Abstract
Background
Cue reactivity, the ability of cues associated with addictive substances to induce seeking and withdrawal, is a major contributor to addiction. Although human imaging studies show that cigarette-associated cues simultaneously activate the insula and the orbitofrontal cortex and evoke craving, how these activities functionally contribute to distinct elements of cue reactivity remains unclear. Moreover, it remains unclear whether the simultaneous activation of these cortical regions reflects coordinated functional connectivity or parallel processing.
Methods
We selectively lesioned the insula or orbitofrontal cortex with the excitotoxin ibotenic acid in mice, and their approach to nicotine-associated cues (n = 6–13/group) and avoidance of withdrawal-associated cues (n = 5–12/group) were separately examined in place conditioning paradigms. We additionally tested the role of these two cortical structures in approach to food-associated cues (n = 6–7/group) and avoidance of LiCl-associated cues (n = 6–7/group).
Results
Our data show a double dissociation in which excitotoxic lesions of the insula and orbitofrontal cortex selectively disrupted nicotine-induced cue approach and withdrawal-induced cue avoidance, respectively. These effects were not entirely generalized to approach to food-associated cues or avoidance of LiCl-associated cues.
Conclusions
Our data provide functional evidence that cue reactivity seen in addiction includes unique neuroanatomically dissociable elements and suggest that the simultaneous activation of these two cortical regions in response to smoking-related cues does not necessarily indicate functional connectivity.
Keywords: cue reactivity, incentive, nicotine, withdrawal, insula, orbitofrontal cortex
Introduction
Approach/avoidance is a fundamental principle governing behavior to maximize the survival of organisms. Environmental stimuli exert a powerful control over this dimension of behavior. This process, termed cue reactivity, together with craving as its subjective motivation, is thought to be a significant contributor to addiction (1–4). Ex-smokers report exposure to smoking-related cues and strong craving as factors for lapses (5). In laboratory settings, smoking-related cues evoke craving and smoking (6,7).
Functional imaging studies have consistently demonstrated that in smokers, exposure to smoking-associated cues simultaneously activates, among others, both the insula (INS) and the orbitofrontal cortex (OFC) and evokes subjective reports of craving (8–11). However, it remains unclear whether coordinated activation of these two cortical regions indicates functional connections or independent, parallel processes of the various elements of craving.
This issue is further complicated by the potentially multiple motivational processes of cue reactivity. In experimental set-ups where cues are conditioned with smoking, such cues evoke both desire to smoke and withdrawal (6,7,12). Cue reactivity can be interpreted as indicating that smoking-related cues evoke incentive seeking for nicotine’s effects, avoidance of withdrawal, or both (1). Questionnaires used to define craving include elements of both processes (13,14). Moreover, it is not certain whether smokers are aware of the exact motivational state underlying craving and are capable of accurately reporting their real motivational processes.
Cue reactivity can be experimentally established in humans under Pavlovian contingency and such cues are used as classically conditioned stimuli (15–17). Like human cue reactivity, the rodent place conditioning paradigm utilizes Pavlovian conditioning and is thought to model the whole process of cue reactivity --including the impact of nicotine or withdrawal, coding of these effects in association with cues, and retrieval and sustained expression of this memory (18). This paradigm can independently reveal both drug-induced cue approach and withdrawal-induced cue avoidance, by separately pairing cues with nicotine to induce approach (19) or with precipitated withdrawal to induce avoidance (20,21). Moreover, predictive validity has been satisfied for both nicotine-associated cue approach and withdrawal-associated cue avoidance, as anti-craving agents (22,23) attenuate the development of both conditioned behaviors (24–26).
The motivationally complex nature of craving prompted us to investigate the relative roles played by the INS and the OFC in cue-evoked incentive and withdrawal processes. Here, we lesioned the INS or OFC in mice with the fiber-sparing excitotoxin ibotenic acid and separately tested for nicotine cue approach and withdrawal-induced cue avoidance in Pavlovian place conditioning paradigms. Our data indicate that these two cortical regions independently contribute to two distinct motivational processes of nicotine cue reactivity.
Methods and Materials
Animals
Male C57BL/6J mice were used (Jackson Laboratory, Bar Harbor, ME). Surgery was done at the age of 4 weeks and behavioral analysis began at the age of 5 weeks. This age period is the developmental stage at which mice exhibit signs of sexual maturation (27). Mice were maintained on a 14-h light/10-h dark cycle with food and water available ad libitum unless otherwise specified. Animal handling and use followed a protocol approved by the Animal Care and Use Committee of the Albert Einstein College of Medicine, in accordance with NIH guidelines.
Drugs
(-)-Nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO) for injection was dissolved in physiological saline at a concentration of 0.1 free base mg ml−1 and pH was adjusted to 6.8–7.6. Mecamylamine hydrochloride (Sigma-Aldrich) was dissolved in physiological saline at a concentration of 1.25 free base mg ml−1. LiCl (Sigma-Aldrich) was dissolved in water at a concentration of 40 free base mg ml−1. Nicotine and mecamylamine injections were given subcutaneously (s.c.) at a volume of 2 ml kg−1. LiCl was injected intraperitoneally (i.p.) at a volume of 6 ml kg−1. For the induction of nicotine dependence, alkaline (-)-nicotine free-base solution (200 free base μg ml−1, Sigma-Aldrich) was prepared in 0.33% (wt/vol) saccharin water.
