Abstract
Purpose
To investigate whether the association between physical activity and serum 25-hydroxyvitamin D (25(OH)D) concentrations is independent of sun exposure, body size, and other potential explanatory variables.
Methods
Using data from a sample of 1,343 postmenopausal women, from the Women’s Health Initiative, linear regression was used to examine the associations of duration (minutes/week) of recreational activity and of yard work with 25(OH)D concentrations (nmol/L).
Results
In age-adjusted analyses, positive associations were observed between 25(OH)D concentrations and both duration of recreational physical activity (β=0.71, SE(0.09), P<0.001) and yard work (β=0.36, SE(0.10), P=0.004). After further adjustment for vitamin D intake, self-reported sunlight exposure, waist circumference, and season of blood draw, 25(OH)D was significantly associated with recreational activity (β=0.21, SE(0.09), P=0.014) but not with yard work (β=0.18, SE(0.09), P=0.061). Interactions were observed between season and both recreational activity (Pinteraction=0.082) and yard work (Pinteraction=0.038) such that these activity-25(OH)D associations were greater during summer/fall compared to winter/spring. Self-reported sunlight exposure and measures of body size did not modify the associations.
Conclusion
The observed age-adjusted activity-25(OH)D associations were attenuated after adjusting for explanatory variables and were modified by season of blood draw. Adopting a lifestyle that incorporates outdoor physical activity during summer/fall, consuming recommended amounts of vitamin D, and maintaining a healthy weight may improve or maintain vitamin D status in postmenopausal women.
Keywords: 25-hydroxyvitamin D, vitamin D, serum, sunlight exposure, physical activity, epidemiology, women
INTRODUCTION
The role of vitamin D in the etiology and prevention of chronic disease, such as cancer, osteoporosis, and autoimmune diseases, is of growing interest among healthcare providers and in public health settings (1). Identifying modifiable determinants of vitamin D status, therefore, may have broad population health benefits. Some of the most commonly reported predictors of sufficient 25-hydroxyvitamin D (25(OH)D) status include age, race, dietary and supplemental vitamin D intake, sunlight exposure, body size, and physical activity (2–9).
The observed positive association between physical activity and 25(OH)D status is most likely explained by increased sunlight exposure (1) and decreased body size (10) in more physically active individuals, though few studies have reported data comparing outdoor and indoor activities (11–13). Alternately, it has been hypothesized that the mechanics of physical activity may affect calciotropic hormone levels (reviewed in 14) through increasing post-activity calcium absorption and circulating vitamin D levels (15–16), most likely to help maintain or increase bone mineral density. Additionally, in postmenopausal women, physical activity may increase 25(OH)D concentrations through an IGF-1 mediated process (17–19).
Using data from the Carotenoids in Age-Related Eye Disease Study (CAREDS), an ancillary study of the Women’s Health Initiative Observational Cohort Study (WHI-OS), we investigated whether physical activity among postmenopausal women is associated with serum 25(OH)D concentration, independent of sunlight exposure, body size, or other known factors (e.g., age) associated with both physical activity and vitamin D status . This knowledge can help elucidate the role that promoting physical activity could have in maintaining a healthy vitamin D status (1).
The purpose of this cross-sectional study was to determine if duration (minutes/week) of recreational physical activity and of yard work were associated with serum 25(OH)D concentrations (nmol/L). Additionally, we examined if the association between duration of physical activity and 25(OH)D concentrations was 1) modified by measures of sunlight exposure and body size and 2) explained by other extraneous factors.
MATERIALS AND METHODS
Study sample
The WHI-OS, a prospective cohort study, assessed morbidity and mortality in 93,676 postmenopausal women (50–79 years) recruited from 40 sites throughout the United States (1993–1998) (20–22). CAREDS is an ancillary study of the WHI-OS that examined the association between dietary intake of carotenoids, lutein and zeaxanthin, and the prevalence of age-related eye disease including macular degeneration (23). Participants with baseline carotenoid intake >78th or <28th percentiles were eligible for CAREDS and were recruited (2001–2004) from three WHI-OS centers in Madison, WI, Iowa City, IA, and Portland, OR (n=3,143). Demographic and other health-related data did not differ between the overall sample of women enrolled in WHI-OS and the subsample enrolled in CAREDS (23). The study was approved by the Institutional Review Board at each research center and women provided written informed consent to participate in study activities.
Among women eligible for enrollment in CAREDS, 96 (3.05%) died or were lost to follow-up, leaving 3,047 women who were invited to participate, of whom 1,042 (34.2%) declined. Out of the 2,005 women who agreed to enroll, 1,894 (94.5%) completed the CAREDS study visits and 1,475 had serum 25(OH)D concentrations available. Of these, women with missing data for physical activity (n=20), sunlight exposure (n=37), body mass index (BMI) (n=7), or other covariate data (n=7); and women who self-reported or were missing data on malabsorbtive conditions (e.g., Crohn’s disease) at WHI-OS baseline (n=75), which could affect absorption of vitamin D, were excluded. The final sample for analyses included 1,343 women.
Women who were included in this study (n=1,343) were significantly younger (mean: 62.87 vs. 64.38 years, P<0.05) and expended less energy from yard work (mean: 5.77 vs. 6.97 MET-hours/week, P<0.05) compared to women who were excluded (n=662). No statistically significant differences were found with respect to recreational physical activity, sunlight exposure, body size, education level, clinic center, or hormone use.
