Abstract
Even though hepatitis B virus (HBV) vaccines effectively prevent new cases of HBV infection, with approximately 350 million patients worldwide, chronic HBV infection remains a major health problem because of the associated complications (such as liver cirrhosis and hepatocellular carcinoma) and the limited treatment options. Immunotherapy has the potential to effectively control HBV replication. In this current study, we found that recombinant lentivectors could induce potent HBV surface antigen (HBsAg) specific T cell responses and humoral immune responses. Tagging the HBsAg with immunoglobulin Fc fragment further substantially increased the HBsAg specific immune responses. Remarkably, the HBS-Fc-lv lentivector could effectively break immune tolerance and induce potent HBsAg specific adaptive immune responses in HBsAg transgenic (Tg) mice with low serum level of HBsAg. More importantly, the induction of HBsAg specific immune responses in Tg mice accompanied seroconversion from HBsAg to anti-HBsAg antibody (anti-HBsAb). Our study demonstrated the potential of utilizing lentivector to treat chronic HBV infection following reduction of viral load with antiviral drug therapy.
Keywords: Lentivector, Hepatitis B virus, Vaccines, Genetic immunization, Immunotherapy, Chronic HBV infection
1. Introduction
Wide application of protein based hepatitis B virus (HBV) vaccines has been successful in preventing new cases of HBV infections [1]. However, for more than 350 millions of chronic carriers who have already been infected with HBV, the treatment options are limited. Interferon is effective only in a small proportion of chronic HBV patients. Nucleoside and nucleotide analogue antiviral drugs are only virostatic rather viricidal, and their use is limited by the life-long dependence on these drugs, the risk of emerging drug resistant viruses [2], and side effects [3]. Chronic HBV infection without treatment can lead to severe liver diseases including liver cirrhosis and hepatocellular carcinoma. The idea of utilizing immunotherapy to control HBV replication is supported by findings that patients who recovered from acute HBV infections usually contained high levels of polyclonal T cell responses against multiple HBV Ags [4–5] and that bone marrow transplantation of anti-HBV immunity to recipient could cure chronic HBV infections [6]. Prophylactic HBV vaccines and dendritic cell (DC) based vaccines have been examined for their potential to treat chronic HBV infection [7–8]. Even though they are well tolerated in chronic HBV patients, their effectiveness remains limited. DNA based HBV vaccines were found to be capable of eliciting CD8 T cell responses in one animal model [9], but they failed to break immune tolerance in another HBV transgenic (Tg) mouse model [10]. Although DC based vaccine could break tolerance and induce immune responses in Tg mice [10–11], their effect on converting the HBV serum markers was not certain, possibly because the magnitude of immune responses was low [10]. In clinical trial, the protein and DNA based immunotherapy of chronic HBV infection were found not to be effective [7, 12–14]. Thus, there is a need to develop more potent immunization approaches for the purpose of treating chronic HBV infection.
We and others previously found that recombinant lentivectors could induce potent T cell immunity against self tumor Ag and model Ag [15–18] possibly because they can effectively transduce DCs and directly prime naïve T cells by skin derived DCs in vivo [19–21]. Lentivector immunization stimulates much higher CD8 T cell immune responses than DNA vaccines and other viral vectors [19]. Recently, Collins and colleagues demonstrated that non-integrating lentivector immunization could effectively stimulate anti-HBs antibody (Ab) in the circulation and T cell responses in naïve mice [22]. However, no attempt was made to study if lentivector could be also effective in the presence of HBsAg and if such immune responses could result in therapeutic benefits.
Therefore, in the current study, we investigated the potential of lentivector immunization to induce HBV surface (HBs) Ag specific immune responses and whether lentivector immunization can break tolerance to induce HBsAg specific immune responses in HBsAg Tg mice. We found that lentivector immunization elicited potent HBsAg specific CD8 T cell responses. Furthermore, tagging HBsAg with immunoglobulin (Ig) Fc fragment markedly enhanced CD8 immune responses and stimulated CD4 T cell responses and anti-HBsAb. Importantly, lentivector immunization also induced HBsAg specific adaptive immune responses in the Tg mice expressing low level of HBsAg even though failed to break tolerance in the Tg mice with high level of HBsAg. Our data suggest that lentivector expressing Fc tagged HBsAg is a potent immunization vehicle for stimulating HBsAg specific adaptive immune responses and may be capable of inducing HBsAg specific immune responses in the presence of low level of HBsAg, implicating the potential of using lentivector for immunotherapy of chronic HBV infection following reducing the viral antigen load with antiviral treatment.
2. Materials and Methods
2.1. Cell lines and mice
293T cells were purchased from American Tissue and Cell Collection (ATCC, Manassas, VA) and maintained in complete DMEM media. Fc receptor γ-chain (FcRγ) knockout mice [23] were purchased from Taconic (Germantown, NY). The HBsAg transgenic mice (C57BL/6J-Tg (Alb1HBV)44Bri/J) constitutively expressing HBsAg in the liver were purchased from Jackson Laboratory (Bar Harbor, ME) and bred in the Laboratory Animal Services (LAS) of the Medical College of Georgia. C57BL/6 mice were obtained from either Taconic or the National Cancer Institute (Frederick, MD). All mice were housed under SPF conditions and used at 6–10 weeks old. Animal care protocols were approved by the IACUC of the Medical College of Georgia.
2.2. Lentivector preparation and immunization
Plasmid pRC/CMV-HBS (ayw) was kindly provided by Aldevron LLC (Fargo, ND). HBV small surface Ag (HBS) and HBS-IgG2a Fc fusion genes (HBS-Fc) were obtained by overlapping PCR using pRC/CMV-HBS and murine IgG2a as templates. The stop codon was deleted and the rest of HBS gene was fused in frame to the Fc fragment gene so that HBS-Fc fusion Ag gene can be created. Sequences were verified. Recombinant lentivector plasmid was originally purchased from Invitrogen (San Diego, CA) and modified as previously reported [20]. Lentivector HBS-lv and HBS-Fc-lv plasmids were constructed by replacing the TRP1 gene in TRP1-lv [15] with the HBS gene or the HBS-Fc fusion gene using restriction sites of BamHI and XhoI. Lentivectors were prepared and titered as described previously [20]. For immunization, 1.5 × 107 transduction units (TU) of HBS-lv or HBS-Fc-lv were injected on the footpad. Plasmid DNA immunization was conducted by intramuscular injection (via tibialis anterior muscle) of 100µg of plasmid pRC/CMV-HBS DNA. Two injections (weekly) were conducted. For in vitro transduction of 293T cells with lentivector, 5×106 TU of HBS-lv or HBS-Fc-lv were used to transduce the cells in 6-well plate.