Surgery
Mice were anesthetized with sodium pentobarbital (40 mg kg−1, i.p., Sigma Aldrich) and placed into a stereotaxic apparatus (MyNeuroLab, Richmond, IL). We used the excitotoxin ibotenic acid to produce localized lesions without damaging fibers of passage (28). Ibotenic acid (Sigma-Aldrich) was dissolved in sterile 0.1 M phosphate buffered saline at a concentration of 10 μg μl−1, and an amount ranging from 0.5–2.0 μg was bilaterally injected. Sham mice received bilateral injections of the vehicle. The coordinates used for the targeted regions were as follows: INS, anterior-posterior (AP) 1.98 mm, medial-lateral (ML) 2.25 mm, dorsal-ventral (DV) -3.5 mm; OFC, AP 2.46 mm, ML 0.75 mm, DV -3.0 mm. Separate control groups of mice received lesions 1.5 mm dorsal to the target regions. Animals were allowed 5–7 days to recover from surgery.
Behavioral Testing
The apparatus used was a rectangular Plexiglas box composed of three distinct compartments (29,30) . Two large compartments (24.5 cm × 18 cm × 33 cm) had distinguishable visual and tactile cues: one compartment had black-and-white striped walls and a wire mesh floor with 2.1 × 2.1-mm openings and was lit at 3.12 lux; the other compartment had gray walls and a wire mesh floor with 3.7 × 3.7-mm openings and was lit at 1.40 lux. These two large compartments were separated by a central compartment (13 cm × 18 cm × 33 cm). Each large compartment was divided from the center compartment by a guillotine door (18 cm × 37 cm). The guillotine doors were opened 5 cm above the floor, and the mice were allowed to explore the three compartments freely during pre- and post-conditioning test days.
We designed our experiment so that each conditioned behavior was optimally induced. Surgery was done at the age of 4 weeks and behavioral analysis began at the age of 5 weeks. Consistent with the literature (31–37), we found that nicotine induced reliable cue approach in adolescent, but not adult C57BL/6J mice, in our pilot studies. For withdrawal-induced cue avoidance, mice started to receive chronic nicotine administration at 5 weeks of age and were conditioned with withdrawal at 7 weeks of age. We found that withdrawal-induced cue avoidance was most robustly induced at this age (38), consistent with studies reporting that precipitated withdrawal induces robust cue avoidance in rats after, but not during, adolescence (39,40).
For nicotine-induced cue approach (Fig. 1A) and LiCl-induced cue avoidance (Fig. 1A), animals were allowed to explore the apparatus for 15 min on the first day, and time spent in either side compartment was recorded. Like precipitated withdrawal, LiCl induces cue avoidance that is mediated by the brain (41–44). This group was used to test the specificity of lesion effects on avoidance. On the second day, animals received an injection of nicotine (0 and 0.2 free base mg kg−1, s.c.) or LiCl (0 and 240 free base mg kg−1, i.p.) and were immediately confined to either of the two distinct place conditioning compartments in two 30-min sessions at least 5 h apart. This dose of nicotine was chosen because it induces the most robust cue approach under our experimental condition (29,30). Nicotine was always given when animals were confined to the initially non-preferred compartment while LiCl pairings were arranged such that on a group basis, mice showed equal preference for the compartments to be paired with drug and vehicle. The order of injections was counterbalanced between all animals. From days 3–12, animals were allowed to freely explore the apparatus for 15 min per day, and time spent in each side compartment was recorded by an experimenter blinded to the specific treatment.
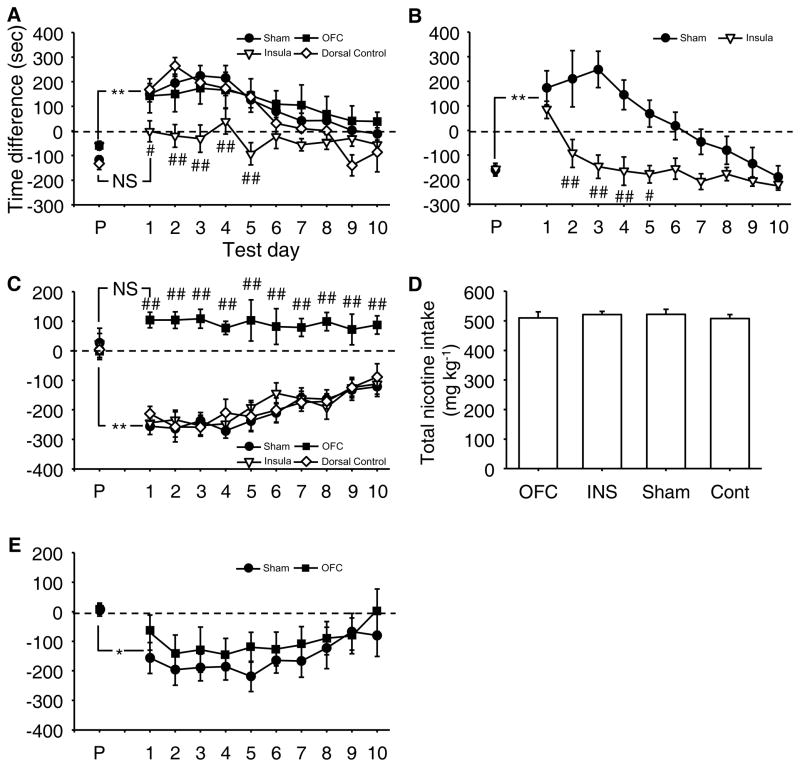
Figure 1.
