Abstract
Endophytic microorganisms are to be found in virtually every plant on earth. These organisms reside in the living tissues of the host plant and do so in a variety of relationships, ranging from symbiotic to slightly pathogenic. Because of what appears to be their contribution to the host plant, the endophytes may produce a plethora of substances of potential use to modern medicine, agriculture, and industry. Novel antibiotics, antimycotics, immunosuppressants, and anticancer compounds are only a few examples of what has been found after the isolation, culture, purification, and characterization of some choice endophytes in the recent past. The potential prospects of finding new drugs that may be effective candidates for treating newly developing diseases in humans, plants, and animals are great.
INTRODUCTION
The need for new and useful compounds to provide assistance and relief in all aspects of the human condition is ever growing. Drug resistance in bacteria, the appearance of life-threading viruses, the recurring problems with disease in persons with organ transplants, and the tremendous increase in the incidence of fungal infections in the world's population each only underscore our inadequacy to cope with these medical problems. Added to this are enormous difficulties in raising enough food on certain areas of the Earth to support local human populations. Environmental degradation, loss of biodiversity, and spoilage of land and water also add to problems facing mankind. Endophytes, microorganisms that reside in the tissues of living plants, are relatively unstudied and potential sources of novel natural products for exploitation in medicine, agriculture, and industry. It is noteworthy that, of the nearly 300,000 plant species that exist on the earth, each individual plant is host to one or more endophytes. Only a few these plants have ever been completely studied relative to their endophytic biology. Consequently, the opportunity to find new and interesting endophytic microorganisms among myriads of plants in different settings and ecosystems is great. The intent of this review is to provide insights into the presence of endophytes in nature, the products that they make, and how some of these organisms are beginning to show some potential for human use. The majority of the report discusses the rationale, methods, and examples of a plethora of endophytes isolated and studied in the authors' laboratory over the course of many years. This review, however, also includes some specific examples that illustrate the work of others in this emerging field of bioprospecting the microbes of the world's rainforests.
NEEDS FOR NEW MEDICINES AND AGROCHEMICAL AGENTS
There is a general call for new antibiotics, chemotherapeutic agents, and agrochemicals that are highly effective, possess low toxicity, and have a minor environmental impact. This search is driven by the development of resistance in infectious microorganisms (e.g., species of Staphylococcus, Mycobacterium, and Streptococcus) to existing compounds and by the menacing presence of naturally resistant organisms. The ingress to the human population of new diseases such as AIDS and severe acute respiratory syndrome requires the discovery and development of new drugs to combat them. Not only do diseases such as AIDS require drugs that target them specifically, but so do new therapies for treating ancillary infections which are a consequence of a weakened immune system. Furthermore, others who are immunocompromised (e.g., cancer and organ transplant patients) are at risk for opportunistic pathogens, such as Aspergillus spp., Cryptococcus spp., and Candida spp., that normally are not major problems in the human population. In addition, more drugs are needed to efficiently treat parasitic protozoan and nematodal infections, such as malaria, leishmaniasis, trypanomiasis, and filariasis. Malaria alone is more effective in claiming lives each year than any other single infectious agent with the exception of the AIDS virus and Mycobacterium tuberculosis (45). Finally, because of safety and environmental problems, many synthetic agricultural agents have been and currently are being targeted for removal from the market, which creates a need to find alternative ways to control farm pests and pathogens (16). Novel natural products and the organisms that make them offer opportunities for innovation in drug and agrochemical discovery. Exciting possibilities exist for those who are willing to venture into the wild and unexplored territories of the world to experience the excitement and thrill of engaging in the discovery of endophytes, their biology, and their potential usefulness.
NATURAL PRODUCTS AND TRADITIONAL APPROACHES IN MEDICINE
Natural products are naturally derived metabolites and/or by-products from microorganisms, plants, or animals (2). These products have been exploited for human use for thousands of years, and plants have been the chief source of compounds used for medicine. Even today the largest users of traditional medicines are the Chinese, with more than 5,000 plants and plant products in their pharmacopoeia (5). In fact, the world's best known and most universally used medicinal is aspirin (salicylic acid), which has its natural origins from the glycoside salicin which is found in many species of the plant genera Salix and Populus. Examples abound of natural-product use, especially in small native populations in a myriad of remote locations on Earth. For instance, certain tribal groups in the Amazon basin, the highland peoples of Papua New Guinea, and the Aborigines of Australia each has identified certain plants to provide relief of symptoms varying from head colds to massive wounds and intestinal ailments (30). History also shows that now-extinct civilizations had also discovered the benefits of medicinal plants. In fact, nearly 3,000 years ago, the Mayans used fungi grown on roasted green corn to treat intestinal ailments (10). More recently, the Benedictine monks (800 AD) began to apply Papaver somniferum as an anesthetic and pain reliever as the Greeks had done for years before (20). Many people, in past times, realized that leaf, root, and stem concoctions had the potential to help them. These plant products, in general, enhanced the quality of life, reduced pain and suffering, and provided relief, even though an understanding of the chemical nature of bioactive compounds in these complex mixtures and how they functioned remained a mystery.
It was not until Pasteur discovered that fermentation is caused by living cells that people seriously began to investigate microbes as a source for bioactive natural products. Then, scientific serendipity and the power of observation provided the impetus to Fleming to usher in the antibiotic era via the discovery of penicillin from the fungus Penicillium notatum. Since then, people have been engaged in the discovery and application of microbial metabolites with activity against both plant and human pathogens. Furthermore, the discovery of a plethora of microbes for applications that span a broad spectrum of utility in medicine (e.g., anticancer and immunosuppressant functions), agriculture and industry is now practical because of the development of novel and sophisticated screening processes in both medicine and agriculture. These processes use individual organisms, cells, enzymes, and site-directed techniques, many times in automated arrays, resulting in the rapid detection of promising leads for product development.
Even with untold centuries of human experience behind us and a movement into a modern era of chemistry and automation, natural-product-based compounds have had an immense impact on modern medicine since about 40% of prescription drugs are based on them. Furthermore, 49% of the new chemical products registered by the U.S. Food and Drug Administration are natural products or derivatives thereof (9). Excluding biologics, between 1989 and 1995, 60% of approved drugs and pre-new drug application candidates were of natural origin (20). From 1983 to 1994, over 60% of all approved cancer drugs and cancer drugs at the pre-new drug application stage were of natural origin, as were 78% of all newly approved antibacterial agents (13). In fact, the world's first billion-dollar anticancer drug, paclitaxel (Taxol), is a natural product derived from the yew tree (69). Many other examples abound that illustrate the value and importance of natural products in modern civilizations.
Recently, however, natural-product research efforts have lost popularity in many major drug companies and, in some cases, have been replaced entirely by combinatorial chemistry, which is the automated synthesis of structurally related small molecules (6). In addition, many drug companies have developed interests in making products that have a larger potential profit base than anti-infectious drugs. These include compounds that provide social benefits, that reduce the symptoms of allergies and arthritis, or that can soothe the stomach. It appears that this loss of interest can be attributed to the enormous effort and expense that is required to pick and choose a biological source and then to isolate active natural products, decipher their structures, and begin the long road to product development (20). It is also apparent that combinatorial chemistry and other synthetic chemistries revolving around certain basic chemical structures are now serving as a never-ending source of products to feed the screening robots of the drug industry. Within many large pharmaceutical companies, progress of professionals is primarily based upon numbers of compounds that can be produced and sent to the screening machines. This tends to work against the numerous steps needed even to find one compound in natural-product discovery. It is important to realize that the primary purpose of combinatorial chemistry should be to complement and assist the efforts of natural-product drug discovery and development, not to supersede it (20). The natural product often serves as a lead molecule whose activity can be enhanced by manipulation through combinatorial and synthetic chemistry. Natural products have been the traditional pathfinder compounds, offering an untold diversity of chemical structures unparalleled by even the largest combinatorial databases.
ENDOPHYTES
It may also be true that a reduction in interest in natural products for use in drug development has happened as a result of people growing weary of dealing with the traditional sources of bioactive compounds, including plants of the temperate zones and microbes from a plethora of soil samples gathered in different parts of the world by armies of collectors. In other words, why do something different (working on endophytic microbes) when robots, combinatorial chemistry, and molecular biology have arrived on the scene? Furthermore, the logic and rationale for time and effort spent on drug discovery using a target site-directed approach have been overwhelming.
While combinatorial synthesis produces compounds at random, secondary metabolites, defined as low-molecular-weight compounds not required for growth in pure culture, are produced as an adaptation for specific functions in nature (17). Schutz (51) notes that certain microbial metabolites seem to be characteristic of certain biotopes, on both an environmental as well as organismal level. Accordingly, it appears that the search for novel secondary metabolites should center on organisms that inhabit unique biotopes. Thus, it behooves the investigator to carefully study and select the biological source before proceeding, rather than to have a totally random approach in the biological source material. Careful study also indicates that organisms and their biotopes that are subjected to constant metabolic and environmental interactions should produce even more secondary metabolites (51). Endophytes are microbes that inhabit such biotopes, namely, higher plants, which is why they are currently considered to be a wellspring of novel secondary metabolites offering the potential for medical, agricultural, and/or industrial exploitation. Currently, endophytes are viewed as an outstanding source of bioactive natural products because there are so many of them occupying literally millions of unique biological niches (higher plants) growing in so many unusual environments. Thus, it appears that these biotypical factors can be important in plant selection, since they may govern the novelty and biological activity of the products associated with endophytic microbes.