Serum vitamin D
A fasting venous blood sample was obtained at WHI-OS baseline and serum was stored at −80°C. The Diasorin LIAISON® chemiluminescence method (Heartland Assays, Inc. (Ames, IA)) was used to assay serum 25(OH)D concentrations. Two percent of the assayed samples had insufficient serum volume for the LIAISON® method and were run using a standard radioimmunoassay (Diasorin, Stillwater, MN). Distributions of 25(OH)D were similar between methods. Assays included blind duplicates for 328 paired participants, which were used to calculate a coefficient of variation of 8.9%. Based on previous studies (9, 24–25), 25(OH)D concentrations of <50, 50–<75, and ≥75 nmol/L were used to define categories of deficiency, insufficiency, and sufficiency, respectively.
Data collection
Physical activity
Usual physical activity, without reference to a specific timeframe, was assessed with a self-administered questionnaire previously shown to be valid and reliable (26–27). Participants reported usual duration (<20, 20–39, 40–59, or >0 minutes/day) and frequency (days/week) for recreational physical activities, including walking, mild (e.g., golf), moderate (e.g., biking), and strenuous (e.g., jogging) physical activity. Weekly duration (minutes/week) of each activity was estimated by multiplying median duration (minutes/day) by frequency (days/week). These values were then summed to compute total weekly recreational activity duration for all activities combined. Location (indoor/outdoor) of recreational activities was not queried.
Duration of yard work (minutes/week) was examined separately from recreational activity since this physical activity was done outdoors and could facilitate sunlight exposure. Yard work was defined as activities that were done in the yard including raking, mowing, shoveling snow, and gardening. Frequency (months/year) and duration (hours/week during the months the participant was engaged in yard work) of yard work were assessed with a self-administered questionnaire. To estimate overall duration (minutes/week) of yard work, the following equation was used: duration (hours/week) × frequency (months/year) × ((60 minutes/hour)/(12 months/year)).
Sunlight exposure
A questionnaire, administered at CAREDS baseline (2001–2004), was used to collect information about the time (<1, 1–3, or >3 hours per day) spent in direct sunlight during peak daily sun times (10 AM–4 PM) on weekdays and weekends from April to September for each location in which the participant lived since the age of 18. From this information, a retrospective estimate of sunlight exposure at the time of baseline enrollment in WHI-OS (1993–1998), concurrent to serum 25(OH)D assessment, was obtained. Additionally, season of blood draw (summer/fall: June–November, winter/spring: December-May; hereafter referred to as season) at WHI-OS baseline was examined in this study as a proxy measure of the availability of solar radiation (28).
Body size measures
Height (m) was measured with a stadiometer and weight (kg) with a calibrated balance beam or digital scale and from these measures BMI (kg/m2) was calculated (20) and categorized as underweight/normal (<24.99 kg/m2), overweight (25.00–29.99 kg/m2), and obese (≥30.00 kg/m2) (29). Waist circumference was measured with a tape measure, halfway between the iliac crest and the bottom of the rib cage, to the nearest 0.1 cm (30) and was dichotomized using a standard clinical cutoff (≤88 and >88 cm) (20, 31).
Other potential confounders
Total vitamin D intake (mcg) included vitamin D intake from diet and supplements, estimated from the validated WHI food frequency questionnaire and supplement use questionnaire, respectively (32–34). Self-reported age, race/ethnicity, education, smoking, alcohol use, and use of hormone therapy also were obtained at WHI-OS baseline (20).
Statistical analysis
Descriptive statistics were computed for 25(OH)D concentrations and duration of physical activity, and Spearman correlations were computed to examine the crude activity-25(OH)D associations. Differences in mean 25(OH)D concentrations and duration of recreational physical activity by demographic and lifestyle characteristics were examined using analysis of variance.
Multiple linear regression was used to examine the activity-25(OH)D associations with recreational physical activity and yard work, separately, while controlling for the effect of potential confounding factors. Since the physical activity variables were not normally distributed, a square root transformation was applied prior to analysis. Regression coefficients were considered significant at P<0.05. Measures of sunlight exposure (self-reported sunlight exposure and season) and body size (BMI and waist circumference) were examined as potential effect modifiers of the activity-25(OH)D association and interaction product terms were considered significant if P<0.10.
All models were adjusted minimally for age and further adjusted for potential confounding factors that were significantly related in univariate analyses to both 25(OH)D and duration of physical activity, and that changed the regression coefficient for physical activity by 10% or more when added to the linear regression model. Vitamin D intake, sunlight exposure, season, waist circumference, and BMI met these criteria.
Waist circumference and BMI were strongly correlated (r=0.82), thus we chose not to include them both simultaneously in regression analyses. When examined as potential confounding factors, the percent change in the regression coefficient for physical activity was slightly greater for waist circumference than BMI and thus we chose to only adjust for waist circumference. When primary analyses were repeated with BMI instead, the findings were materially the same as those seen for waist circumference.
All statistical analyses were performed with SAS® version 9.2 (35).
