Abstract
Mature B cells generate protective immunity by undergoing immunoglobulin (Ig) class switching and somatic hypermutation, two Ig gene-diversifying processes that usually require cognate interactions with T cells that express CD40 ligand. This T cell-dependent pathway provides immunological memory but is relatively slow to occur. Thus it must be integrated with a faster, T cell-independent pathway for B cell activation through CD40 ligand-like molecules that are released by innate immune cells in response to microbial products. Here we discuss recent advances in our understanding of the interplay between the innate immune system and B cells, particularly at the mucosal interface. We also review the role of innate signals in the regulation of Ig diversification and production
B cells: at the interface between innate and adaptive immunity
The mammalian immune system comprises of innate and adaptive branches that integrate to mount sophisticated responses that combat invading pathogens, while preserving homeostasis at mucosal sites colonized by non-invasive commensal bacteria [1]. Dendritic cells (DCs), monocytes, macrophages, granulocytes, natural killer (NK) cells and epithelial cells of the innate immune system mediate fast but non-specific responses after recognizing generic microbial structures through germline gene-encoded receptors, often referred to as pattern recognition receptors (PRRs) [2, 3]. In contrast, T and B cells of the adaptive immune system mediate specific, but temporally delayed responses, after recognizing discrete antigenic epitopes through somatically recombined receptors [4]. Remarkably, the innate and adaptive immune systems are functionally linked by mechanisms that were originally predicted by Charles Janeway Jr. in his unified model of the immune response [2]. According to this model, innate immune cells instruct T and B cells to initiate adaptive immune responses upon sensing highly conserved microbial molecular signatures through PRRs, including Toll-like receptors (TLRs) [5–7]. However, TLRs might not be the only PRRs capable of delivering instructing signals to T and B cells; indeed some studies did not confirm a role for TLRs in the control of adaptive immunity [8]. One possibility is that the innate immune system utilizes distinct PRR signaling pathways in relation to the specific composition of the immunizing antigen [9].
Similar to innate immune cells, B cells also express TLRs [10]. B cell-intrinsic TLRs seem to cooperate with adaptive Ig receptors not only to achieve rapid humoral immunity, but also to preserve long-term humoral memory [11–13]. This cooperation is particularly developed in extrafollicular B cells that are positioned at the interface between the sterile milieu of the body and the external environment [14, 15]. Such “frontline” B cells include peritoneal B-1 cells and splenic marginal zone (MZ) B cells [16]. In the mouse, B-1 cells constitute a distinct lineage of self-renewing B cells that are produced during fetal life and are mostly localized in the peritoneal cavity, spleen and intestine [17]. B-1 cells generate innate (or “natural”) adaptive immunity by spontaneously releasing large amounts of polyspecific IgM and IgA antibodies that provide a first line of defense against viral and bacterial infections [17]. Recent findings indicate that humans also have a subset of B cells that are functionally equivalent to mouse B-1 cells [18]. Similar to B-1 cells, MZ B cells express polyspecific antibodies that recognize T cell-independent (TI) antigens with low affinity [14, 19, 20]. These antigens do not require CD4+ T helper cells to activate B cells and include microbial TLR ligands (type-1) and microbial polysaccharides with repetitive structure (type-2) [21].
Importantly, both B-1 and MZ B cells are characterized by a state of active readiness, which involves elevated TLR expression, as well as expression of “innate” Ig receptors with poorly diversified antigen-binding variable regions that can recognize multiple highly conserved microbial products [14, 19, 20]. B-1 and MZ B cells also show elevated responsiveness to B cell-activating factor of the TNF family (BAFF) and a proliferation-inducing ligand (APRIL) [22, 23], two B cell-stimulating factors released by innate immune cells in response to microbial TLR ligands [24–26]. Unlike extrafollicular B-1 and MZ B cells, follicular B cells (also called B-2 cells) produce monospecific IgM, IgG and IgA antibodies that recognize T cell-dependent (TD) antigens with high affinity. These antigens include microbial proteins and typically require CD4+ T helper cells to activate B cells. However, growing evidence indicates that conventional follicular B cells can also utilize BAFF and APRIL to initiate rapid Ig responses, particularly at the mucosal interface [27, 28]. In this review, we discuss the mechanisms by which TLR ligands cooperate with BAFF and APRIL to induce Ig diversification and production in systemic and mucosal B cells.
Ig diversification and production
Ig gene diversification is essential for B cells to mount protective humoral responses. Mature B cells diversify their Ig genes through class switch recombination (CSR) and somatic hypermutation (SHM), two processes that require the DNA-editing enzyme activation-induced cytidine deaminase (AID) [29]. SHM introduces point mutations within V(D)J exons, thereby providing the structural correlate for selection of high-affinity Ig mutants by antigen [30], whereas CSR replaces constant μ (Cμ) and Cδ exons encoding IgM and IgD with Cγ, Cα, or Cε exons encoding IgG, IgA, or IgE, thereby providing antibodies with novel effector functions without changing antigen specificity [31]. In humans, a non-canonical form of CSR from Cμ to Cδ also exists and generates B cells specialized in IgD production [32]. Most antigens initiate CSR and SHM in the germinal center of lymphoid follicles [33], a specialized microenvironment that fosters antigen-driven cognate interactions between B cells and CD4+ T helper cells that express CD40 ligand (CD40L) [34].
Engagement of CD40 on B cells by trimeric CD40L causes recruitment of TNF receptor-associated factor 2 (TRAF2), TRAF3, TRAF5 and TRAF6 adaptor proteins to the cytoplasmic tail of CD40 [35]. Such recruitment is followed by activation of an IκB kinase (IKK) complex that triggers phosphorylation and proteasomal degradation of inhibitor of NF-κB (IκB) [36]. The resulting IκB-free p50–p65 and p50-c-Rel NF-kB complexes translocate from the cytoplasm to the nucleus to initiate germline Ig gene transcription as well as transcription of the gene encoding AID [31]. Germline Ig gene transcription renders the intronic switch (S) regions, which are located upstream of each of the Cγ, Cα, or Cε genes, a substrate of AID, which in turns generates multiple DNA lesions that are ultimately processed into double-stranded DNA breaks [31]. Fusion of DNA breaks within Sμ and a recombining Sγ, Sα, or Sε region induces looping-out deletion of the intervening DNA, thereby deleting Cμ and Cδ while juxtaposing Cγ, Cα, or Cε to the VDJ exon [31]. The mechanism by which AID targets the cryptic switch-like σδ region upstream of Cδ remains unknown [32, 37].
