Abstract
In this report a new method is introduced for simultaneous detection of the metabolism of two 13C-labeled subtracts in brain in vivo. We recognized and experimentally demonstrated that when a 13C-labeled substrate generates [1,2-13C2]acetylCoA ([1-13C]acetylCoA) only, glutamate C5, glutamine C5 and apsartate C4 doublets (singlets) are formed exclusively, regardless of the number of turns of the tricarboxylic acid cycle. We utilized the large one-bond 13C-13C homonuclear J coupling between a carboxylic/amide carbon and an aliphatic carbon (~50 Hz) and demonstrated that it is feasible to simultaneously detect the labeling of brain metabolites by two different substrates using different isotopomer signals of the same carbon atom. Uniformly labeled glucose was used to generate the doublets and a second substrate ([2-13C] lactate or [1,3-13C2]β-hydroxybutyrate or [1-13C] acetate) was used to generate the singlets. It was shown that contribution to cerebral metabolism from different substrates can be simultaneously measured in vivo.
Keywords: cerebral energy metabolism, in vivo 13C MRS, carboxylic/amide spectral region, ketone body, lactate
1. Introduction
Glucose is considered as the predominant energy substrate utilized by the mature brain to fuel its activities and functions (Pellerin and Magistretti, 2003). At rest, the adult brain comprising only about 2~3% of the body weight, accounts for approximately 25% and 16% of total body glucose and oxygen utilization respectively (Owen et al., 1967; Guzman and Blazquez, 2001; Henderson, 2008). Thus, glucose oxidation provides a major energy source for the brain function (Sibson et al, 1997; Pellerin and Magistretti, 2003). However, the brain does not use glucose as its only energy source (e.g., glycogen is found to be an energy reserve of significance; Cruz and Dienel, 2002; Choi et al, 1999), particularly during ontogenic development, fasting and diabetes. Under a variety of pathological conditions, ketone bodies including β-hydroxybutyrate (BHB) and acetoacetate (AcAc), lactate and free fatty acids can be used as alternative fuels for energy production (O'Neal R et al., 1966; Lundquist et al., 1973; Hawkins, 1986; Edmond, 1992; Shen et al, 1998; Guzman and Blazquez, 2001; Lebon et al., 2002; Al-Mamun et al., 2009). Sometimes, even alcohol can act as an indirect or abnormal cerebral oxidative energy substrate in subjects of chronic alcoholism (Zakhari, 2006) due to its direct byproduct acetate being metabolized by glial cells in the brain. Even for glucose per se, there is still considerable controversy regarding compartmentalization of glucose oxidation (Aubert et al., 2005; Occhipinti et al., 2009; Cerdán et al., 2006). In recent decades, there has been a long-standing interest in compartmentalization, energy metabolism and neurotransmission in the brain. However, partly due to technical difficulties, the majority studies in this field have focused on detecting the cerebral metabolism of a single substrate in the brain (e.g., Cremer, 1964; O'Neal R et al., 1966; Hassel et al., 1995; Sibson et al, 1997; Waniewski and Martin, 1998), and few studies have investigated the simultaneous metabolism of multiple energy substrates.
The carbon-13 (13C) nucleus, a stable isotope of carbon with 1.1% natural abundance, is nuclear magnetic resonance (NMR) detectable. 13C magnetic resonance spectroscopy (MRS) combined with 13C-labeled substrates allows for nonradioactive and noninvasive measurement of energy production and the metabolic fluxes in the brain because dynamic 13C incorporation into the tricarboxylic acid (TCA) cycle and several different brain metabolites can be detected. Compared with in vitro or ex vivo 13C NMR studies of brain extracts, in vivo 13C MRS studies allow for continuous and nondestructive monitoring of 13C incorporation from 13C-labeled precursors into the various carbon positions of metabolites such as TCA cycle intermediates and amino acids under different physiological and pathophysiological conditions. The chemical shift of 13C spans the alkyl (0~160 ppm) and carboxylic/amide (160~220 ppm) regions (Wilson, 1987; Rumpel, 2008), and the former especially the range from 20 to 60 ppm has been broadly used (Behar et al., 1986; Beckmann et al., 1991; de Graaf, 2007) for in vivo 13C MRS. However, simultaneous detection of cerebral metabolism of two different substrates in the aliphatic 13C spectral region is technically challenging because of the complex isotopomer signals generated by aliphatic carbons (e.g., Xu and Shen, 2006), which may lead to significant spectral overlap. In contrast to aliphatic carbons, carboxylic and amide carbons are located at an end of the carbon skeleton of a molecule. 13C signals of carboxylic and amide carbons can only form a singlet (doublet) when its neighboring carbon is 12C (13C), leading to significant spectral simplification. Here we show that this natural spectral simplification can be utilized to simultaneously detect the metabolism of two 13C-labeled subtracts in brain using in vivo 13C MRS. We used uniformly labeled glucose to generate the doublets and a second substrate ([2-13C] lactate or [1,3-13C2]BHB or [1-13C] acetate) to generate the singlets and demonstrated that contribution to cerebral metabolism from different substrates can be determined in vivo.
2. Materials and Methods
2.1. Hardware
All MRS experiments were performed on a Bruker microimaging spectrometer (Bruker Biospin, Billerica, MA, USA) interfaced to an 11.7 Tesla 89-mm bore vertical magnet (Magnex Scientific, Abingdon, UK). This magnet is equipped with a 57-mm i.d. gradient (Mini 0.5; Bruker Biospin, Billerica, MA, USA) with a maximum gradient strength of 3.0 G/mm and a rise time of 100 μs for in vivo studies of adult rats. The 1H and 13C radio frequency (RF) coils were coplanar and made of single-sided printed circuit board. The inner loop is the 13C coil, with an inner diameter and conductor width of 10.8 and 4.3 mm, respectively. The outer loop is the 1H coil with an inner diameter and conductor width of 23.6 and 5.4 mm, respectively. No noise injection was found in the 13C channel due to proton decoupling. The lower end of the system was an aluminum interface box through which RF cables, ventilation tubes, rectal thermal probe, and catheters were connected. A similarly designed RF probe/animal handling system for proton and Proton-Observed, Carbon-13-Edited spectroscopy experiments of rat brain using this vertical magnet have been previously described (Li and Shen, 2005; Xu and Shen, 2006). The loaded isolation between 1H and 13C coils (S21), which have a large frequency separation of 375 MHz, is −30.6 dB at 125 MHz and −31.2 dB at 500 MHz, respectively, and the standard Bruker filters were used. A broadband low-pass filter was used in the 13C channel whose insertion loss at 125 MHz was less than 0.5 dB. Its rejection at 500 MHz was greater than 80 dB. In the proton channel, a broadband high-pass filter was used with an insertion loss at 500 MHz of less than 0.5 dB, and rejection at 125 MHz greater than 60 dB. The integrated RF coils/head-holder system is capable of rat head fixation (with two ear pins and a bite bar), body support, physiology maintenance, coil tuning, and RF shielding. The pulse-acquire sequence (Li et al., 2009) was applied in the study to measure 13C MRS signals in the rat brain with stochastic 1H decoupling. Anatomical images were acquired using the three-slice (coronal, horizontal, and sagittal) scout rapid acquisition with Rapid Acquisition with Relaxation Enhancement (RARE) imaging method (field of view = 2.5 × 2.5 cm2, slice thickness = 1 mm, TR/TE = 200/15 ms, rare factor = 8, data matrix = 128×128).
