Abstract
AMP-activated protein kinase (AMPK) is a cellular energy sensor that responds to low endogenous energy by stimulating fatty acid oxidation (through inactivation of acetyl-CoA carboxylase (ACC)) and food intake. Fasting generally stimulates phosphorylation of AMPK (pAMPK) and ACC (pACC), but it is unclear how AMPK and ACC react to a long-term fast (i.e. hibernation). We performed Western blots for total and pAMPK and pACC on tissues from a species of hibernator (Callospermophilus lateralis) after short-term summer fasting (1–5 days) and long-term winter fasting (3 months). Winter animals were sacrificed during hibernation at low body temperature (torpid, Tb~5°C) or at normal high Tb(euthermic, Tb~37°C). We found a general increase in pAMPK in most tissues (liver, muscle, white adipose tissue (WAT), but not hypothalamus) and pACC in all tissues after a short-term summer fast. Response of AMPK and ACC to a long-term winter fast differed by tissue—in liver, there was no difference in total or pAMPK or pACC between groups, but in muscle, WAT and BAT, euthermic GMGS had lower relative abundance of pAMPK and pACC than torpid animals. Therefore, AMPK may be an important energy sensor at all points in hibernator’s circannual cycles of food intake and Tb.
Keywords: ACC, AMPK, fasting, hibernation
1. Introduction
AMP-activated protein kinase (AMPK) is an important metabolic regulator that senses decreasing cellular energy status and whose activation stimulates ATP producing pathways, such as fatty acid oxidation and glycolysis, and blocks anabolic processes (fatty acid biosynthesis (Andersson et al., 2004). AMPK is an intracellular heterotrimeric enzyme and is activated by phosphorylation when exposed to high AMP:ATP ratios experienced during periods of low cellular energy (Carling & Hardie, 1989; Hardie et al., 2006). Acetyl-CoA carboxylase (ACC) is a multi-domain enzyme that catalyzes the conversion of acetyl-CoA to malonyl-CoA, which is an important step in lipid biosynthesis. ACC is inactivated through reversible phosphorylation by AMPK. Inhibition of ACC decreases malonyl-CoA availability; as high malonyl-CoA levels are needed to inhibit carnitine palmitoyl transferase-1 (CPT1) and decrease mitochondrial oxidation of fatty acids, the inhibition of ACC by AMPK allows increased fatty acid oxidation.
AMPK has distinct and various effects on peripheral and central tissues. In peripheral tissues, AMPK regulates a variety of metabolic pathways that support catabolic (ATP-producing) processes and downregulate anabolic processes. Phosphorylated AMPK (pAMPK) stimulates fatty acid oxidation in various tissues, inhibits fatty acid synthesis in liver and adipose tissue, and inhibits protein synthesis in liver and muscle (Xue & Kahn, 2006). During fasting, AMPK in peripheral tissues reacts in different ways. In mice and rats, short-term fasting (19–39 h) generally results in an increase of pAMPK and pACC in WAT, but not in liver or muscle tissue (Kajita et al., 2008; Sponarova et al., 2005). In contrast, long-term caloric restriction in Wistar rats appeared to decrease pAMPK in the liver, but had no effect on pACC (To et al., 2007). Centrally, Sprague-Dawley rats fasted for two days demonstrated increased pAMPK in the ventromedial area of the hypothalamus (Murphy et al., 2009); several other studies have reported similar results in other areas of the hypothalamus (for a review, see Minokoshi et al., 2004).
Hibernation is a life history strategy during which animals voluntarily fast for 6–8 months out of the year. Mammals that hibernate (hibernators) exhibit a robust circannual cycle of fluctuating food intake. In spring and summer, hibernators are normophagic (food intake similar to that of non-hibernating rodent), but in autumn, animals become hyperphagic (eating 2–3 times as much food as during spring and summer). At the end of this hyperphagic period, hibernators decrease and eventually completely cease food intake (aphagia) and become heterothermic, dropping their body temperature (Tb) to approximate ambient temperature (Ta) during multi-day torpor bouts throughout the winter season. These multi-day torpor bouts are interrupted by brief periods (12–24 h) of euthermia (interbout arousals) after which animals return to low tissue temperature (torpid). During torpor bouts, hibernators maintain very low cardiovascular and metabolic rates and metabolize almost entirely fat (Dark, 2005). As an animal re-warms to euthermia for an interbout arousal, it increases metabolic rate and metabolizes primarily carbohydrates until Tb reaches ~16°C, at which point it switches back to primarily lipid metabolism for the remainder of the arousal period (Snapp& Heller, 1981). Interbout arousals are the most energetically expensive part of a torpor bout, with 85–90% of a hibernator’s energy budget fueling these arousals (Wang, 1978). Hibernators may remain heterothermic and aphagic from late August to mid May (Florant et al., unpub. data).
The effects of short-term fasting on AMPK in various tissues have been investigated extensively with conflicting results, but few have studied the effects of AMPK during the long-term fast exhibited by hibernating mammals. In one such study, Horman et al. (2005) found that during the hibernation season, AMPK was activated in white adipose tissue (WAT) in thirteen-lined ground squirrels (Spermophilus tridecemlineatus, now Ictidomys tridecemlineatus after Helgen et al., 2009), but not in liver, muscle, brown adipose tissue (BAT) or brain as compared to summer euthermic ground squirrels. However, this experiment did not differentiate between the torpid and winter euthermic (interbout arousal) stages of hibernation, and measured central AMPK activity without specifying brain region. Information on how AMPK acts in specific areas of the brain, such as hypothalamus, where AMPK acts as a fuel sensor, is lacking in the hibernation literature. Recent experiments by Florant et al. (2010) demonstrated that central infusion of the AMPK agonist 5-aminoimidazole-4-carboxamide 1 B-D-ribofuranoside (AICAR) into the third ventricle of winter aphagic marmots caused an initiation of food intake, consistent with AMPK’s role in increasing food intake.
In order to elucidate the effects of both long and short-term fasting on AMPK and ACC in hibernators, we compared these enzymes in tissues from a locally abundant species of hibernator (the golden-mantled ground squirrel, Callospermophilus lateralis) after a short-term (1–5 days) fast in the summer and during a long-term fast (3 months) in the winter while either torpid or euthermic. We hypothesized that a summer fast would increase the active form of AMPK (pAMPK) and the inactivated form of ACC (pACC) in liver, muscle, WAT, and in the hypothalamus as squirrels metabolized endogenous energy stores. In winter animals, we hypothesized that euthermic squirrels would have higher total and phosphorylated levels of AMPK and ACC than would torpid animals in liver, muscle, WAT, BAT, and in the arcuate nucleus of the hypothalamus (ARC) since these enzymes are broken down fairly quickly, and protein synthesis during torpor (while animals are at low tissue temperature) is minimal.