RESULTS
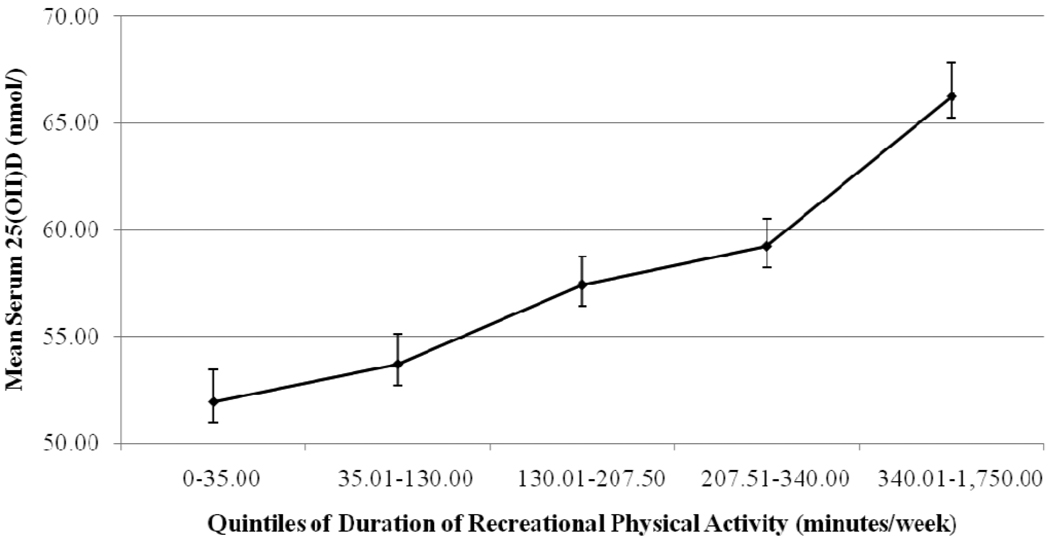
The average 25(OH)D concentration among all participants was 57.7 nmol/L (SE=0.65), with 39.24% of the sample vitamin D deficient (<50 nmol/L), 39.99% insufficient (50–75 nmol/L) and 20.77% sufficient (≥75 nmol/L). The average weekly duration of recreational physical activity was 207.77 minutes/week (SE=5.26) and yard work was 86.48 minutes/week (SE=3.47). Recreational physical activity (r=0.21, P<0.001) and yard work (r=0.10, P=0.004) were each correlated with 25(OH)D and were correlated with each other (r=0.14, P<0.001). The prevalences of inactivity (0 minutes/week) were 11.43% for recreational physical activity and 35.82% for yard work. Figure 1 illustrates the significant trend for higher 25(OH)D across incremental recreational physical activity quintile medians (P<0.001). A similar trend was observed for 25(OH)D and yard work (not shown).
Figure 1. Mean (SE) Serum 25(OH)D (nmol/L) by Quintiles of Duration of Recreational Physical Activity (minutes/week).
Mean serum 25-hydroxyvitamin D (25(OH)D) concentrations and standard errors are plotted for postmenopausal women in each quintile of duration of recreational physical activity: the Carotenoids in Age-Related Eye Disease Study (CAREDS), N=1,343. Test of linear trend across quintile medians, P<0.001.
Average 25(OH)D concentrations were higher among women who resided in Wisconsin, were of Caucasian race/ethnicity, had more years of education, drank alcohol more frequently, consumed more vitamin D from foods and supplements, were leaner, spent more time in the sun, and had their blood draw in the summer/fall (Table 1). Average duration of recreational physical activity was also greater among women with the same characteristics; although no differences were observed in duration of activity and racial/ethnic group, and duration of activity was lowest among current smokers.
Table 1.
Mean values of serum 25 (OH)D concentrations (nmol/L) and recreational physical activity duration (minutes/week) for postmenopausal women in the Carotenoids in Age-Related Eye Disease Study (CAREDS) by WHI-OS baseline (1993–1998) characteristics, N=1,343.
| Characteristics: | n | Serum 25(OH)D (nmol/L) Mean (SE) |
P-value* | Recreational Physical Activity (min/week) Mean (SE) |
P-value* |
|---|---|---|---|---|---|
| Age | 0.231 | 0.446 | |||
| 50–59 | 445 | 59.3 (1.14) | 210.30 (9.24) | ||
| 60–69 | 618 | 56.7 (0.98) | 202.11 (7.31) | ||
| 70–79 | 280 | 57.5 (1.36) | 216.22 (12.70) | ||
| Clinic Center | 0.010 | <0.001 | |||
| Iowa | 433 | 56.5 (1.10) | 172.26 (7.81) | ||
| Oregon | 439 | 56.0 (1.16) | 199.99 (8.90) | ||
| Wisconsin | 471 | 60.4 (1.12) | 247.65 (9.95) | ||
| Race/Ethnicity | 0.020 | 0.793 | |||
| Caucasian | 1,311 | 58.0 (0.66) | 207.40 (5.27) | ||
| Non-Caucasian | 32 | 48.0 (3.59) | 222.73 (47.43) | ||
| Education (highest level completed) | 0.016 | <0.001 | |||
| High school diploma/GED | 313 | 54.9 (1.36) | 169.15 (9.87) | ||
| Vocational training/some college | 505 | 57.4 (1.04) | 199.11 (8.45) | ||
| College degree | 133 | 57.1 (1.90) | 217.59 (16.66) | ||
| Post-graduate coursework/degree | 392 | 60.6 (1.27) | 246.42 (10.29) | ||
| Smoking Status | 0.58 | 0.029 | |||
| Never Smoked | 769 | 58.3 (0.87) | 200.52 (7.14) | ||
| Past Smoker | 529 | 56.5 (1.03) | 222.48 (8.12) | ||
| Current Smoker | 45 | 57.0 (3.96) | 158.72 (23.77) | ||
| Alcohol Intake | <0.001 | <0.001 | |||
| Nondrinker | 141 | 54.3 (1.92) | 197.54 (19.49) | ||
| Past drinker | 224 | 57.4 (1.60) | 189.80 (11.85) | ||
| <1 drink per month | 175 | 52.8 (1.66) | 167.80 (12.60) | ||
| <1 drinker per week | 282 | 56.2 (1.36) | 192.11 (10.49) | ||
| 1 to <7 drinks per week | 378 | 60.0 (1.26) | 241.43 (10.65) | ||
| 7+ drinks per week | 143 | 64.6 (2.16) | 236.80 (15.20) | ||
| Hormone Use Status: | 0.082 | 0.270 | |||