2.2. In vivo MR Procedures
After experimental animals were placed in the scanner, RARE images were acquired to ensure the animal brain and the RF coils were properly positioned. The gradient isocenter of the RF probe/animal handling system in a Mini 0.5 gradient was about 0–1 mm posterior to bregma. The rat brain was shimmed automatically using the FASTMAP/FLATNESS method (Chen et al., 2004) as described previously
A two-dimensionally localized stimulated-echo acquisition mode (STEAM) method was used to calibrate the proton pulse (Shen et al., 1999). Overhauser enhancement (NOE) between carboxylic/amide carbon and nearby protons (Pearson et al., 1975) was generated using a train of nominal 180° proton pulses spaced 200 ms apart. Because carbon in the carboxylic/amide carbon is only coupled to protons via very weak long-range 1H-13C scalar couplings, 13C-labeled chemicals appearing in this chemical shift region can be effectively decoupled at a very low RF power using stochastic decoupling (Li et al., 2007; Yang et al., 2009). TR = 18.75s, Nominal flip angle = 45°. 1H decoupling used a pseudo noise (stochastic) decoupling scheme with constant γB2 amplitude and randomly inverted phases (Ernst, 1966; Li et al., 2007) and a repetition unit of 0.2–0.4 ms. The pseudo noise decoupling scheme allowed effective broadband proton decoupling at 11.7 T with a TR (18750 ms)-averaged forward decoupling power at < 6 mW (γB2 < ~274 Hz calibrated at the gradient isocenter.). Our previous results have shown that this decoupling scheme can effectively decouple all protons that are coupled to the observed 13C spins (from lactate H3 1.32 ppm to glutamine HE at 7.60 ppm; Yang et al, 2009). All 13C MRS data except for the baseline were acquired after the initiation of infusion of 13C-labeled substrates. The time domain data were zero-filled to 16 K. The resolution enhancing exponential line broadening factor (lb = −15) and the Gaussian broadening factor (gb = 0.12) were applied before Fourier transform. Zero-order and a small first-order phase corrections were made.
2.3. Animal preparation
Animals were used in accordance to protocols approved by the National Institute of Mental Health (NIMH) Intramural Research Program Animal Care and Use Committee, National Institutes of Health (NIH). During the experimental process, all efforts were made to minimize animal suffering, to reduce the number of animals used, and to use alternatives in vivo techniques whenever available. Animals (adult Sprague-Dawley rats, Taconic Laboratories, Germantown, NY, USA) were kept under conditions of constant temperature (21°C) and humidity (50%), with a 12-hour light/dark cycle and free access to food and water 24 hrs before the experiments. 13C-enriched [13C6]-D-glucose and sodium [1-13C]acetate were purchased from Cambridge Isotope Laboratories, Inc (Andover, MA, USA); sodium [1, 3-13C2]BHB and sodium [2-13C]lactate solutions (45~55% w/w in H2O) were obtained from Isotec., Sigma Aldrich (St. Louis, MO, USA). Enrichment of all 13C-labeled chemicals was 99%.
All animals ((body weight (BW) = 193–237 g) fasted for 24 hrs were divided into seven groups from A to G. The rats were orally intubated and mechanically ventilated (SAR-830/AP, CWE, Inc., Ardmore, PA, USA) with a mixture of ~70% N2O, 30% O2 and 1.5% isoflurane. The left femoral artery was cannulated for periodically sampling arterial blood to monitor blood gases (pO2, pCO2), pH, and glucose concentration using a blood analyzer (Bayer Rapidlab 860, East Walpole, MA, USA), and for monitoring arterial blood pressure levels. The isolateral (left) vein was also cannulated for intravenous infusion or co-infusion of 13C-labeled chemicals.
Three animals (Group A, n = 3) were given intravenous infusion of 0.75 M [13C6]-D-glucose. The intravenous infusion protocol consists of an initial bolus of 75.5 mg/min/kg BW of 0.75 M [13C6]-D-glucose in the first 10 min followed by the same solution at approximately 28.5 mg/min/kg BW. Plasma glucose level was constantly monitored and maintained at 16.0~19.0 mM. Animals in Group B (n = 3) received intravenous infusion of 0.6 M [2-13C]lactate (pH = 7.0) with a initial bolus of 26.0 mg/min/kg BW followed by infusing the same solution at 10.2 mg/min/kg BW. Group C animals (n = 10) received intravenous co-infusion of 0.6 M [2-13C]lactate and 0.75 M [13C6]-D-glucose. The glucose and lactate infusion protocols were the same as those for Group A and B respectively. Group D animals (n = 3) were used for intravenous infusion of 0.8 M [1, 3-13C2]BHB (pH = 7.0) with a initial bolus of 60.1 mg/min/kg BW followed by the same solution at 13.3 mg/min/kg BW. Animals in Group E (n = 9) received intravenous co-infusion of [13C6]-D-glucose and [1, 3-13C2]BHB. The glucose and BHB infusion protocols were the same as those for Group A and D respectively. Animals in Group F (n = 4) received intravenous infusion of 0.9 M [1-13C]acetate (pH = 7.0) with a initial bolus of 18.7 mg/min/kg BW followed by infusion the same solution at 7.1 mg/min/kg BW. Animals in Group G (n = 10) were subjected to intravenous co-infusion of 0.9 M [1-13C]acetate and 0.75 M [13C6]-D-glucose. The glucose and acetate infusion protocols were the same as those for Group A and F respectively. Throughout all experiments, normal physiological conditions were maintained according to results obtained from the blood analyzer (pH ~7.4, pCO2 ~35 mmHg and pO2 > 100 mmHg), respiration monitor (SurgicalVet, SIMS BCI, Inc. Wisconsin, USA), and by real-time regulation of ventilation. Alterations in blood pressure, heart rate, and respiration changes were also monitored (BIOPAC System, Inc. GenuineIntel, Goleta, CA). An external pump for heat exchange by circulating warm water (BayVoltex, Modesto, CA, USA) was used to maintain body temperature at 37.8 ± 0.5 °C.
