Abstract
Dopaminergic compounds often affect the unlearned behaviors of preweanling and adult rats differently, although the brain regions underlying these age-dependent behavioral effects have not been specified. A candidate brain region is the dorsal caudate-putamen (CPu); thus, a goal of the present study was to determine whether D1 and D2 receptors in the dorsal CPu are capable of modulating the unlearned behaviors of preweanling rats. In Experiments 1 and 2, selective and nonselective dopamine agonists were bilaterally microinjected into the dorsal CPu on postnatal day (PD) 18 and both locomotor activity and stereotypy were measured. In Experiment 3, the functional coupling of D1 and D2 receptors was assessed by microinjecting the D1 agonist SKF-82958 and the D2/D3 agonist quinpirole either alone or in combination. In Experiments 4 and 5, quinpirole and the D1 receptor antagonist SCH-23390, or SKF-82958 and the D2 receptor antagonist raclopride, were co-administered into the dorsal CPu to further assess whether a functional D1 or D2 receptor system is necessary for the expression of quinpirole- or SKF-82958-induced behaviors. Results showed that selective stimulation of D1 or D2 receptors in the dorsal CPu increased both the locomotor activity and stereotypy of preweanling rats. Receptor coupling was evident on PD 18 because co-administration of a subthreshold dose of SKF-82958 and quinpirole produced more locomotor activity than either agonist alone. Lastly, the dopamine antagonist experiments showed that both D1 and D2 receptor systems must be functional for SKF-82958- or quinpirole-induced locomotor activity to be fully manifested. When the present data are compared to results from non-ontogenetic studies, it appears that pharmacological manipulation of D1 and D2 receptors in the dorsal CPu affects the behavior of preweanling and adult rats in a generally similar manner, although some important age-dependent differences are apparent. For example, D1 and/or D2 agonists preferentially induce locomotor activity, and not intense stereotypy, in younger animals.
Keywords: caudate-putamen, stereotypy, locomotor activity, quinpirole, SKF-82958, ontogeny
INTRODUCTION
Early studies investigating the neural substrates of unlearned motor behavior showed that the nucleus accumbens is important for mediating locomotor activity in adult rats (Plaznik et al., 1989; Delfs et al., 1990), whereas the caudate-putamen (CPu) is necessary for stereotyped behaviors (Carr and White, 1984; Kelley et al., 1988; Bordi et al., 1989). In a notable example, Staton and Solomon (1984) reported that microinjecting amphetamine into the nucleus accumbens of adult rats caused a robust increase in locomotion but not stereotypy, while infusing amphetamine into the CPu induced stereotypy but not locomotion. Although recent research typically provides results consistent with this dichotomy (Allen and Winn, 1995; Neisewander et al., 1995; Hauber and Münkle, 1997; Schildein et al., 1998; Canales et al., 2000; Ikemoto, 2002; Waszczak et al., 2002), a number of studies suggest that the behavioral specificity of striatal structures is more varied than was initially reported. For example, orofacial stereotypies and sniffing are evident when cocaine or the D2/D3 agonist quinpirole is microinjected into the nucleus accumbens of adult rats (Koene et al., 1993; Canales and Iversen, 1998; Neisewander et al., 1998), and both locomotor activity and rearing have been observed when apomorphine or amphetamine is infused into various subregions of the CPu (Dickson et al., 1994; Carrera et al., 1998; Dias et al., 2006).
The receptor subtypes responsible for mediating unlearned behavior have been intensively studied. Intraaccumbal infusions of SKF-38393 (a partial D1 agonist) or SKF-82958 (a full D1 agonist) stimulate moderate amounts of locomotor activity in the adult rat (Meyer, 1993; Meyer et al., 1993; Swanson et al., 1997), while quinpirole causes a relatively smaller increase in locomotion (Dreher and Jackson, 1989; Van Hartesveldt et al., 1992; Gong et al., 1999; but see Mogenson and Wu, 1991; Canales and Iversen, 2000). In the CPu, SKF-82958 infusions produce only mild stereotypy (Kreipke and Walker, 2004; Krolewski et al., 2005), whereas quinpirole causes a more pronounced stereotypic response (Delfs and Kelley, 1990). This quinpirole-induced stereotypy requires concurrent D1 receptor stimulation because stereotyped behaviors are not evident when quinpirole and the selective D1 receptor antagonist SCH-23390 are co-administered into the CPu (Waszczak et al., 2002). Not surprisingly, combined treatment with SKF-38393 and quinpirole produces robust stereotypy in the adult rat, which is approximately equivalent to the stereotypy induced by indirect dopamine agonists like amphetamine or cocaine (Bordi and Meller, 1989; Waszczak et al., 2002).
Although the neural control of motor behavior has been studied in detail in the adult rat, the ontogeny of dopamine-mediated motor systems has been examined much less thoroughly. In general, systemic administration of a dopamine agonist produces quantitatively greater behavioral effects in adulthood than during the preweanling or adolescent periods (for reviews, see Spear, 1979; Shalaby and Spear, 1980; Spear and Brake, 1983; Andersen, 2003). Even so, direct and indirect dopamine agonists are capable of inducing forward locomotion by as early as postnatal day (PD) 3 (Shalaby and Spear, 1980; Camp and Rudy, 1987; Moody and Spear, 1992) and coupling between D1 and D2 receptors is apparent at the same age (Moody and Spear, 1992; see also McDougall et al., 1990; Byrnes and Bruno, 1994). Whether the intensity of dopamine agonist-induced stereotypy differs between preweanling and adult rats is uncertain (e.g., see Abrams and Bruno, 1992; Moody and Spear, 1992; Cortez et al., 2010), but adolescent mice exhibit substantially less stereotypy than adults (Adriani and Laviola, 2000). Although few microinjection experiments have been conducted during early ontogeny, available evidence indicates that dopamine agonists differentially affect the behavior of preweanling and adult rats. Specifically, bilateral infusions of the nonselective dopamine agonist R-propylnorapomorphine (NPA) into the dorsal CPu causes robust locomotor activity on PD 18 (Charntikov et al., 2008); whereas, microinjecting NPA into the same brain region of adult rats causes minimal locomotor activity but moderately intense oral and sniffing stereotypies (Bordi et al., 1989). Charntikov et al. (2008) did not measure stereotyped behaviors, so it is uncertain whether infusing NPA into the dorsal CPu induces stereotypy, as well as increased locomotion, in the preweanling rat.
The purpose of the present study was to assess the importance of D1 and D2 receptors in the dorsal CPu for the locomotor activity and stereotyped behaviors of male and female preweanling rats. In the first experiment, the behavioral effects of nonselectively stimulating dopamine receptors was examined by bilaterally administering NPA or a “cocktail” of SKF-82958 and quinpirole. NPA was used because it is a full agonist at D1 and D2 receptors (Goldman and Kebabian, 1984; Meller et al., 1987; Bordi et al., 1989), whereas SKF-82958 is a full agonist at D1 receptors and quinpirole is a full agonist at D2/D3 receptors. Locomotor activity was quantified as well as three measures of stereotypy: repetitive motor movements, head-down sniffing, and behavioral intensity. In the second experiment, the effects of selectively stimulating D1 or D2 receptors were assessed by measuring locomotor activity and stereotypy after bilateral infusions of SKF-82958, quinpirole, or the partial D1 agonist SKF-38393. SKF-38393 was included because much of the initial ontogenetic research examining dopamine receptor subtypes used this D1 agonist (e.g., McDougall et al., 1990; Moody and Spear, 1992; Byrnes and Bruno, 1994; Shieh and Walters, 1996; Sobrian et al., 2003). In the third experiment, the coupling of D1 and D2 receptors in the dorsal CPu was assessed by infusing SKF-82958 and quinpirole either alone or in combination. In the final experiments, quinpirole and the D1 receptor antagonist SCH-23390, or SKF-82958 and the D2 receptor antagonist raclopride, were co-administered into the dorsal CPu in order to determine whether a functioning D1 or D2 receptor system is necessary for the expression of quinpirole- or SKF-82958-induced behaviors, respectively. In all cases, behavioral testing was done on PD 18 because (a) systemic administration of dopamine agonists does not induce adult-like behavioral responses until weaning or later (Moody and Spear, 1992) and (b) rats of the late preweanling period (approx. PD 17–PD 21) can be more directly compared to adult rats than can younger age groups (i.e., preweanling rats are freely mobile, their auditory canals and eyes are open, and thermoregulation is more adult-like than at earlier ages).
EXPERIMENTAL PROCEDURES
Animals
Subjects were 297 male and female rats of Sprague-Dawley descent (Charles River, Hollister, CA, USA) that were born and bred at California State University, San Bernardino (CSUSB). Litters were culled to 10 pups at three days of age. Preweanling rats were kept with the dam and littermates throughout behavioral testing and were housed in large polycarbonate maternity cages (56 × 34 × 22 cm) with wire lids. Food and water were freely available. The colony room was maintained at 22–24°C and kept under a 12-h light/dark cycle, with behavioral testing occurring during the light phase of the cycle. Subjects were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
Apparatus
Behavioral testing was done in activity monitoring chambers (25.5 × 25.5 × 41 cm, L × W × H) that consisted of acrylic walls, a plastic floor, and an open top (Coulbourn Instruments, Allentown, PA, USA). Each chamber included an X–Y photobeam array, with 16 photocells and detectors, that was used to determine distance traveled (a measure of locomotor activity) and repetitive motor movements (a measure of stereotypy). Photobeam resolution was 0.76 cm, with the position of each rat being determined every 100 ms.
