Abstract
Over the last two decades, consequences of HIV infection of the CNS on disease severity and clinical neuropsychiatric manifestations have changed. These changes are due, in part, to improved control of peripheral infection by new anti-retroviral medications and more efficient CNS penetration of combination anti-retroviral therapies (cART). While the life spans of HIV-infected patients have been prolonged with successful cART, the spectrum of cognitive alterations observed in these patients has broadened. Recent studies report that there does not appear to be a single prototypical pattern of neuropsychological impairment associated with HIV, but rather it includes diverse manifestations. Some co-morbidities such as substance abuse or depression, likely play significant roles in the neuropsychiatric profiles of some HIV-infected patients. Newly recognized factors contributing to neurocognitive impairments include ageing and unanticipated side effects from cART. Likewise, disturbances in neuroendocrine functioning are emerging as potentially important contributors to HIV-associated neurocognitive alterations. A retrospective review of clinical data from a small cohort of HIV-infected patients admitted to the psychiatric unit of an inner city hospital indicates that thyroid stimulating hormone levels were abnormal in 27% of the patients. Our data from analyses of post-mortem tissues from HIV patients show for the first time HIV infection of the hypothalamus and altered levels of thyroid hormone processing enzymes. Decreased vasopressin and oxytocin immunoreactivity in hypothalamic neurons was also observed. Thus, HIV infection of the CNS may contribute to changes in hypothalamic hormone signaling, thereby resulting in abnormal hypothalamic-pituitary-thyroid axis feedback and neuropsychiatric dysfunction.
Introduction
Over the past two decades, significant advances in understanding the etiology, pathophysiology, and treatment of HIV-related cognitive alterations have resulted in a large preclinical and clinical body of research. In this context, the development of highly active anti-retroviral therapy (HAART) has altered the neuropathological profiles of HIV-associated CNS disease (Langford et al., 2003; Vallat-Decouvelaere et al., 2003) since systemic viral burdens are largely controlled in adherent HIV patients. Likewise, the evolution of more efficient CNS-penetrating combination anti-retroviral therapy (cART) has contributed in part to changing CNS-related HIV disorders. Numerous emerging co-morbid conditions such as substance abuse, ageing, and even cART-related disturbances pose new challenges to healthcare providers treating HIV patients.
Disturbances in the hypothalamic-pituitary-thyroid (HPT) axis have emerged as a complication among some individuals with HIV infection and/or substance abuse (SA) (Zirilli et al., 2008). HPT dysfunction in the absence of HIV infection or SA commonly causes mood disorders, depression and even dementia. In the HAART-era, depression, mild to moderate cognitive alterations, and in some cases, dementia are continuing observations in a significant proportion of HIV patients. Substance abuse increases not only the risk of becoming infected by HIV, but also exacerbates cognitive dysfunctions leading to loss of independence and decreased quality of life. Strong evidence suggesting roles of HIV and SA in HPT dysregulation in significantly large patient populations warrants investigation into this emerging syndrome. Several studies suggest a link between SA and HPT dysfunction (Teoh et al., 1993; Budziszewska et al., 1996; Vescovi and Pezzarossa, 1999). Likewise, more recent studies report links between HIV infection and HPT dysfunction (Beltran et al., 2003; Kumar et al., 2003; Wiener et al., 2008; Zirilli et al., 2008) (Wilson et al., 1996) and some even recommend thyroid function screening in all HIV patients, especially those on HAART (Bongiovanni et al., 2006; Madeddu et al., 2006). Some studies however, report no association between HIV and thyroid function (Madge et al., 2007). Despite the extensive extant literature on CNS involvement seen with HIV infection, few studies have investigated the role of the HPT neuroendocrine feed back loop. Based on our clinical observation of unexplained hypothyroidism in a cohort of substance abusing, HIV positive psychiatric inpatients and neuropathological examination of post-mortem hypothalamic tissues from six HIV encephalitic (HIVE) patients, we suggest that HIV may interfere with hormone signaling in the hypothalamus leading to disruptions in the HPT axis. We report findings from our preliminary studies and provide a brief review of the literature regarding potential contributions of HIV and substance abuse on the HPT.
In this context, we offer the hypothesis that HIV infection of the CNS may affect hypothalamic signaling that in turn disrupts thyroid hormone processing or signaling between neurons and glia. The presence of HIV and its neuropathological correlate, HIVE, have been illustrated in frontal cortex, basal ganglia and hippocampus of some HIV patients (Langford et al., 2006). Only one report published in 1993 addressed the presence of HIV in the hypothalamus (Purba et al., 1993). In this study, the numbers of vasopressin and oxytocin positive neurons in the hypothalamus from 20 AIDS patients and 10 controls were examined. A 40% decrease in the number of oxytocin immunoreactive neurons was observed in the AIDS group compared with controls, and no significant changes were observed in vasopressin expressing neurons. On the other hand, no evidence of inflammation was detected (Purba et al., 1993). We report for the first time, HIVE in the hypothalamus as evidenced by microglial nodules, multinucleated giant cells, perivascular cuffing by inflammatory cells and the presence of HIV-infected cells. Alterations in levels of type-2 deiodinase (D2), type-3 deiodinase (D3) and neurogranin were also observed in hypothalamic tissues from HIV patients compared to sero-negative patients. In the hypothalamus, thyroxin (T4) is converted to tri-iodothyronine (T3) by D2 enzyme and transported to neurons (Figure 1) (Fliers et al., 2006). In neurons, T3 can bind to thyroid receptors to regulate thyroid releasing hormone expression, or it may be degraded to inactive forms T2 or rT3 by neuronal D3. Thus, levels of cellular D2 and D3 regulate levels of T3 and T4. Changes in D2 or D3 may contribute to alterations in HPT functioning, but the clinical significance of these changes is unknown.
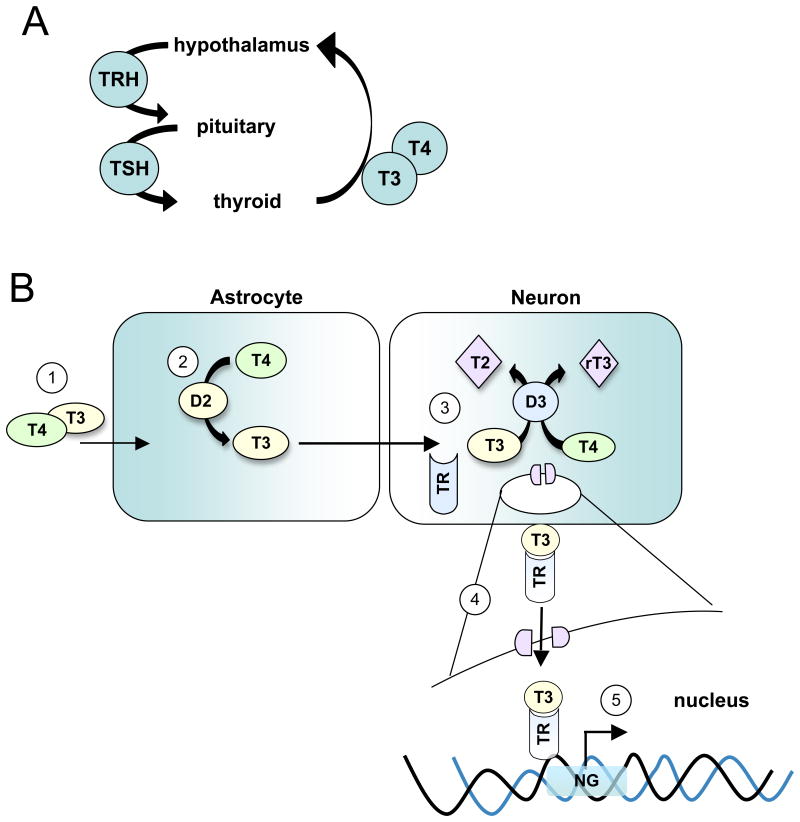
Figure 1.