Experimental protocol. A) Nicotine-induced conditioned cue approach (0 and 0.2 mg free base kg−1, s.c.) and LiCl-induced conditioned cue avoidance (LiCl salt, 0 and 240 mg kg−1, i.p.). An acute nicotine or LiCl injection was not given on the test days. B) Food (sweetened condensed milk)-induced conditioned cue approach. Food and an empty cup were alternately given in each compartment during conditioning sessions. C) Mecamylamine-induced conditioned cue avoidance (0 and 2.5 mg free base kg−1, s.c.). The black horizontal line indicates exposure to oral nicotine (200 μg free base ml−1) in the home cage. Black box, 15-min drug-free testing session; P, pre-conditioning test; T, post-conditioning test; grey box, 30-min conditioning (C) session.
For food-induced cue approach (Fig. 1B), mice were singly housed and, through limited access to regular food pellets, maintained at approximately 80% free feeding weight. Mice were habituated with 2 ml of food reward (sweetened condensed milk diluted 1:1 with water, America’s Choice, Montvale, MA) per day in their home cages for 3 days. The animal’s pre-existing bias toward the two compartments was tested for 15 min on the first test day. On days 2–7, mice were confined to one of the compartments for 30 min with either a cup containing 2 ml of food reward or an empty cup, alternating each day. Three conditioning days with food were given, because our pilot studies showed that this number of pairings was needed to induce food cue approach comparable to nicotine cue approach. The order of exposure was counterbalanced between the mice. The food was always placed in the initially non-preferred compartment, and no food was provided when mice were placed in the initially preferred compartment during conditioning. On days 8–17, mice were allowed to freely explore the apparatus for 15 min, with one empty food cup present in each of the two large compartments. An experimenter blinded to the specific treatment recorded time spent in each side compartment.
We followed our published procedure for withdrawal-induced cue avoidance (Fig. 1C) (38). Briefly, mice were singly housed in a home cage with a single bottle containing 200μg ml−1 nicotine in a 0.33% saccharin solution. Every 3 days, the mice and bottles were weighed and fresh nicotine solution was provided. On the 14th day, animals were pretested in the place conditioning apparatus. On the 15th day, animals received mecamylamine (0 and 2.5 mg free base kg−1, s.c.) immediately before two 30-min sessions at least 5 h apart. This nAChR antagonist has been used to precipitate withdrawal in the place conditioning paradigm in rats and mice chronically exposed to nicotine (20,21). The order of injections, as well as the drug pairings, was counterbalanced between animals so that the animals, as a group, did not show a pre-existing bias to either compartment. From the 16th to 25th day, animals were allowed to freely explore the apparatus for 15 min per day, and time spent in each side compartment was recorded by an experimenter blinded to the specific treatment. Nicotine intake was measured in home cages and the total volume up to the pre-conditioning test day was analyzed.
Histology
Following completion of testing, animals were anesthetized with pentobarbital (62.5 mg kg−1, i.p.) and transcardially perfused with physiological saline followed by 4% paraformaldehyde in 0.1 M Na-K phosphate buffer (PB). The brains were removed and postfixed in 4% paraformaldehyde for 1–2 h and cryoprotected in 20% glycerol in PB overnight. Brains were sliced coronally at 50 μm on a freezing microtome, and sections were kept in PB with sodium azide (0.1%). We used mitogen-associated protein type 2 (MAP2) staining to delineate the extent of lesions. Because this protein is found mainly in dendrites, a lack of MAP2 staining is indicative of neuronal cell death (28,45). Moreover, unlike Nissl staining, this marker does not label glial proliferation within a lesioned area, thereby showing neuronal loss as a clear lack of any staining. Free-floating sections were treated for 10 min with 3% H2O2 in 0.01 M Na phosphate buffer containing 0.2% Triton X-100 and 0.9% NaCl (PBS, pH 7.4) and for 30 min with 5% normal goat serum. Following washes with PBS (3 × 10 min), sections were incubated in rabbit MAP2 antibody (1:2,000, Millipore, Billerica, MA) for 48 h at 4°C. Sections were washed in PBS (3 × 10 min), incubated in goat anti-rabbit IgG (1:500, Vector Laboratories, Burlingame, CA) for 1 h, and subsequently in avidin-biotin-peroxidase complex (1:170, Vector Laboratories) for 1 h. Each of these steps was followed by three washes in PBS except for the last wash in PB. Reaction between H2O2 (0.003%) and horseradish peroxidase conjugated with biotin oxidized diaminobenzidine (DAB; Sigma-Aldrich, St. Louis, MO) yielded brown deposits.
Statistics
Data were analyzed as the time spent in the drug-paired compartment minus the time spent in the vehicle-paired compartment. All data are presented as the mean ± standard error of the mean (SEM). Statistical significance was determined by two-way ANOVA followed by the Newman–Keuls post hoc test. Minimum significance was set at 5%. The Jarque-Bera test showed that JB values ranged from 0.009–4.41, demonstrating the normality of all groups (p = 0.111–0.996).