2.3. Intracellular staining of cytokines
To measure cytokines, single cell suspensions from peripheral blood were stimulated for 3hrs with 1µg/ml of HBS peptides (S190–198) or overnight with recombinant HBsAg protein (5µg/ml) with 10µ/ml of hIL-2 (Prospec, Rehovot, Israel), in the presence of GolgiStop (BD Bioscience, San Diego, CA). To measure the cytokine production of liver infiltrating T cells, the liver single cell suspension was enriched for T cells with 40% Percoll solution (GE-healthcare Bioscience AB, Uppsala, Sweden) after collagenase treatment as previously described [24]. Cells were then stimulated with peptides. Intracellular staining of IFN-γ was performed [20]. Surface staining included Thy1.2, CD4 and CD8. Cells were collected using a FACScanto system (BD Bioscience, San Jose, CA). Data were analyzed using FCS Express V3 software (De Novo Software, Ontario, Canada).
2.4. In vivo killing assay
To measure the cytolytic function of CD8 T cells, in vivo killing assay was performed as described previously [20, 25]. Briefly, HBS peptide pulsed (targets) and non-pulsed (control) mouse splenocytes were labeled with 5µM or 0.5µM 5- (and -6)-carboxyfluorescein diacetate succinimidyl ester (CFSE), respectively, and then injected into mice. After 12 hours, splenocytes were collected from mice and the specific lysis of target cells were examined and calculated as described previously [20].
2.5. ELISA
To compare the HBsAg expression in vitro, 293T cells were transduced with lentivector HBS-lv or HBS-Fc-lv. Forty-eight hours later, supernatants (2.5ml) from the transfected 293T cells were collected and used directly for ELISA. To lyze the transduced 293T cells, 250µl of Triton X-/SDS lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% SDS, 1% Triton X100, 1 × protease inhibitor cocktail of BD Biosciences) was added. The cell lysates were then diluted with PBS (1:2) before measurement. To measure the HBsAg level, serum was collected from HBsAg Tg mice pre and post immunization. To detect HBsAg in the liver, liver samples (20mg) were disaggregated and homogenized in 500µl of Triton X-/SDS lysis buffer and the cell lysate was diluted with PBS (1:2). Then, HBsAg in the serum, cell lysate and supernatants, and liver samples were detected by using HBsAg ELISA kit according to the protocol suggested by the manufacturer (DiaSorin, Stillwater, MN). The anti-HBsAb in the serum was detected by using the anti-HBs detection kit from DiaSorin (Stillwater, MN).
2.6 Detection of serum alanine aminotransferase (sALT)
To examine the liver enzyme, mouse serum was collected from immunized or untreated control HBsAglow Tg mice. The serum sALT was determined by using the ALT reagents from Teco Diagnostics (Anaheim, CA) according to the protocol of manufacturer.
2.7 Statistical analysis
All statistical analyses were done using unpaired t-test (GraphPad Software Inc, La Jolla, CA).
3. Results and Discussion
3.1. Tagging the HBsAg with Ig Fc fragment markedly increases the HBsAg level in the lentivector transduced 293T cells
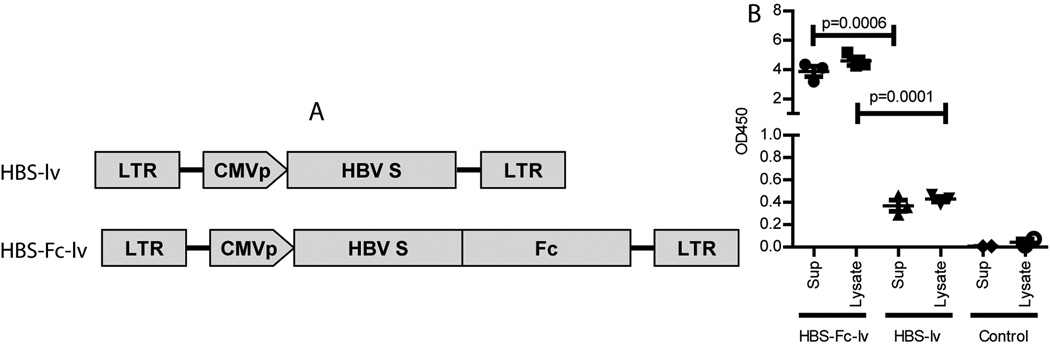
To investigate if lentivector could be utilized to stimulate HBV specific immune responses, the HBV (type ayw) small S gene (HBs) was cloned into recombinant lentivector. Since Ig Fc fragment was found to enhance the immunization effect of plasmid DNA [26], we tagged the HBsAg with mouse IgG2a Fc fragment. The two recombinant lentivectors were designated as HBS-lv and HBS-Fc-lv, respectively (Fig. 1A). To determine the expression and secretion of HBsAg and HBs-Fc fusion Ag, 293T cells were transduced with lentivector HBS-lv or HBS-Fc-lv. HBsAg in the supernatant and cell lysate were determined by ELISA. The level of HBsAg or HBS-Fc Ag was presented as the absolute amount of OD450 in the supernatant or in the cell lysate. We found that Fc fragment tagging at the C-terminal of protein increased the HBsAg level by 10 folds in both the supernatant and cell lysate of transduced 293T cells (Fig.1B). The data were similar to previous report that of N-terminal fusion of Fc fragment could increase expression and secretion of protein in mammalian cells [27]. However, the mechanism was not clear. Nevertheless, Fc tagging increases the availability of Ag and thus likely enhances the immune responses.
Fig.1.
Tagging of HBsAg with the Fc fragment of mouse IgG2a increases the HBsAg level. A. Schematic structure of recombinant lentivector HBS-lv and HBS-Fc-lv. HBsAg gene and HBS-Fc fusion gene were inserted behind the CMV promoter in the recombinant lentivector. B. Increase of the expression and secretion of HBsAg after transduction. 293T cells were transduced with either HBS-lv or HBS-Fc-lv lentivector. Untransduced 293T cells were used as control. Two days after transduction, HBsAg level in the supernatant and cell lysate was examined by ELISA. The data value was presented as OD450 in the total cell lysate or supernatant. The mean±SEM was indicated on the figure by the central value and error bar (95% confidence limit). Statistiacal anlysis was done using unpaired t-test. The experiment was repeated three times with similar data.