| Never Used | 443 | 55.9 (1.17) | 199.03 (8.98) | ||
| Past User | 183 | 57.0 (1.75) | 197.75 (14.13) | ||
| Current User | 717 | 59.1 (0.88) | 215.72 (7.30) | ||
| Vitamin D Intake from foods | |||||
| and supplements (mcg/day) | <0.001 | <0.001 | |||
| 0.35–4.55 | 268 | 46.9 (1.45) | 179.92 (11.16) | ||
| 4.56–9.52 | 269 | 53.1 (1.32) | 185.44 (10.59) | ||
| 9.53–13.94 | 269 | 58.2 (1.41) | 198.52 (9.82) | ||
| 13.95–17.89 | 269 | 62.3 (1.39) | 221.11 (13.00) | ||
| 17.90–61.48 | 268 | 68.3 (1.39) | 253.92 (13.38) | ||
| BMI (kg/m2) | <0.001 | <0.001 | |||
| Underweight/Normal (<24.99) | 492 | 65.4 (1.17) | 256.09 (9.65) | ||
| Overweight (25.00–29.99) | 487 | 55.9 (0.97) | 199.79 (8.08) | ||
| Obese (≥ 30.00) | 364 | 49.8 (1.11) | 153.13 (8.56) | ||
| Waist Circumference (cm) | <0.001 | <0.001 | |||
| ≤88 | 902 | 61.5 (0.81) | 236.73 (6.78) | ||
| >88 | 441 | 50.1 (1.00) | 148.52 (7.26) | ||
|
Self-reported sunlight exposure (hrs/week) † |
<0.001 | <0.001 | |||
| < 1 | 503 | 54.3 (1.00) | 179.20 (7.71) | ||
| 1–3 | 702 | 59.0 (0.92) | 212.35 (7.12) | ||
| > 3 | 138 | 63.5 (2.16) | 288.57 (21.34) | ||
| Season of blood draw‡ | <0.001 | 0.023 | |||
| Summer/Fall | 750 | 63.0 (0.86) | 227.75 (7.61) | ||
| Winter/Spring | 593 | 51.0 (0.93) | 182.50 (6.89) |
Analysis of variance (ANOVA) was used to compute P-values for which statistical significance was set at an alpha level of 0.20.
Sunlight exposure at WHI-OS baseline (1993–1998) was retrospectively estimated at CAREDS baseline (2001–2004).
Summer/Fall: June–November; Winter/Spring: December-May
In age-adjusted linear regression analyses, both recreational physical activity and yard work were positively associated (Ps<0.01) with 25(OH)D concentrations (Table 2). These associations were attenuated with the addition of each covariate (vitamin D intake, self-reported sunlight exposure and waist circumference), with waist circumference having the largest effect on the regression coefficient. The activity-25(OH)D association remained significant for recreational physical activity (P=0.002) but was no longer significant for yard work (P=0.298). Additional adjustment for season further attenuated the relationship with recreational activity and slightly strengthened the association with yard work.
Table 2.
Regression coefficient (β), standard error (SE) and p-value for physical activity from linear regression analysis of physical activity (minutes/week) on serum 25(OH)D (nmol/L) adjusted* for various covariates: the Carotenoids in Age-Related Eye Disease Study (CAREDS), N=1,343.
| Independent Variable | β (SE) | P-value |
|---|---|---|
| Recreational Physical Activity (min/week) | ||
| Age-adjusted | 0.71 (0.09) | <0.001 |
| Adjusted for age + vitamin D intake | 0.57 (0.09) | <0.001 |
| Adjusted for age + vitamin D intake + self-reported sunlight exposure | 0.52 (0.09) | <0.001 |
| Adjusted for age + vitamin D intake + self-reported sunlight exposure + waist circumference | 0.28 (0.09) | 0.002 |
| Adjusted for age + vitamin D intake + self-reported sunlight exposure + waist circumference + season | 0.21 (0.09) | 0.014 |
| Yard Work (min/week) | ||
| Age-adjusted | 0.36 (0.10) | 0.004 |
| Adjusted for age + vitamin D intake | 0.33 (0.10) | 0.006 |
| Adjusted for age + vitamin D intake + self-reported sunlight exposure | 0.23 (0.10) | 0.020 |
| Adjusted for age + vitamin D intake + self-reported sunlight exposure + waist circumference | 0.10 (0.10) | 0.298 |
| Adjusted for age + vitamin D intake + self-reported sunlight exposure + waist circumference + season | 0.18 (0.09) | 0.061 |
The following variables were added to the linear regression models: age (years), vitamin D intake from food and supplements combined (mcg/day), self-reported sunlight exposure ((<1, 1–3, or >3 hours per day), waist circumference (cm), and season (summer/fall [June–November] and winter/spring [December-May]).