3. Results
3.1. Intravenous infusion [13C6]-D-glucose and [2-13C]lactate
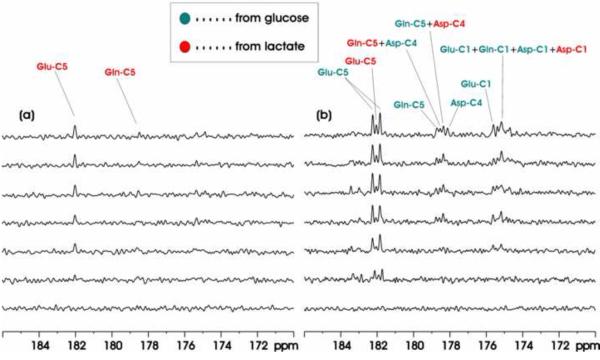
Figure 1 shows in vivo proton decoupled 13C MRS time-course spectra acquired from one Group A animal brain during intravenous infusion of [13C6]-D-glucose. Each spectrum corresponds to 20-minute signal averaging. 13C MRS baseline from the rat brain (bottom trace) shows no detectable signals in the carboxylic/amide 13C spectral region because there is no lipid interference in the spectral region of interest. Due to the homonuclear J coupling effect between the 4th and 5th 13C from glutamate, glutamate C5 appears as a clear doublet in the carboxylic/amide 13C spectral region. This is expected because the glutamate 13C4–13C5 moiety always comes from [1,2-13C2]acetylCoA regardless of the number of turns of the TCA cycle. For example, in the second turn of the TCA cycle, glutamate C4–C5 is replaced by newly arrived 13C-13C from [1,2-13C2]acetylCoA. As a result, glutamate C5 singlet cannot be formed when [1,2-13C2]acetylCoA is the only labeled input to the TCA cycle. Glutamine C5 (178.5 ppm), and aspartate C4 (178.4 ppm) also appear as doublets because the covalent bond between the two carbons in [1,2-13C2]acetylCoA remains intact when these two 13C labels are transferred to glutamine and aspartate. Glutamate C1 (175.3 ppm), aspartate C1 (175.1 ppm) and Glutamine C1 (174.8 ppm), however, can form both doublets and singlets although the two acetyl carbons of acetylCoA are derived from fully labeled [13C6]-D-glucose. This is because, when CO2 is formed, the original covalent bond between the two acetyl carbons in acetylCoA is broken up. The break-off of CO2 can lead to glutamate, glutamine and aspartate C1 singlets because the C2 carbons in these molecules may still be unlabeled. When glutamate, glutamine and aspartate are fully turned over, all carbons become 13C-labeled. Therefore, glutamate, glutamine and aspartate C1 singlets are formed only during the 12C → 13C turnover period. With relatively long infusion time, all metabolites signals except bicarbonate are doublets in the carboxylic/amide spectral region. The 13C-labeled bicarbonate HCO3− (161.0 ppm) was also detected (not shown in the figure). Similar results were obtained from all animals of group A. The glutamate C5, glutamine C5, aspartate C4 resonance peaks as well as the two largest peaks (Glu C1, Glu C1 +Gln C1 + Asp C1) in the C1 region were labeled in Fig. 1.
Figure 1.
Baseline (bottom trace) and time-course (120 minutes) 13C MRS spectra acquired from an individual rat brain during intravenous infusion of [13C6]-D-glucose. Each spectrum was averaged for 20 minutes. Parameters for data processing: lb = −15, gb = 0.12. Glu-C5: glutamate C5, Gln-C5: glutamine C5, Asp-C4: aspartate C4, Glu-C1: glutamate C1, Gln-C1: glutamine C1.
When [2-13C]lactate entered into the brain, it is converted into [1-13C]acetylCoA by the actions of lactate dehydrogenase and pyruvate dehydrogenase. The 13C label is subsequently incorporated into glutamate C5 (182.0 ppm), glutamine C5 (178.5 ppm) and aspartate C4 (178.3 ppm) during the first turn of the TCA cycle. For exactly the same reason stated above, the glutamate 12C4–13C5 moiety always comes from [1-13C]acetylCoA regardless of the number of turns of the TCA cycle. Therefore, glutamate C5, glutamine C5 and aspartate C4 doublets cannot be formed when [1-13C]acetylCoA is the only labeled input to the TCA cycle. When [1-13C]acetylCoA is the only labeled input to the TCA cycle glutamate, glutamine and aspartate C1 doublets cannot be formed, either. Fig. 2(a) shows in vivo proton decoupled 13C MRS time-course spectra acquired from one Group B animal brain during intravenous infusion of [2-13C]lactate. Only singlets were observed as expected. Fig. 2(b) shows the corresponding result of co-infusion of [13C6]-D-glucose and [2-13C]lactate acquired from a Group C animal. In the co-infusion experiment, glutamate C5, glutamine C5 and aspartate C4 doublets are originated from [13C6]-D-glucose while glutamate C5, glutamine C5 and aspartate C4 singlets are originated from [2-13C]lactate. Fig. 2(b) clearly demonstrated the feasibility of simultaneously detecting the metabolism of two different substrates if one substrate (e.g., [13C6]-D-glucose) only produces [1,2-13C2]acetylCoA and the other (e.g., [2-13C]lactate) only produces [1-13C]acetylCoA.
Figure 2.
(a) Baseline (bottom trace) and time-course (120 minutes) 13C MRS spectra acquired from an individual rat brain during intravenous infusion of [2-13C] lactate. (b) Baseline (bottom trace) and time-course (120 minutes) 13C MRS spectra acquired from an individual rat brain during intravenous co-infusion of [2-13C]lactate and [13C6]-D-glucose. Each individual spectrum was averaged for 20 minutes. Parameters for data processing: lb = −15, gb = 0.12. Glu-C5: glutamate C5, Gln-C5: glutamine C5, Asp-C4: aspartate C4, Glu-C1: glutamate C1, Asp-C1: aspartate C1, Gln-C1: glutamine C1. Green: resonance lines originated from [13C6]-D-glucose; Red: resonance lines originated from [2-13C] lactate.