Results
Lesions were centered within each target (see Fig. 2). INS lesions were located within the entire antero-posterior extent (Fig. 2A,C). Lesions of the OFC included, throughout the anterior-posterior extent, the medial, ventral, and lateral subregions (Fig. 2B,D). Because we noted that lesions were often extended along the cannula tracks, dorsally above the target regions, we tested separate groups of mice that received lesions just above the targets; they were mostly located in the primary and secondary motor, primary somatosensory, cingulate, prelimbic, and frontal association cortices (Fig. 2A,B).
Figure 2.
Schematic of ibotenic acid-induced lesions of the A) insula and B) orbitofrontal cortex. Red-toned areas represent individual lesions. The darker red areas represent areas lesioned in more than one mouse. Grey areas represent individual lesions of “dorsal control” groups. Representative photographs of coronal sections showing MAP2 staining. Lesioned areas are devoid of staining in the insula (C) and the orbitofrontal cortex (D). INS, insula; OFC, orbitofrontal cortex; M1, primary motor cortex; M2, secondary motor cortex; S1, primary somatosensory cortex; Cg, cingulate cortex; PrL, prelimbic cortex; FrA, frontal association cortex; CL, claustrum; MO, medial orbitofrontal cortex; VO, ventral orbitofrontal cortex; LO, lateral orbitofrontal cortex. Scale bar: 500 μm.
Mice with INS lesions showed no approach, but mice with sham (Sham), OFC-lesions, and lesions in areas dorsal to the INS (Dorsal control) did (Fig. 3A; Group, F(3,30)=3.43, p=0.0295). Moreover, all groups, except for the INS group, increased their preference for a compartment following conditioning with nicotine compared to a pre-conditioning day (see P, pre-conditioning day), and their preference gradually extinguished (Day, F(10,300)=25.36, p<0.0001; Group x Day, F(30,300)=3.28, p<0.0001).
Figure 3.
Impact of cortical lesions on cue approach and avoidance. A) Nicotine-induced cue approach. N=6–13/group. B) Food-induced cue approach. N=7 (Sham) and 6 (Insula). C) Withdrawal-induced cue avoidance. N=5–12/group. D) Total nicotine intake of groups used for withdrawal-induced cue avoidance. OFC, orbitofrontal group; INS, insula group; Cont, control group that received lesions dorsal to the orbitofrontal cortex. E) LiCl-induced cue avoidance. N=7 (Sham) and 6 (orbitofrontal). X axis in A-C and E, pre-conditioning (P) and 10 postconditioning test days (Test day). Y axis in A, C and E, difference in time spent in the drug-paired and saline-paired compartments (sec). Y axis in B, difference in time spent in the food-paired and unpaired compartments (sec). # and ## (p<0.05 and p<0.01) represent statistically significant difference from sham group; * and ** represent a statistically significant difference from the pre-conditioning test at 5 and 1% levels, respectively. NS indicates no difference from the pre-conditioning test day.
To determine whether the effects of INS lesions can be generalized to natural incentive cue approach, we tested INS-lesioned mice in food-induced cue approach (Fig. 3B). Both sham and INS-lesioned mice increased their preference for a food-paired compartment on the first post-conditioning test day. However, while sham-operated mice maintained cue approach from the second post-conditioning day, INS-lesioned mice did not (Group, F(1,11)=18.64, p=0.0012; Day, F(10,110)=11.70, p<0.0001; Group x Day, F(10,110)=4.02, p=0.0001).
We next examined the role of these two cortical regions in withdrawal-induced cue avoidance. Precipitated withdrawal established conditioned cue avoidance in Sham mice and mice with lesions to the INS and areas dorsal to the OFC (Dorsal control). By contrast, OFC-lesioned mice failed to show this conditioned behavior (Fig. 3C; Group, F(3,27)=24.82, p<0.0001). All but the OFC group showed avoidance following conditioning compared to the pre-conditioning day, and gradually decreased this avoidance during post-conditioning test days (i.e., extinction) (Day, F(10,270)=12.40, p<0.0001; Group x Day, F(30,270)=3.39, p<0.0001). All groups of mice consumed indistinguishable amounts of nicotine (Group, F(3,27) = 0.17, p=0.9182; Fig. 3D).
To determine the specificity of the deficit, we tested mice with OFC lesions for LiCl-induced cue avoidance. Compared to the pre-conditioning test day, this drug equally induced cue avoidance in both OFC- and sham-lesioned mice, which equally extinguished during postconditioning test days (Fig. 3E; Group, F(1,11)=0.57, p=0.46; Day, F(10,110)=5.87, p<0.0001; Group x Day, F(10,110)=0.52, p=0.87).
Discussion
The present study separated cue reactivity into seeking of incentive stimuli and avoidance of withdrawal and demonstrated that the INS and OFC are independently required, through a dissociable manner, for these separate elements of craving. Our finding is consistent with the idea that cue-evoked craving is not a unitary process (13,14,46) and further suggests parallel processing of the two elements of craving at the level of the two cortical regions.
We designed our experiments to optimally induce nicotine-associated cue approach and withdrawal-associated cue avoidance in the place conditioning paradigm. It has been noted that nicotine induces cue approach more reliably in the biased design --in which an initially non-preferred compartment is paired with nicotine --compared to the non-biased procedure (19). Our pilot studies were consistent with this claim and we observed consistent cue approach when nicotine was paired with an initially non-preferred compartment in C57BL/6J mice; the non-biased design failed to establish statistically reliable cue approach in C57BL/6J mice.