Drugs
R(−)-propylnorapomorphine hydrochloride (NPA) was dissolved in distilled water containing 0.1% metabisulfite (an antioxidant), whereas (±)-SKF-38393 hydrochloride, (−)-quinpirole hydrochloride, R(+)-SCH-23390 hydrochloride, and S(−)-raclopride (+)-tartrate salt were dissolved in distilled water. (±)-SKF-82958 hydrobromide (also called chloro-APB hydrobromide) was dissolved in 10% dimethyl sulfoxide (DMSO) in distilled water. In some treatment conditions, SKF-82958 and quinpirole, raclopride and SKF-82958, or SCH-23390 and quinpirole were administered together in a “cocktail” solution. All solutions were prepared prior to testing and were administered at a volume of 0.5 μl per side. Drugs were purchased from Sigma (St. Louis, MO, USA).
Surgery
On PD 16, anesthesia was induced by isoflurane (2.5–5%) mixed with oxygen. A topical lidocaine solution (1%) was applied to the scalp and ibuprofen (2 mg/kg IP) was administered. Rats were placed in a Cunningham Neonatal Rat Adapter attached to a standard Kopf stereotaxic apparatus and the scalp was incised to reveal the skull. Two craniotomies were performed and a stainless steel double guide cannula (22 gauge; Plastics One, Roanoke, VA, USA) was implanted in the dorsal CPu (A/P +5.3, M/L ±2.8, D/V +6.0 mm from the interaural line). Guide cannulae were fixed in place using cyanoacrylate gel followed by dental cement. Stainless steel stylets (Plastics One) were used to seal guide cannulae until time of testing. After surgery, rats were allowed to recover away from the dam in temperature-controlled chambers (37°C). Upon becoming fully responsive, rats were returned to their dam and littermates. Stereotaxic coordinates are from the developing rat brain atlas of Sherwood and Timiras (1970).
Procedures
Experiment 1: effects of NPA or combined SKF-82958/quinpirole treatment
On PD 18, rats (N = 32) were transported to the testing room and placed in activity chambers for a 20-min “chamber habituation” phase. Rats were then injected (IP) with saline and immediately returned to the activity chambers for a 20-min “saline habituation” phase [this procedure replicates the methods from a previous study (Charntikov et al., 2008)]. Rats were removed from the testing chamber and the stainless steel stylets were replaced by infusion cannulae (Plastics One) which extended 1 mm below the guide cannulae. Hamilton microsyringes (10 μl) attached to a dual infusion pump were used to bilaterally microinject distilled water vehicle, 10% DMSO vehicle, NPA (20 μg), a moderate-dose “cocktail” of SKF-82958 (3 μg) plus quinpirole (10 μg), or a high-dose “cocktail” of SKF-82958 (10 μg) plus quinpirole (20 μg) at a volume of 0.5 μl per side. Drugs were delivered at a constant rate over a 60 s period and the infusion cannulae were subsequently left in place for an additional 60 s. After drug infusion, rats were returned to the activity chambers for a 40 min “testing” phase.
Experiment 2: effects of SKF-82958, quinpirole, or SKF-38393 alone
On PD 18, rats (N = 88) were allowed to habituate to the activity chambers and saline injection in the same manner as just described. After 40 min, rats were removed from the testing chamber and were given bilateral microinjections of distilled water vehicle, 10% DMSO vehicle, the full D1 receptor agonist SKF-82958 (1, 3, or 10 μg), or the full D2/D3 agonist quinpirole (10, 20, or 30 μg). For comparison purposes, additional groups of rats were given bilateral infusions of the partial D1 agonist SKF-38393 (10, 20, or 30 μg) or distilled water vehicle. Rats were then returned to the activity chambers for an additional 40 min of behavioral testing.
Experiment 3: effects of SKF-82958 and quinpirole administered either alone or in combination
For this experiment, the chamber habituation phase lasted 40 min and rats (N = 64) did not receive a systemic injection of saline on PD 18. This modified procedure was used to ensure that the IP saline injection was not affecting behavior during the testing phase. After the 40-min chamber habituation period, rats were given bilateral microinjections of distilled water vehicle, 10% DMSO vehicle, SKF-82958 (1 or 3 μg), quinpirole (1 or 3 μg), a low-dose “cocktail” of SKF-82958 (1 μg) plus quinpirole (1 μg), or a moderate-dose “cocktail” of SKF-82958 (3 μg) plus quinpirole (3 μg). Rats were then returned to the activity chambers for an additional 40 min of behavioral testing.
Experiment 4: effects of combined quinpirole/SCH-23390 treatment
The chamber habituation phase occurred on PD 18 and was the same as described for Experiment 3. After the 40-min habituation period, rats (N = 36) were removed from the activity chambers and given bilateral microinjections of distilled water vehicle, SCH-23390 (1 or 5 μg), quinpirole (10 μg), or combined administrations of quinpirole (10 μg) plus SCH-23390 (1 or 5 μg). Rats were then returned to the activity chambers for an additional 40 min of behavioral testing.
Experiment 5: effects of combined SKF-82958/raclopride treatment
Procedures were the same as described for Experiment 3, except that rats (N = 48) were given bilateral microinjections of distilled water vehicle, 10% DMSO vehicle, raclopride (1 or 5 μg), SKF-82958 (3 μg), or combined administrations of SKF-82958 (3 μg) plus raclopride (1 or 5 μg) on PD 18.
Behavioral assessment
Distance traveled (a measure of horizontal locomotor activity) and repetitive motor movements (a measure of stereotypy) were assessed continuously across the 80-min session (i.e, 40 min of habituation and 40 min of drug testing). Repetitive motor movements were defined as the total number of repetitive coordinate changes on the X–Y axes that occurred within 2 s (three back and forth movements were required before the behavior qualified as a repetitive motor movement). In additon to these automated measures, behavior was recorded via ceiling-mounted hard disk cameras (JVC, model GZ-MG670) and data were later coded by observers blind to treatment conditions. Discrete behaviors (e.g., head-down sniffing and grooming) were quantified using the fixed interval momentary time sampling method described by Cameron et al. (1988). Using this technique, the presence or absence of a particular behavior was determined at 20 s intervals during each 5-min time block. The videotaped behavioral data were also quantified using a behavioral intensity scale developed by Creese and Iversen (1973). Specifically, rats were observed once every 5 min (for a 1-min period) and behavior was coded using the following rating system: “0 = asleep or inactive, 1 = normal exploratory activity (no repetitive behaviors), 2 = discontinuous activity with prominent (repetitive) sniffing or rearing, 3 = continuous activity with repetitive sniffing and rearing in a fixed path, 4 = continuous activity with repetitive sniffing and rearing in the same location, 5 = continuous activity with repetitive chewing or gnawing in a fixed path, 6 = continuous activity with repetitive chewing or gnawing in the same location.”
Histology
After behavioral testing, rats were given an overdose of sodium pentobarbital and brains were fixed in 4% paraformaldehyde. Brains were cryoprotected in a 20% sucrose solution, sectioned coronally (70 μm) using a cryostat, and then stained with thionin. Histological assessment of cannula placements was done by observers blind to drug treatment conditions. Overall, 90.2% of rats (268 out of 297) had proper cannula placements in the dorsal CPu. Data from animals with inappropriate guide cannula placements were not included in the statistical analyses. Replacement rats were added to groups missing subjects, thus experiments had either 6 subjects (Experiment 4) or 8 subjects (Experiments 1, 2, 3, and 5) per group. Cannula placements of rats included in the statistical analyses are shown in Fig. 1.
Fig. 1.
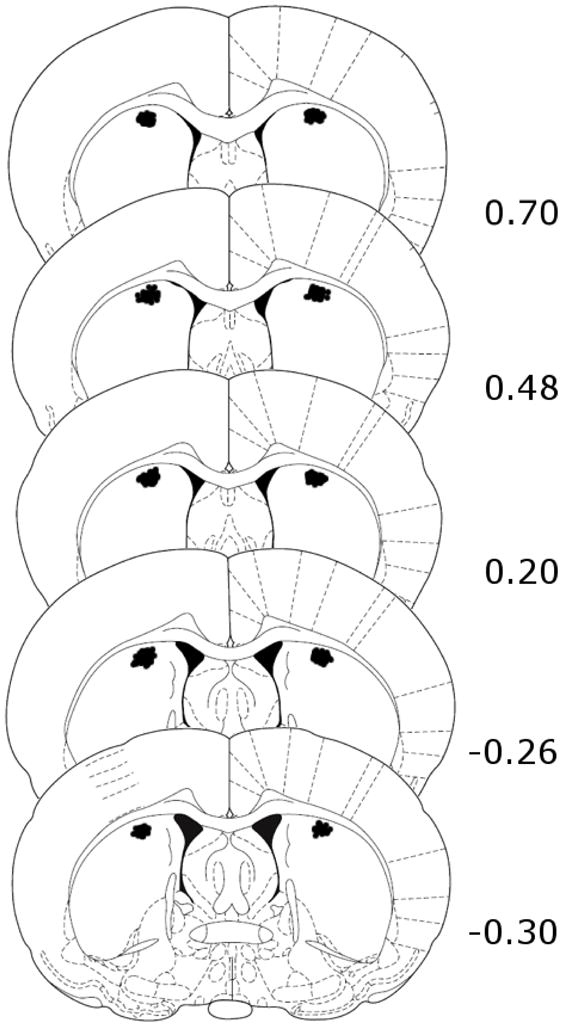
Schematic representations of cannula placements in the dorsal CPu of preweanling rats from Experiments 1–4. In all cases, numbers on the right indicate distance (mm) from Bregma using coordinates from the rat brain atlas of Paxinos and Watson (1998).