Ig responses through TD and TI pathways
High-affinity and class-switched B cells that emerge from germinal centers undergo selection by antigen exposed on follicular dendritic cells (FDCs) and thereafter differentiate into long-lived memory B cells, which enter the circulation, and Ig-secreting plasma cells, which home to the bone marrow or mucosal effector sites such as the intestine [38]. This TD pathway requires at least 5–7 days to develop, a delay that could prove fatal in the presence of blood-borne or mucosal infections. To compensate for this limitation, B-1 cells and MZ B cells generate IgM, as well as IgG and IgA antibodies through a faster TI pathway that involves recognition of highly conserved microbial products by low-affinity Ig receptors and TLRs [15–17, 19]. DC and epithelial cells also recognize microbial products and in response release BAFF and APRIL, two CD40L-related CSR-inducing factors that cooperate with Ig and TLR ligands to activate B cells [24–26, 39]. The sequence of events leading to TI activation of innate B cells remains unclear, but one possibility is that TI antigens such as LPS or CpG DNA predominantly trigger TLRs when present at high concentration [40]. When present at low concentration, TI antigens may engage both TLRs and innate Ig receptors [40]. In this context, microenvironmental co-signals from BAFF and APRIL receptors would integrate with signals from Ig receptors and TLRs to optimize B cell survival, Ig production, Ig diversification and plasma cell differentiation [41].
Although TI pathways usually generate unmutated Igs that recognize multiple microbial antigens with low affinity, TI pathways can also give rise to hypermutated Igs with relatively high affinity for specific microbial antigens, at least in humans. Indeed, human splenic MZ B cells express V(D)J genes that contain somatic mutations, albeit to a lesser degree than germinal center B cells, and encode Igs that recognize polysaccharides from encapsulated bacteria [14, 42]. The mechanism by which human MZ B cells undergo SHM remains unknown, but growing evidence suggests the involvement of CD40-independent signals from microbial TLR ligands [43]. Consistent with their ability to trigger AID expression and CSR [12, 13, 44, 45], signals from TLRs induce SHM in precursors of human MZ B cells [46–48]. Similarly, TLRs elicit SHM in extrafollicular B cells from autoimmune-prone mice [49, 50] and in a subset of bone marrow B cells from wild type mice [51, 52]. The involvement of BAFF and APRIL in TI pathways for SHM is currently unknown, but seems probable, because BAFF and APRIL cooperate with TLR ligands to induce AID expression in B cells [39, 45, 53].
The participation of PRRs other than TLRs in Ig CSR, SHM and production seems likely, although only few data are currently available. Similar to TLRs [54], NOD (nucleotide oligomerization domain)-like receptors (NLRs) might play an important role in Ig responses against respiratory viruses [55]. These viruses can elicit neutralizing Ig responses in a TI manner [56], but the role of NLRs in TI Ig production remains unknown. Also mannose C-type lectin receptors (MCLRs) such as dendritic cell-specific ICAM3 grabbing nonintegrin (DC-SIGN) can deliver TI activating signals to a subset of B cells upon exposure to viral glycoproteins such as gp120 from HIV envelope [57]. Clearly, further studies are needed to fully understand the role of NLRs, MCLRs and other PRRs in the activation of systemic and mucosal B cells.
Innate Ig-inducing factors in mucosal B cells
In addition to enhancing the antigen presenting function of DCs [58], TLRs stimulate DCs, as well as macrophages, granulocytes and epithelial cells, to release BAFF and APRIL [27, 28, 32, 45, 53, 59]. These innate CD40L-related molecules cooperate with TLR ligands to induce CSR from IgM to IgA in “frontline” B cells that inhabit the mucosal interface and namely the intestine [15, 25, 60]. The intestinal mucosa is inhabited by large communities of commensal bacteria that are kept outside the sterile milieu of our body by various immune strategies that generate protection without causing inflammation. A notable non-inflammatory strategy for mucosal defense involves TI production of IgA antibodies by follicular and extrafollicular B cells.
Mucosal follicular B cells
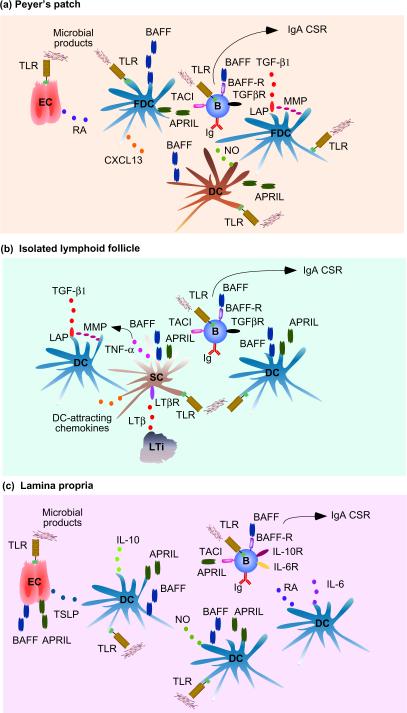
IgA is the most abundant antibody in intestinal secretions and its production mainly takes place in mucosal lymphoid follicles such as Peyer's patches. Together with isolated lymphoid follicles, Peyer's patches have a less stringent requirement for cognate T-B cell interaction than systemic lymphoid follicles [61–63]. Instead, mucosal follicles are highly dependent on innate microbial signals, as lack of the TLR-associated adaptor protein myeloid differentiation primary response gene 88 (MyD88) disrupts intestinal IgA responses [27, 28, 64]. Recent evidence shows that microbial signals stimulate IgA production in B cells from Peyer's patches through a mechanism involving FDCs (Figure 1a). Indeed, signals from TLRs trigger FDC release of BAFF and APRIL as well as production of matrix metalloproteases (MMPs) that promote cleavage of active transforming growth factor-β 1 (TGF-β1) from a membrane-bound precursor known as latency-associated peptide [27, 28]. By activating SMAD transcription factors, TGF-β1 initiates germline Cα gene transcription, which is essential to initiate IgA CSR [25]. TLR-activated FDCs also release the chemokine CXCL13, which attracts follicular B cells expressing the chemokine receptor CXCR5 [27, 28]. Intestinal FDCs become particularly proficient in these responses as a result of their exposure to retinoic acid [28], a vitamin A-derived immuneoregulatory molecule produced by epithelial cells in response to microbial TLR ligands [65].
