Abstract
The study of macromolecular interactions by sedimentation equilibrium is a highly technical method that requires great care in both the experimental design and data analysis. The complexity of the interacting system that can be analyzed is only limited by the ability to deconvolute the exponential contributions of each of the species to the overall concentration gradient. This is achieved in part through the use of multi-signal data collection and the implementation of soft mass conservation. We illustrate the use of these constraints in SEDPHAT through the study of an A + B + B ⇌ AB + B ⇌ ABB system and highlight some of the technical challenges that arise. We show that both the multi-signal analysis and mass conservation result in a precise and robust data analysis and discuss improvements that can be obtained through the inclusion of data from other methods such as sedimentation velocity and isothermal titration calorimetry.
1. Introduction
Macromolecular interactions, such as protein-protein, protein-oligosaccharide, protein-nucleic acid and nucleic acid-nucleic acid interactions are intrinsic to all cellular processes. Their characterization and understanding represents one of the primary endeavors of biochemistry and drug discovery. The biophysical solution studies of these interactions usually involves the determination of both the affinity of interaction and the stoichiometry of the complex through a direct or indirect measure of the concentrations of free and bound species. Traditional methods for the study of such reversible interactions include analytical ultracentrifugation, equilibrium dialysis, gel electrophoresis, size exclusion chromatography, light scattering, differential scanning calorimetry, isothermal titration calorimetry, surface plasmon resonance and various spectroscopic methods [1]. Of these, analytical ultracentrifugation is perhaps one of the oldest techniques still in use [2 – 4]. It is also one of the most versatile, particularly given the recent improvements in the sensitivity of the detection systems and the continuing developments in the computational methods for data analysis [5 – 8].
Analytical ultracentrifugation includes the methods of sedimentation equilibrium and sedimentation velocity. In both cases the macromolecular solutions of interest are subjected to a high gravitational field and the resulting changes in the concentration distribution are monitored in real time using various optical methods. Sedimentation equilibrium experiments are usually carried out at low rotor speeds such that the flux of sedimenting macromolecules is balanced by the flux of their diffusion. The time-invariant concentration gradient obtained, described simply based on first principles and equilibrium thermodynamics, can be used to provide information on the macromolecular molar mass and in the case of interacting systems the interaction affinity and stoichiometry. Conversely, sedimentation velocity experiments are usually carried out at high rotor speeds providing information on the transport behavior of the macromolecules in solution. For interacting systems, the sedimentation velocity boundaries will depend on the hydrodynamic properties of each of the macromolecular species, as well as the reaction kinetics [9 – 11]. Recent advances in the understanding of these systems and the deconvolution of the sedimentation velocity profiles result in a method complementary to sedimentation equilibrium [5, 6, 9 – 15].
1.1. Sedimentation equilibrium
Sedimentation equilibrium is one of the most effective methods for the characterization of macromolecular interactions - the determination of the molecular mass by sedimentation equilibrium does not depend on the macromolecular shape and the reaction kinetics does not feature in the data analysis, even though it influences the time to reach equilibrium. Applications of this method, which recently include the analysis of receptor-receptor and receptor-ligand interactions [16 – 18], the self-association of various regulatory proteins and receptors [19 – 25], and the interaction of various proteins with DNA, RNA and RNA/DNA hybrids [26 – 30], altogether demonstrate its usefulness for determining both the affinity and stoichiometry of interacting systems. Further illustration on the use of sedimentation equilibrium can be found in earlier and more comprehensive reviews [8, 31 – 35] and references cited therein.
1.1.1 Principles and considerations
In sedimentation equilibrium the flux of sedimenting macromolecules is balanced by the flux of their diffusion resulting in the establishment of a time-invariant concentration gradient. At equilibrium the chemical potential of the solution is constant, resulting in a concentration distribution that has the following exponential form for a single ideal macromolecule:
| Equation 1.1 |
where r is the radial distance from the center of rotation, ω the angular velocity of the rotor, T the absolute temperature, R the molar gas constant, ro an arbitrary reference point, such as the meniscus or cell bottom, M2 the molecular mass of the macromolecule and ∂ρ/∂c2 the density increment of the macromolecule at constant chemical potential. M2∂ρ/∂c2 represents the buoyant molecular mass which takes into account the Archimedean displacement of a volume of water corresponding to the volume of the sedimenting species, including contributions from macromolecular hydration and macromolecular-cosolvent interactions. Using the three-component formalism developed by Eisenberg [36 – 39] it can be shown that at constant chemical potential:
| Equation 1.2 |
where ρ is the solution density, v1, v2, v3 are the partial specific volumes of water, the macromolecule and the buffer cosolutes, respectively, and B1 and B3 represent the quantities of bound or excluded water and buffer cosolutes in g/g of macromolecule, respectively. In cases where the macromolecules are not highly charged, the solution density is low and cosolutes such as glycerol or detergents are absent, the contributions of B1 and B3 are essentially zero and this equation reduces to the familiar:
| Equation 1.3 |
Traditionally, sedimentation equilibrium has been used to determine molecular mass. For proteins this has been possible through the calculation of the partial specific volume v2 based on their amino acid composition and this parameter, along with the buffer density, can now be calculated using the program SEDNTERP [40]. Partial specific volumes can also be estimated for other macromolecules, such as carbohydrates [41 – 42] and glycoproteins [43 – 44] based on their composition, using Traube’s additivity rules [45]. In addition, these rules in conjunction with the atomic partial molar volumes published by Durchschlag and Zipper [46 – 47] allow for estimates of the partial specific volumes of macromolecules other than proteins, carbohydrates and their conjugates. In the case of nucleic acids and other highly charged polyelectrolytes, however, care needs to be exercised as the partial specific volumes are very dependent on both the buffer composition and ionic strength [48 – 49]. In these cases the effective partial specific volume φ’, which incorporates contributions from the B1 and B3 terms, needs to be determined experimentally by densimetry or sedimentation equilibrium [26 – 29]. For the purposes of this work, we will consider dilute solutions and buffer cosolutes such that solutes behave ideally and that M2∂ρ/∂c2 can be replaced simply by M2(1 − v2ρ) or the functional M2(1 − φ’ρ).
1.1.2 Application to interacting systems
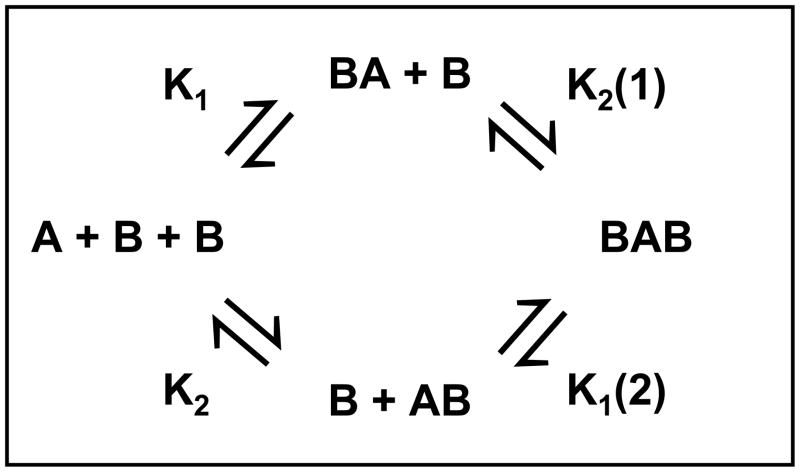
The study of interacting systems by sedimentation equilibrium relies on the fact that at sedimentation equilibrium the system of interest is also at chemical equilibrium and that the concentrations of the various components are related by the laws of mass action. In many respects sedimentation equilibrium, unlike other methods used to characterize interacting systems, provides a true test for whether a system is at chemical equilibrium and in fact truly reversible - in setting up the equilibrium concentration gradient one is able to assay concentration regimes in which both the complex and the free components are favored. Furthermore, reversibility can be tested by simply changing the rotor speed and perturbing the concentration gradient. The application of sedimentation equilibrium to the study of self-associating and interacting systems has been the subject of previous reviews [8, 31 – 35]. In this work we will only consider the special case of an A + B + B ⇌ AB + B ⇌ ABB interacting system, where macromolecule A has two distinct binding sites for macromolecule B. As shown in Figure 1, the microscopic association constants K1 and K2 describe the binding of B to free A on either site (designated 1 and 2) to form complexes AB and BA, respectively. The microscopic association constants describing the binding of a second B species are designated K2(1) and K1(2), where K2(1) describes the binding of the B species to site 2 on A, with a B already bound to site 1 [50]. As sedimentation equilibrium only distinguishes the associating species based on their buoyant molecular mass, one cannot discriminate between complexes AB and BA, and thus between the two sets of microscopic association constants. For this reason, sedimentation equilibrium data analysis usually distinguishes between the formation of complexes AB, BA and BAB through the association constants Ka1 and Ka2:
| Equation 1.4 |
where cA and cB are the concentrations of free A and B, respectively and cAB, cBA and cBAB are the concentrations of the respective complexes. In the special case where A has two symmetric binding sites for B, the microscopic association constants K1 and K2 become equal, as do K2(1) and K1(2). Based on these equalities, K1 = K2 = K01 and K2(1) = K1(2) = K12 one can relate the microscopic association constants to the macroscopic association constants Ka1 and Ka2 through:
| Equation 1.5 |
where Ka1 is the association constant for the formation of complexes (AB + BA) from free A and free B, and Ka2 is the association constant for the formation of complex BAB from (AB + BA) and B. If the binding of the first macromolecule B does not influence the binding of the second macromolecule B, then K1 = K2 = K2(1) = K1(2) from which it follows that Ka1 = 4Ka2. In a positively cooperative system, where the binding of the first species B increases the affinity for the binding of the second B species, it follows that Ka1 < 4Ka2.
Figure 1. Reaction scheme for a BAB system.
Reaction scheme describing the association of STI (A) with α-chymotrypsin (B) in which the microscopic association constants K1, K2, K2(1), and K1(2) are defined.
A sedimentation equilibrium experiment carried out on a mixture of species A and B, at a concentration in which all complexes are populated, will lead to the formation of a concentration gradient for all four species such that:
| Equation 1.6 |
where ctot represents the overall concentration, cn is the concentration of species n, Mn* the buoyant molecular mass of species n and H is ω2/2RT. In these experiments it is generally assumed that the buoyant molecular masses of the complexes are simply the sum of the buoyant molecular masses of the components. Furthermore, as the various species are also in chemical equilibrium, their concentrations are related by the laws of mass action:
| Equation 1.7 |
This equation provides the basis for fitting the A + B + B ⇌ AB + B ⇌ ABB interacting system under consideration. This model is referred to as ‘A + B + B <-> {AB} + B <-> ABB; with two symmetric sites, macroscopic K’ in the program SEDPHAT [6] utilized in this study. Its implementation is discussed in the following section. Two distinct binding sites for B on A can also be analyzed by SEDPHAT using the model ‘A + B + B <-> AB + B <-> BA + B <-> BAB; with two non-symmetric sites, microscopic K’ but only in association with other methods such as sedimentation velocity or isothermal titration calorimetry where one can distinguish the complexes AB and BA.