Since the discovery of endophytes in Darnel, Germany, in 1904 (65), various investigators have defined endophytes in different ways, which is usually dependent on the perspective from which the endophytes were being isolated and subsequently examined. Bacon and White give an inclusive and widely accepted definition of endophytes—“microbes that colonize living, internal tissues of plants without causing any immediate, overt negative effects” (1). While the symptomless nature of endophyte occupation in plant tissue has prompted focus on symbiotic or mutualistic relationships between endophytes and their hosts, the observed biodiversity of endophytes suggests they can also be aggressive saprophytes or opportunistic pathogens. Both fungi and bacteria are the most common microbes existing as endophytes. It seems that other microbial forms, e.g., mycoplasmas and archaebacteria, most certainly exist in plants as endophytes, but no evidence for them has yet been presented. The most frequently isolated endophytes are the fungi. It turns out that the vast majority of plants have not been studied for their endophytes. Thus, enormous opportunities exist for the recovery of novel fungal forms, taxa, and biotypes. Hawksworth and Rossman estimated there may be as many as 1 million different fungal species, yet only about 100,000 have been described (26). As more evidence accumulates, estimates keep rising as to the actual number of fungal species. For instance, Dreyfuss and Chapela estimate there may be at least 1 million species of endophytic fungi alone (18). It seems obvious that endophytes are a rich and reliable source of genetic diversity and novel, undescribed species. Finally, in our experience, novel microbes usually have associated with them novel natural products. This fact alone helps eliminate the problems of dereplication in compound discovery.
Rationale for Plant Selection
It is important to understand the methods and rationale used to provide the best opportunities to isolate novel endophytic microorganisms as well as ones making novel bioactive products. Thus, since the number of plant species in the world is so great, creative and imaginative strategies must be used to quickly narrow the search for endophytes displaying bioactivity (44).
A specific rationale for the collection of each plant for endophyte isolation and natural-product discovery is used. Several reasonable hypotheses govern this plant selection strategy and these are as follows. (i) Plants from unique environmental settings, especially those with an unusual biology, and possessing novel strategies for survival are seriously considered for study. (ii) Plants that have an ethnobotanical history (use by indigenous peoples) that are related to the specific uses or applications of interest are selected for study. These plants are chosen either by direct contact with local peoples or via local literature. Ultimately, it may be learned that the healing powers of the botanical source, in fact, may have nothing to do with the natural products of the plant, but of the endophyte (inhabiting the plant). (iii) Plants that are endemic, that have an unusual longevity, or that have occupied a certain ancient land mass, such as Gonwanaland, are also more likely to lodge endophytes with active natural products than other plants. (iv) Plants growing in areas of great biodiversity also have the prospect of housing endophytes with great biodiversity.
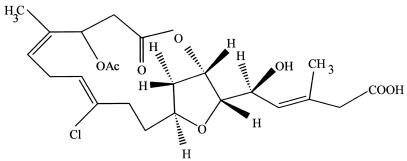
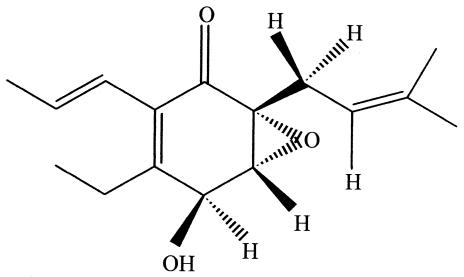
Just as plants from a distinct environmental setting are considered to be a promising source of novel endophytes and their compounds, so too are plants with an unconventional biology. For example, an aquatic plant, Rhyncholacis penicillata, was collected from a river system in Southwest Venezuela where the harsh aquatic environment subjected the plant to constant beating by virtue of rushing waters, debris, and tumbling rocks and pebbles (61). This created many portals through which common phytopathogenic oomycetes could enter the plant. Still, the plant population appeared to be healthy, possibly due to protection from an endophytic product. This was the environmental biological clue used to pick this plant for a comprehensive study of its endophytes. Eventually, a potent antifungal strain of Serratia marcescens was recovered from R. penicillata and was shown to produce oocydin A, a novel antioomycetous compound having the properties of a chlorinated macrocyclic lactone (Fig. 1.). It is conceivable that the production of oocydin A by S. marcescens is directly related to the endophyte's relationship with its higher plant host. Currently, oocydin A is being considered for agriculture use to control the ever-threatening presence of oomyceteous fungi such as Pythium and Phytophthora.
FIG. 1.
Oocydin A, a chlorinated macrocyclic lactone from a strain of Serratia marcescens isolated from R. penicillata.
Plants with ethnobotanical history, as mentioned above, also are likely candidates for study, since the medical uses to which the plant may have been selected relates more to its population of endophytes than to the plant biochemistry itself. For example, a sample of the snakevine, Kennedia nigriscans, from the Northern Territory of Australia was selected for study since its sap has traditionally been used as bush medicine for many years. In fact, this area was selected for plant sampling since it has been home to one of the world's long-standing civilizations—the Australian Aborigines. The snakevine is harvested, crushed, and heated in an aqueous brew by local Aborigines in southwest Arnhemland to treat cuts, wounds, and infections. As it turned out, the plant contained a novel endophyte, Streptomyces sp. strain NRRL 30562, that produces wide-spectrum novel peptide antibiotics called munumbicins (discussed below) (12). It is reasonable to assume that the healing processes, as discovered by indigenous peoples, might be facilitated by compounds produced by one or more specific plant-associated endophytes as well as the plant products themselves.
In addition, it is noteworthy that some plants generating bioactive natural products have associated endophytes that produce the same natural products. Such is the case with paclitaxel, a highly functionalized diterpenoid and famed anticancer agent that is found in each of the world's yew tree species (Taxus spp.) (64). In 1993, a novel paclitaxel-producing fungus, Taxomyces andreanae, from the yew Taxus brevifolia was isolated and characterized (58).
Endophytes and Biodiversity
Of the myriad of ecosystems on earth, those having the greatest biodiversity seem to be the ones also having endophytes with the greatest number and the most biodiverse microorganisms. Tropical and temperate rainforests are the most biologically diverse terrestrial ecosystems on earth. The most threatened of these spots cover only 1.44% of the land's surface, yet they harbor more than 60% of the world's terrestrial biodiversity (44). As such, one would expect that areas of high plant endemicity also possess specific endophytes that may have evolved with the endemic plant species.
Ultimately, biological diversity implies chemical diversity because of the constant chemical innovation that exists in ecosystems where the evolutionary race to survive is the most active. Tropical rainforests are a remarkable example of this type of environment. Competition is great, resources are limited, and selection pressure is at its peak. This gives rise to a high probability that rainforests are a source of novel molecular structures and biologically active compounds (49). Bills et al. (6) describe a metabolic distinction between tropical and temperate endophytes through statistical data which compares the number of bioactive natural products isolated from endophytes of tropical regions to the number of those isolated from endophytes of temperate origin. Not only did they find that tropical endophytes provide more active natural products than temperate endophytes, but they also noted that a significantly higher number of tropical endophytes produced a larger number of active secondary metabolites than did fungi from other tropical substrata (6). This observation suggests the importance of the host plant in influencing the general metabolism of endophytic microbes.
Endophytes and Phytochemistry
Tan and Zou believe the reason why some endophytes produce certain phytochemicals originally characteristic of the host might be related to a genetic recombination of the endophyte with the host that occurs in evolutionary time (65). This is a concept that was originally proposed as a mechanism to explain why the endophytic fungus T. andreanae may be producing paclitaxel (53). Thus, if endophytes can produce the same rare and important bioactive compounds as their host plants, this would not only reduce the need to harvest slow-growing and possibly rare plants but also preserve the world's ever-diminishing biodiversity. Furthermore, it is recognized that a microbial source of a valued product may be easier and more economical to produce, effectively reducing its market price.
