Abstract
Aging is associated with ED. Although age-related ED is attributed largely to increased oxidative stress and endothelial dysfunction in the penis, the molecular mechanisms underlying this effect are not fully defined. We evaluated whether endothelial nitric oxide synthase (eNOS) uncoupling in the aged rat penis is a contributing mechanism. Correlatively, we evaluated the effect of replacement with eNOS cofactor tetrahydrobiopterin (BH4) on erectile function in the aged rats. Male Fischer 344 ‘young’ (4-month-old) and ‘aged’ (19-month-old) rats were treated with a BH4 precursor sepiapterin (10 mg/kg intraperitoneally) or vehicle for 4 days. After 1-day washout, erectile function was assessed in response to electrical stimulation of the cavernous nerve. Endothelial dysfunction (eNOS uncoupling) and oxidative stress (thiobarbituric acid reactive substances, TBARS) were measured by conducting western blot in penes samples. Erectile response was significantly reduced in aged rats, whereas eNOS uncoupling and TBARS production were significantly increased in the aged rat penis compared with young rats. Sepiapterin significantly improved erectile response in aged rats and prevented increase in TBARS production, but did not affect eNOS uncoupling in the penis of aged rats. These findings suggest that aging induces eNOS uncoupling in the penis, resulting in increased oxidative stress and ED.
Keywords: erection, oxidative stress, penis, tetrahydrobiopterin
Introduction
ED is highly associated with aging.1–3 According to the Massachusetts Male Aging Study, 52% of men beyond 40 years of age have some degree of ED and it is projected to affect 322 million men worldwide by 2025.4 Age-related ED is characterized by decreased nerve and endothelium-mediated corpus cavernosum relaxation,5 decreased androgen levels,6 pathological remodeling of the pudendal artery,7 reduction in smooth muscle content,8–11 impaired growth factor and cytokine signaling12,13 and upregulation of the RhoA/Rho-kinase contractile pathway14 in the penis. Decreased endothelial signaling is attributed to impaired endothelial nitric oxide synthase (eNOS) expression,2,15–17 decreased eNOS phosphorylation,17 decreased content of the nitric oxide synthase (NOS) substrate L-arginine,18 excessive cGMP degradation by upregulated PDE5,19 reduced activation of protein kinase G-I by cGMP20 and increased oxidative stress.21 However, the source of reactive oxygen species in the aged penis is not known and the mechanisms of decreased endothelial nitric oxide signaling are only partially elucidated.
Oxidative stress is an important factor contributing to endothelial dysfunction associated with aging. Nicotinamide adenine dinucleotide phosphate oxidases, xanthine oxidase, cyclooxygenases, mitochondrial electron transport and eNOS uncoupling are potential vascular sources of reactive oxygen species.22 eNOS uncoupling is a switch in the enzymes' activity from a nitric oxide- to a predominantly superoxide-producing enzyme when tetrahydrobiopterin (BH4), an essential eNOS cofactor, is at suboptimal levels.23,24 Decreased nitric oxide production and increased superoxide production by uncoupled eNOS further enforce oxidative stress by perpetuating eNOS uncoupling through peroxynitrite formation, thereby resulting in chronic endothelial dysfunction. Although eNOS uncoupling has been largely observed in vitro and in the general diseased vasculature,23,24 including that of the penis,25,26 its role in the regulation of eNOS function and dysfunction in the penis, and the functional relevance of eNOS uncoupling in ED associated with aging, are unknown. The aim of this study was to investigate whether eNOS uncoupling serves as a potential basis for increased oxidative stress in the penis, contributing to ED, using the aged-rat model. Correlatively, we evaluated BH4 replacement as an intervention for restoring erectile function.
Materials and methods
Animal model
Male Fischer 344 ‘aged’ (19-month-old, 14 rats) and ‘young’ (4-month-old, 14 rats) rats were used (National Institute of Aging, Bethesda, MD, USA). Rats were injected intraperitoneally with sepiapterin, a BH4 precursor (10 mg/kg, Sigma Aldrich, St Louis, MO, USA) or vehicle (3.3% DMSO/saline) for 4 consecutive days. This time period was chosen on the basis of previous studies in non-penile vasculature, which demonstrated improvement in eNOS-mediated responses by sepiapterin.27,28 Erectile function and penes collection for molecular studies were performed after a 1-day washout period. All animal procedures were conducted in accordance with the ethical standards of the Johns Hopkins University School of Medicine Guidelines for the Care and Use of Animals.
