLPR and CVR only in a negative stress condition (31, 32), the effects of the 5-HTTLPR in only the negative audience condition were directly assessed to allow for comparisons with prior work.
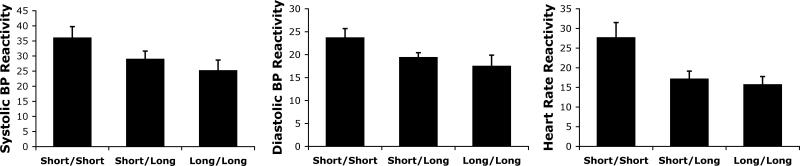
According to a one-way ANCOVA (using baseline value and ethnicity as the covariates) there was a significant main effect of the 5-HTTLPR on each of the CVR dependent measures (Figure 1; SBP reactivity: (F(2,53) =3.25, p =.047, η2 =.11; DBP reactivity (F(2,53) =3.08, p =.054, η2 =.1; and HR reactivity (F(2,53) =5.94, p =.005, η2 =.18). Post-test comparisons revealed that for each cardiovascular measure, the short/short genotype group had greater reactivity than the long/long genotype group (SBP reactivity: F(1,53)= 5.99, p =.018; DBP reactivity: F(1,53) = 4.14, p = .027; HR reactivity: F(1,53) = 8.31, p = .006) as well as the short/long group (SBP reactivity: F(1,53) =3.34, p =.073; DBP reactivity: F(1,53) = 4.07, p = .049; HR reactivity: F(1,53) = 9.34, p = .004), while the differences between the short/long and long/long groups were not significant (all p's > .27).
Figure 1.
Relationship between the 5-HTTLPR and systolic blood pressure reactivity (mm Hg), diastolic blood pressure reactivity (mm Hg), and heart rate reactivity (beats per minute) in the negative evaluative audience condition. Error bars denote S.E.M.
To ensure that these significant effects in the negative audience condition were not due to population stratification, the relationship between the 5-HTTLPR and cardiovascular reactivity was assessed separately for each ethnic grouping. Although the associations in this subdivided sample were not significant due to the reduced power, the qualitative pattern of reactivity was the same with the short/short individuals having the greatest SBP, DBP, and HR reactivity in each ethnic group (for descriptive statistics, see Supplemental Digital Content).
To assess the interactive effects of audience condition and genotype (Table 1), a two-way ANCOVA was conducted for each dependent measure. For HR reactivity, there was a significant interaction between the 5-HTTLPR and audience condition (F(4,172) = 4.13, p = .003, η2 = .088). Tests of simple effects revealed that the short/short individuals in the negative audience condition had significantly greater reactivity than the short/short individuals in the no audience condition (F(1,172) = 29.81, p < .0001) and the positive audience condition (F(1,172) = 14.72, p < .001).
Table 1.
Means and standard deviations (SD) for each genotype in the different audience conditions.
| SBP Reactivity (mm Hg) | DBP Reactivity (mm Hg) | HR Reactivity (Beats/minute) |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| No Audience Condition | ||||||
| Short/Short (n = 27) | 23.07 | 10.00 | 16.85b | 6.46 | 11.15b | 7.37 |
| Short/Long (n = 26) | 23.77 | 10.54 | 16.31 | 6.57 | 14.04 | 8.98 |
| Long/Long (n = 11) | 21.45 | 16.91 | 19.18 | 6.01 | 11.18 | 12.43 |
| Positive Evaluative Audience Condition | ||||||
|---|---|---|---|---|---|---|
| Short/Short (n = 22) | 31.73 | 9.01 | 21.91 | 6.34 | 15.68b | 7.70 |
| Short/Long (n = 24) | 31.63 | 7.41 | 22.21 | 4.50 | 16.42 | 8.40 |
| Long/Long (n = 13) | 28.77 | 12.15 | 18.46 | 7.34 | 19.62 | 9.19 |
| Negative Evaluative Audience Condition | ||||||
|---|---|---|---|---|---|---|
| Short/Short (n = 18) | 36.17 | 15.10 | 23.78 | 8.14 | 27.78 | 15.78 |
| Short/Long (n = 29) | 29.14a* | 13.47 | 19.48a | 5.15 | 17.28a | 10.06 |
| Long/Long (n = 12) | 25.33a | 11.52 | 17.58a | 7.98 | 15.83a | 6.64 |
Significantly different from short/short genotype
denotes marginally significant difference
Significantly different from same genotype in negative audience condition
For DBP reactivity, the interaction between genotype and audience condition approached significance (F(4,172) = 2.13, p = .079) with the short/short individuals in the negative audience condition exhibiting greater reactivity than the short/short individuals in the no audience condition (F(1.172) = 11.37, p < .001), but not the positive audience condition (F(1,172) = .78, p = .38).