All aspects of the biology and interrelatedness of endophytes with their respective hosts is a vastly underinvestigated and exciting field. Thus, more background information on a given plant species and its microorganismal biology would be exceedingly helpful in directing the search for bioactive products. Currently, no one is quite certain of the role of endophytes in nature and what appears to be their relationship to various host plant species. While some endophytic fungi appear to be ubiquitous (e.g., Fusarium spp., Pestalotiopsis spp., and Xylaria spp.), one cannot definitively state that endophytes are truly host specific or even systemic within plants any more than one can assume that their associations with plants are chance encounters. Frequently, many endophytes (biotypes) of the same species are isolated from the same plant and only one of the endophytes will produce a highly biologically active compound in culture (38). A great deal of uncertainty also exists between what an endophyte produces in culture and what it may produce in nature. It does seem apparent that the production of certain bioactive compounds by the endophyte in situ may facilitate the domination of its biological niche within the plant or even provide protection to the plant from harmful invading pathogens. This may be especially true if the bioactive product of the endophyte is unique to it and is not produced by the host. Seemingly, this would more easily facilitate the study of the role of the endophyte and its role in the plant. Furthermore, little information exists relative to the biochemistry and physiology of the interactions of the endophyte with its host plant. It would seem that many factors changing in the host as related to the season and age, environment, and location may influence the biology of the endophyte. Indeed, further research at the molecular level must be conducted in the field to study endophyte interactions and ecology. These interactions are probably all chemically mediated for some purpose in nature. An ecological awareness of the role these organisms play in nature will provide the best clues for targeting particular types of endophytic bioactivity with the greatest potential for bioprospecting.
Collection and Isolation Techniques of Endophytes
After a plant is selected for study, it is identified, and its location is plotted using a global positioning device. Small stem pieces are cut from the plant and placed in sealed plastic bags after excess moisture is removed. Every attempt is made to store the materials at 4°C until isolation procedures can begin (52-60).
In the laboratory, plant materials are thoroughly surface treated with 70% ethanol, sometimes they are flamed, and ultimately they are air dried under a laminar-flow hood. This is done in order to eliminate surface-contaminating microbes. Then, with a sterile knife blade, outer tissues are removed from the samples and the inner tissues are carefully excised and placed on water agar plates. After several days of incubation, hyphal tips of the fungi are removed and transferred to potato dextrose agar. Bacterial forms also emerge from the plant tissues, including, on rare occasions, certain Streptomyces spp. The endophytes are encouraged to sporulate on specific plant materials and are eventually identified via standard morphological and molecular biological techniques and methods. Eventually, when an endophyte is acquired in pure culture, it is tested for its ability to be grown in shake or still culture by the use of various media and growth conditions. It is also immediately placed in storage under various conditions, including 15% glycerol at −70°C. Ultimately, once appropriate growth conditions are found, the microbe is fermented and extracted and the bioactive compound(s) is isolated and characterized. Virtually all of the common and advanced procedures for product isolation and characterization are utilized in order to acquire the product(s) of interest. Central to the processes of isolation is the establishment of one or more bioassays that will guide the compound purification processes. One cannot put too much emphasis on this point since the ultimate success of any natural-product isolation activity is directly related to the development or selection of appropriate bioassay procedures. These can involve target organisms, enzymes, tissues, or model chemical systems that relate to the purpose for which the new compound is needed.
NATURAL PRODUCTS FROM ENDOPHYTIC MICROBES
The following section shows some examples of natural products obtained from endophytic microbes and their potential in the pharmaceutical and agrochemical arenas.
Endophytic Microbial Products as Antibiotics
Antibiotics are defined as low-molecular-weight organic natural products made by microorganisms that are active at low concentration against other microorganisms (17). Often, endophytes are a source of these antibiotics. Natural products from endophytic microbes have been observed to inhibit or kill a wide variety of harmful disease-causing agents including, but not limited to, phytopathogens, as well as bacteria, fungi, viruses, and protozoans that affect humans and animals.
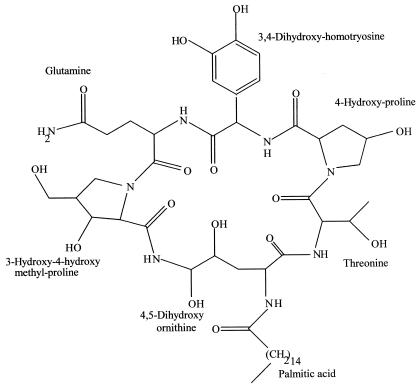
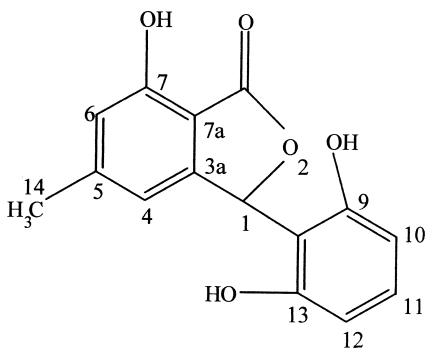
Cryptosporiopsis quercina is the imperfect stage of Pezicula cinnamomea, a fungus commonly associated with hardwood species in Europe. It was isolated as an endophyte from Tripterigeum wilfordii, a medicinal plant native to Eurasia (62). On petri plates, C. quercina demonstrated excellent antifungal activity against some important human fungal pathogens—Candida albicans and Trichophyton spp. A unique peptide antimycotic, termed cryptocandin, was isolated and characterized from C. quercina (62). This compound contains a number of peculiar hydroxylated amino acids and a novel amino acid: 3-hydroxy-4-hydroxy methyl proline (Fig. 2). The bioactive compound is related to the known antimycotics, the echinocandins and the pneumocandins (67). As is generally true not one but several bioactive and related compounds are produced by a microbe. Thus, other antifungal agents related to cryptocandin are also produced by C. quercina. Cryptocandin is also active against a number of plant-pathogenic fungi, including Sclerotinia sclerotiorum and Botrytis cinerea. Cryptocandin and its related compounds are currently being considered for use against a number of fungi causing diseases of skin and nails.
FIG. 2.
Cryptocandin A, an antifungal peptide obtained from the endophytic fungus C. quercina.
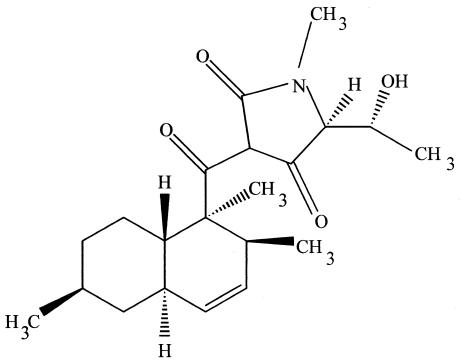
Cryptocin, a unique tetramic acid, is also produced by C. quercina (see above) (37) (Fig. 3). This unusual compound possesses potent activity against Pyricularia oryzae as well as a number of other plant-pathogenic fungi (37). The compound was generally ineffective against a general array of human-pathogenic fungi. Nevertheless, with MICs of this compound for P. oryzae being 0.39 μg/ml, this compound is being examined as a natural chemical control agent for rice blast and is being used as a base model to synthesize other antifungal compounds.
FIG. 3.
Cryptocin, a tetramic acid antifungal compound also found in C. quercina.
The ecomycins are produced by Pseudomonas viridiflava (43). P. viridiflava is a member of a group of plant-associated fluorescent bacteria. It is generally associated with the leaves of many grass species and is located on and within the tissues (43). The ecomycins represent a family of novel lipopeptides and have molecular weights of 1,153 and 1,181. Besides common amino acids such as alanine, serine, threonine, and glycine, some unusual amino acids are also involved in the structure of the ecomycins, including homoserine and β-hydroxyaspartic acid. The ecomycins are active against such human-pathogenic fungi as Cryptococcus neoformans and C. albicans.
Another group of antifungal compounds is the pseudomycins, produced by a plant-associated pseudomonad (3, 25). The pseudomycins represent a family of lipopeptides that are active against a variety of plant- and human-pathogenic fungi. Some of the notable target organisms include C. albicans, C. neoformans, and a variety of plant-pathogenic fungi, including Ceratocystis ulmi (the Dutch elm disease pathogen) and Mycosphaerella fijiensis (the causal agent of Black Sigatoka disease of banana) (25; G. Strobel, unpublished data). The key conserved part of the pseudomycins is a cyclic nonapeptide. The terminal carboxyl group of l-chlorothreonine closes the macrocyclic ring on the OH group of the N-terminal serine. Variety is added to this family of compounds by virtue of N-acetylation by one of a series of fatty acids, including 3,4-dihydroxydecanoate or 3-hydroxytetradecanoate and others (3). The pseudomycins contain several nontraditional amino acids, including l-chlorothreonine, l-hydroxy aspartic acid, and both d- and l-diaminobutryic acid. The molecules are candidates for use in human medicine especially after structural modification has successfully removed mammalian toxicity (74). Although the pseudomycins are also effective against a number of ascomycetous fungi, they are also being considered for agricultural use.
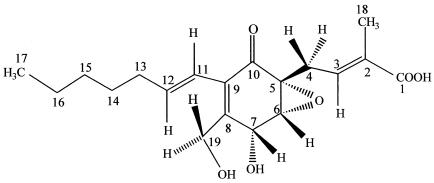
As mentioned elsewhere, Pestalotiopsis microspora is a common rainforest endophyte (55, 56). It turns out that enormous biochemical diversity does exist in this endophytic fungus, and as such there seem to be many secondary metabolites produced by a myriad of strains of this widely dispersed fungus. One such secondary metabolite is ambuic acid, an antifungal agent which has been recently described from several isolates of P. microspora found as representative isolates in many of the world's rainforests (39) (Fig. 4). In fact, this compound and another endophyte product, terrein, have been used as models to develop new solid-state nuclear magnetic resonance (NMR) tensor methods to assist in the characterization of molecular stereochemistry of organic molecules (22, 24).