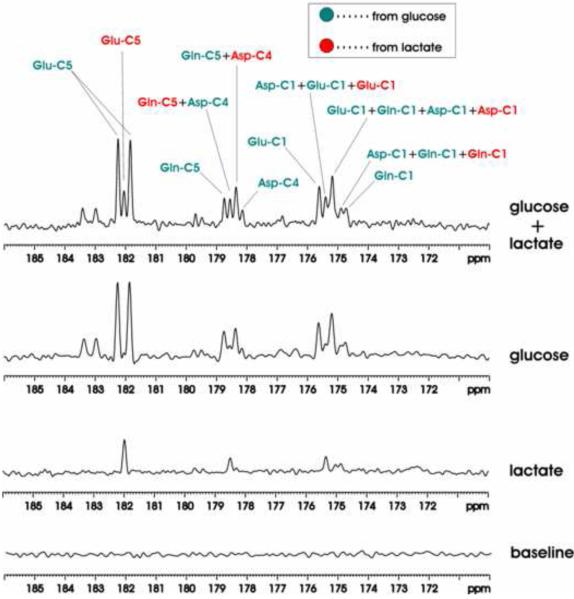
Fig. 3 shows 13C MRS baseline and spectra accumulated over the 0–180 minutes infusion period. As expected, only singlets of glutamate, glutamine and aspartate were detected during intravenous infusion of [2-13C]lactate; during intravenous infusion of [13C6]-D-glucose only doublets were detected for glutamate C5, glutamine C5 and aspartate C4. The same conclusion holds when spectra from all animals in Group A and B were summed respectively. The summed co-infusion spectrum demonstrates that co-infusion of differently labeled substrates and the detection of signals in the carboxylic/amide 13C spectral region allow clear separation of contribution to glutamate, glutamine and aspartate from the different substrates. As shown by Fig. 2(b) and the co-infusion spectrum of Fig. 3, the large homonuclear 13C-13C J coupling between an aliphatic carbon and a carboxylic or an amide carbon and the lack of interference from other one-bond couplings allow a clear separation of signals originated from singly and doubly labeled substrates. At 11.7 Tesla, the chemical shift separation between glutamine C5 and aspartate C4 is one half of the one-bond J coupling between an aliphatic carbon and a carboxylic/amide carbon. In Fig. 2(b) and Fig. 3, a pseudo quartet was detected in the 178–179 ppm region, allowing easy separation of contributions to glutamine C5 and aspartate C4 from different substrates. In the C1 region, singlets originated from singly labeled [2-13C]lactate and doublets and singlets originated from [13C6]-D-glucose were detected in the summed co-infusion spectrum of Fig. 3.
Figure 3.
Summed in vivo 13C MRS spectra. Baseline: no significant interfering signals were detected in the spectral region of interest. Lactate: only singlets of glutamate, glutamine and aspartate were detected during intravenous infusion of [2-13C]lactate. Glucose: only doublets were detected for glutamate C5, glutamine C5 and aspartate C4 during intravenous infusion of [13C6]-D-glucose. Glucose+lactate: both doublets and singlets were detected for glutamate C5, glutamine C5 and aspartate C4 during intravenous co-infusion of [13C6]-D-glucose and [2-13C]lactate. Each spectrum was averaged over the 0~180 minutes infusion period. Lactate: [2-13C] lactate, glucose: [13C6]-D-glucose. Parameters for data processing: lb = −15, gb = 0.12. Glu-C5: glutamate C5, Gln-C5: glutamine C5, Asp-C4: aspartate C4, Glu-C1: glutamate C1, Asp-C1: aspartate C1, Gln-C1: glutamine C1. Green: resonance lines originated from [13C6]-D-glucose; Red: resonance lines originated from [2-13C] lactate.
3.2. Intravenous infusion of [13C6]-D-glucose and [1,3-13C2]BHB
Like [2-13C]lactate, the ketone body [1,3-13C2]BHB is also converted into [1-13C]acetylCoA in brain. Fig. 4 shows the in vivo 13C MRS time-course spectra acquired from individual animals in Groups D and E, respectively. In Fig. 4(a), only singlets were generated in the carboxylic/amide 13C spectral region. With co-infusion of [13C6]-D-glucose and [1,3-13C2]BHB glutamate C5, glutamine C5 and aspartate C4 doublets are originated from [13C6]-D-glucose; glutamate C5, glutamine C5 and aspartate C4 singlets are originated from [1,3-13C2]BHB (see Fig. 4(b)). Similar to co-infusion of [13C6]-D-glucose and [2-13C]lactate, a pseudo quartet was detected in the 178–179 ppm region at 11.7 Tesla during co-infusion of [13C6]-D-glucose and [1,3-13C2]BHB. Similar results were also obtained for Group F and Group G animals.
Figure 4.
(a) Baseline (bottom trace) and time-course (120 minutes) 13C MRS spectra acquired from an individual rat brain during intravenous infusion of [1,3-13C2] BHB. (b) Baseline (bottom trace) and time-course (120 minutes) 13C MRS spectra acquired from an individual rat brain during intravenous co-infusion of [1,3-13C2] BHB and [13C6]-D-glucose. Each individual spectrum was averaged for 20 minutes. Parameters for data processing: lb = −15, gb = 0.12. Glu-C5: glutamate C5, Gln-C5: glutamine C5, Asp-C4: aspartate C4, Glu-C1: glutamate C1, Asp-C1: aspartate C1, Gln-C1: glutamine C1. Green: resonance lines originated from [13C6]-D-glucose; Red: resonance lines originated from [2-13C] lactate.