For SBP reactivity, there was not a significant interaction between audience condition and the 5-HTTLPR ((F(4,172) = 1.04, ns).
Tests of Sex Differences
Because prior work (31) has found that the 5-HTTLPR can interact with sex to affect HR reactivity, we also assessed whether the effects of genotype and condition were moderated by sex. There was a significant three-way interaction between the 5-HTTLPR, sex, and audience condition (F(4,163) = 3.71, p = .006, η2 = .083) for HR reactivity. Dividing by sex to decompose this interaction revealed that there was no interaction between audience condition and genotype (F(4,163) = .44, p = .78) for males. For females, there was a significant two-way interaction between genotype and audience condition (F(4,163) = 7.61, p < .0001). There was a significant main effect of the 5-HTTLPR in the negative audience condition (F(2,163) = 17.35, p < .001), but not in the no audience (F(2,163) = .73, p = .582) or positive audience condition (F(2,163) = .54, p = .58). In the negative audience condition (Figure 2), females with the short/short genotype were significantly more reactive than females with the short/long (p < .05) or long/long genotype (p < .05).
Figure 2.
Relationship between the 5-HTTLPR and heart rate reactivity (beats per minute) in the negative evaluative audience condition, separated by gender. Error bars denote S.E.M.
There was not a significant interaction between the 5-HTTLPR, sex, and audience condition for either SBP reactivity (F(4,163) = .4, p = .81) or DBP reactivity (F(4,163) = .94, p = .44).
The 5-HTTLPR and Cardiovascular Recovery
To assess the effects of audience condition and genotype on cardiovascular recovery, each respective cardiovascular measure five minutes after the termination of the stress task was subtracted from baseline levels prior to the task. There was not a significant effect of audience condition upon DBP (2,178) = .56, p = .57) or HR recovery (F(2,178) = 2.18, p = .12), but there was an audience effect upon SBP (F(2,178) = 7.97, p < .0001, η2 = .08) recovery. In the positive audience condition, SBP remained higher than in the control condition (F(1,180) =14.59, p < .0001) and the negative audience condition (F(1,180) = 5.14, p = .025).
With respect to the 5-HTTLPR, there was no interaction between genotype and audience condition for SBP recovery (F(4,172) = .38, p = .82) or HR recovery (F(4,172) = 1.41, p = .23). However, there was a significant interaction between the 5-HTTLPR and audience condition for DBP recovery (Figure 3; F(4,172) = 3.42, p = .01, η2 = .07). Short/short individuals in the negative audience condition had significantly higher DBP than long/long individuals (F(1,172) = 6.27, p = .013) and short/long individuals (F(1,172) = 3.13, p = .079) in the same audience condition. Short/short individuals in the negative audience condition also had less recovery than the short/short individuals in the no audience (F(1,172) = 4.27, p = .04) or positive audience (F(1,172) = 5.71, p = .018) conditions. The difference between the long/long individuals in the negative audience condition and the no audience condition approached significance (F(1,172) = 3.47, p = .064). All other comparisons were not significant (p's > .28).