FIG. 4.
Ambuic acid, a highly functionalized cyclohexenone produced by a number of isolates of P. microspora found in rainforests around the world. This compound possesses antifungal activity. Ambuic acid has also served as a model to develop new solid-state NMR methods for the structural determination of organic substances (22, 24).
A strain of P. microspora was also isolated from the endangered tree Torreya taxifolia and produces several compounds that have antifungal activity, including pestaloside, an aromatic β glucoside, and two pyrones: pestalopyrone and hydroxypestalopyrone (34). These products also possess phytotoxic properties. Other newly isolated secondary products obtained from P. microspora (endophytic on T. brevifolia) include two new caryophyllene sesquiterpenes—pestalotiopsins A and B (46). Other novel sesquiterpenes produced by this fungus are 2-α-hydroxydimeninol and a highly functionalized humulane (47, 48). Variation in the amount and kinds of products found in this fungus depends on both the cultural conditions of the organism as well as the original plant source from which it was isolated.
A newly described species of Pestalotiopsis, namely, Pestalotiopsis jesteri, from the Sepik River area of Papua New Guinea, produces jesterone and hydroxy-jesterone, which exhibit antifungal activity against a variety of plant-pathogenic fungi (36). Jesterone, subsequently, has been prepared by organic synthesis with complete retention of biological activity (29) (Fig. 5).
FIG. 5.
Jesterone, a cyclohexenone epoxide from P. jesteri that has antioomycete activity.
Phomopsichalasin, a metabolite from an endophytic Phomopsis sp., represents the first cytochalasin-type compound with a three-ring system replacing the cytochalasin macrolide ring. This metabolite mainly exhibits antibacterial activity in disk diffusion assays (at a concentration of 4 μg/disk) against Bacillus subtilis (12-mm zone of inhibition), Salmonella enterica serovar Gallinarum (11-mm zone of inhibition), and Staphylococcus aureus (8-mm zone of inhibition). It also displays a moderate activity against the yeast Candida tropicalis (8-mm zone of inhibition) (28).
An endophytic Fusarium sp. from the plant Selaginella pallescens, collected in the Guanacaste Conservation Area of Costa Rica, was screened for antifungal activity. A new pentaketide antifungal agent, CR377, was isolated from the culture broth of the fungus and showed potent activity against C. albicans in agar diffusion assays performed on fungal lawns (8).
Colletotric acid, a metabolite of Colletotrichum gloeosporioides, an endophytic fungus in Artemisia mongolica, displays antimicrobial activity against bacteria as well as against the fungus Helminthsporium sativum (75). Another Colletotrichum sp., isolated from Artemisia annua, produces bioactive metabolites that showed varied antimicrobial activity as well. A. annua is a traditional Chinese herb that is well recognized for its synthesis of artemisinin (an antimalarial drug) and its ability to inhabit many geographically different areas. The Colletotrichum sp. found in A. annua produced not only metabolites with activity against human-pathogenic fungi and bacteria but also metabolites that were fungistatic to plant-pathogenic fungi (42).
In addition to plants such as A. annua producing antimalarial compounds, some endophytes have shown powerful activity against protozoal diseases as well. Wide-spectrum antibiotics are produced by Streptomyces sp. strain NRRL 30562, an endophyte in K. nigriscans (12). These antibiotics, called munumbicins, possess widely differing biological activities, depending on the target organism. In general, the munumbicins demonstrate activity against gram-positive bacteria such as Bacillus anthracis and multidrug-resistant M. tuberculosis as well as a number of other drug-resistant bacteria. However, the most impressive biological activity of any of the munumbicins is that of munumbicin D against the malarial parasite Plasmodium falciparum, for which the 50% inhibitory concentration is 4.5 ± 0.07 ng ml−1 (12). The munumbicins are highly functionalized peptides, each containing threonine, aspartic acid (or asparagine), and glutamic acid (or glutamine). Since the peptides are yellowish orange in color, they also contain one or more chromophoric groups. Their masses range from 1,269 to 1,326 Da. The isolation of an endophytic streptomycete, Streptomyces sp. strain NRRL 30562, represents an important clue in providing one of the first examples of plants serving as reservoirs of actinomycetes, which are the world's primary source of antibiotics. However, in the past, virtually all of them used for modern antibiotic production had been isolated from soils. Now, more than 30 of these are on hand as endophytes, and many possess antibiotic activity (U. Castillo and G. Strobel, unpublished data). In fact, endophytic actinomycetes are now being tested and seriously considered for use in controlling plant diseases (31).
Another endophytic streptomycete (NRRL 30566), from a fern-leaved Grevillea tree (Grevillea pteridifolia) growing in the Northern Territory of Australia, produces, in culture, novel antibiotics called kakadumycins (11). Each of these antibiotics contains, by virtue of their amino acid compositions, alanine, serine, and an unknown amino acid. Kakadumycin A has wide-spectrum antibiotic activity similar to that of munumbicin D, especially against gram-positive bacteria, and it generally displays better bioactivity than echinomycin. For instance, the MIC of kakdumycin A for B. anthracis strains is 0.2 to 0.3 μg per ml in contrast to that of echinomycin, which is 1.0 to 1.2 μg per ml. Both echinomycin and kakadumycin A have impressive activity against P. falciparum, with 50% lethal doses in the range of 7 to 10 ng/ml (11). Kakadumycin A and echinomycin are related by virtue of their very similar chemistries (amino acid content and quinoxaline rings) but differ slightly with respect to their elemental compositions, aspects of their spectral qualities, and biological activities (11). This is yet another example of an endophytic actinomycete having promising antibiotic properties.
Antiviral Compounds
Another fascinating use of antibiotic products from endophytic fungi is the inhibition of viruses. Two novel human cytomegalovirus protease inhibitors, cytonic acids A and B, have been isolated from the solid-state fermentation of the endophytic fungus Cytonaema sp. Their structures as p-tridepside isomers were elucidated by mass spectrometry and NMR methods (21). It is apparent that the potential for the discovery of compounds, from endophytes, having antiviral activity is in its infancy. The fact, however, that some compounds have been found is promising. The main limitation in compound discovery is probably related to the absence of appropriate antiviral screening systems in most compound discovery programs.
Volatile Antibiotics from Endophytes
Muscodor albus is a newly described endophytic fungus obtained from small limbs of Cinnamomum zeylanicum (cinnamon tree) (70). This xylariaceaous (non-spore-producing) fungus effectively inhibits and kills certain other fungi and bacteria by producing a mixture of volatile compounds (59). The majority of these compounds have been identified by gas chromatography-mass spectrometry, synthesized or acquired, and then ultimately made into an artificial mixture. This mixture mimicked the antibiotic effects of the volatile compounds produced by the fungus. It was also used to gain positive identification of the ingredients of the fungal volatile compounds (59). Each of the five classes of volatile compounds produced by the fungus had some inhibitory effect against the test fungi and bacteria, but none was lethal. However, collectively they acted synergistically to cause death in a broad range of plant- and human-pathogenic fungi and bacteria. The most effective class of inhibitory compounds was the esters, of which isoamyl acetate was the most biologically active. The ecological implications and potential practical benefits of the “mycofumigation” effects of M. albus are very promising given the fact that soil fumigation utilizing methyl bromide will soon be illegal in the United States. The potential use of mycofumigation to treat soil, seeds, and plants may soon be a reality. In fact, this organism is already on the market for the decontamination of human wastes.
Using M. albus as a screening tool, it has now been possible to isolate other endophtyic fungi that produce volatile antibiotics. The newly described Muscodor roseus was twice obtained from tree species growing in the Northern Territory of Australia. This fungus is just as effective in causing inhibition and death of test microbes in the laboratory as M. albus (71). In addition, for the first time, a nonmuscodor species, a Gliocladium sp., was discovered to be a volatile antibiotic producer. The volatile components of this organism are totally different from those of either M. albus or M. roseus. In fact, the most abundant volatile inhibitor is [8]annulene, formerly used as a rocket fuel and discovered for the first time as a natural product in an endophytic fungus (54). The bioactivity of the volatile compounds of Gliocladium sp. is not as good or comprehensive as those of the Muscodor spp. (54).
