Abstract
Teichoic acids (TAs) are major wall and membrane components of most gram-positive bacteria. With few exceptions, they are polymers of glycerol-phosphate or ribitol-phosphate to which are attached glycosyl and d-alanyl ester residues. Wall TA is attached to peptidoglycan via a linkage unit, whereas lipoteichoic acid is attached to glycolipid intercalated in the membrane. Together with peptidoglycan, these polymers make up a polyanionic matrix that functions in (i) cation homeostasis; (ii) trafficking of ions, nutrients, proteins, and antibiotics; (iii) regulation of autolysins; and (iv) presentation of envelope proteins. The esterification of TAs with d-alanyl esters provides a means of modulating the net anionic charge, determining the cationic binding capacity, and displaying cations in the wall. This review addresses the structures and functions of d-alanyl-TAs, the d-alanylation system encoded by the dlt operon, and the roles of TAs in cell growth. The importance of dlt in the physiology of many organisms is illustrated by the variety of mutant phenotypes. In addition, advances in our understanding of d-alanyl ester function in virulence and host-mediated responses have been made possible through targeted mutagenesis of dlt. Studies of the mechanism of d-alanylation have identified two potential targets of antibacterial action and provided possible screening reactions for designing novel agents targeted to d-alanyl-TA synthesis.
INTRODUCTION
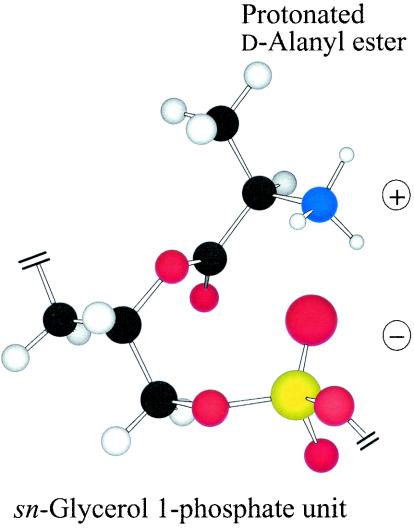
The wall of the gram-positive bacterium constitutes a multifaceted fabric that is essential for survival, shape, and integrity (493). Macromolecular assemblies of cross-linked peptidoglycan (murein), polyanionic teichoic acids (TAs), and surface proteins function within this envelope. TAs are composed of wall teichoic acid (WTA) and lipoteichoic acid (LTA) (24, 30, 33, 36, 158, 160, 291, 489, 499). WTA is covalently linked to the peptidoglycan, whereas LTA is a macroamphiphile with its glycolipid anchored in the membrane and its poly(glycerophosphate) (Gro-P) chain extending into the wall. Protonated d-alanyl ester residues (Fig. 1), one of the principal substituents of TAs in many low-G+C gram-positive bacteria, are covalently linked to these chains and provide counterions for determining the net anionic charge of the TA.
FIG. 1.
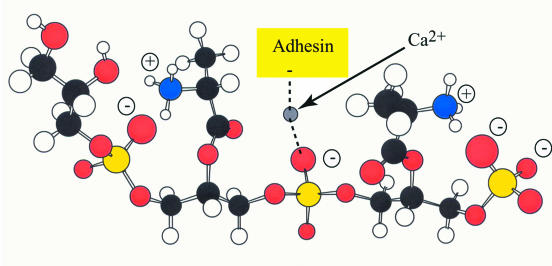
Protonated d-alanyl ester substituent linked to the 2′ hydroxyl of a Gro-P unit (sn-glycerol 1-phosphate). Ion pairing of the phosphodiester with the protonated amino group occurs on rotation of the phosphodiester linkage.
Together with peptidoglycan, WTA and LTA make up a polyanionic network or matrix that provides functions relating to the elasticity, porosity, tensile strength, and electrostatic steering of the envelope (15, 76, 125, 130, 284, 319, 474). This matrix is a polyelectrolyte gel with ion-exchange properties required for not only maintaining metal cation homeostasis and control but also assisting in the “trafficking” of ions, nutrients, proteins, and antibiotics (14, 35, 127, 199, 230, 291, 319, 483). The wall matrix is also responsible, in part, for the permeability of proteins (123, 125), the linkage of wall proteins (334, 357), and the presentation of peptidoglycan hydrolases (autolysins) and adhesins (173, 445), as well as being one of the determinants of cell surface hydrophobicity (426). Under conditions of chemiosmosis, a proton gradient further defines the ionic properties of this matrix (256, 268). Within this complex continuum of anionic charge, peptidoglycan provides its stress-bearing role against turgor pressure (267). Thus, the envelope is an organelle that provides the necessary functions needed for cellular growth of the gram-positive cell in its biological niche.
Although not all gram-positive bacteria have conventional LTA and WTA, those that lack these polymers generally have functionally similar anionic ones (413, 466). For example, lipomannan is found in place of LTA in Micrococcus luteus (379, 404). Its polyanionic character is determined by succinyl groups esterified to the mannosyl residues. In another example, growth of Bacillus subtilis in phosphate-limited medium results in the replacement of WTA with teichuronic acid, a phosphorus-free polysaccharide containing uronic acid residues (145). Each of these examples illustrates the importance of a wall anionic polymer(s) during the growth of the organism.
Both the structures and biosyntheses of WTA and LTA have been well characterized (22, 158, 160, 201, 293a, 401, 489). However, the functions of TAs within the wall matrix have been more difficult to define. The d-alanyl esters of these polymers, resulting from a single d-alanine incorporation system encoded by the dlt (for “d-alanyl-LTA”) operon (209, 367, 383), constitute important substituents for modulating the properties of the envelope in many species. For this reason, knowledge of these ester residues is essential for understanding the functions of TAs in bacterial physiology as well as in host-mediated responses.
The goals of this article are (i) to summarize the structures and functions of d-alanyl-TAs in the envelope; (ii) to describe the mechanism of d-alanine incorporation into TAs; and (iii) to review the role of the d-alanyl esters in antibiotic action, pathogenesis, adhesion, biofilm formation, and virulence. New insights are emerging which provide a greater understanding of the role played by these esters both in the growth of the bacterium and in their function in host-mediated responses. The ability to isolate mutants deficient in d-alanyl esters by targeted mutagenesis provides a tool for addressing these goals. In addition, an understanding of the d-alanine incorporation system provides screening reactions for designing novel antibacterial agents targeted to d-alanyl-TA synthesis.
Since the WTA and LTA of Streptococcus pneumoniae contain phosphorylcholine substituents instead of d-alanyl esters, the review does not address the TAs of this organism; the reader is directed to reference 164.
TEICHOIC ACIDS
Structures of WTA and LTA
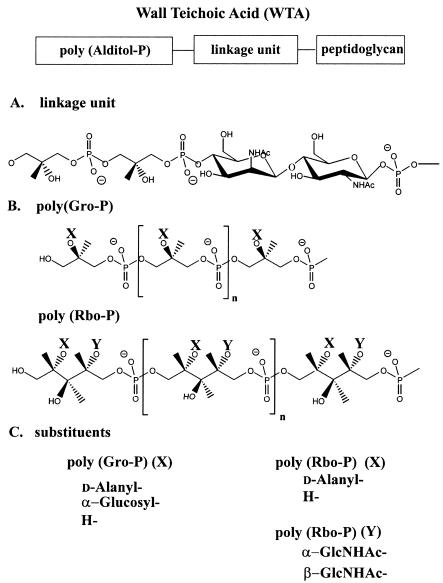
A review of the literature reveals a wide structural diversity of WTAs in gram-positive bacteria (22, 32, 149, 239, 355). Some of this diversity is confined to the presence and nature of the glycosyl substituents, d-alanyl esters, and repeating units (monomers) (11, 33, 201). The monomers are joined via anionic phosphodiester linkages to form linear chains that constitute 30 to 60% of the cell wall. Two examples are 1,3-glycerol-phosphate (-Gro-P-) and 1,5 d-ribitol-phosphate (-Rbo-P-) (11, 33). WTA is attached to peptidoglycan via the linkage unit (Gro-P)2 or 3ManNAc(β1-4)GlcNAc-P to C-6 of the MurNAc residues (Fig. 2A) (9, 102, 205, 273).
FIG. 2.
WTA. (A) Linkage unit. (B) Poly(Gro-P)(sn-glycerol 3-P) moiety from B. subtilis 168 and poly(Rbo-P) from S. aureus H. (C) Substituents on poly(Rbo-P) and poly(Gro-P) characteristic of these bacteria.
The major WTA from B. subtilis 168 is d-alanyl-[α-d-glucosylated poly(Gro-P)], with a chain length of 53 residues (range, 45 to 60) (Fig. 2B) (32, 133, 393). The degree of α-d-glucosylation is 0.8, but this has been shown to depend on the age of the cells and the Pi concentration in the growth medium (68, 190). This bacterium also contains a minor WTA, poly (3-O-β-Glu-GalNAc) (441). The genus Bacillus contains WTA with a variety of repeating units (22, 239). For examples, B. subtilis 168, B. subtilis W23, and Bacillus coagulans contain the monomers -Gro-P-, -Rbo-P-, -6-Gal(α1-2)GroP-, respectively.
Staphylococci also contain either -Gro-P- or -Rbo-P- as the repeating unit of WTA. For example, Staphylococcus aureus H contains d-alanyl-[α,β-GlcNAc-poly(Rbo-P)] glycosylated on position 4 of the d-ribitol in either an α- or β-linkage (Fig. 2C) (38, 40). S. aureus Copenhagen contains this WTA with 15% α- and 85% β-GlcNAc-poly(Rbo-P) WTA (430). On the other hand, Staphylococcus cohnii contains poly(Gro-P) WTA with glucosyl substituents (149). Although most WTAs conform to the substituted poly(1,5-Rbo-P) or poly(1,3-Gro-P) structures, there are exceptions; these include examples in which the repeating unit is either -Gro-P-glycosyl-P- or -GlcNAc-P- (18, 20, 149). In addition, other species contain arabitol-P (e.g., Agromyces cerinus) (440) or erythritol-P (e.g., Glycomyces tenuis) (403).
Without exception, the alanyl esters of TA are of the d-configuration (25). In the poly(Rbo-P) WTA of S. aureus H, the d-alanyl ester is found at position 2 of the -Rbo-P-monomer (39, 346). In this WTA, a phosphodiester anionic linkage and the vicinal 3′-OH of the ribitol flank the d-alanyl ester. In contrast, two phosphodiester linkages flank the d-alanyl ester of poly(Gro-P) TAs. When the 2′-OH of glycerol is substituted by a glycosyl unit, e.g., in group D streptococci, the d-alanyl esters are substituents on the sugar (496).
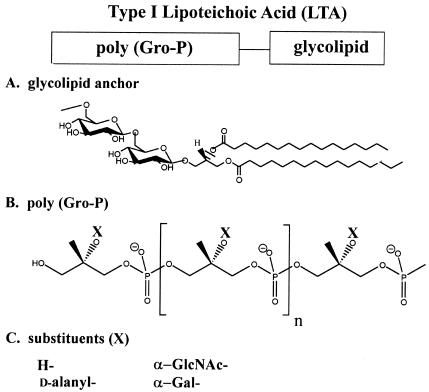
Many LTAs, originally named membrane TAs (17, 31, 291), are macroamphiphiles composed of poly(Gro-P) (attached to C-6 of the nonreducing glucosyl of the glycolipid anchor [type I] [160, 170, 476, 498, 499]). The glycolipid is Glc(β1-6)Glc(β1-3)(gentiobiosyl)diacyl-Gro in staphylococci, bacilli, and streptococci (Fig. 3A) (135, 160, 428). The chain length of poly(Gro-P) (Fig. 3B) varies from 14 to 33 and from 5 to 50 Gro-P units in LTA isolated from Enterococcus faecalis and Lactobacillus rhamnosus ATCC 7469, respectively (301, 394). The polydispersity of LTA in these organisms confirmed that observed originally in Streptococcus agalactiae (331). In contrast to this LTA type, Lactococcus garvieae and Clostridium innocuum contain type II and type III LTA, respectively. Type II LTA has a -GalGal-Gro-P- repeating unit, while type III LTA has a -Gal-Gro-P- repeating unit (160). Type I LTA occurs in 84 of 86 strains of oral streptococci (the exceptions are Streptococcus mitis and Streptococcus oralis) (221). Streptococcus sp. strain DSM 8747, which is closely related to S. pneumoniae (with phosphorylcholine TAs), contains type I LTA with an average chain length of 10 Gro-P units partially substituted by d-alanyl esters (417). The chain length distribution varies from 7 to 17, and the glycolipid anchor is a rare 3-O-(β-d-galactofuranosyl)-1,2-diacylglycerol (417). Based on the side chain substituents of LTA, Bacillus strains/species are divided into group A and group B. In group A (six members), α-GlcNAc is linked to the -Gro-P- repeating unit, while in group B (five members) α-Gal is linked (Fig. 3C) (239). B. subtilis is an example of group A, and Bacillus megaterium is an example of group B. Neither LTA nor a related anionic polymer was found in Bacillus circulans and Bacillus polymyxa (240). Comparative studies of 13 species of lactobacilli revealed that 8 contain both LTA and WTA while 5 have only LTA (41, 254). All possess a type I d-alanyl-poly(Gro-P)[Glc(β1-6)Gal(α1-2)Glc(α1-3) diacylglycerol] (353). A significant fraction of the glycolipid anchor is also Glc(β1-6)Gal(α1-2)6-O-acyl-6Glc(α1-3) diacylglycerol (167, 353). While this example implies a defined structure, Fischer (159) has emphasized that microheterogeneity of LTAs is the result of several variables: (i) fatty acid composition, (ii) kind and extent of glycosyl substitution, (iii) length of hydrophilic chain; and (iv) degree of d-alanylation.
FIG. 3.
Type I LTA. (A) Glycolipid anchor. (B) Poly(Gro-P) (sn-glycerol 1-P). (C) Substituents (X).