Diagram of the signaling pathway of T4 and T3 from the blood stream to the cells of the brain for enzymatic processing. A) Simplified HPT feedback loop. In disease, disruptions in HPT signaling may occur at one or more of the three components (hypothalamus, pituitary or thyroid gland) of the feedback loop. Likewise the disruptions may occur in interpretation of incoming stimuli, or in output response from stimuli. B) 1) T3 and T4 are produced by the thyroid gland and transported via the circulatory system to the hypothalamus. 2) T4 and T3 enter tanycytes (specialized astrocytes) and T4 is processed to T3 by D2. 3) T3 is transported to neurons where it is processed by D3 to its inactive forms, T2 or rT3. 4) Remaining T3 may bind to TR and translocate to the nucleus. 5) In the nucleus, the T3/TR complex can bind to the promoter element of the neurogranin gene to active its transcription. HPT, hypothalamic-pituitary-thyroid; T4, thyroxine; T3, tri-iodothyronine; D2, deiodinase-2; D3, deiodinase-3; rT3, reversed T3; T2, 3,5-Diiodo-L-thyronine; TR, nuclear thyroid hormone receptor; NG, neurogranin.
Changes in levels of D2 and D3 observed hypothalamic tissues from HIVE patients might be related to HIV infection and to the presence of encephalitis. Altered levels of D2, D3, oxytocin and vasopressin observed in hypothalamic tissue from HIVE patients may contribute in part, to the alterations in circulating thyroid hormone levels in life. In summary, our data provide compelling evidence for potential HIV-mediated alterations in hypothalamic signaling in the HPT feedback loop that may result in abnormal thyroid hormone levels.
Materials and Methods
Participants
Patients admitted to the psychiatric unit of an area hospital were considered for this study. Participants were included on the basis of the following criteria: 1) meeting DSM-IV criteria for their psychiatric diagnosis, 2) substance use within the past 6 months, and a self report of drug abuse or dependence confirmed by positive urine toxin screen, 3) previous diagnosis for HIV/AIDS by standard clinical methods (positive antibody and RT PCR for plasma viral load), and 4) ages 18-55. A retrospective chart review was conducted of 128 HIV+ patients treated between 2007 and 2009. Data including TSH levels, HIV plasma viral burden, cART and thyroid disease history, gender, ethnicity (race), and age were collected. The Temple University Institutional Review Board approved all studies.
Acquisition and Neuropathological Examination of Brain Tissue
The National NeuroAIDS Tissue Consortium (NNTC) provided post-mortem human brain tissues. Hypothalamic brain sections from 4 male HIVE patients and 2 age matched, male, HIV negative, non-drug user patients were examined. Exclusion criteria included patients with a history of non-HIV related CNS medical disorders (neurosyphilis, schizophrenia, head trauma, etc). Inclusion criteria included cases with confirmed HIVE and history of SA, with cocaine reported as the drug of choice. The mean age of subjects was 39 (± 5 years). The diagnosis of HIVE was based on criteria established by the American Academy of Neurology and the HIV Neurobehavioral Research Center group (Langford et al., 2002; Del Valle and Pina-Oviedo, 2006).
Immunohistochemical Analyses
Five μm sections of formalin fixed, paraffin embedded tissues from the hypothalamus were deparaffinized in xylene and rehydrated through descending grades of ethanol up to water. After non-enzymatic antigen retrieval in 0.01M sodium citrate buffer (pH 6.0) for 30 min at 97 °C in a vacuum oven, and quenching of endogenous peroxidase with 3% H2O2 in methanol for 20 min, hematoxylin and eosin staining was performed for routine histological evaluation (Langford et al., 2003; Rearden et al., 2008). Overnight incubation with polyclonal antibodies against vasopressin (1:25, Abcam, Cambridge, MA), oxytocin (1:25, Abcam) or with monoclonal anti-p24 antibody (1:5, DAKO) was followed by incubation with biotinylated goat anti-rabbit secondary antibody (1:200, Vector Laboratories). Slides were developed with avidin-biotin D-horseradish peroxidase (HRP) (Vector) and diaminobenzidine (DAB, 0.2 mg/ml) in 50 mM Tris (pH 7.4). Sections were counterstained with hematoxylin, dehydrated in ethanol, cleared with xylene and mounted with permount.
Immunofluorescence labeling
Serial tissue sections from the hypothalamus were processed as described for immunohistochemical analyses with the following modifications for fluorescence labeling. Antibodies included anti-deiodinase 2 (D2) (1:50, Santa Cruz Biotechnology, CA), anti-deiodinase 3 (D3) (1:50, Novus Biologicals, Littleton, CO), or anti-MAP2 (1:500, Covance, Emeryville, CA), anti-glial fibrillary protein (GFAP) (1:500, Chemicon International, Temecula, CA). Sections were blocked, incubated with the primary antibodies overnight in a humidified chamber at room temperature, rinsed three times with PBS, then incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:500) or rhodamine-tagged secondary antibodies (1:200) for 2h at room temperature in the dark. After washing with PBS, the sections were re-blocked and incubated overnight at room temperature in a humidified chamber the second primary antibody. After washing, sections were incubated with the second secondary antibody for 2h at room temperature in the dark. Finally, sections were cover-slipped with an aqueous based mounting media containing DAPI for nuclear labeling (Vectashield, Vector Laboratories), and visualized with a Nikon ultraviolet inverted microscope and processed with deconvolution software (Slidebook 4.0, Intelligent Imaging, Denver, Colorado). Deconvolution was performed using SlideBook4 software, allowing acquisition of multiple 0.2 μm thick digital sections and 3-D reconstruction of the image.
Western Analyses
Western analyses were conducted on snap frozen blocks from human brain tissues from the hypothalamic region. Frozen tissue was homogenized with mechanical dounce disruption on ice in HEPES buffer (TNN buffer; 1mM HEPES, 5 mM benzamidine, 2 mM 2-mercaptoethanol, 3 mM EDTA, 0.5 mM magnesium sulfate, 0.05% sodium azide, 1 mM sodium orthovanadate and 0.01 mg/ml leupeptin). Total proteins were isolated from tissues and standard Western analyses were performed as previously described (Langford et al., 2003; Langford et al., 2004). Briefly, protein concentrations were determined by the Bradford assay and equal concentrations of protein were loaded per well. Proteins were separated by electrophoresis for 1h at 200 V on 4-12% Bis-Tris NuPage Gels (Invitrogen, Carlsbad, CA). After transferring onto nitrocellulose membranes, proteins of interest were detected with primary antibodies against D2 (1:1000, Novus), D3 (1:1000, Novus), or a loading control, tubulin (1:1000, BD Biotechnologies, San Jose, CA) overnight at 4°C. Membranes were then incubated with appropriate secondary anti-mouse or -rabbit antibodies (1:10,000) for 1h and developed with Western Lightning Chemiluminescence Reagent Plus kit (Amersham, UK). Data were analyzed using one-way analysis of variance (ANOVA) with post hoc Dunnett's or Tukey-Kramer using GraphPad Prism (GraphPad Software Inc, San Diego, CA) and results were expressed as mean ± SEM, n ! 3.