Physiologic erection studies
Animals were anesthetized with intraperitoneal injection of 50 mg/kg ketamine/5 mg/kg xylazine. A bipolar electrode attached to a Grass Instruments S48 stimulator (Grass Instruments, Quincy, MA, USA) was placed around the cavernous nerve, as described.17 The right carotid artery was cannulated with polyethylene tubing (PE-50), anticoagulated with 20 U/ml heparin solution for continuous monitoring of systemic blood pressure. To measure intracavernosal pressure (ICP), the shaft of the penis was denuded of skin and fascia and the left corpus cavernosum was perforated with a 27-gauge needle connected through PE-50 to a pressure transducer (DI-190; Dataq Instruments, Akron, OH, USA), as described.17 ICP was measured at increasing voltages. ICP responses were calculated as stimulated ICP above baseline pressure, expressed per mean arterial pressure (MAP) using MATLAB software (Mathworks, Natick, MA, USA).
Low-temperature sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Minced penile tissue was homogenized and partially purified for NOS by affinity binding to 2′, 5′-ADP Sepharose, an NADP structural analog immobilized on Sepharose 4B, which binds and immobilizes enzymes requiring this cofactor.29 Partially purified NOS samples were resolved on 4–20% Tris gels at low temperature (below 15 °C) and transferred to polyvinylidene difluoride membrane. Membranes were probed with polyclonal rabbit anti-eNOS antibody (BD Transduction Laboratories, San Diego, CA, USA) at 1:700 dilution.25 eNOS uncoupling is inversely related to the ratio of active eNOS dimers to inactive eNOS monomers. The ratio was determined with arbitrary units and expressed relative to the ratio for vehicle-treated young rats.
Thiobarbituric acid reactive substances (TBARS) production
Thiobarbituric acid reactive substance (TBARS) assay, which evaluates lipid peroxidation, serves as an indicator of oxidative stress in tissues. The rat penis was pulverized and resuspended at a concentration of 100 mg/ml in phosphate buffered saline. TBARS production was measured according to the manufacturer's instructions using a TBARS assay kit (ZeptoMetrix Corp., Buffalo, NY, USA), as described.25 Samples were analyzed spectrofluorometrically in a microplate reader (BMG Labtech, Durham, NC, USA). Results were expressed relative to vehicle-treated young rats.
Statistical analysis
Statistical analysis was performed by using one-way analysis of variance, followed by Newman–Keuls multiple comparison test or by t-test when appropriate. The data were expressed as the mean ± s.e.m. A value of P<0.05 was considered to be statistically significant.
Results
Effect of aging and sepiapterin treatment on erectile function
Erectile response, expressed as ICP/MAP, was significantly (P<0.05) decreased in aged rats compared with young rats at all voltages of electrical stimulation of the cavernous nerve (Figure 1). Sepiapterin treatment significantly (P<0.05) increased the ICP/MAP ratio in aged rats at 2 and 4 V, compared with aged vehicle-treated rats. Sepiapterin treatment did not affect the ICP/MAP ratio in young rats (Figure 1).
Figure 1.