Figure 3.
Relationship between the 5-HTTLPR and DBP recovery (mm Hg) in the negative evaluative audience condition. Error bars denote S.E.M.
With respect to sex differences, there was a significant main effect of sex upon SBP recovery (F(1,163) = 4.38, p = .038, η2 = .03; Males: M = 8.99, SD = 6.69; Females: M = 6.79, SD = 6.25). However the interaction of sex, audience condition and the 5-HTTLPR was not significant (F(4,163) = 1.66, p = .16). For DBP recovery and HR recovery the main effect of sex was not significant (p's > .16), nor were the three-way interactions (p's > .54).
Discussion
The results of this study demonstrate a significant relationship between the 5-HTTLPR and cardiovascular reactivity to negative social evaluation, as predicted. Under conditions of high socially evaluative threat, there was a graded genotype-dependent relationship between the 5-HTTLPR and both SBP and DBP: Individuals with the short/short genotype were most reactive, followed by those with the short/long genotype followed by those with the long/long genotype, who were the least reactive. The DBP reactivity of short/short individuals in the negative audience condition was significantly greater than the no audience condition. This heightened reactivity persisted five minutes after the stressor, as DBP levels of the short/short individuals in the negative audience condition remained significantly higher than those of short/short individuals in the no audience condition. The heightened reactivity to negative social evaluation of short/short individuals suggests that the 5-HTTLPR is particularly associated with the degree of reactivity to social threat rather than just reactivity to stressors in general.
With respect to HR reactivity, a similar pattern was seen with the short/short genotype being especially reactive to negative social evaluation. The HR reactivity results were moderated by gender, however. Women showed significantly greater HR reactivity as a function of the 5-HTTLPR, whereas the results for HR reactivity were not significant for men.
In terms of mechanisms, the 5-HTTLPR-related differential reactivity to negative social cues could be a result of psychological factors operating at multiple stages in the information processing stream. At the initial stages of processing, the heightened response of short/short individuals to threatening and disapproving social signals is consistent with recent findings of a 5-HTTLPR-related attentional bias to threatening stimuli. In studies of attentional allocation using the dot-probe task with threatening words (41) or pictures (42), short allele carriers, relative to long/long individuals, have a negative bias, focusing greater attention on threatening stimuli and less on positive stimuli. In adolescents, a 5-HTTLPR short allele-dependent bias towards angry faces and away from happy faces has also been found (43). The degree of vigilance to socially threatening information, such as angry faces (44) or disapproving faces (45), has been found to be associated with the cortisol response to psychological stress in the laboratory (44) and workplace (45). This bias reflecting greater attention to socially threatening stimuli may help explain the particular association of the 5-HTTLPR with CVR only in the negative evaluative audience condition, but not the positive evaluation condition or the no audience condition, where such social cues were not present.
The 5-HTTLPR related differential reactivity to the differing social cues could also be occurring at later stages of processing. Neuroimaging studies of social threat indicate that short allele carriers have greater amygdala reactivity to negative facial expressions than long/long individuals (46). As amygdala reactivity correlates with the degree of CVR to a stressor (47), it is likely that the amygdala is involved in the 5-HTTLPR-associated differences in CVR to the different experimental conditions in the present study.
In terms of later stages of processing, the 5-HTTLPR may also be associated with impaired emotion regulation capabilities. That the DBP of short/short individuals remained elevated after the negative social evaluation suggests that these individuals were less able to engage psychological processes that would effectively dampen this reactivity. Taken together, these data are consistent with the 5-HTTLPR affecting the degree of reactivity to social threats at multiple psychological levels.