3.2 Recombinant lentivector stimulates potent HBsAg specific CD8 T cell immune responses
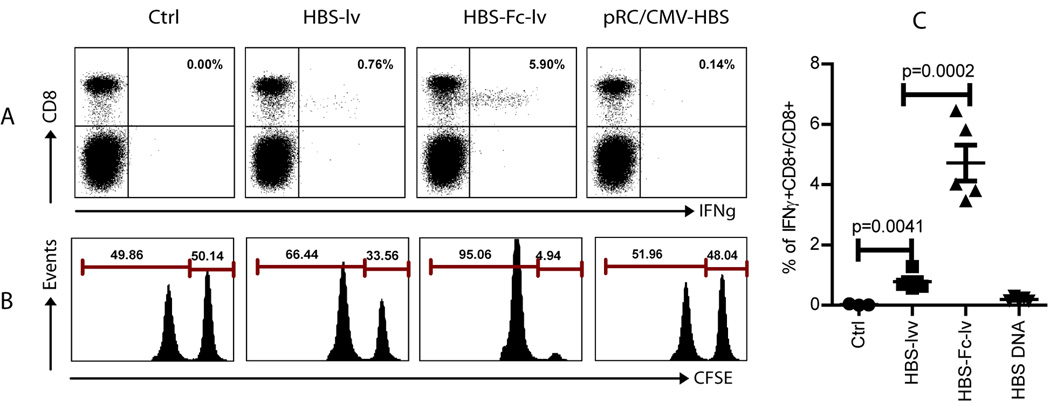
To study the efficacy of CD8 T cell responses stimulated by lentivectors and plasmid DNA immunization, C57BL/6 mice were immunized with either plasmid DNA or recombinant lentivectors HBS-lv and HBS-Fc-lv. CD8 T cell response was examined two weeks later. We found that two injections of plasmid DNA elicited a weak immune response (0.1~0.2% of CD8 T cells were IFNγ+). On the other hand, one injection of lentivector HBS-lv induced moderate CD8 T cell responses with 0.5~1% of CD8 cells being IFNγ+. Remarkably, one immunization with HBS-Fc-lv expressing HBS-Fc fusion Ag stimulated the most potent T cell responses with ~6% of CD8 T cells producing IFNγ (Fig.2A and 2C). In addition, the in vivo killing data demonstrated that nearly all the HBS peptide (S190–198) pulsed target cells were eliminated in the HBS-Fc-lv immunized mice (Fig. 2B.), demonstrating potent HBsAg specific cytolytic function after HBS-Fc-lv immunization. In contrast, only partial killing effect was found in HBS-lv immunized mice and nearly no in vivo killing of target cells was detected in DNA immunized mice. Results from both assays suggest that lentivector expressing HBs-Fc fusion Ag elicits much more potent CD8 T cell responses in the immunized C57BL/6 mice.
Fig.2.
Lentivector HBS-Fc-lv immunization stimulates potent CD8 T cell responses. Female C57BL/6 mice (5 mice in each group) were immunized with lentivector HBS-lv or HBS-Fc-lv on footpad. For DNA immunization, intramuscular injection was performed. Two weeks later, immune responses were examined. A. Intracellular staining of the IFNγ among CD8 T cells in the peripheral blood after brief (3hr) ex vivo stimulation with HBS peptide. The numbers on the upper right quadrant represent the percentage of IFNγ producing CD8 T cells out of total CD8 T cells. B. The in vivo CTL activity was assayed on day 14 by in vivo killing assay. Only the CFSE+ cells were shown in the histogram. The numbers represent the percentage of each CFSE+ cell population. The experiment was reproduced at least 5 times with similar observation. C. A summary of the IFNγ+ CD8 from five mice in each group is presented. The mean±SEM was indicated on the figure by the central value and error bar (95% confidence limit). Unpaired t-test was used for statistical analysis.
3.3. Lentivector immunization also induces CD4 T cell responses and humoral immune responses
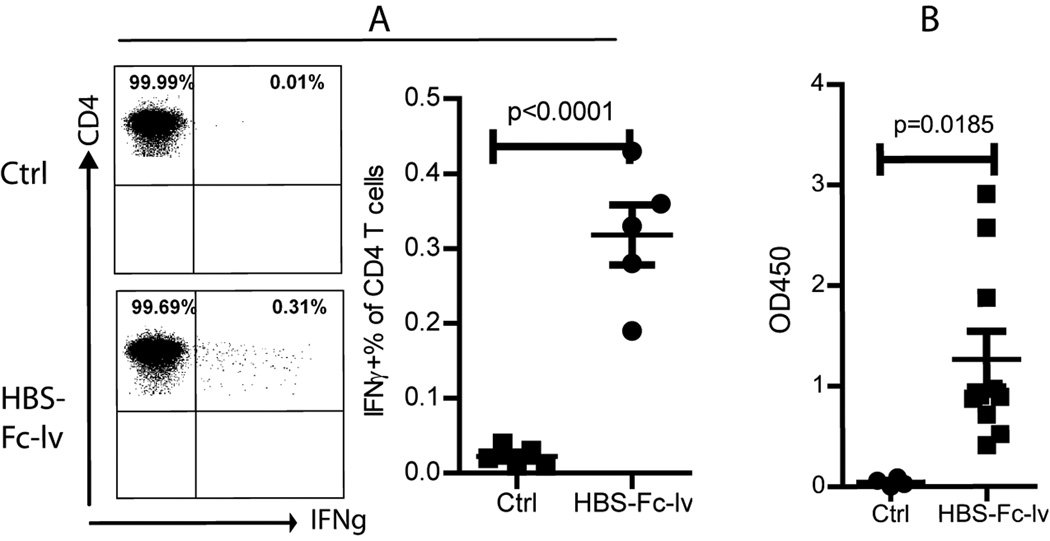
Lentivector is not effective in stimulating CD4 T cell responses if the Ag is synthesized and remains inside the cells [18]. Since HBS-Fc fusion Ag is a secretary protein and high level of Ag can be detected in the supernatant (Fig.1), we speculate that CD4 T cell responses can be induced after HBS-Fc-lv immunization. Because no MHC II (I-Ab) restricted HBS epitope has been identified thus far, we utilized whole HBsAg recombinant protein for in vitro stimulation. Two weeks after immunization with HBS-Fc-lv, using intracellular staining, we could detect IFNγ producing CD4 T cells after brief ex vivo HBsAg stimulation in the HBS-Fc-lv immunized mice, suggesting that HBS-Fc-lv immunization also stimulated CD4 T cell responses (Fig. 3A). On the other hand, immunization with HBS-lv expressing HBsAg without Fc tagging did not stimulate measurable CD4 T cell responses (data not shown).