Table 3 shows the activity-25(OH)D associations stratified by measures of sunlight exposure and body size. Season significantly modified the age-adjusted associations of serum 25(OH)D with both recreational physical activity (P for interaction=0.082) and yard work (P for interaction=0.038). The age-adjusted associations of recreational physical activity with serum 25(OH)D were significant in each season, but were greatest during the summer/fall months when ultraviolet B (UVB) exposure is greatest in Northern latitudes. The age-adjusted associations between yard work and serum 25(OH)D were significant during summer/fall but not during winter/spring. After adjusting the season-stratified models for additional covariates, the regression coefficients for recreational physical activity and yard work remained significant during the summer/fall, but not during the winter/spring. The associations between 25(OH)D and physical activity were not significantly modified by self-reported sunlight exposure or waist circumference (Ps>0.10).
Table 3.
Regression coefficient (β), standard error (SE) and p-value for physical activity from linear regression of physical activity minutes/week) on serum 25(OH)D (nmol/L) stratified by measures of sunlight exposure and body size: the Carotenoids in Age-Related Eye Disease Study (CAREDS), N=1,343.
| Independent Variable | Age-Adjusted Models | Multivariate Model† | |||
|---|---|---|---|---|---|
| n | β (SE) | P-value | β (SE) | P-value | |
| Recreational Physical Activity (min/week) | |||||
| Season | |||||
| Summer/Fall | 750 | 0.75 (0.11) | <0.001 | 0.32 (0.11) | 0.005 |
| Winter/Spring | 593 | 0.44 (0.14) | 0.001 | 0.06 (0.13) | 0.640 |
| P-value for interaction | 0.082 | 0.100 | |||
| Self-reported Sunlight Exposure (hrs/week) | |||||
| < 1 | 503 | 0.58 (0.14) | <0.001 | 0.18 (0.13) | 0.162 |
| 1–3 | 702 | 0.76 (0.13) | <0.001 | 0.29 (0.13) | 0.021 |
| > 3 | 138 | 0.46 (0.27) | 0.088 | −0.05 (0.27) | 0.865 |
| P-value for interaction | 0.541 | 0.627 | |||
| Waist circumference (cm) | |||||
| ≤ 88 | 902 | 0.55 (0.11) | <0.001 | 0.27 (0.11) | 0.012 |
| > 88 | 441 | 0.65 (0.15) | <0.001 | 0.26 (0.14) | 0.067 |
| P-value for interaction | 0.653 | 0.999 | |||
| Yard work (min/week) | |||||
| Season | |||||
| Summer/Fall | 750 | 0.61 (0.13) | <0.001 | 0.35 (0.13) | 0.006 |
| Winter/Spring | 593 | 0.20 (0.14) | 0.169 | −0.06 (0.14) | 0.663 |
| P-value for interaction | 0.038 | 0.019 | |||
| Self-reported Sunlight Exposure (hrs/week) | |||||
| < 1 | 503 | 0.31 (0.18) | 0.087 | 0.26 (0.16) | 0.098 |
| 1–3 | 702 | 0.23 (0.15) | 0.116 | 0.10 (0.13) | 0.427 |
| > 3 | 138 | 0.26 (0.27) | 0.343 | 0.19 (0.26) | 0.480 |
| P-value for interaction | 0.934 | 0.737 | |||
| Waist circumference (cm) | |||||
| ≤ 88 | 902 | 0.26 (0.12) | 0.038 | 0.30 (0.11) | 0.009 |
| > 88 | 441 | 0.34 (0.17) | 0.040 | 0.18 (0.15) | 0.223 |
| P-value for interaction | 0.725 | 0.960 | |||
Adjusted age (years), vitamin D intake from food and supplements combined (mcg/day), self-reported sunlight exposure (<1, 1–3, or >3 hours per day), waist circumference (cm), and season (summer/fall [June–November] and winter/spring [December-May]). Analyses stratified by self-reported sunlight exposure were not adjusted for self-reported sunlight exposure.
DISCUSSION
Similar to previous studies (5–6, 8–9, 11–12, 16, 36–49), we found positive activity-25(OH)D associations with recreational physical activity and yard work. Differences in vitamin D intake, self-reported sunlight exposure, waist circumference, and season accounted for much of the observed activity-25(OH)D associations. Further adjustment of age-adjusted activity-25(OH)D associations for these covariates reduced the magnitude of associations by at least 50%.
A stronger activity-25(OH)D relationship was observed with recreational physical activity than yard work. One would hypothesize that the association between 25(OH)D and physical activity would be strongest in an activity (yard work) conducted exclusively outside. It is possible that unmeasured behaviors might have confounded the association with yard work. Women may have worn more clothing when they participated in yard work (subsequently minimizing their dermal vitamin D production) than when they participated in recreational physical activity. However, we could not examine this further because data regarding the amount of clothing worn during physical activity was not collected in this study. Alternatively, the weaker association with yard work may have been explained by a narrower distribution of time spent in activity among women conducting yard work (~7 minutes/day to a little over 1 ½ hours per day) than among those engaged in recreational physical activity (~10 minutes/day to ~ 4 hours per day).