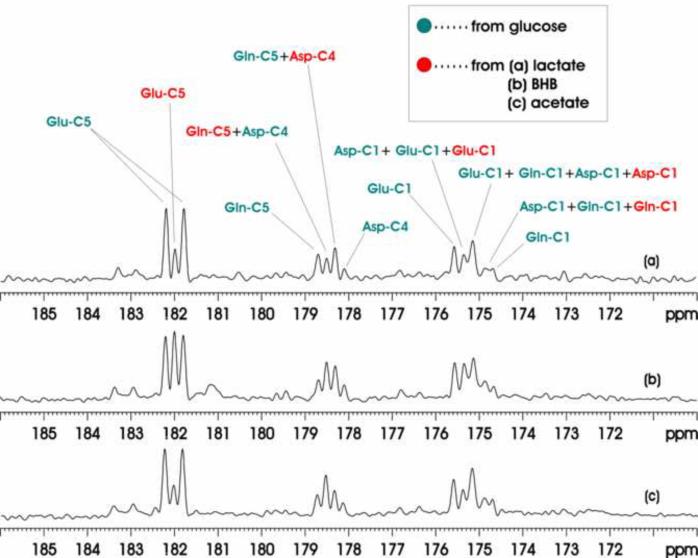
3.3 Comparison of intravenous co-infusions of [13C6]-D-glucose, [2-13C]lactate, [1,3-13C2]BHB and [1-13C]acetate
Fig. 5 shows 13C MRS spectra accumulated over the 0–180 minutes infusion period for three different co-infusion experiments: [13C6]-D-glucose + [2-13C]lactate (top trace), [13C6]-D-glucose + [1,3-13C2]BHB (middle trace), [13C6]-D-glucose + [1-13C]acetate (bottom trace). Among [13C6]-D-glucose, [2-13C]lactate, [1,3-13C2]BHB and [1-13C]acetate, [1-13C]acetate is the only glia-specific substrates. [1-13C]acetate labels glutamine C5 in glial cells first before the labels is transferred to neuronal glutamate. As a result in the bottom trace of Fig. 5, the signal intensity of glutamine C5 singlet is much higher than that of glutamate C5 singlet. In contrast, none of the rest of the substrates shows such a reversal of the precursor-product relationship between glutamate and glutamine. Quantitative analysis of the glutamate C5, glutamine C5 and aspartate C4 singlets and doublets revealed the expected brain's preference for glucose as the respiration fuel. At 180 min after the initiation of co-infusion, [GluC5]Glucose/[GluC5]Lactate, [GlnC5]Glucose/[GlnC5]Lactate and [GluC5 + GlnC5 + AspC4]Glucose/[GluC5 + GlnC5 + AspC4]Lactate ratios from Group C animals were found to be (4.23 ± 0.30, 7.50 ± 0.31, 5.11 ± 0.58):1 (see Table 1). The results for Group E and Group G animals were also listed in Table 1.
Figure 5.
Accumulated in vivo 13C MRS spectra of intravenous co-infusions of [13C6]-D-glucose + [2-13C]lactate (a), [13C6]-D-glucose + [1,3-13C2]BHB [(b), and [13C6]-D-glucose + [1-13C]acetate (c). Each spectrum was averaged over the 0–180 minutes infusion period from an individual rat brain. Parameters for data processing: lb = −15, gb = 0.12. Glu-C5: glutamate C5, Gln-C5: glutamine C5, Asp-C4: aspartate C4, Glu-C1: glutamate C1, Asp-C1: aspartate C1, Gln-C1: glutamine C1. Green: resonance lines originated from [13C6]-D-glucose; Red: resonance lines originated from [2-13C] lactate.
Table 1.
Contribution to cerebral metabolism from different 13C-labeled substrates
| Co-infusion | [GluC5]Glc/[GluC5]2nd* | [GlnC5]Glc/[GlnC5]2nd | [GluC5+GlnC5+ AspC4]Glc/[GluC5+GlnC5+AspC4]2nd |
|---|---|---|---|
| Glc+Lac (n=10) | 4.23 ± 0.30 | 7.50 ± 0.31 | 5.11 ± 0.58 |
| Glc+BHB (n=9) | 2.34 ± 0.21 | 3.77 ± 0.49 | 3.16 ± 0.20 |
| Glc+Ac (n=10) | 5.24 ± 0.48 | 1.80 ± 0.23 | 3.54 ± 0.34 |
Concentration ratio of 13C-labeled metabolites at 180 min after the initiation of co-infusion. “2nd” represents lactate (Lac) or BHB or acetate (Ac). All ratios were reported as mean ± SD.
4. Discussion
4.1. Observation of cerebral metabolism by in vivo 13C MRS in the carboxylic/amide region
In vivo 13C MRS combined with the administration of 13C-labeled substrates has become a powerful tool for studies of cerebral energy metabolism due to its nonradioactive and nondestructive features. In the present study, we tried to observe the cerebral metabolism of different energy substrates in the carboxylic/amide 13C spectral region. The results showed that the major difficulties associated with decoupling the large 1H-13C scalar coupling (120–150 Hz) for alkyl carbons of multiple brain metabolites can be ignored in the carboxylic/amide 13C spectral region because the carboxylic/amide carbons are only remotely coupled to protons. As such, complete decoupling was achieved using very low RF power. An additional advantage of this method is there are no overlapping signals from scalp lipids in the spectral region of interest (174–183 ppm) even when conventional localization techniques are not used. This is in contrast to the commonly used aliphatic 13C spectral region where signals from scalp lipids dominate and may interfere with metabolite signals. Since carboxylic and amide carbons are located at an end of the carbon skeleton of a molecule 13C signals of carboxylic and amide carbons can only form a singlet or a doublet. This is in contrast to aliphatic carbons that may form complex 13C isotopomer signals, leading to significant spectral overlap (Xu and Shen, 2006). The results of this study showed that the natural spectral simplification in the carboxylic/amide 13C spectral region can be taken advantage of to simultaneously detect the metabolism of two 13C-labeled subtracts in brain in vivo. The simultaneous detection of cerebral metabolism in the carboxylic/amide 13C spectral region is further helped by the fact that the one-bond 13C-13C homonuclear J coupling between a carboxylic/amide carbon and an aliphatic carbon (~50 Hz) is quite larger than the homonuclear J coupling between two aliphatic carbons (~30 Hz). In our study we only used uniformly labeled glucose to generate glutamate C5, glutamine C5 and aspartate C4 doublets and another substrate ([2-13C] lactate or [1,3-13C2]BHB or [1-13C] acetate) to generate singlets. Other choices or combinations of substrates are also possible. As long as one substrate only generates [1,2-13C2]acetylCoA and the other only generates ([1-13C]acetylCoA) simultaneous detection of cerebral metabolism of these two substrates in the carboxylic/amide 13C spectral region can be performed using in vivo 13C MRS.
In this study, signal intensity ratios (Table 1) were used to quantify brain's preference of respiration fuels (Malloy et al, 1988). For metabolic modeling of the kinetics of 13C labeling of different istopomers absolulte quantification of the detected carboxylic/amide 13C signals will be needed, which is beyond the scope of this report.