1.1.2 Mass conservation and multi-signal analysis constraints
The analysis of sedimentation equilibrium data for interacting systems requires a deconvolution of the various exponential terms shown in Equation 1.7; in this manner one determines the concentrations of the free species and complexes at each radial position r and consequently the equilibrium constants. The analysis of these multi-exponential equations is usually an ill-defined problem with a complex error surface [51, 52]. However, the appropriate experimental setup procedures and the use of both soft mass conservation and a multi-wavelength analysis lead to analysis constraints that result in rapid convergence of the data fits and high precision in the parameters obtained. The experimental design and use of these various constraints, as implemented in SEDPHAT, for the characterization of interacting systems by sedimentation equilibrium have been previously discussed [33, 52, 53]. The application of key constraints is briefly discussed in this work.
The buoyant molecular masses of species A and B, MA* and MB* respectively, need to be determined under the same experimental conditions used for the study of the interacting system and fixed in the data analysis. Prior to sedimentation equilibrium it is also good practice to characterize the behavior of each single species by sedimentation velocity in order to verify that these in fact behave as single ideal solutes. In cases where either species self-associates to form higher-order species, the equilibrium constant describing this self-association will need to be established by sedimentation equilibrium and a calculated value of the partial specific volume will be required to determine the equilibrium constant [33].
Multi-wavelength data collection and analysis is a powerful tool to distinguish between various species based on their spectral signatures. In this method absorbance data are collected at various wavelengths along with interference data, when possible. To avoid issues with wavelength reproducibility, absorbance data are collected at wavelengths corresponding to minima and maxima in the absorption spectrum. Typically, values of 250 and 280 nm are used for protein-protein interactions essentially distinguishing the proteins based on their relative tryptophan and tyrosine content. Data can also be collected at 230 nm as this corresponds to a sharp emission peak of the flash lamp source [54], even though the protein absorption spectrum exhibits a steep dependence on the wavelength in this region. By collecting absorbance data at 230, 250 and 280 nm, one also alleviates some of the constraints in the protein concentration range that can be studied resulting from the fact that absorbance is only linear at 0 to 1.5 optical densities. Equation 1.8 is then used to describe the interference and absorbance data collected at various wavelengths - for example, in the case of a single solute A, the total signal aA,λ(r) for each wavelength is related to the concentration cA(ro) through the extinction coefficient εA,λ and the path-length l:
| Equation 1.8 |
where bλ represents a baseline correction term. Therefore, prior to the analysis of the interacting system, each component needs to be characterized in terms of its spectral signature using the ‘A (Single species of interacting system)’ model implemented in SEDPHAT. In addition to the buoyant molecular mass, this analysis also determines the values of εA,λ at the various wavelengths studied based on the fixed extinction coefficient at 280 nm or the interference optics signal increment. The values of εA,λ and εB,λ obtained will be used as fixed parameters in the global multi-signal analysis described by Equation 1.9, which further assumes that the extinction coefficient of the complex at each wavelength is the sum of the extinction coefficients of the components:
| Equation 1.9 |
Another important constraint in the dissection of Equation 1.9 is the use of soft mass conservation, in which the total concentrations of species A and B are floated as independent parameters. In a manner similar to hard mass conservation originally introduced by Lewis [55, 56], this initially accounts for the known loading concentrations of the species A and B. However, by allowing both the initial concentrations and cell bottom parameters to float, the method accounts for the uncertainty in the value of the loading concentrations, possible losses of material observed in the course of the experiment and the significant amounts of non-observable material at the bottom of the cell where the concentration gradients are steep. It is assumed that the redistribution of material that occurs when the rotor speeds are changed leaves the total amount of material invariant and that there is no sample degradation or loss of material over time [57]. Even though this does not reduce the number of fitting parameters, rather it replaces the reference concentrations cA(ro) and cB(ro) as fitting parameters with the known loading concentrations of A and B, this constraint acts as a guide towards the correct fit [58, 59]. Combined with data collection at various rotor speeds and multiple wavelengths, soft mass conservation provides for a well defined sedimentation equilibrium data analysis that aids in the measurement of the populations of the free and complex species. As sedimentation equilibrium resolves species based on their buoyant molecular mass, the use of mass conservation further expands the range of useful mass ratios for species A and B by judicious utilization of the known loading concentration - for example one can now distinguish between cA(r) and cB(r) in cases where MA* and MB* are equal, as well as distinguish between cB(r) and cAB(r) in cases where MA* is much smaller than MB*. Advantages of these constraints are also apparent in situations where one of the binding partners has an exceedingly low extinction coefficient or in cases where the loading ratios of species A and B are very different from the expected complex stroichiometry [58].
1.1.3 Implementation
Sedimentation equilibrium is a very technical method and the study of interacting systems using this means requires a careful experimental setup to implement the advantageous constraints of mass conservation and multi-signal analysis. All of the interacting species need to be individually characterized prior to the study and analysis of their appropriately designed mixtures, in this way no information or assumptions are required with respect to their partial specific volumes. In this work we describe a set of sedimentation equilibrium experiments designed to characterize the interaction of α-chymotrypsin with soybean trypsin inhibitor (STI) and demonstrate that this method, as implemented in SEDPHAT, is both robust and precise. We also highlight some technical challenges with respect to the data analysis and accuracy of the method. This particular interaction was chosen as it utilizes inexpensive and commercially available components, and thus can be readily reproduced by the interested reader. It also illustrates the usefulness of this method in distinguishing between STI (species A) and α-chymotrypsin (species B) given the similarity in the buoyant molecular masses (MA*/MB* = 0.81) and large differences in the extinction coefficients at 280 nm (εA/εB = 0.34). Furthermore, it is an interaction that has been previously studied by sedimentation equilibrium [60] and well characterized by both static and dynamic light scattering [61, 62]. In order to simplify the analysis, buffer conditions were chosen such that the α-chymotrypsin does not dimerize [62] or undergo autolysis [63, 64]. In this manner an A + B + B ⇌ AB + B ⇌ ABB model as represented by Equation 1.9 can be used for data fitting. As in the studies described by Attri and Minton [61], we initially assume that the two α-chymotrypsin sites on the inhibitor have approximately equal affinity, this way the value of Ka2 is fixed to ¼ that of Ka1; that is log10[Ka2/Ka1] = −0.60206
2. Materials and Methods
2.1 Protein purification and characterization
Purified α-chymotrypsin (Cat. No. 1475) and soybean trypsin inhibitor (STI) (Cat. No. 3570) were purchased from Worthington Biochemical Corporation. Approximately 21 mg of each were dissolved in 0.2M sodium chloride and 0.05M sodium phosphate (pH = 6.7) and further purified by size exclusion chromatography on a HiPrep 16/60 Sephacryl S-200 HR column (Cat. No. 17-1166-01, GE Healthcare) at 0.5 mL/min in the same buffer. Fractions corresponding to the peak center were both dialyzed against the same 0.2M sodium chloride and 0.05M sodium phosphate (pH = 6.7) buffer and used as stock solutions for sedimentation experiments. α-chymotrypsin corresponds to the mature chymotrypsinogen A (Uniprot accession number P00766) lacking residues 14 – 15 and 147 – 148 of the propeptide. Based on the amino acid sequence α-chymotrypsin is characterized by the following parameters as determined in SEDNTERP 1.09 [40]: a molecular mass M of 25,197.7 Da, an extinction coefficient at 280 nm ε280 of 50,590 M−1cm−1 and partial specific volumes of 0.7329 and 0.7287 cm3g−1 at 20.0 and 10.0°C, respectively. STI corresponds to the processed peptide of trypsin inhibitor A (residues 25 – 205, Uniprot accession number P01070), it has a calculated molecular mass M of 20,090.7 Da and a calculated extinction coefficient at 280 nm ε280 of 17,210 M−1cm−1. Partial specific volumes at 20.0 and 10.0°C are 0.7336 and 0.7294 cm3g−1, respectively. The identity of the soybean trypsin inhibitor was confirmed by N-terminal protein sequencing (15 residues) using a Procise 492c LC Protein Sequencer (Applied Biosystems) and the molecular masses of both purified proteins were confirmed by MALDI-TOF on a Voyager-DE mass spectrometer (Perseptive Biosystems).
2.2 Sedimentation velocity
Sedimentation velocity experiments on the individual proteins were conducted at 20.0°C on a Beckman Coulter Proteome XL-I analytical ultracentrifuge using both the absorbance and Rayleigh interference optical detection systems. Samples of α–chymotrypsin, prepared by dilution into the dialysis buffer, were studied at loading concentrations of 5.3, 10, 18, 34 and 68 μM; whereas samples of STI were studied at loading concentrations of 21, 41 and 82 μM. 2-channel, 3 mm path-length sector shaped cells were used for the α-chymotrypsin at 68 μM and STI at 82 μM (100 μL), otherwise samples were loaded into 2-channel, 12 mm path-length sector shaped cells (400 μL). 100 scans were acquired at 7 minute intervals and a rotor speed of 50 krpm; absorbance data were collected as single absorbance measurements at 280 nm using a radial spacing of 0.003 cm. Data were analyzed in SEDFIT 12.1b [5] in terms of a continuous c(s) distribution; when required the use of a buffer mismatch model was implemented in order to account for slight meniscus mismatches [65]. Solution densities ρ and viscosities η were calculated using the program SEDNTERP 1.09 [40]. The c(s) analyses were carried out using an s range of 0.5 to 5 with a linear resolution of 100 and confidence levels of 0.68. In all cases, excellent fits were observed with root mean square deviations (rmsd) ranging from 0.0032 – 0.0053 absorbance units and 0.0029 – 0.0051 fringes. Sedimentation coefficients were corrected to standard conditions at 20.0°C in water, s20,w.