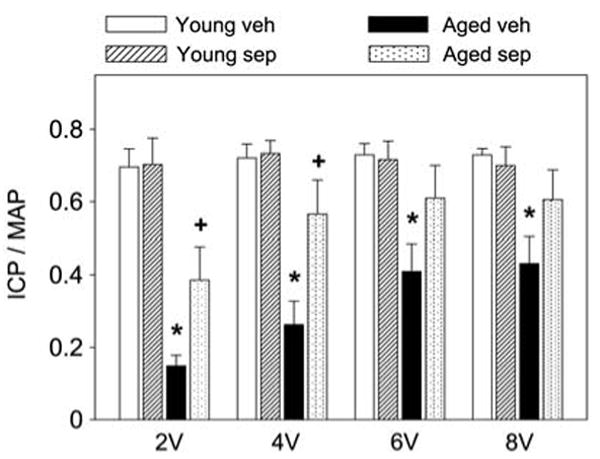
Effect of aging and sepiapterin treatment on penile erection. Erectile response is indicated by intracavernosal pressure (ICP) indexed to systemic blood pressure (mean arterial pressure, MAP). Each bar represents the mean ± s.e.m. of 4–6 rats; *P<0.05 vs young vehicle; +P<0.05 vs aged vehicle. Veh, vehicle; Sep, sepiapterin.
Effect of aging and sepiapterin treatment on eNOS uncoupling
Aged rat penes exhibited significantly decreased (P<0.05) ratio of eNOS dimers to monomers, compared with young rat penes (Figure 2), indicating increased eNOS uncoupling in the aged rat penis. Sepiapterin treatment did not affect eNOS uncoupling in the penis of young or aged rats compared with vehicle-treated rats (Figure 2).
Figure 2.
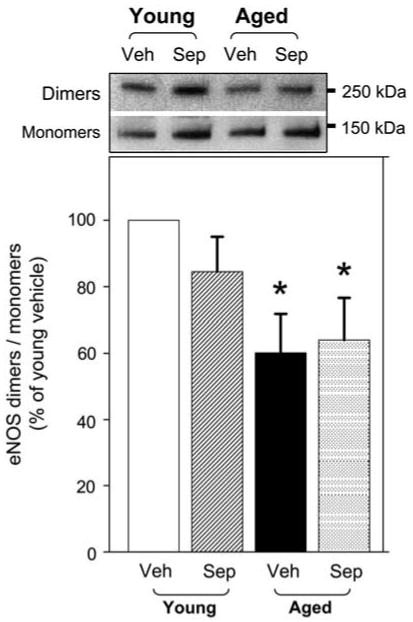
Effect of aging and sepiapterin treatment on endothelial nitric oxide synthase (eNOS) dimers/monomers in the penis. Upper panel is a representation of western immunoblot of the dimers/monomers in the penis of young vehicle-, young sepiapterin-, aged vehicle- and aged sepiapterin-treated rats. Lower panel represents quantitative analysis of eNOS dimer/monomer ratio. Each bar represents the mean ± s.e.m. of 4–6 rats; *P<0.05 vs young vehicle. Veh, vehicle; Sep, sepiapterin.
Effect of aging and sepiapterin treatment on TBARS production
The levels of TBARS were significantly elevated (P<0.05) in penes of aged rats compared with young rats (Figure 3). Treatment of aged rats with sepiapterin prevented increases in TBARS production in the penis, although it did not reverse elevated TBARS levels (P>0.5). Treatment of young rats with sepiapterin did not affect TBARS production in penes.
Figure 3.
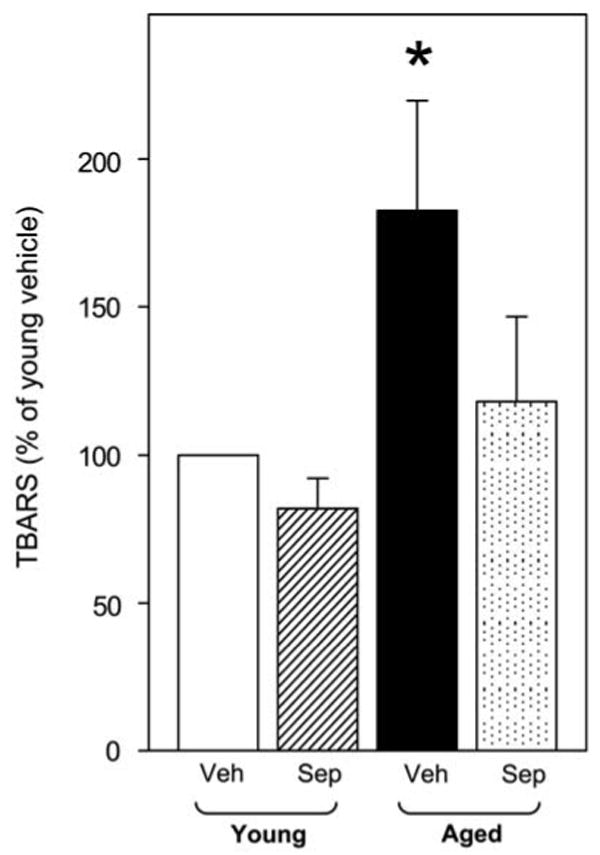
Effect of aging and sepiapterin treatment on thiobarbituric acid reactive substances, (TBARS) production in the penis of young vehicle-, young sepiapterin-, aged vehicle- and aged sepiapterin-treated rats. Each bar represents the mean ± -s.e.m. of 6–7 rats; *P<0.05 vs young vehicle. Veh, vehicle; Sep, sepiapterin.