The increase in HR reactivity in the negative audience condition was seen only in females with the short/short genotype, which is consistent with a prior study (31). Sex differences in HR reactivity are not without precedent, as an early meta-analysis found that women responded to laboratory stressors with greater HR reactivity than men (48). Subsequent research examining the gender relevance of the stressor task as an explanation for such differences in CVR, including the social or non-social nature of the task, has led to mixed results (49, 50). Based on the findings presented here, consideration of genotype in future studies may help to clarify some of these differences. The greater HR reactivity of short/short females is consistent with previously reported 5-HTTLPR-related sex differences using other dependent measures, which have shown that short/short females are more affected by stress than short/short males (51, 52).
As noted, a prior study found that individuals with the long/long genotype had greater CVR to the recall of an emotional event (32), results that are potentially discrepant with the pattern in the current study and with previous studies (31). One potential resolution to this discrepancy is that the long allele adversely affects CVR via peripheral mechanisms, while the short allele increases CVR via central mechanisms (53). In so far as aging affects peripheral cardiovascular processes associated with the 5-HTTLPR, such as platelet activation (54), the older age of the sample in the Williams et al study (32) may provide a potential explanation. In contrast, in the younger sample studied here the 5-HTTLPR may be more associated with central responses to the stressor. Evidence for this supposition is that the 5-HTTLPR was also associated with cortisol reactivity in this sample (55) which is likely to be a reflection of greater neural activation in response to the stressor. Ultimately, further studies with different psychological stress paradigms are needed to clarify the central versus peripheral roles of the 5-HTTLPR and serotonin system in modulating CVR.
Future research at the molecular level may also help to clarify the relationship between the serotonin transporter gene and CVR. Other polymorphisms in the SCL6A4 promoter (rs25531; (56); rs25532; (57)) as well as 3’region (rs3813034; (58) have been demonstrated to affect SLC6A4 expression and may modulate the association between the 5-HTTLPR and CVR.
A limitation of this study is the heterogeneous ethnic composition of the sample, which raises concerns of potential population stratification artifacts due to the differences in allele frequency between ethnic groups (59). As one precautionary means to guard against such effects, self-reported ethnicity was entered as a covariate in the analyses (60). Furthermore, the qualitative relationship between the 5-HTTLPR and CVR was similar across the different ethnic groups, suggesting that the 5-HTTLPR was functioning similarly in each ethnic group. This is consistent with the vast majority of replicated gene-phenotype associations, which have been shown to be similar across ethnic groups (61).
Conclusion
The 5-HTTLPR moderates cardiovascular responsivity to stress marked by social threat and disapproval. These results are consistent with a growing body of literature suggesting 5-HTTLPR involvement in setting sensitivity to the social environment (34) and reactivity to social threat in particular. The combination of 5-HTTLPR-related CVR seen in the present study, as well as cortisol reactivity seen in prior work (55), is indicative of the broad effects the serotonin system can have upon multiple health-relevant physiological pathways. This widespread physiological influence of the serotonin system, in concert with its sensitivity to social experience, suggests that the serotonin system may be a critical link by which both interpersonal and societal level social factors influence health.
Supplementary Material
Acknowledgments
This research was supported by a grant from the National Institute on Aging (AG030309) to S.E.T.
Acronyms
- 5-HTTLPR
Serotonin Transporter Gene Linked Polymorphic Region
- CVR
Cardiovascular Reactivity
- SBP
Systolic Blood Pressure
- DBP
Diastolic Blood Pressure
- HR
Heart Rate
- HPA
Hypothalamic-Pituitary-Adrenal
Footnotes
Financial Disclosure: The authors declare no biomedical financial interests or potential conflicts of interest.
The relationship between the 5-HTTLPR and cortisol responses to social stress in this sample have been published previously (41).