4.2. [2-13C] lactate and [13C6]-D-glucose
Brain may not use glucose as the only energy source especially under pathological conditions such as hypoxia and ischemia. Lactate can become a significant fuel source and occupy a special position in energy metabolism of the brain, and it may be required energetically to support synaptic function (Prichard, 1991; Boumezbeur et al., 2010). The exact role of lactate in brain energy metabolism and glutamatergic neurotransmission has been controversial (Waagepetersen et al., 1998; Bouzier-Sore et al., 2006; Schurr, 2006; Uehara et al., 2008). Measuring lactate in the brain is important for studying blood flow, consumption of glucose and oxygen, and brain activities (Urrila et al., 2004; Mangia et al., 2009). MRS detection of lactate usually involves measuring its methyl proton signal at 1.32 ppm in proton MRS spectra. With intravenous infusion of [1-13C] or [1,6-13C2]glucose the methyl carbon of lactate (C3, 21.0 ppm) can also be detected using conventional 13C MRS that measures 13C labels in the aliphatic 13C spectral region (Shen et al., 1999). In our study, [2-13C]lactate was administered. The lactate C2 signal at 69.3 ppm was also observed (data not shown). We found that cerebral metabolism of [2-13C]lactate leads to 13C labeling of glutamate, glutamine, aspartate and bicarbonate. Using [13C6]-D-glucose to generate [1,2-13C2]acetylCoA and [2-13C]lactate to generate [1-13C]acetylCoA our results showed that the metabolism of glucose and lactate in brain can be simultaneously measured. Table 1 showed that brains' selection of respiration fuels may be quantitatively measured by our in vivo 13C MRS method. Comparing the accumulated 13C MRS spectra with infusion of [2-13C] lactate, [13C6]-D-glucose, and co-infusion of [2-13C] lactate and [13C6]-D-glucose (Fig. 3 and Table 1), it is interesting to see that lactate has a significant contribution to brain energy metabolism even at the high blood glucose level of 18.5 ± 1.7 mM (Group C).
4.3. [1,3-13C2]BHB and [13C6]-D-glucose
A substantial body of evidence indicates that during ontogenic development, fasting and diabetes, ketone body including BHB, AcAc and acetone can serve as an alternative fuels for energy production in the brain (Shen et al, 1998; Magistretti et al., 1999; Guzman and Blazquez, 2001; Pan et al, 2002; Bouzier-Sore et al., 2003). Previous experimental and clinical studies confirmed that ketone bodies can be used to treat diseases such as epilepsy, Alzheimer's disease, Parkinson's disease, Friedreich's ataxia, cerebral ischemia, and hypoxia (Guzman and Blazquez, 2001; Veech et al., 2001; Guzman and Blazquez, 2004). BHB is able to replace glucose as an energy substrate and to preserve neuronal integrity and stability during glucose deprivation or glycolytic inhibition (Nehlig, 2004). Because both BHB C1 and C3 become acetyl C1 carbon of acetylCoA we used [1,3-13C2]BHB to double the sensitivity of detecting BHB metabolism. The resonances of BHB C1 and C3 are located at 181.2 and 66.8 ppm. Its C1 peak can be appreciated in Figs. 4 and 5, which does not overlap with metabolite signals of interest. BHB is known to easily enter both neuronal and glial compartments. Previous tissue culture studies have estimated that (Sykes et al., 1986) approximately 60% of BHB entered into neurons is oxidized, whereas this is only 20% in glial cells. During the infusion of [1,3-13C2]BHB both glutamate and glutamine were found to be readily labeled by 13C (Fig. 4(a)). With co-infusion of [1,3-13C2]BHB and [13C6]-D-glucose brain's selection of fuels can be quantified (Table 1).
4.4. [1-13C]acetate and [13C6]-D-glucose
Previous studies indicate that acetate, the so-called simplest of fats (Ebert et al., 2003) is oxidized in the brain as an energy substrate (Bluml et al, 2002; Lebon et al., 2002). It has been found to be predominantly or exclusively metabolized in glial cells (Nicklas and Clarke, 1969; Badar-Goffer et al., 1990; Cerdan et al., 1990; Waniewski and Martin, 1998; Cruz et al., 2005). Therefore, acetate is usually considered as a marker in the investigation of glial metabolism in the brain (Muir et al., 1986; Waniewski and Martin, 1998). Systemic administration of specific 13C-enriched glucose and/or acetate has been used to study brain energy metabolism and glutamatergic neurotransmission with 1H-decoupled 13C MRS (Taylor et al., 1996; Bluml et al., 2002; Yang et al, 2007). In the present study, [1-13C]acetate produced glutamate, glutamine and aspartate singlets. The resonance signal of [1-13C]acetate appears at 182.15 ppm which partially overlaps with glutamate C5 even at 11.7 Tesla. In separate studies using [2-13C] and [1,2-13C2]acetate we have confirmed that our infusion protocol did not lead to detectable acetate C2 signal at 24.5 ppm. As noted earlier, we observed that the signal intensity of glutamine C5 singlet is much higher than that of glutamate C5 singlet in both Group F and Group G animals. This reversal of the precursor-product relationship between glutamate and glutamine has been detected in the literature (Bluml et al, 2002; Lebon et al, 2002; Yang et al., 2007). Acetate is directly metabolized in glial cells. As a result, glutamine, which resides predominantly in glial cells, is labeled by 13C-labeled acetate first. Via the glutamate-glutamine cycle pathway glutamate, which resides predominantly in glutamatergic neurons, is labeled as a consequence of glutamine hydrolysis by the action of phosphate-activated glutaminase. In Fig. 5 (bottom trace), it was shown that 13C label incorporation from glucose and acetate into glutamate, glutamine and aspartate can be simultaneously detected based on different carboxylic/amide carbon isotopomers.
4.5. Conclusions
The present study has demonstrated the feasibility of detecting metabolism of different energy substrates in brain simultaneously by means of analyzing in vivo 13C MRS results from the carboxylic/amide region when different specific 13C-labeled chemicals are administered. An effort to apply this strategy to studying the human brain is currently in progress, which will be helpful for the investigation of brain energy metabolism and neurotransmission under different physiological and pathological conditions.
Acknowledgements
The authors are grateful to Dr. Su Xu, Mr. Christopher Johnson, Drs. Steve Li, Yan Zhang, Jan Willem van der Veen and BuSik Park for their valuable help, and Ioline Henter for editing the manuscript. This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, US Department of Health and Human Services (IRP-NIMH-NIH-DHHS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/conflict of interest This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (NIMH-NIH). The authors have no conflict of interest to disclose, financial or otherwise.