In the high-G+C (>55 mol%) subdivision, LTA is generally replaced by lipoglycans (160, 466). For example, Bifidobacterium bifidum contains a macroamphiphile with single Gro-P units attached to the glycan backbone by phosphodiester linkages and substituted with l-alanyl esters (156, 238). The lipoglycan of M. luteus is a mannan substituted with succinyl substituents esterified to approximately 25% of the mannose residues. Sutcliffe (463) proposed that the diversity of these cell surface components might be useful in classification of the high-G+C and low-G+C (<50 mol%) gram-positive bacteria.
Pathways of LTA and WTA Biosynthesis
With the exception of d-alanyl esters, WTA and LTA are assembled via different pathways (489). For example, in organisms that have the repeating unit -Gro-P- for both LTA and WTA (such as B. subtilis 168), the units are derived from different sources. WTA contains sn-glycerol 3-phosphate derived from CDP-glycerol (78, 201), whereas LTA contains sn-glycerol 1-phosphate derived from phosphatidylglycerol (147, 176, 315). Because of the different origins of the Gro-P units, the chains are not stereoisomerically identical. Thus, LTA is not a precursor of WTA.
WTA assembly.
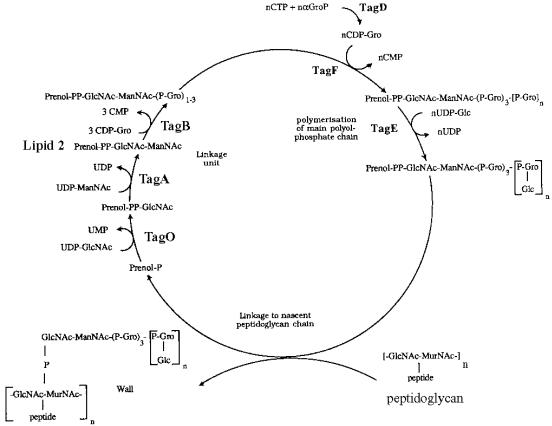
The assembly of WTA requires four phases: (i) synthesis of the (Gro-P)3-ManNAc-GlcNAc-PP-polyprenol carrier (linkage unit carrier) (9, 200, 201, 502), (ii) polymerization of the poly(alditol-P) on this lipid intermediate (78, 154, 183, 198, 205, 294, 326, 431), (iii) glycosylation of this moiety (184), and (iv) attachment of the WTA linkage unit to peptidoglycan. Undecaprenol phosphate is the carrier lipid on which this linkage intermediate is assembled prior to attachment (69, 201, 502). This lipid is identical to that involved in peptidoglycan synthesis (490). Chain elongation in WTA assembly occurs by successive addition of monomer from either CDP-glycerol or CDP-ribitol to the terminal alditol-P that is distal to the linkage unit (235, 259).
The genes (tagABDFEGHO) encoding the enzymes for the assembly of WTA in B. subtilis 168 have been isolated and characterized (Fig. 4) (293a, 401). These are organized into two divergently transcribed operons (divergon), tagAB and tagDEF (224, 329). The one-gene operon tagO and the two-gene operon tag GH, involved in WTA translocation, are both independent of the above divergon (293a, 451). tagF encodes the polymerase responsible for formation of the poly(Gro-P) moiety from CDP-glycerol (399, 431), tagD encodes the glycerol 3-phosphate cytidyltransferase (382), and tagE encodes the enzyme for glucosylation of the poly(Gro-P) from UDP-glucose (Fig. 4). The regulatory elements of the divergon, two σA-controlled promoters, are further modulated by signals coupled to cell division as well as to growth phase, media richness, Pi concentration, and temperature (328). Evidence pointing to differences in the septal and cylindrical wall in this strain may be correlated with the differential control of WTA synthesis determined by the regulatory elements of this divergon (328). Growth of strains with temperature-sensitive tag mutations (exception tagE) at the restrictive temperature caused an immediate cessation of WTA synthesis, while the synthesis of LTA and phospholipid was not affected (398, 402). In contrast to tagF, no conditional lethal phenotypes were observed for tagE. Thus, glucosylation is not essential for growth. Interestingly, the synthesis of the poly(Rbo-P) WTA of B. subtilis W23 is directed by the tar genes, also organized in two divergently transcribed operons but with different regulation from that observed in B. subtilis 168 (292). In W-23 four promoters are needed for poly(Rbo-P) WTA synthesis, whereas in B. subtilis 168 two promoters are needed for poly(Gro-P) WTA synthesis.
FIG. 4.
Assembly of the glucosylated poly(Gro-P) WTA of B. subtilis 168. TagD, TagE, and TagF are enzymes in WTA synthesis, and TagO, TagA, and TagB participate in linkage unit synthesis. Reprinted from reference 399 and amended with permission of the authors and publisher.
The synthesis of the linkage unit requires the sequential transfer of GlcNAc-1-phosphate from UDP-GlcNAc (TagO) and ManNAc from UDP-ManNAc (TagA) to polyprenyl phosphate to form lipid 2 (Fig. 4) (9, 205, 293b, 451, 502). Completion of the linkage unit is accomplished by the addition of two or three Gro-P units from CDP-glycerol to this lipid (TagB). In early studies of a putative LTA carrier (LTC) (154, 326), it was observed that poly(Rbo-P) polymerase from S. aureus is strongly inhibited by d-alanyl esters (157, 271). While the role of this LTC in WTA synthesis has not been supported (157, 335), the inhibitory effect of d-alanyl ester residues on the polymerase (169, 271) remains a distinct possibility in regulating the synthesis of WTA on the linkage unit (157). Both the LTC and the linkage unit contain at least two or three Gro-P units. A similar type of inhibitory effect has also been observed in Bacillus cereus, where the glycosyltransferase is inhibited by the d-alanyl esters of the LTA acceptor (442). Short-chain, lipophilic LTA readily accepts d-alanyl esters (90, 155), and thus the (Gro-P)n moiety of the linkage unit lipid, analogous to the terminus of LTC, may also accept d-alanyl esters, resulting in the inhibition of the poly(Rbo-P) or poly(Gro-P) polymerases. Under conditions of high d-alanylation, the assembly of WTA on the linkage unit may be inhibited. Alternatively, under conditions of low d-alanylation, the assembly of WTA may be enhanced. This putative regulatory mechanism clearly warrants further study.
LTA synthesis.
Biosynthesis occurs via the transfer of Gro-P units from phosphatidylglycerol with the formation of elongated LTA and diacylglycerol (80, 147, 272, 469). In Enterococccus hirae ATCC 9790, Gro-P moieties are added sequentially to the glycolipid acceptor, phosphatidylkojibiosyldiacylglycerol (176). This observation is consistent with the in vivo pulse-chase experiments that implicated phosphatidylglycerol as the donor of Gro-P units (147, 185). On the other hand, the discovery of a series of oligophosphoglycerophospholipids derived from phosphatidylglycerol in Streptococcus sanguis suggested that these lipids may be intermediates in the assembly of LTA and that the mechanism of assembly may differ from that observed in E. hirae (92). One of these lipids, phosphatidylglycerophosphoglycerol, is formed from two molecules of phosphatidylglycerol. The resulting diacylglycerol is phosphorylated by diacylglycerol (diglyceride) kinase and reutilized for phosphatidylglycerol synthesis (469). The elongation of the poly(Gro-P) moiety would appear to occur by the distal (external) addition of Gro-P units (80). Using sequential cleavage by phosphodiesterase/phosphatase, Taron et al. (469) found that the newest Gro-P units added were the ones first cleaved from the in vitro-synthesized LTA. Distal addition can occur only while the growing poly(Gro-P) moiety is in contact with the cytoplasmic membrane. The polydispersity of the LTA chain length suggests that chain growth may terminate at any point in elongation (300). Comparative studies of LTA glycosylation in B. subtilis further illustrate aspects of structural diversity. Glycosylation of the -Gro-P- repeating unit (three strains, group A) is the result of GlcNAc transferases that utilize β-GlcNAc-P-polyprenol (240, 443). Another intermediate, α-GlcNAc-P-polyprenol, is the precursor of β-linked glycosyl groups in several Bacillus species. The mechanism of elongation, the organization of this assembly system, and the attachment of the glycolipid anchor are not well understood.
It was hypothesized that a specific glycerol phosphotransferase targets the glycolipid with a single Gro-P unit and that this modified glycolipid would serve as the growing point for the assembly of the poly(Gro-P) chain (161, 272, 417). In Streptococcus sp. strain DSM 8747, the least abundant glycolipid (monohexosyldiacylglycerol) is selected of the four for the membrane anchor (417). Several short-chain d-alanyl-lipophilic LTAs that could participate in this targeting process have been described (71, 287). To test this mechanism, mutants deficient in glycolipid anchor were sought. The ypfP gene encoding the diglucosyldiacylglycerol synthase, responsible for synthesis of this anchor, was isolated and characterized from S. aureus and B. subtilis (249, 250, 260). Inactivation of ypfP resulted in LTA with diacylglycerol as the membrane anchor (260), an LTA similar to that described by Chiu et al. (92). In 1976, Button and Hemmings (79) observed the loss of the glycolipid anchor in a phosphoglucomutase mutant of Bacillus licheniformis. The poly(Gro-P) was linked, instead, to diacylglycerol. In at least two organisms, B. coagulans and B. megaterium, the glycolipid is also normally replaced by diacylglycerol as the LTA anchor (160, 239). While not proven, these observations are consistent with the assembly of the poly(Gro-P) moiety on phosphatidylglycerol with subsequent transfer to the glycolipid anchor.
The synthesis of peptidoglycan, LTA, and WTA occurs within the proton gradient of the membrane-wall matrix of the growing cell (247, 257). Harrington and Baddiley (204) found that the ionophore valinomycin disrupted this gradient and thereby inhibited the synthesis of WTA and peptidoglycan. In contrast, this ionophore had no effect on LTA assembly. Since each of the phases of WTA synthesis was detected on the outer leaflet of the protoplast membrane (50), it was suggested that an enzyme complex rotates or reorients between the inner and outer leaflets. At least one of these phases most probably utilizes a translocation step facilitated by the ATP-binding cassette transporter (TagGH) encoded by the tagGH operon (293). The dependence of WTA synthesis on the proton gradient (34) is consistent with an energy requirement for a transport system necessary for the translocation of intermediates across the membrane. Whether the proton gradient is coupled to the rotation of an enzyme complex or used to drive a transport system in the translocation of a polymer is not known.
Topography of TAs
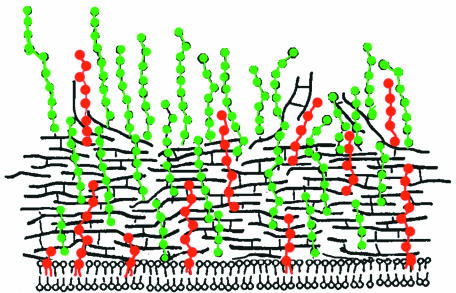
Archibald et al. (19) proposed that the chains of WTA in B. subtilis are arranged perpendicular to the surface of the wall. A fraction (50%) of this WTA is located in a “fluffy”-layer region beyond the wall (63, 132). It was postulated that this fibrous layer reflects a region of autolysin-catalyzed degradation of outer nonstressed peptidoglycan (189). A similar organization was also observed in many strains of S. aureus (23, 478). This topography is illustrated in Fig. 5.
FIG. 5.
Topography of WTA and LTA in S. aureus. Peptidoglycan (black lines) and WTA (green symbols) adapted with permission of the author and publisher of reference 478. Topology of LTA (red symbols) and WTA derived from references 19, 158, 189, 481, and 500.
Many bacteriophages show binding specificity for accessible WTA (12). By using pulsed incorporation of WTA in B. subtilis, it was observed that phage bind initially only to the inner surface of the wall (7, 13). Maximal binding to the cell surface occurred after 0.75 to 1 generation. These findings indicated that the assembly of WTA initiates at the wall-membrane interface at many sites and that this is followed by movement of covalently linked WTA-peptidoglycan through the thickness of the cylindrical wall. At longer times after the pulse, phage binding was detected only at the polar region. Using differential staining of WTA and teichuronic acid, Merad et al. (339) established that in the transition from phosphate-limited to phosphate-replete growth, the new WTA is evenly distributed along the inner surface of the cylindrical region of the B. subtilis cell. Therefore, insertion of new WTA occurs at the membrane concurrently with peptidoglycan in an “inside-to-outside” growth mode (119, 151, 269, 327, 339, 396, 397), with a slower appearance in the cell poles (96).
LTA is found at the interface of the cytoplasmic membrane and wall (157, 291, 489). Using immunoelectron microscopy, Aasjord and Grov (1) established that LTA in S. aureus Cowan not only is attached to the membrane but also penetrates the wall. In Lactobacillus fermentum, an organism that does not contain WTA, one portion of the LTA is exposed on the cell surface while a second portion is concentrated at the membrane (481). The surface-oriented LTA is responsible for the serological specificity of this species (41). Based on turnover experiments, the LTA is released from the cell surface during growth (107, 344, 499). This transient LTA is noncovalently associated with wall components (e.g., peptidoglycan and proteins) through ionic interactions (157, 230). In group A streptococci, the glycolipid moiety of the LTA becomes surface oriented as a result of interaction with the M protein. Thus, one of the determinants of cellular hydrophobicity in this bacterium is the anchoring of LTA with the hydrophobic moiety to the medium (107, 344, 373).