Data analysis
Repeated measures (5-min time blocks) analyses of variance (ANOVAs) were used for statistical analysis of distance traveled, repetitive motor movements, and head-down sniffing data. Because of ongoing experimental manipulations, separate ANOVAs were used to analyze time blocks 1–4 (chamber habituation), 5–8 (saline habituation), and 9–16 (drug testing). For Experiment 3, data from the low-dose drug groups (1 μg quinpirole, 1 μg SKF-82958, and 1 μg quinpirole plus 1 μg SKF-82958) were analyzed separately from the moderate-dose groups (3 μg quinpirole, 3 μg SKF-82958, and 3 μg quinpirole plus 3 μg SKF-82958). When required, significant higher order interactions were further analyzed using one-way ANOVAs, while Tukey tests (P<0.05) were used for making post hoc comparisons. Behavioral intensity data were analyzed using the Kruskal-Wallis (KW) nonparametric statistic, with Dunn’s tests (P<0.05) being used for post comparisons (Dunn, 1964; Siegel and Castellan, 1988). Distilled water vehicle and 10% DMSO vehicle had similar effects on the various behavioral measures, therefore data from the two vehicle groups were combined for each experiment.
Litter effects were minimized by assigning one subject from each litter to a particular group (for a discussion of litter effects, see Zorrilla, 1997). In situations where this procedure was not possible (i.e., during the habituation phases), a single litter mean was calculated from multiple littermates assigned to the same group (Holson and Pearce, 1992; Zorrilla, 1997). Preliminary analyses indicated that distance traveled scores did not differ according to sex, so this variable was not included in the final analyses.
RESULTS
Experiment 1: effects of NPA or combined SKF-82958/quinpirole treatment
Distance traveled
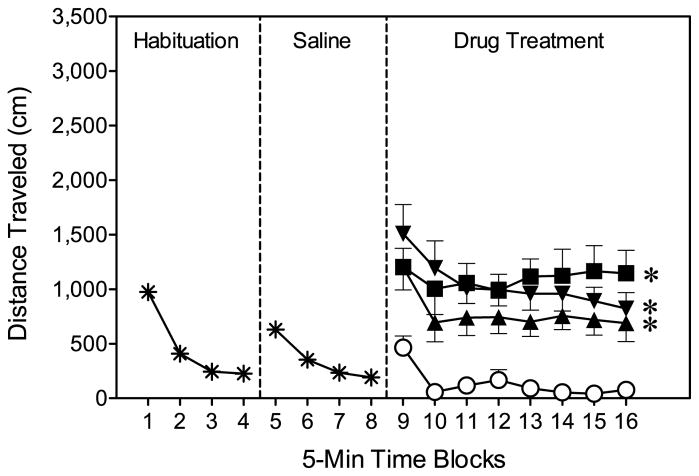
During the chamber and saline habituation phases (i.e., time blocks 1–4 and 5–8), distance traveled scores were elevated immediately after rats were placed in the activity chambers, but scores then declined to a stable baseline (Fig. 2, left and middle panels) [Time Block effects, F(3, 105)=76.25, P<0.001, F(3, 105)=19.10, P<0.001, respectively]. Microinjecting vehicle into the CPu at time block 9 had minimal effects on locomotor activity, because distance traveled scores remained at a low, stable rate on time blocks 9–16 (Fig. 2, right panel). In contrast, bilateral infusions of either 20 μg NPA or a “cocktail” of SKF-82958 (3 μg/10 μg) plus quinpirole (10 μg/20 μg) caused a significant increase in distance traveled scores [Drug main effect, F(3, 28)=12.87, P<0.001 and Tukey tests]. The various dopamine agonist-treated groups did not differ among themselves, nor did the drug and time variables interact to affect performance.
Fig. 2.
Mean distance traveled (±SEM) during the 80-min behavioral testing session on PD 18 (n = 8 per group) of Experiment 1. At the conclusion of time block 8 (indicated by the second dashed line), rats received bilateral CPu infusions of vehicle, NPA (20 μg), or a “cocktail” of SKF-82958 (3 μg/10 μg) plus quinpirole (10 μg/20 μg). (○) Vehicle; (▼) SKF-82958 (3 μg) plus quinpirole (10 μg); (▲) SKF-82958 (10 μg) plus quinpirole (20 μg); (■) 20 μg NPA.
* Significantly different from the vehicle group when collapsed across time blocks 9–16.
Repetitive motor movements
Across time blocks 1–4 and 5–8 there was a progressive decline in repetitive motor movements (Fig. 3, left panel) [Time Block effects, F(3, 105)=24.80, P<0.001, F(3, 105)=22.44, P<0.001, respectively]. When compared to vehicle controls, the repetitive motor movements of preweanling rats were increased by NPA (20 μg) and both dose combinations of SKF-82958/quinpirole (Fig. 3, right panel) [Drug main effect, F(3, 28)=25.71, P<0.001 and Tukey tests]. The effects of drug treatment varied according to time block [Drug × Time Block interaction, F(21, 196)=2.42, P<0.001], because none of the dopamine agonist-treated groups differed from vehicle controls on time block 9. Across the remainder of the testing session (i.e., time blocks 10–16), the NPA- and SKF-82958/quinpirole-treated rats exhibited significantly more repetitive motor movements than vehicle controls [Tukey tests].
Fig. 3.
Mean time (s) engaged in repetitive motor movements (±SEM) during the 80-min behavioral testing session (n = 8 per group) of Experiment 1. These are the same rats as described in Fig. 2. (○) Vehicle; (▼) SKF-82958 (3 μg) plus quinpirole (10 μg); (▲) SKF-82958 (10 μg) plus quinpirole (20 μg); (■) 20 μg NPA.
* Significantly different from the vehicle group when collapsed across time blocks 9–16.
† Significantly different from the vehicle group at the same time block.
Head-down sniffing
Head-down sniffing (i.e., stereotyped sniffing) was infrequently observed during the habituation phases (< 1 occurrence per 5 min time block). Infusing either NPA (20 μg) alone or a “cocktail” of SKF-82958 (3 μg/10 μg) plus quinpirole (10 μg/20 μg) significantly increased head-down sniffing relative to vehicle controls (Table 1) [Drug main effect, F(3, 28)=42.30, P<0.001 and Tukey tests]. Head-down sniffing counts did not vary among the agonist-treated groups.
Table 1.
Mean (±SEM) head-down sniffing counts and behavioral intensity scores of dopamine agonist-treated preweanling rats on time blocks 9–16 (Experiment 1)
| Drug Infusion | Sniffing | Behavioral Intensity |
|---|---|---|
| Vehicle | 0.26 (±0.13) | 0.16 (±0.04) |
| 3 μg SKF-82958/10 μg Quinpirole | 11.55 (±1.55)* | 2.92 (±0.15)* |
| 10 μg SKF-82958/20 μg Quinpirole | 10.78 (±0.76)* | 2.69 (±0.28)* |
| 20 μg NPA | 11.14 (±0.95)* | 3.03 (±0.15)* |
Values represent mean (±SEM) head-down sniffing and behavioral intensity scores per 5-min time block collapsed across the final 40-min testing phase.
Significantly different from the vehicle group.
Behavioral intensity
The ability of dopamine agonists to induce stereotypy in preweanling rats was also assessed using a behavioral intensity scale (Creese and Iversen, 1973). During the habituation phases, behavioral intensity scores approached zero (data not shown). Behavioral intensity scores were significantly elevated after dopamine agonist infusions (i.e., on time blocks 9–16; Table 1) [Drug effect, KW=18.54, P<0.001 and Dunn’s tests], with NPA and either dose combination of SKF-82958 (3 μg/10 μg) plus quinpirole (10 μg/20 μg) producing a similar enhancement in behavioral intensity.
Experiment 2: effects of SKF-82958, quinpirole, or SKF-38393 alone
Distance traveled
Distance traveled scores declined across each of the two habituation phases (Fig. 4, left and middle panels) [Time Block effects, F(3, 189)=172.92, P<0.001, F(3, 165)=58.45, P<0.001, respectively]. Rats receiving vehicle or 1 μg SKF-82958 exhibited similar low levels of locomotor activity (Fig. 4, lower graph, right panel); however, infusing 3 or 10 μg SKF-82958 into the dorsal CPu significantly increased distance traveled scores relative to vehicle controls [Dose main effect, F(3, 28)=12.15, P<0.001 and Tukey tests]. Analyses of individual time blocks showed that rats receiving 3 or 10 μg SKF-82958 had greater distance traveled scores than vehicle controls on time blocks 10–16 [Dose × Time Block interaction, F(21, 196)=5.00, P<0.001 and Tukey tests]. Rats infused with 1 μg SKF-82958 transiently exhibited more locomotor activity than vehicle controls, but this was restricted to time blocks 10–12 [Tukey tests].
Fig. 4.
Mean distance traveled (±SEM) during the 80-min behavioral testing session on PD 18 (n = 8 per group) of Experiment 2. At the conclusion of time block 8 (indicated by the second dashed line), rats received bilateral CPu infusions of vehicle, SKF-82958 (1, 3, or 10 μg), or quinpirole (10, 20, or 30 μg).
* Significantly different from the vehicle group when collapsed across time blocks 9–16.
** Significantly different from all other groups when collapsed across time blocks 9–16.
† Significantly different from the vehicle group at the same time block.
‡ Significantly different from all other groups at the same time block.
^ Significantly different from the 1 μg SKF-82958 group.
Infusing quinpirole into the dorsal CPu significantly increased distance traveled scores relative to vehicle controls (Fig. 4, upper graph, right panel). More specifically, 10 μg quinpirole caused the greatest increase in distance traveled scores, while 20 and 30 μg quinpirole produced an intermediate amount of locomotor activity that was significantly different from both the vehicle and 10 μg quinpirole groups [Dose main effect, F(3, 28)=16.64, P<0.001 and Tukey tests]. When assessed across individual time blocks, 10 μg quinpirole produced significantly more locomotor activity than 20 or 30 μg quinpirole on time blocks 10–13 [Dose × Time Block interaction, F(21, 196)=3.04, P<0.001 and Tukey tests]. Each of the quinpirole groups differed from the vehicle controls on time blocks 9–16 [Tukey tests].