Discussion
This study demonstrates that aging is associated with eNOS uncoupling in the penis, which serves as a source of increased oxidative stress and contributes to the pathophysiology of age-related ED. Our findings support the contention that oxidative stress generated by eNOS uncoupling is a mechanism for ED associated with aging. Pharmacologic supplementation with sepiapterin, a precursor of an essential eNOS cofactor BH4, prevented increase in oxidative stress in the aged penis and preserved erectile function of the aged rats, but did not prevent eNOS uncoupling. The latter effect of sepiapterin may be due to its antioxidant effect unrelated to eNOS function or to centrally mediated effects on neuroregulatory control of penile erection.
A critical determinant of the coupled versus uncoupled state of eNOS is its cofactor BH4. BH4 maintains and stabilizes the active dimeric form of eNOS, increases its affinity for substrateL-arginine, maintains the heme prosthetic group in its redox active form and provides one electron required for the multistep oxidation of L-arginine for NO production.23 Several studies in general vasculature point to the importance of eNOS uncoupling in the pathogenesis of aging. Arterioles of aged rats30 and mice31 exhibit reduced BH4 levels associated with a decline in blood flow-induced vasodilation, whereas increasing BH4 levels with sepiapterin increases vasodilation.32 Brachial artery dilatation in older people has also been shown to be increased by an acute bolus of BH4 supplementation.33 The results of this study show, for the first time, that eNOS uncoupling occurs with aging in the rat penis, thus establishing a molecular mechanism of reactive oxygen species production in the penis associated with aging. We extend these observations by showing its functional relevance, in that eNOS uncoupling in the aged rat penis is associated with ED.
Several studies demonstrate that eNOS protein expression is unchanged or, counterintuitively, increased in the penis of aged rats exhibiting ED.2,8,13,17 These findings may be explained by eNOS uncoupling, which decreases the abundance of the active dimeric form of eNOS, whereas total eNOS expression is retained, or even increased, but mainly in the monomeric form.
Our results reveal that sepiapterin supplementation prevented increase in oxidative stress, but did not decrease oxidative stress in the penis of aged rats. TBARS assay, an established and widely adopted method for measuring lipid oxidation,34 may not be sensitive and reproducible enough, providing high variability. Future studies will be needed to pursue more sensitive assay of lipid peroxidation and oxidative stress, such as C11-boron dipyrromethene difluoride, a fluorescent probe for measuring lipid peroxidation and antioxidant efficacy;35 4-hydroxy-2-nonenal, a marker of protein peroxidation;36 or direct measurements of superoxide and reactive oxygen species production.