Endophytic Fungal Products as Anticancer Agents
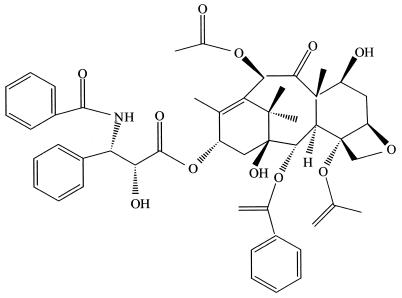
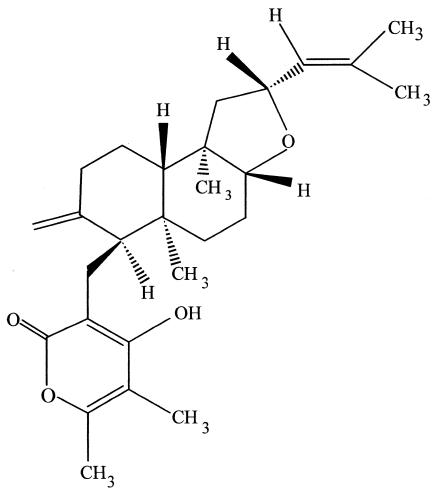
Paclitaxel and some of its derivatives represent the first major group of anticancer agents that is produced by endophytes (Fig. 6). Paclitaxel, a highly functionalized diterpenoid, is found in each of the world's yew (Taxus) species (64). The mode of action of paclitaxel is to preclude tubulin molecules from depolymerizing during the processes of cell division (50). This compound is the world's first billion-dollar anticancer drug. It is used to treat a number of other human tissue-proliferating diseases as well. The presence of paclitaxel in yew species prompted the study of their endophytes. By the early 1990s, however, no endophytic fungi had been isolated from any of the world's representative yew species. After several years of effort, a novel paclitaxel-producing endophytic fungus, T. andreanae, was discovered in T. brevifolia (58). The most critical line of evidence for the presence of paclitaxel in the culture fluids of this fungus was the electrospray mass spectrum of the putative paclitaxel isolated from T. andreanae. In electrospray mass spectroscopy, paclitaxel usually gives two peaks, one at a mass of 854, which is (M + H+), and the other at 876, which is (M + Na+), and fungal paclitaxel had a mass spectrum identical to that of authentic paclitaxel (53). Then, 14C labeling studies irrefutably showed the presence of fungus-derived paclitaxel in the culture medium (53). This early work set the stage for a more comprehensive examination of the ability of other Taxus species and other plants to yield endophytes producing paclitaxel.
FIG. 6.
Paclitaxel, the world's first billion-dollar anticancer drug, is produced by many endophytic fungi. It, too, possesses outstanding antioomycete activity.
Some of the most commonly found endophytes of the world's yews are Pestalotiopsis spp. (55). One of the most commonly isolated endophytic species is P. microspora (56). An examination of the endophytes of Taxus wallichiana yielded P. microspora, and a preliminary monoclonal antibody test indicated that it might produce paclitaxel (55). After preparative thin-layer chromatography, a compound was isolated and shown by spectroscopic techniques to be paclitaxel. Labeled (14C) paclitaxel was produced by this organism from several 14C precursors that had been administered to it (55). Furthermore, several other P. microspora isolates were obtained from bald cypress in South Carolina and also shown to produce paclitaxel (38). This was the first indication that endophytes residing in plants other than Taxus spp. were producing paclitaxel. Therefore, a specific search was conducted for paclitaxel-producing endophytes on continents not known for any indigenous Taxus spp. This included an examination of the prospects that paclitaxel-producing endophytes exist in South America and Australia. From the extremely rare, and previously thought to be extinct, Wollemi pine (Wollemia nobilis), Pestalotiopsis guepini was isolated, which was shown to produce paclitaxel (63). Also, quite surprisingly, a rubiaceous plant, Maguireothamnus speciosus, yielded a novel fungus, Seimatoantlerium tepuiense, that produces paclitaxel. This endemic plant grows on the tops of the tepuis in the Venzuelan-Guyana region in southwestern Venezuela (61). Furthermore, fungal paclitaxel production has also been noted in a Periconia sp. (40) and in Seimatoantlerium nepalense, another novel endophytic fungal species (4). Simply, it appears that the distribution of those fungi making paclitaxel is worldwide and not confined to endophytes of yews. The ecological and physiological explanation for the wide distribution of fungi that make paclitaxel seems to be related to the fact that paclitaxel is a fungicide and the organisms with the most sensitivity to it are plant pathogens such as Pythium spp. and Phytophthora spp. (72). These pythiaceous organisms are some of the world's most important plant pathogens and are strong competitors with endophytic fungi for niches within plants. In fact, their sensitivity to paclitaxel is based on their interaction with tubulin in a manner identical to that in rapidly dividing human cancer cells (50). Thus, bona fide endophytes may be producing paclitaxel to protect their respective host plant from degradation and disease caused by these pathogens.
As time has passed, other investigators have also made observations on paclitaxel production by endophytes, including the discovery of paclitaxel production by a Tubercularia sp. isolated from southern Chinese yew (Taxus mairei) in the Fujian province of southeastern China (68). At least three endophytes of T. wallichiana produce paclitaxel, including Sporormia minima and a Trichothecium sp. (52). By the use of high-performance liquid chromatography and electrospray mass spectroscopy, paclitaxel has been discovered in Corylus avellana cv. Gasaway (filbert) (27). Several fungal endophytes of filbert produce paclitaxel in culture (27). It is important to note, however, that paclitaxel production by all endophytes in culture is in the range of submicrograms to micrograms per liter. Also, commonly, endophytic fungi will attenuate paclitaxel production in culture. It is possible, however, to recover paclitaxel production in attenuated cultures if certain activator compounds are added to the medium (40). Efforts are being made to determine the feasibility of making microbial paclitaxel a commercial possibility.
Torreyanic acid, a selectively cytotoxic quinone dimer (anticancer agent), was isolated from a P. microspora strain. This strain was originally obtained as an endophyte associated with the endangered tree T. taxifolia (Florida torreya) as mentioned above (33). Torreyanic acid was tested in several cancer cell lines, and it demonstrated 5 to 10 times more potency in those lines that are sensitive to protein kinase C agonists and causes cell death by apoptosis. Recently, a complete synthesis of torreyanic acid has been successfully completed using the application of a biomimetic oxidation-dimerization cascade (35).
The alkaloids are also commonly found in endophytic fungi. Such fungal genera as Xylaria, Phoma, Hypoxylon, and Chalara are representative producers of a relatively large group of substances known as the cytochalasins, of which over 20 are now known (66). Many of these compounds possess antitumor and antibiotic activities, but because of their cellular toxicity they have not been developed into pharmaceuticals. Three novel cytochalasins have recently been reported from a Rhinocladiella sp. as an endophyte on Tripterygium wilfordii. These compounds have antitumor activity and have been identified as 22-oxa-[12]-cytochalasins (66). Thus, it is not uncommon to find one or more cytochalasins in endophytic fungi, and workers in this field need to be alerted to the fact that redundancy in discovery does occur. Chemical redundancy (dereplication) usually occurs with certain groups of organisms on which previous studies have already established the chemical identity of major biologically active compounds. For instance, as with the cytochalasins, they are commonly associated with the xylariaceaous fungi.
Products from Endophytes as Antioxidants
Two compounds, pestacin and isopestacin, have been obtained from culture fluids of P. microspora, an endophyte isolated from a combretaceaous plant, Terminalia morobensis, growing in the Sepik River drainage of Papua New Guinea (23, 60). Both pestacin and isopestacin display antimicrobial as well as antioxidant activity. Isopestacin was suspected of antioxidant activity based on its structural similarity to the flavonoids (Fig. 7). Electron spin resonance spectroscopy measurements confirmed this antioxidant activity; the compound is able to scavenge superoxide and hydroxyl free radicals in solution (60). Pestacin was later described from the same culture fluid, occurring naturally as a racemic mixture and also possessing potent antioxidant activity (23) (Fig. 8). Proposed antioxidant activity of pestacin arose primarily via cleavage of an unusually reactive C—H bond and to a lesser extent, though O—H abstraction (56). The antioxidant activity of pestacin is at least 1 order of magnitude greater than that of trolox, a vitamin E derivative (23).
FIG. 7.
Isopestacin, an antioxidant produced by an endophytic P. microspora strain isolated from T. morobensis growing on the north coast of Papua New Guinea.
FIG. 8.
Pestacin is also produced by the same fungus as that in Fig. 7, and it, too, is an antioxidant.
Products of Endophytes with Insecticidal Activities
Bioinsecticides are only a small part of the insecticide field, but their market is increasing (16). Several endophytes are known to have anti-insect properties. Nodulisporic acids, novel indole diterpenes that exhibit potent insecticidal properties against the larvae of the blowfly, work by activating insect glutamate-gated chloride channels. The first nodulisporic compounds were isolated from an endophyte, a Nodulisporium sp., from the plant Bontia daphnoides. This discovery has since resulted in an intensive search for more Nodulisporium spp. or other producers of more-potent nodulisporic acid analogues (16). Insect toxins have also been isolated from an unidentified endophytic fungus from wintergreen (Gaultheria procumbens). The two new compounds, 5-hydroxy-2-(1′-hydroxy-5′-methyl-4′-hexenyl)benzofuran and 5-hydroxy-2-(1′-oxo-5′-methyl-4′-hexenyl)benzofuran, both show toxicity to spruce budworm, and the latter is also toxic to the larvae of spruce budworm (19). Another endophytic fungus, Muscodor vitigenus, isolated from a liana (Paullina paullinioides), yields naphthalene as its major product. Naphthalene, the active ingredient in common mothballs, is a widely exploited insect repellant. M. vitigenus shows promising preliminary results as an insect deterrent and has exhibited potent insect repellency against the wheat stem sawfly (Cephus cinctus) (14, 15). As the world becomes wary of ecological damage done by synthetic insecticides, endophytic research continues for the discovery of powerful, selective, and safe alternatives.