References
- Al-Mamun M, Goto K, Chiba S, Sano H. Responses of plasma acetate metabolism to hop (Humulus lupulus L.) in sheep. Int J Biol Sci. 2009;5:287–92. doi: 10.7150/ijbs.5.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert A, Costalat R, Magistretti PJ, Pellerin L. Brain lactate kinetics: Modeling evidence for neuronal lactate uptake upon activation. Proc Natl Sci U S A. 2005;102:16448–53. doi: 10.1073/pnas.0505427102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badar-Goffer RS, Bachelard HS, Morris PG. Cerebral metabolism of acetate and glucose studied by 13C-n.m.r. spectroscopy. A technique for investigating metabolic compartmentation in the brain. Biochem J. 1990;266:133–9. doi: 10.1042/bj2660133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann N, Turkalj I, Seelig J, Keller U. 13C NMR for the assessment of human brain glucose metabolism in vivo. Biochemistry. 1991;30:6362–6. doi: 10.1021/bi00240a002. [DOI] [PubMed] [Google Scholar]
- Behar KL, Petroff OA, Prichard JW, Alger JR, Shulman RG. Detection of metabolites in rabbit brain by 13C NMR spectroscopy following administration of [1-13C]glucose. Magn Reson Med. 1986;3:911–20. doi: 10.1002/mrm.1910030611. [DOI] [PubMed] [Google Scholar]
- Bluml S, Moreno-Torres A, Shic F, Nguy CH, Ross BD. Tricarboxylic acid cycle of glia in the in vivo human brain. NMR Biomed. 2002;15:1–5. doi: 10.1002/nbm.725. [DOI] [PubMed] [Google Scholar]
- Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, et al. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2010;30:13983–91. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Serres S, Canioni P, Merle M. Lactate involvement in neuron-glia metabolic interaction: (13)C-NMR spectroscopy contribution. Biochimie. 2003;85:841–8. doi: 10.1016/j.biochi.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM, Pellerin L. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci. 2006;24:1687–94. doi: 10.1111/j.1460-9568.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- Cerdán S, Kunnecke B, Seelig J. Cerebral metabolism of [1,2-13C2]acetate as detected by in vivo and in vitro 13C NMR. J Biol Chem. 1990;265:12916–26. [PubMed] [Google Scholar]
- Cerdán S, Rodrigues TB, Sierra A, Benito M, Fonseca LL, Fonseca CP, et al. The redox switch/redox coupling hypothesis. Neurochem Int. 2006;48:523–30. doi: 10.1016/j.neuint.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Chen Z, Li SS, Yang J, Letizia D, Shen J. Measurement and automatic correction of high-order B0 inhomogeneity in the rat brain at 11.7 Tesla. Magn Reson Imaging. 2004;22:835–42. doi: 10.1016/j.mri.2004.01.062. [DOI] [PubMed] [Google Scholar]
- Choi IY, Tkác I, Ugurbil K, Gruetter R. Noninvasive measurements of [1-13C]glycogen concentrations and metabolism in rat brain in vivo. J Neurochem. 1999;73:1300–8. doi: 10.1046/j.1471-4159.1999.0731300.x. [DOI] [PubMed] [Google Scholar]
- Cremer JE. Amino Acid Metabolism in Rat Brain Studied with 14C-Labelled Glucose. J Neurochem. 1964;11:165–85. doi: 10.1111/j.1471-4159.1964.tb06127.x. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Dienel GA. High glycogen levels in brains of rats with minimal environmental stimuli: implications for metabolic contributions of working astrocytes. J Cereb Blood Flow Metab. 2002;22:1476–89. doi: 10.1097/01.WCB.0000034362.37277.C0. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Lasater A, Zielke HR, Dienel GA. Activation of astrocytes in brain of conscious rats during acoustic stimulation: acetate utilization in working brain. J Neurochem. 2005;92:934–47. doi: 10.1111/j.1471-4159.2004.02935.x. [DOI] [PubMed] [Google Scholar]
- de Graaf RA. In vivo NMR spectroscopy: principles and techniques. John Wiley & Sons; Hoboken, NJ: 2007. [Google Scholar]
- Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2003;23:5928–35. doi: 10.1523/JNEUROSCI.23-13-05928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst RR. Nuclear magnetic double resonance with an incoherent radio-frequency field. J Chem Phys. 1966;45:3845–61. [Google Scholar]
- Edmond J. Energy metabolism in developing brain cells. Can J Physiol Pharmacol. 1992;70(Suppl):S118–29. doi: 10.1139/y92-253. [DOI] [PubMed] [Google Scholar]
- Guzman M, Blazquez C. Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol Metab. 2001;12:169–73. doi: 10.1016/s1043-2760(00)00370-2. [DOI] [PubMed] [Google Scholar]
- Guzman M, Blazquez C. Ketone body synthesis in the brain: possible neuroprotective effects. Prostaglandins Leukot Essent Fatty Acids. 2004;70:287–92. doi: 10.1016/j.plefa.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Hassel B, Sonnewald U, Fonnum F. Glial-neuronal interactions as studied by cerebral metabolism of [2-13C]acetate and [1-13C]glucose: an ex vivo 13C NMR spectroscopic study. J Neurochem. 1995;64:2773–82. doi: 10.1046/j.1471-4159.1995.64062773.x. [DOI] [PubMed] [Google Scholar]
- Hawkins RA. Transport of essential nutrients across the blood-brain barrier of individual structures. Fed Proc. 1986;45:2055–9. [PubMed] [Google Scholar]
- Henderson ST. Ketone bodies as a therapeutic for Alzheimer's disease. Neurotherapeutics. 2008;5:470–80. doi: 10.1016/j.nurt.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, et al. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22:1523–31. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Shen J. Integrated RF probe for in vivo multinuclear spectroscopy and functional imaging of rat brain using an 11.7 Tesla 89 mm bore vertical microimager. Magma. 2005;18:119–27. doi: 10.1007/s10334-005-0103-y. [DOI] [PubMed] [Google Scholar]
- Li S, Yang J, Shen J. Novel strategy for cerebral 13C MRS using very low RF power for proton decoupling. Magn Reson Med. 2007;57:265–71. doi: 10.1002/mrm.21148. [DOI] [PubMed] [Google Scholar]
- Li S, Zhang Y, Wang S, Yang J, Ferraris Araneta M, Farris A, et al. In vivo 13C magnetic resonance spectroscopy of human brain on a clinical 3 T scanner using [2-13C]glucose infusion and low-power stochastic decoupling. Magn Reson Med. 2009;62:565–73. doi: 10.1002/mrm.22044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist F, Sestoft L, Damgaard SE, Clausen JP, Trap-Jensen J. Utilization of acetate in the human forearm during exercise after ethanol ingestion. J Clin Invest. 1973;52:3231–5. doi: 10.1172/JCI107523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–7. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Malloy CR, Sherry AD, Jeffrey FM. Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy. J Biol Chem. 1988;263:6964–71. [PubMed] [Google Scholar]