TAs and the Glycocalyx
Many bacteria are enveloped with an additional matrix of polymers known as the glycocalyx (104, 150, 179, 288). This matrix is distal to the wall peptidoglycan and in some cases includes an S layer, capsule, or slime layer. These highly hydrated (99%) structures play roles in adherence, access of macromolecules and ions, and virulence. In several bacteria, coalescence of adjacent glycocalyces leads to biofilm formation (150, 179, 444). The glycocalyx is composed of exopolysaccharides (467), WTA and LTA (74, 234, 376), wall-associated proteins (253), and a variety of membrane constituents (179). A major fraction of the LTA-antibodies-protein A-gold complexes in group B streptococci is located within the glycocalyx (capsule) and organized as long, fibrous threads spanning this structure (376). These threads appear to be fibrillar in the glycocalyces of several organisms. Electron microscopy with the cationic dye ruthenium red defined regions of anionic sites on the fibrils from S. aureus ATCC 6538P (321). In Staphylococcus epidermidis, the solid component of the slime layer is approximately 80% (wt/wt) TA and 20% protein (234). Based on immunochemical labeling studies and LTA turnover experiments, Wicken et al. (497, 501) concluded that LTA would be expected to be a component of the glycocalyx and thus to play an important role in its function. It was proposed that the spatial divisions of the wall and glycocalyx are not rigid but “represent regions in a continuum and individual types of cell-wall-associated polymers may be distributed across the continuum both spatially and also temporally if they are in transit” (501).
d-ALANYL ESTER
Content in WTA and LTA and Relationship to Growth Conditions
The d-alanyl ester content in WTA and LTA is highly variable. The molar ratios of d-alanine to P (degree of d-alanylation) in LTA from a variety of species vary from not detectable to 0.88 (157, 158, 165, 428). WTA generally has a lower ratio of d-alanine to P than does LTA. For example, the ratio of d-alanylation (WTA/LTA) in S. aureus is 0.75 (161, 312). Growth of B. subtilis in two media of different richness resulted in ratios of 0.2 and 0.5 (383).
Although these esters are constituents of TAs in many bacteria, some bacteria appear to lack d-alanyl esters: e.g. E. hirae ATCC 9790 (157), Micrococcus varians ATCC 29750 (157), S. cohnii (428), and S. pneumoniae (158). TA from actinomycetes also contains no d-alanyl esters (355). Type II and type III LTAs do not contain d-alanyl esters. Iwasaki et al. (239) found that LTAs of Bacillus lacking the glycolipid anchors are also deficient in d-alanyl esters. Thus, d-alanyl ester substituents are generally found in the low-G+C subdivision of gram-positive bacteria, mostly those containing type I LTAs.
The d-alanyl ester contents of LTA and WTA of S. aureus and B. subtilis are a function of the pH of the growth medium (21, 145, 146, 312). In the former, the ester content varies from 0.75 at pH 6.1 to 0.07 at pH 8.1, and thus at the higher pH only 9% of the ester content was observed (312). MacArthur and Archibald (312) reasoned that this observation might be incompatible with a regulatory function of d-alanyl-LTA. It was proposed that the newly synthesized d-alanyl-LTA at pH 8.1 is highly substituted and that the ester groups are subsequently lost by base-catalyzed hydrolysis. The newly synthesized molecules would fulfill the functions that require the presence of d-alanyl esters, while the alanine-free molecules would be excreted or utilized when esters are not required. Growth of B. subtilis in media of decreasing pH (i.e., 7 to 5) resulted in a progressive increase in the d-alanyl ester content of WTA (146). Ellwood and Tempest (145) concluded that the increased ester content is necessary for the proper functioning of TA in the cell at higher proton concentrations. This conclusion may be important for interpreting the observation that S. mutans deficient in d-alanine esters loses its acid tolerance response (65).
In addition to medium pH, the degree of d-alanylation is a function of the temperature (233, 370). For example, growth of the facultative thermophile B. coagulans at 55°C resulted in a threefold decrease in ester content compared to cells grown at 37°C. In addition, sublethal heating of S. aureus resulted in a loss of 65% of the d-alanyl ester content of TA. “Repaired” cells contained four times more d-alanine than did the freshly heated cells (233).
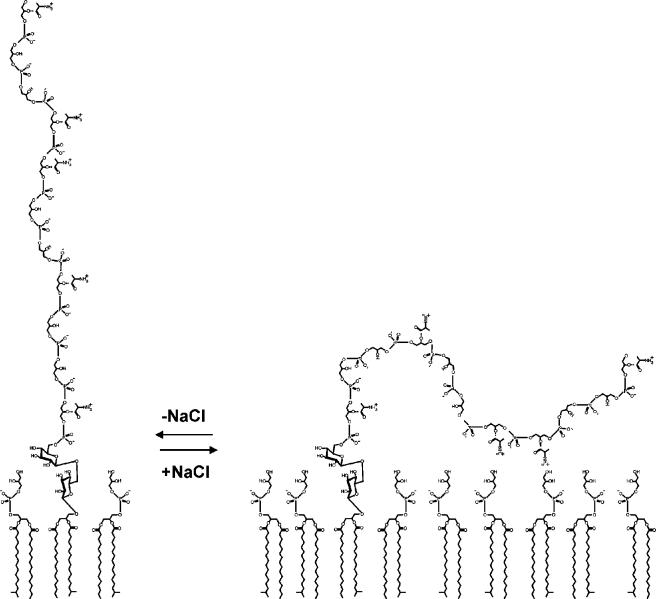
The degree of d-alanylation is also affected by growth on media containing increasing concentrations of NaCl. For example, d-alanylation of S. aureus LTA decreased from 0.71 to 0.33 (d-alanine/P) as the NaCl concentration was increased from 0.2 to 7.5% (171, 172). Koch et al. (270) suggested that the high concentration of NaCl directly affects one of the enzymes involved in the synthesis of d-alanyl-LTA. Both NaCl and KCl markedly decreased the d-alanylation of membrane-associated LTA when measured in an in vitro system from L. rhamnosus (414).
In contrast to separate biosynthetic pathways for WTA and LTA, the d-alanyl-ester substituents of WTA are derived from those of d-alanyl-LTA (197). This is consistent with a single dlt operon encoding the machinery of the d-alanine incorporation system (383). One of the remarkable features of the d-alanyl esters of TA is their dynamic turnover. For example, a t1/2 of 37 min was observed for d-alanyl-LTA in S. aureus growing at pH 7. It was postulated that the turnover is an enzyme-catalyzed process (197). In toluene-treated cells of this organism, the d-alanyl esters are lost from LTA and replaced by the ATP-dependent incorporation of new ones (270). “Reesterification” of vacant sites in LTA and WTA maintains the d-alanyl ester content of both TAs. The rate of d-alanyl-LTA synthesis is correlated with the rates of ester loss that occurs through transfer to WTA and through “base-catalyzed” hydrolysis. Thus, from both in vitro and in vivo pulse-chase experiments, it was concluded that the d-alanyl esters of LTA are the precursors of those in WTA (197, 270).
Of major importance to this review is the observation that the growth of B. subtilis under conditions of Mg2+ limitation resulted in elevated levels of WTA (143). Organisms grown under these limiting conditions have a higher capacity and affinity for binding Mg2+ (145). Higher growth rates in Mg2+-limited cells also resulted in an increased amount of TA in the wall. These observations strongly support a requirement for TA in cation assimilation and scavenging from the environment.
Chemical Reactivity
Knowledge of the chemical reactivity and stability of the d-alanyl esters is important for understanding the functions, distribution, and transacylation of these esters in the envelope. They are unusually labile at alkaline pH. For example, at pH 8 and 37°C, the t1/2 of d-alanyl-LTA from L. rhamnosus is 3.9 h (90). In contrast, the t1/2 at pH 6 and 37°C is >10,000 h. When making stability measurements of d-alanyl-LTA from S. aureus, it was observed that >98% of the ester content was recovered after 9 h at 25°C in the pH range from 4.0 to 6.8 (168) but that only 3% of the content was recovered when d-alanyl-LTA was incubated at pH 8. These in vitro studies are consistent with the in vivo growth experiments described in the previous section. Esterification of the carboxyl group of alanine increases the acidity of the protonated amino group by ∼2 pKa units (101). Thus, in the pH range from 7 to 8, the concentration of the nonprotonated amino function is increased in d-alanyl-LTA compared with that of the zwitterionic amino acid.
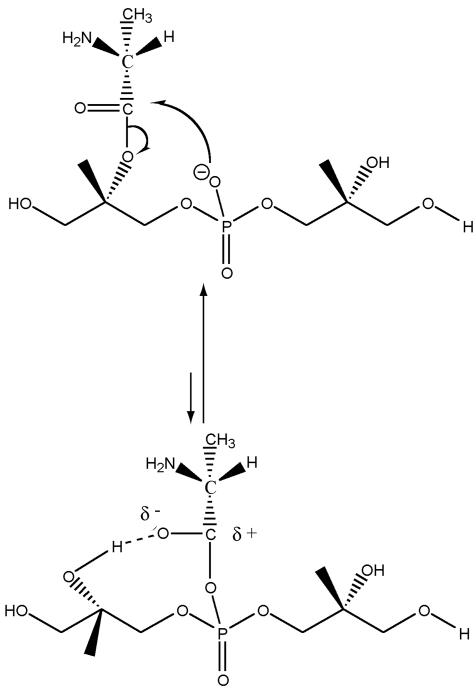
To explain the enhanced base-catalyzed hydrolysis of d-alanyl-LTA, we suggest that in the unprotonated form, the d-alanyl ester forms a transient d-alanyl-phosphodiester intermediate stabilized by a hydrogen bonding to an adjacent 2′-hydroxyl of a Gro-P unit (Fig. 6). This structure would be more susceptible to base-catalyzed hydrolysis and thus would account for the lability of the d-alanyl ester at pHs of >7. Under conditions of protonation, the amino group forms an ion pair with the anionic phosphodiester, hindering the formation of this intermediate and resulting in increased stability of the d-alanyl ester in the range from pH 5 to 7. Thus, the features that determine the reactivity and stability of the ester provide a basis for interpreting a number of experimental observations to be described in this review.
FIG. 6.
Formation of the acyl phosphodiester intermediate. Stabilization by hydrogen bonding of the C-2 hydroxyl of glycerol increases the electrophilicity of the carbonyl carbon. Protonation of the d-alanyl ester would result in ion pair formation with the phosphodiester and thus would inhibit the formation of the intermediate.
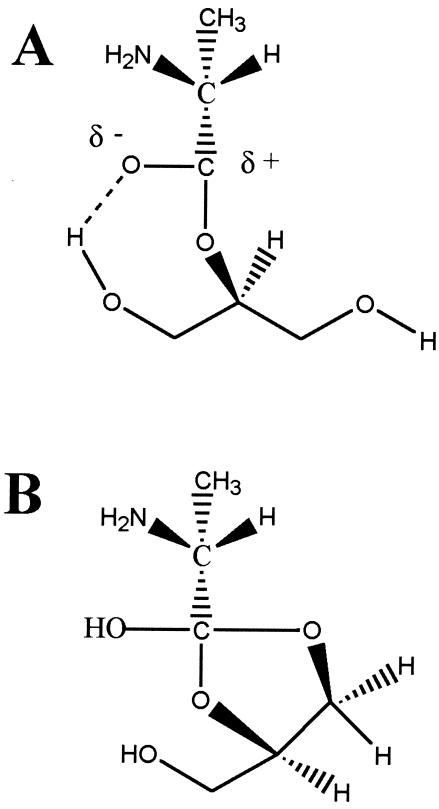
The vicinal hydroxyl groups in d-alanyl-glycerol play a role in determining the stability of this ester (90, 168). Compared with the stability of d-alanyl-LTA from L. rhamnosus at pH 6.0, the t1/2 of d-alanyl-glycerol is 8.8 h at 37°C. The instability of d-alanyl-glycerol at this pH can be partially explained by the inductive effect of the hydroxyl groups that increase the electrophilicity of the carbonyl carbon (75). In addition, hydrogen bonding of the -OH to the oxygen atom of the carbonyl (75, 504) enhances its electrophilicity (Fig. 7A). The ease of acyl migration is illustrated by the postulated cyclic ortho diester shown in Fig. 7B. These suggestions are consistent with the chemical reactivities of hydroxyethyl-d,l-alanate and phosphohydroxylethyl-d,l-alanate in 0.1 M hydroxylamine at pH 7.4 and 37°C (439).
FIG. 7.
(A) Enhanced electrophilicity of the carbonyl carbon in d-alanyl-glycerol by hydrogen bonding to the C-1 hydroxyl of glycerol. (B) Migration of the d-alanyl ester via the cyclic ortho ester intermediate. These structures are based on those proposed for acyl mobility and reactivity in aminoacyl-tRNA (75, 192, 504).
The reactivity of phosphohydroxyethyl-d,l-alanate in 0.1 M hydroxylamine at pH 7.4 is similar to that of the type 1 LTA (439). In the pH range from 5 to 6, the anionic linkages apparently shield the carbonyl carbon from nucleophilic attack, resulting in a greater stability of the ester while maintaining its reactivity. In d-alanyl-poly (Rbo-P) WTA, the d-alanyl ester is flanked by both a phosphodiester anionic linkage and a vicinal OH group. Thus, the stability of this ester is different from that of d-alanyl poly(Gro-P) LTA and d-alanyl-glycerol. In group D streptococci, the d-alanyl esters of LTA are found on the glucosyl units at either position 3 or position 4 (496). Since no cis-vicinal OH groups are present, these esters are appreciably more stable to alkali than are those of type I d-alanyl-LTA.