2.3 Sedimentation equilibrium
Sedimentation equilibrium experiments were carried out at 10.0°C on a Beckman Optima XL-A analytical ultracentrifuge. Samples of α-chymotrypsin were studied at loading concentrations of 4.5, 8.4, 16.7, 22.3 and 27.9 μM, whereas samples of STI were studied at loading concentrations of 12.6, 24.4 and 48.8 μM. Mixtures of the two proteins were studied at loading stoichiometries of 1.7:1, 0.85:1 and 0.42:1 α-chymotrypsin:STI with STI concentrations ranging from 0.7 to 13.7 μM (see Figure 4). All samples were loaded into 6-channel, 12 mm path-length cells (130 μL). Data were acquired at 17, 25, 33 and 41 krpm, as an average of 4 absorbance measurements at 230, 250 and 280 nm using a radial spacing of 0.001 cm. Experiments were started at the lowest rotor speed by taking scans at 6 hour intervals and testing for equilibrium by inspection of the sedimentation profiles using the program WINMATCH. Once equilibrium was established, the rotor speed was increased and the process repeated – at all rotor speeds studied Sedimentation equilibrium was achieved within 48 hours. It is critical, especially in the case of interacting systems, to establish the attainment of sedimentation equilibrium by this method - data which are not at equilibrium can still be analyzed with good fits only to obtain erroneous results.
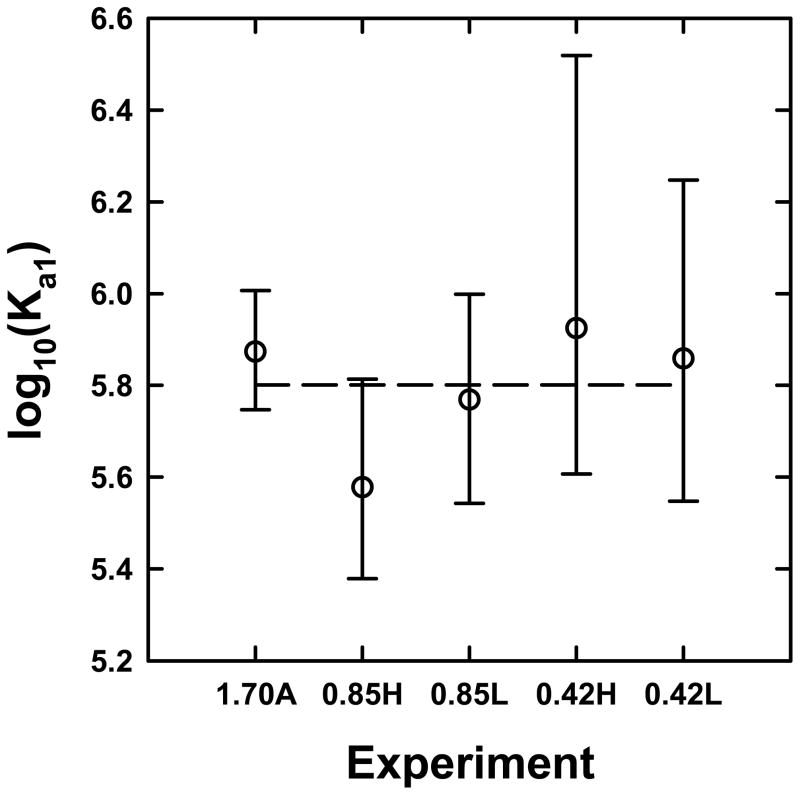
Figure 4. Sedimentation equilibrium analyses are robust and precise.
(A) Values of log10(Ka1) obtained for various data sets corresponding to STI and α-chymotrypsin loading concentrations of - (1.70A) 0.7, 1.3, 2.0, 2.0, 4.0, 8.0 μM STI and 1.7 equivalents of α-chymotrypsin, (0.85H) 2.7, 5.1, 10.2 μM STI and 0.85 equivalents of α-chymotrypsin, (0.85L) 0.7, 1.4, 2.7 μM STI and 0.85 equivalents of α-chymotrypsin, (0.42H) 3.7, 6.8, 13.7 μM STI and 0.42 equivalents of α-chymotrypsin, (0.42L) 0.9, 1.8, 3.7 μM STI and 0.42 equivalents of α-chymotrypsin. STI concentrations were allowed to float but the STI:α-chymotrypsin ratio was fixed. The error bars represent the 95% confidence limits of log10(Ka1) and the dashed line represents the average value of log10(Ka1).
Data collected for the individual proteins collected at different speeds, loading concentrations and wavelengths were analyzed globally in terms of a single species of interacting system, A, model in SEDPHAT 8.2 [6] with the implementation of mass conservation. In a similar fashion, data for the α-chymotrypsin and STI mixtures were analyzed in terms of an A + B + B ⇌ AB + B ⇌ ABB with two symmetric sites model in SEDPHAT 8.2 with mass conservation. Errors in the molecular masses of the individual species and the equilibrium constants were determined using the method of F-statistics with a confidence level of 95%. Samples of α-chymotrypsin and STI were also characterized by sedimentation equilibrium at 10.0°C on a Beckman Coulter Proteome XL-I analytical ultracentrifuge at loading concentrations of 8.4, 16.7, 27.9, 55.8 and 83.7 μM and 24.6 and 49.7 μM, respectively. 3 mm path-length double-sector cells were used for the three highest α-chymotrypsin concentrations.
3. Results and Discussion
3.1 Macromolecular characterization
3.1.1 Characterization by sedimentation velocity
The purity and homogeneity of the interacting macromolecules are critical to a successful sedimentation equilibrium experiment. It is very important, therefore, that each macromolecule be purified extensively and characterized as being pure prior to the sedimentation experiments. Size exclusion chromatography is routinely used in our laboratory as a last step in purification - in addition to confirming the monodispersity of the sample preparation, the method also results in buffer exchange which is especially important in instances where dialysis is not carried out. Accordingly, the commercially available STI and α-chymotrypsin used in these studies were purified in this manner; in both cases traces of protein dimer or higher mass impurities were removed, as were traces of lower mass absorbing impurities in the case of α-chymotrypsin (data not shown). The purity of the preparations was further confirmed by sedimentation velocity (Figure 2A) as this provides a rapid method for the ultimate characterization of the preparation prior to sedimentation equilibrium. As is routine, in cases where enough sample is available, we carried out these experiments at various protein loading concentrations and showed that both the STI and α-chymotrypsin are single species at all concentrations - the STI is characterized by an average s20,w of 2.10 ± 0.007 S, an estimated mass of 20.4 ± 0.7 kDa and a frictional ratio f/fo of 1.28. Similarly, the α-chymotrypsin is characterized by an average s20,w of 2.59 ± 0.02 S, an estimated mass of 25.8 ± 0.7 kDa and a frictional ratio f/fo of 1.21, consistent with published data [66]. These data demonstrate that both samples are monodisperse with molecular masses corresponding to those expected based on their amino acid sequence. Furthermore these data show that neither the STI nor the α-chymotrypsin undergo a reversible self-association in the concentration range studied.
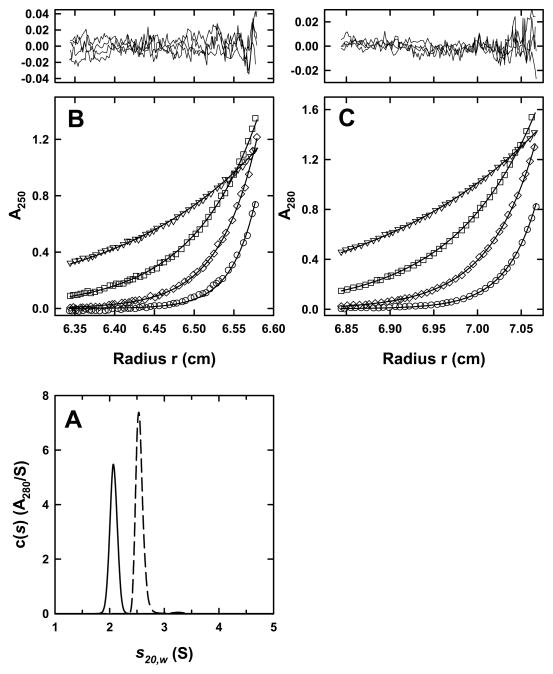
Figure 2. STI and α-chymotrypsin are monomers.
(A) c(s) distribution profiles for STI (solid line) and α-chymotrypsin (dashed line) based on sedimentation velocity absorbance data collected at 50 krpm, 20.0°C and loading concentrations of 41 and 17 μM, respectively. (B) Sedimentation equilibrium data for α-chymotrypsin at a loading concentration of 31 μM shown as a distribution of A250 at equilibrium. Data were collected at 10.0°C and rotor speeds of 17 (triangles), 25 (squares), 33 (diamonds) and 41 (circles) krpm. The results are analyzed globally along with data collected at 280 nm and various other loading concentrations for the best single component using mass conservation. Best fits are shown as a line through the experimental points and the combined residuals are shown above the plot. A best fit rmsd of 0.011 absorbance units is obtained. For clarity only every third experimental data point is shown. (C) Sedimentation equilibrium data for STI at a loading concentration of 53 μM shown as a distribution of A280 at equilibrium. Data were collected as in (B) and analyzed globally along with data collected at 250 nm and various other concentrations for the best single component using mass conservation. Best fits are shown as a line through the experimental points and the combined residuals are shown above the plot. A best fit rmsd of 0.0064 absorbance units is obtained. For clarity only every third experimental data point is shown.
3.1.2 Characterization by sedimentation equilibrium
Having established the purity and homogeneity of the preparations, both proteins were analyzed by sedimentation equilibrium at various rotor speeds with absorbance data collected at 280, 250 and 230 nm. When possible, interference data were also collected, along with water blanks at each rotor speed used. As the cells need to be mechanically stabilized (‘aged’) prior to the run, these experiments can only be carried out using cells that can be loaded using external ports [53]. The different rotor speeds utilized in the characterization of the individual components are the same as those used to study the interacting system. At least three rotor speeds are recommended - the lowest rotor speed corresponds to a concentration gradient such that the concentration at the cell bottom is approximately three times the concentration at the meniscus. The highest rotor speed should correspond to meniscus depletion and a concentration at the cell bottom which is at least ten times the loading concentration [33]. The appropriate rotor speeds were determined using the ‘estimate equilibrium rotor speeds’ calculator function in SEDFIT, with the highest expected mass value (the (α-chymotrypsin)2STI complex) used to estimate the lowest rotor speed and the lowest mass value (STI) used to estimate the highest rotor speed. Based on these calculations, we used rotor speeds of 17, 25, 33 and 41 krpm for the 130 μL volumes (~ 3.2 mm column height) and six channel centerpieces utilized on the XL-A, noting that in these calculations the outermost channels were considered. Rotor speeds of 14, 21, 28 and 35 krpm were used for the 170 μL volumes (~ 4.5 mm column height) and two sector centerpieces utilized on the XL-I.