Supplemental Digital Content: Way and Taylor Population Stratification Text.doc
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orth-Gomer K, Rosengren A, Wilhelmsen L. Lack of social support and incidence of coronary heart disease in middle-aged Swedish men. Psychosomatic Medicine. 1993;55:37–43. doi: 10.1097/00006842-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan G, Salonen J, Cohen R, Brand R, LEONARD S. Social connections and mortality from all causes and from cardiovascular disease: prospective evidence from eastern Finland. American Journal of Epidemiology. 1988;128:370–80. doi: 10.1093/oxfordjournals.aje.a114977. [DOI] [PubMed] [Google Scholar]
- 3.Raikkonen K, Matthews K, Kuller L. Trajectory of psychological risk and incident hypertension in middle-aged women. Hypertension. 2001;38:798–802. [PubMed] [Google Scholar]
- 4.Orth-Gomer K, Wamala S, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman M. Marital stress worsens prognosis in women with coronary heart disease: The Stockholm Female Coronary Risk Study. JAMA. 2000;284:3008–14. doi: 10.1001/jama.284.23.3008. [DOI] [PubMed] [Google Scholar]
- 5.Felitti M, Vincent J, Anda M, Robert F, Nordenberg M. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults:: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 6.Manuck S. Cardiovascular reactivity in cardiovascular disease: Once more unto the breach. International Journal of Behavioral Medicine. 1994;1:4–31. doi: 10.1207/s15327558ijbm0101_2. [DOI] [PubMed] [Google Scholar]
- 7.Matthews K, Zhu S, Tucker D, Whooley M. Blood pressure reactivity to psychological stress and coronary calcification in the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2006;47:391–5. doi: 10.1161/01.HYP.0000200713.44895.38. [DOI] [PubMed] [Google Scholar]
- 8.Treiber F, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic medicine. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Chida Y, Steptoe A. Greater Cardiovascular Responses to Laboratory Mental Stress Are Associated With Poor Subsequent Cardiovascular Risk Status. A Meta-Analysis of Prospective Evidence. Hypertension. doi: 10.1161/HYPERTENSIONAHA.109.146621. in press. [DOI] [PubMed] [Google Scholar]
- 10.Woodall K, Matthews K. Familial environment associated with Type A behaviors and psychophysiological responses to stress in children. Health Psychology. 1989;8:403–26. doi: 10.1037//0278-6133.8.4.403. [DOI] [PubMed] [Google Scholar]
- 11.El-Sheikh M. Children's emotional and physiological responses to interadult angry behavior: The role of history of interparental hostility. Journal of Abnormal Child Psychology. 1994;22:661–78. doi: 10.1007/BF02171994. [DOI] [PubMed] [Google Scholar]
- 12.Taylor S, Lerner J, Sage R, Lehman B, Seeman T. Early environment, emotions, responses to stress, and health. Journal of Personality. 2004;72:1365–94. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- 13.Carels R, Szczepanski R, Blumenthal J, Sherwood A. Blood pressure reactivity and marital distress in employed women. Psychosomatic medicine. 1998;60:639–43. doi: 10.1097/00006842-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Ljung T, Ahlberg A, Holm G, Friberg P, Andersson B, Eriksson E, Bjorntorp P. Treatment of abdominally obese men with a serotonin reuptake inhibitor: a pilot study. Journal of internal medicine. 2001;250:219–24. doi: 10.1046/j.1365-2796.2001.00881.x. [DOI] [PubMed] [Google Scholar]
- 15.Golding M, Kotlyar M, Carson S, Hoyler S, Lazarus C, Davidson C, Guzzo J, Sontz E, Garbutt J. Effects of paroxetine on cardiovascular response to mental stress in subjects with a history of coronary artery disease and no psychiatric diagnoses. Psychopharmacology. 2005;182:321–6. doi: 10.1007/s00213-005-0075-7. [DOI] [PubMed] [Google Scholar]