Figure 1.
BAFF and APRIL signaling networks in B cells. (a) TLR-activated epithelial cells (ECs) from Peyer's patches release retinoic acid (RA), which “primes” TLR-activated FDCs to produce BAFF, APRIL, the B cell-attracting chemokine CXCL13 as well as MMPs. These enzymes generate active TGF-β1 from latency-associated peptide (LAP). As well as producing additional BAFF and APRIL, TLR-activated TipDCs release nitric oxide (NO), which up-regulates TGF-β1 receptor (TGFβR) on B cells. In the presence of B cell-intrinsic TLR signals, engagement of TACI, BAFF-R and TGFβR by BAFF, APRIL and TGFβ stimulates IgA CSR and secretion. (b) Lymphoid tissue inducer (LTi) cells from isolated lymphoid follicles release LTβ, which enhances production of TNF and DC-attracting chemokines in local TLR-activated stromal cells (SCs). TNF up-regulates the expression of MMPs and release of TGF-β1 in TLR-activated DCs, which also secrete BAFF and APRIL as TLR-activated SCs do. BAFF, APRIL and TGF-β stimulate follicular B cells as in (a). (c) TLR-activated ECs from the lamina propria release BAFF, APRIL and TSLP, which elicits BAFF, APRIL as well as IL-10 production, in TLR-activated myeloid DCs. NO from TLR-activated TipDCs further enhances DC production of BAFF and APRIL. Another DC subset releases RA and IL-6, which promote IgA secretion. BAFF, APRIL and TGF-β stimulate follicular B cells as in (a) together with engagement of IL-10R, IL-6R and retinoic acid receptor (not shown). APRIL, a proliferation-inducing ligand; BAFF, B cell-activating factor of the TNF family; CSR, class switch recombination; DC, dendritic cell; FDC, follicular dendritic cell; MMP, matrix metalloprotease; TACI, transmembrane activator and calcium-modulating ciclophilin-ligand interactor; TLR, Toll-like receptor.
Together with B cell-intrinsic signals from TLRs, signals from BAFF, APRIL and TGF-β1 receptors would not only enhance local TD IgA responses [66, 67], but would also initiate IgA CSR and production in a TI manner [28, 66]. In Peyer's patches, innate IgA responses are further augmented by DCs that express TNF and inducible nitric oxide synthase (TipDCs) [64, 68]. When activated by TLR ligands, TipDCs release BAFF, and APRIL, as well as nitric oxide, a compound that up-regulates the expression of the type-II TGF-β receptor on follicular B cells [64]. In isolated lymphoid follicles (Figure 1b), lymphotoxin β (LTβ) from TLR-activated lymphoid tissue-inducer cells stimulates local stromal cells to release tumor necrosis factor (TNF) as well as DC-attracting chemokines such as CCL19 and CCL21 [27]. In these DCs, TNF promotes production of active TGF-β1 [27]. In the presence of TLRs signals, BAFF and APRIL are produced by stromal cells and DCs, and cooperate with TGF-β1 to induce TI IgA CSR and production [27].
Mucosal extrafollicular B cells
Extrafollicular B cells from the intestinal lamina propria can also undergo IgA CSR and production in response to microbial products, although less efficiently and at a lower frequency than follicular B cells [15, 25, 69, 70]. In the lamina propria (Figure 1c), B cells undergo IgA CSR and production in response to BAFF, APRIL, IL-6, IL-10, TGF-β1 and retinoic acidproduced by TipDCs and a subset of DCs expressing TLR5 (a PRR for microbial flagellin) but not other TLRs [53, 70, 71]. Signals from TLRs also stimulate TipDCs to release nitric oxide, which in turn enhances TipDC production of BAFF and APRIL in an autocrine manner [64]. Additional innate factors involved in the induction and/or amplification of TI antibody responses are IFN-α/β and thymic stromal lymphopoietin (TSLP). IFN-α/β is an antiviral and immunoenhancing cytokine produced by plasmacytoid DCs in response to TLR ligands [72]. In addition to augmenting BAFF and APRIL release by myeloid DCs [73], IFN-α/β increases the expression of TLR7 (a receptor for viral single-stranded RNA) in naive and memory B cells, thereby accelerating TLR7-induced plasma cell formation and Ig production [74, 75]. TSLP is an IL-7-like cytokine produced by epithelial cells that up-regulates production of BAFF and APRIL in myeloid DCs [45, 53, 76]. Interestingly, TSLP also augments myeloid DC release of IL-10, an anti-inflammatory cytokine that promotes IgA CSR and production in human B cells [45, 53, 77].