Statistical analyses comparing the quinpirole and SKF-82958 groups showed that rats infused with 10 μg quinpirole had greater distance traveled scores than rats infused with 1–10 μg SKF-82958 (Fig. 3) [Drug × Dose interaction, F(3, 56)=9.97, P<0.001 and Tukey tests]. Unexpectedly, microinjecting the partial D1 agonist SKF-38393 (10–30 μg) into the dorsal CPu did not affect the distance traveled scores of preweanling rats (Table 2) [Dose main effect, F(1, 28=0.77, NS].
Table 2.
Mean (±SEM) distance traveled (DT), repetitive motor movements (RMM), head-down sniffing counts, and behavioral intensity scores of vehicle and SKF-38393-treated preweanling rats collapsed across time blocks 9–16 (Experiment 2)
| Drug Infusion | DT | RMM | Sniffing | Behavioral Intensity |
|---|---|---|---|---|
| Vehicle | 808 (±141) | 166.6 (±20) | 0.23 (±0.09) | 0.08 (±0.05) |
| 10 μg SKF-38393 | 1,227 (±329) | 295.6 (±55) | 0.51 (±0.16) | 0.17 (±0.07) |
| 20 μg SKF-38393 | 830 (±300) | 220.1 (±72) | 0.40 (±0.12) | 0.12 (±0.08) |
| 30 μg SKF-38393 | 737 (±192) | 168.7 (±37) | 0.29 (±0.06) | 0.07 (±0.05) |
Distance traveled and repetitive motor movement values represent mean total scores collapsed across the final 40-min testing phase, whereas head-down sniffing and behavioral intensity values represent mean scores per 5-min time block during the final 40-min testing phase.
Repetitive motor movements
Repetitive motor movements declined across the two habituation phases (Fig. 5, left and middle panels), with some stabilization occurring on time blocks 7 and 8 [Time Block effects, F(3, 189)=33.50, P<0.001, F(3, 189)=40.36, P<0.001, respectively]. Bilateral infusions of SKF-82958 into the dorsal CPu significantly increased repetitive motor movements in a dose-related fashion (Fig. 5, lower graph, right panel). Rats receiving 10 μg SKF-82958 spent the most time engaged in repetitive motor movements, whereas rats receiving 1 or 3 μg SKF-82958 were intermediate between the vehicle and high-dose group [Dose main effect, F(3, 28)=55.65, P<0.001 and Tukey tests]. Relative to vehicle, the two higher doses of SKF-82958 (3 or 10 μg) increased repetitive motor movements on time blocks 10–16, while 1 μg SKF-82958 caused a significant elevation on time blocks 10–14 [Dose × Time Block interaction, F(21, 196)=7.30, P<0.001 and Tukey tests]. Among the SKF-82958 groups, the 10 μg dose induced more repetitive motor movements on time blocks 12–16 than the two lower doses of SKF-82958; and 3 μg SKF-82958 induced more repetitive motor movements than the 1 μg dose on time blocks 14–16 [Tukey tests].
Fig. 5.
Mean time (s) engaged in repetitive motor movements (±SEM) during the 80-min behavioral testing session (n = 8 per group) of Experiment 2. These are the same rats as described in Fig. 4.
* Significantly different from the vehicle group when collapsed across time blocks 9–16.
** Significantly different from all other groups when collapsed across time blocks 9–16.
† Significantly different from the vehicle group at the same time block.
‡ Significantly different from all other groups at the same time block.
^ Significantly different from the 1 μg SKF-82958 groups at the same time block.
Bilateral administration of quinpirole also increased repetitive motor movements (Fig. 5, upper graph, right panel). In this case, all doses of quinpirole (10, 20, and 30 μg) enhanced repetitive motor movements, with none of the individual quinpirole groups differing amongst themselves [Dose main effect, F(3, 28)=37.20, P<0.001 and Tukey tests]. The effects of quinpirole did not vary significantly across time blocks 9–16 [Dose × Time Block interaction, F(21, 196)=1.29, NS].
Direct comparisons between the full D1 and D2 agonists showed that rats infused with 10 μg SKF-82958 into the dorsal CPu had more repetitive motor movements than rats given 10–30 μg quinpirole (Fig. 4) [Drug × Dose interaction, F(3, 56)=4.75, P<0.01 and Tukey tests]. Consistent with the distance traveled data, infusing SKF-38393 (10, 20, or 30 μg) into the dorsal CPu did not alter the repetitive motor movements of preweanling rats (Table 2) [Drug main effect, F(3, 28)=1.46, NS].
Head-down sniffing
SKF-82958 produced a dose-dependent increase in head-down sniffing, with each successive dose of the D1 agonist producing progressively more sniffing counts than the lesser dose (Table 3) [Dose main effect, F(3, 28)=43.19, P<0.001 and Tukey tests]. Quinpirole also increased the head-down sniffing of preweanling rats (Table 3), but in this case the two higher doses of quinpirole (20 and 30 μg) produced more head-down sniffing than the 10 μg dose [Dose main effect, F(3, 28)=69.70, P<0.001 and Tukey tests].
Table 3.
Mean (±SEM) head-down sniffing counts and behavioral intensity scores of SKF-82958- or quinpirole-treated rats on time blocks 9–16 (Experiment 2)
| Drug Infusion | Sniffing | Behavioral Intensity |
|---|---|---|
| Vehicle | 0.55 (±0.65) | 0.18 (±0.07) |
| 1 μg SKF-82958 | 2.92 (±0.48)* | 0.91 (±0.11) |
| 3 μg SKF-82958 | 5.82 (±0.53)* † | 1.80 (±0.17)* |
| 10 μg SKF-82958 | 7.77 (±0.62)* † ‡ | 2.14 (±0.23)* |
|
| ||
| Vehicle | 0.23 (±0.09) | 0.08 (±0.05) |
| 10 μg Quinpirole | 7.17 (±0.72)* | 2.54 (±0.10)* |
| 20 μg Quinpirole | 9.30 (±0.57)* ^ | 2.38 (±0.17)* |
| 30 μg Quinpirole | 9.71 (±0.50)* ^ | 2.29 (±0.19)* |
Values represent mean (±SEM) head-down sniffing and behavioral intensity scores per 5-min time block collapsed across the final 40-min testing phase.
Significantly different from the vehicle group;
significantly different from the 1 μg SKF-82958 group;
significantly different from the 3 μg SKF-82958 group;
significantly different from the 10 μg quinpirole group.
Infusing rats with 20 or 30 μg quinpirole produced more head-down sniffing than the highest dose of SKF-82958 tested (10 μg) [Drug × Dose interaction, F(3, 56)=7.98, P<0.001 and Tukey tests]. Head-down sniffing was not affected by SKF-38393 infusions (Table 2).
Behavioral intensity
The two higher doses of SKF-82958 (3 and 10 μg) significantly increased the behavioral intensity scores of preweanling rats (Table 3) [Dose effect, KW=24.39, P<0.001 and Dunn’s tests]. Quinpirole also enhanced behavioral intensity scores on PD 18 (Table 3) but, in this case, all three doses of quinpirole (10, 20, or 30 μg) produced a similar increase in behavioral intensity [Dose effect, KW=17.90, P<0.001 and Dunn’s tests].
Direct comparisons between the SKF-82958 and quinpirole groups showed that 10 μg quinpirole resulted in greater behavioral intensity scores than 1 μg SKF-82958 (Table 3) [Drug effect, KW=50.10, P<0.001 and Dunn’s tests]; however, higher doses of SKF-82958 (3 and 10 μg) and quinpirole (20 and 30 μg) did not differentially affect behavioral intensity. Microinjecting SKF-38393 (10, 20, or 30 μg) into the dorsal CPu did not alter the behavioral intensity scores of preweanling rats (Table 2) [Dose effect, KW=2.33, NS].
Experiment 3: effects of SKF-82958 and quinpirole administered either alone or in combination
Distance traveled
Overall, distance traveled scores declined across the first three time blocks of the chamber habituation phase and then stabilized (Fig. 6, left panels) [Time Block effect, F(7, 441)=106.21, P<0.001]. In terms of the low-dose groups (Fig. 6, bottom graph), bilaterally infusing a “cocktail” of 1 μg SKF-82958/quinpirole into the dorsal CPu produced greater distance traveled scores than 1 μg SKF-82958 alone, 1 μg quinpirole alone, or vehicle [Drug main effect, F(3, 28)=9.10, P<0.001 and Tukey tests]. Differences between the 1 μg SKF-82958/quinpirole and vehicle groups were statistically significant on time blocks 10–16; whereas, the 1 μg SKF-82958/quinpirole group had greater distance traveled scores than all other low-dose groups on time blocks 11, 13, 14, and 16 [Drug × Time Block interaction, F(21, 196)=5.00, P<0.001 and Tukey tests]. Among the groups given a single agonist, rats injected with 1 μg SKF-82958 had greater distance traveled scores than vehicle controls on time blocks 10 and 11 [Tukey tests].
Fig. 6.
Mean distance traveled (±SEM) during the 80-min behavioral testing session on PD 18 (n = 8 per group) of Experiment 3. At the conclusion of time block 8 (indicated by the dashed line), rats received bilateral CPu infusions of vehicle, SKF-82958 (1 or 3 μg), quinpirole (1 or 3 μg), or a “cocktail” of SKF-82958 (1 μg/3 μg) plus quinpirole (1 μg/3 μg).
* Significantly different from the vehicle group when collapsed across time blocks 9–16.
** Significantly different from all other groups when collapsed across time blocks 9–16.
† Significantly different from the vehicle group at the same time block.
‡ Significantly different from all other groups at the same time block.
^ Significantly different from the 3 μg SKF-82958 groups at the same time block.