Antidiabetic Agents from Rainforest Fungi
A nonpeptidal fungal metabolite (L-783,281) was isolated from an endophytic fungus (Pseudomassaria sp.) collected from an African rainforest near Kinshasa in the Democratic Republic of the Congo (73). This compound acts as an insulin mimetic and, unlike insulin, is not destroyed in the digestive tract and may be given orally. Oral administration of L-783,281 to two mouse models of diabetes resulted in significant lowering of blood glucose levels. These results may lead to new therapies for diabetes (73).
Immunosuppressive Compounds from Endophytes
Immunosuppressive drugs are used today to prevent allograft rejection in transplant patients, and in the future they could be used to treat autoimmune diseases such a rheumatoid arthritis and insulin-dependent diabetes. The endophytic fungus Fusarium subglutinans, isolated from T. wilfordii, produces the immunosuppressive but noncytotoxic diterpene pyrones subglutinol A and B (32) (Fig. 9). Subglutinol A and B are equipotent in the mixed lymphocyte reaction assay and thymocyte proliferation assay, with a 50% inhibitory concentration of 0.1 μM. In the same assay systems, the famed immunosuppressant drug cyclosporine is roughly as potent in the mixed lymphocyte reaction assay and 104 more potent in the thymocyte proliferation assay. Still, the lack of toxicity associated with subglutinols A and B suggests that they should be explored in greater detail (32).
FIG. 9.
Subglutinol A, an immunosuppressant, is produced by an endophytic F. subglutinans strain.
The Microbiology Department at Sandoz Ltd. developed a computer-aided evaluation program to screen and evaluate fungi for bioactivity. The program can recognize and eliminate from study common fungi producing known compounds and thereby direct attention to the evaluation of rare samples, which are more likely to produce metabolites with novel bioactivity. This approach resulted in the discovery of the fungus Tolypocladium inflatum, from which cyclosporine, a hugely beneficial immunosuppressant, was isolated (7). This example perfectly depicts the current aim of many investigators to seek out rare endophytes from interesting and uncommon hosts and environments.
Surprising Results from Molecular Biological Studies of P. microspora
Of some compelling interest is an explanation as to how the genes for paclitaxel production may have been acquired by P. microspora (43, 53). Although the complete answer to this question is not at hand, some other relevant genetic studies have been done with this organism. P. microspora Ne 32 is one of the most easily genetically transformable fungi that has been studied to date. In vivo addition of telomeric repeats to foreign DNA generates extrachromosomal DNAs in this fungus (41). Repeats of the telomeric sequence 5′-TTAGGG-3′ were appended to nontelomeric transforming DNA termini. The new DNAs, carrying foreign genes and the telomeric repeats, replicated independently of the chromosome and expressed the information carried by the foreign genes. The addition of telomeric repeats to foreign DNA is unusual among fungi. This finding may have important implications in the biology of P. microspora Ne 32 since it explains at least one mechanism by which new DNA can be captured by this organism and eventually expressed and replicated. Such a mechanism may begin to explain how the enormous biochemical variation may have arisen in this fungus (38). Also, this initial work represents a framework to aid in the understanding of how this fungus may adapt itself to the environment of its plant hosts and suggests that the uptake of plant DNA into its own genome may occur.
CONCLUDING STATEMENTS
Endophytes are a poorly investigated group of microorganisms that represent an abundant and dependable source of bioactive and chemically novel compounds with potential for exploitation in a wide variety of medical, agricultural, and industrial arenas. The mechanisms through which endophytes exist and respond to their surroundings must be better understood in order to be more predictive about which higher plants to seek, study, and spend time isolating microfloral components. This may facilitate the product discovery processes.
Although work on the utilization of this vast resource of poorly understood microorganisms has just begun, it has already become obvious that an enormous potential for organism, product, and utilitarian discovery in this field holds exciting promise. This is witnessed by the discovery of a wide range of products and microorganisms that already hold an inkling for future prospects as mentioned in this report. It is important for all involved in this work to realize the importance of acquiring the necessary permits from governmental, local, and other sources to pick and transport plant materials (especially from abroad) from which endophytes are to be eventually isolated. In addition to this aspect of the work is the added activity of producing the necessary agreements and financial sharing arrangements with indigenous peoples or governments in case a product does develop an income stream.
Certainly, one of the major problems facing the future of endophyte biology and natural-product discovery is the rapid diminishment of rainforests, which hold the greatest possible resource for acquiring novel microorganisms and their products. The total land mass of the world that currently supports rainforests is about equal to the area of the United States (44). Each year, an area the size of Vermont or larger is lost to clearing, harvesting, fire, agricultural development, mining, or other human-oriented activities. It is estimated that only 12 to 15% of what were the original rainforests in the “hot spots of biodiversity,” existing 1,000 to 2,000 years ago, are currently present on the earth (44). Few have ever expressed information or opinions about what is happening with regard to the potential loss of microbial diversity as entire plant species disappear. It can only be guessed that this loss is also happening, perhaps with the same frequency as the loss of mega-life forms, especially since certain microorganisms may have developed unique specific symbiotic relationships with their plant hosts. Thus, when a plant species disappears, so too does its entire suite of associated endophytes. Multistep processes are needed now to secure information and life forms before they continue to be lost. Areas of the planet that represent unique places housing biodiversity need immediate preservation. Countries need to establish information bases of their biodiversity and at the same time begin to make national collections of microorganisms that live in these areas.
Acknowledgments
We thank Gene Ford and David Ezra for helpful discussions. We also appreciate Don Mathre, David Daisy, and Doug Beauregard for critically reviewing the manuscript.
We express appreciation to the National Science Foundation, the U.S. Department of Agriculture, Novozymes Biotech, the NIH, the BARD Foundation of Israel, and the R&D Board of the State of Montana and the Montana Agricultural Experiment Station for providing financial support for some of the work reviewed in this report.
REFERENCES
- 1.Bacon, C. W., and J. F. White. 2000. Microbial endophytes. Marcel Dekker Inc., New York, N.Y.
- 2.Baker, D., U. Mocek, and C. Garr. 2000. Natural products vs. combinatorials: a case study, p. 66-72. In S. K. Wrigley, M. A. Hayes, R. Thomas, E. J. T. Chrystal, and N. Nicholson (ed.), Biodiversity: new leads for pharmaceutical and agrochemical industries. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 3.Ballio, A., F. Bossa, P. DiGiogio, P. Ferranti, M. Paci, P. Pucci, A. Scaloni, A. Segre, and G. A. Strobel. 1994. Structure of the pseudomycins, new lipodepsipeptides produced by Pseudomonas syringae MSU 16H. FEBS Lett. 355:96-100. [DOI] [PubMed] [Google Scholar]
- 4.Bashyal, B., J. Y. Li, G. A. Strobel, and W. M. Hess. 1999. Seimatoantlerium nepalense, an endophytic taxol producing coelomycete from Himalayan yew (Taxus wallichiana). Mycotaxon 72:33-42. [Google Scholar]
- 5.Bensky, D., and A. Gamble. 1993. Chinese herbal medicine. Materia medica, new ed. Eastland Press Inc., Seattle, Wash.
- 6.Bills, G., A. Dombrowski, F. Pelaez, J. Polishook, and Z. An. 2002. Recent and future discoveries of pharmacologically active metabolites from tropical fungi, p. 165-194. In R. Watling, J. C. Frankland, A. M. Ainsworth, S. Issac, and C. H. Robinson. (ed.), Tropical mycology: micromycetes, vol. 2. CABI Publishing, New York, N.Y.
- 7.Borel, J. F., and Z. L. Kis. 1991. The discovery and development of cyclosporine. Transplant. Proc. 23:1867-1874. [PubMed] [Google Scholar]
- 8.Brady, S. F., and J. Clardy. 2000. CR377, a new pentaketide antifungal agent isolated from an endophytic fungus. J. Nat. Prod. 63:1447-1448. [DOI] [PubMed] [Google Scholar]
- 9.Brewer, S. 2000. The relationship between natural products and synthetic chemistry in the discovery process, p. 59-65. In S. K. Wrigley, M. A. Hayes, R. Thomas, E. J. T. Chrystal, and N. Nicholson (ed.), Biodiversity: new leads for pharmaceutical and agrochemical industries. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 10.Buss, T., and M. A. Hayes. 2000. Mushrooms, microbes and medicines, p. 75-85. In S. K. Wrigley, M. A. Hayes, R. Thomas, E. J. T. Chrystal, and N. Nicholson (ed.), Biodiversity: new leads for pharmaceutical and agrochemical industries. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 11.Castillo, U., J. K. Harper, G. A. Strobel, J. Sears, K. Alesi, E. Ford, J. Lin, M. Hunter, M. Maranta, H. Ge, D. Yaver, J. B. Jensen, H. Porter, R. Robison, D. Millar, W. M. Hess, M. Condron, and D. Teplow. 2003. Kakadumycins, novel antibiotics from Streptomyces sp. NRRL 30566, an endophyte of Grevillea pteridifolia. FEMS Lett. 224:183-190. [DOI] [PubMed] [Google Scholar]
- 12.Castillo, U. F., G. A. Strobel, E. J. Ford, W. M. Hess, H. Porter, J. B Jensen, H. Albert, R. Robison, M. A. Condron, D. B. Teplow, D. Stevens, and D. Yaver. 2002. Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscans. Microbiology 148:2675-2685. [DOI] [PubMed] [Google Scholar]
- 13.Concepcion, G. P., J. E. Lazaro, and K. D. Hyde. 2001. Screening for bioactive novel compounds, p. 93-130. In S. B. Pointing and K. D. Hyde (ed.), Bio-exploitation of filamentous fungi. Fungal Diversity Press, Hong Kong, Hong Kong.