Innate Ig-inducing signaling pathways
TACI and TLRs
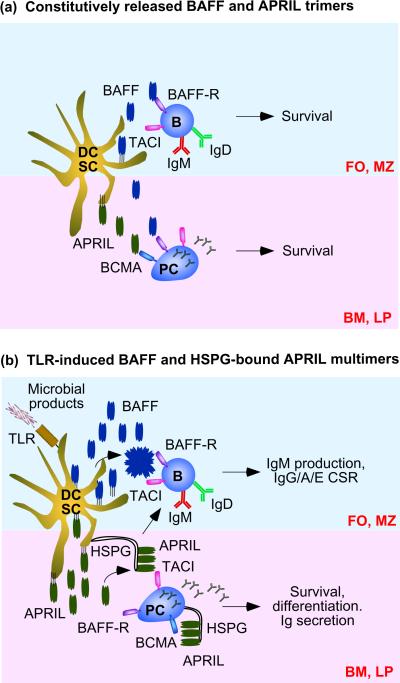
BAFF and APRIL trigger CD40-independent IgM production, CSR and plasma cell survival and differentiation by engaging transmembrane activator and calcium-modulating ciclophilin-ligand interactor (TACI) [22, 39, 64, 66, 67, 73, 78–80]. This receptor triggers productive signals only after undergoing high order oligomerization in response to soluble capsid-like BAFF 60-mers (20 trimers) or cell-bound APRIL oligomers that are associated with heparan sulphate proteoglycans [26]. Such multimeric ligands would be mostly released by innate immune cells in response to microbial TLR ligands and might be critical for TACI to initiate TI Ig responses (Box 1). Under steady-state conditions, however, innate immune cells and stromal cells would instead release soluble BAFF and APRIL trimers (Figure 2a), which deliver powerful survival signals to B cells and plasma cells via the BAFF receptor (BAFF-R) and B cell maturation antigen (BCMA) for which APRIL is a ligand [26]. Indeed, BAFF-R and BCMA have less stringent oligomerization requirements than TACI [26]. In addition to favoring expression of membrane-bound versus soluble BAFF and APRIL multimers (Figure 2b), microbial TLR ligands also induce up-regulation of TACI expression on B cells, particularly on B-1 and MZ B cells specialized in fast Ig responses [23, 39, 81–83]. TLRs further cooperate with TACI by converging on NF-κB signalling (Figure 3), which is critical for the induction of AICDA and germline Ig gene transcription, two events essential for CSR [25]. Such cooperation might explain why TACI requires B cell-intrinsic co-signals from TLRs to efficiently activate Ig production [39].
Figure 2.
Modalities of B cell activation by BAFF and APRIL. (a) Constitutive DC and stromal cell (SC) release of soluble BAFF trimers (upper half) stimulates survival in follicular (FO) and MZ B cells. BAFF trimers predominantly elicit signals through BAFF-R. Constitutive DC and SC release of APRIL (bottom half) supports plasma cell survival in the bone marrow (BM) and perhaps intestinal lamina propria (LP). APRIL trimers predominantly signal through BCMA. (b) TLR-induced DC and stromal cell (SC) release of abundant BAFF and APRIL trimers causes formation of virus-like BAFF multimers and HSPG-bound APRIL multimers, which induce IgM production, IgG IgA and IgE CSR, Ig secretion, survival and differentiation by signaling through TACI as well as BCMA and BAFF-R in B cells and plasma cells. HSPGs are in particularly expressed on SCs and plasma cells. APRIL, a proliferation-inducing ligand; BAFF, B cell-activating factor of the TNF family; BCMA, B cell maturation antigen; CSR, class switch recombination; DC, dendritic cell; HSPG, heparan sulphate proteoglycan; MZ, marginal zone; TACI, transmembrane activator and calcium-modulating ciclophilin-ligand interactor; TLR, Toll-like receptor.
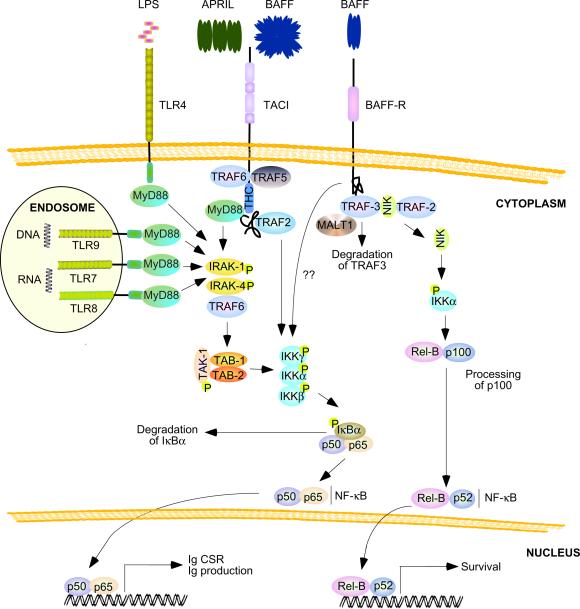
Figure 3.
BAFF and APRIL signaling pathways in B cells. Soluble BAFF multimers or cell-bound APRIL multimers trigger recruitment of TRAF2, TRAF5 and TRAF6 to TACI, thereby activating an IKK complex that mediates phosphorylation of IκBα, followed by proteasomal degradation of IκBα. In the absence of IκBα, p50–p65 and p50c-Rel (not shown), NF-κB dimers translocate from the cytoplasm to the nucleus, where they activate genes involved in Ig CSR and production. A TACI highly conserved (THC) domain also recruits MyD88 similarly to TLR4 (expressed by mouse but not human B cells), TLR7, TLR8 and TLR9, which are receptors for microbial lipolpolysaccharide (LPS), single-stranded RNA and CpG-rich DNA. These TLRs are particularly abundant in B cells and recruit MyD88 through a TIR domain. MyD88 signals from TACI and TLRs activate IKK through IRAK-1, IRAK-4, TRAF6, TAK-1, TAB-1 (TAK-1-binding protein 1), and TAB-2 (TAK-1-binding protein 2). Soluble BAFF trimers induce recruitment of TRAF3 to BAFF-R, followed by TRAF3 degradation through a mechanism involving interaction of TRAF3 with TRAF2 and MALT1. In the absence of TRAF3, NIK elicits phosphorylation of p100, followed by processing of p100 to p52 and translocation of p52-RelB complexes from the cytoplasm to the nucleus, where they activate genes involved in B cell survival. BAFF-R can also activate the canonical NF-κB pathway through a mechanism that remains unclear. CSR, class switch recombination; IkB, inhibitor of NF-κB; IKK, IκB kinase; IRAK, IL-1 receptor-associated kinase; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein 1; MyD88, myeloid differentiation primary response gene 88; NIK, NF-κB-inducing kinase; TIR doman, Toll-IL-1 receptor domain; TRAF, TNF receptor-associated factor.