2. Materials and methods
2.1. Animals
Adult golden-mantled ground squirrels (GMGS; Callospermophilus lateralis)) were live trapped in Larimer and Gunnison Counties, Colorado, and brought to the animal facility at Colorado State University. All animal procedures were conducted in accordance with the guide for the care and use of animals as indicated by the National Institutes of Health and all protocols received prior approval by the Animal Care and Use Committee of CSU. Animals were kept in a temperature-controlled room under natural photoperiod (Paragon Sun Tracker EC72ST;Invensys Controls, Carol Stream, IL, USA) and with food (Teklad 8604 Rodent Diet) and water available ad libitum. Food intake was measured weekly. Ambient temperature (Ta) in the animal room was gradually decreased from 18°C in July to 12°C in August to approximate natural conditions. In September, Ta was decreased to 5°C, and GMGS were kept in constant darkness as they began to undergo torpor. GMGS were randomly assigned to two groups: summer-fast and winter-fast animals. Summer-fast animals were either fed ad lib (control, n=6) or fasted for one (n=3), three (n=7), or five (n=4) days, then anesthetized and tissues removed. Body mass was measured immediately before food was removed, and again at time of euthanasia. Briefly, animals were anesthetized with a ketamine-acepromazine-xylazine cocktail (75%:15%:10% respectively), weighed, and the majority of animals were decapitated. Two control animals and three 3-day fast animals were transcardially perfused with ~50 mL saline followed with 4% paraformaldehyde in 0.1M PB. Prior to perfusion, tissues were removed, flash-frozen in liquid nitrogen, and stored at −80°C. All tissues were removed and flash frozen sufficiently rapidly to avoid exposure to anoxia. After perfusion, brains were removed and post-fixed in 4% paraformaldehyde overnight, soaked in 30% sucrose solution for 2 days, and stored in cryoprotectant at 4°C until sectioning. Brains were sectioned coronally at 30 µm using a cryostat, and slices containing the arcuate nucleus (determined from a ground squirrel atlas (Joseph et al., 1966)) were retained and stored in cryoprotectant at 4°C.
Winter-fast GMGS were allowed to hibernate until January, and were then euthanized either during an interbout arousal (euthermic, Tb~37°C, n=7) or at low tissue temperature (torpid, Tb~5°C, n=8). All winter-fast animals had been completely aphagic since October. Euthermic animals were roused to euthermia by physical manipulation, allowed to remain euthermic for 2 h, and then euthanized as described above. Three winter-euthermic animals and two winter-torpid animals were transcardially perfused as described above, and the remaining animals were decapitated. Torpid animals had low Tb verified by skin, mouth, and blood temperature readings by thermocouple (Thermochron), and then were decapitated or perfused without anesthesia while at low tissue temperature. Prior to perfusion, tissues were removed and treated as described above.
2.2. Hypothalamic dissection
In all non-perfused animals, brains were removed immediately following decapitation, flash-frozen in 2-methylbutanol and stored at −80°C until use. Hypothalami from all brains were dissected out (using stereotaxic ground squirrel brain atlas by Joseph et al. (1966)) and homogenized in 0.5mL lysis buffer with protease inhibitor cocktail, centrifuged, and the supernatant removed. Homogenates were frozen at −80°C until assayed.
2.3. Western blots
Tissues (liver, muscle, WAT, BAT) were homogenized in 1mL ice-cold lysis buffer containing a protease inhibitor cocktail, centrifuged, and the supernatant removed. Protein concentration was determined by BCA assay, and Western blots were performed. In summer animals, liver, muscle, WAT, and total hypothalamus were analyzed, and in winter animals, liver, muscle, WAT, and BAT were analyzed by Western blot. Briefly, sample proteins were separated by SDS-PAGE gel and transferred to nitrocellulose. 100µg of protein were added to each gel, and β-actin or GAPDH were used as loading controls. The membranes were blocked in TBS with 5% milk powder and incubated overnight on an orbital shaker at 4°C in primary antibody (diluted 1:1000 for pAMPK, AMPK, pACC, ACC, β-actin, and GAPDH). Antibodies were obtained from Cell Signaling (Phospho-AMPKα (Thr172) Rabbit mAb #2531, AMPKα (23A3) Rabbit mAb #2603, Phospho-Acetyl-CoA Carboxylase (Ser79) Antibody #3661, Acetyl-CoA Carboxylase (C83B10) Rabbit mAb # 3676, β-actin Antibody # 4967, GAPDH Antibody #2118). After washing in TBST, membranes were incubated at room temperature for 1 hr in HRP-conjugated secondary antibody (1:2000) and HRP-conjugated anti-biotin antibody (1:1000). After further washing in TBST, membranes were developed by chemiluminescence (Amersham ECL Plus from GE Healthcare), and imaged on a STORM. Protein relative abundance was quantified using ImageQuant and normalized against β-actin (for all summer tissues, and for liver, WAT, and BAT in winter tissues) or GAPDH (for winter muscle).
2.4. Immunohistochemistry
ARC-containing brain slices from summer control (fed, n=2), summer 3-day fasted (n=3), winter euthermic (n=3), and winter torpid (n=2) animals were analyzed for pAMPK using immunohistochemistry (IHC). Slices were labeled with antibodies against the nuclear pAMPK protein, which was detected through the use of avidin-biotin-immunoperoxidase and nickel-enhanced diaminobenzidine. Therefore, pAMPK-immunoreactive (pAMPK-ir) cells were identified by their dark blue/black nuclei. Before IHC was performed, brain slices were washed in phosphate buffer, plated onto positively charged glass slides (Superfrost Plus Slides; Fisher Scientific), and dried for 48 h. Slices were antigen-unmasked by boiling in 10M, pH 6.0, sodium citrate solution (Fisher 5279) for 10 min. After cooling, slices were washed in 1X Tris Buffered Saline/0.1% Triton X-100 solution. They were then exposed to a 1% hydrogen peroxide solution (Sigma) for ten minutes, and blocked in 6% normal goat serum (NGS, Jackson Laboratories) solution for one h. Slides were incubated with primary antibody (1:50 dilution of phospho-AMPK alpha (Thr 172) 40H9 rabbit mAb (Cell Signaling Technology, #2535)) for 48 h at 4°C. They were then incubated for one h in a 1:1000 dilution of biotinylated goat anti-rabbit IgG secondary antibody (Vector Laboratories, Burlingame, CA, USA). Avidin-Biotin complex (ABC, 1:1000, Vector Laboratories) was applied for one h, and visualization was accomplished using 1% 3,3’-diaminobenzidine (DAB), 0.003% hydrogen peroxide, and 0.02% nickel sulfate. The reaction was stopped after ten minutes with TBS. IHC controls included omission of the primary antibody from the immunostaining protocol, the absence of which completely eliminated immunoreactivity for the corresponding antigen.
2.5. Analysis of IHC protein immunoreactivity
All physiological states were analyzed for pAMPK in the ARC. Brain slices from each animal were arranged rostrally to caudally on slides for visualization by IHC. For each animal, four random slices (representative of different rostral to caudal areas of the arcuate nucleus) were chosen for analysis. In each of these slices, a unilateral ARC was chosen to be micrographed by a Zeiss Axioskop microscope. Since pAMPK is a nuclear protein, cells immunoreactive for this neuropeptide were easily identified by the reaction product within the nuclear compartment. Number of cells were manually counted using Image J software (National Institute of Health). A digital grid was placed on top of each micrograph, and a medial-ventral portion of each arcuate nucleus was counted. The same arcuate portion and total area were analyzed for each slice. Cell counts from four unilateral arcuate nuclei per animal were averaged, and then these mean counts were averaged within a physiological state (summer fed, summer fasted, winter euthermic, or winter torpid) to determine differences between groups.
2.6. Statistics
Statistics were performed using Graph Pad Prism 5. Differences between fasting states were determined using a one-way ANOVA followed by a Bonferroni post-test for multiple comparisons, while differences between torpid and euthermic GMGS were determined using Student’s t-test coupled with an F test for homogeneity of variance. All results were considered significant at p≤0.05.
