Abstract
Background
Prominent neurobiological theories of addiction posit a central role for aberrant mesolimbic dopamine release, but disagree as to whether repeated drug experience blunts or enhances this system. While drug withdrawal diminishes dopamine release, drug sensitization augments mesolimbic function, and both processes have been linked to drug-seeking. One possibility is that the dopamine system can rapidly switch from dampened to enhanced release depending upon the specific drug-predictive environment. To test this, we examined dopamine release when cues signaled delayed cocaine delivery versus imminent cocaine self-administration.
Methods
Fast-scan cyclic voltammetry was used to examine real-time dopamine release while simultaneously monitoring behavioral indices of aversion as rats experienced a sweet taste cue that predicted delayed cocaine availability and during self-administration. Further, the impact of cues signaling delayed drug availability on intracranial self-stimulation (ICSS), a broad measure of reward function, was assessed.
Results
We observed decreased mesolimbic dopamine concentrations, decreased reward sensitivity, and negative affect in response to the cocaine-predictive taste cue that signaled delayed cocaine availability. Importantly, dopamine concentration rapidly switched to elevated levels to cues signaling imminent cocaine delivery in the subsequent self-administration session.
Conclusions
These findings reveal rapid, bivalent contextual control over brain reward processing, affect, and motivated behavior and have implications for mechanisms mediating substance abuse.
Keywords: dopamine, aversion, reward, affect, cocaine, addiction
A challenge in treating psychostimulant abuse lies in how certain environments promote drug consumption despite the substance abuser’s attempts to remain abstinent. Stimuli associated with cocaine promote craving in humans and induce relapse in animal models, indicating that associative mechanisms are critical to the regulation of drug-seeking (1). Several influential theories of psychostimulant addiction have emerged from such findings (1–6), with each emphasizing a critical role for conditioned stimuli in the addiction cycle.
Hedonic dysregulation is one of the earliest known repercussions of drug-taking thought to promote its ongoing use (7, 8). This mood state involves the emergence of negative feelings that disrupt the abuser’s occupational and social life, ultimately promoting craving and relapse (9–11). Understanding the neural basis of such a state is challenging, as it involves the emergence of emotions that may regulate drug-seeking (10), but are difficult to assay in non-human models. Moreover, for negative affect to remain a motivating force that drives relapse, cocaine-predictive stimuli should be able to elicit this aversive state. While cocaine-cues have been shown to induce negative affect (11), the position that this influences drug-seeking remains controversial (1, 4, 12).
Previously, we reported behavioral and electrophysiological signs of negative affect for a taste cue that predicted delayed cocaine availability (13). Specifically, we observed a predictive relationship between the expression of aversion for a taste cue that predicts cocaine, and the subsequent motivation to self-administer the drug. It is clear that such taste-drug designs capture both the reinforcing and aversive aspects of drug-taking (14) but the mechanisms and motivational consequences are unclear (15, 16). Understanding these mechanisms may reveal how drug-predictive stimuli may both positively and negatively influence reward processing and motivation.
Here we examined rapid (subsecond) dopamine release to a taste cue that signaled delayed cocaine availability versus to cues that signaled imminent cocaine delivery during self-administration. First, we examined taste reactivity elicited by taste stimuli that predicted delayed cocaine availability. As stereotyped ingestive responses are modifiable by learned associations, taste reactivity reflects the perceived palatability of a stimulus and is a convenient measure of an animal’s affective state (13, 17). We assessed a neurochemical consequence of this aversive state by examining its impact on real-time dopamine release. Proper dopamine function in the nucleus accumbens (NAc) regulates motivated behaviors (18) and is essential for conditioned motivation (2). While dopamine transmission does not directly mediate taste perception (19), dopamine rapidly increases in the NAc during the presentation of rewarding stimuli and typically decreases during aversive events (20–22). Moreover, both ventral and dorsal striatal dopamine regulate cocaine-seeking (23) and are thought to become dysregulated during drug withdrawal (7, 24, 25). Second, we examined rapid dopamine release to cues that signaled imminent cocaine delivery during self-administration. Given the close relationship between dynamic dopamine function and the processing of rewarding and aversive events, we hypothesized that rapid and opposing changes in dopamine release would be observed to drug-associated cues depending upon whether they signaled delayed versus imminent cocaine availability. Further, the behavioral impact of cues signaling delayed drug availability on reward function was assessed with intracranial self-stimulation (ICSS).
Methods and Materials
Subjects
Twenty-four male Sprague-Dawley rats (300–350g) were individually housed with ad libitum food and water with a 12/12 hr light/dark cycle. All experiments were conducted in the light phase and approved by the Institutional Animal Care and Use Committee at the University of North Carolina, Chapel Hill.