- 16.DeVane C, Ware M, Emmanuel N, Brawman-Mintzer O, Morton W, Villarreal G, Lydiard R. Evaluation of the efficacy, safety and physiological effects of fluvoxamine in social phobia. International clinical psychopharmacology. 1999;14:345–51. doi: 10.1097/00004850-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Tucker P, Smith K, Marx B, Jones D, Miranda R, Jr, Lensgraf J. Fluvoxamine reduces physiologic reactivity to trauma scripts in posttraumatic stress disorder. Journal of Clinical Psychopharmacology. 2000;20:367–72. doi: 10.1097/00004714-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Straneva-Meuse P, Light K, Allen M, Golding M, Girdler S. Bupropion and paroxetine differentially influence cardiovascular and neuroendocrine responses to stress in depressed patients. Journal of Affective Disorders. 2004;79:51–61. doi: 10.1016/S0165-0327(02)00352-X. [DOI] [PubMed] [Google Scholar]
- 19.Corchs F, Nutt D, Hood S, Bernik M. Serotonin and Sensitivity to Trauma-Related Exposure in Selective Serotonin Reuptake Inhibitors-Recovered Posttraumatic Stress Disorder. Biological Psychiatry. 2009;66:17–24. doi: 10.1016/j.biopsych.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg B, Tolliver T, Huang S, Li Q, Bengel D, Murphy D. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. American Journal of Medical Genetics. 1999;88:83–7. [PubMed] [Google Scholar]
- 22.Javors M, Seneviratne C, Roache J, Ait-Daoud N, Bergeson S, Walss-Bass M, Akhtar F, Johnson B. Platelet serotonin uptake and paroxetine binding among allelic genotypes of the serotonin transporter in alcoholics. Progress in Neuropsychopharmacology & Biological Psychiatry. 2005;29:7–13. doi: 10.1016/j.pnpbp.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson G, Gutknecht L, Cohen D, Brailly-Tabard S, Cohen J, Ferrari P, Roubertoux P, Tordjman S. Serotonin transporter promoter variants in autism: functional effects and relationship to platelet hyperserotonemia. Molecular psychiatry. 2002;7:831–6. doi: 10.1038/sj.mp.4001099. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser R, Muller-Oerlinghausen B, Filler D, Tremblay P, Berghofer A, Roots I, Brockmoller J. Correlation between serotonin uptake in human blood platelets with the 44-bp polymorphism and the 17-bp variable number of tandem repeat of the serotonin transporter. American Journal of Medical Genetics. 2002;114:323–8. doi: 10.1002/ajmg.10119. [DOI] [PubMed] [Google Scholar]
- 25.Whale R, Quested D, Laver D, Harrison P, Cowen P. Serotonin transporter (5-HTT) promoter genotype may influence the prolactin response to clomipramine. Psychopharmacology. 2000;150:120–2. doi: 10.1007/s002130000432. [DOI] [PubMed] [Google Scholar]
- 26.Reist C, Mazzanti C, Vu R, Tran D, Goldman D. Serotonin transporter promoter polymorphism is associated with attenuated prolactin response to fenfluramine. American Journal of Medical Genetics. 2001;105:363–8. doi: 10.1002/ajmg.1360. [DOI] [PubMed] [Google Scholar]
- 27.Smith G, Lotrich F, Malhotra A, Lee A, Ma Y, Kramer E, Gregersen P, Eidelberg D, Pollock B. Effects of serotonin transporter promoter polymorphisms on serotonin function. Neuropsychopharmacology. 2004;29:2226–34. doi: 10.1038/sj.npp.1300552. [DOI] [PubMed] [Google Scholar]
- 28.Muldoon M, Sved A, Flory J, Perel J, Matthews K, Manuck S. Inverse relationship between fenfluramine-induced prolactin release and blood pressure in humans. Hypertension. 1998;32:972–5. doi: 10.1161/01.hyp.32.6.972. [DOI] [PubMed] [Google Scholar]
- 29.Muldoon M, Mackey R, Korytkowski M, Flory J, Pollock B, Manuck S. The metabolic syndrome is associated with reduced central serotonergic responsivity in healthy community volunteers. Journal of Clinical Endocrinology & Metabolism. 2006;91:718–21. doi: 10.1210/jc.2005-1654. [DOI] [PubMed] [Google Scholar]