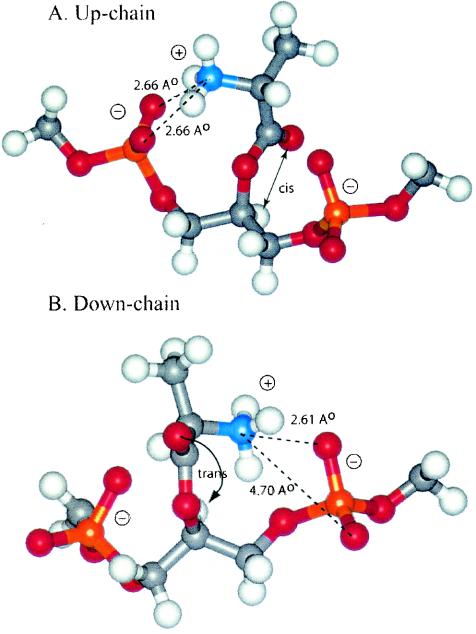
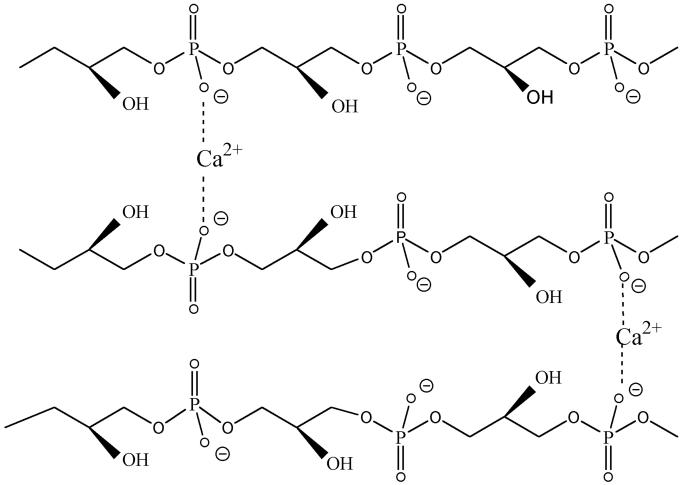
In 31P nuclear magnetic resonance (NMR) spectra of L. rhamnosus LTA substituted with d-alanine, phosphorus has two additional peaks (0.57 and 0.62 ppm) in addition to the primary resonance at 0.96 ppm. These shifts would appear to be the result of differences in ion pairing between the amino function of the chiral d-alanyl residue and the two adjacent phosphodiester anionic linkages (45). Using MNDO-PM3 predictions, Arnold and Neuhaus modeled two energy-minimized conformations, one in which the protonated amino group forms ion pairs with the up-chain phosphodiester and one in which the protonated group forms ion pairs with the down-chain phosphodiester (F. Arnold and F. Neuhaus, unpublished observation) (Fig. 8). In the up-chain ion pair, the protonated amino group can form symmetrical hydrogen bonds with the nonbridging oxygens, while in the down-chain ion pair, one hydrogen bond would appear to be formed with the nonbridging oxygen and one is formed with the bridging oxygen of the phosphodiester linkage. Thus, the rotational characteristics of the d-alanyl ester, determined by the cis-trans orientation of the carbonyl oxygen and the C-2 proton of the glycerol, allow for the different interactions in the two conformations. While this review emphasizes the role of the d-alanyl ester in decreasing the net anionic charge of TA, another physical feature that has not been quantitated is the hydrophobic effect contributed by the d-alanyl residues to LTA and WTA. As described below, the increasing lipophilicity of the LTA determined by d-alanyl esters may play a significant role in a number of host responses.
FIG. 8.
Predicted conformations of the d-alanyl ester on (Gro-P)2Gro. Two conformations are shown: up-chain ion pairing (A) and down-chain ion pairing (B). In panel A, the N O distances are both 2.66 Å. In panel B, the corresponding distances are 2.61 and 4.70 Å. In panel A, the carbonyl oxygen and the C-2 proton of glycerol are cis, and in panel B they are trans. Resonance stabilization in the ester linkage determines a rotational barrier (495) between the two conformers. For this figure, the flanking glycerol residues are truncated. The conformations were calculated by the semi-empirical molecular orbital method, MNDO-PM3 (457, 458; Arnold and Neuhaus, unpublished).
Distribution in LTA
A key feature of the d-alanyl esters of isolated LTA is their even distribution along the poly(alditol-P) chain. Fischer et al. found that the esterification of LTA from S. aureus occurs within a relatively narrow substitution range and that no alanine-free species are present (168, 171). Stepwise hydrolysis of the LTA revealed a uniform (even) distribution of the d-alanyl esters (168). In additional studies it was observed by hydrophobic interaction chromatography that the molar ratio of d-alanine to P increased from 0.54 to 0.81 as the length of the hydrophilic chain decreased from 39 to 16 Gro-P units (159, 162). An inverse relationship was also found with E. faecalis LTA by using anion-exchange chromatography (302). As the chain length increased, the ratio decreased from 0.53 to 0.23. These observations imply that shorter-chain LTA has a higher degree of d-alanylation than does longer-chain LTA. The implications of this observation are not understood. In Lactobacillus lactis Kiel, the distribution of the d-alanyl esters is correlated with the random distribution of the α-d-galactopyranosyl residues of the LTA (434).
A random distribution of d-alanyl esters was also deduced for LTA from L. fermentum and Enterococcus faecium by using 31P NMR (45). d-Alanine incorporation in toluene-treated cells of L. rhamnosus also did not show a gradient of d-alanyl ester label along the poly(Gro-P) backbone even though a gradient of Gro-P addition was easily observed during LTA synthesis (91). The random distribution of d-alanyl esters would appear to have significant implications for the transacylation and redistribution of these esters within the wall.
We propose that transacylation of d-alanyl esters could occur during the isolation and purification of LTA and that the apparent even or random distribution of the esters may, in fact, be the result of events occurring after cell death. From the time of LTA isolation to the time of measuring the distribution of d-alanyl esters, a variety of manipulations, e.g., extraction, purification, and concentrating steps, have been performed. While transacylation has not been measured at each of these steps, migration of d-alanyl esters from short-chain LTA to long-chain LTA was easily measured in protein-free LTA micelles (91). Therefore, the distribution of d-alanyl esters in the TA at the time of exponential or balanced cell growth is probably not really known. It is conceivable that during the time of growth a gradient of d-alanyl esters across the wall may exist, determined by a variety of factors, e.g. the proton gradient (see “Functions of teichoic acids” below). At the time of cell death, this gradient collapses and redistribution of the esters occurs. Clearly, additional experiments are required to assess the importance of this proposal.
SYNTHESIS OF d-ALANYL-LTA
Overview
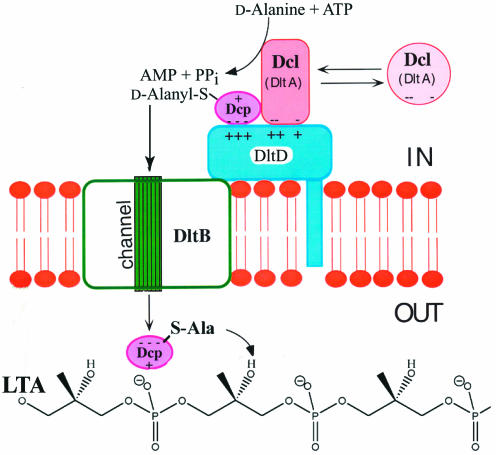
The synthesis of d-alanyl-LTA requires four proteins that are encoded by the dlt operon (Fig. 9). Two of these are the 56-kDa d-alanine:d-alanyl carrier protein ligase (AMP forming) (Dcl) and the 8.8-kDa d-alanyl carrier protein (Dcp). In addition to the genes encoding Dcl (dltA) and Dcp (dltC), dltB and dltD of this operon encode a transport protein (DltB) and a membane protein (DltD) that ensures the ligation of d-alanine to Dcp. Thus, incorporation of d-alanine is accomplished in the two-step reaction sequence:
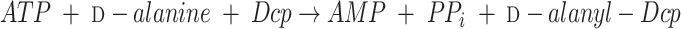 |
(1) |
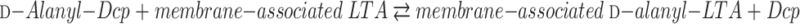 |
(2) |
FIG. 9.
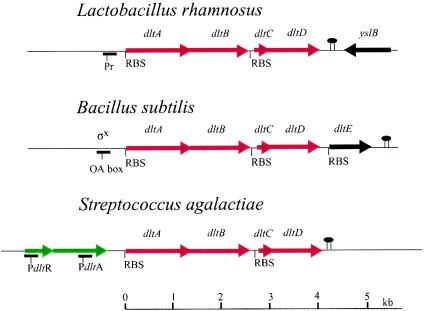
Comparison of the dlt operons from L. rhamnosus, B. subtilis 168, and S. agalactiae. The accession numbers are AF192553 (U43894), X73124, and AJ291784, respectively. In addition, the sequences for dlt from L. monocytogenes (AJ012255), S. mutans (AF051356; AF049357), S. aureus (AF101234; D86240), S. pneumoniae R6 (AE008562), L. lactis (AE006358), S. xylosus (AF032440), S. pyogenes (AE004092), S. gordonii DL1 subsp. Challis (AF059609), and L. plantarum (NC_004567) have been determined. For alignment and comparison of the dlt proteins, the http://genolist.pasteur.fr/SubtiList site is invaluable. Each of the red genes is common to all dlt operons. The green genes in S. agalactiae represent a novel two-component regulatory system (405). The genes in black are not required for d-alanylation. • is the rho-independent terminator.
In this sequence, d-alanyl-Dcp provides the essential link between the ligase (Dcl) and the d-alanylation of LTA. Transfer of the activated d-alanine from this intermediate requires that the acceptor LTA be membrane associated.
Proteins of the dlt Operon
In 1960, Baddiley and Neuhaus (43) detected an enzyme that activates d-alanine by using a pyrophosphoryl cleavage of ATP. Isolation of the gene encoding the activating enzyme (dltA) (Fig. 9) from L. rhamnosus ATCC 7469 provided the key for identifying the role of this enzyme in d-alanine incorporation (209, 367). The enzyme is a member of a large protein family that both activates and transfers amino or fatty acids via a 4′-phosphopantetheine prosthetic group of a carrier protein or coenzyme A CoA (262). It contains 7 of the 10 highly conserved consensus sequences (A2, A3, A4, A5, A7, A8, and A10) of the nonribosomal peptide synthetases (adenylation domain) (209) described by Konz and Marahiel (278). A heat-stable protein, which was formerly designated the d-alanine:membrane acceptor ligase (309, 414), contains this prosthetic group and functions as the d-alanyl carrier protein (Dcp) (117, 211). Thus, Dcl (DltA) not only activates d-alanine but also ligates the activated ester to the 4′-phosphopantetheine prosthetic group of the carrier protein. Therefore, the activating enzyme is now designated d-alanine:Dcp ligase (AMP forming).
In addition to dltA and dltC, the operon (Fig. 9) contains two additional genes, dltB and dltD. The hydropathy profile of DltB shows a pattern of 12 putative membrane-spanning domains (367). A BLAST search with DltB from L. rhamnosus identified regions of DltB with sequence similarity to a variety of transport proteins in the major facilitator superfamily and ATP-binding cassette family. Three of these proteins include proton antiporters that pump compounds (e.g., tetracycline, glycerol 3-phosphate, and gluconate) from the cytosol at the expense of the proton motive force. Amiloride, a pyrazinoylguanidine inhibitor of Na+ channels (263), prevents the synthesis of d-alanyl-LTA in the in vitro incorporation system when Na+ is the only monovalent cation (367). No effect was observed when K+ replaced Na+. While it is not established, we suggest that one of the functions of DltB is the secretion of unfolded d-alanyl-Dcp. The reversibility of the thermal denaturation of Dcp (485) is consistent with this suggestion.
Comparison of DltB with a variety of O-acyltransferases identified two conserved motifs that may also link this transport protein to a superfamily of membrane-bound O-acyltransferases (219). It is of interest that this family also includes AlgI, involved in the O acetylation of alginate in Pseudomonas aeruginosa (174, 175). Based on low homology to transferases requiring polyprenol, it was proposed that undecaprenol-P might be an intermediate membrane acceptor in the d-alanine incorporation system (211). However, compelling evidence for d-alanyl-P-polyprenol is lacking, and it has therefore been concluded that this lipid is not an intermediate in d-alanine incorporation (F. C. Neuhaus, unpublished observations). Whether DltB functions in the actual secretion of d-alanyl-Dcp, whether it functions as an acyltransferase, or whether it is bifunctional is not known.
The membrane protein, DltD, functions in the selection of the correct carrier protein, Dcp, for ligation with d-alanine (reaction 1 [above]) and in the hydrolysis of mischarged d-alanyl-ACPs (118). As shown in Fig. 10, it is proposed that DltD facilitates the binding of Dcp and Dcl for ligation of Dcp with d-alanine. The hydrophobic N-terminal sequence of DltD is required to anchor this protein to the membrane, most probably the inner leaflet (118). It had been proposed that DltD functions in the final esterification step (383, 389). However, attempts to implicate DltD as a catalyst in reaction 2 (d-alanyl transfer to membrane-associated LTA) have been unsuccessful (F. C. Neuhaus, unpublished observations). Thus, the protein complex utilizing DltD to bind the cytosolic components, Dcl, Dcp, ATP and d-alanine described in Fig. 10 guarantees the specific ligation of Dcp with d-alanine.
FIG. 10.
Model for the incorporation of d-alanyl ester residues into membrane-associated LTA. DltD provides binding sites for Dcp and Dcl on the cytoplasmic leaflet. DltB provides a putative channel for the secretion of d-alanyl-Dcp to the periplasm where d-alanylation occurs.
Based on this proposal, one of the paradoxes of the d-alanine incorporation system is explained (Fig. 10). It was observed that the Km for d-alanine, as measured in the assay of the isolated d-alanine-activating enzyme, is 70 mM (43). In contrast, the Km for d-alanine in the synthesis of d-alanyl-LTA is 18 μM (309). Thus, it would appear that the binding of Dcl and Dcp to membrane-associated DltD enhances the affinity of Dcl for d-alanine. This change in Km resulting from the binding of Dcl to DltD would further ensure that Dcp is ligated with d-alanine at the cytosolic concentrations of this amino acid.
d-Alanylation of LTA
The transfer of the d-alanyl residue from d-alanyl-Dcp to LTA requires only that the acceptor LTA be membrane associated (211) (reaction 2). None of the acyl carrier proteins (ACPs) involved in fatty acid metabolism replace the requirement for Dcp, even though Dcl ligates d-alanine to ACPs in the absence of DltD (211, 367). A distinct membrane-acceptor:d-alanyl transferase that catalyzes reaction 2 has not been detected.
Incubation of d-alanyl-Dcp with micellar LTA (Na+ form) resulted in the time-dependent hydrolysis of d-alanyl-Dcp (261). In contrast, d-alanyl-ACP is not hydrolyzed. It was proposed that d-alanyl-Dcp forms a complex with the poly(Gro-P) moiety of LTA and that within this complex a “thioesterase-like” enzyme mimic occurs. Based on the specificity for d-alanyl-Dcp in the d-alanylation of LTA and this “thioesterase-like” reaction, we suggest the presence of a specific binding site in Dcp for LTA (261). Thus, while d-alanyl-Dcp is hydrolyzed in the presence of isolated micellar LTA, transfer of the d-alanyl residue occurs only when membrane-associated LTA is used (211).