Conditioning procedures
Experimenter Delivered Cocaine
Naïve rats were implanted with intraoral catheters as described previously (13). After recovery, rats were given daily drug conditioning sessions (Figure 1A). In each session mildly water deprived rats were intraorally infused with a grape or orange Kool Aid™ flavored 0.15% saccharin solution, the Paired tastant, (0.2 ml delivered through a solenoid over 3.5 s/trial, approximately 1 trial/min, for 45 trials), that predicted an investigator delivered injection of cocaine (20mg/kg, ip). The following day these rats were intraorally infused with the other flavored 0.15% saccharin solution, the Unpaired tastant that predicted an ip saline injection. Rats received 10 taste-drug pairings. After training, all rats underwent voltammetry surgery, described previously (21). After one week of recovery rats were tested, as in training, while voltammetric measurements were made. In the test session, rats were placed in the operant chamber (Med Associates, St. Albans, VT) and tethered to a headstage for voltammetric recordings. Rats were intraorally infused, with the Unpaired and then the Paired tastant (0.2 ml delivered over 3.5 s/trial for 45 trials each) while orofacial responses were recorded on digital video for later analysis. Twelve additional rats served as controls and were tested for naïve responses to 0.15% saccharin or 0.001M quinine (see Supplement). Six of these rats were prepared for voltammetric recordings in the shell, and 6 in the core.
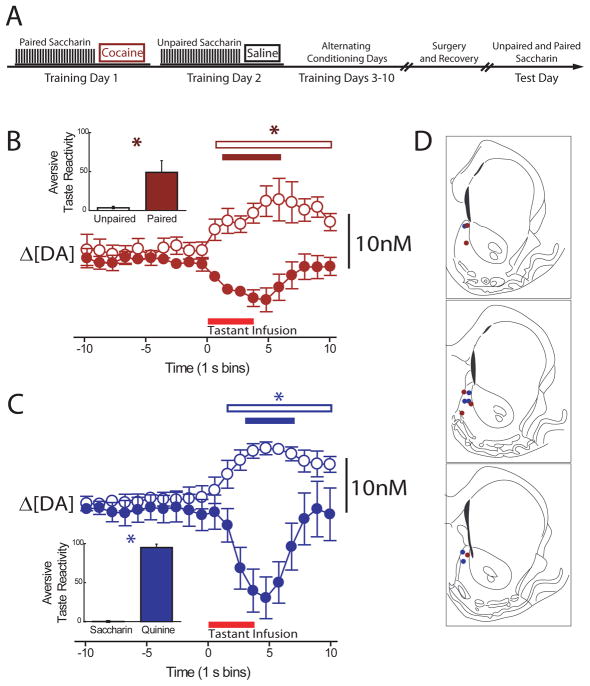
Figure 1.
Aversive conditioning and reduced dopamine release for a cocaine-predictive stimulus. (A) Diagram of cocaine-conditioning regimen. (B) Intraoral infusions of a palatable saccharin solution (unpaired, open circles) elicited dopamine release (mean ± s.e.m.), while an equally palatable but distinctively flavored saccharin solution that predicted a cocaine injection (paired, closed circles) decreased dopamine. Inset: Mean counts of aversive taste reactivity events were significantly greater for the cocaine-paired, but not the unpaired saccharin solution, P<0.05. (C) Dopamine increased during infusion of saccharin (open circles) and decreased during aversive quinine delivery (closed circles). Inset: Mean counts of aversion were significantly elevated for the quinine solution, not the saccharin solution, P<0.05. (D) Electrode placements were in the NAc shell. Asterisks over open and closed bars indicate duration of significant differences for unpaired and paired groups, respectively.
Self-Administered Cocaine with Audiovisual Cues
Mildly water deprived rats were trained in daily sessions to press a lever for water. Following acquisition, intraoral and intrajugular catheter surgeries were conducted. One week later, rats were tested for retention of lever pressing for water. Rats were then given daily drug conditioning sessions (Figure 2A). In each session, rats were intraorally infused with a 0.15% saccharin solution, (0.2 ml delivered through a solenoid over 3.5 s/trial for 45 trials) that predicted cocaine access (2 h access to 0.33 mg/infusion). Specifically, animals were trained to self-administer cocaine during daily 2 h sessions in the operant conditioning chamber as described previously (13). Lever depression on an FR1 schedule resulted in intravenous cocaine delivery (0.33 mg/infusion) over 6 s. Drug delivery was signaled by termination of the cue light and simultaneous onset of a tone (67 db, 1 kHz) houselight (25 W) stimulus complex (20 s). Rats received 4 daily taste-drug pairings. The following day, rats were tested. Rats were placed in the operant chamber and tethered to a headstage for voltammetric recordings. In the test session, as in training, rats were infused with the saccharin tastant, while orofacial movements were recorded for analysis. Immediately following the taste session, the cocaine self-administration session began and rats had the opportunity to press a lever for cocaine, as in training, while voltammetric recordings continued.