Data collected for α-chymotrypsin at various rotor speeds, absorbance wavelengths and concentrations were sorted in SEDFIT and assembled into a series of SEDPHAT experimental files. Each SEDPHAT experimental file comprises multiple absorbance scans, collected at the same wavelength, for a single channel acquired at various rotor speeds. In the analysis, various experimental files corresponding to the same channel (Figure 2B) are assigned identical values for the sample concentration, cell meniscus and cell bottom positions – in this manner the data sets are ‘linked’ in SEDPHAT [52]. In order to implement the analysis with soft mass conservation, the sample concentrations were initialized to values corresponding to those expected based on the A280 measured prior to the experiment and these, along with the cell bottom positions were allowed to freely refine during data fitting. The extinction coefficient at 280 nm was fixed, whereas the values for the extinction coefficients at 250 and 230 nm were allowed to refine; 230 nm data for the highest concentrations were not considered in the analysis due to high absorbance values. Data were analyzed in SEDPHAT using an ‘A (single species of interacting system)’ model implementing mass conservation and a baseline correction for each rotor speed. Good fits are obtained with an overall reduced χ2 of 4.90 and rmsd values ranging from 0.0043 to 0.0173 - this fit returns a molecular mass of 23.3 ± 0.6 kDa, consistent with a monomeric α-chymotrypsin, and overall concentrations which are reproducibly ~ 15% larger than expected (Figure 2B). As noted by others [52] we found that data collected at 230 nm show poorer fits; ignoring these data leads to much better fits with an overall reduced χ2 of 2.83 and rmsd values ranging from 0.0043 to 0.0111 - the molecular mass, sample concentrations and extinction coefficient at 250 nm remain identical, within the error of the method. Data collected for STI were analyzed in a similar manner; good fits are obtained with an overall reduced χ2 of 3.00 and rmsd values ranging from 0.0051 to 0.0121 returning a molecular mass of 19.3 ± 0.4 kDa, consistent with a monomeric STI, and overall concentrations which are reproducibly ~ 11% larger than expected (Figure 2C). Once again, data collected at 230 nm showed poorer fits; ignoring these data leads to better fits with an overall reduced χ2 of 1.92 and rmsd values ranging from 0.0044 to 0.0093. A molecular mass of 19.8 ± 0.4 kDa is obtained with essentially identical concentrations and extinction coefficient at 250 nm. In the absence of mass conservation, identical values of the molecular masses are obtained with much better data fits corresponding to overall reduced χ2 of 1.53 and 1.92 for the STI and α-chymotrypsin, respectively.
It should be noted that a reduced χ2 is used as a measure of the goodness of fit in SEDPHAT. This essentially represents the summation of the weighted sum of the squared errors for each individual experiment divided by the number of degrees of freedom. The variance of the experimental observations used to weight the sum of squared errors for each experimental file is not a value based on the experimental data; rather it is a value obtained from the experimental parameter box in SEDPHAT, specifically the noise field. This value is typically 0.005 absorbance units or fringes for sedimentation equilibrium data. As this value is different from the actual experimental variance, the numerical value of the reduced χ2 will deviate from the expected value of 1.0 for a perfect fit. Consequently we compare the relative values of the reduced χ2 when assessing different best-fit models and parameters and typically find that reduced χ2 values can range from less than 1.0 to approximately 5.0 for good sedimentation equilibrium data fits. The values of the rmsd for each individual experiment and the radial distributions of the residuals provide additional criteria for assessing the goodness of fit. In the case of good data fits, the rmsd errors usually represent the noise for data acquisition and for absorbance data collected at 280 or 250 nm we usually find this varies from 0.003 to 0.006 absorbance units. The higher rmsd observed for data collected at 230 nm are consistent with the increased noise observed at this wavelength, based on an analysis of the raw sedimentation equilibrium data files. It has been noted that very low levels of apparently non-random noise can also be observed when modeling sedimentation equilibrium data, particularly in the case of interference data [52], this contribution will add to overall rmsd error.
3.1.3 Extinction coefficients and interference signal increments
Absorbance data collected at 280 and 250 nm on an XL-I led to identical observations - for α-chymotrypsin a molecular mass of 24.8 ± 0.4 kDa was obtained along with an extinction coefficient at 250 nm of 19,740 M−1cm−1 (cf. XL-A ε250 values with and without inclusion of the 230 nm data are 20,130 and 19,860 M−1cm−1, respectively); for STI a molecular mass of 19.8 ± 0.4 kDa was obtained along with an extinction coefficient at 250 nm of 9,040 M−1cm−1 (cf. XL-A ε250 values obtained above are 8,820 and 8,760 M−1cm−1). In both cases the concentrations returned from the fit were within ± 9% of those calculated spectrophotometrically and best fit cell bottoms ranged from 7.192 to 7.216 cm (data not shown). The use of the interference optics in the XL-I raises a possible problem with respect to the determination of the protein concentrations used in these studies. Fixing the extinction coefficient for absorbance data collected at 280 nm leads to interference molar signal increments of 80,230 and 70,760 M−1cm−1 for the α-chymotrypsin and STI, respectively, values which are in agreement with the signal increments observed by sedimentation velocity (Figure 2A). The molar signal increment εJ for the interference optics is related to the refractive index increment dn/dc in cm3g−1 as follows:
| Equation 3.1 |
where M is the molecular mass and λ the wavelength in cm (655 × 10−7 cm in our particular case). Both these εJ values are larger than expected based on a consensus dn/dc of 0.185 cm3g−1. In the particular case of α-chymotrypsin a dn/dc of 0.185 cm3g−1 has recently been determined at 589 nm [61]; correcting for the wavelength dispersion [67] an expected εJ of 70,400 M−1cm−1 is calculated. This value would indicate that the extinction coefficient at 280 nm is either too large, or that there are other contributions to dn/dc. The extinction coefficient ε280 of 50,590 M−1cm−1 calculated in SEDNTERP is quite close to the published value of 51,400 M−1cm−1 based on a molecular mass of 25,197.7 Da [68]. This is not the case for the STI where the calculated value of 17,210 M−1cm−1 is significantly smaller than the value of 18,970 M−1cm−1 recalculated based on published data [69]. For the purposes of the analysis of the interacting system, which are based exclusively on absorbance data, we have used the extinction coefficients based on values calculated in SEDNTERP. In many of the recombinant systems studied in our laboratory, the values of the signal increment εJ and calculated extinction coefficient ε280 are usually in good agreement with a consensus dn/dc value of 0.185 cm3g−1. In such cases, it is customary to use the value of εJ as a standard and derive the various absorbance extinction coefficients, provided that the protein is not glycosylated or modified in some other form [70]. Were it not for the inexplicable differences in the interference and absorbance signal increments, the interference detection system would have aided significantly in the multi-signal analysis as the εJ values differentiate STI and α-chymotrypsin rather differently from ε280.
We have now shown that both components do not undergo degradation or aggregation in the course of the experiment and we have determined many of the fixed parameters used in the analysis of the interacting system. For α-chymotrypsin we determined a buoyant molecular mass of 6,271 Da and extinction coefficients of 19,910 and 156,570 M−1cm−1 at 250 and 230 nm, respectively. Similarly for STI we determined an experimental buoyant molecular mass of 5,118 Da and extinction coefficients of 8,870 and 54,090 M−1cm−1 at 250 and 230 nm, respectively. The buoyant molecular masses result in slightly larger effective partial specific volumes of 0.741 (α-chymotrypsin) and 0.735 (STI) cm3g−1 than calculated. Also the ratios of the extinction coefficients at 280 and 250 nm (ε280/ε250) of 2.54 (α-chymotrypsin) and 1.94 (STI) allow for a spectral resolution of these two species based on their different amino acid content. This is not the case for data collected at 230 nm as essentially indistinguishable ε280/ε230 ratios of 0.323 and 0.318 are determined for the α-chymotrypsin and STI, respectively. These particular ratios differ significantly from those determined on either a Nanodrop ND-1000 or Agilent 8453 spectrophotometer (Table 1), presumably due to the different optical geometries of the absorption system [71] and the fact that 230 nm is on a steep part of the protein absorbance curve. For this reason, along with the fact that poorer fits were consistently obtained for data collected at 230 nm, we chose not to consider the 230 nm absorbance data in the analysis.
Table 1.
Optical absorbance properties of STI and α-chymotrypsina
| Protein | ε280/ε250 | ε280/ε230 | ||||
|---|---|---|---|---|---|---|
| XLA/XLI | ND1000 | AG8453 | XLA/XLI | ND1000 | AG8453 | |
| Chymotrypsin | 2.54 | 2.62 | 2.83 | 0.323 | 0.336 | 0.225 |
| STI | 1.94 | 2.20 | 2.23 | 0.318 | 0.212 | 0.180 |
Optical absorbance properties of STI and α-chymotrypsin obtained from a mass conservation multi-signal analysis on the XL-A and XL-I analytical ultracentrifuges. These are compared to A280/A250 and A280/A230 ratios obtained for protein stock solutions (ND1000) and serial dilutions (AG8453) on Nanodrop ND-1000 and Agilent 8453 spectrophotometers.
3.2 Interacting system
3.2.1 Experimental setup
STI binds two molecules of α-chymotrypsin with similar affinities corresponding to a Kd of approximately 1 μM depending on the temperature, pH and ionic strength [60 – 62]. Based on this we designed sedimentation equilibrium experiments for the XL-A with six STI loading concentrations ranging from 0.7 to 8.0 μM and 1.7 stoichiometric equivalents of α-chymotrypsin. These concentrations, prepared by dilution of an STI: α-chymotrypsin stock, cover the linear 0 – 1.5 optical density range when combining 280 and 250 nm absorbance data. In designing the experiments care is taken to use a concentration series at a fixed stoichiometric ratio of components and/or a series of samples having different loading ratios of the components. The concentrations used should be such that the sedimentation equilibrium gradients will encompass a range both above and below the expected Kd of the interaction. Furthermore, loading ratios should be such that one component does not overwhelm – precisely-determined stoichiometric ratios of approximately 2:1, 1:1 and 1:2 are typically used. In instances where the complex can be purified at high concentrations by methods such as size exclusion chromatography, a dilution series may be used to prepare samples. As in the case of the single species analysis, experimental data collected on the XL-A were sorted in SEDFIT and assembled into SEDPHAT. Experiment files corresponding to the same channel were linked in terms of their sample concentration and cell meniscus and bottom positions, with the cell meniscus fixed at a position corresponding to the optical artifact where the meniscus is customarily found. Data were analyzed in SEDPHAT using an ‘A + B + B <-> {AB} + B <-> ABB; with two symmetric sites, macroscopic K’ model implementing mass conservation and a small baseline correction for each rotor speed. Parameters such as the buoyant molecular masses of A and B, along with their extinction coefficients determined from the single species analysis were fixed. The STI concentrations were initialized to values corresponding to those expected based on the A280 measured spectrophotometrically prior to the experiment and the ratio of the α-chymotrypsin to STI was fixed at 1.7. Both the initial STI concentrations and value of the cell bottom were allowed to float as part of soft mass conservation. The value of the parameter log10[Ka2/Ka1] was fixed at −0.60206, based on the assumption that the two binding sites on STI have identical affinities for α-chymotrypsin and the binding of one α-chymotrypsin species does not affect the binding of the other (i.e. no cooperativity). Using a combination of simplex, Marquardt-Levenberg and simulated annealing minimization routines a best fit parameter log10[Ka1] of 5.79 was obtained with rmsd ranging from 0.0033 to 0.0129 for each of the experiment files and an overall reduced χ2 of 2.45. In order to improve on the fit TI noise was implemented; we found that the TI noise was essentially flat for all experimental files resulting in a log10[Ka1] of 5.87 ± 0.13 - excellent fits were observed with rmsd ranging from 0.0023 to 0.0108 and an overall reduced χ2 of 1.72 (Figure 3A).