The biased design has been criticized on the grounds that it fails to isolate the rewarding effects of drugs and might be contaminated by other effects of drugs (47). However, there is no compelling reason to isolate “reward” alone. First, nicotine preference in humans might include effects other than reward (e.g., reduction of baseline depression or anxiety) (48–50). Second, compared to cue-evoked craving, reward and positive reinforcement might not play a significant role in daily smoking or relapse and might not contribute to motives that uniquely characterize addiction and dependence (51,52). Third, cue-evoked craving includes not only the approach-inducing effects of drugs but also the entire Pavlovian conditioning process (see Introduction).
Withdrawal-induced avoidance has been demonstrated in the non-biased design in rats (20) and in the biased design where mecamylamine-precipitated withdrawal was paired with an initially preferred compartment in mice (21,25). We tested cue avoidance in the non-biased design for two reasons. First, we have reported that robust withdrawal-cue avoidance can be induced in the non-biased procedure (38). Second, because OFC inactivation has been shown to both potentiate and attenuate many cocaine-related effects in self-administration (53–58), we needed a design that would allow us to evaluate both possibilities. If withdrawal is conditioned with an initially preferred compartment in the biased design, any effect other than avoidance would be difficult to detect due to a ceiling effect; if mecamylamine is paired with the initially non-preferred compartment, a floor effect would make it difficult, if not impossible, to reveal any conditioned avoidance.
Because INS lesions impaired nicotine cue approach in the biased design, and OFC lesions impaired withdrawal cue avoidance in the non-biased design, the differential effects might be due to this procedural difference, rather than some functional differences of these two cortical regions in nicotine cue approach and withdrawal cue avoidance. Obviously, our conclusions are limited to these experimental conditions. However, these designs were adapted to optimally induce conditioned behaviors. Moreover, there is no reliable way to make the experimental designs of cue approach and cue avoidance equal. Even if the non-biased (or biased) place conditioning procedure was used for both preference and avoidance, there would remain a number of procedural differences (e.g., nicotine vs. withdrawal and presence vs. absence of chronic nicotine infusion). Nonetheless, the effects of INS and OFC lesions were evaluated within the identical condition and lesion effects were clearly dissociated within each procedure; INS, but not OFC lesions impaired nicotine cue approach and OFC, but not INS lesions impaired withdrawal cue avoidance. Moreover, the effects of OFC lesions were not specific to the non-biased design, as such lesions impaired withdrawal cue avoidance but not LiCl-induced cue avoidance in the non-biased design. Similarly, the effects of INS lesions on nicotine- and food-cue approach were not identical despite that fact that both were done in the biased design.
INS-lesioned mice did not express nicotine cue approach from the first test day. By contrast, such mice acquired food-cue approach and expressed it normally on the first testing day, but failed to maintain the behavior from the second extinction day. Because the duration and level of nicotine and food cue approach were indistinguishable in sham mice, the subtle difference in the way INS lesions affect these two types of incentive cue approach is unlikely to reflect a difference in the strength of conditioning. One possibility is that the INS is differentially involved in the acquisition and retrieval of nicotine and food cue approach. Alternatively or additionally, INS lesions might have accelerated extinction of nicotine cue approach more rapidly than food cue approach by more rapidly establishing the new learning of cue-no incentive on the first post-conditioning day. More work is needed to ascertain how the INS contributes to the acquisition, expression, and extinction of nicotine and food cue approach.
Our observations that INS lesions eliminated nicotine cue approach, and extinction diminished cue approach over a course of 10 days, support the validity of this rodent behavior as a model of craving. The vast majority of smokers relapse within the first 8 days (59) and experience the most intense craving during this period, but progressively less craving thereafter (60–63). Moreover, cues indicative of cigarettes activate the INS (8–11,64–66). This activation is functionally significant, as smokers with widespread damage, including damage to the INS, are significantly more likely to have less craving and quit smoking (67) (but see (68)). Together with our observation that INS lesions had no effect on withdrawal-induced cue avoidance, it could be suggested that the INS functionally mediates the cue-guided incentive motivation component, but not the avoidance of withdrawal component, of craving.
While the functional role of the INS in other forms of addiction in humans is not well understood, the INS has been shown to contribute to cue reactivity with amphetamine in the place conditioning paradigm (44) and cue-evoked reinstatement of nicotine (69) and cocaine (70) self-administration in rats. Such a role might be embedded in other functions ascribed to this structure, including interoceptive representation (i.e., bodily states), subjective feelings (e.g., emotion), and cognitive functions. Alternatively, incentive motivation might be one additional function to which this region contributes.
The INS is likely to be selectively required for the prolonged expression of cue-food association; it is not critical for acquisition of cue-food association or its acute expression. Expression of cue-food association during extinction could include heterogeneous elements. Cues indicative of food activate the INS in humans (71). Such activation might selectively reflect its role in the maintenance of cue reactivity.