3. Results
3.1. Summer short-term fast
Animals fasted for 1, 3, and 5 days lost progressively and significantly more mass than ad-lib fed controls, which gained mass over the 5 days over which the experiment took place. Control animals gained 19g (±9.3g), while 1-day fast animals lost a mean of 18g (±7.5g), 3-day fast animals lost a mean of 36g (±3.2g), and 5-day fast animals lost a mean of 49.5g (±6.2g).
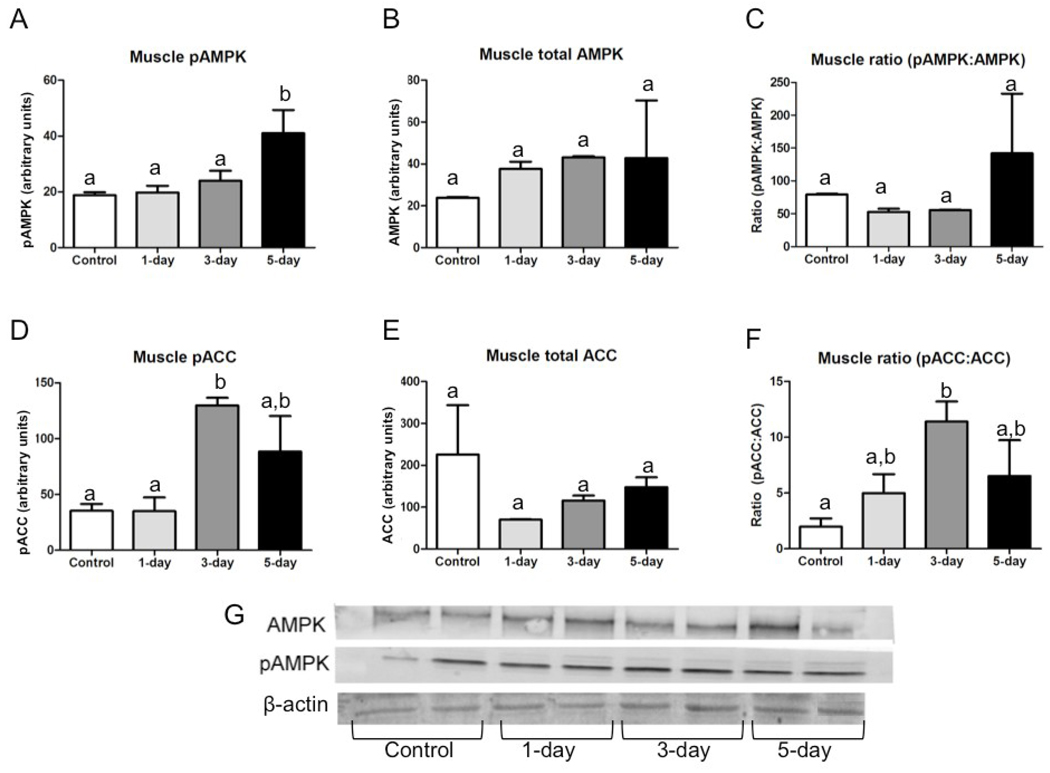
The response of AMPK and ACC to a short-term summer fast depended on phosphorylation state and tissue type. In muscle, pAMPK relative abundance increased significantly from control levels by day five of fasting (Fig. 1A). There were no differences between fasting states in either total AMPK or the pAMPK:AMPK ratio in muscle (Fig. 1B & 1C). Muscle pACC increased significantly with three days of fasting (Fig. 1D), but there was no difference in total ACC between fasting states (Fig. 1E). The ratio of pACC:ACC increased significantly over control levels by day three of fasting (Fig. 1F).
Fig. 1.
Total and phosphorylated AMPK and ACC in muscle of animals fasted 0, 1, 3, or 5 days in July (n=3–4 per group); all bars are group means (relative abundance of protein normalized to β-actin)± SEM. Bars with different lower-case letters are statistically different (p≤0.05). (A) Relative abundance of phosphorylated AMPK; (B) Relative abundance of total AMPK; (C) Proportion of phosphorylated to total AMPK; (D) Relative abundance of phosphorylated ACC; (E) Relative abundance of total ACC; (F) Proportion of phosphorylated to total ACC; (G) Representative Western blots for pAMPK, AMPK, and β-actin in muscle.
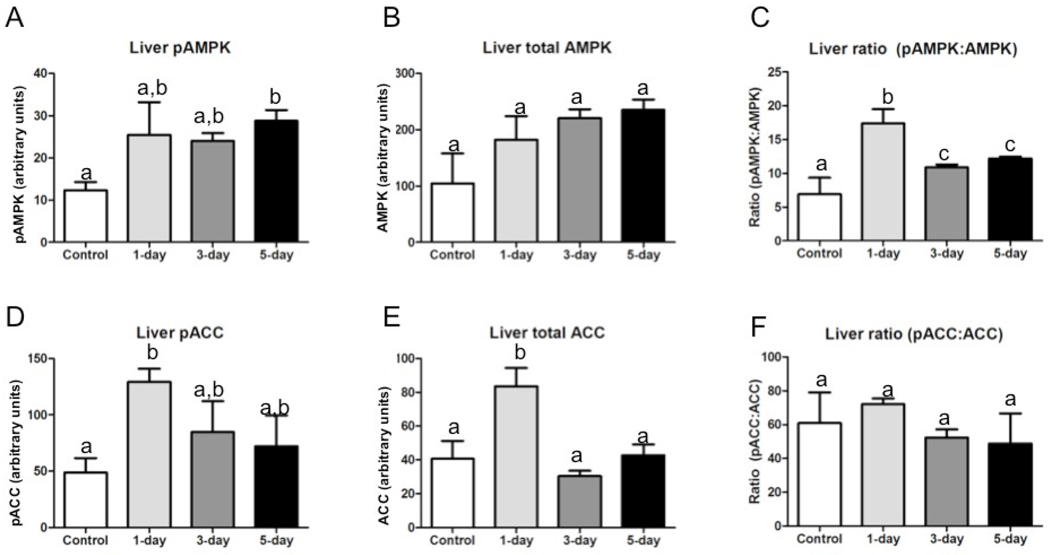
In liver, fasting increased pAMPK significantly over ad-lib fed controls by day 5 of fasting, but had no significant effect on total AMPK (Fig.2A &2B). The proportion of pAMPK to AMPK increased significantly after one day of fasting; the proportion decreased after 3 and 5 days of fasting, but still remained elevated compared with controls (Fig.2C). Both pACC and total ACC were increased significantly after one day of fasting, but decreased to control levels by fasting days three and five (Fig.2D &2E). There was no difference in proportion of pACC to ACC between fasting states (Fig.2F).
Fig. 2.
Total and phosphorylated AMPK and ACC in liver of animals fasted 0, 1, 3, or 5 days in July. (n=3–4 per group); all bars are group means (relative abundance of protein normalized to β-actin)± SEM. Bars with different lower-case letters are statistically different (p≤0.05). (A) Relative abundance of phosphorylated AMPK; (B) Relative abundance of total AMPK; (C) Proportion of phosphorylated to total AMPK; (D) Relative abundance of phosphorylated ACC; (E) Relative abundance of total ACC; (F) Proportion of phosphorylated to total ACC.