The lack of effect of sepiapterin on preventing eNOS uncoupling in the penis of aged animals, as observed in this study, implies that sepiapterin, at the dose and time-frame used in this study, preserved erectile function through the mechanisms independent of its effects on eNOS activity. In addition to its NOS cofactor role, BH4 is a potent antioxidant and its loss might compromise redox balance by reducing the pool of antioxidants available to counteract an oxidant stress.23,24 It is possible that sepiapterin may provide a localized antioxidant effect within caveolae, which may positively impact erectile function. GTP cyclohydrolase I, a rate-limiting enzyme in de novo BH4 biosynthesis, has been localized to the caveolae, along with eNOS and caveolin-1.37 The location of GTP cyclohydrolase I in the oxidant-sensitive caveolae implies that its product, BH4, may protect the integrity of caveolae under conditions of oxidative stress. It remains to be determined whether this may be the mechanism of sepiapterin's effect on the improvement of penile erection with aging. At present the mechanism of BH4-mediated improvement in erectile function in the aged rats cannot be explained. It is possible that the lack of ability of sepiapterin to restore coupled eNOS in the penis of aged rats was due to its inadequate concentration, inability to restore BH4 content or that other mechanisms, in addition to reduced BH4 levels, contribute to eNOS uncoupling in the penis with aging. In addition, as these studies were conducted in a 4-day time frame, future experiments will be required to determine if long-term treatment with sepiapterin preserves coupled eNOS function. The young rats in this study showed no change in erectile function or TBARS levels with sepiapterin treatment, indicating that oxidative stress is absent in the penes of young, healthy rats.
Conversely, in addition to serving as a NOS cofactor and antioxidant, BH4 is required for the activity of tyrosine hydroxylase, tryptophan hydroxylase and phenylalanine hydroxylase, enzymes required for the synthesis of neurotransmitters dopamine and serotonin, and hydroxylation of phenylalanine to tyrosine, respectively. BH4 deficiency is accompanied by deficiencies of dopamine and serotonin within the central nervous system and overabundance of phenylalanine, which may cause central nervous system abnormalities.38 It is thus possible that BH4 deficiency with aging affects central regulatory mechanisms of penile erection, whereas BH4 supplementation reverses this effect. Future studies may be done to determine the effect of BH4 on centrally mediated penile erection, physiologically and pathophysiologically.
Little is known about neuronal NOS uncoupling in vivo, for example, how it is regulated or its physiological and pathophysiological implications. One paper recently demonstrated that diabetes reduced BH4 levels, neuronal NOSα expression and neuronal NOS dimers in the female rat gastric antrun, in parallel with delaying gastric emptying and reducing nitrergic relaxation of pyloric strips, which was all restored by BH4 supplementation.39 Whether neuronal NOS uncoupling in the penis contributes to age-related ED warrants future investigations.
Conclusion
Aging induces eNOS uncoupling in the penis, resulting in increased oxidative stress and ED. Sepiapterin improves age-related ED, possibly through its antioxidant effect, whereas it has no effect on eNOS uncoupling. Further studies are warranted to evaluate whether reversal of eNOS uncoupling in the aged penis improves erectile response, whether BH4 is deficient in the aged rat penis and what mechanisms underlie BH4 depletion with aging.
Acknowledgments
This work was supported by NIH/NIDDK grant DK075782 to Biljana Musicki.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Garban H, Vernet D, Freedman A, Rajfer J, Gonzalez-Cadavid N. Effect of aging on nitric oxide-mediated penile erection in rats. Am J Physiol. 1995;268:H467–H475. doi: 10.1152/ajpheart.1995.268.1.H467. [DOI] [PubMed] [Google Scholar]