Fig.3.
Lentivector HBS-Fc-lv immunization elicits CD4 T cell responses and humoral immune responses. A. To examine the CD4 T cell responses, peripheral blood cells were stimulated with HBsAg whole protein before intracellular staining of IFNγ and surface staining of CD4. Only the Thy1.2+CD4+ T cells were shown. The numbers indicate the percentage of each cell population. A summarized data from 5 mice was also presented. B. The humoral immune response was determined by the anti-HBsAb level using ELISA. The data value was presented as OD450/ml of serum. A summary of 10 immunized mice was presented. The mean±SEM was indicated on the figure by the central value and error bar (95% confidence limit). Unpaired t-test was used for analysis.
To examine the humoral immune responses after HBS-Fc-lv immunization, we determined the anti-HBsAb in the serum of HBS-Fc-lv immunized mice. Our data showed that anti-HBsAb could be detected in all immunized mice even though a wide variation was observed among different animals (Fig. 3B).
In summary, the above data demonstrate that HBS-Fc-lv lentivector immunization not only stimulates potent HBsAg specific CD8 responses, but also CD4 responses and humoral responses in C57BL/6 mice, which is normally considered a low responder to HBV vaccines [28].
3.4. Enhancement of CD4 and humoral responses, but not CD8 responses after HBS-Fc-lv immunization is mediated by Fc receptor
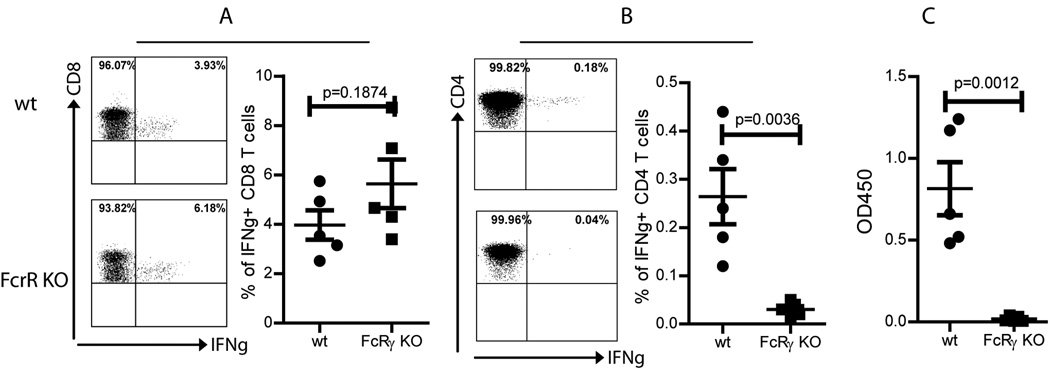
One of the commonly cited mechanisms of how Ig Fc fragment enhance CD8 T cell responses is that Fc receptor on DCs can enhance Ag cross presentation by taking up secreted Ag in the extracelluar surrounding in a autocrine/paracrine fashion [29]. Ag-Ab complex can be taken up by DCs after binding Fc receptor and then be processed and presented via MHC I and II molecules. Ag-Ab protein complex has been shown to generate enhanced HBsAg specific immune responses in animal [30] and in human patients [31] although no mechanisms were studied. To examine if the increase of CD8 T cell responses after HBS-Fc-lv immunization was indeed through Fc receptor, wild type (WT) mice and Fc receptor γ-chain (FcRγ) knockout (KO) mice were immunized with HBS-Fc-lv. The CD8 T cell responses were examined two weeks following immunization. As demonstrated in Fig. 4A, HBS-Fc-lv immunization stimulated equally potent CD8 T cell responses in FcRγ KO and WT mice, suggesting that the enhancement of the primary CD8 T cell responses by Fc fragment tagging is not mediated by Fc receptor. That is not a surprise because the main pathway for CD8 T cell activation after lentivector immunization is via the MHC I molecule restricted presentation of endogenously synthesized protein by transduced skin DCs [19] and the Fc receptor mediated cross presentation may only play a minor role in activating CD8 T cell responses. The enhancement of CD8 T cell response by Fc tagging may be related to the increased level of HBsAg as demonstrated in Fig.1. In contrast, CD4 T cell response was severely inhibited in the FcRγ KO mice (Fig.4B). The exact mechanism is not known. Because the MHC II restricted pathway is utilized mainly to process and present foreign (extracellular) Ag to activate CD4 T cells, it is likely that Fc receptor mediates foreign Ag uptake, processing, and presentation. Fc receptor knockout, thus, should mainly affect CD4 T cell responses.
Fig.4.
The CD4 and humoral immune responses but not CD8 T cell responses are affected by the Fc receptor. To determine if the HBS-Fc-lv induced immune responses were mediated by Fc receptor, five wt C57BL/6 mice and FcRγ KO (also on C57BL/6 background) mice were immunized with HBS-Fc-lv. A. The CD8 T cell response was not affected by Fc receptor knockout. B and C. CD4 T cell response and humoral immune response were severely compromised in the Fc receptor KO mice. Only the indicated cell population was shown in the dot plot. The anti-HBs Ab value was presented as OD450/ml of serum. A summary of data from 5 mice was presented. The mean±SEM was indicated on the figure by the central value and error bar (95% confidence limit). Unpaired t-test was used for statistical analysis.
CD4 T cell help is critical for humoral immune responses [32]. We, next, examined the anti-HBsAb in the FcRγ KO and WT mice following HBS-Fc-lv immunization. While anti-HBsAb can be easily detected in WT mice, there is no anti-HBs Ab detected in the FcRγ KO mice (Fig.4C), suggesting that both CD4 and humoral immune responses induced by lentivector HBS-Fc-lv immunization are mediated and affected by Fc receptor.