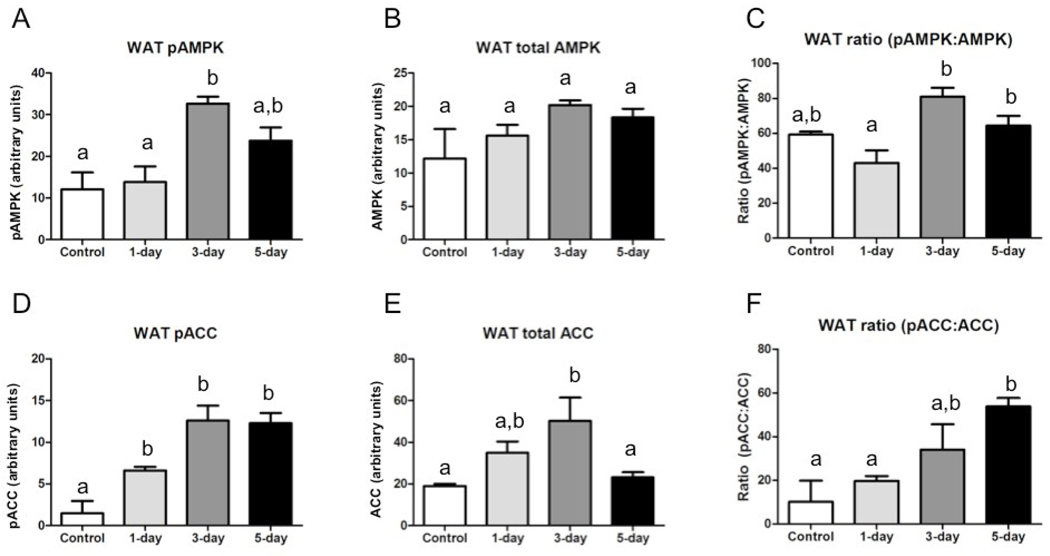
In WAT, pAMPK increased significantly over control levels by fasting day three (Fig.3A). There were no differences between groups in total AMPK (Fig.3B), but the proportion of pAMPK to AMPK was significantly higher in 3-day and 5-day fasted animals than in 1-day fasted animals (Fig.3C). Relative abundance of pACC increased significantly by day one of fasting and remained elevated in subsequent groups (Fig.3D). Total ACC was significantly increased in 3-day fasted animals compared with controls (Fig.3E), and the proportion of pACC to ACC was increased significantly over controls by day five of fasting (Fig.3F).
Fig. 3.
Total and phosphorylated AMPK and ACC in WAT of animals fasted 0, 1, 3, or 5 days in July (n=3–4 per group); all bars are group means (relative abundance of protein normalized to β-actin)± SEM. Bars with different lower-case letters are statistically different (p≤0.05). (A) Relative abundance of phosphorylated AMPK; (B) Relative abundance of total AMPK; (C) Proportion of phosphorylated to total AMPK; (D) Relative abundance of phosphorylated ACC; (E) Relative abundance of total ACC; (F) Proportion of phosphorylated to total ACC.
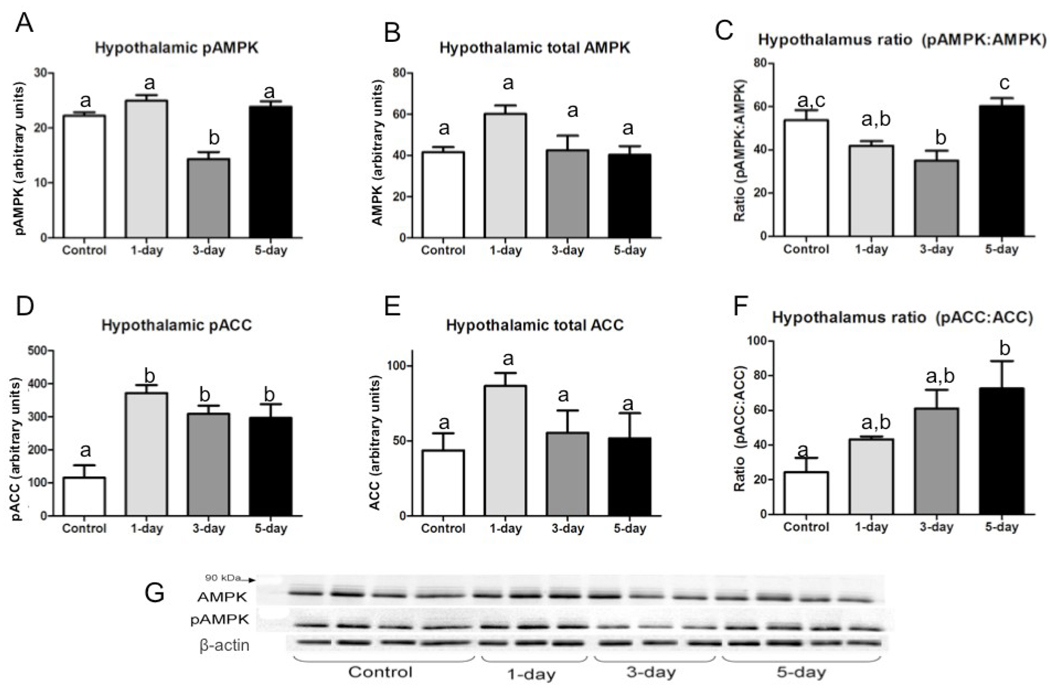
In the hypothalamus, pAMPK increased slightly with one day of fasting (p=0.07), decreased from control levels after three days of fasting, and increased to control levels again by day five of fasting (Fig.4A). There was no difference in relative abundance of total AMPK between fasting states (Fig.4B).The overall proportion of pAMPK to AMPK decreased significantly from controls on day three of fasting, then increased significantly between days three and five to return to control levels (Fig.4C). Relative abundance of pACC increased significantly with one day of fasting and remained elevated compared with controls for all five fasting days (Fig.4D). There were no significant differences in total ACC between fasting states (Fig. 4E), so the proportion of pACC to ACC increased with fasting, becoming significant by day five (Fig.4F).
Fig. 4.
Total and phosphorylated AMPK and ACC in hypothalamus of animals fasted 0, 1, 3, or 5 days in July (n=3–4 per group); all bars are group means (relative abundance of protein normalized to β-actin)± SEM. Bars with different lower-case letters are statistically different (p≤0.05). (A) Relative abundance of phosphorylated AMPK; (B) Relative abundance of total AMPK; (C) Proportion of phosphorylated to total AMPK; (D) Relative abundance of phosphorylated ACC; (E) Relative abundance of total ACC; (F) Proportion of phosphorylated to total ACC; (G) Representative Western blots for pAMPK, AMPK, and β-actin in hypothalamus.
3.2. Winter-fasted animals
Total and phosphorylated levels of AMPK and ACC were compared between two physiological states in winter-fasted animals: torpid and euthermic. In liver, there were no significant differences between states for either total or phosphorylated AMPK or ACC (data not shown).