The interconnectivity of TACI with TLR pathways may have further layers of complexity (Figure 3). Engagement of TACI by APRIL triggers recruitment of the TLR-associated adaptor protein MyD88 to a highly conserved cytoplasmic domain of TACI distinct from the canonical cytoplasmic Toll-IL-1 receptor (TIR) domain of TLRs [39]. Interaction of TACI with MyD88 is followed by activation of a TLR-like pathway that involves IL-1 receptor-associated kinase-1 (IRAK-1), IRAK-4, TRAF6, TGF-β-associated kinase 1 (TAK1) and IKK [39]. As seen in the CD40 pathway [35], TACI- or TLR-activated IKK induces nuclear translocation of NF-κB by triggering phosphorylation and degradation of IκBα [39]. TACI further activates NF-κB by recruiting TRAF2 [84], an adaptor protein that plays an important role in CSR [85]. In general, these findings suggest that TACI and TLRs converge on MyD88 and TRAF adaptor proteins to optimize Ig diversification and production in frontline B cells.
BAFF-R and BCMA
BAFF and APRIL further activate B cells through BAFF-R and BCMA [24, 26]. The mandatory role of BAFF and BAFF-R in the survival of peripheral B cells is well known [24, 26]. Yet, BAFF-R also plays an important role in cell growth (Box 2) as well as Ig diversification and production. Indeed, mouse B cells lacking BAFF-R show less induction of AID expression and CSR upon exposure to BAFF, suggesting that BAFF-R delivers Ig-diversifying in addition to B cell survival signals [78]. This possibility would be consistent with in vivo data pointing to a role of BAFF-R in both follicular and extrafollicular Ig responses [86, 87]. Signals from BAFF-R mostly rely on ligand-induced recruitment of TRAF3 to BAFF-R (Figure 3), which is followed by degradation of TRAF-3 through a mechanism involving interaction of TRAF3 with TRAF2 and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1) [88–91]. In the absence of TRAF3, the enzyme NF-κB-inducing kinase (NIK) activates an alternative NF-κB pathway that involves proteosomal degradation of p100 to p52 and translocation of p52-RelB complexes from the cytoplasm to the nucleus [90–93]. While essential for the activation of genes involved in B cell survival, p52 and RelB are not important for the induction of genes mediating CSR [94], suggesting that BAFF-R utilizes an alternative pathway to up-regulate AID expression and germline Ig gene transcription [78]. Consistent with this possibility, BAFF-R can also activate the canonical NF-κB pathway through a mechanism that remains unclear [73, 95].
As for BCMA, this receptor enhances plasma cell survival, plasma cell differentiation and B cell antigen presentation by recruiting TRAF proteins and activating NF-κB [96–98]. The role of BCMA in Ig diversification and production is unknown, but lack of BCMA does not seem to affect the induction of CSR by BAFF or APRIL [78]. Yet, BCMA might regulate post-CSR events, including the differentiation and survival of mucosal IgA-secreting plasma cells [99]. Consistent with this possibility, intestinal epithelial cells and DCs express large amounts of APRIL [53], which mediates plasma cell survival through BCMA [100, 101].
Immunodeficiencies involving TACI, BAFF-R and TLRs
Consistent with an important role of TACI in Ig diversification and production, some individuals with deleterious TACI substitutions suffer from recurrent infections by encapsulated bacteria and have less serum IgM, IgG and IgA (hypogammaglobulinemia) and impaired IgG responses to TI antigens such as capsular polysaccharides [102–104] (see also Box 1). Also patients with deleterious BAFF-R substitutions suffer from recurrent infections and hypogammaglobulinemia, and also have less circulating B cells [105], which are normal or increased even in TACI-deficient patients [102, 103]. No primary immunodeficiencies involving BCMA have been identified thus far.
Unlike patients with deleterious TACI substitutions, patients lacking MyD88 or IRAK-4 (a kinase downstream of MyD88) have recurrent infections with pyogenic bacteria, including encapsulated bacteria, but do not develop hypogammaglobulinemia and their responses to TI antigens such as capsular polysaccharides are impaired only sporadically in vivo [39, 106, 107]. However, human TLRs and TACI recruit both MyD88 and IRAK-4 to trigger TI Ig diversification and production in vitro [12]. One possibility is that human TLRs and TACI utilize a MyD88-independent pathways to initiate TI Ig responses and a MyD88-dependent pathways to sustain TI Ig responses over time. Alternatively, MyD88-dependent pathways might be important to optimize the class and affinity of TI Ig responses. A better understanding of these issues would require a systematic analysis of intestinal and respiratory Ig responses in patients with deleterious TACI, MyD88 or IRAK-4 substitutions. In mice, lack of TLRs or MyD88 impairs intestinal TI IgA production [28, 64], whereas lack of TACI impairs systemic TI IgM, IgG and IgA production [108], but has unclear effects on respiratory TI IgA production [67]. In light of these findings, it is likely that the contribution of TLR and TACI to mucosal immunity varies depending on the type of antigen (viral versus bacterial, soluble versus particulate) and the route of immune challenge.
Concluding remarks
B cells represent a unique component of the adaptive immune system as they express both specific Ig receptors and non-specific PRRs, including TLRs. This dual recognition system facilitates the initiation of Ig diversification and production through either a TD pathway involving CD40L or a TI pathway involving BAFF and APRIL. TD and TI pathways are not rigidly compartmentalized, as both require co-signals from Ig receptors, cytokine receptors and TLRs for efficient B cell activation. In addition, there is crosstalk between TD and TI signals to optimize Ig diversification, Ig production and memory B cell survival [11, 53, 66]. Although the heterogeneity and plasticity of the B cell signaling systems reviewed here might seem complex, more details of the role of innate signals in the generation of immunoprotective and immunoregulatory B cells continue to emerge [54, 109]. Given the key role of B cells in generating immunity and homeostasis at mucosal sites, a better understanding of innate B cell-stimulating signaling networks will help to develop novel or more effective vaccines against mucosal pathogens, including human immunodeficiency virus [60]. Innate B cell-stimulating signals are further implicated in the survival and expansion of autoreactive and neoplastic B cells [24, 26, 110] and therefore a more complete knowledge of such signals will also be critical to devise better treatments against autoimmune disorders and lymphoid tumors.