Similar to previous studies (11, 38, 47), season modified the activity-25(OH)D associations such that the associations were only significant for summer/fall. All participants of this study resided above 40°N, a latitude at which the amount of UVB photons reaching the earth from November through February (50) is limited. If physical activity was associated with 25(OH)D concentrations independent of sunlight exposure one would expect to also observe an association during winter/spring, the time of year when dermal production of 25(OH)D is limited by reduced exposure to UVB (50). Thus, the observed activity-25(OH)D associations largely reflect the effect of sunlight exposure during outdoor physical activity. We found no evidence to suggest that physical activity, independent of the other factors accounted for in this study, contributes to variation in 25(OH)D concentrations.
This is one of the first studies to specifically investigate the activity-25(OH)D association in postmenopausal women, while also considering the impact of potential explanatory factors of this association. The majority of previous studies has only presented physical activity as a determinant of 25(OH) D status (5–6, 8–9, 12, 37–44, 46–49) and did not examine their association in more depth. However, using data from the Third National Health and Nutrition Examination Survey (NHANES III), Scragg et al. (11) found a positive association between the self-reported frequency of outdoor, but not indoor, leisure activities during the past month and 25(OH)D, even after adjustment for age, sex, race/ethnicity, BMI, month of year, and vitamin D intake from food and supplements. When stratified by month of examination, stronger associations were seen between outdoor activity and serum 25(OH)D during the summer/fall compared to winter/spring. The results of the present study in older women are consistent with those reported by Scragg et al., suggesting that seasonal variation in sunlight exposure likely explains a large amount of the activity-25(OH)D association.
Several aspects of our study expand on the findings reported by Scragg et al. (11). First, Scragg et al.’s activity questionnaire did not allow for quantifying the dose of activity because only frequency, and not duration, of activities done in the past month was measured. Our activity exposure is based both on frequency and on duration, which likely results in a better assessment of activity dose. Second, NHANES III’s activity questionnaire did not assess the location of activities. Instead, the investigators classified self-reported activities as indoor or outdoor based on their judgment of the likely location of the activity, leading to potential misclassification of activity location. Although our study also did not assess specific location for recreational physical activity, we additionally queried about yard work which allowed us to examine the effect of a known outdoor activity on 25(OH)D. Third, individual level information on sunlight exposure was not available in the study by Scragg et al. Instead, the investigators used month of clinical examination as a proxy measure for sunlight exposure, which was further complicated by NHANES collecting data (e.g., blood draws) at northern latitudes in the summer/fall and at southern latitudes in the winter/spring. In our study, self-reported sunlight exposure was assessed and all women resided at a similar latitude (>40°N). These data, together with season of blood draw, provides a more complete assessment of overall sunlight UVB exposure than seasonality alone. However, we found that self-reported sunlight exposure did not modify any of the activity-25(OH)D associations, and only partially explained the observed associations after adjustment in the multivariate models.
Body size appears to be a relevant confounder of the observed association because body size tends to be inversely related to physical activity levels (10) and inversely related to 25(OH)D levels (1); the latter most likely due to the sequestration of vitamin D in fat tissue (51). This association was attenuated by 48–57% when waist circumference was added to multivariate models. It appears that regardless of body size, higher physical activity levels are associated with higher serum 25(OH)D concentrations.
There are several limitations to this study. Our measures of physical activity and sunlight exposure were subjective and retrospective, and thus might have resulted in measurement error. Participants were asked to provide an estimate of their usual recreational physical activity and yard work habits; however a specific recall timeframe or season was not delineated. Other than yard work, we could not distinguish between indoor and outdoor activity because location was not assessed. The physical activity questionnaire did not provide sufficient detail to differentiate between activities according to weight-bearing characteristics and this information could be relevant for examining how activity, related specifically to increasing bone health, affects 25(OH)D concentrations.
Self-reported sunlight exposure might not have been observed as an effect modifier or a stronger confounder, because of measurement error in sunlight exposure assessment. On CAREDS’ baseline (2001–2004) questionnaires, women retrospectively estimated their average sunlight exposure during WHI-OS baseline (1993–1998). Changes in sunlight exposure behaviors from WHI-OS baseline to CAREDS baseline may have affected the accuracy of women’s recall. Furthermore, the questionnaire required women to report a single estimate of their activities and behaviors which contributed to sunlight exposure for each location in which they lived since they were 18 and this estimate would not have captured any variation in sunlight exposure for the duration of time at each location.
The women in our study have similar demographics, medical characteristics, physical activity levels and serum 25(OH)D as other women in the WHI-OS. Notably, women who participated in WHI-OS were highly educated volunteers and were likely healthier than the general population. Additionally, compared to national estimates, our participants were more active than women of similar age (52). Greater than 30% of women 50 years and above reported being inactive from 1993–1998 according to the Behavioral Risk Factor Surveillance System survey data (52), whereas only about 12% of our sample was inactive. The relatively low prevalence of inactivity in our study sample of older women may reflect greater measurement error using questionnaire-based activity assessment in our study than in national surveillance programs, or it may further indicate that our study sample tended to be more health conscious than women of similar age in the overall population.
In conclusion, a better understanding of the activity-25(OH)D association could have important public health implications. Research shows that physical activity, vitamin D intake, sunlight exposure, and decreased body size are modifiable predictors of a healthy 25(OH)D status (1, 9). Therefore, incorporating these factors into one’s lifestyle could help to maintain a healthy 25(OH)D status which has been associated with reduced risk for disease (e.g., cancer, osteoporosis) (1). Our findings emphasize the relevance of these issues among a subgroup of postmenopausal women in whom levels of physical activity and serum vitamin D tend to be lower than recommended.
ACKNOWLEDGEMENTS
We thank the women who generously contributed their time to participate in the CAREDS.