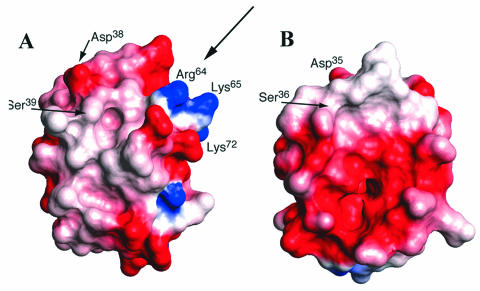
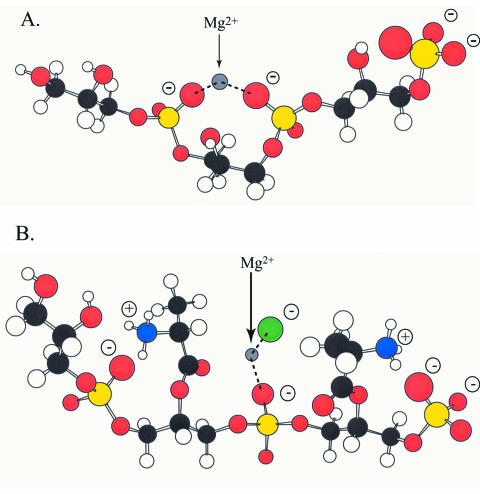
Structural studies of Dcp by multidimensional heteronuclear NMR provided the basis for concluding that the carrier protein is a homologue (three-helix bundle) of the ACPs involved in fatty acid, polyketide, and nonribosomal peptide syntheses (485) (Fig. 11). These studies also provided a basis for defining two sites on Dcp: (i) the phosphopantetheine prosthetic group linked to Ser39 at the N terminal of helix II, which is recognized by Dcl, and (ii) a putative binding site utilizing invariant Arg64 for binding the poly(Gro-P) moiety of LTA. The first site is modified by AcpS, the 4′-phosphopantetheine transferase of primary metabolism in B. subtilis (117, 349). The second site, which encompasses a 310-helix (helix II′) spanning residues Asp63-Trp67 of Dcp, is, in part, made up of Arg64. The conserved Trp67 plays an important role in positioning helix II′, which in turn determines the orientation of Arg64 for participation in the binding of Dcp to the poly(Gro-P) moiety of LTA. This invariant cationic surface residue is missing from ACPs involved in fatty acid metabolism (Fig. 12, arrow).
FIG. 11.
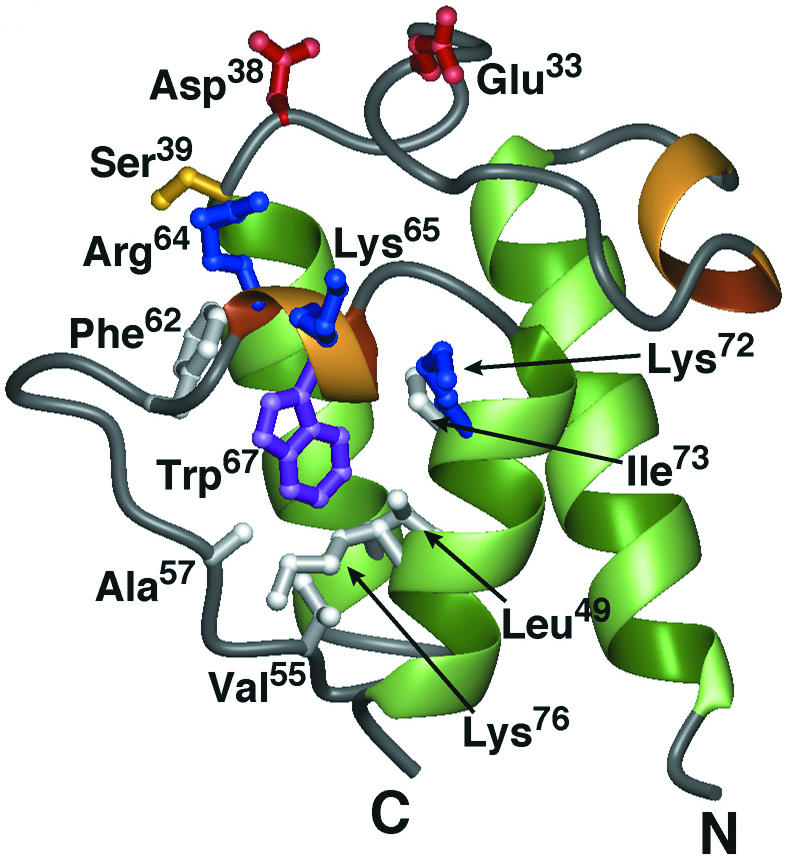
Ribbon diagram of the minimized average structure of apo-Dcp (PDP entry 1HQB). Residues shown in white bury the Trp67 side chain (purple) in the hydrophobic core. Other key residues include the conserved Glu33 and Asp38(red) and Ser39 (yellow), as well as a cluster of basic residues (blue) proximal to the phosphopantetheine attachment site (Arg64, Lys65, and Lys72). Reprinted from reference 485 with permission of the publisher.
FIG. 12.
Putative binding site for the poly(Gro-P) moiety on apo-Dcp. Surface representations, colored according to electrostatic potential, are shown for apo-Dcp (A) and AcpP (PDP entry 1ACP, model 1) (B). Arg64 is conserved in all Dcp proteins, whereas Lys65 in L. rhamnosus Dcp is not conserved. Reprinted from reference 485 with permission of the publisher.
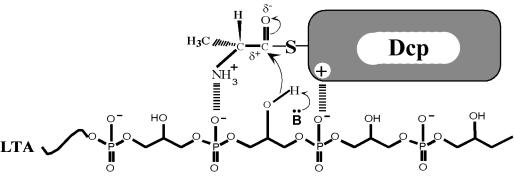
It is proposed that the transacylation of the activated d-alanyl ester residue from d-alanyl-Dcp to membrane-associated LTA occurs by nucleophilic attack of the 2′-glycerol hydroxyl (R-O:) on the electrophilic carbonyl of d-alanyl-Dcp (261) (Fig. 13). While the binding site on d-alanyl-Dcp is not completely defined, it is further suggested that its interaction with membrane-associated LTA positions the nucleophile (R-O:) for transacylation of the d-alanyl ester to LTA. Thus, the proposed mechanism for reaction 2 does not require a putative membrane acceptor:d-alanyl transferase; only d-alanyl-Dcp and membrane-associated LTA are required.
FIG. 13.
Proposed mechanism for the formation of membrane-associated d-alanyl-LTA from d-alanyl-Dcp. B·· indicates an unknown proton acceptor for generating nucleophile. The electrostatic interaction between d-alanyl-Dcp and the phosphodiester anion may be due to Arg64. Reprinted from reference 261 with permission.
Transacylation of d-Alanyl Esters
Two features of the d-alanyl esters linked to LTA are that (i) they are precursors of the d-alanyl esters of WTA and (ii) the esters of both WTA and LTA are evenly distributed along their backbone chains in isolated polymers. Based on these features, it was proposed that the esterification of LTA with d-alanine occurs in one of two modes: (i) addition at random or (ii) addition at at specified loci in the poly(Gro-P) chain followed by redistribution of the ester residues to other loci. If (ii) occurs, a mechanism for distributing or transacylating d-alanyl esters between and along TAs must exist. Two observations have provided insights into this process.
In 1985, Childs et al. (91) observed the nonenzymatic transacylation of d-alanyl esters from short-chain d-[14C]alanyl-lipophilic LTA to long-chain hydrophilic LTA. This transacylation required neither ATP nor the components of the d-alanine incorporation system. No evidence for an enzyme-catalyzed transacylation reaction was detected. The only prerequisite for this reaction was the assembly of the donor and acceptor LTA species into a micelle. Since this transacylation was described in micelles of pure LTA with high packing density, the topological organization most probably does not reflect that in the cellular membrane. However, as noted in “Distribution in LTA” (above), this process may play a role in redistributing the d-alanyl esters during purification and isolation procedures, resulting in their uniform or even distribution.
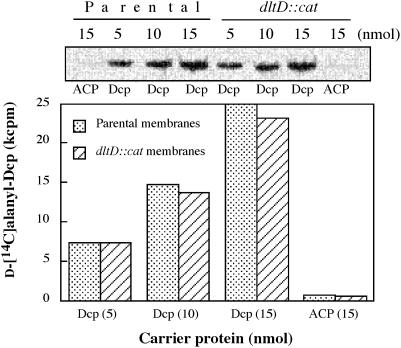
A second observation that provides an insight into the transacylation process was detected in further studies of reaction 2. It was discovered that the d-alanyl esters of membrane-associated d-alanyl LTA are transferred to Dcp in the reversal of this reaction (261) (Fig. 14). Reversal is consistent with the high chemical reactivity of these esters. As in the case of the forward reaction, the reverse is also specific for Dcp. Membranes prepared from Lactobacillus casei 102S with inactivated dltD also synthesize d-[14C]alanyl-Dcp from membrane-associated d-[14C]alanyl-LTA. Thus, the observations presented in Fig. 14 indicate not only that reaction 2 is reversible but also that DltD is not a catalyst in this reaction.
FIG. 14.
Effect of Dcp concentration on the formation of d-Alanyl-Dcp from membrane-associated d-alanyl-LTA. The reaction mixture contained 20 μg of membrane-associated d-[14C]alanyl-LTA and the indicated amounts of Dcp or ACP in 15 μl of reaction mixture. In mixtures containing dltD::cat membranes, dltD was insertionally inactivated (118). The amounts of d-[14C]alanyl-Dcp formed were quantitated by nondenaturing polyacrylamide gel electrophoresis by the method of Heaton and Neuhaus (211). Reproduced from reference 261 with permission.
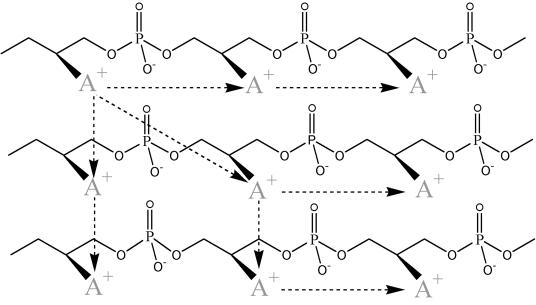
Assuming that d-alanyl-Dcp is secreted (translocated) from the inner leaflet of the cytoplasmic membrane to the wall matrix by DltB, two reactions may occur: (i) d-alanylation of LTA and (ii) d-alanylation of the resulting Dcp with d-alanyl ester from an adjacent LTA molecule followed by d-alanylation at yet another LTA site. Thus, transacylation (ii) of d-alanyl residues by this mechanism will distribute the d-alanyl esters along and among the LTA molecules of the wall. As illustrated schematically in Fig. 15, it is proposed that this process of inter- and intrachain transacylation is responsible for modulating the net anionic charge and lipophilicity of the hydrophilic LTA chain. In this way, perturbation of the d-alanyl ester content by either acylation or deacylation at one location in the membrane can be translated to an adjacent location (365). The proposal does not account for differences in the degree of d-alanylation of short-chain and long-chain LTA noted above.
FIG. 15.
Transacylation of d-alanyl ester residues along and among the chains of LTA and WTA. Interchain and intrachain transacylation is illustrated as a mechanism for distributing the esters and for the formation of d-alanyl-WTA from d-alanyl-LTA. Whether this process occurs by the mechanism described in reference 91 or that described in reference 261 has not been determined. A+ represents the d-alanyl ester.
LTA is located primarily at the membrane-wall interface, with poly(Gro-P) chains extending into the wall. This topography is consistent with the observations by Haas et al. (197), who found that the d-alanyl esters of WTA are derived from those of d-alanyl-LTA. Dcp in the membrane-wall matrix can be utilized for d-alanyl-Dcp synthesis from membrane-associated d-alanyl-LTA. By this mechanism, Dcp can “catalyze” the transfer of the d-alanyl ester from LTA to WTA. The thermodynamic feasibility of this process for distributing esters between and along molecules of LTA and WTA is not well understood.
Organization and Regulation of the dlt Operon
The dlt operon has been characterized in 12 species (Fig. 9). Each of these contains four genes, dltABCD, in a novel organization. dltA and dltB, as well as dltC and dltD, overlap by either 1 or 4 bp. Overlapping stop and start codons in the two pairs of genes are characteristic of most dlt operons examined and, thus, may be the basis for the mechanism of translational coupling that coordinates the expression of these functionally related proteins (375). In addition to dltABCD, Glaser et al. (186) identified a fifth gene (dltE) encoding an oxidoreductase in B. subtilis (Fig. 9). However, inactivation of dltE did not inhibit d-alanylation (383). It has not been established whether a fifth gene encoding an acetyl hydrolase is required for d-alanylation in Streptococcus pyogenes. From these studies and the position of the rho-independent terminator, it was concluded that dlt contains a minimum of four genes encoding the machinery required for d-alanylation.
The dlt operon was also identified in the genome of S. pneumoniae R6 (44). Since this species contains phosphorylcholine esters instead of d-alanyl esters in both LTA and WTA (164), the observation was unexpected. The organization of the operon in this organism is identical to that described above. Gene expression studies indicated that mRNAs of each dlt gene are synthesized under normal laboratory growth conditions (228). Whether there is an additional constituent of the envelope that is d-alanylated remains to be established.
Our understanding of the regulatory elements that control the expression of the dlt operon is fragmentary No single regulatory paradigm has been found that can be applied to the expression of all dlt operons. Apparently, the multiplicity of elements reflects the fact that individual species are adapted to different growth requirements, stresses, and habitats. Nevertheless, a comparison of these elements has provided some insights into the regulation of dlt.
The dlt operon in B. subtilis is part of the σx regulon (213). σx-dependent promoters precede a variety of genes that affect the composition or metabolism of the cell envelope. In addition to the PX promoter, another regulatory sequence that controls its expression is the global regulator SpoOA, targeted to a DNA-binding recognition sequence, the “OA box,” located downstream of the σx-dependent promoter (383). In addition to control by SpoOA, AbrB functions in the temporal regulation of dlt transcription. This complex regulatory system reflects, in part, the sporulation capability of this organism and the fact that spore LTA contains no detectable d-alanyl ester. In contrast, the L. rhamnosus dlt operon contains a single putative promoter region (−10 and −35 with a 20-bp spacer) similar to those reported for Lactobacillus species (86, 209).