In terms of the moderate-dose groups (Fig. 6, upper graph), rats receiving 3 μg quinpirole alone had significantly greater distance traveled scores than vehicle controls [Drug main effect, F(3, 28)=27.64, P<0.001 and Tukey tests]. Rats receiving 3 μg SKF-82958 alone or a “cocktail” of 3 μg SKF-82958/quinpirole were intermediate between, and significantly different from, the quinpirole and vehicle groups [Tukey tests]. The 3 μg quinpirole group exhibited more distance traveled than the two other drug groups on time blocks 11–16 and more distance traveled than the vehicle controls on time blocks 10–16 [Drug × Time Block interaction, F(21, 196)=13.43, P<0.001 and Tukey tests]. Curiously, rats given 3 μg quinpirole exhibited less locomotion than all other groups on time block 9. Rats administered a “cocktail” of 3 μg SKF-82958/quinpirole had greater distance traveled scores than vehicle-treated rats on time blocks 10–14 [Tukey tests].
Repetitive motor movements
Repetitive motor movements showed a progressive decline across the chamber habituation phase (data not shown) [Time Block effect, F(7, 441)=38.49, P<0.001]. Rats given bilateral infusions of 1 μg SKF-82958, 1 μg quinpirole, or a “cocktail” of 1 μg SKF-82958/quinpirole into the dorsal CPu exhibited greater repetitive motor movement scores than vehicle controls (Table 4) [Drug main effect, F(3, 28)=12.65, P<0.001 and Tukey tests]. Among the moderate-dose groups, rats microinjected with 3 μg SKF-82958 alone or a “cocktail” of 3 μg SKF-82958/quinpirole had more repetitive motor movements than vehicle-treated rats [Drug main effect, F(3, 28)=16.54, P<0.001 and Tukey tests]. Rats treated with 3 μg quinpirole alone did not differ significantly from the vehicle group.
Table 4.
Mean (±SEM) repetitive motor movements (RMM), head-down sniffing counts, and behavioral intensity scores of drug-treated preweanling rats collapsed across time blocks 9–16 (Experiment 5)
| Drug Infusion | RMM | Sniffing | Behavioral Intensity |
|---|---|---|---|
| Vehicle | 221.4 (±43) | 0.50 (±0.17) | 0.28 (±0.09) |
| 1 μg SKF-82958 | 663.8 (±66)* | 2.14 (±0.34)* | 1.42 (±0.25)* |
| 1 μg Quinpirole | 460.6 (±62)* | 1.28 (±0.41)† | 0.95 (±0.26) |
| 1 μg SKF/1 μg Quin | 633.0 (±54)* | 3.53 (±0.58)* | 2.03 (±0.25)* |
|
| |||
| Vehicle | 388.6 (±65) | 1.08 (±0.22) | 0.34 (±0.07) |
| 3 μg SKF-82958 | 1,024.5 (±81)* | 5.62 (±0.88)* † | 2.01 (±0.30)* |
| 3 μg Quinpirole | 645.6 (±56) | 5.70 (±0.72)* † | 2.44 (±0.11)* |
| 3 μg SKF/3 μg Quin | 831.2 (±63)* | 10.76 (±1.48)* | 3.06 (±0.18)* |
Repetitive motor movement values represent mean total scores collapsed across the final 40-min testing phase, whereas head-down sniffing and behavioral intensity values represent mean scores per 5-min time block during the final 40-min testing phase.
Significantly different from the vehicle group.
Significantly different from the SKF/Quin group in the same dose range.
Head-down sniffing
In terms of the low-dose groups, head-down sniffing data were similar in pattern to the repetitive motor movement data (Table 4). Specifically, rats microinjected with 1 μg SKF-82958 or a “cocktail” of 1 μg SKF-82958/quinpirole had significantly more head-down sniffing counts than rats given vehicle [Drug main effect, F(3, 28)=10.36, P<0.001 and Tukey tests]. Head-down sniffing counts of the 1 μg SKF-82958/quinpirole group were also elevated relative to rats receiving 1 μg quinpirole alone [Tukey tests].
Among the moderate-dose groups, the D1 and D2 agonists had a more conspicuous additive effect (Table 4). That is, rats microinjected with a “cocktail” of 3 μg SKF-82958 plus 3 μg quinpirole had significantly more head-down sniffing counts than rats infused with either agonist alone [Drug main effect, F(3, 28)=17.65, P<0.001 and Tukey tests]. Conversely, both SKF-82958 (3 μg) and quinpirole (3 μg), when administered alone, produced more head-down sniffing than vehicle [Tukey tests].
Behavioral intensity
As with head-down sniffing, behavioral intensity scores of rats infused with 1 μg SKF-82958 or a “cocktail” of 1 μg SKF-82958/quinpirole were significantly greater than rats given bilateral microinjections of vehicle (Table 4) [Drug effect, KW=16.60, P<0.001 and Dunn’s tests]. In the case of the moderate dose groups, rats infused with either agonist alone or a “cocktail” of 3 μg SKF-82958/quinpirole had greater behavioral intensity scores than vehicle controls (Table 4) [Drug effect, KW=22.33, P<0.001 and Dunn’s tests]. Moderate doses of the D1 and D2 agonists did not have an additive effect on behavioral intensity scores, because combined treatment with 3 μg SKF-82958/quinpirole produced only a nonsignificant elevation in behavioral intensity scores when compared to rats treated with 3 μg SKF-82958 or 3 μg quinpirole alone.
Experiment 4: effects of combined quinpirole/SCH-23390 treatment
Distance traveled
During the chamber habituation phase (i.e., time blocks 1–8), distance traveled scores declined from the first to the second time block and then stabilized (Fig. 7, left panel) [Time Block effect, F(7, 245)=57.49, P<0.001]. Overall, rats receiving quinpirole infusions into the dorsal CPu exhibited the most locomotor activity, whereas rats microinjected with a “cocktail” of quinpirole plus SCH-23390 (1 or 5 μg) had distance traveled scores that were intermediate between the quinpirole and vehicle groups (Fig. 7, right panel) [Drug main effect, F(5, 30)=30.04, P<0.001 and Tukey tests]. With the exception of time blocks 9 and 12, distance traveled scores of the quinpirole and quinpirole/SCH-23390 groups differed on all time blocks [Drug × Time Block interaction, F(35, 210)=3.16, P<0.001 and Tukey tests]. In contrast, vehicle controls only differed from the quinpirole/SCH-23390 (1 μg) group on time blocks 10–13 and from the quinpirole/SCH-23390 (5 μg) group on time blocks 11 and 12 [Tukey tests]. Distance traveled scores were not affected by SCH-23390 alone, perhaps because of a “floor” effect that precluded a significant reduction in behavior below the vehicle controls.
Fig. 7.
Mean distance traveled (±SEM) during the 80-min behavioral testing session on PD 18 (n = 6 per group) of Experiment 4. At the conclusion of time block 8 (indicated by the dashed line), rats received bilateral CPu infusions of vehicle, SCH-23390 (1 or 5 μg), quinpirole (10 μg), or a “cocktail” of quinpirole (10 μg) plus SCH-23390 (1 or 5 μg).
* Significantly different from the vehicle group when collapsed across time blocks 9–16.
** Significantly different from all other groups when collapsed across time blocks 9–16.
† Significantly different from the vehicle group at the same time block.
‡ Significantly different from all other groups at the same time block.
Repetitive motor movements
Repetitive motor movements showed a progressive decline across the chamber habituation phase (data not shown) [Time Block effect, F(7, 245)=17.50, P<0.001]. During the testing phase, rats given bilateral infusions of quinpirole (10 μg) exhibited significantly more repetitive motor movements than vehicle controls (Table 5) [Drug main effect, F(5, 30)=44.52, P<0.001 and Tukey tests]. Interestingly, co-administering quinpirole and SCH-23390 (1 or 5 μg) did not reduce repetitive motor movements relative to the quinpirole group. Rats microinjected with SCH-23390 alone (1 or 5 μg) did not differ from vehicle controls.
Table 5.
Mean (±SEM) repetitive motor movements (RMM), head-down sniffing counts, and behavioral intensity scores of drug-treated preweanling rats collapsed across time blocks 9–16 (Experiment 3)
| Drug Infusion | RMM | Sniffing | Behavioral Intensity |
|---|---|---|---|
| Vehicle | 223.7 (±54) | 0.30 (±0.10) | 0.18 (±0.14) |
| 10 μg Quinpirole | 677.9 (±34)* | 6.99 (±0.61)* | 2.83 (±0.07)* |
| 1 μg SCH-23390 | 114.4 (±22)† | 0.09 (±0.03)† | 0.03 (±0.02)† |
| 5 μg SCH-23390 | 77.7 (±22)† | 0.84 (±0.74)† | 0.22 (±0.21)† |
| 1 μg SCH-23390/Quinpirole (10 μg) | 615.2 (±40)* | 4.92 (±1.10)* | 1.50 (±0.39) |
| 5 μg SCH-23390/Quinpirole (10 μg) | 670.5 (±67)* | 6.99 (±0.61)* | 1.38 (±0.19) |
Repetitive motor movement values represent mean total scores collapsed across the final 40-min testing phase, whereas head-down sniffing and behavioral intensity values represent mean scores per 5-min time block during the final 40-min testing phase.
Significantly different from the vehicle group.
Significantly different from the 10 μg quinpirole group.
Head-down sniffing
Head-down sniffing was not observed during the habituation phase (data not shown). As with repetitive motor movements, bilaterally infusing quinpirole into the dorsal CPu significantly enhanced head-down sniffing during the testing phase (Table 5). This quinpirole-induced effect was apparent regardless of whether SCH-23390 (1 or 5 μg) was co-administered with the D2 agonist [Drug main effect, F(5, 30)=26.19, P<0.001 and Tukey tests]. SCH-23390 alone did not alter head-down sniffing relative to the vehicle group.