- 2.Haas C, Seftel AD, Razmjouei K, Ganz MB, Hampel N, Ferguson K. Erectile dysfunction in aging: upregulation of endothelial nitric oxide synthase. Urology. 1998;51:516–522. doi: 10.1016/s0090-4295(97)00715-2. [DOI] [PubMed] [Google Scholar]
- 3.Melman A, Biggs G, Davies K, Zhao W, Tar MT, Christ GJ. Gene transfer with a vector expressing Maxi-K from a smooth muscle-specific promoter restores erectile function in the aging rat. Gene Ther. 2008;15:364–370. doi: 10.1038/sj.gt.3303093. [DOI] [PubMed] [Google Scholar]
- 4.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 5.Carrier S, Nagaraju P, Morgan DM, Baba K, Nunes L, Lue TF. Age decreases nitric oxide synthase-containing nerve fibers in the rat penis. J Urol. 1997;157:1088–1092. [PubMed] [Google Scholar]
- 6.Aversa A, Bruzziches R, Francomano D, Natali M, Gareri P, Spera G. Endothelial dysfunction and erectile dysfunction in the aging man. Int J Urol. 2010;17:38–47. doi: 10.1111/j.1442-2042.2009.02426.x. [DOI] [PubMed] [Google Scholar]
- 7.Hannan JL, Blaser MC, Oldfield L, Pang JJ, Adams SM, Pang SC, et al. Morphological and functional evidence for the contribution of the pudendal artery in aging-induced erectile dysfunction. J Sex Med. 2010;7:3373–3384. doi: 10.1111/j.1743-6109.2010.01920.x. [DOI] [PubMed] [Google Scholar]
- 8.Bakircioglu ME, Sievert KD, Nunes L, Lau A, Lin CS, Lue TF. Decreased trabecular smooth muscle and caveolin-1 expression in the penile tissue of aged rats. J Urol. 2001;166:734–738. [PubMed] [Google Scholar]
- 9.Davila HH, Rajfer J, Gonzalez-Cadavid NF. Corporal venoocclusive dysfunction in the aging rat: evaluation by cavernosometry and cavernosography. Urology. 2004;64:1261–1266. doi: 10.1016/j.urology.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Ferrini MG, Magee TR, Vernet D, Rajfer J, Gonzalez-Cadavid NF. Aging-related expression of inducible nitric oxide synthase and markers of tissue damage in the rat penis. Biol Reprod. 2001;64:974–982. doi: 10.1095/biolreprod64.3.974. [DOI] [PubMed] [Google Scholar]
- 11.Ferrini MG, Davila H, Valente EG, Gonzalez-Cadavid NF, Rajfer J. Aging-related induction of inducible nitric oxide synthase (NOS2A) is vasculo-protective in the arterial media. Cardiovasc Res. 2004;61:796–805. doi: 10.1016/j.cardiores.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Pu XY, Wang XH, Gao WC, Yang ZH, Li SL, Wang HP, et al. Insulin-like growth factor-1 restores erectile function in aged rats: modulation the integrity of smooth muscle and nitric oxide-cyclic guanosine monophosphate signaling activity. J Sex Med. 2008;5:1345–1354. doi: 10.1111/j.1743-6109.2008.00817.x. [DOI] [PubMed] [Google Scholar]
- 13.Rajasekaran M, Kasyan A, Jain A, Kim SW, Monga M. Altered growth factor expression in the aging penis: the Brown-Norway rat model. J Androl. 2002;23:393–399. [PubMed] [Google Scholar]
- 14.Jin L, Liu T, Lagoda GA, Champion HC, Bivalacqua TJ, Burnett AL. Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. FASEB J. 2006;20:536–538. doi: 10.1096/fj.05-4232fje. [DOI] [PubMed] [Google Scholar]
- 15.Champion HC, Bivalacqua TJ, Hyman AL, Ignarro LJ, Hellstrom WJ, Kadowitz PJ. Gene transfer of endothelial nitric oxide synthase to the penis augments erectile responses in the aged rat. Proc Natl Acad Sci USA. 1999;96:11648–11652. doi: 10.1073/pnas.96.20.11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bivalacqua TJ, Champion HC, Mehta YS, Abdel-Mageed AB, Sikka SC, Ignarro LJ, et al. Adenoviral gene transfer of endothelial nitric oxide synthase (eNOS) to the penis improves age-related erectile dysfunction in the rat. Int J Impot Res. 2000;12(3 suppl):S8–S17. doi: 10.1038/sj.ijir.3900556. [DOI] [PubMed] [Google Scholar]
- 17.Musicki B, Kramer MF, Becker RE, Burnett AL. Age-related changes in phosphorylation of endothelial nitric oxide synthase in the rat penis. J Sex Med. 2005;2:347–357. doi: 10.1111/j.1743-6109.2005.20349.x. [DOI] [PubMed] [Google Scholar]
- 18.Bivalacqua TJ, Burnett AL, Hellstrom WJ, Champion HC. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: influence of in vivo gene therapy of anti-arginase. Am J Physiol Heart Circ Physiol. 2007;292:H1340–H1351. doi: 10.1152/ajpheart.00121.2005. [DOI] [PubMed] [Google Scholar]