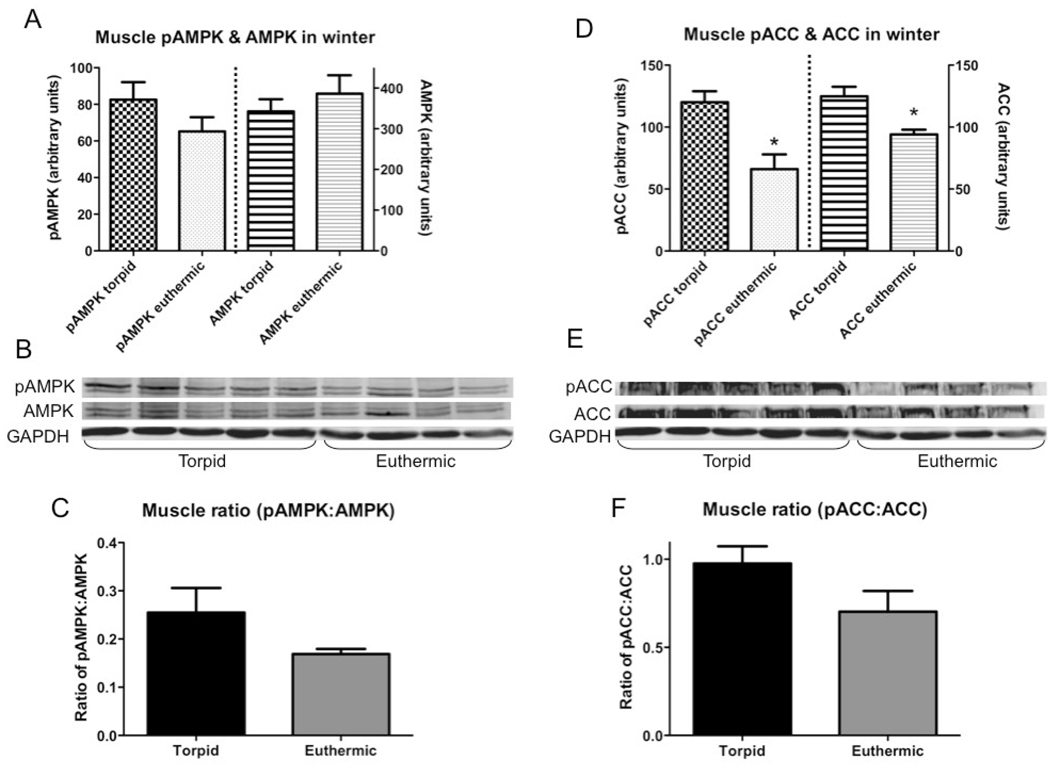
In muscle, euthermic animals generally had lower levels of enzymes than torpid animals, but there were no significant differences in AMPK between groups (Fig.5). Relative abundance of pAMPK was slightly (but not statistically) lower in euthermic animals (p=0.08), and the ratio of pAMPK:AMPK was slightly lower in euthermic than in torpid animals (p=0.07) (Fig.5A &5C). Both phosphorylated and total ACC were significantly lower in euthermic than torpid animals (p≤0.05) (Fig.5D &5F).
Fig. 5.
Total and phosphorylated AMPK and ACC in muscle of animals sacrificed in January either in torpor (torpid, n=4) or during an interbout arousal (euthermic, n=5); all bars are group means ± SEM. (A) Effect of hibernation state on muscle AMPK (total and phosphorylated, normalized against GAPDH); (B) Representative Western blots for pAMPK, AMPK, and GAPDH in muscle; (C) Ratio of phosphorylated to total AMPK (proportion of pAMPK:AMPK); (D) Effect of hibernation state on muscle ACC (total and phosphorylated, normalized against GAPDH); (E) Representative Western blots for pACC, ACC, and GAPDH in muscle; (F) Ratio of phosphorylated to total ACC (proportion of pACC:ACC). * = euthermic significantly different from torpid (p≤0.05).
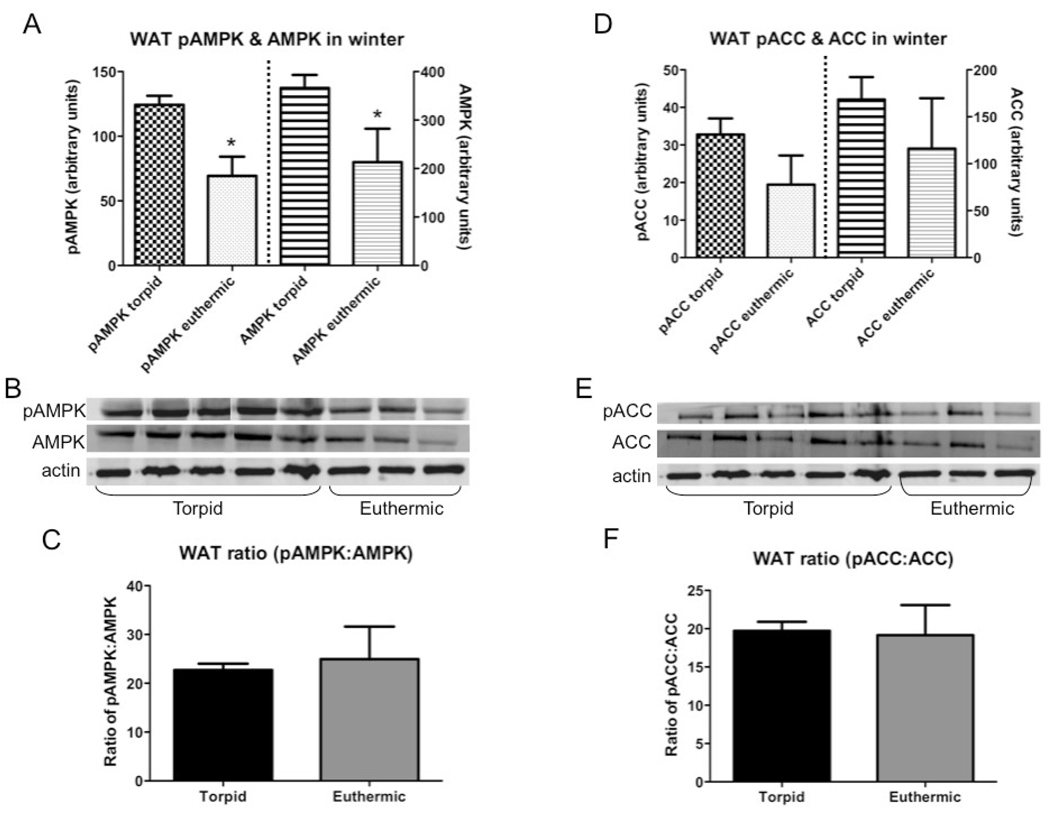
In WAT, euthermic animals had significantly (p≤0.05) less pAMPK and total AMPK than torpid animals (Fig.6A). There were no significant differences in total or phosphorylated ACC in WAT between states, or in pAMPK:AMPK or pACC:ACC ratios (Fig.6C, 6D, 6F).
Fig. 6.
Total and phosphorylated AMPK and ACC in WAT of animals sacrificed in January either in torpor (torpid, n=4) or during an interbout arousal (euthermic, n=5); all bars are group means ± SEM. (A) Effect of hibernation state on WAT AMPK (total and phosphorylated, normalized against β-actin); (B) Representative Western blots for pAMPK, AMPK, and actin in WAT; (C) Ratio of phosphorylated to total AMPK (proportion of pAMPK:AMPK); (D) Effect of hibernation state on WAT ACC (total and phosphorylated, normalized against β-actin); (E) Representative Western blots for pACC, ACC, and actin in WAT; (F) Ratio of phosphorylated to total ACC (proportion of pACC:ACC). * = euthermic significantly different from torpid (p≤0.05).