Box 1. TACI delivers Ig-inducing signals to B cells.
TACI triggers CD40-independent Ig CSR and production by activating the classical NF-κB pathway in B cells [39, 73, 111–113]. Multiples lines of evidence show the importance of TACI in the induction of Ig responses in vivo. In mice, TACI deficiency reduces serum IgA under steady-state conditions and impairs IgM, IgG and IgA responses to TI antigens [108]. Consistent with this, APRIL-deficient mice show decreased intestinal IgA production [114], whereas APRIL-transgenic mice show enhanced IgM and IgG responses to TI antigens [115]. Notably, lack of TACI also increases peripheral B cell numbers [108, 116], possibly because TACI inhibits BAFF-R and CD40 signaling in addition to stimulating CD40-independent Ig production [117]. In humans, deleterious mutations of one or both TACI alleles are associated with about 8% of cases of common variable immunodeficiency (CVID), a primary immunodeficiency that predisposes to bacterial infections by impairing steady state as well as antigen-induced IgM, IgG and/or IgA production [102, 104]. Unlike TACI-deficient mice, CVID patients with heterozygous or homozygous mutations affecting the extracellular or transmembrane domain of TACI show defects in both TI and TD arms of humoral immunity, indicating that TACI is more important for Ig production in humans than is in mice [103]. Although generally impairing Ig production in vitro [39, 102], TACI substitutions do not always cause disease in vivo. Indeed, about 2% of the normal human population carries a mutant TACI allele [118]. In addition, CVID develops most of the times in the absence of TACI substitutions and a heterozygous TACI substitution did not segregate with disease in one case of familial CVID [102, 103, 118, 119]. Furthermore, in the case of two brothers with identical homozygous TACI substitution, CVID was present only in one brother, whereas the other brother had low serum Igs but no disease [102]. Thus, although having a negative impact on Ig production, TACI substitutions seem to require additional genetic and/or environmental factors to cause disease.
Box 2. BAFF-R delivers growth signals to B cells.
BAFF-R facilitates the growth (i.e., increase of volume) of B cells by activating the protein kinase mammalian target of rapamycin (mTOR) in the context of mTOR complex 1 (mTORC1), a signaling module that also comprises regulatory associated protein of mTOR (Raptor) [120]. BAFF activates mTORC1 through protein kinase AKT1, also called protein kinase B (PKB) [120, 121]. BAFF phosphorylates AKT1 via phosphoinositide 3 kinase (PI3K)-mediated activation of phosphoinositide-dependent kinase 1 (PDK1) [120]. BAFF would further elicit PI3K-independent phosphorylation of AKT1 through protein kinase C β (PKCβ) and mTOR complex 2 (mTORC2), a signaling module that comprises rapamycin insensitive companion of mTOR (Rictor) [120–122]. Well-characterized downstream targets of mTORC1 are S6 kinase 1 (S6K1) and eukaryotic initiator factor 4E (EIF4E) binding protein 1 (4EBP1) [120, 122]. After being phosphorylated by mTORC1, S6K1 activates ribosomes and augments protein synthesis, whereas 4EBP1 releases EIF4E, which then promotes mRNA translation [120, 122]. Interestingly, 4EBP1 is also phosphorylated by PIM2, a kinase induced by signaling via NF-κB2, highlighting the presence of a close interplay between BAFF-induced survival and growth signals in B cells [120]. In this regard, it must also be noted that the PI3K-AKT1 pathway downstream of BAFF up-regulates the transcription of the gene encoding myeloid cell leukemia-1 (MCL-1), a short-lived member of the B cell lymphoma-2 (BCL-2) protein family critical for the survival of peripheral B cells [120]. Furthermore, BAFF increases the translation of MCL1 by activating mTORC1 and mitigates the destabilization of MCL-1 by activating AKT1, which in turn relieves the negative regulation of MCL-1 as imposed by glycogen synthase kinase β (GSKβ) [120]. In conclusion, alternative NF-κB signaling, PI3K-AKT1-mTOR signaling and up-regulated expression of MCL-1 are critical events in the induction of survival and growth signals in B cells exposed to BAFF. It remains unknown whether the PI3K-AKT1-mTOR pathway also contributes to Ig diversification and production.