3.5 Lentivector immunization could break tolerance in HBsAg Tg mice when the serum level of HBsAg is low
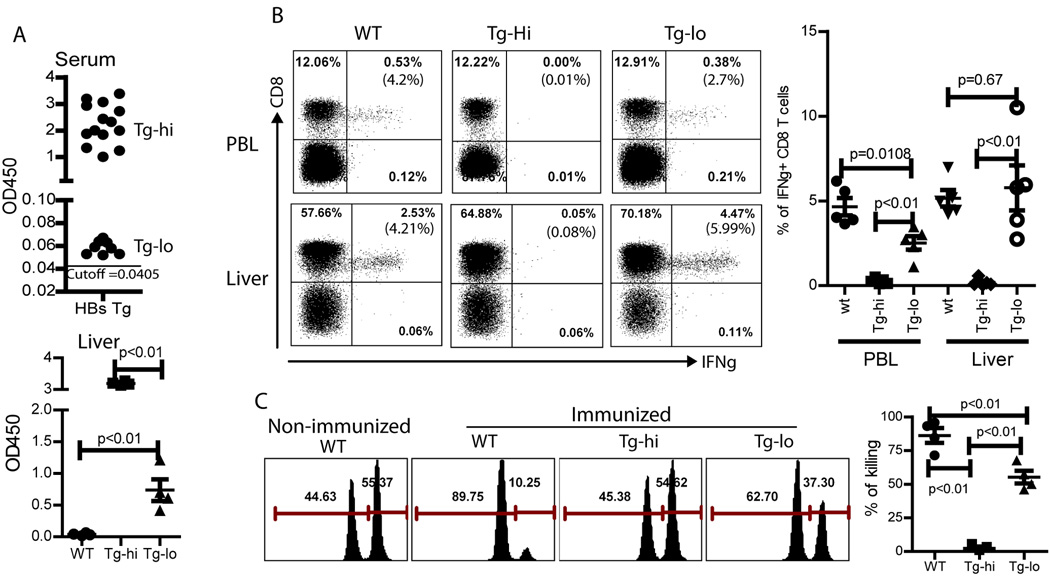
Although the above data demonstrated that lentivector immunization could stimulate potent HBsAg specific adaptive immune responses in naïve mice, for therapeutic purpose, lentivector immunization must be capable of eliciting HBsAg specific immune responses in the presence of HBsAg, a situation that exists in chronic HBV infection. To examine if lentivector HBS-Fc-lv immunization could break immune tolerance in the presence of HBsAg, we utilized the HBsAg Tg mice, in which the expression of HBsAg is under the control of Albumin promoter in hepatocytes and there is a high level of HBsAg in the blood, mimicking the chronic HBV patients’ conditions [33]. When the HBsAg Tg mice were bred, two groups of offspring, HBsAghigh and HBsAglow Tg mice, were observed (upper section of Fig.5A). To make sure that the HBsAglow Tg mice are indeed expressing HBsAg, we examined the HBsAg level in the liver tissue. We found that the liver of the HBsAglow Tg mice contained a definitive level of HBsAg, on average of 0.75 OD450 (lower section of Fig.5A), confirming that HBsAglow Tg mice were indeed expressing HBsAg even though the level was significantly lower than that of HBsAghigh Tg mice.
Fig.5.
HBS-Fc-lv immunization can break immune tolerance in Tg mice expressing low level of HBsAg. A. Offspring of HBsAg Tg mice from Jackson Laboratory could be divided into two groups based on the serum level of HBsAg (upper figure 5A): HBsAghigh (OD450>1.0) and HBsAglow (0.1>OD450>0.05) mice; the cutoff value of OD450 is 0.0405. The HBsAg level in the liver of HBsAg Tg mice was also determined. Although significantly lower than HBsAghigh, Tg-lo mouse liver contained a definitive level of HBsAg (lower part of Fig 5A). B. Following lentivector HBS-Fc-lv immunization, CD8 T cell immune responses were detected in peripheral blood and liver of both WT (normal C57BL/6) mice and HBsAglow Tg mice. However, no CD8 response was detected in the HBsAghi Tg mice. A summary of 5 mice was also presented. Unpaired t-test was utilized for analysis. C. In vivo CTL activity was determined by in vivo killing assay. The Ctrl mice (normal C57BL/6 without treatment) and the HBsAghi Tg mice did not show any in vivo killing activity), while immunized WT mice had high level of CTL activity. A lower level of in vivo killing activity was detected in the HBsAglow Tg mice. A summary of 4 mice was also shown. The mean±SEM was indicated on the figure by the central value and error bar (95% confidence limit). Unpaired t-test was used for analysis.
Following HBS-Fc-lv immunization, while potent HBsAg specific CD8 T cell responses was detected in WT mice, no HBsAg specific CD8 T cell responses were induced in the HBsAghigh Tg mice. However, in the HBsAglow Tg mice, HBS-Fc-lv immunization elicited HBsAg specific CD8 T cell responses that were detected although the responses was lower compared to WT mice (Fig.5B). A relatively higher percent of HBsAg specific CD8 T cell response was detected in the liver compared to the peripheral blood of the Tg mice. This observation is consistent with our previous finding that T cell infiltration and accumulation are Ag driven and dependent [34]. To examine if the activated CD8 T cells in the HBsAglow Tg mice maintain their cytolytic function, we conducted in vivo killing assay. Not surprisingly, there was no detectable in vivo killing activity in the HBsAghigh Tg mice. We found that a significant in vivo killing activity could be detected in the HBsAglow Tg mice even though at a significantly reduced level compared to wt mice. These data suggest that CD8 cytotoxic T cells maintain their target killing activity possibly at a reduced level. Whether the CD8 T cells have a compromised effector function on per cell basis as previously reported in the HBV polymerase specific CD8 T cells by Kakimi et al [35] requires further studies by specifically gating on the HBsAg specific T cells.