Results
Abnormal thyroid hormones levels
Our pilot data describe a cohort of 128 patients treated at an inner city psychiatric hospital. All patients in the cohort had an Axis I diagnosis including at least one of the following: major depressive disorder (all categories), psychosis (NOS), psychosis, schizophrenia, bipolar or post-traumatic stress disorder and co-morbid substance abuse or dependence [Table I]. Forty-two percent of the population were African American, 29% Hispanic/Latino, 6% Caucasian, and 23% were of undefined race. Of the 128 HIV patients, 27% had abnormal TSH levels, compared to the 4.3% in national normative estimates in the US population (n= 17,353) (Hollowell et al., 2002). The abnormal range for TSH in these studies was < 0.5 mU/L or > 4 mU/L (Biondi and Cooper, 2008). Of the 27% (N = 35) with abnormal TSH levels, approximately 50% of these patients' TSH levels were < 0.5 mU/L and 50% were > 4 mU/L. Thirty percent of the patients with TSH levels in the hypothyroid range and 40% in the hyperthyroid range, respectively, were confirmed for substance abuse/dependence. Of these patients, one had thyroiditis and had normal TSH levels. Four had acquired hypothyoridism, but only one of the four had TSH levels above 4 uM/L. Our data show that a significant percentage (33%) of minority patients, both male and female infected with HIV had abnormal TSH levels. Of the remaining patients with abnormal TSH levels, 60% were of undefined race and 7% were Caucasian. No correlation was observed between either viral load or CD4 counts and abnormal TSH levels. Thirty percent of patients in our pilot data set were positive for the use of one or more substances including cocaine, opioids, marijuana and benzodiazepine (Table I). Of the 30% of patients positive for substance use/abuse, 21% of patients were positive for cocaine plus at least one other drug, and 11% were positive for cocaine alone (Table I). Likewise, 9% of patients were positive for opioids plus at least one other drug, and 3% were positive for opioids alone. Five percent of patients were positive for marijuana plus at least one other drug, and 2% were positive for marijuana alone. Seven percent of patients were positive for benzodiazepine plus at least one other drug, and 3% were positive for benzodiazepine alone. Surprisingly, no patients tested positive for phencyclidine or amphetamines. The potential contribution of these drugs to TSH levels is unknown. These pilot data support findings from previous studies (Bongiovanni et al., 2006; Madeddu et al., 2006; Madge et al., 2007) demonstrating a need to investigate HPT dysfunction in individuals with SA disorders and HIV.
Table I. HIV patient population data.
M/F, male/female; TSH, thyroid stimulating hormone; PCP, phencyclidine. Numbers in parentheses represent the percentage of patients with single drug use; while the numbers to the left of parentheses represent a positive result for the given drug plus at least one other drug.
| N = 128 | % |
|---|---|
| Race | |
| African American | 42 |
| Hispanic/Latino | 29 |
| Caucasian | 6 |
| Undefined | 23 |
| Gender (M/F) | 57/43 |
| Abnormal TSH | 27 |
| Substance Use | 30 |
| Cocaine | 21 (11) |
| Opioids | 9 (3) |
| Marijuana | 5 (2) |
| Benzodiazepine | 7 (3) |
| PCP, Amphetamine | 0 (0) |
Hypothalamic Neuropathology in HIVE
We report for the first time, the presence of HIV in the hypothalamus of patients suffering from HIVE and AIDS with a history of SA (Figure 2). A previous report from 1993 reported significant loss of oxytocin immunoreactive neurons in the hypothalamus of HIV patients, with no evidence of HIVE (Purba et al., 1993). Examination of hypothalamic tissues from 4 HIVE patients revealed hallmarks of HIVE including microglial nodules, reactive astrocytes (Figure 2A), multinucleated giant cells (Figure 2B, lower inset), and perivascular cuffing of inflammatory cells (Figure 2B, upper inset, and 2C). HIV antigen-p24 immunoreactive macrophages were present associated with small vessels in the hypothalamus, as well (Figure 2D-F, arrowheads).
Figure 2.
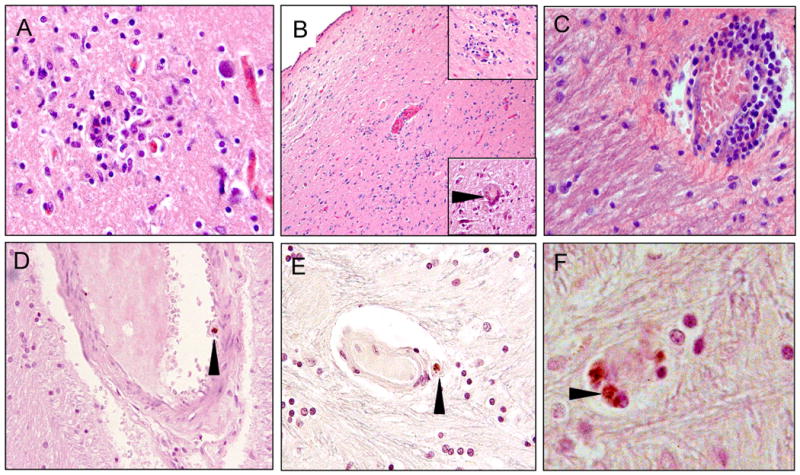
Neuropathological alterations in hypothalamic sections from the brains of HIVE+ patients. A) microglial nodule at the grey/white matter junction; B) low magnification of the periventricular region of the 3rd ventricle. The top right corner inset shows inflammatory cell infiltration surrounding small vessels. The lower right corner inset shows a multinucleated giant cell (arrowhead); C) perivascular cuffing by infiltrating macrophages and lymphocytes with expansion of the perivascular space in the white matter; D) p24 immunoreactive macrophage in the vascular space of a small artery of the blood brain barrier (arrowhead); E) p24 immunoreactive macrophage adjacent to a small vessel (arrowhead); F) higher magnification of 2-3 p24 immunoreactive cells (arrowhead) associated with a disrupted microvessel. Magnification for A, C, D, E is 40×, B is 10× (insets are 40×), F is 100×.
Expression of D2 and D3 in the hypothalamus
To confirm the presence of hypothalamus-specific thyroid enzymes, double immunofluorescence labeling with GFAP and D2 for astrocytes, and MAP2 and D3 for neurons was conducted in hypothalamic brain tissues. As expected, GFAP (red) and D2 (green) are co-expressed by the same cells, although not all astrocytes (red) express D2 (Figure 3A, arrowhead). Likewise, co-expression of MAP2 and D3 is evident in neurons (Figure 3B, arrowhead), but not all MAP2 (green) positive neurons are expressing for D3 (red). To illustrate cell-type specificity, double labeling of astrocytes with GFAP (red) and of neurons with MAP2 (green) shows distinctly different cell populations (Figure 3C). Levels of D2 and D3 in hypothalamic tissues from HIV negative and HIVE patients were next assessed by Western analyses.