WHI Investigators
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Haleh Sangi-Haghpeykar; (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence S. Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Erin LeBlanc; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael S. Simon.
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Amy E. Millen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
SOURCES OF SUPPORT
This research was supported by grants EY13018 and EY016886 from the National Institutes of Health and by Research to Prevent Blindness. It was part of the Carotenoids and Age-Related Eye Disease Study (CAREDS), an ancillary study of the Women’s Health Initiative (WHI).
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
LIST OF ABBREVIATIONS
- 25(OH)D
25-hydroxvitamin D
- CAREDS
Carotenoids in Age-Related Eye Disease Study
- BMI
body mass index
- NHANES III
Third National Health and Nutrition Examination Survey
- UVB
ultraviolet B
- WHI-OS
Women’s Health Initiative Observational Cohort Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: None;
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 3.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macdonald HM, Mavroeidi A, Barr RJ, Black AJ, Fraser WD, Reid DM. Vitamin D status in postmenopausal women living at higher latitudes in the UK in relation to bone health, overweight, sunlight exposure and dietary vitamin D. Bone. 2008;42:996–1003. doi: 10.1016/j.bone.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Liu Y, Rimm EB, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 7.Rizzoli R, Eisman JA, Norquist J, Ljunggren O, Krishnarajah G, Lim SK, et al. Risk factors for vitamin D inadequacy among women with osteoporosis: an international epidemiological study. Int J Clin Pract. 2006;60:1013–1019. doi: 10.1111/j.1742-1241.2006.01066.x. [DOI] [PubMed] [Google Scholar]
- 8.Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93:3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millen AE, Wactawski-Wende J, Pettinger M, Melamed ML, Tylavsky FA, Liu S, et al. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women's Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr. 2010;91:1324–1335. doi: 10.3945/ajcn.2009.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brock DW, Thomas O, Cowan CD, Allison DB, Gaesser GA, Hunter GR. Association between insufficiently physically active and the prevalence of obesity in the United States. J Phys Act Health. 2009;6:1–5. doi: 10.1123/jpah.6.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scragg R, Camargo CA., Jr Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2008;168:577–586. doi: 10.1093/aje/kwn163. discussion 587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florez H, Martinez R, Chacra W, Strickman-Stein N, Levis S. Outdoor exercise reduces the risk of hypovitaminosis D in the obese. J Steroid Biochem Mol Biol. 2007;103:679–681. doi: 10.1016/j.jsbmb.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E. Serum 25-hydroxyvitamin D3 is related to physical activity and ethnicity but not obesity in a multicultural workforce. Aust N Z J Med. 1995;25:218–223. doi: 10.1111/j.1445-5994.1995.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 14.Maimoun L, Sultan C. Effect of physical activity on calcium homeostasis and calciotropic hormones: a review. Calcif Tissue Int. 2009;85:277–286. doi: 10.1007/s00223-009-9277-z. [DOI] [PubMed] [Google Scholar]
- 15.Yeh JK, Aloia JF, Yasumura S. Effect of physical activity on calcium and phosphorus metabolism in the rat. Am J Physiol. 1989;256:E1–E6. doi: 10.1152/ajpendo.1989.256.1.E1. [DOI] [PubMed] [Google Scholar]
- 16.Zittermann A, Sabatschus O, Jantzen S, Platen P, Danz A, Dimitriou T, et al. Exercise-trained young men have higher calcium absorption rates and plasma calcitriol levels compared with age-matched sedentary controls. Calcif Tissue Int. 2000;67:215–219. doi: 10.1007/s002230001132. [DOI] [PubMed] [Google Scholar]
- 17.Barnes BB, Chang-Claude J, Flesch-Janys D, Kinscherf R, Schmidt M, Slanger T, et al. Cancer risk factors associated with insulin-like growth factor (IGF)-I and IGF-binding protein-3 levels in healthy women: effect modification by menopausal status. Cancer Causes Control. 2009 doi: 10.1007/s10552-009-9393-0. [DOI] [PubMed] [Google Scholar]
- 18.Gomez JM. The role of insulin-like growth factor I components in the regulation of vitamin D. Curr Pharm Biotechnol. 2006;7:125–132. doi: 10.2174/138920106776597621. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad AM, Thomas J, Clewes A, Hopkins MT, Guzder R, Ibrahim H, et al. Effects of growth hormone replacement on parathyroid hormone sensitivity and bone mineral metabolism. J Clin Endocrinol Metab. 2003;88:2860–2868. doi: 10.1210/jc.2002-021787. [DOI] [PubMed] [Google Scholar]
- 20.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 21.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 22.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 23.Mares JA, LaRowe TL, Snodderly DM, Moeller SM, Gruber MJ, Klein ML, et al. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women's Health Initiative. Am J Clin Nutr. 2006;84:1107–1122. doi: 10.1093/ajcn/84.5.1107. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF. Optimal vitamin D status for the prevention and treatment of osteoporosis. Drugs Aging. 2007;24:1017–1029. doi: 10.2165/00002512-200724120-00005. [DOI] [PubMed] [Google Scholar]
- 25.Hollis BW, Wagner CL, Drezner MK, Binkley NC. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J Steroid Biochem Mol Biol. 2007;103:631–634. doi: 10.1016/j.jsbmb.2006.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test-retest reliability of the Women's Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41:530–538. doi: 10.1249/MSS.0b013e31818ace55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettee Gabriel K, McClain JJ, Lee CD, Swan PD, Alvar BA, Mitros MR, et al. Evaluation of physical activity measures used in middle-aged women. Med Sci Sports Exerc. 2009;41:1403–1412. doi: 10.1249/MSS.0b013e31819b2482. [DOI] [PubMed] [Google Scholar]
- 28.NOAA. What causes the seasons? [Accessed January 8, 2010]; http://www.crh.noaa.gov/fsd/astro/season.php.