- 14.Daisy, B., G. Strobel, D. Ezra, U. Castillo, G. Baird, and W. M. Hess. 2002. Muscodor vitigenus anam. sp. nov., an endophyte from Paullinia paullinioides. Mycotaxon 84:39-50. [Google Scholar]
- 15.Daisy, B. H., G. A. Strobel, U. Castillo, D. Ezra, J. Sears, D. Weaver, and J. B. Runyon. 2002. Naphthalene, an insect repellent, is produced by Muscodor vitigenus, a novel endophytic fungus. Microbiology 148:3737-3741. [DOI] [PubMed] [Google Scholar]
- 16.Demain, A. L. 2000. Microbial natural products: a past with a future, p. 3-16. In S. K. Wrigley, M. A. Hayes, R. Thomas, E. J. T. Chrystal, and N. Nicholson (ed.), Biodiversity: new leads for pharmaceutical and agrochemical industries. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 17.Demain, A. L. 1981. Industrial microbiology. Science 214:987-994. [DOI] [PubMed] [Google Scholar]
- 18.Dreyfuss, M. M., and I. H. Chapela. 1994. Potential of fungi in the discovery of novel, low-molecular weight pharmaceuticals, p. 49-80. In V. P. Gullo (ed.), The discovery of natural products with therapeutic potential. Butterworth-Heinemann, London, United Kingdom. [DOI] [PubMed]
- 19.Findlay, J. A., S. Bethelezi, G. Li, and M. Sevek. 1997. Insect toxins from an endophyte fungus from wintergreen. J. Nat. Prod. 60:1214-1215. [Google Scholar]
- 20.Grabley, S., and R. Thiericke (ed.). 1999. Drug discovery from nature, p. 3-33. Springer-Verlag. Berlin, Germany.
- 21.Guo, B., J. Dai, S. Ng, Y. Huang, C. Leong, W. Ong, and B. K. Carte. 2000. Cytonic acids A and B: novel tridepside inhibitors of hCMV protease from the endophytic fungus Cytonaema species. J. Nat. Prod. 63:602-604. [DOI] [PubMed] [Google Scholar]
- 22.Harper, J., A. E. Mulgrew, J. Y. Li, D. H. Barich, G. A. Strobel, and D. M. Grant. 2001. Characterization of stereochemistry and molecular confirmation using solid state NMR tensors. J. Am. Chem. Soc. 123:9837-9842. [DOI] [PubMed] [Google Scholar]
- 23.Harper, J. K., E. J. Ford, G. A. Strobel, A. Arif, D. M. Grant, J. Porco, D. P. Tomer, and K. Oneill. 2003. Pestacin: a 1,3-dihydro isobenzofuran from Pestalotiopsis microspora possessing antioxidant and antimycotic activities. Tetrahedron 59:2471-2476. [Google Scholar]
- 24.Harper, J. K., D. H. Barich, J. Z. Hu, G. A. Strobel, and D. M. Grant. 2003. Stereochemical analysis by solid-state NMR: structural predictions in ambuic acids J. Org. Chem. 68:4609-4614. [DOI] [PubMed] [Google Scholar]
- 25.Harrison, L., D. Teplow, M. Rinaldi, and G. A. Strobel. 1991. Pseudomycins, a family of novel peptides from Pseudomonas syringae, possessing broad spectrum antifungal activity. J. Gen. Microbiol. 137:2857-2865. [DOI] [PubMed] [Google Scholar]
- 26.Hawksworth, D. C., and A. Y. Rossman. 1987. Where are the undescribed fungi? Phytopathology 87:888-891. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman, A., W. Khan, J. Worapong, G. Strobel, D. Griffin, B. Arbogast, D. Borofsky, R. B. Boone, L. Ning, P. Zheng, and L. Daley. 1998. Bioprospecting for taxol in angiosperm plant extracts. Spectroscopy 13:22-32. [Google Scholar]
- 28.Horn, W. S., M. S. J. Simmonds, R. E. Schwartz, and W. M. Blaney. 1995. Phomopsichalasin, a novel antimicrobial agent from an endophytic Phomopsis sp. Tetrahedron 14:3969-3978. [Google Scholar]
- 29.Hu, Y., L. Chaomin, B. Kulkarni, G. Strobel, E. Lobkovsky, R. Torczynski, and J. Porco. 2001. Exploring chemical diversity of epoxyquinoid natural products: synthesis and biological activity of jesterone and related molecules. Org. Lett. 3:1649-1652. [DOI] [PubMed] [Google Scholar]
- 30.Isaacs, J. 2002. Aboriginal food and herbal medicine. New Holland Press, Sydney, Australia.
- 31.Kunoh, H. 2002. Endophytic actinomycetes: attractive biocontrol agents. J. Gen. Plant Pathol. 68:249-252. [Google Scholar]
- 32.Lee, J., E. Lobkovsky, N. B. Pliam, G. A. Strobel, and J. Clardy. 1995. Subglutinols A and B: immunosuppressive compounds from the endophytic fungus Fusarium subglutinans. J. Org. Chem. 60:7076-7077. [Google Scholar]
- 33.Lee, J. C., G. A. Strobel, E. Lobkovsky, and J. C. Clardy. 1996. Torreyanic acid: a selectively cytotoxic quinone dimer from the endophytic fungus Pestalotiopsis microspora. J. Org. Chem. 61:3232-3233. [Google Scholar]
- 34.Lee, J. C., X. Yang, M. Schwartz G. Strobel, and J. Clardy. 1995. The relationship between an endangered North American tree and an endophytic fungus. Chem. Biol. 2:721-727. [DOI] [PubMed] [Google Scholar]
- 35.Li, C., R. P. Johnson, and J. A. Porco. 2003. Total synthesis of the quinine epoxide dimer (+)-torreyanic acid: application of a biomimetic oxidation/electrocyclization/Diels-Alder dimerization cascade. J. Am. Chem. Soc. 125:5059-5106. [DOI] [PubMed] [Google Scholar]
- 36.Li, J. Y., and G. A. Strobel. 2001. Jesterone and hydroxy-jesterone antioomycetcyclohexenenone epoxides from the endophytic fungus Pestalotiopsis jesteri. Phytochemistry 57:261-265. [DOI] [PubMed] [Google Scholar]
- 37.Li, J. Y., G. A. Strobel, J. K. Harper, E. Lobkovsky, and J. Clardy. 2000. Cryptocin, a potent tetramic acid antimycotic from the endophytic fungus Cryptosporiopsis cf. quercina. Org. Lett. 2:767-770. [DOI] [PubMed] [Google Scholar]
- 38.Li, J. Y., G. A. Strobel, R. Sidhu, W. M. Hess, and E. Ford. 1996. Endophytic taxol producing fungi from Bald Cypress Taxodium distichum. Microbiology 142:2223-2226. [DOI] [PubMed] [Google Scholar]
- 39.Li, J. Y., J. K. Harper, D. M. Grant, B. O. Tombe, B. Bashyal, W. M. Hess, and G. A. Strobel. 2001. Ambuic acid, a highly functionalized cyclohexenone with antifungal activity from Pestalotiopsis spp. and Monochaetia sp. Phytochemistry 56:463-468. [DOI] [PubMed] [Google Scholar]
- 40.Li, J. Y., R. S. Sidhu, E. Ford, W. M. Hess, and G. A. Strobel. 1998. The induction of taxol production in the endophytic fungus Periconia sp. from Torreya grandifolia. J. Ind. Microbiol. 20:259-264. [Google Scholar]
- 41.Long, N. E., E. D. Smidmansky, A. J. Archer, and G. A. Strobel. 1998. In vivo addition of telomeric repeats to foreign DNA generates chromosomal DNAs in the taxol-producing fungus Pestalotiopsis microspora. Fungal Genet. Biol. 24:335-344. [DOI] [PubMed] [Google Scholar]
- 42.Lu, H., W. X. Zou, J. C. Meng, J. Hu, and R. X. Tan. 2000. New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant Sci. 151:67-73. [Google Scholar]
- 43.Miller, R. V., C. M. Miller, D. Garton-Kinney, B. Redgrave, J. Sears, M. Condron, D. Teplow, and G. A. Strobel. 1998. Ecomycins, unique antimycotics from Pseudomonas viridiflava. J. Appl. Microbiol. 84:937-944. [DOI] [PubMed] [Google Scholar]
- 44.Mittermeier, R. A., N. Meyers, P. R. Gil, and C. G. Mittermeier. 1999. Hotspots: Earth's biologically richest and most endangered ecoregions. Toppan Printing Co., Tokyo, Japan.