Figure 3.
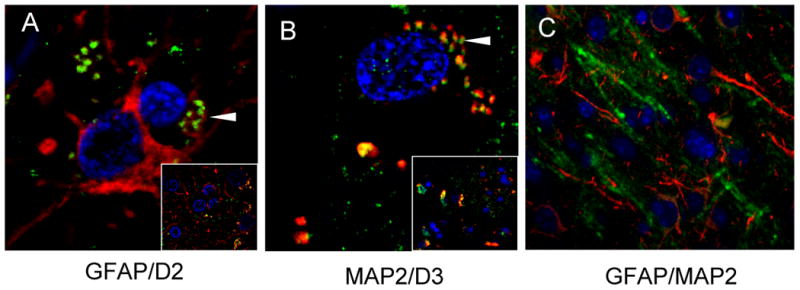
Immunofluorescence detection of the expression of thyroid hormone enzymes in post-mortem tissue from periventricular hypothalamic sections at the level of the 3rd ventricle from a representative HIVE patient. A) High magnification (100×) of astrocytes expressing both GFAP (red) and D2 (green). The arrowhead indicates a region of co-expression. The inset shows the lower power image of the same field of view (40×). B) High magnification (100×) of a neuron expressing both MAP2 (green) and D3 (red). The arrowhead indicates a region of co-expression. The inset shows the lower power image of the same field of view (40×). C) Hypothalamic tissue of an HIVE patient immunolabeled with GFAP (red) for astrocytes and MAP2 for neurons (green) showing no co-expression (40×). Nuclei are stained blue with DAPI. HIVE, HIV encephalitis; D2, deiodinase-2; D3, deiodinase-3; GFAP glial fibrillary acidic protein; MAP2, microtubule-associated protein-2.
D2 protein, which is responsible for converting T4 to T3 (Figure 1), is expressed as α and β isoforms, as a result of splicing variations (Croteau et al., 1996; Salvatore et al., 1996). Both isoforms are detected in hypothalamic tissues from HIV negative and HIVE patients (Figure 4A, upper panel). Western analyses show that compared to the 2 control cases, D2 levels in the 4 HIVE cases are increased in the hypothalamus (Figure 4B). The D2 doublet representing α and β isoforms is detected at approximately 31- and 34-kDa, respectively. Interestingly, expression levels of the α-isoform are significantly increased in each of the HIVE cases (Figure 4B, compare the darker bars to one another), while the β-isoform levels remain fairly stable (compare the lighter bars to one another). D3, which converts T3 to inactive rT3 or T2, is decreased in HIVE cases compared to HIV negative controls (Figure 4A, C).
Figure 4.
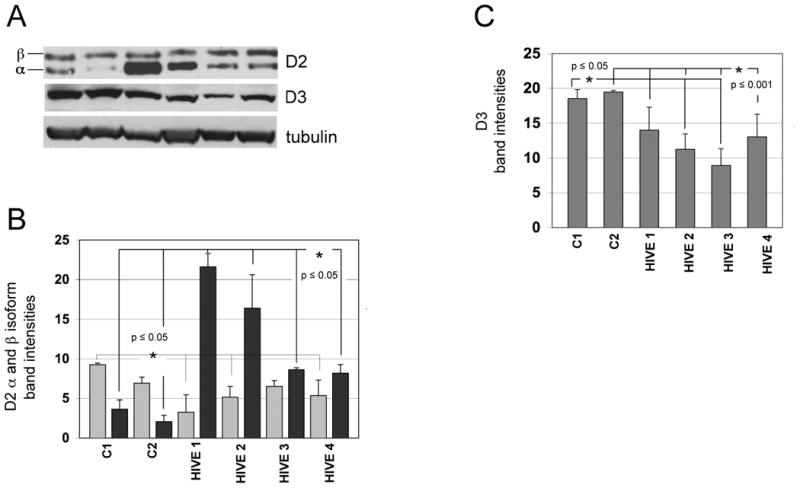
Western analyses of D2 and D3 expression levels in the hypothalamus from control and HIVE patients. A) Representative Western blot from two HIV negative controls (lanes 1 and 2) and four HIVE cases (lanes 3-6). D2 is detected as a doublet reported to represent β (upper band) and α (lower band) isoforms. B) Lighter bars represent β-D2 expression levels and darker bars represent α-D2 expression levels. C) D3 expression levels. Band densities were calculated after normalization to tubulin levels in each lane. Levels of D2 and D3 in HIVE cases were analyzed by one-way ANOVA with Tukey Kramer post hoc when compared to controls, C1 or C2. D2, deiodinase-2; D3, deiodinase-3; HIVE, HIV encephalitis.
In support of previous findings, 81% fewer oxytocin (Figure 5A, B) and 52% fewer vasopressin (5C, D) immunoreactive neurons were present in the hypothalamus from the HIVE patients compared to HIV negative controls. Compared to a representative HIV negative case, (Figure 5A), in HIVE, the numbers of oxytocin immunoreactive neurons were significantly fewer (Figure 5B). Sparse oxytocin immunoreactivity was associated with dystrophic neuronal cell bodies (arrow), but also with infiltrating macrophages surrounding microvessels (arrowhead). Likewise, loss of vasopressin immunoreactive neurons was also observed in the HIVE cases (Figure 5D) compared to the HIV negative cases (5C). Several vasopressin immunoreactive cells are observed in association with macrophages (arrowhead) and within a microglial nodule (Figure 5D, inset), suggesting that vasopressin positive cells maybe undergoing phagocytosis.
Figure 5.