3.6 Lentivector HBS-Fc-iv immunization results in seroconversion in the HBsAglow Tg mice
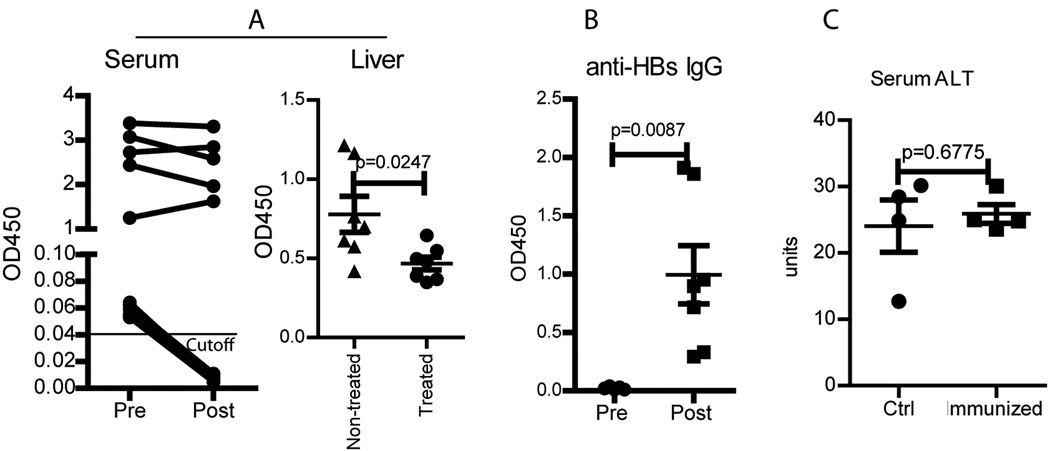
To study if the HBsAg specific immune responses in the HBsAglow Tg mice would result in clinical benefits, we first examined the change of HBsAg in the serum. We found that the HBsAg level in the serum of HBsAglow Tg mice decreased to negative after HBS-Fc-lv immunization (left column of Fig.6A). On the other hand, consistent with the absence of HBsAg specific T cell responses, the HBsAg level remained unchanged in the HBsAghigh Tg mice following HBS-Fc-lv immunization. Furthermore, lentivector immunization also decreased the HBsAg level in the liver of HBsAglow Tg mice (right column of Fig.6A). More importantly, anti-HBsAb could be easily detected in the serum (Fig.6B), suggesting that seroconversion of HBsAg to anti-HBsAb occurred in the immunized HBsAglow Tg mice. These data indicate that the lentivector HBS-Fc-lv immunization can result in seroconversion of HBsAg to anti-HBsAb in HBsAglow Tg mice.
Fig.6.
HBS-Fc-lv immunization results in seroconversion of HBsAg to anti-HBsAb. A. HBS-Fc-lv immunization reduced the serum HBsAg in the HBsAglow Tg mice to become negative (left column). Although it was unable to fully convert liver HBsAg to negative, immunization also decreased the HBsAg level compared to untreated HBsAglow Tg mice. B. Anti-HBs Ab could be detected in the HBsAglow Tg mouse serum after but not before HBS-Fc-lv immunization. Data from 7 mice was presented. The ELISA data value was presented as OD450/ml of serum. C. Compared to control (non-immunized) HBsAglow Tg mice, no significantly increase of serum ALT was found in the immunized HBsAglow Tg mice. The mean±SEM was indicated on the figure by the central value and error bar (95% confidence limit). Statistical analysis was performed using unpaired t-test.
Although in vivo CTL activity was detected (Fig.5C) in HBsAglow Tg mice, there was no obvious increase of the liver enzyme ALT in the serum (Fig.6C), suggesting that there is not sufficient killing of HBsAg expressing hepatocytes. The reasons are not clear but can be due to either the low number or low activity of CD8 T cells in the liver. Even though the data in Fig.5B demonstrated that a high percent of the liver CD8 T cells was IFNγ+ after ex vivo stimulation with HBS peptides, the absolute number of T cells in the liver remained low (data not shown). In fact, it required a whole liver to enrich sufficient cells to measure their IFNγ production after ex vivo stimulation with peptides. In addition, it is not known if the activity of killing peptide-pulsed targets (Fig.5C) can truly reflect the killing activity of CD8 T cells against hepatocytes, especially when the hepatocytes express low amount of HBsAg. These data suggest that more potent immune responses may be required in order to effectively reduce the HBsAg level in the liver via eradicating the HBsAg expressing hepatocytes. However, this may be an overstatement of the difficulty in treating chronic HBV infection since in true HBV infections, the major antiviral effect come from the non-cytolytic effect of cytokines expressed by activated T cells [5]. The reduction of HBsAg level in the liver (right column of Fig.6A) is consistent with this idea and indicates that it is possible to achieve viral control without obvious lysis of hepatocytes. Therefore, even though not all of the HBV expressing cells are eliminated by cytotoxic T lymphocytes, HBV viral replication may be suppressed or controlled by CD8 cytotoxic T lymphocytes and CD4 T cells in a non-cytolytic pathway. This hypothesis may be further examined in a HBV Tg mouse model containing the entire HBV genome and replication competent viruses [10] in the future.
A number of viral vectors including modified vaccinia virus [36]-, measles virus [37]-, and vesicular stomatitis virus [38]- based vectors have been tested for inducing HBV specific immune responses. Compared to protein based vaccines, recombinant viral vectors have the advantages of effectively activating both cellular and humoral immune responses. Unlike most other viral vectors, lentivector encode no viral proteins except the desired transgene Ag, thus, the immune responses can be more focused on the intended Ag. Lentivector is also replication defective and thus can be safer. In addition, lentivector can effectively transduce dendrtic cells, which directly prime naïve CD8 T cells for a prolonged period of time possibly due to its lack of interference with the function of transduced dendritic cells [19, 39]. Lentivector immunization induce more potent immune responses [19] and long-lasting memory responses [40]. In the current study, we provided the evidences that HBS-Fc-lv immunization could break immune tolerance in HBsAglow Tg mice and generated HBsAg specific immune responses and resulted in seroconversion of HBsAg to anti-HBsAb. We are aware that the immunological conditions in chronic HBV infection (even after the viral load is reduced by antiviral treatment) may be different from the HBsAglow Tg mice, which has the de novo low level Ag. For example, animals with initial high viral load or chronic HBV patients with high viral titers may deplete the viral specific T cells during development in the thymus. However, it is also possible that regeneration of novel T cell from the thymus after viral load reduction may be able to replete the T cell repertoire with HBV specific T cells and render patients the capability of developing HBV specific immunity in responding to immunization. The recent findings that combination therapy of antiviral drugs and immunization can break the immune tolerance in woodchuck hepatitis model provide strong evidences for this theory [41]. The positive data from using HBsAglow Tg mice in our study suggests that it may be possible to generate HBV specific immune responses with HBS-Fc-lv immunization after the HBV viral load is decreased by antiviral drugs. Further experiments in the HBV replication competent Tg mice are needed to examine this hypothesis and such researches could lead to a clinically applicable procedure to treat HBV patients in the future.