Behavioral intensity
Microinjecting quinpirole into the dorsal CPu significantly increased behavioral intensity scores relative to rats receiving vehicle or SCH-23390 (1 or 5 μg) alone (Table 5) [Drug effect, KW=25.63, P<0.001 and Dunn’s tests]. Co-administering quinpirole plus SCH-23390 (1 or 5 μg) produced behavioral intensity scores that were intermediate between the vehicle and quinpirole groups but significantly different from neither.
Experiment 5: effects of combined SKF-82958/raclopride treatment
Distance traveled
Similar to the previous experiment, distance traveled scores declined from the first to the second time block of the chamber habituation phase and then stabilized (Fig. 8, left panel) [Time Block effect, F(7, 406)=74.63, P<0.001]. Microinjecting SKF-82958 into the dorsal CPu significantly increased distance traveled scores relative to all other groups (Fig. 8, right panel) [Drug main effect, F(7, 406)=19.77, P<0.001 and Tukey tests]. Raclopride completely attenuated SKF-82958-induced locomotor activity, because rats infused with vehicle, raclopride (1 or 5 μg) alone, or a “cocktail” of SKF-82958 (3 μg) plus raclopride (1 or 5 μg) exhibited similar low rates of locomotor activity.
Fig. 8.
Mean distance traveled (±SEM) during the 80-min behavioral testing session on PD 18 (n = 8 per group) of Experiment 5. At the conclusion of time block 8 (indicated by the dashed line), rats received bilateral CPu infusions of vehicle, raclopride (1 or 5 μg), SKF-82958 (3 μg), or a “cocktail” of SKF-82958 (3 μg) plus raclopride (1 or 5 μg).
** Significantly different from all other groups when collapsed across time blocks 9–16.
Repetitive motor movements
Repetitive motor movements declined in a step-wise fashion across the chamber habituation phase (data not shown) [Time Block effect, F(7, 406)=29.24, P<0.001]. Rats given SKF-82958 (3 μg) infusions had more repetitive motor movements than all other groups (Table 6) [Drug main effect, F(7, 406)=4.56, P<0.001 and Tukey tests]. At the lowest dose tested, raclopride (1 μg) only marginally inhibited SKF-82958-induced repetitive motor movements, because the scores of the SKF-82958/raclopride (1 μg) group were significantly greater than the vehicle group [Tukey tests]. In contrast, the vehicle, raclopride (1 or 5 μg) alone, and SKF-82958/raclopride (5 μg) groups did not differ significantly amongst themselves.
Table 6.
Mean (±SEM) repetitive motor movements (RMM), head-down sniffing counts, and behavioral intensity scores of drug-treated preweanling rats collapsed across time blocks 9–16 (Experiment 4)
| Drug Infusion | RMM | Sniffing | Behavioral Intensity |
|---|---|---|---|
| Vehicle | 258.0 (±54) | 0.69 (±0.36) | 0.26 (±0.10) |
| 3 μg SKF-82958 | 1,005.0 (±32)* | 5.26 (±0.34)* | 1.97 (±0.03)* |
| 1 μg Raclopride | 244.9 (±76)† | 0.71 (±0.28)† | 0.42 (±0.18)† |
| 5 μg Raclopride | 167.2 (±30)† | 0.26 (±0.09)† | 0.16 (±0.10)† |
| 1 μg Rac/SKF-82958 (3 μg) | 490.4 (±47)* † | 2.06 (±0.27)* † | 0.56 (±0.17)† |
| 5 μg Rac/SKF-82958 (3 μg) | 438.6 (±62)† | 1.76 (±0.27)† | 0.55 (±0.18)† |
Repetitive motor movement values represent mean total scores collapsed across the final 40-min testing phase, whereas head-down sniffing and behavioral intensity values represent mean scores per 5-min time block during the final 40-min testing phase.
Significantly different from the vehicle group.
Significantly different from the 3 μg SKF-82958 group.
Head-down sniffing
The head-down sniffing data provided a pattern of results that was identical to the repetitive motor movement data (Table 6). Specifically, rats microinjected with 3 μg SKF-82958 had significantly more head-down sniffing counts than all other groups [Drug main effect, F(5, 42)=41.84, P<0.001 and Tukey tests], with rats in the SKF-82958/raclopride (1 μg) group being intermediate between, and significantly different from, the vehicle and SKF-82958 groups [Tukey tests]. Rats injected with raclopride (1 or 5 μg) alone did not differ from vehicle controls.
Behavioral intensity
During the testing phase, behavioral intensity scores of the SKF-82958-treated rats were significantly greater than all other groups (Table 6) [Drug effect, KW=24.81, P<0.001 and Dunn’s tests]. Rats given vehicle infusions into the dorsal CPu did not differ from rats given raclopride (1 or 5 μg) alone or rats given a “cocktail” of SKF-82958 plus raclopride (1 or 5 μg).
DISCUSSION
As hypothesized, nonselective stimulation of dopamine receptors in the dorsal CPu significantly increased the locomotor activity of preweanling rats. More specifically, infusing 20 μg NPA or various “cocktail” solutions of SKF-82958/quinpirole into the dorsal CPu caused a robust increase in locomotion on PD 18. The same dopamine agonists also enhanced stereotypic responding regardless of whether stereotypy data were generated by activity monitors or were quantified by human observers. For example, rats treated with NPA or a high-dose “cocktail” of SKF-82958/quinpirole exhibited intense head-down sniffing, an increase in repetitive motor movements, and elevated behavioral intensity scores. In fact, the locomotor activity and stereotypy induced by NPA (20 μg) or a “cocktail” of SKF-82958 (3 μg/10 μg) plus quinpirole (10 μg/20 μg) were quantitatively similar. Lower dose combinations of SKF-82958/quinpirole (1 or 3 μg of each) produced substantial amounts of locomotor activity, but relatively less stereotypy. When these results are considered together, it is clear that concurrent stimulation of D1 and D2 receptors in the dorsal CPu enhanced both locomotor activity and stereotypy, with stereotypy being preferentially expressed as receptor occupancy increased.
Ontogenetic studies have consistently shown that systemic administration of the D2 agonist quinpirole produces both locomotor activity and stereotyped sniffing in preweanling rats of various ages (from at least PD 3 through PD 22) (McDougall et al., 1990; Moody and Spear, 1992; Byrnes and Bruno, 1994; Van Hartesveldt et al., 1994; Sobrian et al., 2003). Results from the present study indicate that the dorsal CPu alone is potentially sufficient for producing these D2 agonist-induced effects, although other subregions of the CPu are likely to share similar behavior-inducing properties. Specifically, infusing 3–10 μg quinpirole into the dorsal CPu resulted in substantial locomotor activity on PD 18, whereas infusing 20 or 30 μg quinpirole caused a smaller, but still significant, increase in locomotion. Opposite effects occurred with head-down sniffing, because the greater doses of quinpirole (20 and 30 μg) produced more head-down sniffing counts than 10 μg quinpirole. This pattern of results can be explained by “behavioral competition” (e.g., Morelli et al., 1980; Bordi et al., 1989), because dose-dependent increases in stereotypy may have begun to overshadow the locomotor activating properties of quinpirole.
In the present study, microinjecting the full D1 agonist SKF-82958 into the dorsal CPu increased both locomotor activity and stereotypy in preweanling rats. The 3 μg dose of SKF-82958 produced the greatest increase in distance traveled scores (Fig. 4 and 8), whereas a larger dose of SKF-82958 (10 μg) stimulated more stereotypy (i.e., repetitive motor movements, head-down sniffing, and behavioral intensity scores). This pattern of behavior accords well with studies utilizing systemic administration techniques, because subcutaneous and intraperitoneal injections of the partial D1 agonist SKF-38393, in doses ranging from 0.01–30 mg/kg, increase the locomotor activity of preweanling rats (McDougall et al., 1990; Moody and Spear, 1992; Byrnes and Bruno, 1994; Shieh and Walters, 1996; but see Sobrian et al., 2003). These D1 agonist-induced effects are not limited to locomotion, because peripheral administration of SKF-38393 also stimulates the sniffing response throughout the preweanling period (Moody and Spear, 1992; Byrnes and Bruno, 1994; Sobrian et al., 2003).
When considering these past results using partial and full D1 agonists, it is surprising that microinjecting SKF-38393 (10–30 μg) into the dorsal CPu did not increase the locomotor activity or stereotypy of preweanling rats. This is especially the case since SKF-82958 (3–10 μg) dramatically increased both classes of behavior in the same aged animals. For example, preweanling rats treated with 10 μg SKF-82958 exhibited much greater distance traveled scores (M = 6,368 cm) and head-down sniffing counts (M = 7.77) than rats infused with 10 μg SKF-38393 (distance traveled, M = 1,227 cm; head-down sniffing, M = 0.51). Researchers infusing SKF-38393 into various striatal structures of adult rats have also occasionally observed some discrepancies in their data. Most notably, Delfs and Kelley (1990) reported that microinjecting high doses of SKF-38393 (up to 30 μg) into the ventrolateral striatum induced substantial tissue damage in the brains of adult rats. This SKF-38393-induced cell damage was so profound that it blocked the normal stimulatory effects of the drug (Delfs and Kelley, 1990). If young rats are particularly susceptible to the potential toxic effects of SKF-38393, it would explain why microinjecting SKF-82958 and SKF-38393 into the dorsal CPu of preweanling rats produced such dramatically different behavioral results. Alternatively, these discrepant findings may be due to differences in the intrinsic activity (Km) of the drugs: SKF-82958 is a full agonist at the D1 receptor (O’Boyle et al., 1989), while SKF-38393 is a partial agonist (Setler et al., 1978; O’Boyle et al., 1989). In the present case, the relevance of these differences in intrinsic activity is uncertain, since systemically administered SKF-38393 is capable of inducing both locomotor activity and stereotypy in preweanling rats (McDougall et al., 1990; Moody and Spear, 1992; Byrnes and Bruno, 1994; Shieh and Walters, 1996). Lastly, it remains possible that infusing lower doses of SKF-38393 into the dorsal CPu might induce locomotor activity and stereotypy on PD 18.