- 30.Muldoon M, Mackey R, Sutton-Tyrrell K, Flory J, Pollock B, Manuck S. Lower central serotonergic responsivity is associated with preclinical carotid artery atherosclerosis. Stroke. 2007;38:2228–33. doi: 10.1161/STROKEAHA.106.477638. [DOI] [PubMed] [Google Scholar]
- 31.McCaffery J, Bleil M, Pogue-Geile M, Ferrell R, Manuck S. Allelic variation in the serotonin transporter gene-linked polymorphic region (5-HTTLPR) and cardiovascular reactivity in young adult male and female twins of European-American descent. Psychosomatic medicine. 2003;65:721–8. doi: 10.1097/01.psy.0000088585.67365.1d. [DOI] [PubMed] [Google Scholar]
- 32.Williams R, Marchuk D, Siegler I, Barefoot J, Helms M, Brummett B, Surwit R, Lane J, Kuhn C, Gadde K. Childhood Socioeconomic Status and Serotonin Transporter Gene Polymorphism Enhance Cardiovascular Reactivity to Mental Stress. Psychosomatic medicine. 2008;70:32–9. doi: 10.1097/PSY.0b013e31815f66c3. [DOI] [PubMed] [Google Scholar]
- 33.Gruenewald T, Kemeny M, Aziz N, Fahey J. Acute threat to the social self: Shame, social self-esteem, and cortisol activity. Psychosomatic medicine. 2004;66:915–24. doi: 10.1097/01.psy.0000143639.61693.ef. [DOI] [PubMed] [Google Scholar]
- 34.Way BM, Taylor SE. Social influences on health: Is serotonin a critical mediator? Psychosomatic Medicine. 2010;72:107–12. doi: 10.1097/PSY.0b013e3181ce6a7d. [DOI] [PubMed] [Google Scholar]
- 35.Way BM, Gurbaxani BM. A genetics primer for social health research. Social and Personality Psychology Compass. 2008;2:785–816. [Google Scholar]
- 36.Kirschbaum C, Pirke KM, Hellhammer DH. The‘Trier Social Stress Test’-a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 37.Taylor S, Seeman T, Eisenberger N, Kozanian T, Moore A, Moons W. Effects of a supportive or unsupportive audience on biological and psychological responses to stress. Journal of Personality and Social Psychology. 2010;98:47–56. doi: 10.1037/a0016563. [DOI] [PubMed] [Google Scholar]
- 38.Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60:671–6. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Kamarck T, Jennings J, Debski T, Glickman-Weiss E, Johnson P, Eddy M, Manuck S. Reliable measures of behaviorally-evoked cardiovascular reactivity from a PC-based test battery: Results from student and community samples. Psychophysiology. 1992;29:17–28. doi: 10.1111/j.1469-8986.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 40.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 41.Beevers C, Gibb B, McGeary J, Miller I. Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. Journal of Abnormal Psychology. 2007;116:208–12. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- 42.Fox E, Ridgewell A, Ashwin C. Looking on the bright side: biased attention and the human serotonin transporter gene. Proceedings of the Royal Society B: Biological Sciences. 2009;276:1747–51. doi: 10.1098/rspb.2008.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.PÈrez-Edgar K, Bar-Haim Y, McDermott J, Gorodetsky E, Hodgkinson C, Goldman D, Ernst M, Pine D, Fox N. Variations in the serotonin-transporter gene are associated with attention bias patterns to positive and negative emotion faces. Biological Psychology. 2010;83:269–71. doi: 10.1016/j.biopsycho.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Honk J, Tuiten A, van den Hout M, Koppeschaar H, Thijssen J, de Haan E, Verbaten R. Conscious and preconscious selective attention to social threat: Different neuroendocrine response patterns. Psychoneuroendocrinology. 2000;25:577–91. doi: 10.1016/s0306-4530(00)00011-1. [DOI] [PubMed] [Google Scholar]