While smoking-related cues activate the OFC and induce craving (8–10,72–75), our data suggest that this activation is functionally required for processing of the cue-evoked withdrawal avoidance component, but not the cue-evoked incentive component, of craving. It should be noted that withdrawal cue avoidance includes a state of dependence, induction of withdrawal, association between cues and withdrawal, and acute and maintained retrieval/expression of this association. Given that abstinence alone also is associated with activation of this cortical region in smokers (76), a future challenge is to identify the exact process of withdrawal cue reactivity for which this cortical region is functionally required.
As our paradigm does not require reversal or devaluation, such functions of the OFC (77) do not offer a plausible explanation for our finding. The cortical region might additionally be involved in some aspects of withdrawal-associated cue reactivity. Although this region is implicated in processing of cues that signal aversive stimuli in humans (78), mice with OFC lesions showed normal LiCl-induced cue avoidance. This cortical region might not equally contribute to all types of aversive cue learning.
As the functional specification among corticolimbic regions might not be identical between humans and mice, caution is needed in comparing activation of regions in humans and effects of damage to the regions in rodents. With this interpretative caveat, it is still interesting to observe that human and rodent data are not entirely consistent. Among corticolimbic regions, the cingulate cortex is another cortical area that is activated by smoking-associated cues (8,9,11,64–66,72–74,79). However, excitotoxic lesions of this area have little effect on cue approach with cocaine, morphine, or amphetamine in rodents (80). We also observed that six out of eight “dorsal control” mice had lesions in this cortical area (see Fig. 2B) but exhibited normal withdrawal-associated cue avoidance. It remains unclear what functional role the cingulate cortex plays in cue reactivity.
Other corticolimbic regions have been shown to be activated by smoking-related cues. The medial prefrontal region responds to smoking-related cues in smokers (9,75). The rodent medial prefrontal cortex has been implicated in cue approaches with morphine and cocaine (80,81). Smoking-related cues activate the amygdala and hippocampus in smokers (10,11,74,82). In rats, excitotoxic lesions of the basolateral amygdaloid complex impair cue approach induced by amphetamine (83) and cocaine (84,85), and cue avoidance induced by withdrawal in morphine-dependent rats (86). Cocaine-associated cue approach has been shown to be reduced by excitotoxic lesions of the dorsal hippocampus (87). As the role of these structures in nicotine cue reactivity has not been examined in rodents, a future challenge is to fully understand how these structures, together with the INS and OFC, orchestrate craving through cue-evoked incentive motivation and withdrawal in addictions to nicotine and other addictive substances.
Given that pre-existing traits are a determinant for addiction susceptibility (50,88) and cue reactivity is seen in addiction with many other substances (2), our observations provide a framework to further elucidate the way in which pre-existing activity levels of the various cortical regions contribute to susceptibility to many forms of cue reactivity in addiction.
Acknowledgments
This work was partly supported by a grant from the NIH (R01DA024330) to NH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. We thank M. Lee, K. Harper, G. Suzuki, and G. Kang for their help in blinding experimental groups.
Footnotes
Financial Disclosure
The authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- 2.Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- 3.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- 4.Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- 5.Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- 6.Droungas A, Ehrman RN, Childress AR, O'Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict Behav. 1995;20:657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- 7.Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp Clin Psychopharmacol. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- 8.Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 9.McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- 10.Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O'Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein A, Greif J, Yemini Z, Lerman H, Weizman A, Even-Sapir E. Attenuation of cue-induced smoking urges and brain reward activity in smokers treated successfully with bupropion. J Psychopharmacol. 2010;24:829–838. doi: 10.1177/0269881109105456. [DOI] [PubMed] [Google Scholar]
- 12.Payne TJ, Smith PO, Sturges LV, Holleran SA. Reactivity to smoking cues: mediating roles of nicotine dependence and duration of deprivation. Addict Behav. 1996;21:139–154. doi: 10.1016/0306-4603(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 13.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 14.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 15.Mucha RF, Pauli P, Angrilli A. Conditioned responses elicited by experimentally produced cues for smoking. Can J Physiol Pharmacol. 1998;76:259–268. [PubMed] [Google Scholar]
- 16.Lazev AB, Herzog TA, Brandon TH. Classical conditions of environmental cues to cigarette smoking. Exp Clin Psychopharmacol. 1999;7:56–63. doi: 10.1037//1064-1297.7.1.56. [DOI] [PubMed] [Google Scholar]
- 17.Hogarth L, Duka T. Human nicotine conditioning requires explicit contingency knowledge: is addictive behaviour cognitively mediated? Psychopharmacology (Berl) 2006;184:553–566. doi: 10.1007/s00213-005-0150-0. [DOI] [PubMed] [Google Scholar]
- 18.White NM, Hiroi N. Amphetamine cue preference and the neurobiology of drug-seeking. Seminars in the Neurosiences. 1993;5:329–336. [Google Scholar]