Interestingly, bilateral stimulation of D2 receptors in the dorsal CPu caused a greater locomotor response than if D1 receptors were selectively stimulated (Fig. 4 and 6). This finding is not a consequence of using an insufficient dose of SKF-82958, because the locomotor activity produced by 1–10 μg SKF-82958 fits an inverted “U-shaped” pattern (i.e., 3 μg induces more locomotion than 1 or 10 μg). Thus, substantially higher or lower doses of SKF-82958 would not be expected to induce more locomotor activity than 3 μg SKF-82958 (or 10 μg quinpirole). In terms of stereotypy, we found that 10 μg SKF-82958 produced more repetitive motor movements than any dose of quinpirole (10–30 μg); however, 20 and 30 μg quinpirole stimulated more head-down sniffing than 1–10 μg SKF-82958. In this case, interpretation of the results is limited since it is possible that higher doses of SKF-82958 (> 10 μg) would further increase the head-down sniffing of preweanling rats. In comparison, adult rats only exhibit mild stereotypy when a D1 agonist is infused into the CPu (Kreipke and Walker, 2004; Krolewski et al., 2005), whereas a more intense stereotypic response results from D2 receptor stimulation (Delfs and Kelley, 1990).
To determine whether D1 and D2 receptors in the dorsal CPu are functionally coupled during the late preweanling period: (a) low and moderate doses of SKF-82958 and quinpirole were administered either alone or in combination (Experiment 3); and (b) selective D1 or D2 receptors antagonists were co-administered with the opposite selective dopamine agonist (e.g., SCH-23390 and quinpirole were co-administered). In terms of locomotor activity, receptor coupling was apparent because combined treatment with a near subthreshold dose (1 μg) of SKF-82958/quinpirole produced a potentiated locomotor response when compared to the effects of either agonist alone (Figure 6). Infusing a higher dose (3 μg) of SKF-82958/quinpirole into the dorsal CPu caused stereotypy, rather than locomotor activity, to be preferentially expressed. Dopamine antagonist experiments also indicate that the D1 and D2 receptors mediating locomotor activity are functionally coupled by PD 18, because SCH-23390 (1 or 5 μg) partially attenuated quinpirole-stimulated locomotor activity, whereas both doses of raclopride (1 or 5 μg) blocked SKF-82958-induced locomotion. In terms of stereotypy, it is more uncertain whether D1 and D2 receptors in the dorsal CPu are cooperating to mediate this class of behavior. On the one hand, combined treatment with either low (1 μg) or moderate (3 μg) doses of SKF-82958/quinpirole did not cause a potentiated increase in repetitive motor movements or behavioral intensity scores (Table 4). On the other hand, statistical analysis of head-down sniffing showed that a “cocktail” of 3 μg SKF-82958/quinpirole produced significantly more sniffing counts than SKF-82958 or quinpirole alone. A similar inconsistency was observed with the D1 antagonist data, since SCH-23390 attenuated quinpirole-induced increases in behavioral intensity but not repetitive motor movements or head-down sniffing. Results using the D2 antagonist are more clear, because raclopride caused a partial reduction in SKF-82958-induced repetitive motor movements, head-down sniffing, and behavioral intensity. In general, these results suggest that D1 and D2 receptors systems in the dorsal CPu cooperate when mediating both locomotor activity and stereotypy during the late preweanling period (see also McDougall et al., 1990; Moody and Spear, 1992; Byrnes and Bruno, 1994). In the case of stereotypy, the degree of receptor coupling may be related to the intensity or type of stereotyped behavior being measured.
In conclusion, infusing nonselective and selective dopamine agonists into the CPu tends to affect preweanling (i.e., PD 18) and adult rats similarly. Commonalities during the two ontogenetic periods include the following findings: (a) microinjecting SKF-82958 into the CPu increases the locomotor activity of both preweanling and adult rats (present study; Kreipke and Walker, 2004; Krolewski et al., 2005); (b) stereotypy is enhanced after infusing SKF-82958, quinpirole, or a combination of the two drugs into the CPu (present study; Bordi and Meller, 1989; Canales and Iversen, 1998; Waszczak et al., 2002; Kreipke and Walker, 2004; Krolewski et al., 2005); (c) SCH-23390 infusions reduce D2 agonist-induced behaviors at both ages, although D1 antagonist-induced effects are more pronounced in adult rats (present study; Waszczak et al., 2002); and (d) intra-CPu infusions of a D2 receptor antagonist attenuate D1 agonist-induced behaviors in preweanling and adult rats (present study; Dreher and Jackson, 1989; Waszczak et al., 2002). Possible incongruities between the two ontogenetic periods are evident after selective D2 receptor activation, because intra-CPu infusions of quinpirole produced a dramatic increase in the locomotor activity of preweanling rats (Fig. 4), while causing either a reduction (Bordi and Meller, 1989; Canales and Iversen, 1998) or a subtle, biphasic increase in the locomotor activity of adult rats (Van Hartesveldt et al., 1992). Another ontogenetic difference is apparent when D1 and D2 receptors are co-activated. More specifically, microinjecting either a nonselective dopamine agonist (e.g., NPA or apomorphine) or a combination of selective D1 and D2 agonists (e.g., SKF-82958 plus quinpirole) into the dorsal CPu of adult rats causes an intensification of stereotypy (e.g., gnawing and biting) and a pronounced reduction in locomotor activity (Bordi and Meller, 1989; Waszczak et al., 2002). In contrast, preweanling rats show only a modest intensification of stereotypic behaviors and continue to exhibit a robust locomotor response under the same experimental conditions (Fig. 2 and 6). It is possible that intense stereotypy is not typically an accessible component of the young rats’ behavioral repertoire, thus microinjecting D1 and/or D2 agonists into the CPu may preferentially induce locomotor activity, and not intense stereotypy, in younger animals.
Acknowledgments
This research was supported by NIGMS research grant GM073842 (SAM) and NIGMS training grant DA025319 (TD and FAV).
Abbreviations
- ANOVA
Analysis of variance
- CPu
caudate-putamen
- DMSO
dimethyl sulfoxide
- IP
intraperitoneal
- KW
Kruskal-Wallis
- NPA
R-propylnorapomorphine
- PD
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams DR, Bruno JP. Ontogeny of apomorphine-induced stereotypy and its D1 and D2 receptor mediation in rats depleted of dopamine as neonates. Dev Psychobiol. 1992;25:475–495. doi: 10.1002/dev.420250703. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. A unique hormonal and behavioral responsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–346. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Allen LF, Winn P. Excitotoxic lesions of the pedunculopontine tegmental nucleus disinhibit orofacial behaviours stimulated by microinjections of d-amphetamine into rat ventrolateral caudate-putamen. Exp Brain Res. 1995;104:262–274. doi: 10.1007/BF00242012. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Bordi F, Carr KD, Meller E. Stereotypies elicited by injection of N-propylnorapomorphine into striatal subregions and nucleus accumbens. Brain Res. 1989;489:205–215. doi: 10.1016/0006-8993(89)90852-4. [DOI] [PubMed] [Google Scholar]
- Bordi F, Meller E. Enhanced behavioral stereotypies elicited by intrastriatal injection of D1 and D2 dopamine agonists in intact rats. Brain Res. 1989;504:276–283. doi: 10.1016/0006-8993(89)91368-1. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Bruno JP. Development of uncoupling between D1- and D2-mediated motor behavior in rats depleted of dopamine as neonates. Dev Psychobiol. 1994;27:409–424. doi: 10.1002/dev.420270608. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Crosbie J, Crocker AD. A fixed interval momentary sampling method for assessing on-going behaviours induced by dopamine receptor agonists. Prog Neuro-Psychopharmacol Biol Psychi. 1988;12:595–606. doi: 10.1016/0278-5846(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Behavioral activation in infant rats: pharmacological evidence for dopaminergic mediation. Psychobiology. 1987;15:317–328. [Google Scholar]
- Canales JJ, Gilmour G, Iversen SD. The role of nigral and thalamic output pathways in the expression of oral stereotypies induced by amphetamine injections into the striatum. Brain Res. 2000;856:176–183. doi: 10.1016/s0006-8993(99)02344-6. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Iversen SD. Behavioural topography in the striatum: differential effects of quinpirole and D-amphetamine microinjections. Eur J Pharmacol. 1998;362:111–119. doi: 10.1016/s0014-2999(98)00752-3. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Iversen SD. Dynamic dopamine receptor interactions in the core and shell of nucleus accumbens differentially coordinate the expression of unconditioned motor behaviors. Synapse. 2000;36:297–306. doi: 10.1002/(SICI)1098-2396(20000615)36:4<297::AID-SYN6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Carr GD, White NM. The relationship between stereotypy and memory improvement produced by amphetamine. Psychopharmacology. 1984;82:203–209. doi: 10.1007/BF00427774. [DOI] [PubMed] [Google Scholar]
- Carrera PM, Brunhara FC, Schwarting RK, Tomaz C. Drug conditioning induced by intrastriatal apomorphine administration. Brain Res. 1998;790:60–66. doi: 10.1016/s0006-8993(98)00047-x. [DOI] [PubMed] [Google Scholar]