Poyart et al. (405) discovered two regulatory genes, dltR and dltS, upstream of the dlt operon in S. agalactiae (Fig. 9). These encode putative regulatory and sensor proteins of a two-component regulatory system. Based on primer extension analysis, two promoters were detected, PdltA, located in the 3′ extremity of dltS, and PdltR, located upstream of dltR. The efficiency of PdltR is six times that of PdltA, and so it was concluded that the dlt operon is transcribed mainly from the PdltR promoter. This two-component system modulates expression of the operon and would appear to sense an environmental or external signal related to the absence of d-alanyl esters in LTA (405). In Lactobacillus plantarum, dlt includes an upstream gene, pbpX, that encodes a putative d,d-carboxypeptidase. Using the dltA and pbpX probes, Emmanuelle et al. demonstrated that the five-gene cluster was transcribed as a single polycistronic mRNA (P. Emmanuelle, P. Hols, M. Kleerebezem, R. Leer, C. J. P. Boonaert, and J. Delcour, Abstr. Belg. Soc. Microbiol., p. 17, 2001). The expression of enzymes that function in both peptidoglycan and TA assembly represents a novel link in the syntheses of these polymers.
One of the missing pieces of information is a putative signal molecule that would play a role in regulating the expression of dlt. Poyart et al. (405) considered the amount of available d-alanine in the cytoplasm to be such a regulator. Under conditions of nutrient starvation, the decreased availability of d-alanine might initiate derepression of dlt. Inactivation of the alanine racemase gene provides a mechanism for controlling the amount of available d-alanine. In contrast to gram-negative bacteria, there is only one gene encoding the racemase (alr) in B. subtilis (153), L. plantarum (222), S. aureus (282), and Listeria monocytogenes (472). Kullik et al. (282) observed that the S. aureus alr mutant, which synthesizes a fraction of its d-alanine via the d-glutamic acid:aminotransferase (dat), had 40% of the parental d-alanyl ester content in LTA while the ester content of WTA remained unchanged. This unexpected result does not appear to support a role of d-alanine in derepression, nor does it support the fact that d-alanyl esters of d-alanyl-LTA are the precursor of those in d-alanyl-WTA.
Transcription profiling in S. aureus provided the basis for identifying genes regulated by the agr (for “accessory gene regulator”) locus (138). This is one of several loci involved in regulating the expression of virulence factors in this organism. One of these factors, encoded by dltD, is downregulated 58-fold in an agr-dependent manner. Thus, dltD, which is potentially repressed by agr, may provide a clue to the broader regulatory network that functions to control the expression of the machinery required for d-alanylation. Whether dltABC is also under the agr control locus was not established. A common regulatory theme is not apparent from our comparison of the dlt operons from various species; therefore, functions of d-alanyl esters may be uniquely determined in each organism for growth and adaptation to their respective habitats.
TARGETED MUTAGENESIS
Inactivation of the dlt Operon in B. subtilis
A variety of pleomorphic mutants from L. rhamnosus, partially deficient in d-alanyl ester content, was isolated by using chemical mutagenesis (371). However, since it was not known whether these mutations were in an isogenic background or whether a single mutation was, in fact, responsible for the observed phenotype, the observations were difficult to interpret (371). Earlier observations with a stable L-phase variant of S. pyogenes showed that the LTA was deficient in d-alanyl ester content (448). An analysis of the d-alanine incorporation system from this variant indicated that the L-form membrane does not function as an acceptor even though LTA is present. Thus, while the L-form contains Dcl and Dcp, the membrane-associated LTA does not accept the activated d-alanine (89). Whether the d-alanyl ester deficiency plays a role in determining the stabilized L-form was not established.
With the identification of the dlt operon, it became feasible to inactivate each of the dlt genes with an integrational plasmid and hence to correlate d-alanyl ester deficiency with a specific phenotype. Inactivation of dltA, dltB, dltC, or dltD in B. subtilis resulted in mutants with d-alanyl ester deficiency in both LTA and WTA (383). Of the possible phenotypes examined, only enhanced autolysis and increased susceptibility to methicillin were observed (491, 492). Each bound more of the positively charged cytochrome c, reflecting an increase in TA anionic binding sites. All other growth parameters (basic metabolism, cellular content of phosphorus-containing compounds, ultrastructure, cell separation, and formation of flagella) were normal.
d-Alanyl ester-deficient mutants of B. subtilis restored the protein secretion deficiency resulting from defective PrsA (236) and enhanced the production of recombinant proteins (473). For example, a 2.5-fold increase in the level of plasmid-encoded Bacillus anthracis protective antigen was observed in the deficient mutant of B. subtilis. The extracytoplasmic lipoprotein PrsA is a peptidyl-prolyl cis-trans isomerase that assists in the folding of secreted polypeptides (277). It was suggested that the increased net anionic charge in the deficient mutant suppresses the mutation encoding defective PrsA by promoting the stabilization and folding of wall proteins through the increased binding of Ca2+ and Mg2+ to TAs. In characterizing the dynamics of this process, Chambert and Petit-Glatron (85) examined the rates of α-amylase and levansucrase folding in the presence of the TA mimics, polyphosphates of various chain lengths. While levansucrase folded rapidly in the presence of polyphosphate, α-amylase required Ca2+ in addition to polyphosphate. Using a DltA− mutant of B. licheniformis, Craynest et al. (112) observed a 1.5- to 7-fold increase in the secretion of heterologous cyclodextrin glycosyltransferase. Thus, enhancement of protein folding in d-alanyl ester-deficient mutants and in the presence of TA mimics further emphasizes the role of metal ion binding, e.g Ca2+, in the secretion and translocation of proteins (391, 483).
Even though the reported phenotypes are identical for each dlt mutant from B. subtilis, the targeted insertions are not always correlated with inactivation of a single gene in the operon. For example, the integration of pLT65A into dltA results in the disruption of the dlt transcriptional unit (383). Therefore, the complexities of translational coupling and interrupted transcription may also compromise expression of downstream genes of the dlt operon.
Inactivation of the dlt Operon in Other Gram-Positive Bacteria
In S. aureus and Staphylococcus xylosus, inactivation of dlt by either random transposon or targeted mutagenesis results in increased sensitivity of these bacteria to defensins, protegrins, tachyplesins, magainin II, and other cationic peptides (389). The enhanced sensitivity to these host defense peptides is correlated with the higher net polyanionic charge of the TA in the d-alanyl ester-deficient mutant. On the other hand, parental strains bearing additional plasmid-located copies of dlt acquire increased resistance to these cationic peptides. It was proposed that many pathogenic bacteria utilize TAs esterified with d-alanine as a protection mechanism against these host peptides (386, 387, 389).
The Dlt− mutant of S. aureus was 8- and 50-fold more sensitive to gallidermin and nisin, respectively. Resistance to these lantibiotics was restored by complementation with the plasmid bearing dlt (389). In contrast, resistance to nisin was not correlated with the d-alanyl ester content in either Streptococcus bovis or L. monocytogenes (115, 317). In the former, nisin-resistant cells have more LTA than do nisin-sensitive cells, while in the latter less anionic phosphatidylglycerol and cardiolipin were found in the resistant strain (111, 317). Thus, other mechanisms of acquisition of resistance to this lantibiotic have also been defined.
Insertional mutagenesis of dltA in Streptococcus gordonii DL1 (Challis) resulted in a loss of intrageneric coaggregation and in the formation of pleomorphs (97). These strains were characterized by aberrant septation, a lower growth rate, and defective cell separation. Inactivation of dltC in Streptococcus mutans resulted in a loss of acid tolerance and in a lower growth rate (65). The mutant is characterized by unequal polar caps and is devoid of a fibrous matrix on the cell surface. Protons are more permeable in the mutant than in the parental strain, an observation correlated with the loss of acid tolerance. Insertion of Tn916 94 nucleotides upstream of the ribosome-binding site in the S. mutans dlt operon resulted in the defective synthesis of intracellular polysaccharides (IPS) as well as a loss of d-alanyl esters (454). IPS are glycogen-like polymers synthesized by proteins encoded by glgP, glgA, and glgD. Further studies of an insertion into dltA revealed that both operons are coordinately regulated and may be part of the same regulon (S. Selgrade, N. Donovan, K. Wagner, and G. Spatafora, J. Dent. Res. vol. 81 [special issue], abstr. 0093, 2002). The expression of dlt is growth phase dependent and modulated by carbohydrates internalized via the phosphoenolpyruvate phosphotransferase system (PTS). When non-PTS sugars are the sole carbohydrate source, the operon is expressed constitutively. With sucrose as a carbon source, expression of the dlt transcript is maximal. Spatafora et al. (454) observed that the regulated expression of the dlt operon is cell density dependent, subject to regulatory control by PTS sugars, and is coordinately regulated with the glg operon for IPS synthesis.
Mutants of L. lactis defective for dltD expression grow slowly, have increased UV sensitivity, and form longer chains than does the parental strain (139). In addition, two mutations in dltD suppress the acid stress resistance of RelA and AcrR mutants (F. Rallu and E. Maguin, personal communication cited in reference 122a). relA encodes ppGPP synthetase (408), and acr encodes a regulator of ion efflux pumps. When dltD was inactivated in L. casei 102S, increased cellular length (1.6-fold) and enhanced antimicrobial sensitivities to cetyltrimethylammonium bromide and chlorhexidine were observed (118).
While it is apparent from these different phenotypes that the d-alanyl esters of LTA play important roles in the physiology of the individual species, there is no single phenotype or theme that is common to all species examined. Aberrant cell formation (pleomorphs) resulting from inactivation of dlt was observed in S. gordonii (97), S. mutans (65), and S. agalactiae (clumping phenotype) (405). In contrast, no changes in morphology were observed in the dlt mutants of S. aureus, S. xylosus (389), B. subtilis (383), and L. monocytogenes (2). In a different approach, earlier efforts to implicate d-alanyl esters in the morphogenetic program of B. subtilis also were not successful. For example, the outgrowth of B. subtilis spores provided a system for studying wall substituents during two synchronous cycles of cell division (68). While these studies indicated that the syntheses of TA and peptidoglycan are coordinated during cell growth and division, no correlation between ester-linked d-alanine and the stage of growth was found. Therefore, these observations do not argue for a unified role of d-alanyl esters in the morphogenetic programs of bacteria containing d-alanyl-TA.
Mutants Defective in LTA and WTA Assembly
WTA plays an essential role in the growth and morphology of B. subtilis 168. For example, rod mutants (66, 67, 420, 421) that have temperature-sensitive defects in the assembly of WTA undergo a rod-to-sphere transition at the restrictive termperature. Growth of a tagF1 (rodC1) strain with a defect in the Gro-P transferase TagF (Fig. 4) (399) at this temperature gave WTA with chains approximately 8 units long, in contrast to chain lengths of approximately 53 residues when the strain was grown at the permissive temperature (393). This strain contained only 16% of its WTA when grown at the restrictive temperature. Thus, while the mutant grew with the decreased amount of WTA, the morphology changed from a rod to a sphere.
In 1989, Mauël et al. (330) established that the genes encoding WTA assembly in B. subtilis are essential for growth in phosphate-replete media (293, 293a, 400). Using a mutant strain with a deletion of tagD in B. subtilis, Bhavsar et al. (57, 58) showed a full phenotypic rescue on expression of a complementing plasmid copy of tagD under tight transcriptional control with xylose. These results, which define the indispensable role of WTA in phosphate-replete medium, show a progression of phenotypic changes on depletion of TagD (Fig. 4): (i) deviations from rod to curved shape; (ii) enlargement to irregular, bloated spheres; (iii) aberrant cell division evident in malformed septa; and (iv) thickened peptidoglycan and cell lysis. In a detailed analysis of the B. subtilis genome, it was concluded that tagA, tagB, tagD, and tagO are essential for linkage unit synthesis and that tagF, tagG, and tagH are essential for chain polymerization, translocation, and linkage to peptidoglycan (265). These results clearly support a requirement for polyanionic WTA in the growth of this organism in phosphate-replete medium.
The requirement for anionic wall polymers is also supported by the observation that B. subtilis grown in phosphate-limited media replaces its WTA, but not its LTA, with teichuronic acid (145, 190, 489). Most of the enzymes involved in WTA synthesis are almost undetectable during balanced growth of strain W23 at low concentrations of Pi in chemostat cultures, while at 4 mM Pi they are maximally expressed (87, 88). The teichuronic acid operon (tua) belongs to the Pho regulon (231), and hence phosphate limitation induces its transcription (286, 452). The transcriptional regulator, PhoP∼P, plays a key role in the activation of tuaA, the first gene in the operon, and in the repression of tagA and tagD (407). The gene (tagO) which encodes the enzyme for synthesizing undecaprenyl-PP-GlcNAc, involved in the formation of the WTA linkage unit, also represents a pivotal element in the phosphate switch between WTA and teichuronic acid syntheses (451). The interdependence between WTA and teichuronic acid syntheses ensures a constant level of anionic charge in the wall of this bacterium as well as ensuring a reserve phosphate source (190).
Growth of phosphate-limited cultures of B. subtilis in the presence of NaCl reverses the WTA-teichuronic acid switching system (144, 145). For example, as the NaCl concentration increases from near zero to 6% in the medium, the wall phosphorus concentration increased 10-fold, reflecting an increase in WTA content. Even though the need to conserve phosphate was detected, the culture reverts to WTA synthesis in the presence of Na+, most probably to achieve more competitive binding of Mg2+ and assimilation of divalent cations. As described below, this observation is the basis for a suggested control system that may participate in the regulation of WTA assembly.
Park et al. (381) isolated three groups of bacteriophage-resistant mutants from S. aureus that are deficient in either WTA or a specific component of WTA. One of these, strain 52A5, has both a reduced capacity and a lower affinity for cations (320, 377). It grows 30% slower than the wild type, and cell separation is defective. While these results have been cited as evidence that WTA does not have a high affinity for divalent metal cations, the results do not negate the role of TA in the binding and assimulation of metal cations.