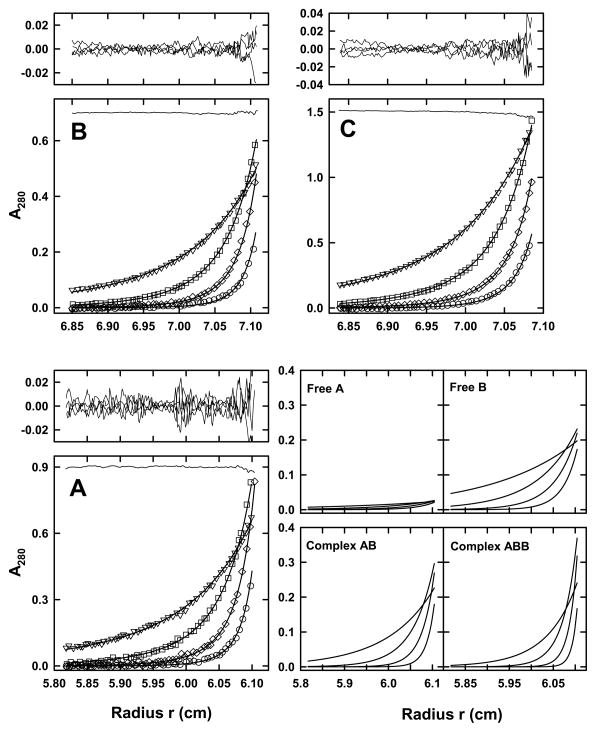
Figure 3. STI and α-chymotrypsin interact to form 1:1 and 1:2 complexes.
(A) Sedimentation equilibrium data for a mixture of 2.5 μM STI and 1.7 molar equivalents of α-chymotrypsin shown as a distribution of A280 at equilibrium. Data were collected at 10.0°C and rotor speeds of 17 (triangles), 25 (squares), 33 (diamonds) and 41 (circles) krpm. The results are analyzed globally along with data collected at 280 nm and various other loading concentrations in terms of an A + B + B <-> AB + B <-> ABB model using mass conservation, as described in the text. Best fits are shown as a line through the experimental points and the combined residuals are shown above the plot. A best fit rmsd of 0.0072 absorbance units is obtained; the TI noise profile is shown shifted by +0.9 absorbance units. For clarity only every third experimental data point is shown. The panels on the right show the contributions of each of the free species and complexes to the overall sedimentation equilibrium profiles at the various rotor speeds. (B) Sedimentation equilibrium data for a mixture of 3.3 μM STI and 0.85 molar equivalents of α-chymotrypsin shown as a distribution of A280 at equilibrium. Data were collected and analyzed as in (A). Best fits are shown as a line through the experimental points and the combined residuals are shown above the plot. A best fit rmsd of 0.0047 absorbance units is obtained; the TI noise profile is shown shifted by +0.7 absorbance units. (C) Sedimentation equilibrium data for a mixture of 14.6 μM STI and 0.42 molar equivalents of α-chymotrypsin shown as a distribution of A280 at equilibrium. Data were collected and analyzed as in (A). Best fits are shown as a line through the experimental points and the combined residuals are shown above the plot. A best fit rmsd of 0.0075 absorbance units is obtained; the TI noise profile is shown shifted by +1.5 absorbance units.
It has been noted that the buoyant molecular masses of species A and B were fixed in the analysis – this is achieved by setting the partial specific volume in the experimental parameters box to zero and using values of the buoyant molecular masses for the molecular mass parameters in the global analysis parameters window. In this manner SEDPHAT calculates correct values for the buoyant masses of species A, B and their complexes. An alternate approach would be to use a fixed, non-zero value for the partial specific volume (v*) in all the experiment files and adjust the molecular masses of species A and B to MA′ and MB′, respectively, such that the products MA′(1 − v*ρ) and MB′(1 − v*ρ) correspond to the experimentally determined buoyant molecular masses for species A and B, respectively.
3.2.2 TI and RI noise
The TI noise is a radially dependent baseline common to each experimental multi-rotor speed data set and is used to account for systematic noise (e.g. spikes and other features) in the data. Such noise usually arises from dust or scratches on the windows in the case of absorption optics, or imperfections on any optical surface in interference optical data [52]. The use of TI noise needs to be implemented judiciously as this can become highly correlated with the model used. In the case of absorbance data, we generally implement this noise parameter once data have been fit in terms of the best model - care is also taken to ensure that this noise correction is essentially flat, except when it accounts for spikes clearly visible in the data. Similarly, for interference data which have been corrected using water blanks, the TI noise profiles need to be essentially flat and featureless [52]. As in the case of the single species analysis, we utilized a time dependent, radial independent noise (RI noise) correction, which accounts for flat baselines that depend on the rotor speed. This is done to account for the residuals which are offset vertically relative to each other when data are analyzed using a common baseline parameter for each file. These corrections are typically small and usually necessary when multi-wavelength analyses are carried out.
3.2.3 Validation and model refinement
The experimental log10[Ka1] value of 5.87 corresponds to dissociation constants Kd1 of 1.34 (+ 0.45, − 0.35) μM and Kd2 of 5.4 (+ 1.8, − 1.4) μM, in broad agreement with previously published values [60 – 62]. As the determination of the equilibrium constants requires populations of both free and complex species, it is essential to test whether all such species are present in solution. Using the ‘Show distribution of extra free or complex species’ option in the ‘Display’ sub-menu of SEDPHAT we confirmed that all of the A, B, AB and BAB species are populated in this analysis (Figure 3A). Due to the very different extinction coefficients at 280 nm, the overall contribution of free STI is small compared to that of either the free α-chymotrypsin or any of the complexes. This would usually represent a problem for data analysis, but mass conservation ensures that the overall ratio of α-chymotrypsin to STI is fixed at 1.7. In order to account for possible pipetting errors in the preparation of the 1.7:1 α-chymotrypsin:STI stock solution, the analysis was further refined by floating the ratio of B to A from the initial value of 1.7. This returns a statistically indistinguishable best fit log10[Ka1] value of 5.89 with an overall reduced χ2 of 1.72; the decrease in the best fit B:A ratio of 1.63 (i.e. 96% of the original value) is compensated for by slight increases of the total STI concentrations.
The analyses carried out so far do not consider the possibility that fractions of either the STI or α-chymotrypsin are incompetent for binding. Static light scattering studies carried out in phosphate buffered saline (pH = 7.2) at 20.0°C returned a best fit log10[Ka1] of 6.2 ± 0.1 [61]; however better fits were obtained with a log10[Ka1] of 6.8 ± 0.3 and 30% of the α-chymotrypsin incompetent for binding. Similarly, dynamic light scattering studies carried out at 25.0°C in 0.2M sodium chloride and 0.05M sodium phosphate (pH = 6.7) return a log10[Ka1] of 6.3 ± 0.1 with 38% of the α-chymotrypsin inactive for binding [62]. The log10[Ka1] value of 5.9 ± 0.2 at 10.0°C, assumes that the proportion of incompetent α-chymotrypsin remains unchanged. In view of this, data were reanalyzed by also fitting for the fraction of incompetent value of α-chymotrypsin. Using only RI noise and fixing the molar fraction of α-chymotrypsin:STI at 1.7, the best fit analysis returns a value of 5.7% for the amount of incompetent α-chymotrypsin with a log10[Ka1] of 5.90 and a reduced χ2 of 2.37. Implementation of TI noise results in no incompetent α-chymotrypsin from which we conclude that no more than 6% of the α-chymotrypsin is incompetent for binding to STI, consistent with the original observation that at least 94% of the α-chymotrypsin is active for STI binding at pH 6.7 [72].
3.2.4 Sedimentation equilibrium analysis is precise and robust
The experiments described so far were carried out using nearly stoichiometric ratios of STI and α-chymotrypsin. Analysis of sedimentation equilibrium data for interacting systems is usually more challenging when mixtures are far from the preferred stoichiometry [58]. However, both mass conservation and the multi-wavelength analysis aid tremendously in addressing this issue. To demonstrate this we carried out four more sets of experiments in the presence of limiting amounts of α-chymotrypsin; data analysis carried out in a manner analogous to that described above led to excellent fits (Figures 3B and C) resulting in essentially identical values of log10[Ka1] (Figure 4). Altogether an average log10[Ka1] of 5.80 ± 0.14 corresponding to a Kd1 of 1.6 μM is obtained, demonstrating that the combined soft mass conservation and multi-wavelength analysis lead to high precision in the determination of the value of log10[Ka1]. As in the first study, there was no evidence for any incompetent fraction of α-chymotrypsin in all cases. For the sake of simplicity the model implemented assumes that both α-chymotrypsin binding sites are symmetric and equivalent. However, studies by Bösterling and Quast [73] have shown that these two binding sites are in fact not equivalent and that one site binds to trypsin with high affinity (Kd ~ 3 pM). By blocking this tryptic binding site, these authors demonstrate that the two α-chymotrypsin binding sites on STI have essentially the same affinity at pH 7.5 with dissociation constants of 0.9 and 0.45 μM for the tryptic and non-tryptic binding sites, respectively. In order to test for this, data were reanalyzed by removing the log10[Ka2/Ka1] = −0.60206 constraint; in all cases larger values of this parameter were obtained resulting in an average log10[Ka1] of 5.4 ± 0.3 and log10[Ka2/Ka1] of 0.0 ± 0.4, corresponding to Kd1 and Kd2 values of 4 (+3, −2) and 4 (+16, −3) μM, respectively. Even though this led to better data fits the overall reduced χ2 obtained were not statistically distinguishable from the best fits with log10[Ka2/Ka1] of −0.60206 ( i.e. the 95% confidence limits on χ2 overlapped with the χ2 observed in the latter case), illustrating the importance of testing for the statistical significance of incremental improvements to a model used for data fitting.