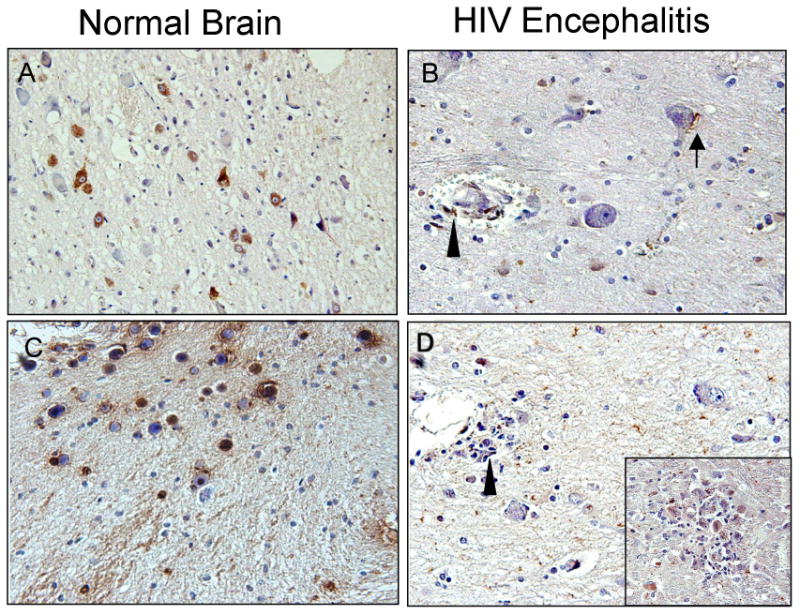
Immunohistochemical analyses of oxytocin and vasopressin expression in hypothalamic tissues from HIVE patients. Oxytocin (A, B) and Vasopressin (C, D) immunoreactivity patterns in the paraventricular nucleus of the hypothalamic regions of a representative HIV negative patient and an HIVE patient. Immunoreactive neurons are indicated by diaminobenzidine staining (brown) and sections are counterstained with hematoxylin. A) Abundant oxytocin+ neurons are present in the normal brain; B) significantly less immunoreactivity is observed in the HIVE patient with a few oxytocin+ cells associated with infiltrating macrophages surrounding disrupted vascular (arrowhead) and dystrophic neuronal cells (arrow). C) Abundant vasopressin immunoreactivity is observed in a HIV negative control case; D) Significantly less vasopressin immunoreactivity is detected in the HIVE case, with scattered immunopositive cells throughout the section. Magnification is 40×. Compared to a representative HIV negative case, (A), in HIVE the numbers of oxytocin immunoreactive neurons were significantly fewer (B). Sparse oxytocin immunoreactivity was associated with dystrophic neuronal cell bodies (arrow) but also with infiltrating macrophages surrounding microvessels (arrowhead). Likewise, loss of vasopressin immunoreactive neurons was also observed in the HIVE case (D) compared to the HIV negative case (C). Several vasopressin immunoreactive cells are observed in association with macrophages (arrowhead) and within a microglial nodule (D, inset), suggesting that vasopressin positive cells maybe undergoing phagocytosis. OXY, oxytocin; VSP, vasopressin; HIVE, HIV encephalitis.
Taken together, our data show that a considerable percentage (27%) of a cohort of HIV patients with SA disorder have abnormal thyroid hormone levels, and over 50% of these patients are male. In addition, evidence of HIVE and HIV-infected macrophages was observed in the hypothalamus of patients who died from AIDS. Levels of the thyroid hormones, D2 and D3, were significantly altered in HIVE patients compared to HIV negative control cases, and oxytocin and vasopressin levels were decreased, as well. Based on data from hypothalamic tissues from this proof-of-concept pilot investigation, more extensive studies with a larger number of cases are required to confirm our findings.
DISCUSSION and Brief Literature Review
Since the first case of AIDS appeared in the United States in Los Angeles June 5, 1981, (CDC, 1981) understanding the full impact of HIV infection on CNS functioning has been elusive. Although progressive dementia was recognized as a significant complication of HIV CNS infection, early clinical investigations focused primarily on decreased immune function status, opportunistic infections, neoplasms, the exceedingly high and rapid death rate, and public health debates on how to best control the spread of the infection. The fact that the disease had a CNS component was observed, but thought to be largely a neurocognitive disorder. A report published in the New York Times on August 28, 1987 (www.nytimes.com/1987/08/28/US) cited Dr. A. Fauci, of the NIH, who reported that blocking neuroleukin might explain some of the symptoms of AIDS dementia, but did not account for all the changes seen in the brains of AIDS patients. Six months later, in February 1988, NIH scientists speculated a toxic viral protein produced by the AIDS virus might be “partly responsible” for developing AIDS dementia (Washington Post Science Section 2/16/88). A commentary by Richard Price and Bruce Brew published in the Journal of Infectious Diseases (Price and Brew, 1988) claimed that the most common CNS complication of HIV was a neurological syndrome, AIDS Dementia Complex, (ADC) that resulted at least in part from the direct effects of HIV-1 on the brain. They concluded that ADC was widespread among AIDS patients, but largely under recognized and misunderstood by clinicians, researchers and patients.
Co-morbid conditions continue to emerge in the HIV+ population and include SA, ageing, endocrine and even cART-related disturbances. Recognition and treatment of these conditions and their potential contributions to rate of disease progression, and confounding issues such as depression, remain challenging to healthcare providers. Among the newly recognized complications faced by HIV-infected patients are disturbances in the HPT axis (Zirilli et al., 2008).
HPT dysfunction commonly causes mood disorders, depression and even dementia. In the HAART-era, depression, mild to moderate cognitive alterations, and in some cases dementia are continuing observations in a significant proportion of HIV patients. Many studies illustrate links between HIV infection and HPT functioning, and between SA and HPT functioning (Teoh et al., 1993; Budziszewska et al., 1996; Vescovi and Pezzarossa, 1999) (Beltran et al., 2003; Kumar et al., 2003; Wiener et al., 2008; Zirilli et al., 2008) (Wilson et al., 1996) (Bongiovanni et al., 2006; Madeddu et al., 2006). One study reports no association between HIV and thyroid function (Madge et al., 2007). Despite the extensive extant literature on CNS involvement seen with HIV infection, few studies have investigated the role of the HPT neuroendocrine feed back loop.
Thyroid Feedback Loop
In brief, the hypothalamus responds to low blood levels of thyroid hormones, tri-iodothyronine (T3) and thyroxine (T4) by producing thyroid releasing hormone (TRH) that in turn induces production and release of thyroid stimulating hormone (TSH) by the pituitary gland. Circulating TSH stimulates the thyroid gland to then produce T3 and T4. T3 and T4 are taken up by astrocytes in the hypothalamus. T4 is converted to T3 by type-2 deiodinase (D2) enzyme and transported to neurons (Figure 1) (Fliers et al., 2006). In neurons, T3 can bind to thyroid receptors to regulate TRH expression or it may be degraded to inactive forms T2 or rT3 by neuronal type-3 deiodinase (D3). In this manner, levels of cellular D2 and D3 regulate levels of T3 and T4. Cytoplasmic T3 binds to its nuclear receptor TR1α or TRβ and travels to the nucleus where it activates transcription of target genes containing hormone response elements. One gene target is neurogranin that contains a thyroid responsive element in its first intron (Martinez de Arrieta et al., 1999) and is involved in synaptic plasticity and learning (Pak et al., 2000). Several studies suggest that neurological symptoms of adult onset hypothyroidism may be related to changes in synaptoplasticity, (Alzoubi et al., 2005; Sui et al., 2006) but the molecular basis for these changes is unknown.
Non-thyroidal Illness in Disease
In general, three thyroid function test patterns are observed in hypothyroidism and include: overt, subclinical and euthyroid-sick-syndrome or non-thyroidal illness (NTI). Low levels of T4 and subsequent increases in TSH levels usually characterize overt hypothyroidism. Subclinical hypothyroidism has a prevalence of approximately 4.3% in the general population and usually presents as slightly elevated TSH levels (approximately between 4.5 and 10 mU/L) with no change in T4 (Hollowell et al., 2002).