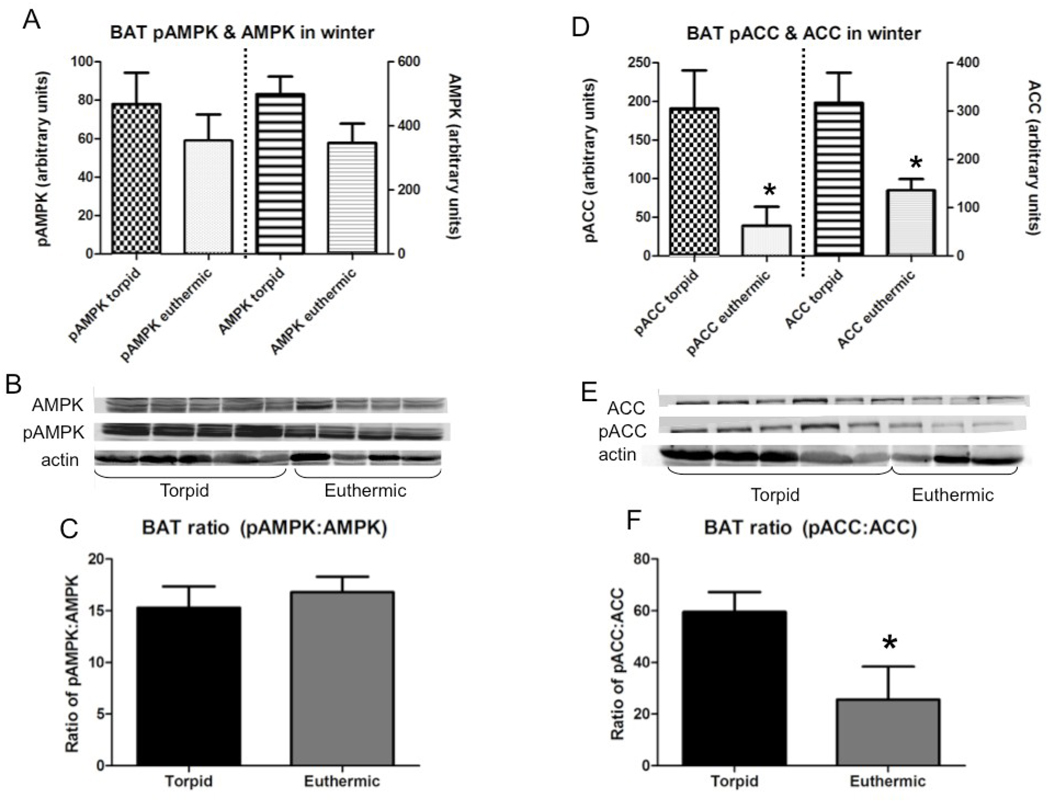
In BAT, there were no differences in total or phosphorylated AMPK between states (Fig.7A), but both pACC and total ACC were lower in euthermic than in torpid animals (Fig.7D). There was no difference in the proportion of pAMPK to AMPK between states (Fig.7C), but the pACC:ACC ratio was significantly lower in euthermic than in torpid animals (Fig. 7F).
Fig. 7.
Total and phosphorylated AMPK and ACC in BAT of animals sacrificed in January either in torpor (torpid, n=4) or during an interbout arousal (euthermic, n=5); all bars are group means ±SEM. (A) Effect of hibernation state on BAT AMPK (total and phosphorylated, normalized against β-actin); (B) Representative Western blots for pAMPK, AMPK, and actin in BAT; (C) Ratio of phosphorylated to total AMPK (proportion of pAMPK:AMPK); (D) Effect of hibernation state on BAT ACC (total and phosphorylated, normalized against β-actin); (E) Representative Western blots for pACC, ACC, and actin in BAT; (F) Ratio of phosphorylated to total ACC (proportion of pACC:ACC). * = euthermic significantly different from torpid (p≤0.05).
3.3. pAMPK immunoreactivity
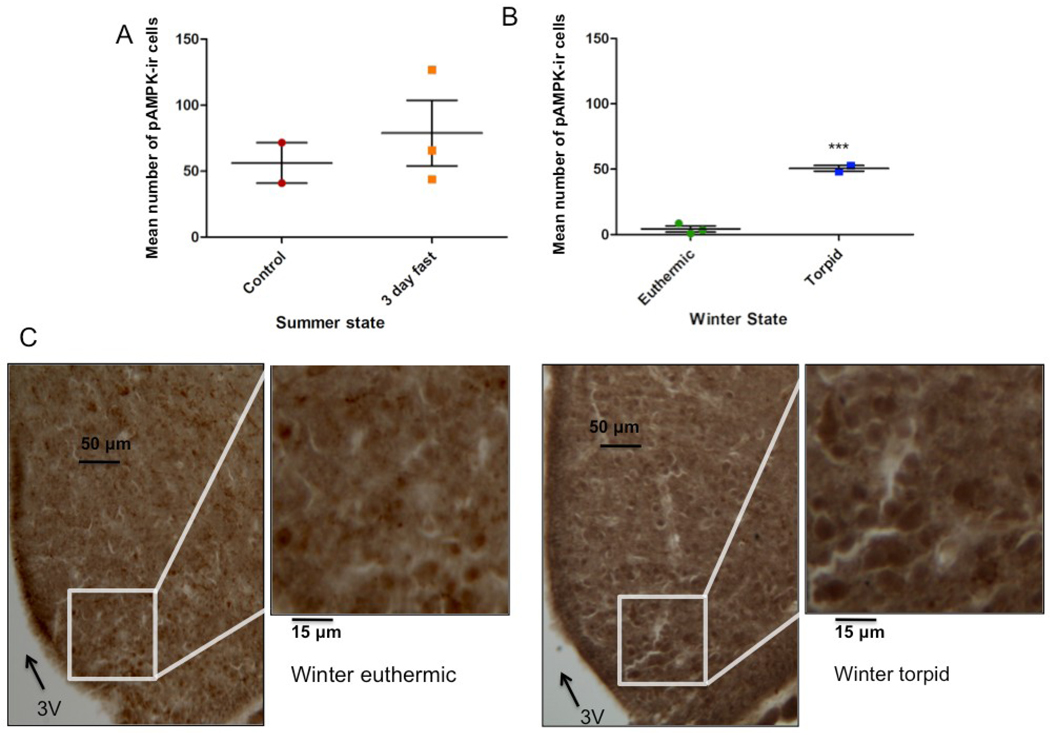
Relative pAMPK immunoreactivity was established in ARC from squirrels in all physiological states by IHC. There was a slight trend towards higher pAMPK immunoreactivity in summer 3-day fast animals compared to the summer fed group, though this was not significant (Fig. 8A). The ARC from winter torpid animals showed higher pAMPK immunoreactivity than the winter euthermic group (Figs. 8B &8C). Additionally, the combined summer group (summer fasted and summer fed) had higher pAMPK immunoreactivity than the combined winter states (winter euthermic and winter torpid) (p=0.0405, data not shown). The combined winter group also showed a trend towards lower pAMPK immunoreactivity compared to the summer fasted animals (p=0.056), but there was no difference between winter groups and summer controls (p=0.1693).
Fig. 8.
pAMPK immunoreactivity changes with physiological state and season. (A) Mean counts of pAMPK-ir cells in ARC of summer animals. Each point represents a mean cell count for one animal; long horizontal lines signify group means for summer control (n=2) vs. summer 3-day fast (n=3); (B) Mean counts of pAMPK-ir cells in ARC of winter animals, euthermic (n=3) vs. torpid (n=2). ***= p=0.0008; (C) Representative photomicrographs of pAMPK immunoreactivity in winter euthermic (left) and winter torpid (right) animals; arrow denotes third ventricle (3V); magnification bars: 50µm in large pictures, 15µm in insets.