4. Conclusion
In the study, we found that lentivector induced potent HBsAg specific CD8 T cell responses. IgG2a Fc fragment tagging further enhanced the HBsAg specific adaptive immunity including CD8, CD4 responses, and humoral responses. Importantly, lentivector immunization could induce immune responses in the HBsAg Tg mice with low level of HBsAg and decreased the HBsAg level to become negative in the mouse serum. Remarkably, lentivector immunization also converted the mice into anti-HBsAb positive, suggesting that it may be possible to break immune tolerance when the viral Ag load is low, which can be achievable by antiviral drugs in clinical setting. Thus, such immunotherapy has the potential of working together with antiviral therapy to establish the immune responses to control HBV infection. Further investigations are warranted to explore this potential.
Acknowledgement
The authors are grateful for the helpful discussion and insightful comments from Dr. Tracy McGaha of Immunotherapy Center, Medical College of Georgia and Dr. Ju-Tao Guo of the Drexel Institute for Biotechnology and Virology Research, Drexel University.
Glossary
- HBV
hepatitis B virus
- HBsAg
HBV Surface Ag
- anti-HBsAb
anti-HBsAg antibody
- DC
dendritic cells
- FcR
Fc receptor
Footnotes
Researches are supported by NIH grant R01 CA116444 and the Distinguished Investigator of Vaccine Research Fund from Georgia Research Alliance (GRA) to Dr. Yukai He, and by the National Basic Research Program (973 Program), Ministry of Science and Technology, China to Dr. Gui-qiang Wang.
References
- 1.Chang MH. Impact of hepatitis B vaccination on hepatitis B disease and nucleic acid testing in high-prevalence populations. J Clin Virol. 2006 May;36 Suppl 1:S45–S50. doi: 10.1016/s1386-6532(06)80008-9. [DOI] [PubMed] [Google Scholar]
- 2.Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology. 2009 Nov;137(5):1593–1608. e1–e2. doi: 10.1053/j.gastro.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 3.Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology. 2009 May;49(5 Suppl):S185–S195. doi: 10.1002/hep.22885. [DOI] [PubMed] [Google Scholar]
- 4.Penna A, Chisari FV, Bertoletti A, Missale G, Fowler P, Giuberti T, et al. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J Exp Med. 1991 Dec 1;174(6):1565–1570. doi: 10.1084/jem.174.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 6.Lau GK, Suri D, Liang R, Rigopoulou EI, Thomas MG, Mullerova I, et al. Resolution of chronic hepatitis B and anti-HBs seroconversion in humans by adoptive transfer of immunity to hepatitis B core antigen. Gastroenterology. 2002 Mar;122(3):614–624. doi: 10.1053/gast.2002.31887. [DOI] [PubMed] [Google Scholar]
- 7.Akbar SM, Horiike N, Onji M. Immune therapy including dendritic cell based therapy in chronic hepatitis B virus infection. World J Gastroenterol. 2006 May 14;12(18):2876–2883. doi: 10.3748/wjg.v12.i18.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michel ML, Mancini-Bourgine M. Therapeutic vaccination against chronic hepatitis B virus infection. J Clin Virol. 2005 Dec;34 Suppl 1:S108–S114. doi: 10.1016/s1386-6532(05)80019-8. [DOI] [PubMed] [Google Scholar]
- 9.Mancini M, Hadchouel M, Davis HL, Whalen RG, Tiollais P, Michel ML. DNA-mediated immunization in a transgenic mouse model of the hepatitis B surface antigen chronic carrier state. Proc Natl Acad Sci U S A. 1996 Oct 29;93(22):12496–12501. doi: 10.1073/pnas.93.22.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu Y, Guidotti LG, Fowler P, Chisari FV. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J Immunol. 1998 Nov 1;161(9):4520–4529. [PubMed] [Google Scholar]
- 11.Akbar SM, Furukawa S, Hasebe A, Horiike N, Michitaka K, Onji M. Production and efficacy of a dendritic cell-based therapeutic vaccine for murine chronic hepatitis B virus carrierer. Int J Mol Med. 2004 Aug;14(2):295–299. [PubMed] [Google Scholar]
- 12.Yalcin K, Danis R, Degertekin H, Alp MN, Tekes S, Budak T. The lack of effect of therapeutic vaccination with a pre-S2/S HBV vaccine in the immune tolerant phase of chronic HBV infection. J Clin Gastroenterol. 2003 Oct;37(4):330–335. doi: 10.1097/00004836-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Yalcin K, Acar M, Degertekin H. Specific hepatitis B vaccine therapy in inactive HBsAg carriers: a randomized controlled trial. Infection. 2003 Aug;31(4):221–225. doi: 10.1007/s15010-003-3187-1. [DOI] [PubMed] [Google Scholar]
- 14.Dikici B, Bosnak M, Ucmak H, Dagli A, Ece A, Haspolat K. Failure of therapeutic vaccination using hepatitis B surface antigen vaccine in the immunotolerant phase of children with chronic hepatitis B infection. J Gastroenterol Hepatol. 2003 Feb;18(2):218–222. doi: 10.1046/j.1440-1746.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Peng Y, Mi M, Guevara-Patino J, Munn DH, Fu N, et al. Lentivector immunization stimulates potent CD8 T cell responses against melanoma self-antigen tyrosinase-related protein 1 and generates antitumor immunity in mice. J Immunol. 2009 May 15;182(10):5960–5969. doi: 10.4049/jimmunol.0900008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esslinger C, Chapatte L, Finke D, Miconnet I, Guillaume P, Levy F, et al. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses. J Clin Invest. 2003 Jun;111(11):1673–1681. doi: 10.1172/JCI17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dullaers M, Van Meirvenne S, Heirman C, Straetman L, Bonehill A, Aerts JL, et al. Induction of effective therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors. Gene Ther. 2006 Apr;13(7):630–640. doi: 10.1038/sj.gt.3302697. [DOI] [PubMed] [Google Scholar]