3.2.5 Importance of mass conservation
In this analysis, we implemented both mass conservation and a multi-signal analysis in order to determine a precise value of the equilibrium constant. Of these constraints, mass conservation is the most important - a global analysis of only the 280 nm absorbance data with mass conservation led to identical results. In the case of the mixtures containing 1.7 molar equivalents of α-chymotrypsin to STI a log10[Ka1] value of 5.90 ± 0.15 was obtained with excellent data fits - rmsd values ranged from 0.0023 to 0.0090 with an overall reduced χ2 of 1.45. The value of log10[Ka1] is identical to that obtained earlier. Similar observations are made for data sets in which different loading ratios of α-chymotrypsin to STI are used, resulting in an average log10[Ka1] of 5.76 ± 0.12. This is indistinguishable from the value of 5.80 ± 0.14 obtained with the multi-wavelength analysis. Removal of the mass conservation constraints for the 280 nm data set with 1.7 molar equivalents of α-chymotrypsin also leads to a best fit log10[Ka1] of 5.90 with a reduced χ2 of 1.19 – a value obtained by using the best-fit mass conservation parameters as initial estimates. In an attempt to define the error limits of log10[Ka1] we found that the error surface along this parameter was rather shallow with various local minima depending on the now uncorrelated values of cA(ro) and cB(ro) at each rotor speed. Furthermore, data fitting using non-optimized parameters resulted in fits which represented local minima on the error surface, illustrating the critical role that mass conservation plays as a constraint in shaping the error surface and guiding the fit towards the global error minimum. As the radial position for the cell bottom rb is one parameter which is floated in mass conservation, we found it instructive to evaluate the values obtained from the best-fits - in all cases the value of rb was part of the optical artifact observed for the cell bottom. In addition, the values obtained from all data sets covered a rather narrow range with averages corresponding to 6.162, 6.658 and 7.151 cm for the inner, middle and outer channels, respectively, of the six channel centerpiece. The standard errors of the averages were very small, each less than 0.005 cm, noting however that a small change in the value of rb will result in a disproportionate change in the value of the total loading concentrations.
3.2.6 Model testing using sedimentation equilibrium
Based on previously published reports [61 – 62] sedimentation equilibrium data were analyzed in terms of an A + B + B ⇌ AB + B ⇌ ABB system with two symmetric binding sites on A. The validity of this model was verified by sedimentation equilibrium based on the excellent fits observed and reproducibility of published data. Unlike this particular example, it is now customary to also analyze the interacting system by sedimentation velocity – as in sedimentation equilibrium this method has the ability to determine the number and size of macromolecular complexes, as well as provide a measure of the interaction affinities [6, 9–12, 14]. In this manner one is able to rapidly assess the model required for the analysis of the sedimentation equilibrium data and design the appropriate experiments (i.e. loading stoichiometries and concentrations) prior to data collection. This is particularly helpful in the absence of any prior knowledge of the interacting system. However, before the recent advances in sedimentation velocity, sedimentation equilibrium experiments were routinely used to distinguish between various models [43, 55, 56, 74, 75]. For example, using data collected for mixtures containing 1.7 molar equivalents of α-chymotrypsin to STI clearly we demonstrate that system cannot be modeled in terms of an A + B ⇌ AB equilibrium. A multi-signal, mass conservation analysis in terms of this model returns poor data fits with an overall reduced χ2 of 4.49 and rmsd values for each experimental file ranging from 0.0026 to 0.021. The residuals were clearly systematic and some of the TI noise profiles were curved, indicating that these are highly correlated to the choice of model (data not shown). The value of log10[Ka1] obtained is 10, indicative of saturation of the STI binding site - this observation does not correlate with preliminary analyses showing that the weight average molecular mass increases as the loading STI concentrations of the mixture are increased from 0.7 to 8.0 μM. To discount possible correlations from the TI noise, data were analyzed using only RI noise to obtain poorer fits with an overall reduced χ2 of 11.67 and a log10[Ka1] of 10 once again demonstrating that the A + B ⇌ AB model is not valid. A similar analysis in terms of an A + B + B + B ⇌ AB + B + B ⇌ ABB + B ⇌ ABBB where A has three symmetric binding sites for B, however leads to excellent fits. Constraining the ratios of log10[Ka2/Ka1] and log10[Ka3/Ka1] to −0.47710 and −0.95420, respectively (i.e. no cooperativity) results in an overall reduced χ2 of 1.70 and a log10[Ka1] of 5.62. This particular fit is statistically indistinguishable from the correct best fit for the A + B + B ⇌ AB + B ⇌ ABB interacting system. Inspection of the data, however, shows that the ABBB species is not populated at the two lowest loading concentrations indicating that one would need to collect data at even higher concentrations to really distinguish between these models solely by sedimentation equilibrium.
4 Conclusions
Sedimentation equilibrium is a highly technical method requiring great care both in the experimental design and data analysis, providing in return results that are both robust and precise. In the case of interacting systems, the overall signal representing the concentration gradients generated in the gravitational field contains contributions from all of the free and complex species. The study of interacting systems, namely the determination of the complex stoichiometries and binding affinities, therefore necessitates the deconvolution of the exponential contributions for each of the interacting species. This is achieved through the appropriate experimental design, the use of a multi-signal analysis and judicious choice of wavelengths, and the implementation of mass conservation. As demonstrated for the α-chymotrypsin and STI interaction, this requires multiple experiments covering a broad concentration range and/or different loading ratios of the components such that all species are appropriately populated. In this manner it is also possible to distinguish between the various possible models that can be used to describe the interacting system.
It is critical that each of the interacting species be purified to homogeneity and matched to the required buffer using either size exclusion chromatography and/or dialysis. As sedimentation equilibrium is particularly sensitive to the presence of impurities and traces of non-specific aggregates, it is necessary to characterize the individual components by sedimentation velocity. In many ways, sedimentation velocity is complementary to sedimentation equilibrium and the combination of both methods can be extremely valuable in determining the best model to describe the interaction. In our laboratory we now routinely combine both techniques to characterize the individual components, as well as study the interacting system. Based on recent, unpublished studies of three self-associating proteins we found that identical results (within the 95% confidence limits) are obtained by sedimentation equilibrium and sedimentation velocity, using weight average sedimentation coefficient isotherms derived from c(s) analyses [6, 9, 76]. In fact, sedimentation velocity can be particularly useful when one of the interacting components contains trace amounts of impurities that do not interact with the other component. The contributions of the impurities can be readily discounted when constructing weight average sedimentation coefficient or population isotherms from the continuous c(s) distributions. Alternatively, partial boundary modeling of the sedimentation velocity profiles can be implemented [14]. In a sedimentation equilibrium experiment these impurities will also contribute to the concentration gradient and their contribution may be difficult to remove even though SEDPHAT allows for the inclusion of a non-participating species in the model. Conversely, we find that high speed sedimentation equilibrium is preferable over sedimentation velocity when studying the interaction of small peptides, in part due to the maximum rotor speeds accessible in the analytical ultracentrifuge.
In addition to analytical ultracentrifugation data, SEDPHAT can also analyze isothermal titration calorimetric data [77], as well as binding isotherms derived from sedimentation velocity, surface plasmon resonance and various spectroscopic methods. These can be included, along with the sedimentation equilibrium data, as part of a global analysis placing further constraints and extending the number of useful models that can be analyzed in this manner. For example, we have analyzed the interaction between α-chymotrypsin and STI in terms of an A + B + B ⇌ AB + B ⇌ ABB system, where A has two symmetrical sites for B. A similar model with two non-symmetric sites in which the microscopic K1 and K2 values are determined would have perhaps been more appropriate, but this analysis would require input from a biophysical method that can distinguish between complexes AB and BA. Such data could be obtained from sedimentation velocity or isothermal titration calorimetry and modeled globally in SEDPHAT in conjunction with sedimentation equilibrium data, thus illustrating the versatility of this method.
Overall, sedimentation equilibrium is a very sensitive and precise method that can be used in the analysis of various macromolecular interactions - as in other methods, the accuracy of the method is dependent on the accuracy of the extinction coefficients or signal increments ε used to determine concentrations. Furthermore, the limits of the affinities that can be measured by this method are determined by the extinction coefficient or signal increment ε and the onset of thermodynamic and hydrodynamic non-ideality. Practically this translates to affinities of the order of 100 nM (Kd ~ 1/(10ε)) through to the low mM range for protein-protein and protein-nucleic acid interactions. Binding affinities of the order of 1 – 10 μM, as demonstrated in this work, are particularly well-suited for study by sedimentation equilibrium. Other advantages afforded by this particular study include the unlimited availability of essentially pure materials, ideally sedimenting macromolecules and the use of buffer solutes that do not interact with the proteins of interest. Even though recombinant protein expression usually affords more than sufficient material for the study of biologically important macromolecular interactions, there may be instances in which reagent quantities will be quite limiting. In these cases it may be possible to carry out short column sedimentation equilibrium experiments [78]. Furthermore, we have only considered macromolecular solutions that behave ideally, in part because of the low sample concentrations and the presence of sufficient salt to counteract long rang electrostatic interactions. Formalisms have been developed to account for both thermodynamic and hydrodynamic non-ideality in the study of interacting systems by analytical ultracentrifugation [79 – 82]. In addition to behaving ideally, the α-chymotrypsin and STI do not appear to interact appreciably with any of the buffer components and Equation 1.3 holds. As noted in section 1.1.1, in the case of charged polyelectrolytes, such as nucleic acids the B1 and B3 terms can be incorporated into an experimentally determined effective partial specific volume φ’ [26 – 30]. The subsequent analysis of the interaction implicitly assumes that neither B1 nor B3 change significantly upon complex formation. This may not hold true in the case of membrane proteins solubilized using detergents [83 – 86] - the B3 term reflecting the amount of bound detergent may change upon complex formation, thus complicating the data analysis. Even in cases where density matching is employed, namely B3(1 − v3ρ) is zero by virtue of an experimental buffer density such that v3ρ equals to one, interaction affinities may also depend on the detergent concentration used [87 – 88].