- 45.Dandeneau S, Baldwin M, Baccus J, Sakellaropoulo M, Pruessner J. Cutting stress off at the pass: Reducing vigilance and responsiveness to social threat by manipulating attention. Journal of Personality and Social Psychology. 2007;93:651–66. doi: 10.1037/0022-3514.93.4.651. [DOI] [PubMed] [Google Scholar]
- 46.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–52. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 47.Gianaros P, Sheu L, Matthews K, Jennings J, Manuck S, Hariri A. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. Journal of Neuroscience. 2008;28:990–9. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoney C, Davis M, Matthews K. Sex differences in physiological responses to stress and in coronary heart disease: a causal link? Psychophysiology. 2007;24:127–31. doi: 10.1111/j.1469-8986.1987.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 49.Whited M, Larkin K. Sex Differences in Cardiovascular Reactivity. Journal of Psychophysiology. 2009;23:77–84. [Google Scholar]
- 50.Lash S, Gillespie B, Eisler R, Southard D. Sex differences in cardiovascular reactivity: Effects of the gender relevance of the stressor. Health Psychology. 1991;10:392–8. doi: 10.1037//0278-6133.10.6.392. [DOI] [PubMed] [Google Scholar]
- 51.Brummett B, Boyle S, Siegler I, Kuhn C, Ashley-Koch A, Jonassaint C, Z,chner S, Collins A, Williams R. Effects of environmental stress and gender on associations among symptoms of depression and the serotonin transporter gene linked polymorphic region (5-HTTLPR). Behavior genetics. 2008;38:34–43. doi: 10.1007/s10519-007-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–15. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- 53.Williams R. Psychosocial and biobehavioral factors and their interplay in coronary heart disease. Annual Review of Clinical Psychology. 2008;4:349–65. doi: 10.1146/annurev.clinpsy.4.022007.141237. [DOI] [PubMed] [Google Scholar]
- 54.Whyte E, Pollock B, Wagner W, Mulsant B, Ferrell R, Mazumdar S, Reynolds C., III Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. American Journal of Psychiatry. 2001;158:2074–6. doi: 10.1176/appi.ajp.158.12.2074. [DOI] [PubMed] [Google Scholar]
- 55.Way BM, Taylor SE. The Serotonin Transporter Promoter Polymorphism (5-HTTLPR) is Associated with Cortisol Response to Psychosocial Stress. Biological Psychiatry. 2010;67:487–92. doi: 10.1016/j.biopsych.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. The American Journal of Human Genetics. 2006;78:815–26. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wendland JR, Moya PR, Kruse MR, Ren-Patterson RF, Jensen CL, Timpano KR, Murphy DL. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Human Molecular Genetics. 2008;17:717–23. doi: 10.1093/hmg/ddm343. [DOI] [PubMed] [Google Scholar]
- 58.Gyawali S, Subaran R, Weissman M, Hershkowitz D, McKenna M, Talati A, Fyer A, Wickramaratne P, Adams P, Hodge S. Association of a Polyadenylation Polymorphism in the Serotonin Transporter and Panic Disorder. Biological Psychiatry. doi: 10.1016/j.biopsych.2009.10.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hutchison KE, Stallings M, McGeary J, Bryan A. Population stratification in the candidate gene study: fatal threat or red herring? Psychol Bull. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- 60.Liu X, Paterson A, John E, Knight J. The role of self-defined race/ethnicity in population structure control. Annals of Human Genetics. 2006;70:496–505. doi: 10.1111/j.1469-1809.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 61.Ioannidis JP, Ntzani EE, Trikalinos TA. ‘Racial’ differences in genetic effects for complex diseases. Nature Genetics. 2004;36:1312–8. doi: 10.1038/ng1474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.