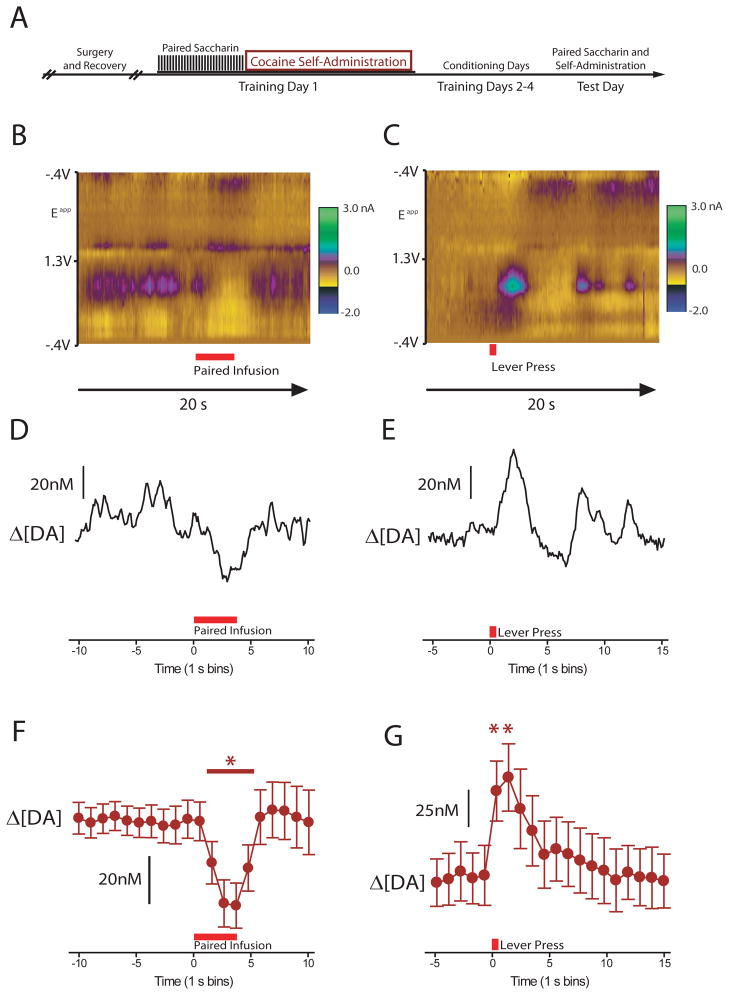
Figure 2.
Dopamine release during tastant infusion and during self-administration. (A) Diagram of cocaine-conditioning regimen. (B,C) Dopamine release to the cocaine-paired taste cue prior to self-administration (B) and relative to the lever press for cocaine (C). Two-dimensional color representation of cyclic voltammetric data collected for 20 s around a single tastant infusion (B) and a single lever press (C). The ordinate is the applied voltage (Eapp) and the abscissa is time (s). Changes in current at the carbon-fiber electrode are indicated in color. Differential DA concentrations determined via principal component analysis are plotted in D and E. (F) The cocaine-paired tastant reduced dopamine concentration across animals (mean ± sem). (G) Dopamine release events were observed immediately following the lever press. Asterisks indicate significant decreases (B) or increases (C) in dopamine concentration.
Self-Administered Cocaine with Taste Cues
Rats were trained and tested in the same self-administration procedure as above except the taste cue was not provided prior to cocaine-self administration, but as a replacement for the audiovisual cues that typically accompany a lever press for cocaine (Figure 3A). In the test session, rats were given 6–10 probe trials in which the same cocaine-predictive tastant was delivered noncontingently. Then rats were allowed to self-administer cocaine with the tastant cue, as in training, while voltammetric recordings were conducted.
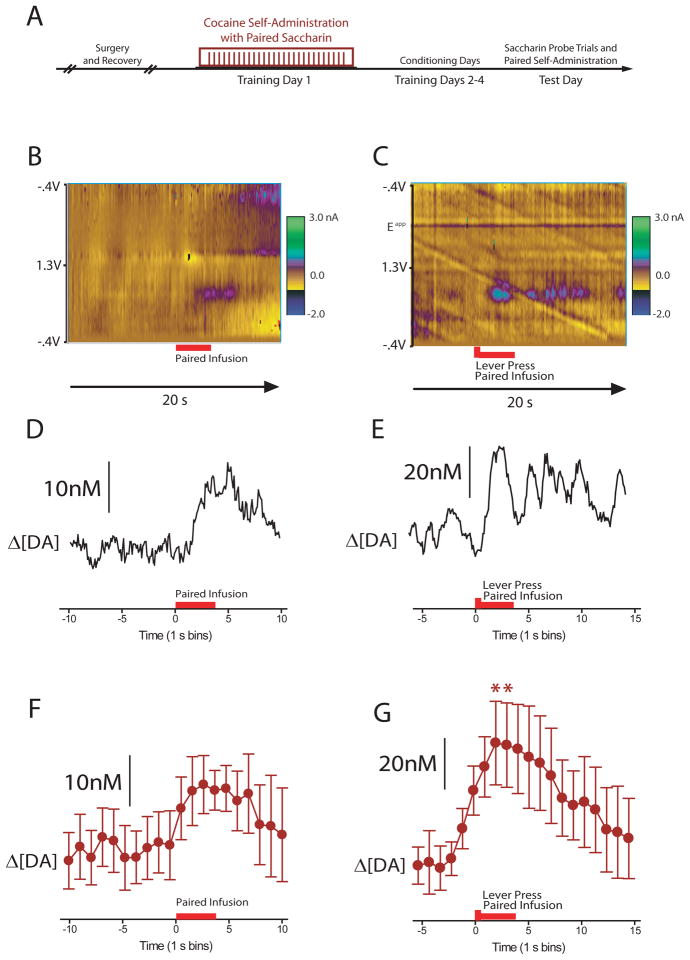
Figure 3.