- 45.National Institutes of Health. 2001. NIAID global health research plan for HIV/AIDS, malaria and tuberculosis. U.S. Department of Health and Human Services, Bethesda, Md.
- 46.Pulici, M., F. Sugawara, H. Koshino, J. Uzawa, S. Yoshida, E. Lobkovsky, and J. Clardy. 1996. Pestalotiopsin-A and pestalotiopsin-B: new caryophyllenes from an endophytic fungus of Taxus brevifolia. J. Org. Chem. 61:2122-2124. [Google Scholar]
- 47.Pulici, M., F. Sugawara, H. Koshino, J. Uzawa, S. Yoshida, E. Lobkovsky, and J. Clardy. 1996. A new isodrimeninol from Pestalotiopsis sp. J. Nat. Prod. 59:47-48. [Google Scholar]
- 48.Pulici, M., F. Sugawara, H. Koshino, J. Uzawa, S. Yoshida, E. Lobkovsky, and J. Clardy. 1996. Metabolites of endophytic fungi of Taxus brevifolia-the first highly functionalized humulane of fungal origin. J. Chem. Res. N. 8:378-379. [Google Scholar]
- 49.Redell, P., and V. Gordon. 2000. Lessons from nature: can ecology provide new leads in the search for novel bioactive chemicals from rainforests?, p. 205-212. In S. K. Wrigley, M. A. Hayes, R. Thomas, E. J. T. Chrystal, and N. Nicholson (ed.), Biodiversity: new leads for pharmaceutical and agrochemical industries. The Royal Society of Chemistry. Cambridge, United Kingdom.
- 50.Schiff, P. B., and S. B. Horowitz. 1980. Taxol stabilizes microtubules in mouse fibroblast cells. Proc. Natl. Acad. Sci. USA 77:1561-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schutz, B. 2001. Endophytic fungi: a source of novel biologically active secondary metabolites, p. 20. In British Mycological Society, international symposium proceedings: bioactive fungal metabolites—impact and exploitation. University of Wales, Swansea, Wales.
- 52.Shrestha, K., G. A. Strobel, S. Prakash, and M. Gewali. 2001. Evidence for paclitaxel from three new endophytic fungi of Himalayan yew of Nepal. Planta Med. 67:374-376. [DOI] [PubMed] [Google Scholar]
- 53.Stierle, A., G. A. Strobel, and D. Stierle. 1993. Taxol and taxane production by Taxomyces andreanae. Science 260:214-216. [DOI] [PubMed] [Google Scholar]
- 54.Stinson, M., D. Ezra, and G. Strobel. 2003. An endophytic Gliocladium sp. of Eucryphia cordifolia producing selective volatile antimicrobial compounds. Plant Sci. 165:913-922. [Google Scholar]
- 55.Strobel, G., X. Yang, J. Sears, R. Kramer, R. S. Sidhu, and W. M. Hess. 1996. Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallichiana. Microbiology 142:435-440. [DOI] [PubMed] [Google Scholar]
- 56.Strobel, G. A. 2002. Microbial gifts from rain forests. Can. J. Plant Pathol. 24:14-20. [Google Scholar]
- 57.Strobel, G. A. 2002. Rainforest endophytes and bioactive products. Crit. Rev. Biotechnol. 22:315-333. [DOI] [PubMed] [Google Scholar]
- 58.Strobel, G. A., A. Stierle, D. Stierle, and W. M. Hess. 1993. Taxomyces andreanae a proposed new taxon for a bulbilliferous hyphomycete associated with Pacific yew. Mycotaxon 47:71-78. [Google Scholar]
- 59.Strobel, G. A., E. Dirksie, J. Sears, and C. Markworth. 2001. Volatile antimicrobials from a novel endophytic fungus. Microbiology 147:2943-2950. [DOI] [PubMed] [Google Scholar]
- 60.Strobel, G. A., E. Ford, J. Worapong, J. K. Harper, A. M. Arif, D. M. Grant, P. C. W. Fung, and K. Chan. 2002. Ispoestacin, an isobenzofuranone from Pestalotiopsis microspora, possessing antifungal and antioxidant activities. Phytochemistry 60:179-183. [DOI] [PubMed] [Google Scholar]
- 61.Strobel, G. A., J. Y. Li, F. Sugawara, H. Koshino, J. Harper, and W. M. Hess. 1999. Oocydin A, a chlorinated macrocyclic lactone with potent anti-oomycete activity from Serratia marcescens. Microbiology 145:3557-3564. [DOI] [PubMed] [Google Scholar]
- 62.Strobel, G. A., R. V. Miller, C. Miller, M. Condron, D. B. Teplow, and W. M. Hess. 1999. Cryptocandin, a potent antimycotic from the endophytic fungus Cryptosporiopsis cf. quercina. Microbiology 145:1919-1926. [DOI] [PubMed] [Google Scholar]
- 63.Strobel, G. A., W. M. Hess, J. Y. Li, E. Ford, J. Sears, R. S. Sidhu, and B. Summerell. 1997. Pestalotiopsis guepinii, a taxol producing endophyte of the Wollemi Pine, Wollemia nobilis. Aust. J. Bot. 45:1073-1082. [Google Scholar]
- 64.Suffness, M. 1995. Taxol, science and applications. CRC Press, Boca Raton, Fla.
- 65.Tan, R. X., and W. X. Zou. 2001. Endophytes: a rich source of functional metabolites. Nat. Prod. Rep. 18:448-459. [DOI] [PubMed] [Google Scholar]
- 66.Wagenaar, M., J. Corwin, G. A. Strobel, and J. Clardy. 2000. Three new chytochalasins produced by an endophytic fungus in the genus Rhinocladiella. J. Nat. Prod. 63:1692-1695. [DOI] [PubMed] [Google Scholar]
- 67.Walsh, T. A. 1992. Inhibitors of β-glucan synthesis, p. 349-373. In J. A. Sutcliffe and N. H. Georgopapadakou (ed.), Emerging targets in antibacterial and antifungal chemotherapy. Chapman & Hall, London, United Kingdom.
- 68.Wang, J., G. Li, H. Lu, Z. Zheng, Y. Huang, and W. Su. 2000. Taxol from Tubercularia sp. strain TF5, an endophytic fungus of Taxus mairei. FEMS Microbiol. Lett. 193:249-253. [DOI] [PubMed] [Google Scholar]
- 69.Wani, M. C., H. L. Taylor, M. E. Wall, P. Goggon, and A. T. McPhail. 1971. Plant antitumor agents, VI. The isolation and structure of taxol, anovel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 93:2325-2327. [DOI] [PubMed] [Google Scholar]
- 70.Worapong, J., G. A. Strobel, E. J. Ford, J. Y. Li, G. Baird, and W. M. Hess. 2001. Muscodor albus gen. et sp. nov., an endophyte from Cinnamomum zeylanicum. Mycotaxon 79:67-79. [Google Scholar]
- 71.Worapong, J., G. A. Strobel, B. Daisy, U. Castillo, G. Baird, and W. M. Hess. 2002. Muscodor roseus anna. nov. an endophyte from Grevillea pteridifolia. Mycotaxon 81:463-475. [Google Scholar]
- 72.Young, D. H., E. J. Michelotti, C. S. Sivendell, and N. E. Krauss. 1992. Antifungal properties of taxol and various analogues. Experientia 48:882-885. [DOI] [PubMed] [Google Scholar]
- 73.Zhang, B., G. Salituro, D. Szalkowski, Z. Li, Y. Zhang, I. Royo, D. Vilella, M. Dez, F. Pelaez, C. Ruby, R. L. Kendall, X. Mao, P. Griffin, J. Calaycay, J. R. Zierath, J. V. Heck, R. G. Smith, and D. E. Moller. 1999. Discovery of small molecule insulin mimetic with antidiabetic activity in mice. Science 284:974-981. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, Y. Z., X. Sun, D. Zechner, B. Sachs, W. Current, J. Gidda, M. Rodriguez, and S. H. Chen. 2001. Synthesis and antifungal activities of novel 3-amido bearing pseudomycin analogs. Bioorg. Med. Chem. 11:903-907. [DOI] [PubMed] [Google Scholar]
- 75.Zou, W. X., J. C. Meng, H. Lu, G. X. Chen, G. X. Shi, T. Y. Zhang, and R. X. Tan. 2000. Metabolites of Colletotrichum gloeosporioides, an endophytic fungus in Artemisia mongolica. J. Nat. Prod. 63:1529-1530. [DOI] [PubMed] [Google Scholar]