Mutants deficient in the poly(Gro-P) moiety of LTA have not been isolated (157, 466). The absence of stable mutants may reflect either the essential role of LTA in growth or the fact that the mechanism of LTA assembly is not completely understood. To define further the functions of LTA, mutant strains deficient in the glycolipid moiety were sought. Glycolipid synthesis in S. aureus is accomplished by diglucosyldiacylglycerol synthase (YpfP) (260). The YpfP− mutant replaces its glycolipid anchor with diacylglycerol-anchored LTA. Under most growth conditions, it was not possible to distinguish the mutant strain from the parental strain. However, differences in glycine sensitivity, viability in 0.85% NaCl, and morphology were observed. These observations not only emphasize the organism's need for polyanionic LTA but also emphasize the importance of the glycolipid anchor.
FUNCTIONS OF TEICHOIC ACIDS
Role of d-Alanyl Esters
Three functions of d-alanyl-TAs have been proposed: (i) to modulate the activities of autolysins, (ii) to maintain cation homeostasis and assist in the assimilation of metal cations for cellular function, and (iii) to define the electromechanical properties of the cell wall. The results presented in this review suggest that these functions may be limited and that depending on the species, additional roles in adhesion, biofilm formation, acid tolerance, intrageneric coaggregation, protein folding, antibiotic resistance, UV sensitivity, and virulence are also important. The multiplicity of these diverse functions is described in this section.
It is curious that nature has chosen d-alanine, a stereoisomer opposite to that in proteins, as a unique metabolite to play roles in both peptidoglycan cross-linking and TA function in the bacterial envelope (210). d-Alanine may be an integral component of a regulatory system connecting the d-alanyl esters of TA on the one hand and the d-Ala-d-Ala moiety of peptidoglycan on the other. It is possible that by sensing and responding to changes in the d-alanine concentration, some bacteria gain a competitive advantage for growth in certain conditions or habitats.
The ease of d-alanyl ester migration noted above strongly suggests that this feature is related to d-alanyl-TA function in the living cell. Although this is not proven, migration or transacylation of the d-alanyl esters to specific locations or regions within the wall matrix provides a unique mechanism for transmitting signals that could determine the activities of proteins requiring a specific ionic microenvironment for function, e.g., an autolysin. Thus, the absence (or presence) of these esters within the wall matrix at specific locations might constitute a targeting mechanism for proteins that are regulated by localized ionic charge. In this way, d-alanyl-LTA is envisaged to be a communicator of cellular needs during growth of the bacterium. Defining the topological features relating to this proposal will require additional experimental methods that are not currently available.
d-Alanyl-LTA has a chemical reactivity that places it in a class of biological molecules known as high-energy intermediates. As an example, aminoacyl-tRNA has a comparable reactivity to that found for the d-alanyl ester of TA. A wall matrix with these covalently linked, activated esters has a potential source of free energy for driving reactions that occur in the wall. The twofold turnover of the d-alanyl ester in one doubling of S. aureus is consistent with this suggestion (166, 197). While coupling of this high-energy intermediate to wall reactions has not been demonstrated, this speculation warrants additional consideration in further studies of the complex biochemistry occurring in the wall.
Although this review addresses the d-alanyl esters of WTA and LTA, it is also possible that these esters acylate other constituents of the envelope. For example, Clark and Young (95) found that d-alanyl esters in B. subtilis not only are linked to TAs but also are covalently linked to two membrane proteins of 80 and 230 kDa. It is also of interest that Surana et al. (462) considered the latter protein to be a candidate for regulating B. subtilis microfiber twist development. While the functions of these proteins have not been established, the fact that dlt is expressed in S. pneumoniae, an organism that does not contain d-alanyl esters in its TAs, suggests that other d-alanyl constituents could also function in wall metabolism. For example, d-alanylation of targeted envelope proteins may result in their activation. A second example is the linkage unit lipid on which WTA is assembled. d-Alanylation of the (Gro-P)n moiety of this lipid (Fig. 2A), an analog of the poly(Gro-P) moiety of LTC, would strongly inhibit WTA synthesis. At low d-alanylation, maximal WTA synthesis would occur. As described, this proposed regulatory system might provide certain species, e.g., S. aureus, with the ability to adjust WTA synthesis for growth under high-salt conditions.
LTA and WTA in the Context of the Envelope
As mentioned in the Introduction, the growing cell possesses a wall with a unique mixture of microenvironments, anion and cation composition, proton gradient, proteins, TAs, and peptidoglycan. Each of these contributes to the functions of the envelope as the cell undergoes growth, binary division, and cell-cell separation. In this milieu, the protonated d-alanyl esters of TA provide the counterions for interaction with the adjacent anionic sites of TAs, peptidoglycan, and proteins. Hydrogen bonds, electrostatic interactions, and van der Waals attractions provide the forces that determine the properties and organization of the TAs as well as the functions of these constituents within the envelope.
The matrix is an elastic polyelectrolyte gel that swells or shrinks in its response to a variety of factors, signals, environmental stresses, and protons (130, 256, 319, 320, 474). The molecular basis for the expansion and contraction of this gel results in part from the charge-charge repulsion of the phosphodiester anionic linkages of TA. Under conditions of maximum repulsion (low ionic strength), hydrodynamic studies indicate that isolated WTA exists in an extended or rigid-rod conformation (133). Using dye-binding and circular dichroic methods, Pal et al. (380) found that the conformation of WTA is helical at low ionic strength and that addition of either Ca2+ or Mg2+ disrupts this structure. At high ionic strength, WTA behaves as a random coil. This reversible transition, rod versus random coil, constitutes one of the features in concert with peptidoglycan that determine the expansion and contraction of the wall. We suggest that this transition also plays a fundamental role in determining the acceptor ability of LTA in the d-alanine incorporation system.
One of the determinants of charge distribution in the envelope of the respiring cell is the proton gradient (83, 247, 256, 257). It was estimated that this gradient extends approximately 2 nm into the wall matrix and can theoretically reduce the pH at the membrane-wall interface by 3 to 4 pH units (266). Kemper et al. (257) suggested that the estimation of this distance might be low when K+ is present. At low pH, under conditions of minimal net charge, the polyelectrolyte gel matrix contracts (high density), while at high pH, under conditions of high anionic charge, maximal expansion is realized (low density) (319, 378). For example, with S. aureus walls, the minimum and maximum volumes are 2.8 and 5.1 ml/g (dry wt), respectively. That the proton gradient plays a major role in the binding of cations and the regulation of autolysin activity has been demonstrated for B. subtilis (82, 83, 247, 257, 479). Competition between protons and mobile counterions in this matrix will determine a gradient of packing density, as well as gradients of cations and active autolysins within the wall of the growing, respiring cell (199, 343).
Of major interest to the goal of this review is the observation by Ou and Marquis (378) that removal of the d-alanyl esters from the WTA of S. aureus causes an expansion of the wall from 5.1 to 10.1 ml/g. Such a volume change is the result of charge-charge repulsion on ester removal and has been designated as resulting from “electromechanical interactions” within the wall (130, 378). Thus, d-alanyl esters, as well as protons, determine the density or compactness of the wall and hence also constitute factors that regulate autolysin and cation binding.
The wall-membrane matrix constitutes a periplasm, even though an actual space is not observed as in gram-negative bacteria (51, 340). Merchante et al. (340) proposed that the negatively charged wall, acting as an external permeability barrier, and the cytoplasmic membrane define the periplasm in the gram-positive cell. On the other hand, Beveridge (51) proposed that “the periplasm resides in and intermingles with the fabric of the gram-positive wall.” According to the latter description, the periplasm consists of the functional components that are associated with this fabric (53). It is this proposal that correlates with many of the observations described in this review.
The periplasm of B. subtilis contains 9.8% of the cellular protein, part of which is attached to the wall fabric by a variety of mechanisms (334, 340). In the respiring organism, the wall has a relatively low pH, distinct from other regions of the cell (83). In addition, the Donnan equilibrium further defines an imbalance or distribution of mobile cations in the wall matrix, which plays a role in cation homeostasis and accumulation as well as establishing an environment for wall-protein function (199, 378). Thus, this polyelectrolyte, composed of peptidoglycan, TAs, and water (412), establishes an environment, different from that of the medium, for the functioning of a fraction of the organism's protein. Gradients of packing density determined by metal cations and protons in this polyelectrolyte are just some of the factors that define the properties of the wall within which the stressed layers of peptidoglycan provide the necessary tensile strength to protect the cell from turgor pressure (268).
d-Alanyl Esters in the Binding of Ligands
Soon after the discovery of TAs (24), it was suggested that their function is to bind the divalent cations Ca2+ and Mg2+ (16, 215). For example, the poly(Rbo-P) WTA from B. subtilis W23 binds Mg2+ in the molar ratio (Mg/P) of 1:1 with an association constant of 0.61 × 103 M−1 (212). To assess the role of d-alanyl esters in binding, WTA samples with various degrees of d-alanylation were examined (21, 289, 290). WTA with a low d-alanyl ester content from Lactobacillus buchneri bound one Mg2+ ion for every two phosphodiester linkages (bidentate binding), with an association constant of 2.7 × 103 M−1 (Fig. 16A) (289). Further studies with WTA from S. aureus showed that the binding of Mg2+ reflects the ratio of d-alanine to P (21, 290). As the ratio increases, the probability of the Mg2+ cation binding to a single phosphodiester linkage (monodentate binding) and to a counterion increases (Fig. 16B) (290). LTA from Streptococcus sanguis (d-alanine/P = 0.49) binds on average either one Ca2+ ion or one Mg2+ ion per phosphodiester linkage (422, 423). The dissociation constants (8.39 mmol/liter for Ca2+ and 15.0 mmol/liter for Mg2+) reflect a preferential binding for Ca2+. Using X-ray photoelectron spectroscopy, Baddiley et al. (42) observed that the d-alanyl ester residues have a marked effect on the nature of the association of Mg2+ in TA from B. licheniformis and L. plantarum. When these esters are present, a fraction of the binding is represented by the higher 2s electron-binding energy. This indicates that a fraction of the cations have a weaker ionic interaction. When esters are absent, a lower 2s binding energy reflects a stronger ionic interaction. It was estimated that 58% of the Mg2+ ions have the higher Mg 2s binding energy and 42% have the lower binding energy in the walls of L. plantarum. Thus, in the absence of d-alanyl esters, bidendate binding occurs between two anionic phosphodiester linkages, while in the presence of esters, monodentate binding of the cation to the linkage occurs (Fig. 16).
FIG. 16.
Bidentate (A) and monodentate (B) binding of the Mg2+ cation by phosphodiester linkages. In panel B, binding to the Cl− counterion would occur. For these structures, the ionic radius of nonhydrated Mg2+ is used. Geometry optimization of structures was performed in Chem3D (Molecular Modeling and Analysis), CambridgeSoft.
Using 31P NMR, Batley et al. (45) observed that the d-alanyl esters of LTA had no effect on the association constant of Mg2+. While this observation contrasts with those by others, the NMR experimental conditions, 10 to 20 mM EDTA and polydisperse aggregates of LTA, may have confused the interpretation of some metal ion binding experiments.
In addition to the phosphodiester linkages provided by LTA and WTA, anionic sites for metal cations are also provided by peptidoglycan. These include the carboxylates of the γ-d-glutamyl and meso-diaminopimelyl (A2pm) residues in the cross-linked glycan, as well as the terminal d-alanine carboxylates of uncross-linked glycan. They also bind cations and thus constitute a fraction of the wall-binding capacity (127). From a series of covalent modifications of walls from B. subtilis 168, it was concluded that while both TA and peptidoglycan each contribute sites for binding metal cations, the latter would appear to provide a larger fraction (55, 131, 324, 453). In contrast, Heckels et al. (212) found that the WTA provides the larger fraction of anionic binding sites in B. subtilis W23, an organism with poly(Rbo-P) WTA. WTA and teichuronic acids are the prime sites of metal binding in B. licheniformis (52). Using similar modification methods, Rose et al. (424) also observed that Ca2+ binding is more phosphate group based in streptococci and more carboxylate group based in several other genera. Therefore, the relative contribution of peptidoglycan and TAs to the binding of cations may depend, in part, on the bacterium examined. Urrutia Mera et al. (479) found that a two- to threefold higher concentration of metal cations is retained in the walls of nonliving B. subtilis cells than in those of living cells. It was speculated that protons compete with metal cations for the anionic sites in the wall matrix. Therefore, not only does the living cell bind fewer metal cations, but also an asymmetric distribution of metal cations may exist, determined in part by the proton gradient.
Since the removal of the d-alanyl esters from the WTA of S. aureus resulted in increased amounts of Mg2+ bound to the wall, it was concluded that the increased Mg2+-binding capacity is the consequence of the decreased ester content (290). Growth of this organism at lower pH values (5 to 6 versus 7 to 7.8) resulted in increased ester content in the wall and a decreased Mg2+-binding capacity (21). Alternatively, growth in 7.5% NaCl gave a 66% reduction in d-alanyl ester content, which resulted in more than a two-fold increase in the Mg2+-binding capacity (215). In addition, as the growth temperature was elevated, Mg2+ binding was increased as d-alanylation was decreased (233, 370). These results provide a clear correlation between d-alanyl ester content and the Mg2+-binding capacity of the wall.
The importance of cation binding in the wall was also demonstrated with partially reconstituted wall-membrane preparations from B. licheniformis (230). Membrane-walls with a full complement of LTA and WTA showed maximal synthesis of WTA in the absence of added Mg2+. When both TAs were removed from the preparation, Mg2+ was required in a narrow concentration range, while addition of LTA to the deficient preparation had a damping effect on the Mg2+ requirement of the enzyme system. TA provides a buffering action for Mg2+ in the vicinity of the membrane, ensuring maximal activity of the WTA synthetic system under widely different external conditions. Hence, TAs are the components of the system that mediate the interaction of cations with the membrane and wall to maintain the optimal ionic environment of the biosynthetic system under investigation. This is one of the key experiments illustrating the role of TAs in maintaining cation homeostasis for cellular function (14, 230).