Dopamine release during the tastant infusion and during self-administration with coincident tastant infusion. (A) Diagram of cocaine-conditioning regimen. (B,C) Dopamine release during a single cocaine-paired taste cue ‘probe’ trial prior to self-administration (B) and relative to a single lever press for cocaine (C). Two-dimensional color representation of cyclic voltammetric data collected for 20 s around a single tastant infusion (B) and a single lever press (C). The ordinate is the applied voltage (Eapp) and the abscissa is time (s). Changes in current at the carbon-fiber electrode are indicated in color. Differential DA concentrations determined via principal component analysis are plotted in D and E. (F) Infusion of the cocaine-paired tastant elevated dopamine concentration across all animals (mean ± s.e.m.). (G) Dopamine release events were also observed immediately following the lever press. Asterisks indicate significant increases in dopamine concentration compared to baseline.
ICSS Threshold Testing
After self-administration training and testing with audiovisual cues as above, rats were given three additional daily habituation sessions in the operant chamber in which they had previously self-administered cocaine. Then rats were trained in this chamber to press a distinct lever for electrical brain stimulation across several days, as in the manner of (26). Each lever press earned a 0.4-s train of square-wave cathodal pulses at a frequency of 60 Hz. The stimulation current (100–300 μA) was adjusted gradually to the lowest value that would sustain reliable responding (at least 40 rewards/min). After the minimal effective current was found for each rat, it was held constant. Each rat was then tested at its minimal effective current with a descending series of 12 stimulation frequencies. Each series comprised 1-min test trials at each frequency. For each frequency, there was an initial 5-s “priming” phase during which 1 noncontingent stimulation was given, followed by a 55-s test phase during which the number of responses was counted. The stimulation frequency was then lowered by 10% (.05 log10 units), and another trial was started. After responding had been evaluated at each of the 12 frequencies, the procedure was repeated such that each rat was given 3 series. Minor adjustments were made to the current for each rat so that only the highest 7–8 frequencies would sustain responding. Testing was initiated after ICSS response rates varied by less than 10% over 3 consecutive daily sessions. During testing, rate-frequency functions were determined in triplicate. These curves were averaged to obtain the baseline (threshold and maximal response rate) parameters. After obtaining baselines, rats received the tastant stimulus that previously predicted access to cocaine self-administration (Paired stimulus) in the same manner as self-administration training. Following this session, rate-frequency functions for ICSS were again determined. The following day, rats were tested in the same manner but received intraoral infusions of water instead of the Paired stimulus.
Surgery and Histology
Voltammetry/ICSS preparation
In preparation of voltammetric recordings, rats were anesthetized with ketamine hydrochloride (100 mg/kg, i.m.) and xylazine hydrochloride (10 mg/kg, i.m.), and were placed in a stereotaxic frame. A guide cannula (Bioanalytical Systems, West Lafayette, IL) over the NAc shell (AP: +1.7mm, ML: +0.8mm, relative to bregma) or NAc core (AP: +1.3mm, ML: +/−1.3) (27) and an Ag/AgCl reference electrode placed contralateral to the guide cannula also was implanted. Stainless-steel skull screws and dental cement were used to secure all items. A bipolar stimulating electrode was then placed just dorsal to the ventral tegmental area (−5.2 AP, 1.0 ML from bregma and 7 mm ventral from the dural surface). A detachable micromanipulator containing a glass-sealed carbon-fiber electrode (75–100 μm exposed tip length, 7 μm diameter, Goodfellow, Oakdale, PA) was inserted into the guide cannula, and the electrode was lowered into the NAc shell or core. The bipolar stimulating electrode was then lowered in 0.2 mm increments until electrically evoked dopamine release was detected at the carbon-fiber electrode in response to a stimulation train (24 biphasic pulses, 60 Hz, 120 μA, 2 ms per phase). The stimulating electrode was then fixed with dental cement and the carbon-fiber electrode was removed.
Intraoral/Intrajugular Catheter Surgery
Intraoral and intrajugular catheter surgeries were performed as in previous reports (13, 21).