4. Discussion
Hibernators require a careful balance of endogenous energy and suppressed metabolic rate in order to survive the winter season. As such, it is important for these animals to be able to sense cellular energy levels even when at low tissue temperature. Animals in deep torpor respond to changes in ambient temperature by increasing their metabolic rate (Geiser & Kenagy, 1988), and some hibernators appear to defend a ‘set-point’ body mass that changes throughout the year (Mrosovsky & Powley, 1977). One sensor of endogenous energy seems to be AMPK; this enzyme is important in food intake regulation and energy balance in most mammals, but has been little studied in hibernators. We compared total and phosphorylated levels of AMPK (as a cellular energy sensor) and ACC (as a downstream controller of fatty acid metabolism) in GMGS that had been fed or fasted in the summer, and in torpid and euthermic winter GMGS.
We found that summer GMGS reacted to fasting in a similar fashion to non-hibernating mammals, generally increasing the phosphorylated (active) form of AMPK with fasting, with a concurrent increase in the inactive form of ACC (pACC) in liver, muscle, and WAT. These increases in the phosphorylated forms of AMPK and ACC were usually not associated with increases in the total relative abundance of these enzymes, effectively resulting in increased percentage of phosphorylation of both enzymes with fasting in most tissues. However, although pACC increased with fasting in the hypothalamus as expected with the increase in fatty acid oxidation that accompanies fasting, there was no associated increase in pAMPK. pAMPK increased slightly (p=0.07) on the first day of fasting, then decreased significantly from control levels on the third day of fasting before increasing again by day five of fasting. In previous research, mice fasted for two days had increased relative abundance of pAMPK in the ventromedial hypothalamus (VMH) and in various hypothalamic areas (Minokoshi et al., 2004; Murphy et al., 2009). We found a slight but non-significant increase in number of pAMPK-ir neurons in the ARC of GMGS fasted for three days. It is possible that this lack of significance is due to our small sample size. Alternatively, it is possible that the brains of hibernators are protected against low energy levels even during summer, and that the increase observed in pAMPK between fasting days three and five was a delayed response to decreased endogenous energy stores. Since AMPK responds to an increase in the endogenous AMP:ATP ratio, it is possible that enough ATP was available in the brains of fasting GMGS that the ratio did not change until after five days of fasting. To clarify this, more research is necessary on the effect of fasting on summer-acclimated hibernators, including a test to assess endogenous energy levels in the hypothalamus during a short-term summer fast.
In comparison with summer animals, winter animals (both euthermic and hibernating) exhibited significantly less pAMPK immunoreactivity in the ARC. This is expected, as metabolic rates are slowed during hibernation; since animals are conserving energy during this time, energetically expensive protein synthesis is downregulated. As pAMPK is involved in the stimulation of food intake, it is likely that phosphorylation of AMPK must be suppressed in order to allow entry into torpor. If AMPK were produced and phosphorylated at high levels during the winter, animals would cease hibernation in order to eat, as shown by Florant et al. (2010). If hibernators reacted to fasting conditions in a manner akin to most mammals, pAMPK levels would be elevated during hibernation, which is essentially a 6–8 month fast. Our data show that this is not the case: pAMPK immunoreactivity was lower in winter GMGS than in animals fasted for three days in the summer. As such, it appears that the amount of pAMPK found during the hibernation season is more similar to that of summer-fed animals than summer-fasted animals. This indicates that, although hibernating mammals are fasting, a mechanism exists to suppress phosphorylation of AMPK, with the effect of preventing arousal and the resumption of food intake until normothermia is regained in the spring.
Studies examining differences in enzyme activation in winter-acclimated hibernator tissues have generally shown enzymes to be downregulated in torpid compared with euthermic tissues. In hearts taken from winter euthermic and summer non-hibernating Richardson’s ground squirrels (Urocitellus richardsonii), ACC activity was decreased in winter animals compared with summer-acclimated squirrels at both 37°C and 5°C. This decrease in activity was associated with a decrease in total ACC relative abundance in winter animals, but was not associated with a change in AMPK activity (Belke et al., 1998). Other enzymes, such as skeletal muscle hexokinase (HK), creatine kinase (CK), and protein kinase C (PKC) have lower activity in torpid than euthermic hibernators, but in the case of HK, this activity is elevated by addition of AMPK (Mehrani & Storey, 1997; Abnous & Storey, 2007; Abnous & Storey, 2008;). In the thirteen-lined ground squirrel (Ictidomys tridecemlineatus), pAMPK relative abundance was lower in livers of winter than summer animals, but increased in WAT in winter animals compared with summer animals. Relative abundanceof pACC was elevated in the BAT of winter animals compared with summer animals, but showed no differences in other tissues (Horman et al., 2005). Occasionally, other enzymes are increased in winter-acclimated animals compared with summer-acclimated animals, including pyruvate dehydrogenase kinase isoenzyme 4 (PDK4), which is upregulated in heart and skeletal muscle and in WAT (Buck et al., 2002). We did not directly compare summer-euthermic and winter-euthermic animals as seen in Horman et al. (2005), but compared state changes within seasons.
Contrary to our hypothesis and to previous research, we found that relative abundance of pAMPK and pACC was generally lower in euthermic compared to torpid GMGS. Our preliminary data indicate that winter torpid animals show higher immunoreactivity of pAMPK in the brain’s arcuate nucleus than do winter euthermic animals. There was no significant difference between total or phosphorylated forms of AMPK or ACC in liver, but total and phosphorylated ACC in muscle were lower in euthermic than in torpid winter animals. Total and phosphorylated AMPK were significantly decreased in WAT of euthermic GMGS compared with torpid animals, while there was no difference in pACC and total ACC between the two groups. In BAT, there was a slight decrease in both pAMPK and total AMPK in euthermic animals, but the difference was significant for both total and pACC in this tissue. Although different relative abundances are seen in the active form of AMPK, it would be instructive to confirm that actual activity of the enzyme is changing with an enzyme activity analysis conducted at different stages of the torpor bout. It is possible that the roles of AMPK and ACC change during periods of greatly fluctuating energy availability (i.e. during the different stages of torpor and arousal),which may explain the state differences we see between AMPK and ACC phosphorylation.
Both WAT and BAT are utilized heavily in the process of arousal to euthermia from torpor. It is possible that in our animals, pAMPK was elevated during torpor in response to low available energy levels, and decreased when more energy became available due to increased metabolic rate during the interbout arousal. Alternatively, it is possible that both AMPK and ACC were maximally phosphorylated during the process of arousal to induce the massive use of fatty acids required to re-warm the animal’s body from 5°C to 36°C over a 60-minute period, and this process of arousal expedited the breakdown of both enzyme forms. Hibernators re-build protein and enzyme stores during the euthermic portion of the interbout arousal (Epperson et al., 2010), so it is possible that at the time we chose to sacrifice the animals (two h after arousal), the protein and energy stores in their tissues had not had the chance to be replenished. In order to clarify this, more research should be performed on enzyme and protein changes during each stage of the torpor bout: during entry into torpor as animals are dropping metabolic rates and Tb, in early torpor (1–2 days after reaching minimum Tb), in late torpor (after animals have been at low Tb for several days or weeks), during arousal (as animals are increasing Tb), and several hours after arousal (animals have been completely euthermic for more than six hours). Each of these stages is physiologically distinct, and may exhibit very different available energy levels, hormones, and enzyme profiles in various tissues as the body is submitted to the stresses of defending a great range of Tb.