- 19.Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki T, Ise Y, Tsuda M, Maeda J, Misawa M. Mecamylamine-precipitated nicotine-withdrawal aversion in rats. Eur J Pharmacol. 1996;314:281–284. doi: 10.1016/s0014-2999(96)00723-6. [DOI] [PubMed] [Google Scholar]
- 21.Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Culbertson CS, Bramen J, Cohen MS, London ED, Olmstead RE, Gan JJ, Costello MR, Shulenberger S, Mandelkern MA, Brody AL. Effect of Bupropion Treatment on Brain Activation Induced by Cigarette-Related Cues in Smokers. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O'Brien CP, Childress AR. Effects of Varenicline on Smoking Cue-Triggered Neural and Craving Responses. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damaj MI, Grabus SD, Navarro HA, Vann RE, Warner JA, King LS, Wiley JL, Blough BE, Lukas RJ, Carroll FI. Effects of hydroxymetabolites of bupropion on nicotine dependence behavior in mice. J Pharmacol Exp Ther. 2010;334:1087–1095. doi: 10.1124/jpet.110.166850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malin DH, Lake JR, Smith TD, Khambati HN, Meyers-Paal RL, Montellano AL, Jennings RE, Erwin DS, Presley SE, Perales BA. Bupropion attenuates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl) 2006;184:494–503. doi: 10.1007/s00213-005-0135-z. [DOI] [PubMed] [Google Scholar]
- 26.Biala G, Staniak N, Budzynska B. Effects of varenicline and mecamylamine on the acquisition, expression, and reinstatement of nicotine-conditioned place preference by drug priming in rats. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:361–370. doi: 10.1007/s00210-010-0498-5. [DOI] [PubMed] [Google Scholar]
- 27.Bronson FH, Dagg CP, Snell GD. In: Biology of the Laboratory Mouse. Green EL, editor. Dover Publications, Inc; New York: 2007. Oneline publication. [Google Scholar]
- 28.Schneider M, Koch M. Behavioral and morphological alterations following neonatal excitotoxic lesions of the medial prefrontal cortex in rats. Exp Neurol. 2005;195:185–198. doi: 10.1016/j.expneurol.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Agatsuma S, Lee M, Zhu H, Chen K, Shih JC, Seif I, Hiroi N. Monoamine oxidase A knockout mice exhibit impaired nicotine preference but normal responses to novel stimuli. Hum Mol Genet. 2006;15:2721–2731. doi: 10.1093/hmg/ddl206. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H, Lee M, Agatsuma S, Hiroi N. Pleiotropic impact of constitutive fosB inactivation on nicotine-induced behavioral alterations and stress-related traits in mice. Hum Mol Genet. 2007;16:820–836. doi: 10.1093/hmg/ddm027. [DOI] [PubMed] [Google Scholar]
- 31.Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- 32.Brielmaier JM, McDonald CG, Smith RF. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicol Teratol. 2007;29:74–80. doi: 10.1016/j.ntt.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 33.Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- 34.Shram MJ, Le AD. Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Behav Brain Res. 2010;206:240–244. doi: 10.1016/j.bbr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Torres OV, Tejeda HA, Natividad LA, O'Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O'Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl) 2009;206:303–312. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- 38.Scott D, Hiroi N. Emergence of dormant conditioned incentive approach by conditioned withdrawal in nicotine addiction. Biol Psychiatry. 2010;68(8):726–732. doi: 10.1016/j.biopsych.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shram MJ, Siu EC, Li Z, Tyndale RF, Le AD. Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology (Berl) 2008;198:181–190. doi: 10.1007/s00213-008-1115-x. [DOI] [PubMed] [Google Scholar]
- 41.Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D'Alessio D. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci. 2000;20:1616–1621. doi: 10.1523/JNEUROSCI.20-04-01616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lachey JL, D'Alessio DA, Rinaman L, Elmquist JK, Drucker DJ, Seeley RJ. The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: differential effects in rats and mice. Endocrinology. 2005;146:458–462. doi: 10.1210/en.2004-0419. [DOI] [PubMed] [Google Scholar]
- 43.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- 45.Katchanov J, Waeber C, Gertz K, Gietz A, Winter B, Bruck W, Dirnagl U, Veh RW, Endres M. Selective neuronal vulnerability following mild focal brain ischemia in the mouse. Brain Pathol. 2003;13:452–464. doi: 10.1111/j.1750-3639.2003.tb00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carr GD, Fibiger H, Phillips AG. In: The neuropharmacological basis of reward. Liebman JM, Cooper SJ, editors. Clarendon Press; Oxford: 1989. pp. 264–319. [Google Scholar]
- 48.Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biol Psychiatry. 2004;55:69–76. doi: 10.1016/s0006-3223(03)00317-2. [DOI] [PubMed] [Google Scholar]
- 49.Di Franza JR, Savageau JA, Fletcher K, Pbert L, O'loughlin J, McNeill AD, Ockene JK, Friedman K, Hazelton J, Wood C, et al. Susceptibility to nicotine dependence: the Development and Assessment of Nicotine Dependence in Youth 2 study. Pediatrics. 2007;120:e974–e983. doi: 10.1542/peds.2007-0027. [DOI] [PubMed] [Google Scholar]