In severe or critical illness, changes in the HPT axis include decreased serum TSH, T3 and T4 and have been suggested to indicate alterations in the HPT set point, as illustrated by decreased TRH in postmortem tissues from the periventricular nuclei of patients who died following critical illness, and who in life displayed decreased T3 serum levels (Fliers et al., 2006). In chronic disease states such as HIV infection, abnormal levels of serum thyroid hormones (T3, T4, or TSH) are commonly reported, with 35% of HIV patients displaying subtle abnormalities in overall thyroid function tests (Grappin et al., 2000; Calza et al., 2002; Beltran et al., 2003; Collazos et al., 2003; Madeddu et al., 2006). Specifically, recent studies report that 1-2% of HIV patients suffer from overt hypothyroidism and the prevalence of subclinical hypothyroidism among HIV-patients, especially those on HAART, is reported to range from 3.5-12.2% (Grappin et al., 2000; Calza et al., 2002; Beltran et al., 2003; Collazos et al., 2003; Madeddu et al., 2006; Madge et al., 2007). This is in contrast to the 0.3 and 4.3% of the general population who suffer from overt and subclinical hypothyroidism, respectively (Hollowell et al., 2002).
HIV and the HPT axis
In HIV infected patients, thyroid abnormities are usually classified as non-thyroidal illness or euthyroid sick syndrome. Characteristics of alterations in thyroid hormones depend to some degree on the stage of HIV disease and severity of AIDS. For example, in advanced AIDS, deiodination processing of T4 to T3 is reduced, and the metabolism to reversed T3 (rT3) is increased, both of which contribute to lower serum free T3 suggesting thyroid dysfunction. TSH levels may or may not change in this scenario, but usually increase slightly during recovery, indicated in part by decreased viral burden and increased CD4 count in HIV patients (see (Hoffmann and Brown, 2007) for review). This pattern is proposed to result from response to illness rather than thyroid dysfunction. For example, in the pre-HAART era, significantly more HIV patients suffered from opportunistic infections (OIs) including Pneumocystis, Cryptococcus, Leishmania, Streptococcus or Staphylococcus, all of which have been reported to be involved in destructive thyroiditis (Sellmeyer and Grunfeld, 1996; Pearce et al., 2003). The development of more efficient CNS penetrating cART has changed the patterns in HIVE and AIDS-related CNS OIs, significantly. Early in the AIDS epidemic, autopsy studies pointed to a high prevalence of these conditions. With the advent of nucleoside reverse transcriptase inhibitors, the prevalence at autopsy of many CNS OIs, declined, while HIVE increased. After the introduction of protease inhibitors, a decline in both HIVE and CNS opportunistic infections was observed. In AIDS patients, studies of neurodegeneration focusing on specific neuronal populations affected have increased our understanding of the mechanisms resulting in cognitive impairment. These studies reported loss primarily of large pyramidal neurons and parvalbumin- and calbindin positive interneurons in the neocortex (Masliah et al., 1997). Subtle neuronal damage was described in the early stages of infection in untreated patients, but more severe HIVE was associated with greater loss of more diverse populations of neurons (Everall et al., 1999). The relationships among HIVE, neurodegeneration, and HIV associated dementia are more complex than degree of neuronal loss, however. For example, not all patients with HIVE develop dementia and not all demented AIDS patients have HIVE. In comparison, most patients with cognitive impairment do have neurodegeneration, and those with a normal neuropsychological profile show preservation of their synaptodendritic organization (Everall et al., 1999). It has also been shown that damage to synapses and dendrites in patients with HIVE are substantial, occurring even in early disease and correlating with brain viral load (Masliah et al., 1997; Everall et al., 1999). More recent studies report that like HIV, SA alone or in combination with HIV infection can damage specific neuronal populations thereby, manifesting in more subtle, but specific cognitive impairments (Langford et al., 2003; Moore et al., 2006).
In this study, we addressed a potential biological and behavioral co-morbid condition to which substance abuse may contribute in individuals living with HIV or at risk for becoming infected. Some co-morbidities associated with substance abuse and/or HIV infection directly affect behavior and cognition, and impact significantly on quality of life, adherence to anti-retroviral therapy and successful drug addiction treatment programs. In this context, alterations in thyroid hormone levels have emerged as a complication among some individuals with substance abuse disorders and/or HIV infection, although the molecular basis for the connection is unknown. HPT dysfunction in the absence of SA or HIV infection commonly causes mood disorders, depression and even dementia. Numerous studies dating as far back as the 1980's and 1990's in HIV+ patient cohorts have addressed possible links among HIV infection, SA and abnormal thyroid hormone levels (LoPresti et al., 1989; Lambert et al., 1990; Brown et al., 1991; Dhopesh et al., 1991; Wilson et al., 1996; Vescovi and Pezzarossa, 1999). Recent studies continue to ask the same questions, and include injection drug abuse and HAART as additional potential contributors to abnormal thyroid levels (Calza et al., 2002; Koutkia et al., 2002; Beltran et al., 2003; Collazos et al., 2003; Quirino et al., 2004; Bongiovanni et al., 2006; Ketsamathi et al., 2006; Madeddu et al., 2006; Afhami et al., 2007; Madge et al., 2007; Wiener et al., 2008). In fact, underlying subclinical hypothyroidism is associated with cocaine withdrawal in addicts (Vescovi and Pezzarossa, 1999). Taken together, conflicting results from such studies illustrate the need for more in depth investigations, particularly at the molecular level.
Disruptions in the HPT feedback loop may originate from one or more of the three components of the feedback loop (hypothalamus, pituitary or thyroid). Misinterpretation of incoming stimuli or in the output response to the stimulus, or both could cause disturbances in the HPT feedback loop (Figure 1A). During disease, a combination of events likely contributes to HPT dysfunction. For example, hypothalamic responses to circulating hormones from the thyroid gland may be abnormal leading to altered T4 and T3 processing by D2 and D3 in astrocytes and neurons in the hypothalamus.
Importantly, over 50% of the subjects in our cohort of HIV patients were male, and 42% were African-American and 29% were Hispanic/Latino, illustrating that in our patients, minority populations predominate (overall, 71%) and may be at increased risk for not only HIV but also to co-morbidities associated with HIV infection and SA.
Of the 128 subjects included in our study, 27% had abnormal TSH levels. Results from a previous study that included 17,353 subjects reported that 4.3% had abnormal TSH level, and that abnormal TSH levels were the least frequent in African Americans compared to Caucasians, with Caucasian females representing the group with most frequent abnormal TSH levels (Hollowell et al., 2002). Our data clearly indicate the need for further investigation into potential thyroid hormone abnormalities in HIV+ male patients with or without substance abuse disorder.
The basis for significant differences observed in expression levels of the α-isoform in the HIVE cases compared to HIV negative controls (Figure 3A, B), are unknown. D2, like D3, contains the rare amino acid selenocysteine encoded by UGA and its translation requires the selenocysteine insertion sequence element in the 3′untranslated region (Gereben et al., 2002). Unlike D3 however, the D2 gene has multiple transcription start sites and a number of factors can influence the protein levels of D2 (Gereben et al., 2002). Alterations in the ratios of D2 mRNA transcripts to protein are reported in Graves disease and in thyroid adenomas (Salvatore et al., 1996), but evidence for preferential regulation of one isoform over another has not been reported previously. Studies address potential biochemical mediators for these observations are underway.