Hibernation is a time of extreme phenotypic and physiological plasticity, and as such represents an intriguing opportunity to study animals under a wide variety of conditions and energy availability. As hypothesized, AMPK changes with physiological condition in a hibernator;therefore, as a sensor of endogenous energy levels, AMPK may be important in hibernators at all stages of their circannual cycle, especially during periods of extreme energy fluctuation experienced during arousal from torpor.
Acknowledgements
Thanks to Cara Ostrom, Kendra Burdett, Ashley Fenn, and Thomas Barnett for help with trapping and animal care, and Yvonne Diaz and Vanessa Selwyn for assistance with Western blots. We thank Denise Pearson of Fox Acres Country Club for allowing us to trap ground squirrels on their property. This work was supported by NIH R25DK067017 to GLF and NIH NS039951 grant to RJH.
List of abbreviations
- ACC
acetyl CoA carboxylase; EC 6.4.1.2
- AMPK
AMP-activated protein kinase; EC 2.7.11.31
- ARC
arcuate nucleus of hypothalamus
- AICAR
5-aminoimidazole-4-carboxamide 1 B-D-ribofuranoside
- BAT
brown adipose tissue
- CPT1
carnitine palmitoyl transferase-1; EC 2.3.1.21
- CK
creatine kinase; EC 2.7.3.2
- GMGS
golden-mantled ground squirrel
- HK
skeletal muscle hexokinase
- IHC
immunohistochemistry
- ir
immunoreactive
- pAMPK
phosphorylated AMPK
- pACC
phosphorylated ACC
- PKA
protein kinase A; EC 2.7.11.11
- PKC
protein kinase C; EC 2.7.11.13
- Ta
ambient temperature
- Tb
body temperature
- VMH
ventromedial hypothalamus
- WAT
white adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abnous K, Storey KB. Regulation of skeletal muscle creatine kinase from a hibernating mammal. Arch. Biochem. Biophys. 2007;467:10–19. doi: 10.1016/j.abb.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Abnous K, Storey KB. Skeletal muscle hexokinase: Regulation in mammalian hibernation. Mol. Cell. Biochem. 2008;319:41–50. doi: 10.1007/s11010-008-9875-5. [DOI] [PubMed] [Google Scholar]
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Belke DD, Wang LCH, Lopaschuk GD. Acetyl-CoA carboxylase control of fatty acid oxidation in hearts from hibernating Richardson’s ground squirrels. Biochim. Biophys. Acta. 1998;1391:25–36. doi: 10.1016/s0005-2760(97)00199-9. [DOI] [PubMed] [Google Scholar]
- Buck MJ, Squire TL, Andrews MT. Coordinate expression of the PDK4 gene: a means of regulating fuel selection in a hibernating mammal. Physiol. Genomics. 2002;8:5–13. doi: 10.1152/physiolgenomics.00076.2001. [DOI] [PubMed] [Google Scholar]
- Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochem. Biophys. Acta. 1989;1012:81–86. doi: 10.1016/0167-4889(89)90014-1. [DOI] [PubMed] [Google Scholar]
- Dark J. Annual lipid cycles in hibernators: integration of physiology and behavior. Annu. Rev. Nutr. 2005;25:469–497. doi: 10.1146/annurev.nutr.25.050304.092514. [DOI] [PubMed] [Google Scholar]
- Epperson LE, Rose JC, bRussell RL, Nikrad MP, Carey HV, Martin SL. Seasonal protein changes support rapid energy production in hibernator brainstem. J. Comp. Physiol. B. 2010;180:599–617. doi: 10.1007/s00360-009-0422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florant GL, Fenn AM, Healy JE, Wilkerson GK, Handa RJ. To eat or not to eat: the effect of AICAR on food intake regulation in yellow-bellied marmots (Marmota flaviventris) J. Exp. Biol. 2010;213:2031–2037. doi: 10.1242/jeb.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser F, Kenagy GJ. Torpor duration in relation to temperature and metabolism in hibernating ground squirrels. Physiol. Zool. 1988;61:442–449. [Google Scholar]
- Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase–development of the energy sensor concept. J. Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgen KM, Cole FR, Helgen LE, Wilson DE. Generic revision in the Holarctic ground squirrel genus Spermophilus. J. Mamm. 2009;90:270–305. [Google Scholar]
- Horman S, Hussain N, Dilworth SM, Storey KB, Rider MH. Evaluation of the role of AMP-activated protein kinase and its downstream targets in mammalian hibernation. Comp. Biochem. Physiol.A. 2005;142:374–382. doi: 10.1016/j.cbpb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Joseph SA, Knigge KA, Kalejs LM, Hoffman RA, Reid P. A stereotaxic atlas of the brain of the 13-line ground squirrel (Citellus tridecemlineatus) Edgewood, MD: Edgewood Arsenal Special Publications; 1966. [Google Scholar]
- Kajita K, Mune T, Ikeda T, Matsumoto M, Uno Y, Sugiyama C, Matsubara K, Morita H, Takemura M, Seishima M, Takeda J, Ishizuka T. Effect of fasting on PPARγ and AMPK activity in adipocytes. Diabetes Res. Clin. Pr. 2008;81:144–149. doi: 10.1016/j.diabres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Mehrani H, Storey KB. Protein kinase C from bat brain: The enzyme from a hibernating mammal. Neurochem. Int. 1997;31:139–150. doi: 10.1016/s0197-0186(96)00130-1. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–564. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Powley TL. Set point for body weight and fat. Behav. Biol. 1977;20:205–223. doi: 10.1016/s0091-6773(77)90773-8. [DOI] [PubMed] [Google Scholar]
- Murphy BA, Fioramonti X, Jochnowitz N, Fakira K, Gagen K, Contie S, Lorsignol A, Penicaud L, Martin WJ, Routh VH. Fasting enhances the response of arcuate neuropeptide Y-glucose-inhibited neurons to decreased extracellular glucose. Am. J. Physiol. Cell. Physiol. 2009;296:C746–C756. doi: 10.1152/ajpcell.00641.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapp BD, Heller HC. Suppression of metabolism during hibernation in ground squirrels. Physiol. Zool. 1981;54:297–307. [Google Scholar]
- Sponarova J, Mustard KJ, Horakova O, Flachs P, Rossmeisl M, Brauner P, Bardova K, Thomason-Hughes M, Braunerova R, Janovska P. Involvement of AMP-activated kinase in fat depot-specific metabolic changes during starvation. FEBS Lett. 2005;579:6105–6100. doi: 10.1016/j.febslet.2005.09.078. [DOI] [PubMed] [Google Scholar]
- To K, Yamaza H, Komatsu T, Hayashida T, Hayashi H, Toyama H, Chiba T, Higami Y, Shimokawa I. Down-regulation of AMP-activated protein kinase by calorie restriction in rat liver. Exp. Gerontol. 2007;42:1063–1071. doi: 10.1016/j.exger.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Wang LCH. Energetic and field aspects of mammalian torpor: the Richardson’s ground squirrel. In: Wang LCH, Hudson JW, editors. Strategies in Cold: Natural Torpidity and Thermogenesis. New York: Academic Press; 1978. pp. 109–146. [Google Scholar]
- Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J. Physiol. 2006;574:73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]