- 19.Musicki B, Champion HC, Becker RE, Liu T, Kramer MF, Burnett AL. Erection capability is potentiated by chronic sildenafil treatment: role of blood flow-induced endothelial nitric oxide synthase phosphorylation. Mol Pharmacol. 2005;8:26–232. doi: 10.1124/mol.104.010678. [DOI] [PubMed] [Google Scholar]
- 20.Hedlund P, Aszodi A, Pfeifer A, Alm P, Hofmann F, Ahmad M, et al. Erectile dysfunction in cylic GMP-dependent kinase I-deficient mice. Proc Natl Acad Sci USA. 2000;97:2349–2354. doi: 10.1073/pnas.030419997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bivalacqua TJ, Armstrong JS, Biggerstaff J, Abdel-Mageed AB, Kadowitz PJ, Hellstrom WJ, et al. Gene transfer of extracellular SOD to the penis reduces O2-* and improves erectile function in aged rats. Am J Physiol Heart Circ Physiol. 2003;284:H1408–H1421. doi: 10.1152/ajpheart.00770.2002. [DOI] [PubMed] [Google Scholar]
- 22.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 23.Channon KM. Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med. 2004;14:323–327. doi: 10.1016/j.tcm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Katusic ZS, d'Uscio LV, Nath KA. Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol Sci. 2009;30:48–54. doi: 10.1016/j.tips.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musicki B, Liu T, Strong T, Jin L, Laughlin MH, Turk JR, et al. Low-fat diet and exercise preserve eNOS regulation and endothelial function in the penis of early atherosclerotic pigs: a molecular analysis. J Sex Med. 2008;5:552–561. doi: 10.1111/j.1743-6109.2007.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musicki B, Liu T, Lagoda GA, Strong TD, Sezen SF, Johnson JM, et al. Hypercholesterolemia-induced erectile dysfunction: endothelial nitric oxide synthase (eNOS) uncoupling in the mouse penis by NAD(P)H oxidase. J Sex Med. 2010;7:3023–3032. doi: 10.1111/j.1743-6109.2010.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Xu J, Song P, Wu Y, Zhang J, Chul Choi H, et al. Acute inhibition of guanosine triphosphate cyclohydrolase 1 uncouples endothelial nitric oxide synthase and elevates blood pressure. Hypertension. 2008;52:484–490. doi: 10.1161/HYPERTENSIONAHA.108.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood KC, Hebbel RP, Lefer DJ, Granger DN. Critical role of endothelial cell-derived nitric oxide synthase in sickle cell disease-induced microvascular dysfunction. Free Radic Biol Med. 2006;40:1443–1453. doi: 10.1016/j.freeradbiomed.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, et al. Akt-dependent phosphorylation of endothelial nitricoxide synthase mediates penile erection. Proc Natl Acad Sci USA. 2002;99:4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of aging and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587:3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. 2009;297:H1829–H1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol. 2008;586:1161–1168. doi: 10.1113/jphysiol.2007.147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Drummen GP, van Liebergen LC, Op den Kamp JA, Post JA. C11-BODIPY(581/591), an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free Radic Biol Med. 2002;33:473–490. doi: 10.1016/s0891-5849(02)00848-1. [DOI] [PubMed] [Google Scholar]
- 36.Catala A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Peterson TE, d'Uscio LV, Cao S, Wang XL, Katusic ZS. Guanosine triphosphate cyclohydrolase I expression and enzymatic activity are present in caveolae of endothelial cells. Hypertension. 2009;53:189–195. doi: 10.1161/HYPERTENSIONAHA.108.115709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyland K. Inherited disorders affecting dopamine and serotonin: critical neurotransmitters derived from aromatic amino acids. J Nutr. 2007;137(6 Suppl 1):1568S–1572S. doi: 10.1093/jn/137.6.1568S. [DOI] [PubMed] [Google Scholar]
- 39.Gangula PR, Mukhopadhyay S, Ravella K, Cai S, Channon KM, Garfield RE, et al. Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2010;298:G692–G699. doi: 10.1152/ajpgi.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