The relationship among HIVE, cognitive impairment and neurodegeneration is complex. Pathologically, the brain is affected by dendritic and synaptic damage, neuronal loss and a spectrum of inflammatory changes (Dawes et al., 2008). Data in support of this possibility show that HIV is present in the hypothalamus and that characteristic hallmarks of HIVE are present, as well. The neuronal populations most severely affected in these regions include large pyramidal neurons in the neocortex, spiny neurons in the putamen, medium-sized neurons in the globus pallidus, and interneurons in the hippocampus (Masliah et al., 1997). The patient from which, the p24 immunolabeling was conducted had been infected with HIV for 33 years at the time of death and the CSF viral burden (75 copies/mL) was low compared to the plasma viral burden (>750,000 copies/mL). These findings suggest that the numerous MGN present in the hypothalamic region could have represent “burnt out” lesions from earlier events in disease progression. Invading HIV+ macrophages associated with microvasculature on the other hand suggest current reseeding of the CNS by circulating HIV.
Our broad hypothesis, that substance abuse and HIV CNS disease contribute to disruptions in the HPT feedback loop and may lead to or exacerbate hypothyroidism is supported by our preliminary data showing evidence of HIVE in the hypothalamus and altered levels of D2 and D3. We propose that these disruptions occur, in part, at the level of hypothalamic signaling and may contribute, in part, to HPT syndrome observed in many HIV + individuals. Left unchecked, these disorders that alone, or in concert contribute to worsening cognitive impairments could lead to poor adherence to HIV medications, increased substance abuse, and more rapid disease progression. More in depth studies to increase our understanding of how altered hypothalamic signaling in patients suffering from SA disorders and/or HIV infection may contribute to declining cognition and disease progression, and/or treatment failures are warranted and may result in more effective treatments and improved clinical outcomes. The impact of pinpointing effects of SA and HIV infection on hypothalamic functioning is significant since this represents a new research area not previously studied.
Acknowledgments
We would like to thank Jeffery Gerbino for assistance with data collection and analyses, and Brittany Tracy for editorial assistance.
This publication was made possible in part by grants from the NIH to:
TDL (NIDAR21029523)
Manhattan HIV Brain Bank: U01MH083501, R24MH59724
Texas NeuroAIDS Research Center U01MH083507, R24 NS45491
National Neurological AIDS Bank 5U01MH083500, NS 38841
California NeuroAIDS Tissue Network U01MH083506, R24MH59745
Statistics and Data Coordinating Center U01MH083545, N01MH32002
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NNTC or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afhami S, Haghpanah V, Heshmat R, Rasoulinejad M, Izadi M, Lashkari A, Tavangar SM, Hajiabdolbaghi M, Mohraz M, Larijani B. Assessment of the factors involving in the development of hypothyroidism in HIV-infected patients: a case-control study. Infection. 2007;35:334–8. doi: 10.1007/s15010-007-6163-3. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Gerges NZ, Alkadhi KA. Levothyroxin restores hypothyroidism-induced impairment of LTP of hippocampal CA1: electrophysiological and molecular studies. Exp Neurol. 2005;195:330–41. doi: 10.1016/j.expneurol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Beltran S, Lescure FX, Desailloud R, Douadi Y, Smail A, El Esper I, Arlot S, Schmit JL. Increased prevalence of hypothyroidism among human immunodeficiency virus-infected patients: a need for screening. Clin Infect Dis. 2003;37:579–83. doi: 10.1086/376626. [DOI] [PubMed] [Google Scholar]
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- Bongiovanni M, Adorni F, Casana M, Tordato F, Tincati C, Cicconi P, Bini T, d'Arminio Monforte A. Subclinical hypothyroidism in HIV-infected subjects. J Antimicrob Chemother. 2006;58:1086–9. doi: 10.1093/jac/dkl360. [DOI] [PubMed] [Google Scholar]
- Brown LS, Jr, Singer F, Killian P. Endocrine complications of AIDS and drug addiction. Endocrinol Metab Clin North Am. 1991;20:655–73. [PubMed] [Google Scholar]
- Budziszewska B, Jaworska-Feil L, Lason W. The effect of repeated amphetamine and cocaine administration on adrenal, gonadal and thyroid hormone levels in the rat plasma. Exp Clin Endocrinol Diabetes. 1996;104:334–8. doi: 10.1055/s-0029-1211463. [DOI] [PubMed] [Google Scholar]
- Calza L, Manfredi R, Chiodo F. Subclinical hypothyroidism in HIV-infected patients receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31:361–3. doi: 10.1097/00126334-200211010-00014. [DOI] [PubMed] [Google Scholar]
- CDC. Pneumocystis Pneumonia- Los Angeles. MMWR Weekly. 1981;30:1–3. [PubMed] [Google Scholar]
- Collazos J, Ibarra S, Mayo J. Thyroid hormones in HIV-infected patients in the highly active antiretroviral therapy era: evidence of an interrelation between the thyroid axis and the immune system. Aids. 2003;17:763–5. doi: 10.1097/00002030-200303280-00019. [DOI] [PubMed] [Google Scholar]
- Croteau W, Davey JC, Galton VA, St Germain DL. Cloning of the mammalian type II iodothyronine deiodinase. A selenoprotein differentially expressed and regulated in human and rat brain and other tissues. J Clin Invest. 1996;98:405–17. doi: 10.1172/JCI118806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes S, Suarez P, Casey CY, Cherner M, Marcotte TD, Letendre S, Grant I, Heaton RK. Variable patterns of neuropsychological performance in HIV-1 infection. J Clin Exp Neuropsychol. 2008;30:613–26. doi: 10.1080/13803390701565225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle L, Pina-Oviedo S. HIV disorders of the brain: pathology and pathogenesis. Front Biosci. 2006;11:718–732. doi: 10.2741/1830. [DOI] [PubMed] [Google Scholar]
- Dhopesh VP, Burke WM, Maany I, Ravi NV. Effect of cocaine on thyroid functions. Am J Drug Alcohol Abuse. 1991;17:423–7. doi: 10.3109/00952999109001601. [DOI] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9:209–17. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliers E, Alkemade A, Wiersinga WM, Swaab DF. Hypothalamic thyroid hormone feedback in health and disease. Prog Brain Res. 2006;153:189–207. doi: 10.1016/S0079-6123(06)53011-0. [DOI] [PubMed] [Google Scholar]
- Gereben B, Kollar A, Harney JW, Larsen PR. The mRNA structure has potent regulatory effects on type 2 iodothyronine deiodinase expression. Mol Endocrinol. 2002;16:1667–79. doi: 10.1210/mend.16.7.0879. [DOI] [PubMed] [Google Scholar]
- Grappin M, Piroth L, Verges B, Sgro C, Mack G, Buisson M, Duong M, Chavanet P, Portier H. Increased prevalence of subclinical hypothyroidism in HIV patients treated with highly active antiretroviral therapy. Aids. 2000;14:1070–2. doi: 10.1097/00002030-200005260-00026. [DOI] [PubMed] [Google Scholar]