- 50.Hiroi N, Scott D. Constitutional mechanisms of vulnerability and resilience to nicotine dependence. Mol Psychiatry. 2009;14:653–667. doi: 10.1038/mp.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piper ME, McCarthy DE, Bolt DM, Smith SS, Lerman C, Benowitz N, Fiore MC, Baker TB. Assessing dimensions of nicotine dependence: an evaluation of the Nicotine Dependence Syndrome Scale (NDSS) and the Wisconsin Inventory of Smoking Dependence Motives (WISDM) Nicotine Tob Res. 2008;10:1009–1020. doi: 10.1080/14622200802097563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piasecki TM, Piper ME, Baker TB, Hunt-Carter EE. WISDM primary and secondary dependence motives: Associations with self-monitored motives for smoking in two college samples. Drug Alcohol Depend. 2010 doi: 10.1016/j.drugalcdep.2010.10.005. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kantak KM, Mashhoon Y, Silverman DN, Janes AC, Goodrich CM. Role of the orbitofrontal cortex and dorsal striatum in regulating the dose-related effects of self-administered cocaine. Behav Brain Res. 2009;201:128–136. doi: 10.1016/j.bbr.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:1370–1381. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grakalic I, Panlilio LV, Quiroz C, Schindler CW. Effects of orbitofrontal cortex lesions on cocaine self-administration. Neuroscience. 2010;165:313–324. doi: 10.1016/j.neuroscience.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 56.Lasseter HC, Wells AM, Xie X, Fuchs RA. Interaction of the Basolateral Amygdala and Orbitofrontal Cortex is Critical for Drug Context-Induced Reinstatement of Cocaine-Seeking Behavior in Rats. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.209. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hutcheson DM, Everitt BJ. The effects of selective orbitofrontal cortex lesions on the acquisition and performance of cue-controlled cocaine seeking in rats. Ann N Y Acad Sci. 2003;1003:410–411. doi: 10.1196/annals.1300.038. [DOI] [PubMed] [Google Scholar]
- 58.Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 60.McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. J Abnorm Psychol. 2006;115:454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- 61.Piasecki TM, Fiore MC, Baker TB. Profiles in discouragement: two studies of variability in the time course of smoking withdrawal symptoms. J Abnorm Psychol. 1998;107:238–251. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- 62.Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB. Smoking withdrawal dynamics in unaided quitters. J Abnorm Psychol. 2000;109:74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- 63.Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. J Abnorm Psychol. 2003;112:3–13. [PubMed] [Google Scholar]
- 64.McClernon FJ, Hutchison KE, Rose JE, Kozink RV. DRD4 VNTR polymorphism is associated with transient fMRI-BOLD responses to smoking cues. Psychopharmacology (Berl) 2007;194:433–441. doi: 10.1007/s00213-007-0860-6. [DOI] [PubMed] [Google Scholar]
- 65.Gloria R, Angelos L, Schaefer HS, Davis JM, Majeskie M, Richmond BS, Curtin JJ, Davidson RJ, Baker TB. An fMRI investigation of the impact of withdrawal on regional brain activity during nicotine anticipation. Psychophysiology. 2009;46:681–693. doi: 10.1111/j.1469-8986.2009.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janes AC, Frederick B, Richardt S, Burbridge C, Merlo-Pich E, Renshaw PF, Evins AE, Fava M, Kaufman MJ. Brain fMRI reactivity to smoking-related images before and during extended smoking abstinence. Exp Clin Psychopharmacol. 2009;17:365–373. doi: 10.1037/a0017797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bienkowski P, Zatorski P, Baranowska A, Ryglewicz D, Sienkiewicz-Jarosz H. Insular lesions and smoking cessation after first-ever ischemic stroke: a 3-month follow-up. Neurosci Lett. 2010;478:161–164. doi: 10.1016/j.neulet.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 69.Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry. 2010;68:265–271. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 70.Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- 71.Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage. 2006;32:1273–1280. doi: 10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- 72.McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- 75.Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, Jens W, Cruz J, Kampman K, Ehrman R, et al. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry. 2008;63:256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gottfried JA, O'Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tzschentke TM, Schmidt WJ. Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci. 1999;11:4099–4109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- 81.Tzschentke TM, Schmidt WJ. Discrete quinolinic acid lesions of the rat prelimbic medial prefrontal cortex affect cocaine- and MK-801-, but not morphine- and amphetamine-induced reward and psychomotor activation as measured with the place preference conditioning paradigm. Behav Brain Res. 1998;97:115–127. doi: 10.1016/s0166-4328(98)00034-5. [DOI] [PubMed] [Google Scholar]
- 82.Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- 83.Hiroi N, White NM. The lateral nucleus of the amygdala mediates expression of the amphetamine-produced conditioned place preference. J Neurosci. 1991;11:2107–2116. doi: 10.1523/JNEUROSCI.11-07-02107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown EE, Fibiger HC. Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference. Psychopharmacology (Berl) 1993;113:123–130. doi: 10.1007/BF02244344. [DOI] [PubMed] [Google Scholar]
- 85.Fuchs RA, Weber SM, Rice HJ, Neisewander JL. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Res. 2002;929:15–25. doi: 10.1016/s0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- 86.Watanabe T, Yamamoto R, Maeda A, Nakagawa T, Minami M, Satoh M. Effects of excitotoxic lesions of the central or basolateral nucleus of the amygdala on naloxone-precipitated withdrawal-induced conditioned place aversion in morphine-dependent rats. Brain Res. 2002;958:423–428. doi: 10.1016/s0006-8993(02)03468-6. [DOI] [PubMed] [Google Scholar]
- 87.Meyers RA, Zavala AR, Neisewander JL. Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport. 2003;14:2127–2131. doi: 10.1097/00001756-200311140-00023. [DOI] [PubMed] [Google Scholar]
- 88.Hiroi N, Agatsuma S. Genetic susceptibility to substance dependence. Molecular Psychiatry. 2005;10:336–344. doi: 10.1038/sj.mp.4001622. [DOI] [PubMed] [Google Scholar]