- Mangia S, Simpson IA, Vannucci SJ, Carruthers A. The in vivo neuron-to-astrocyte lactate shuttle in human brain: evidence from modeling of measured lactate levels during visual stimulation. J Neurochem. 2009;109(Suppl 1):55–62. doi: 10.1111/j.1471-4159.2009.06003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir D, Berl S, Clarke DD. Acetate and fluoroacetate as possible markers for glial metabolism in vivo. Brain Res. 1986;380:336–40. doi: 10.1016/0006-8993(86)90231-3. [DOI] [PubMed] [Google Scholar]
- Nehlig A. Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot Essent Fatty Acids. 2004;70:265–75. doi: 10.1016/j.plefa.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Clarke DD. Decarboxylation studies of glutamate, glutamine, and aspartate from brain labelled with [1-14C]acetate, L-[U-14C]-aspartate, and L-[U-14C]glutamate. J Neurochem. 1969;16:549–58. doi: 10.1111/j.1471-4159.1969.tb06854.x. [DOI] [PubMed] [Google Scholar]
- Occhipinti R, Somersalo E, Calvetti D. Astrocytes as the glucose shunt for glutamatergic neurons at high activity: an in silico study. J Neurophysiol. 2009;101:2528–38. doi: 10.1152/jn.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal RM, Koeppe RE, Williams EI. Utilization in vivo of glucose and volatile fatty acids by sheep brain for the synthesis of acidic amino acids. Biochem J. 1966;101:591–7. doi: 10.1042/bj1010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF., Jr. Brain metabolism during fasting. J Clin Invest. 1967;46:1589–95. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JW, de Graaf RA, Petersen KF, Shulman GI, Hetherington HP, Rothman DL. [2,4-13 C2]-beta-Hydroxybutyrate metabolism in human brain. J Cereb Blood Flow Metab. 2002;22:890–8. doi: 10.1097/00004647-200207000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson H, Gust D, Armitage IM, Huber H, Roberts JD, Stark RE, et al. Nuclear magnetic resonance spectroscopy: reinvestigation of carbon-13 spin-lattice relaxation time measurements of amino acids. Proc Natl Acad Sci U S A. 1975;72:1599–601. doi: 10.1073/pnas.72.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. How to balance the brain energy budget while spending glucose differently. J Physiol. 2003;546:325. doi: 10.1113/jphysiol.2002.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard JW. What the clinician can learn from MRS lactate measurements. NMR Biomed. 1991;4:99–102. doi: 10.1002/nbm.1940040212. [DOI] [PubMed] [Google Scholar]
- Rumpel C. Does burning of harvestinig residues increase soil carbon storage? J Soil Sc Plant Nutr. 2008;8:44–51. [Google Scholar]
- Schurr A. Lactate: the ultimate cerebral oxidative energy substrate? J Cereb Blood Flow Metab. 2006;26:142–52. doi: 10.1038/sj.jcbfm.9600174. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, Shulman RG. In vivo 13C NMR measurements of cerebral glutamine synthesis as evidence for glutamateglutamine cycling. Proc Natl Acad Sci USA. 1997;94:2699–704. doi: 10.1073/pnas.94.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Novotny EJ, Rothman DL. In vivo lactate and beta-hydroxybutyrate editing using a pure-phase refocusing pulse train. Magn Reson Med. 1998;40:783–8. doi: 10.1002/mrm.1910400520. [DOI] [PubMed] [Google Scholar]
- Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, et al. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci U S A. 1999;96:8235–40. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes JE, Lopes-Cardozo M, Van Den Bergh SG. Substrate utilization for energy production and lipid synthesis in oligodendrocyte-enriched cultures prepared from rat brain. Neurochem Int. 1986;8:67–75. doi: 10.1016/0197-0186(86)90102-6. [DOI] [PubMed] [Google Scholar]
- Taylor A, McLean M, Morris P, Bachelard H. Approaches to studies on neuronal/glial relationships by 13C-MRS analysis. Dev Neurosci. 1996;18:434–42. doi: 10.1159/000111438. [DOI] [PubMed] [Google Scholar]
- Uehara T, Sumiyoshi T, Itoh H, Kurata K. Lactate production and neurotransmitters; evidence from microdialysis studies. Pharmacol Biochem Behav. 2008;90:273–81. doi: 10.1016/j.pbb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Urrila AS, Hakkarainen A, Heikkinen S, Vuori K, Stenberg D, Hakkinen AM, et al. Stimulus-induced brain lactate: effects of aging and prolonged wakefulness. J Sleep Res. 2004;13:111–9. doi: 10.1111/j.1365-2869.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF., Jr. Ketone bodies, potential therapeutic uses. IUBMB Life. 2001;51:241–7. doi: 10.1080/152165401753311780. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Bakken IJ, Larsson OM, Sonnewald U, Schousboe A. Comparison of lactate and glucose metabolism in cultured neocortical neurons and astrocytes using 13C-NMR spectroscopy. Dev Neurosci. 1998;20:310–20. doi: 10.1159/000017326. [DOI] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–33. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA. NMR-Techniques and Application in Geochemistry and Soil Chemistry. Pergamin Press; Oxford: 1987. [Google Scholar]
- Xu S, Shen J. In vivo dynamic turnover of cerebral 13C isotopomers from [U-13C]glucose. J Magn Reson. 2006;182:221–8. doi: 10.1016/j.jmr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Yang J, Johnson C, Shen J. Detection of reduced GABA synthesis following inhibition of GABA transaminase using in vivo magnetic resonance signal of [13C]GABA C1. J Neurosci Methods. 2009;182:236–43. doi: 10.1016/j.jneumeth.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li SS, Bacher J, Shen J. Quantification of cortical GABA-glutamine cycling rate using in vivo magnetic resonance signal of [2-13C]GABA derived from glia-specific substrate [2-13C]acetate. Neurochem Int. 2007;50:371–8. doi: 10.1016/j.neuint.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245–54. [PMC free article] [PubMed] [Google Scholar]