- 29.WHO. Report of a WHO consultation. Geneva: 2000. Obesity: Preventing and managing the global epidemic. [PubMed] [Google Scholar]
- 30.Lohman TG, Roche A, R M, editors. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Publications; 1988. [Google Scholar]
- 31.National Heart L, and Blood Institute Expert Panel. Clinical Guidelines on the Identification, Evaulation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. 1998 [Google Scholar]
- 32.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 33.Patterson RE, Kristal AR, Levy L, McLerran D, White E. Validity of methods used to assess vitamin and mineral supplement use. Am J Epidemiol. 1998;148:643–649. doi: 10.1093/aje/148.7.643. [DOI] [PubMed] [Google Scholar]
- 34.Patterson RE, Levy L, Tinker LF, Kristal AR. Evaluation of a simplified vitamin supplement inventory developed for the Women's Health Initiative. Public Health Nutr. 1999;2:273–276. doi: 10.1017/s1368980099000361. [DOI] [PubMed] [Google Scholar]
- 35.Statistical Analysis Software (SAS) 2009 [Google Scholar]
- 36.Bell NH, Godsen RN, Henry DP, Shary J, Epstein S. The effects of muscle-building exercise on vitamin D and mineral metabolism. J Bone Miner Res. 1988;3:369–373. doi: 10.1002/jbmr.5650030402. [DOI] [PubMed] [Google Scholar]
- 37.Arguelles LM, Langman CB, Ariza AJ, Ali FN, Dilley K, Price H, et al. Heritability and environmental factors affecting vitamin D status in rural Chinese adolescent Twins. J Clin Endocrinol Metab. 2009 doi: 10.1210/jc.2008-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brock KE, Graubard BI, Fraser DR, Weinstein SJ, Stolzenberg-Solomon RZ, Lim U, et al. Predictors of vitamin D biochemical status in a large sample of middle-aged male smokers in Finland. Eur J Clin Nutr. 2010 doi: 10.1038/ejcn.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foo LH, Zhang Q, Zhu K, Ma G, Trube A, Greenfield H, et al. Relationship between vitamin D status, body composition and physical exercise of adolescent girls in Beijing. Osteoporos Int. 2009;20:417–425. doi: 10.1007/s00198-008-0667-2. [DOI] [PubMed] [Google Scholar]
- 40.Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62:1079–1089. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 41.Jones G, Dwyer T, Hynes KL, Parameswaran V, Greenaway TM. Vitamin D insufficiency in adolescent males in Southern Tasmania: prevalence, determinants, and relationship to bone turnover markers. Osteoporos Int. 2005;16:636–641. doi: 10.1007/s00198-004-1733-z. [DOI] [PubMed] [Google Scholar]
- 42.Lucas JA, Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, et al. Determinants of vitamin D status in older women living in a subtropical climate. Osteoporos Int. 2005;16:1641–1648. doi: 10.1007/s00198-005-1888-2. [DOI] [PubMed] [Google Scholar]
- 43.Lym YL, Joh HK. Serum 25-hydroxyvitamin D3 is related to fish intake and exercise in Korean adult men. Asia Pac J Clin Nutr. 2009;18:372–376. [PubMed] [Google Scholar]
- 44.Neuhouser ML, Sorensen B, Hollis BW, Ambs A, Ulrich CM, McTiernan A, et al. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am J Clin Nutr. 2008;88:133–139. doi: 10.1093/ajcn/88.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohta H, Kuroda T, Onoe Y, Orito S, Ohara M, Kume M, et al. The impact of lifestyle factors on serum 25-hydroxyvitamin D levels: a cross-sectional study in Japanese women aged 19–25 years. J Bone Miner Metab. 2009;27:682–688. doi: 10.1007/s00774-009-0095-1. [DOI] [PubMed] [Google Scholar]
- 46.Pasco JA, Henry MJ, Nicholson GC, Brennan SL, Kotowicz MA. Behavioural and physical characteristics associated with vitamin D status in women. Bone. 2009;44:1085–1091. doi: 10.1016/j.bone.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 47.Scragg R, Holdaway I, Jackson R, Lim T. Plasma 25-hydroxyvitamin D3 and its relation to physical activity and other heart disease risk factors in the general population. Ann Epidemiol. 1992;2:697–703. doi: 10.1016/1047-2797(92)90014-h. [DOI] [PubMed] [Google Scholar]
- 48.Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E. Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract. 1995;27:181–188. doi: 10.1016/0168-8227(95)01040-k. [DOI] [PubMed] [Google Scholar]
- 49.Tseng M, Giri V, Bruner DW, Giovannucci E. Prevalence and correlates of vitamin D status in African American men. BMC Public Health. 2009;9:191. doi: 10.1186/1471-2458-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 51.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 52.Ham SA, Yore MM, Fulton JE, Kohl HW. Prevalence of no leisure time physical activity - 35 states and the Disctirct of Columbia, 1988–2002. Morbidity and Mortality Weekly Report. 2004;53:82–86. [PubMed] [Google Scholar]