Voltammetric measurements
Following surgery, animals were allowed one week to recover. After recovery, voltammetric recordings were made during the behavioral session as described previously (21). Experiments were conducted when an electrode placement yielded robust (>30:1 signal to noise) electrically evoked dopamine release. Once criteria were met, the electrode was locked in place. Several stimulation trains that varied in number of pulses (4, 6, 12, 24) were administered for the generation of a training set for principal component regression (PCR) for the detection of dopamine and pH changes during the behavioral session (21, 28). Upon completion of each experiment, electrode placement was verified in a manner identical to previous investigations (21).
Statistical analyses
For all analyses the alpha level for significance was 0.05. Repeated measurements of dopamine or behavior were analyzed with repeated-measures ANOVAs, followed by Newman-Keuls post hoc tests of significant effects using commercially available software (Statistica, Tulsa, OK).
Voltammetry
For the taste session, voltammetry data were analyzed 10 s prior, to 10 s after the taste infusion onset. For self-administration, data were analyzed from 5 s prior to 15 s after the lever press for cocaine. Data from each trial were background subtracted using a 0.1 s block at the local minima in the period prior to infusion onset (baseline period). For each rat, data were averaged across the number of trials in each session. The resultant current changes over time were analyzed for dopamine and pH changes using PCR. For the taste session, time was categorized into epochs: baseline (−1.0 to 0s relative to infusion onset), and each 1 s ‘effect’ epoch following the infusion or (0 to 10 s of infusion). For the self-administration session, time was also categorized into epochs: baseline (−1.0 to 0 s relative to lever press for cocaine), and each 1s ‘effect’ epoch following the infusion from 0 to 5 s. The effect epoch was 0 to 15 s when self-administration and tastant infusion were combined. Additionally, trial bin counts were calculated for all trials using NeuroExplorer, (Littleton, MA) to examine baseline changes in dopamine concentration across trials. Dopamine concentration in the first 10 trials was compared to the last 10 trials using a t-test.
ICSS threshold
During testing, ICSS response rates were collected in triplicate for each frequency. These frequency response rates were averaged and then normalized to the frequency that produced each animal’s half-maximal response rate (EF50). This transformation created a relative shape of each animal’s response pattern that was comparable on response rate and threshold frequency for responding. All datasets were then aligned to EF50 and averaged, maintaining the parameters (shape) of the data, minimizing individual differences in response rate and threshold frequency of responding.
Taste Reactivity
Taste reactivity was analyzed in a frame-by-frame analysis using digital video recorded on the test day. Appetitive and aversive taste reactivity was counted using the technique of Grill and Norgren (29) and as described previously (13, 21). Briefly, mouth movements expressed in the 6s following infusion onset that matched a ‘triangle’ shape for a duration exceeding 90 ms were counted as aversive. Instances in which the tongue protruded and crossed the midline were counted as appetitive. Counts for each animal were analyzed with a t-test for both appetitive taste reactivity and aversive taste reactivity.
Results
Prior to investigating cocaine-conditioned effects on dopamine release, we examined the effect of intraoral infusions of palatable and unpalatable taste stimuli on dopamine release in the shell and core subregions of the NAc, as these regions have different roles in the regulation of reward-related behavior (30–32). Measurements were made using fast-scan cyclic voltammetry, an electrochemical technique with the temporal resolution necessary for distinguishing rapid changes in dopamine release (33). Observations of significant alterations of dopamine release in the shell, but not the core (see Supplement) dictated a restriction of subsequent examinations to the shell subregion.
Conditioned dopamine alterations with experimenter-delivered cocaine
Next, we tested whether dopamine release for rewarding stimuli is reflective of sensory properties or can be changed by a taste’s association with cocaine. As 6 rats learned that brief intraoral presentations of a sweet, saccharin solution (presented across a 45 minute period) predicted a 20 mg/kg ip cocaine injection (Figure 1A), the taste became aversive and was orally rejected (Figure 1B, insert, P<0.05). A distinctively flavored but equally palatable sweet taste that was not paired with cocaine remained palatable. Correspondingly, post hoc tests of a significant main effect of epoch indicated that dopamine concentration decreased in the NAc shell for the taste that predicted cocaine (F10,50=3.82, P<0.01), but increased for the non-predictive taste (F10,50=3.42, P<0.01, Figure 1B). This resembled the decline in NAc dopamine concentration (and expression of aversive taste reactivity) observed in naïve animals during infusion of quinine, a bitter, aversive stimulus (Figure 1C), also see (21).