Acknowledgments
This work was supported by the intramural research program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases. The author thanks Dr. Marie-Paule Strub for a careful reading of the manuscript and Dr. Robert Craigie for assistance with the N-terminal protein sequencing. The author also thanks Dr. Gary Felsenfeld and members of his laboratory for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Phizicky EM, Fields S. Protein-protein interactions: Methods for detection and analysis. Microbiol Rev. 1995;59:94–123. doi: 10.1128/mr.59.1.94-123.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svedberg T. The ultra-centrifuge and the study of high-molecular compounds. Nature. 1937;139:1051–1062. [Google Scholar]
- 3.Schachman HK. Ultracentrifugation in biochemistry. Academic Press; New York: 1959. [Google Scholar]
- 4.van Holde KE, Hansen JC. Analytical ultracentrifugation from 1924 to the present: A remarkable history. Chemtracts-Biochem Mol Biol. 1998;11:933–943. [Google Scholar]
- 5.Schuck P. Size distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys J. 2000;78:1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuck P. On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal Biochem. 2003;320:104–124. doi: 10.1016/s0003-2697(03)00289-6. [DOI] [PubMed] [Google Scholar]
- 7.Correia JJ, Alday PH, Sherwood P, Stafford WF. Effect of kinetics on sedimentation profiles and the roles of intermediates. Methods Enzymol. 2009;467:135–161. doi: 10.1016/S0076-6879(09)67006-3. [DOI] [PubMed] [Google Scholar]
- 8.Harding SE, Rowe AJ. Insight into protein-protein interactions from analytical ultracentrifugation. Biochem Soc Trans. 2010;38:901–907. doi: 10.1042/BST0380901. [DOI] [PubMed] [Google Scholar]
- 9.Schuck P. Sedimentation patterns of rapidly reversible protein interactions. Biophys J. 2010;98:2005–2013. doi: 10.1016/j.bpj.2009.12.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuck P. Diffusion of the reaction boundary of rapidly interacting macromolecules in sedimentation velocity. Biophys J. 2010;98:2741–2751. doi: 10.1016/j.bpj.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dam J, Schuck P. Sedimentation velocity analysis of heterogenous protein-protein interactions: sedimentation coefficient distributions c(s) and asymptotic boundary profiles from Gilbert-Jenkins theory. Biophys J. 2005;89:651–666. doi: 10.1529/biophysj.105.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dam J, Velikovsky CA, Mariuzza RA, Urbanke C, Schuck P. Sedimentation velocity analysis of heterogenous protein-protein interactions: Lamm equation modeling and sedimentation coefficient distributions c(s) Biophys J. 2005;89:619–634. doi: 10.1529/biophysj.105.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown PH, Schuck P. Macromolecular size-and-shape distributions by sedimentation velocity analytical ultracentrifugation. Biophys J. 2006;90:4651–4661. doi: 10.1529/biophysj.106.081372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown PH, Balbo A, Schuck P. On the analysis of sedimentation velocity in the study of protein complexes. Eur Biophys J. 2009;38:1079–1099. doi: 10.1007/s00249-009-0514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown PH, Schuck P. A new adaptive grid-size algorithm for the simulation of sedimentation velocity profiles in analytical ultracentrifugation. Comput Phys Commun. 2008;178:105–120. doi: 10.1016/j.cpc.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLellan JS, Zheng X, Hauk G, Ghirlando R, Beachy PA, Leahy DJ. The mode of Hedgehog binding to Ihog homologues is not conserved across different phyla. Nature. 2008;455:979–983. doi: 10.1038/nature07358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonçalves KA, Borges JC, Silva JC, Papa PF, Bressan GC, Torriani IL, Kobarg J. Solution structure of the human signaling protein RACK1. BMC Structural Biology. 2010;10:15. doi: 10.1186/1472-6807-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder GA, Ford J, Torabi-Parizi P, Arthos JA, Schuck P, Colonna M, Sun PD. Characterization of DC-SIGN/R interaction with human immunodeficiency virus type I gp120 and ICAM molecules favors the receptor’s role as an antigen-capturing rather than an adhesion receptor. J Virol. 2005;79:4589–4598. doi: 10.1128/JVI.79.8.4589-4598.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmore MJ, Bieber Urbauer RJ, Minakhin L, Akoyev V, Zolkiewski M, Severinov K, Urbauer JL. Determinants of affinity and activity of the anti-sigma factor AsiA. Biochemistry. 2010;49:6143–6154. doi: 10.1021/bi1002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali SA, Iwabuchi N, Matsui T, Hirota K, Kidokoro S, Arai M, Kuwajima K, Schuck P, Arisaka F. Reversible and fast association equilibria of a molecular chaperone, gp57A, of bacteriophage T4. Biophys J. 2003;85:2606–2618. doi: 10.1016/s0006-3495(03)74683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nöllmann M, Byron O, Stark WM. Behavior of Tn3 resolvase in solution and its interaction with res. Biophys J. 2005;89:1920–1931. doi: 10.1529/biophysj.104.058164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsey JE, Daugherty MA, Kelm RJ. Hydrodynamic studies in the quaternary structure of recombinant mouse Purβ. J Biol Chem. 2007;282:1552–1560. doi: 10.1074/jbc.M609356200. [DOI] [PubMed] [Google Scholar]
- 23.Pesiridis GS, Diamond E, Van Duyne GD. Role of pICLn in methylation of Sm proteins by PRMT5. J Biol Chem. 2009;284:21347–21359. doi: 10.1074/jbc.M109.015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudry C, Plested AJ, Schuck P, Mayer ML. Energetics of glutamate receptor ligand binding domain dimer assembly are modulated by allosteric ions. Proc Natl Acad Sci USA. 2009;106:12329–12334. doi: 10.1073/pnas.0904175106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudry C, Weston MC, Schuck P, Rosenmund C, Mayer ML. Stability of ligand-binding domain dimer assembly controls kainite receptor desensitization. EMBO J. 2009;28:1518–1530. doi: 10.1038/emboj.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowotny M, Cerritelli SM, Ghirlando R, Gaidamakov SA, Crouch RJ, Yang W. Specific recognition of RNA/DNA hybrid and enhancement of human RNase H1 activity by HBD. EMBO J. 2008;27:1172–1181. doi: 10.1038/emboj.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowotny M, Gaidamakov SA, Ghirlando R, Cerritelli SM, Crouch RJ, Yang W. Structure of a human RNase H1 complexed with an RNA/DNA hybrid: insight into HIV reverse transcription. Mol Cell. 2007;28:264–276. doi: 10.1016/j.molcel.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Leonard JN, Ghirlando R, Askins J, Bell JK, Margulies DH, Davies DR, Segal DM. The TLR3 signaling complex forms by cooperative receptor dimerization. Proc Natl Acad Sci USA. 2008;105:258–263. doi: 10.1073/pnas.0710779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carroll KL, Ghirlando R, Ames JM, Corden JL. Interaction of yeast RNA-binding proteins Nrd1 and Nab3 with RNA polymerase II terminator elements. RNA. 2007;13:361–373. doi: 10.1261/rna.338407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh K, Guo F, Van Duyne G. Synapsis of loxP sites by Cre recombinase. J Biol Chem. 2007;282:24004–24016. doi: 10.1074/jbc.M703283200. [DOI] [PubMed] [Google Scholar]
- 31.Minton AP. Quantitative characterization of reversible molecular associations via analytical ultracentrifugation. Anal Biochem. 1990;190:1–6. doi: 10.1016/0003-2697(90)90125-s. [DOI] [PubMed] [Google Scholar]
- 32.Behlke J, Ristau O. Sedimentation equilibrium: a valuable tool to study homologous and heterogeneous interactions of proteins and proteins and nucleic acids. Eur Biophys J. 2003;32:427–431. doi: 10.1007/s00249-003-0318-7. [DOI] [PubMed] [Google Scholar]
- 33.Balbo A, Brown PH, Braswell EH, Schuck P. Measuring protein-protein interactions by equilibrium sedimentation. Curr Protocols Immunol. 2007:18.8.1–18.8.28. doi: 10.1002/0471142735.im1808s79. [DOI] [PubMed] [Google Scholar]
- 34.Stafford WF. Protein-protein and ligand-protein interactions studied by analytical ultracentrifugation. In: Shriver JW, editor. Protein Structure, Stability and Interactions. Vol. 490. Humana Press; 2009. pp. 83–113. [DOI] [PubMed] [Google Scholar]
- 35.Lebowitz J, Lewis MS, Schuck P. Modern analytical ultracentrifugation in protein science: a tutorial review. Protein Sci. 2002;11:2067–2079. doi: 10.1110/ps.0207702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisenberg H. Biological macromolecules and polyelectrolytes in solution. Clarendon Press; Oxford: 1976. [Google Scholar]
- 37.Casassa EF, Eisenberg H. Thermodynamic analysis of multicomponent solutions. Adv Protein Chem. 1964;19:287–395. doi: 10.1016/s0065-3233(08)60191-6. [DOI] [PubMed] [Google Scholar]
- 38.Ebel C, Eisenberg H, Ghirlando R. Probing protein-sugar interactions. Biophys J. 2000;78:385–393. doi: 10.1016/S0006-3495(00)76601-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tardieu A, Vachette P, Gulik A, le Maire M. Biological macromolecules in solvents of variable density: characterization by sedimentation equilibrium, densimetry, and X-ray forward scattering and an application to the 50S ribosomal subunit from Escherichia coli. Biochemistry. 1981;20:4399–4406. doi: 10.1021/bi00518a026. [DOI] [PubMed] [Google Scholar]
- 40.Cole JL, Lary JW, Moody TP, Laue TM. Analytical ultracentrifugation: sedimentation velocity and sedimentation equilibrium. Methods Cell Biol. 2008;84:143–179. doi: 10.1016/S0091-679X(07)84006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbons RA. Physico-chemical methods for the determination of the purity, molecular size and shape of glycoproteins. In: Gottschalk A, editor. Glycoproteins Part A. 2. Elsevier; Amsterdam: Elsevier; Amsterdam: 1972. pp. 128–140. [Google Scholar]
- 42.Durchschlag H. Specific volumes of biological macromolecules and some other molecules of biological interest. In: Hinz HJ, editor. Thermodynamic data for biochemistry and biotechnology. Springer Verlag; Berlin: 1986. pp. 45–128. [Google Scholar]
- 43.Ghirlando R, Keown MB, Mackay GA, Lewis MS, Unkeless JC, Gould HJ. Stoichiometry and thermodynamics of the interaction between the Fc fragment of human IgG1 and its low affinity receptor FcγRIII. Biochemistry. 1995;34:13320–13327. doi: 10.1021/bi00041a007. [DOI] [PubMed] [Google Scholar]