- Charntikov S, Halladay LR, Herbert MS, Marquez EM, McDougall SA. Effects of dorsal striatal infusions of R(−)-propylnorapomorphine (NPA) on κ-opioid-mediated locomotor activity in the young rat: possible role of the indirect pathway. Neuroscience. 2008;155:603–612. doi: 10.1016/j.neuroscience.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez AM, Charntikov S, Der-Ghazarian T, Horn LR, Crawford CA, McDougall SA. Age-dependent effects of κ-opioid receptor stimulation on cocaine-induced stereotyped behaviors and dopamine overflow in the caudate-putamen: an in vivo microdialysis study. Neuroscience. 2010;169:203–213. doi: 10.1016/j.neuroscience.2010.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Iversen SD. Blockage of amphetamine-induced motor stimulation and stereotypy in the adult rat following neonatal treatment with 6-hydroxydopamine. Brain Res. 1973;55:369–382. doi: 10.1016/0006-8993(73)90302-8. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Kelley AE. The role of D1 and D2 dopamine receptors in oral stereotypy induced by dopaminergic stimulation of the ventrolateral striatum. Neuroscience. 1990;39:59–67. doi: 10.1016/0306-4522(90)90221-o. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias FR, Carey RJ, Carrera MP. Conditioned locomotion induced by unilateral intrastriatal administration of apomorphine: D2 receptor activation is critical but not the expression of the unconditioned response. Brain Res. 2006;1083:85–95. doi: 10.1016/j.brainres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Dickson PR, Lang CG, Hinton SC, Kelley AE. Oral stereotypy induced by amphetamine microinjection into striatum: an anatomical mapping study. Neuroscience. 1994;61:81–91. doi: 10.1016/0306-4522(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Dreher JK, Jackson DM. Role of D1 and D2 dopamine receptors in mediating locomotor activity elicited from the nucleus accumbens of rats. Brain Res. 1989;487:267–277. doi: 10.1016/0006-8993(89)90831-7. [DOI] [PubMed] [Google Scholar]
- Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;5:241–252. [Google Scholar]
- Goldman ME, Kebabian JW. Aporphine enantiomers. Interactions with D-1 and D-2 dopamine receptors. Mol Pharmacol. 1984;25:18–23. [PubMed] [Google Scholar]
- Gong W, Neill DB, Lynn M, Justice JB., Jr Dopamine D1/D2 agonists injected into nucleus accumbens and ventral pallidum differentially affect locomotor activity depending on site. Neuroscience. 1999;93:1349–1358. doi: 10.1016/s0306-4522(99)00235-3. [DOI] [PubMed] [Google Scholar]
- Hauber W, Münkle M. Motor depressant effects mediated by dopamine D2 and adenosine A2A receptors in the nucleus accumbens and the caudate-putamen. Eur J Pharmacol. 1997;323:127–131. doi: 10.1016/s0014-2999(97)00040-x. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotox Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, dopamine and D1/D2 agonists. Neuroscience. 2002;113:939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Lang CG, Gauthier AM. Induction of oral stereotypy following amphetamine microinjection into a discrete subregion of the striatum. Psychopharmacology. 1988;95:556–559. doi: 10.1007/BF00172976. [DOI] [PubMed] [Google Scholar]
- Koene P, Prinssen EP, Cools AR. Involvement of the nucleus accumbens in oral behaviour in the freely moving rat. Eur J Pharmacol. 1993;233:151–156. doi: 10.1016/0014-2999(93)90361-k. [DOI] [PubMed] [Google Scholar]
- Kreipke CW, Walker PD. NMDA receptor blockade attenuates locomotion elicited by intrastriatal dopamine D1-receptor stimulation. Synapse. 2004;53:28–35. doi: 10.1002/syn.20035. [DOI] [PubMed] [Google Scholar]
- Krolewski DM, Bishop C, Walker PD. Intrastriatal dopamine D1 receptor agonist-mediated motor behavior is reduced by local neurokinin 1 receptor antagonism. Synapse. 2005;57:1–7. doi: 10.1002/syn.20148. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Arnold TF, Nonneman AJ. Ontogeny of locomotor activity and grooming in the young rat: role of D1 and D2 receptors. Eur J Pharmacol. 1990;186:223–230. doi: 10.1016/0014-2999(90)90437-b. [DOI] [PubMed] [Google Scholar]
- Meller E, Bohmaker K, Namba Y, Friedhoff AJ, Goldstein M. Relationship between receptor occupancy and response at striatal dopamine autoreceptors. Mol Pharmacol. 1987;31:592–598. [PubMed] [Google Scholar]
- Meyer ME. Effects of intraaccumbens dopamine agonist SK&F38393 and antagonist SCH23390 on locomotor activities in rats. Pharmacol Biochem Behav. 1993;45:843–847. doi: 10.1016/0091-3057(93)90130-l. [DOI] [PubMed] [Google Scholar]
- Meyer ME, Van Hartesveldt C, Potter TJ. Locomotor activity following intra-accumbens microinjections of dopamine D1 agonist SK&F 38393 in rats. Synapse. 1993;13:310–314. doi: 10.1002/syn.890130403. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Wu M. Effects of administration of dopamine D2 agonist quinpirole on exploratory locomotion. Brain Res. 1991;551:216–220. doi: 10.1016/0006-8993(91)90935-o. [DOI] [PubMed] [Google Scholar]
- Moody CA, Spear LP. Ontogenetic differences in the psychopharmacological responses to separate and combined stimulation of D1 and D2 dopamine receptors during the neonatal to weanling age period. Psychopharmacology. 1992;106:161–168. doi: 10.1007/BF02801967. [DOI] [PubMed] [Google Scholar]
- Morelli M, Porceddu ML, Di Chiara G. Lesions of substantia nigra by kainic acid: effects on apomorphine-induced stereotyped behaviour. Brain Res. 1980;191:67–78. doi: 10.1016/0006-8993(80)90315-7. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, O’Dell LE, Khroyan TV. Effects of SCH-23390 on dopamine D1 receptor occupancy and locomotion produced by intraaccumbens cocaine infusion. Synapse. 1998;30:194–204. doi: 10.1002/(SICI)1098-2396(199810)30:2<194::AID-SYN9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, O’Dell LE, Redmond JC. Localization of dopamine receptor subtypes occupied by intra-accumbens antagonists that reverse cocaine-induced locomotion. Brain Res. 1995;671:201–212. doi: 10.1016/0006-8993(94)01317-b. [DOI] [PubMed] [Google Scholar]
- O’Boyle KM, Gaitanopoulos DE, Brenner M, Waddington JL. Agonist and antagonist properties of benzazepine and thienpyridine derivatives at the D1 dopamine receptor. Neuropharmacology. 1989;28:401–405. doi: 10.1016/0028-3908(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain: in stereotaxic coordinates. 4. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Plaznik A, Stefanski R, Kostowski W. Interaction between accumbens D1 and D2 receptors regulating rat locomotor activity. Psychopharmacology. 1989;99:558–562. doi: 10.1007/BF00589908. [DOI] [PubMed] [Google Scholar]
- Schildein S, Ǻgmo A, Huston JP, Schwarting RK. Intraaccumbens injections of substance P, morphine and amphetamine: effects on conditioned place preference and behavioral activity. Brain Res. 1998;790:185–194. doi: 10.1016/s0006-8993(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Setler PE, Sarau HM, Zirkle CL, Saunders HL. The central effects of a novel dopamine agonist. Eur J Pharmacol. 1978;50:419–430. doi: 10.1016/0014-2999(78)90148-6. [DOI] [PubMed] [Google Scholar]
- Shalaby IA, Spear LP. Psychopharmacological effects of low and high doses of apomorphine during ontogeny. Eur J Pharmacol. 1980;67:451–459. doi: 10.1016/0014-2999(80)90186-7. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Timiras P. A stereotaxic atlas of the developing rat brain. Berkeley: University of California Press; 1970. [Google Scholar]
- Shieh G-J, Walters DE. Stimulating dopamine D1 receptors increases the locomotor activity of developing rats. Eur J Pharmacol. 1996;311:103–107. doi: 10.1016/0014-2999(96)00417-7. [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ., Jr . Nonparametric statistics for the behavioral sciences. 2. Boston: McGraw-Hill; 1988. [Google Scholar]
- Sobrian SK, Jones BL, Varghese S, Holson RR. Behavioral response profiles following drug challenge with dopamine receptor subtype agonists and antagonists in developing rat. Neurotoxicol Teratol. 2003;25:311–328. doi: 10.1016/s0892-0362(03)00009-6. [DOI] [PubMed] [Google Scholar]
- Spear LP. The use of psychopharmacological procedures to analyse the ontogeny of learning and retention: issues and concerns. In: Spear NE, Campbell BA, editors. Ontogeny of learning and memory. Hillsdale, NJ: Lawrence Erlbaum Associates; 1979. pp. 135–156. [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Staton DM, Solomon PR. Microinjections of d-amphetamine into the nucleus accumbens and caudate-putamen differentially affect stereotypy and locomotion in the rat. Physiol Psychol. 1984;12:159–162. [Google Scholar]
- Swanson CJ, Heath S, Stratford TR, Kelley AE. Differential behavioral responses to dopaminergic stimulation of nucleus accumbens subregions in the rat. Pharmacol Biochem Behav. 1997;58:933–945. doi: 10.1016/s0091-3057(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Van Hartesveldt C, Cottrell GA, Potter T, Meyer ME. Effects of intracerebral quinpirole on locomotion in rats. Eur J Pharmacol. 1992;214:27–32. doi: 10.1016/0014-2999(92)90091-h. [DOI] [PubMed] [Google Scholar]
- Van Hartesveldt C, Meyer ME, Potter TJ. Ontogeny of biphasic locomotor effects of quinpirole. Pharmacol Biochem Behav. 1994;48:781–786. doi: 10.1016/0091-3057(94)90346-8. [DOI] [PubMed] [Google Scholar]
- Waszczak BL, Martin LP, Finlay HE, Zahr N, Stellar JR. Effects of individual and concurrent stimulation of striatal D1 and D2 dopamine receptors on electrophysiological and behavioral output from rat basal ganglia. J Pharmacol Exp Ther. 2002;300:850–861. doi: 10.1124/jpet.300.3.850. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]