- 18.Rowe HM, Lopes L, Ikeda Y, Bailey R, Barde I, Zenke M, et al. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol Ther. 2006 Feb;13(2):310–319. doi: 10.1016/j.ymthe.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 19.He Y, Zhang J, Donahue C, Falo LD., Jr Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity. 2006 May;24(5):643–656. doi: 10.1016/j.immuni.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y, Zhang J, Mi Z, Robbins P, Falo LD., Jr Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol. 2005 Mar 15;174(6):3808–3817. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- 21.Furmanov K, Elnekave M, Lehmann D, Clausen BE, Kotton DN, Hovav AH. The role of skin-derived dendritic cells in CD8+ T cell priming following immunization with lentivectors. J Immunol. 2010 May 1;184(9):4889–4897. doi: 10.4049/jimmunol.0903062. [DOI] [PubMed] [Google Scholar]
- 22.Karwacz K, Mukherjee S, Apolonia L, Blundell MP, Bouma G, Escors D, et al. Nonintegrating lentivector vaccines stimulate prolonged T-cell and antibody responses and are effective in tumor therapy. J Virol. 2009 Apr;83(7):3094–3103. doi: 10.1128/JVI.02519-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994 Feb 11;76(3):519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhou S, Ou R, Huang L, Price GE, Moskophidis D. Differential tissue-specific regulation of antiviral CD8+ T-cell immune responses during chronic viral infection. J Virol. 2004 Apr;78(7):3578–3600. doi: 10.1128/JVI.78.7.3578-3600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barchet W, Oehen S, Klenerman P, Wodarz D, Bocharov G, Lloyd AL, et al. Direct quantitation of rapid elimination of viral antigen-positive lymphocytes by antiviral CD8(+) T cells in vivo. Eur J Immunol. 2000 May;30(5):1356–1363. doi: 10.1002/(SICI)1521-4141(200005)30:5<1356::AID-IMMU1356>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 26.You Z, Huang X, Hester J, Toh HC, Chen SY. Targeting dendritic cells to enhance DNA vaccine potency. Cancer research. 2001 May 1;61(9):3704–3711. [PubMed] [Google Scholar]
- 27.Lo KM, Sudo Y, Chen J, Li Y, Lan Y, Kong SM, et al. High level expression and secretion of Fc-X fusion proteins in mammalian cells. Protein Eng. 1998 Jun;11(6):495–500. doi: 10.1093/protein/11.6.495. [DOI] [PubMed] [Google Scholar]
- 28.Schirmbeck R, Bohm W, Ando K, Chisari FV, Reimann J. Nucleic acid vaccination primes hepatitis B virus surface antigen-specific cytotoxic T lymphocytes in nonresponder mice. J Virol. 1995 Oct;69(10):5929–5934. doi: 10.1128/jvi.69.10.5929-5934.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amigorena S. Fc gamma receptors and cross-presentation in dendritic cells. J Exp Med. 2002 Jan 7;195(1):F1–F3. doi: 10.1084/jem.20011925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng BJ, Ng MH, He LF, Yao X, Chan KW, Yuen KY, et al. Therapeutic efficacy of hepatitis B surface antigen-antibodies-recombinant DNA composite in HBsAg transgenic mice. Vaccine. 2001 Jul 20;19(30):4219–4225. doi: 10.1016/s0264-410x(01)00158-x. [DOI] [PubMed] [Google Scholar]
- 31.Yao X, Zheng B, Zhou J, Xu DZ, Zhao K, Sun SH, et al. Therapeutic effect of hepatitis B surface antigen-antibody complex is associated with cytolytic and non-cytolytic immune responses in hepatitis B patients. Vaccine. 2007 Feb 26;25(10):1771–1779. doi: 10.1016/j.vaccine.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 32.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 33.Chisari FV, Klopchin K, Moriyama T, Pasquinelli C, Dunsford HA, Sell S, et al. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell. 1989 Dec 22;59(6):1145–1156. doi: 10.1016/0092-8674(89)90770-8. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Q, Xiao H, Liu Y, Peng Y, Hong Y, Yagita H, et al. Blockade of programmed death-1 pathway rescues the effector function of tumor-infiltrating T cells and enhances the antitumor efficacy of lentivector immunization. J Immunol. 2010 Nov 1;185(9):5082–5092. doi: 10.4049/jimmunol.1001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakimi K, Isogawa M, Chung J, Sette A, Chisari FV. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol. 2002 Sep;76(17):8609–8620. doi: 10.1128/JVI.76.17.8609-8620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hutchings CL, Gilbert SC, Hill AV, Moore AC. Novel protein and poxvirus-based vaccine combinations for simultaneous induction of humoral and cell-mediated immunity. J Immunol. 2005 Jul 1;175(1):599–606. doi: 10.4049/jimmunol.175.1.599. [DOI] [PubMed] [Google Scholar]
- 37.Reyes-del Valle J, Hodge G, McChesney MB, Cattaneo R. Protective anti-hepatitis B virus responses in rhesus monkeys primed with a vectored measles virus and boosted with a single dose of hepatitis B surface antigen. J Virol. 2009 Sep;83(17):9013–9017. doi: 10.1128/JVI.00906-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cobleigh MA, Buonocore L, Uprichard SL, Rose JK, Robek MD. A vesicular stomatitis virusbased hepatitis B virus vaccine vector provides protection against challenge in a single dose. J Virol. 2010 Aug;84(15):7513–7522. doi: 10.1128/JVI.00200-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Y, Munn D, Falo LD., Jr Recombinant lentivector as a genetic immunization vehicle for antitumor immunity. Expert Rev Vaccines. 2007 Dec;6(6):913–924. doi: 10.1586/14760584.6.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chapatte L, Colombetti S, Cerottini JC, Levy F. Efficient induction of tumor antigen-specific CD8+ memory T cells by recombinant lentivectors. Cancer Res. 2006 Jan 15;66(2):1155–1160. doi: 10.1158/0008-5472.CAN-05-2597. [DOI] [PubMed] [Google Scholar]
- 41.Menne S, Tennant BC, Gerin JL, Cote PJ. Chemoimmunotherapy of chronic hepatitis B virus infection in the woodchuck model overcomes immunologic tolerance and restores T-cell responses to pre-S and S regions of the viral envelope protein. J Virol. 2007 Oct;81(19):10614–10624. doi: 10.1128/JVI.00691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]