- 44.Shire SJ. Beckman publication DS-837. 1992. Determination of molecular weight of glycoproteins by analytical ultracentrifugation. [Google Scholar]
- 45.Hoiland H. Partial molar volumes of biochemical model compounds in aqueous solution. In: Hinz HJ, editor. Thermodynamic data for biochemistry and biotechnology. Springer Verlag; Berlin: 1986. pp. 17–44. [Google Scholar]
- 46.Durchschlag H, Zipper P. Calculation of the partial volume of organic compounds and polymers. Progress Coll Polymer Sci. 1994;94:20–39. [Google Scholar]
- 47.Durchschlag H, Zipper P. Calculation and partial specific volumes and other volumetric properties of small molecules and polymers. J Appl Crystal. 1997;30:803–807. [Google Scholar]
- 48.Cohen G, Eisenberg H. Deoxyribonucleotide solutions: sedimentation in a density gradient, partial specific volumes, density and refractive index increments, and preferential interactions. Biopolymers. 1968;6:1077–1100. doi: 10.1002/bip.1968.360060805. [DOI] [PubMed] [Google Scholar]
- 49.Eisenberg H. Solution properties of DNA: viscometry, sedimentation and light scattering. In: Saenger W, editor. Landolt Börnstein New Series Biophysics - Nucleic Acids 7/1c. Springer Verlag; Berlin: 1990. pp. 257–276. [Google Scholar]
- 50.Weber G. Energetics of ligand binding to proteins. Adv Prot Chem. 1975;29:1–83. doi: 10.1016/s0065-3233(08)60410-6. [DOI] [PubMed] [Google Scholar]
- 51.Provencher SW. Inverse problems in polymer characterization: analysis of polydisperdsity with photon correlation spectroscopy. Makromol Chem. 1979;180:201–209. [Google Scholar]
- 52.Vistica J, Dam J, Balbo A, Yikilmaz E, Mariuzza RA, Rouault TA, Schuck P. Sedimentation equilibrium analysis of protein interactions with global implicit mass conservation constraints and systematic noise decomposition. Anal Biochem. 2004;326:234–256. doi: 10.1016/j.ab.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Balbo A, Schuck P. Analytical ultracentrifugation in the study of protein self-association and heterogeneous protein-protein interactions. In: Golemis EA, Adams PD, editors. Protein-protein interactions. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2005. pp. 253–277. [Google Scholar]
- 54.Laue TM. Beckman publication A-1821A. 1996. Choosing which optical system of the Optima™ XL-I analytical ultracentrifuge to use. [Google Scholar]
- 55.Becerra SP, Kumar A, Lewis MS, Widen SG, Abbotts J, Karawya EM, Hughes SH, Shiloach J, Wilson SH. Protein-protein interactions of HIV-1 reverse transcriptase: implication of central and C-terminal regions in subunit binding. Biochemistry. 1991;30:11707–11719. doi: 10.1021/bi00114a015. [DOI] [PubMed] [Google Scholar]
- 56.Lewis MS, Youle RJ. Ricin subunit association: Thermodynamic and the role of the disulfide bond in toxicity. J Biol Chem. 1986;261:11571–11577. [PubMed] [Google Scholar]
- 57.Roark D. Sedimentation equilibrium techniques: Multispeed analyses and an overspeed procedure. Biophys Chem. 1976;5:185–196. doi: 10.1016/0301-4622(76)80034-8. [DOI] [PubMed] [Google Scholar]
- 58.Philo JS. Sedimentation equilibrium analysis of mixed associations using numerical constraints to impose mass or signal conservation. Methods Enzymol. 2000;321:100–120. doi: 10.1016/s0076-6879(00)21189-0. [DOI] [PubMed] [Google Scholar]
- 59.Johnson ML, Frasier SG. Non-linear least square analysis. Methods Enzymol. 1985;117:301–342. [Google Scholar]
- 60.Quast U, Steffen E. The soybean trypsin inhibitor (Kunitz) is a doubleheaded inhibitor. Hoppe-Seyler’s Z Physiol Chem. 1975;356:617–620. [PubMed] [Google Scholar]
- 61.Attri AK, Minton AP. Composition gradient static light scattering: A new technique for rapid detection and quantitative characterization of reversible macromolecular hetero-associations in solution. Anal Biochem. 2005;346:132–138. doi: 10.1016/j.ab.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 62.Hanlon AD, Larkin MI, Reddick RM. Free-solution, label-free protein-protein interactions characterized by dynamic light scattering. Biophys J. 2010;98:297–304. doi: 10.1016/j.bpj.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar S, Hein GE. Concerning the mechanism of autolysis of α-chymotrypsin. Biochemistry. 1907;9:291–297. doi: 10.1021/bi00804a015. [DOI] [PubMed] [Google Scholar]
- 64.Hofstee BHJ. The rate of chymotrypsin autolysis. Arch Biochem Biophys. 1965;112:224–232. [Google Scholar]
- 65.Zhao H, Brown PH, Balbo A, Fernandez-Alonso MD, Polishchuck N, Chaudhry C, Mayer ML, Ghirlando R, Schuck P. Accounting for solvent signal offsets in the analysis of interferometric sedimentation velocity data. Macromol Biosci. 2010;10:736–745. doi: 10.1002/mabi.200900456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilcox PE, Kraut J, Wade RD, Neurath H. The molecular weight of α-chymotrypsinogen. Biochim Biophys Acta. 1957;24:72–78. doi: 10.1016/0006-3002(57)90147-6. [DOI] [PubMed] [Google Scholar]
- 67.Perlmann GE, Longsworth LG. The specific refractive increment of some purified proteins. J Am Chem Soc. 1948;70:2719–2724. doi: 10.1021/ja01188a027. [DOI] [PubMed] [Google Scholar]
- 68.Morimoto K, Kegeles G. Dimerization and activity of chymotrypsin at pH 4. Biochemistry. 1967;6:3007–3010. doi: 10.1021/bi00862a006. [DOI] [PubMed] [Google Scholar]
- 69.Wu YV, Scheraga HA. Studies of soybean trypsin inhibitor. I Physicochemical properties Biochemistry. 1962;1:698–705. doi: 10.1021/bi00910a025. [DOI] [PubMed] [Google Scholar]
- 70.Padrick SB, Deka RK, Chuang JL, Wynn RM, Chuang DT, Norgard MV, Rosen MK, Brautigam CA. Determination of protein complex stoichiometry through multisignal sedimentation velocity experiments. Anal Biochem. 2010;407:89–103. doi: 10.1016/j.ab.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laue TM. Beckman Application Information publication A-1821A. 1996. Choosing which optical system of the Optima™ XL-I analytical ultracentrifuge to use. [Google Scholar]
- 72.Fersht AR. Conformational equilibria in α- and δ-chymotrypsin: The energetics and importance of the salt bridge. J Mol Biol. 1972;64:497–509. doi: 10.1016/0022-2836(72)90513-x. [DOI] [PubMed] [Google Scholar]
- 73.Bösterling B, Quast U. Soybean trypsin inhibitor (Kunitz) is doubleheaded: Kinetics of the interaction of α-chymotrypsin with each side. Biochim Biophys Acta. 1981;657:58–72. doi: 10.1016/0005-2744(81)90130-3. [DOI] [PubMed] [Google Scholar]
- 74.Muñoz V, Ghirlando R, Blanco FJ, Jas GS, Hofrichter J, Eaton WA. Folding and aggregation kinetics of a β-hairpin. Biochemistry. 2006;45:7023–7035. doi: 10.1021/bi052556a. [DOI] [PubMed] [Google Scholar]
- 75.Bailey MF, Davidson BE, Minton AP, Sawyer WH, Howlett GJ. The effect of self-association on the interaction of the Escherichia coli regulatory protein TyrR with DNA. J Mol Biol. 1996;263:671–684. doi: 10.1006/jmbi.1996.0607. [DOI] [PubMed] [Google Scholar]
- 76.Zhi L, Mans J, Paskow MJ, Brown PH, Schuck P, Jonjić S, Natarajan K, Margulies DH. Direct interaction of the mouse cytomegalovirus m152/gp40 immunoevasin with RAE-1 isoforms. Biochemistry. 2010;49:2443–2453. doi: 10.1021/bi902130j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Houtman JCD, Brown PH, Bowden B, Yamaguchi H, Appella E, Samelson LE, Schuck P. Studying multisite binary and ternary protein interactions by global analysis of isothermal titration calorimetry data in SEDPHAT: Application to adaptor protein complexes in cell signaling. Prot Sci. 2007;16:30–42. doi: 10.1110/ps.062558507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laue TM. Beckman publication DS-835. 1992. Short column sedimentation equilibrium analysis for rapid characterization of macromolecules in solution. [Google Scholar]
- 79.Scott DJ, Winzor DJ. Comparison of methods for characterizing nonideal solute self-association by sedimentation equilibrium. Biophys J. 2009;97:886–896. doi: 10.1016/j.bpj.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scott DJ, Wills PR, Winzor DJ. Allowance for the effect of protein charge in the characterization of nonideal solute self-association by sedimentation equilibrium. Biophys Chem. 2010;149:83–91. doi: 10.1016/j.bpc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 81.Jiménez M, Rivas G, Minton AP. Quantitative characterization of weak self-association in concentrated solutions of immunoglobulin G via the measurement of sedimentation equilibrium and osmotic pressure. Biochemistry. 2007;46:8373–8378. doi: 10.1021/bi7005515. [DOI] [PubMed] [Google Scholar]
- 82.Ang S, Rowe AJ. Evaluation of the information content of sedimentation equilibrium data in self-interacting systems. Macromol Bioscience. 2010;10:798–807. doi: 10.1002/mabi.201000065. [DOI] [PubMed] [Google Scholar]
- 83.le Maire M, Arnou B, Olesen C, Georgin D, Ebel C, Møller JV. Gel chromatography and analytical ultracentrifugation to determine the extent of detergent binding and aggregation, and Stokes radius of membrane proteins using sarcoplasmic reticulum Ca2+-ATPase as an example. Nature Protocols. 2008;3:1782–1795. doi: 10.1038/nprot.2008.177. [DOI] [PubMed] [Google Scholar]
- 84.Nury H, Manon F, Arnou B, le Maire M, Pebay-Peyroula E, Ebel C. Mitochondrial bovine ADP/ATP carrier in detergent is predominantly monomeric but also forms multimeric species. Biochemistry. 2008;47:12319–12331. doi: 10.1021/bi801053m. [DOI] [PubMed] [Google Scholar]
- 85.Lu J, Sharpe S, Ghirlando R, Yau Y-M, Tycko R. Oligomerization state and supramolecular structure of the HIV-1 Vpu protein transmembrane segment in phospholipid bilayers. Protein Sci. 2010;19:1877–1896. doi: 10.1002/pro.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacKenzie KR, Fleming KG. Association energetics of membrane spanning α-helices. Curr Opinion Struct Biol. 2008;18:412–419. doi: 10.1016/j.sbi.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fleming KG. Standardizing the free energy change of transmembrane helix-helix interactions. J Mol Biol. 2002;323:563–571. doi: 10.1016/s0022-2836(02)00920-8. [DOI] [PubMed] [Google Scholar]
- 88.Fleming KG, Engelman DM. Specificity in transmembrane helix-helix interactions can define a hierarchy of stability for sequence variants. Proc Natl Acad Sci USA. 2001;98:14340–14344. doi: 10.1073/pnas.251367498. [DOI] [PMC free article] [PubMed] [Google Scholar]