Acknowledgements
The authors are supported by US National Institutes of Health grants R01 AI-074378 (to A.C.), Ministerio de Ciencia e Innovación grant SAF 2008-02725 (to A.C.), and a Juan de la Cierva fellowship (to I.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sansonetti PJ. War and peace at mucosal surfaces. Nat. Rev. Immunol. 2004;4:953–964. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 4.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Janeway CA., Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 6.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 8.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Lanzavecchia A, Sallusto F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr. Opin. Immunol. 2007;19:268–274. doi: 10.1016/j.coi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Bernasconi NL, et al. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 12.He B, et al. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J. Immunol. 2004;173:4479–4491. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 13.Xu W, et al. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J Immunol. 2008;181:276–287. doi: 10.4049/jimmunol.181.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weill JC, et al. Human marginal zone B cells. Annu.Rev. Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 15.Fagarasan S, et al. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- 16.Fagarasan S, Honjo T. T-Independent immune response: new aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 17.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 18.Griffin DO, et al. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70. J. Exp. Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bendelac A, et al. Autoreactivity by design: innate B and T lymphocytes. Nat. Rev. Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 20.Martin F, Kearney JF. Marginal-zone B cells. Nat. Rev. Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 21.Mond JJ, et al. T cell independent antigens. Curr. Opin. Immunol. 1995;7:349–354. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 22.Balazs M, et al. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 23.Ng LG, et al. BAFF costimulation of Toll-like receptor-activated B-1 cells. Eur. J. Immunol. 2006;36:1837–1846. doi: 10.1002/eji.200635956. [DOI] [PubMed] [Google Scholar]
- 24.Dillon SR, et al. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat. Rev. Drug Discov. 2006;5:235–246. doi: 10.1038/nrd1982. [DOI] [PubMed] [Google Scholar]
- 25.Cerutti A. The regulation of IgA class switching. Nat. Rev. Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackay F, Schneider P. Cracking the BAFF code. Nat. Rev. Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 27.Tsuji M, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, et al. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33:71–83. doi: 10.1016/j.immuni.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Honjo T, et al. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu. Rev. Immunol. 2002;20:165–196. doi: 10.1146/annurev.immunol.20.090501.112049. [DOI] [PubMed] [Google Scholar]
- 30.Peled JU, et al. The biochemistry of somatic hypermutation. Annu. Rev. Immunol. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- 31.Stavnezer J, et al. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen K, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat. Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat. Rev. Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 34.King C, et al. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu. Rev. Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 35.Bishop GA. The multifaceted roles of TRAFs in the regulation of B-cell function. Nat. Rev. Immunol. 2004;4:775–786. doi: 10.1038/nri1462. [DOI] [PubMed] [Google Scholar]
- 36.Siebenlist U, et al. Control of lymphocyte development by nuclear factor-| B. Nat. Rev. Immunol. 2005;5:435–445. doi: 10.1038/nri1629. [DOI] [PubMed] [Google Scholar]
- 37.Chen K, Cerutti A. New insights into the enigma of immunoglobulin D. Immunol. Rev. 2010;237:1–20. doi: 10.1111/j.1600-065X.2010.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu. Rev. Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 39.He B, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat. Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vinuesa CG, Goodnow CC. Immunology: DNA drives autoimmunity. Nature. 2002;416:595–598. doi: 10.1038/416595a. [DOI] [PubMed] [Google Scholar]
- 41.Cerutti A, et al. Immunoglobulin responses at the mucosal tnterface. Annu. Rev. Immunol. 2010 doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weller S, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weller S, et al. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc. Natl. Acad. Sci. U S A. 2001;98:1166–1170. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin L, et al. CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur J Immunol. 2004;34:1483–1487. doi: 10.1002/eji.200324736. [DOI] [PubMed] [Google Scholar]
- 45.Xu W, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 46.Ueda Y, et al. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J. Immunol. 2007;178:3593–3601. doi: 10.4049/jimmunol.178.6.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capolunghi F, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J. Immunol. 2008;180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 48.Aranburu A, et al. TLR ligation triggers somatic hypermutation in transitional B cells inducing the generation of IgM memory B cells. J. Immunol. 2010;185:7293–7301. doi: 10.4049/jimmunol.1002722. [DOI] [PubMed] [Google Scholar]
- 49.William J, et al. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 50.Herlands RA, et al. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao C, et al. T cell-independent somatic hypermutation in murine B cells with an immature phenotype. Immunity. 2004;20:133–144. doi: 10.1016/s1074-7613(04)00019-6. [DOI] [PubMed] [Google Scholar]
- 52.Han JH, et al. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and toll-like receptors. Immunity. 2007;27:64–75. doi: 10.1016/j.immuni.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He B, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 54.Delgado MF, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ichinohe T, et al. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee BO, et al. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J. Immunol. 2005;175:5827–5838. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]
- 57.He B, et al. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol. 2006;176:3931–3941. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 58.Pulendran B, et al. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr. Opin. Immunol. 2008;20:61–67. doi: 10.1016/j.coi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scapini P, et al. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J. Exp. Med. 2003;197:297–302. doi: 10.1084/jem.20021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen K, Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity. 2010;33:479–491. doi: 10.1016/j.immuni.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gardby E, et al. Strong differential regulation of serum and mucosal IgA responses as revealed in CD28-deficient mice using cholera toxin adjuvant. J. Immunol. 2003;170:55–63. doi: 10.4049/jimmunol.170.1.55. [DOI] [PubMed] [Google Scholar]
- 62.Casola S, et al. B cell receptor signal strength determines B cell fate. Nat. Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 63.Bergqvist P, et al. Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers. J. Immunol. 2006;177:7772–7783. doi: 10.4049/jimmunol.177.11.7772. [DOI] [PubMed] [Google Scholar]
- 64.Tezuka H, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 65.Mora JR, et al. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Massacand JC, et al. Intestinal bacteria condition dendritic cells to promote IgA production. PLoS One. 2008;3:e2588. doi: 10.1371/journal.pone.0002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bessa J, et al. Alveolar macrophages and lung dendritic cells sense RNA and drive mucosal IgA responses. J. Immunol. 2009;183:3788–3799. doi: 10.4049/jimmunol.0804004. [DOI] [PubMed] [Google Scholar]