Conditioned dopamine alterations with self-administered cocaine
The learned aversion demonstrates that the taste elicits a conditioned negative affective state prior to cocaine’s pharmacologic actions (13). Decreased dopamine release typically reflects an aversive state (21, 22, 34). Interestingly, cues that predict psychostimulant administration increase dopamine release in the NAc (35–37). Here we used an experimental design that both facilitates aversive conditioning and allows for the behavioral expression of that negative affective state (14, 38). To gain a better understanding of how the conditioned aversive properties of cocaine may influence reward processing and subsequent behavior, and to better model addiction, we incorporated a self-administration design. Across 5 training days, 5 rats were presented with a saccharin-sweetened solution delivered across a 45 minute period prior to a 2 hour cocaine self-administration session. On the 5th day, behavioral responses and dopamine release events were recorded during taste presentation and self-administration (Figure 2A). As anticipated, the sweet taste that predicted future cocaine availability induced a decrease in dopamine concentration (F10,40=4.47, P<0.01, Figure 2B, D, F). Later in the same session, rats had the opportunity to self-administer cocaine. An increase in dopamine concentration was observed as a main effect of epoch (F5,20=2.92, P<0.05), immediately following the lever-press, when animals received an audiovisual stimulus paired with cocaine (Figure 2C, E, G). This pattern of dopamine release has been shown to be related to the cocaine-paired audiovisual stimulus (as opposed to a pharmacological effect of the drug) and is sufficient to promote drug-seeking (36). Thus, we observed context-specific opposing dopaminergic release patterns (from reduced for the taste cue, to elevated for the audiovisual stimulus) during cocaine self-administration.
Self-administered cocaine with immediate taste cues
In these studies we used a taste cue as the drug-predictive stimulus because it allows for the simultaneous assessment of behavioral and neurochemical signs of negative affect. To control for the fact that gustatory and audio/visual conditioned stimuli engage different sensory modalities, we subsequently tested the ability of a taste cue to differentially modulate dopamine release depending on its temporal relationship to cocaine availability. Four rats (n=5 recordings) were trained in a self-administration procedure in which the taste cue was not provided prior to cocaine-self administration, but as a replacement for the audiovisual cues that typically accompany a lever press for cocaine. In the test session, rats were given 6–10 probe trials in which the cocaine-predictive tastant was delivered noncontingently, then rats were allowed to self-administer cocaine (with the tastant cue as in training) while voltammetric recordings were conducted (Figure 3A).
Following daily saccharin-cocaine pairings, a significant main effect of epoch (F(10,40)=4.89, P<0.05), indicated that dopamine was elevated following intraoral saccharin delivery, (Figure 3B, D, F). Dopamine also was elevated following the lever press that triggered both an intraoral saccharin infusion and intravenous cocaine injection, as indicated by a significant main effect of epoch (F(15,60)=3.11, P<0.05, Figure 3C, E, G). Post hoc tests indicated that dopamine was elevated from baseline for 2 s following the lever press.
Dopamine concentration changes across the recording session
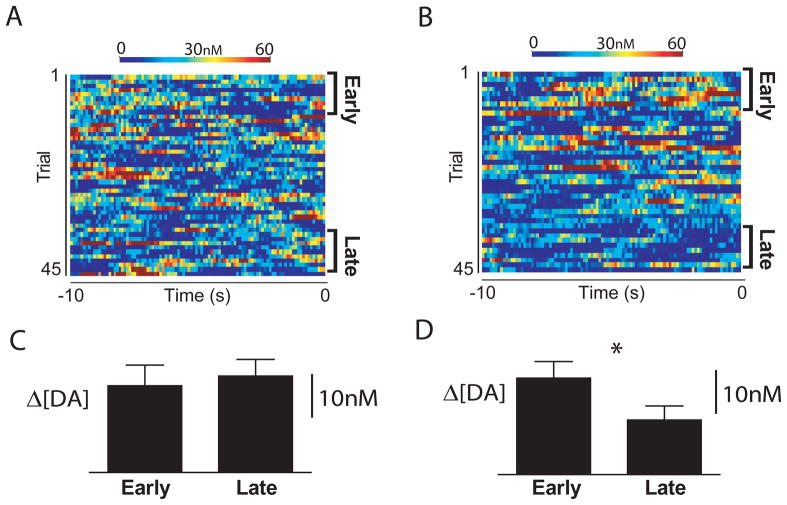
During the period prior to cocaine self-administration, naturally occurring dopamine release events (termed dopamine ‘transients’) were analyzed between tastant infusions. In a session in which a naive animal is intermittently receiving a palatable tastant, transient release events recorded prior to each infusion create a stable time-averaged dopamine concentration (Figure 4A, C). However, in intervals between cocaine-predictive tastant infusions in a design in which the cocaine-predictive taste elicits signs of aversion, high-concentration transient release events were significantly reduced in later trials, resulting in reduced dopamine concentration for animals experiencing the cocaine-predictive taste, even when the tastant was not being infused (Figure 4B, D). Dampened dopamine release coupled to behavioral aversion is consistent with the emergence of a negative affective state (7).
Figure 4.
Dampened dopamine release during the 45-min period prior to cocaine availability. (A) Example of trial-by-trial changes in dopamine concentration during a baseline period prior to intraoral saccharin infusions of the unpaired tastant. High-concentration transients (red) are randomly distributed across time (abscissa) and trials (ordinate). (B) Example of trial-by-trial changes in dopamine concentration transients during the baseline period prior to intraoral infusions of the cocaine-predictive tastant. (C) Average dopamine concentration (mean ± sem) in the baseline period for all animals receiving the unpaired tastant is stable across trials (Early vs Late), P>0.05. (D) Dopamine concentration decreased significantly across trials for rats receiving the cocaine-predictive taste cue, P<0.05.