- Hoffmann CJ, Brown TT. Thyroid function abnormalities in HIV-infected patients. Clin Infect Dis. 2007;45:488–94. doi: 10.1086/519978. [DOI] [PubMed] [Google Scholar]
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- Ketsamathi C, Jongjaroenprasert W, Chailurkit LO, Udomsubpayakul U, Kiertiburanakul S. Prevalence of thyroid dysfunction in Thai HIV-infected patients. Curr HIV Res. 2006;4:463–7. doi: 10.2174/157016206778560036. [DOI] [PubMed] [Google Scholar]
- Koutkia P, Mylonakis E, Levin RM. Human immunodeficiency virus infection and the thyroid. Thyroid. 2002;12:577–82. doi: 10.1089/105072502320288429. [DOI] [PubMed] [Google Scholar]
- Kumar M, Kumar AM, Waldrop D, Antoni MH, Eisdorfer C. HIV-1 infection and its impact on the HPA axis, cytokines, and cognition. Stress. 2003;6:167–72. doi: 10.1080/10253890310001605376. [DOI] [PubMed] [Google Scholar]
- Lambert M, Zech F, De Nayer P, Jamez J, Vandercam B. Elevation of serum thyroxine-binding globulin (but not of cortisol-binding globulin and sex hormone-binding globulin) associated with the progression of human immunodeficiency virus infection. Am J Med. 1990;89:748–51. doi: 10.1016/0002-9343(90)90216-z. [DOI] [PubMed] [Google Scholar]
- Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, Ellis RJ, Marcotte TD, Masliah E. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr. 2003;34:467–74. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- Langford D, Grigorian A, Hurford R, Adame A, Ellis RJ, Hansen L, Masliah E. Altered P-glycoprotein expression in AIDS patients with HIV encephalitis. J Neuropathol Exp Neurol. 2004;63:1038–47. doi: 10.1093/jnen/63.10.1038. [DOI] [PubMed] [Google Scholar]
- Langford D, Marquie-Beck J, de Almeida S, Lazzaretto D, Letendre S, Grant I, McCutchan JA, Masliah E, Ellis RJ. Relationship of antiretroviral treatment to postmortem brain tissue viral load in human immunodeficiency virus-infected patients. J Neurovirol. 2006;12:100–7. doi: 10.1080/13550280600713932. [DOI] [PubMed] [Google Scholar]
- Langford TD, Letendre SL, Marcotte TD, Ellis RJ, McCutchan JA, Grant I, Mallory ME, Hansen LA, Archibald S, Jernigan T, Masliah E. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. Aids. 2002;16:1019–29. doi: 10.1097/00002030-200205030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPresti JS, Fried JC, Spencer CA, Nicoloff JT. Unique alterations of thyroid hormone indices in the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1989;110:970–5. doi: 10.7326/0003-4819-110-12-970. [DOI] [PubMed] [Google Scholar]
- Madeddu G, Spanu A, Chessa F, Calia GM, Lovigu C, Solinas P, Mannazzu M, Falchi A, Mura MS, Madeddu G. Thyroid function in human immunodeficiency virus patients treated with highly active antiretroviral therapy (HAART): a longitudinal study. Clin Endocrinol (Oxf) 2006;64:375–83. doi: 10.1111/j.1365-2265.2006.02472.x. [DOI] [PubMed] [Google Scholar]
- Madge S, Smith CJ, Lampe FC, Thomas M, Johnson MA, Youle M, Vanderpump M. No association between HIV disease and its treatment and thyroid function. HIV Med. 2007;8:22–7. doi: 10.1111/j.1468-1293.2007.00422.x. [DOI] [PubMed] [Google Scholar]
- Martinez de Arrieta C, Morte B, Coloma A, Bernal J. The human RC3 gene homolog, NRGN contains a thyroid hormone-responsive element located in the first intron. Endocrinology. 1999;140:335–43. doi: 10.1210/endo.140.1.6461. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–72. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. Aids. 2006;20:879–87. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Pak JH, Huang FL, Li J, Balschun D, Reymann KG, Chiang C, Westphal H, Huang KP. Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: a study with knockout mice. Proc Natl Acad Sci U S A. 2000;97:11232–7. doi: 10.1073/pnas.210184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348:2646–55. doi: 10.1056/NEJMra021194. [DOI] [PubMed] [Google Scholar]
- Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis. 1988;158:1079–83. doi: 10.1093/infdis/158.5.1079. [DOI] [PubMed] [Google Scholar]
- Purba JS, Hofman MA, Portegies P, Troost D, Swaab DF. Decreased number of oxytocin neurons in the paraventricular nucleus of the human hypothalamus in AIDS. Brain. 1993;116(Pt 4):795–809. doi: 10.1093/brain/116.4.795. [DOI] [PubMed] [Google Scholar]
- Quirino T, Bongiovanni M, Ricci E, Chebat E, Carradori S, Martinelli C, Valsecchi L, Landonio S, Bini T, Bonfanti P. Hypothyroidism in HIV-infected patients who have or have not received HAART. Clin Infect Dis. 2004;38:596–7. doi: 10.1086/381442. [DOI] [PubMed] [Google Scholar]
- Rearden A, Hurford R, Luu N, Kieu E, Sandoval M, Perez-Liz G, Del Valle L, Powell H, Langford TD. Novel expression of PINCH in the central nervous system and its potential as a biomarker for human immunodeficiency virus-associated neurodegeneration. J Neurosci Res. 2008;86:2535–42. doi: 10.1002/jnr.21701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore D, Tu H, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is highly expressed in human thyroid. J Clin Invest. 1996;98:962–8. doi: 10.1172/JCI118880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellmeyer DE, Grunfeld C. Endocrine and metabolic disturbances in human immunodeficiency virus infection and the acquired immune deficiency syndrome. Endocr Rev. 1996;17:518–32. doi: 10.1210/edrv-17-5-518. [DOI] [PubMed] [Google Scholar]
- Sui L, Wang F, Li BM. Adult-onset hypothyroidism impairs paired-pulse facilitation and long-term potentiation of the rat dorsal hippocampo-medial prefrontal cortex pathway in vivo. Brain Res. 2006;1096:53–60. doi: 10.1016/j.brainres.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Teoh SK, Mendelson JH, Woods BT, Mello NK, Hallgring E, Anfinsen P, Douglas A, Mercer G. Pituitary volume in men with concurrent heroin and cocaine dependence. J Clin Endocrinol Metab. 1993;76:1529–32. doi: 10.1210/jcem.76.6.8501161. [DOI] [PubMed] [Google Scholar]
- Vallat-Decouvelaere AV, Chretien F, Lorin de la Grandmaison G, Carlier R, Force G, Gray F. The neuropathology of HIV infection in the era of highly active antiretroviral therapy. Ann Pathol. 2003;23:408–23. [PubMed] [Google Scholar]
- Vescovi PP, Pezzarossa A. Thyrotropin-releasing hormone-induced GH release after cocaine withdrawal in cocaine addicts. Neuropeptides. 1999;33:522–5. doi: 10.1054/npep.1999.0773. [DOI] [PubMed] [Google Scholar]
- Wiener M, Lo Y, Klein RS. Abnormal thyroid function in older men with or at risk for HIV infection. HIV Med. 2008;9:544–9. doi: 10.1111/j.1468-1293.2008.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LD, Truong MP, Barber AR, Aoki TT. Anterior pitutiary and pitutiary-dependent target organ function in men infected with the human immunodeficiency virus. Metabolism. 1996;45:738–46. doi: 10.1016/s0026-0495(96)90140-7. [DOI] [PubMed] [Google Scholar]
- Zirilli L, Orlando G, Diazzi C, Squillace N, Carani C, Guaraldi G, Rochira V. Hypopituitarism and HIV-infection: a new comorbidity in the HAART era. J Endocrinol Invest. 2008;31:33–8. [PubMed] [Google Scholar]