- 68.Serbina NV, et al. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 69.Crouch EE, et al. Regulation of AID expression in the immune response. J. Exp. Med. 2007;204:1145–1156. doi: 10.1084/jem.20061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uematsu S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 71.Fagarasan S, et al. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–643. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 72.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 73.Litinskiy MB, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bekeredjian-Ding IB, et al. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J. Immunol. 2005;174:4043–4050. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 75.Fink K, et al. Early type I interferon-mediated signals on B cells specifically enhance antiviral humoral responses. Eur. J. Immunol. 2006;36:2094–2105. doi: 10.1002/eji.200635993. [DOI] [PubMed] [Google Scholar]
- 76.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat. Immunol. 2006;7:709–714. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 77.Xu W, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat. Immunol. 2009;10:1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castigli E, et al. TACI and BAFF-R mediate isotype switching in B cells. J. Exp. Med. 2005;201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Castigli E, et al. Transmembrane activator and calcium modulator and cyclophilin ligand interactor enhances CD40-driven plasma cell differentiation. J. Allergy Clin. Immunol. 2007;120:885–891. doi: 10.1016/j.jaci.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mantchev GT, et al. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. J. Immunol. 2007;179:2282–2288. doi: 10.4049/jimmunol.179.4.2282. [DOI] [PubMed] [Google Scholar]
- 81.Katsenelson N, et al. Synthetic CpG oligodeoxynucleotides augment BAFF-and APRIL-mediated immunoglobulin secretion. Eur. J. Immunol. 2007;37:1785–1795. doi: 10.1002/eji.200636800. [DOI] [PubMed] [Google Scholar]
- 82.Treml LS, et al. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol. 2007;178:7531–7539. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- 83.Groom JR, et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J. Exp. Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xia XZ, et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J. Exp. Med. 2000;192:137–143. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jabara H, et al. The Binding Site for TRAF2 and TRAF3 but Not for TRAF6 Is Essential for CD40-Mediated Immunoglobulin Class Switching. Immunity. 2002;17:265–276. doi: 10.1016/s1074-7613(02)00394-1. [DOI] [PubMed] [Google Scholar]
- 86.Rahman ZS, et al. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J. Exp. Med. 2003;198:1157–1169. doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shulga-Morskaya S, et al. B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J. Immunol. 2004;173:2331–2341. doi: 10.4049/jimmunol.173.4.2331. [DOI] [PubMed] [Google Scholar]
- 88.Xu LG, Shu HB. TNFR-associated factor-3 is associated with BAFF-R and negatively regulates BAFF-R-mediated NF-kappa B activation and IL-10 production. J. Immunol. 2002;169:6883–6889. doi: 10.4049/jimmunol.169.12.6883. [DOI] [PubMed] [Google Scholar]
- 89.Xie P, et al. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gardam S, et al. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 91.Tusche MW, et al. Differential requirement of MALT1 for BAFF-induced outcomes in B cell subsets. J. Exp. Med. 2009;206:2671–2683. doi: 10.1084/jem.20091802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Claudio E, et al. BAFF-induced NEMO-independent processing of NF-kappaB2 in maturing B cells. Nat. Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 93.Kayagaki N, et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity. 2002;17:515–524. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- 94.Stavnezer J. Antibody class switching. Adv. Immunol. 1996;61:79–146. doi: 10.1016/s0065-2776(08)60866-4. [DOI] [PubMed] [Google Scholar]
- 95.Hatada EN, et al. NF-kappa B1 p50 is required for BLyS attenuation of apoptosis but dispensable for processing of NF-kappa B2 p100 to p52 in quiescent mature B cells. J. Immunol. 2003;171:761–768. doi: 10.4049/jimmunol.171.2.761. [DOI] [PubMed] [Google Scholar]
- 96.Hatzoglou A, et al. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-kappa B, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J. Immunol. 2000;165:1322–1330. doi: 10.4049/jimmunol.165.3.1322. [DOI] [PubMed] [Google Scholar]
- 97.Avery DT, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J. Clin. Invest. 2003;112:286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang M, et al. B cell maturation antigen, the receptor for a proliferation-inducing ligand and B cell-activating factor of the TNF family, induces antigen presentation in B cells. J Immunol. 2005;175:2814–2824. doi: 10.4049/jimmunol.175.5.2814. [DOI] [PubMed] [Google Scholar]
- 99.Hapfelmeier S, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O'Connor BP, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huard B, et al. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J. Clin. Invest. 2008;118:2887–2895. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salzer U, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat. Genet. 2005;37:820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 103.Zhang L, et al. Transmembrane activator and calcium-modulating cyclophilin ligand interactor mutations in common variable immunodeficiency: clinical and immunologic outcomes in heterozygotes. J. Allergy Clin. Immunol. 2007;120:1178–1185. doi: 10.1016/j.jaci.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Castigli E, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat. Genet. 2005;37:829–834. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 105.Warnatz K, et al. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc. Natl. Acad. Sci. USA. 2009;106:13945–13950. doi: 10.1073/pnas.0903543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Picard C, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 107.von Bernuth H, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.von Bulow GU, et al. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–582. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 109.Neves P, et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33:777–790. doi: 10.1016/j.immuni.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 110.Mackay F, Tangye SG. The role of the BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr. Opin. Pharmacol. 2004;4:347–354. doi: 10.1016/j.coph.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 111.Yan M, et al. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nat. Immunol. 2000;1:37–41. doi: 10.1038/76889. [DOI] [PubMed] [Google Scholar]
- 112.Yu G, et al. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat. Immunol. 2000;1:252–256. doi: 10.1038/79802. [DOI] [PubMed] [Google Scholar]
- 113.Castigli E, Geha RS. TACI, isotype switching, CVID and IgAD. Immunol. Res. 2007;38:102–111. doi: 10.1007/s12026-007-8000-2. [DOI] [PubMed] [Google Scholar]
- 114.Castigli E, et al. Impaired IgA class switching in APRIL-deficient mice. Proc. Natl. Acad. Sci. USA. 2004;101:3903–3908. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stein JV, et al. APRIL modulates B and T cell immunity. J. Clin. Invest. 2002;109:1587–1598. doi: 10.1172/JCI15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seshasayee D, et al. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18:279–288. doi: 10.1016/s1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 117.Sakurai D, et al. TACI attenuates antibody production costimulated by BAFF-R and CD40. Eur. J. Immunol. 2007;37:110–118. doi: 10.1002/eji.200636623. [DOI] [PubMed] [Google Scholar]
- 118.Salzer U, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood. 2009;113:1967–1976. doi: 10.1182/blood-2008-02-141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Castigli E, et al. Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA deficiency. Nat. Genet. 2007;39:430–431. doi: 10.1038/ng0407-430. [DOI] [PubMed] [Google Scholar]
- 120.Woodland RT, et al. Multiple signaling pathways promote B lymphocyte stimulator dependent B-cell growth and survival. Blood. 2008;111:750–760. doi: 10.1182/blood-2007-03-077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patke A, et al. BAFF controls B cell metabolic fitness through a PKC beta-and Akt-dependent mechanism. J. Exp. Med. 2006;203:2551–2562. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thomson AW, et al. Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]