Drug-predictive taste stimuli alter ICSS response thresholds
Withdrawal symptoms are specific to drug classes. However, negative affect is a symptom expressed across categories of abused substances (9, 10). This altered emotional state, thought to be the result of dysregulated mesolimbic dopamine function, can be quantified using intracranial self-stimulation (ICSS) (7). ICSS serves as a broad measure of reward function with pronounced decreases in reward sensitivity (detected as elevations in reward threshold) following withdrawal from abused substances (7). We hypothesized that if the cocaine-predictive taste cue, presented across an extended time period, is eliciting a negative affective state, then rats would also demonstrate reduced ICSS sensitivity. Rats trained with a taste cue presented 45 min prior to cocaine availability (n = 8), were subsequently trained to press a distinct lever for ICSS across several days. After training, rats pressed the lever for a descending range of frequencies. After establishing baseline frequency responses, the cocaine-predictive tastant was given, as previously, and the response rates for descending stimulus frequencies were again recorded. A significant condition x frequency interaction (F11,77=9.81, P<0.05), indicated that a rightward shift in the frequency response occurred, demonstrating an elevated ICSS threshold (Figure 5A). This shift was not observed the following day when rats were exposed to a non-predictive water tastant (F11,77=1.67, P>0.05, Figure 5B).
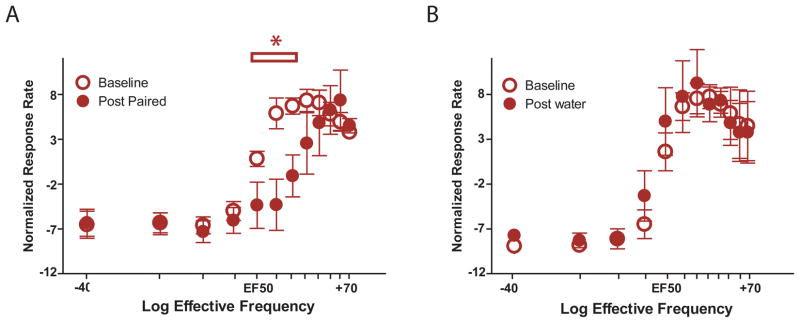
Figure 5.
Taste cues that predict future cocaine availability elevate ICSS thresholds. (A) Baseline ICSS threshold curves were established (open circles, mean ± sem), then rats received infusions of the cocaine-paired tastant, and the curves were re-determined (closed circles). The cocaine-paired tastant right-shifted ICSS thresholds significantly. Asterisks over horizontal bar indicate significant differences in ICSS response rates. (B) Baseline ICSS threshold curves were established (open circles) then all rats received infusions of water and the curves were re-determined (closed circles). Water infusions did not change ICSS response rates.
Discussion
Here, we demonstrate that taste stimuli signaling delayed cocaine availability can induce a negative affective state that alters hedonic sensitivity, brain reward processing, and motivated behavior. We observed the emergence of negative affect and reduced dopamine release using taste cues that signaled delayed cocaine availability when presented prior to cocaine self-administration. Later, in the same session, dopamine release was enhanced to cues that signaled imminent cocaine delivery during self-administration. It should be noted that some associations (like those with visceral malaise) are more readily formed with the sensory modality of taste, rather than vision or hearing (38). However, in this report we demonstrate that a taste cue can either elevate or diminish dopamine release depending on its temporal relationship to the availability of cocaine. This finding is consistent with previous reports demonstrating that close temporal pairings of intraoral sucrose or saccharin delivery with cocaine are not sufficient to produce overt signs of aversion (15, 16). The fact that reduced dopamine release and behavioral aversion were observed in response to the ‘delay,’ but not the ‘immediate’ taste cue suggests that the mechanism of learning across these temporal periods is fundamentally different and motivationally relevant. Ultimately, the conditioning observed here exerted a broad impact on behavior, not only altering affect, but also dopamine release and sensitivity to brain stimulation reward.
These findings complement a large literature indicating that the shell subregion of the NAc has an important role in processing the primary motivating properties of rewarding and aversive stimuli (32, 39). Psychostimulants preferentially induce dopamine release in the shell (31, 40, 41) and animals will self-administer dopamine agonists directly to into this region (42). Pharmacological inhibition of the shell increases motivated behavior (43) and hedonic responses to taste stimuli (44–46). Of course, reward and aversion processing in the NAc is plastic, as environmental conditions may flexibly dictate the current role of the nucleus in processing appetitive or aversive stimuli (21, 47). Consistent with these findings, we observe that dopamine release in this region, but not the core subregion, is rapidly elevated by palatable, and reduced by unpalatable, taste stimuli. Further we show that these rapid fluctuations in release can be altered by devaluation from learned associations, specifically the predictive and temporal relationship of the taste cue to cocaine availability.
Together, the observed alteration in affect, dopaminergic function, and reward processing suggests that conditioned negative affect may influence motivated behavior, perhaps through negative reinforcement as postulated by others (7, 8, 10). That is, drug-seeking may result as an effort to alleviate negative mood, particularly in a situation in which the cue signals that cocaine is not immediately available. This may be analogous to a human cocaine user being shown a drug-predictive cue in the absence of the drug, a design that elicits craving and negative mood (48, 49). Although cocaine use has negative physiological consequences (11) it is unclear how these consequences, which abate hours or days after drug cessation (7), continue to promote drug-seeking. Cue-induced dampening of dopamine release and reduced sensitivity to brain stimulation reward shown in this report present a potential mechanism. The previously-reported predictive relationship between the aversion expressed for a taste cue and subsequent cocaine intake supports this possibility. That is, a cocaine predictive taste cue elicits the behavioral expression of aversion, changes in neuronal firing rates (13), and shown here, reduced dopamine release in the NAc, a brain area critical for the proper expression of motivated behavior. These findings are consistent with the hypothesis that drug-predictive cues can elicit conditioned compensatory responses in opposition to the effects of the drug (50). If a negative affective state can be elicited by a cocaine-predictive cue, then negative reinforcement mechanisms may promote drug-seeking, as predicted by some addiction theories (7, 8).
However, we also observed rapid and pronounced dopamine release during cocaine self-administration and for cues (either tastants or audiovisual) that have been paired with immediate cocaine delivery. In this way, the data also are consistent with the interpretation that enhanced dopaminergic responses to drug-predictive stimuli (that signal imminent drug delivery) reflect incentive and promote drug-seeking (4). In this design, reduced incentive may be present in a ‘delayed drug’ context, but critically, may be quickly reversed by cues that predict ‘immediate drug’ availability. Together, our findings illustrate a rapidly dynamic reward processing system that can switch from dampened to enhanced functionality depending on the specific drug-predictive environment of the animal.
Supplementary Material
Acknowledgments
The authors thank Jessica Briley for assistance in data collection, Parastoo Hashemi, Nina Owesson-White and Pavel Takmakov for assistance with ICSS experiments, and Mitchell Roitman for assistance in the preparation of this manuscript. This research was supported by NIDA DA21055 to RAW, DA023745 to JLJ, DA021979 to JJD, DA21489 to BJA, DA10900 to RMW and DA017318 and DA014339 to RMC.
Footnotes
Financial Disclosures
All authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 2.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 3.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 5.Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, et al. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- 6.Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 7.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 8.Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1993. [Google Scholar]
- 10.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 11.Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- 12.Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- 13.Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Wise RA, Yokel RA, DeWit H. Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science. 1976;191:1273–1275. doi: 10.1126/science.1257748. [DOI] [PubMed] [Google Scholar]
- 15.Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug- induced devaluation of natural rewards. Behav Neurosci. 2002;116:321–333. [PubMed] [Google Scholar]
- 16.Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Rev. 1995;19:143–157. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- 17.Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 18.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 19.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 20.Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- 21.Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- 23.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. Burlington, MA: Elsevier Academic Press; 2005. [Google Scholar]
- 28.Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, et al. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 30.Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Mark Wightman R, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann N Y Acad Sci. 1999;877:71–90. doi: 10.1111/j.1749-6632.1999.tb09262.x. [DOI] [PubMed] [Google Scholar]
- 33.Wightman RM. Detection technologies. Probing cellular chemistry in biological systems with microelectrodes. Science. 2006;311:1570–1574. doi: 10.1126/science.1120027. [DOI] [PubMed] [Google Scholar]
- 34.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 37.Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia J, Koelling RA. Relation of cue to consequence in avoidance learning. Psychon Sci. 1966;4:123–124. [Google Scholar]
- 39.Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierce RC, Kalivas PW. Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther. 1995;275:1019–1029. [PubMed] [Google Scholar]
- 42.Ikemoto S, Qin M, Liu ZH. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell, and olfactory tubercle valid? J Neurosci. 2005;25:5061–5065. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlezon WA, Jr, Wise RA. Microinjections of phencyclidine (PCP) and related drugs into nucleus accumbens shell potentiate medial forebrain bundle brain stimulation reward. Psychopharmacology (Berl) 1996;128:413–420. doi: 10.1007/s002130050151. [DOI] [PubMed] [Google Scholar]
- 44.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 45.Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robbins SJ, Ehrman RN, Childress AR, Cornish JW, O’Brien CP. Mood state and recent cocaine use are not associated with levels of cocaine cue reactivity. Drug Alcohol Depend. 2000;59:33–42. doi: 10.1016/s0376-8716(99)00103-9. [DOI] [PubMed] [Google Scholar]
- 49.Childress A, Ehrman R, McLellan AT, O’Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 1988;81:74–80. [PubMed] [Google Scholar]
- 50.Siegel S, Hinson RE, Krank MD, McCully J. Heroin “overdose” death: contribution of drug-associated environmental cues. Science. 1982;216:436–437. doi: 10.1126/science.7200260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.