The correlation of ligand binding capacity with d-alanyl ester content has also been described for the dlt mutants of several bacteria. For example, the B. subtilis Dlt− mutant has a twofold-higher binding capacity for cytochrome c, a basic protein for measuring binding, than does the wild type (491). A similar increase (four-fold) in the availability of anionic sites was also observed in the d-alanyl ester-deficient mutants of S. aureus (389). Therefore, the ester deficiency in both LTA and WTA leads to the increased availability of anionic sites to bind this basic protein as well as other cationic ligands. Using cytochrome c as a contrast-delivering ligand in electron microscopy, Wecke et al. (491) found, when comparing the mutant and parental strains, that cylindrical walls bind more of this ligand than do cross-walls. Graham and Beveridge (189) also observed a heterogeneous localization of TA anionic sites when using antibody specific for the glycosyl substituents of WTA. Therefore, walls of exponentially growing B. subtilis contain regions of structural differentiation that are determined in part by WTA.
The binding affinities of TAs for Mg2+ and Ca2+ are modest. The interpretation of these results in cation assimilation and binding has been the focus of some discussion (133, 134, 289, 377). However, high affinity for divalent cations in the wall matrix would be counterproductive and thus would work against the corresponding cation transport system of the cell (e.g., the Mg2+ transporter [348]). Nevertheless, the ratio of the Ca2+ concentration in the B. subtilis wall to the medium concentration is 100 to 120 (54, 391). Therefore, binding capacity, as well as binding affinity, plays a role in determining the concentration of cations in the wall. TAs, together with peptidoglycan, function in concert within the wall and glycocalyx to provide these binding sites, which provide the conduit for the trafficking of mono- and divalent cations to the membrane.
While the focus of this review is on the role of protonated amino groups of the d-alanyl esters in determining the net polyanionic charge of the wall, other membrane and wall substituents are also recognized to play a similar role. These include the protonated ɛ-amino groups of l-lysyl-phosphatidylglycerol (229, 388), the protonated 2′-amino groups of de-N-acetylated N-acetylglucosamyl and N-acetylmuramyl residues of peptidoglycan (10, 127, 506), and with the protonated ɛ-amino groups of the l-Lys and amidated A2pm residues of peptidoglycan (27). Amidation of the carboxyl groups of the A2pm residues and γ-d-Glu residues (447, 475) also plays an important role in determining the net anionic charge in many bacteria. It is of interest that the TA of Streptomyces roseoflavus contains l-lysyl esters in place of d-alanyl esters (356). Ion pairing between the protonated amino groups of peptidoglycan and the phosphodiester linkages of TA provides additional neutralization of the anionic charge established by TAs in the wall matrix. This provides an example of the complex interplay between these two major groups of wall polymers.
In accordance with our view of the wall matrix, we envisage divalent cations forming bridges between LTA and WTA, as well as wall proteins and peptidoglycan (Fig. 17). Rose et al. (425) extended this model to include bidentate Ca2+ bridging between cells in plaque. While this organization of bridging cations is difficult to comprehend in detail, let alone to demonstrate, it appears that the wall matrix, with its anionic constituents and metal cations, represents a supramolecular organization that is used by cells for growth and survival.
FIG. 17.
Interchain bidentate bridging of TAs by Ca2+. Up-chain and down-chain ion pairings with protonated d-alanyl esters are not illustrated.
d-Alanyl Esters in Autolysin Control
The inhibitory action of LTA on autolysins (peptidoglycan hydrolyases) had been widely accepted in the literature (100, 172, 223, 461). It was suggested that the degree of d-alanylation influenced this inhibitory activity and that alanine-free LTA and alanine-substituted LTA represented active and inactive forms, respectively, of an autolysin inhibitor (172). The arguments for and against this proposal were summarized for B. subtilis, and it was concluded that there is “no real evidence suggesting that LTA modifies the N-acetylmuramic acid l-alanine amidase activity in vitro or in vivo” (129). Herbold and Glaser (216) found, however, that the high affinity of this amidase for walls requires WTA. The induction of autolysis in Staphylococcus simulans 22 by the cationic lantibiotics Pep5 and nisin suggested that these cationic substances and autolysins compete for the anionic sites on LTA (62). Higher levels of these lantibiotics were required to initiate lysis when the d-alanyl esters were removed. Therefore, the esters would appear to determine the number of binding sites on LTA for autolysins (61, 62). The action of the hemolysin/bacteriocin of E. faecalis (237) was inhibited by the d-alanyl-WTA of this organism (114). Removal of the d-alanyl esters inactivated this inhibitory activity and induced autolysis. Therefore, the number of binding sites for hemolysin/bacteriocin binding in S. simulans would appear to be determined by the degree of d-alanylation. It is recognized that autolysin binding to LTA and WTA, determined in part by d-alanyl esters, constitutes only one of the factors that may regulate and present these potentially lethal hydrolases (82, 83, 129, 216, 445, 446, 450).
The ability to isolate d-alanyl ester-deficient mutants (383) provided a new approach to examination of the role of these esters in the regulation of autolysis (491). However, the observed increase in autolysin activity of the Dlt− mutants from B. subtilis was unexpected, because results with the isolated micellar LTA had indicated that a decreased rate of autolysin action might have been observed. This contradiction was resolved when Wecke et al. (491, 492) suggested that the inhibitory effect of LTA micelles observed in the earlier work is actually the result of autolysin entrapment, preventing lysis.
A physical analysis of the LTA micelle revealed that it is assembled from approximately 150 molecules with the hydrophilic poly(Gro-P) chains extending 8.5 nm from a 5-nm core (micelle diameter, 22 nm) (285). It was proposed that the heavily coiled chains of the micelle trap the cationic autolysin molecules and thus inhibit autolysis (491). However, it was further postulated that the packing density of LTA in these micelles is too high to reflect their actual organization in the bacterial membrane. To address these proposals, LTA was diluted into Triton X-100 micelles and tested as an inhibitor of autolysin action (196; see also reference 99). Under these conditions, the inhibitory activity was abolished. Micelles with a decreased LTA concentration do not trap or sequester autolysins, and therefore the inhibitory action of LTA that had originally been described with high-density micelles may have led to an equivocal conclusion. A molecule of membrane-associated LTA in the cell is surrounded, on average, by eight phospholipid molecules (195). Under these conditions, LTA binds autolysin for presentation to the susceptible peptidoglycan linkages. For species with d-alanyl esters, Wecke et al. (491) proposed that the ester content determines the number of anionic sites on LTA and WTA for autolysin binding. Therefore, according to this hypothesis, the Dlt− strain binds more autolysin, resulting in an increased rate of autolysis.
SUPRAMOLECULAR ORGANIZATION OF LTA AND THE NaCl-ACTIVATED d-ALANYL-DCP “THIOESTERASE”
Several possible roles for the membrane in the d-alanylation of LTA have been considered: (i) to establish a specific conformation or organization of the acceptor LTA, (ii) to contain an enzyme required for d-alanylation, or (iii) to facilitate the formation of a specific LTA complex with other membrane constituents (309, 368, 414). From the evidence presented in this review, conformational and organizational features of LTA in the cell would appear to play a role in the d-alanylation process. In 1974, Doyle et al. (133) noted that the inhibition of d-alanine incorporation by Na+ and K+ (414) might be related to the conformation of the acceptor TA.
In studies of peptidoglyan assembly in gram-positive bacteria, it was recognized that a spatial interrelationship or junction between the membrane and the wall (418) is required for the proper processing and incorporation of nascent glycan (251, 345, 347, 369). This relationship may be determined in part by the intercalation of the poly(Gro-P) moiety of LTA into the peptidoglycan matrix as well as by the bridging of the growing peptidoglycan chains between the membrane and the wall (218, 413, 489). Understanding the role of LTA in this junction is also important to our understanding of the d-alanylation process.
In low-ionic-strength medium, TA exists in the extended or stretched conformation, while in high-ionic-strength medium, screening of the anionic centers results in a decrease of charge repulsion. This transition is illustrated schematically (Fig. 18) with a type I LTA in the absence of other wall components, e.g., peptidoglycan. In a biophysical analysis of type I LTA, Gutberlet et al. (195) observed two types of interaction that occur with LTA in vesicles of dipalmitoyl-sn-glycero-3-phospho-1-glycerol (DPPG) at high ionic strength. The first occurs between the glucosyl hydroxyl groups of the LTA glycolipid anchor and the phosphoglycerol moiety of adjacent DPPG. The second occurs as long-distance interactions between the DPPG headgroup and the LTA poly(Gro-P) chain in random-coil conformation, producing a stabilizing effect on the membrane surface. It is remarkable that a single LTA molecule surrounded by 50 DPPG molecules results in a measurable effect on the phase transition of DPPG. One explanation for this effect is that LTA causes the formation of an unusual gel-fluid transition observed in vesicles of phosphatidylglycerol (415). This transition is accompanied by an increased mobility of the poly(Gro-P) chain at high ionic strength. Therefore, these observations define one of the LTA features that may result in the inhibition of d-alanylation.
FIG. 18.
Scheme illustrating the salt-induced transition in the supramolecular organization of the membrane-associated poly(Gro-P) moiety of LTA. At low ionic strength (−NaCl), the poly(Gro-P) chain assumes a stretched conformation, and at high ionic strength (+NaCl), it forms a random coil. Courtesy of T. Gutberlat (presented at the Spring Colloquium on Molecular Modeling, Darmstadt, Germany, 1995) with permission.
The transition of LTA between the extended and random-coil conformations provides a basis for interpreting the effects of NaCl on the d-alanine incorporation system (414). For example, the addition of 0.25 M NaCl inhibits the incorporation of d-alanine into membrane-associated LTA from d-alanyl-Dcp by 50% while promoting its hydrolysis (261). The salt-induced transition is the consequence of inhibiting charge-charge repulsion in the poly(Gro-P) moiety of LTA. Random-coil formation facilitates either an increased accessibility of the acceptor LTA/d-alanyl-Dcp complex to bulk solvent or an increased mobility of the poly(Gro-P) chain, leading to misalignment of the nucleophile for d-alanylation. Therefore, it is proposed that the NaCl-stimulated “thioesterase activity” is the consequence of electrostatic screening of the poly(Gro-P) moiety of LTA, leading to the random-coil conformation. The result is hydrolysis of the d-alanyl-Dcp by LTA.
The addition of NaCl also inhibits the formation of d-alanyl-Dcp from membrane-associated d-alanyl-LTA and Dcp (reverse of reaction 2). This inhibition is specific for Dcp and does not occur in the presence of the ACPs involved in fatty acid metabolism. The electrostatic screening of LTA by NaCl leads to hydrolysis of d-alanyl-LTA in the presence of Dcp. Thus, one of the features of membrane-associated LTA that is required for either the incorporation of d-alanyl ester from d-alanyl-Dcp or its transfer from d-alanyl-LTA to Dcp would appear to be the extended or stretched conformation of LTA within the environment of the membrane.
31P NMR of whole cells of S. mutans provided an interesting insight into the role of NaCl for determining the conformational mobility of LTA (416). Increasing the ionic strength of the growth medium resulted in a narrowing of the line widths of the resonances characteristic of the LTA phosphodiester linkages. It is speculated that narrower linewidths in these intact cells reflect a higher mobility of the linkages and hence of the LTA molecule. From this in vivo study, it is argued that the addition of salt increases the conformational mobility of the LTA molecule, resulting in an inhibition of acceptor ability. We suggest that d-alanylation of the poly(Gro-P) moiety occurs in the extended or stretched conformation favored under conditions of lower ionic strength. Under these conditions, phosphodiester linkages would have minimal mobility, ensuring alignment of the glycerol nucleophile for transacylation from d-alanyl-Dcp.
d-ALANYL ESTERS AND PATHOGENICITY
Pathogenicity defines the ability of a bacterium to inflict damage on the host (313). In this context, TAs function as inducers of proinflammatory mediators, immunogens, complement activators, adhesins, and mitogens (158, 180, 232, 471). Our goal in this review is to summarize the host responses where the d-alanyl esters of TAs are implicated. Therefore, if one can correlate the pathogenicity of an organism with the ester content of its LTA and WTA, it is conceivable that inhibitors of d-alanylation may be efficacious in modulating some of these responses. There are three roles for polyanionic TAs: (i) d-alanyl-TAs can provide the scaffolding for the presentation of adhesins and surface proteins that initiate the infectious process; (ii) TAs can interact directly, both specifically and nonspecifically, with host receptors to elicit their responses; and (iii) TAs can determine the effectiveness of innate cationic inhibitors, as well as cationic antibacterial agents. The ability to inactivate dlt provides an approach to assess the role(s) of d-alanyl esters in pathogenicity.
Correlation with Antibacterial Action
The correlation of d-alanyl ester content with the actions of cationic antimicrobial peptides, β-lactams, and glycopeptide antibiotics was an unexpected result. For example, Peschel et al. (389) inactivated dlt in S. aureus and S. xylosus and found increased sensitivity to defensins, protegrins, tachyplesins, magainin II, and gallidermin. The enhanced sensitivity to these cationic compounds resulted from the higher net polyanionic charge in the Dlt− cell. One of the d-alanyl ester-deficient mutants was 10-fold more sensitive than the parental strain to the host defensin, HNP1-3. Devine and Hancock (124) have also concluded that since many gram-positive species possess dlt, d-alanylation could be a common mechanism for resisting peptides produced by other gram-positive bacteria (e.g., lactococcin, nisin, and subtilin). The antibacterial activities of the glycopeptides vancomycin, teicoplanin, and balhimycin are also increased in mutants deficient in d-alanyl esters (390).
The bactericidal action of human group IIA phospholipase A2 (PLA2) (pI > 10.5) is facilitated by the increased anionic charge in the gram-positive organism (76). For example, PLA2 has been identified as one of the principal mediators of antistaphylococcal activity in human tears. This protein works in concert with lysozyme and other antibacterial peptides as part of the innate response of the host. Inactivation of dltA increased the sensitivity of S. aureus to PLA2 by 30 to 100-fold (279). Koprivnjak et al. (279) suggested that the increased Ca2+-dependent activity of the bound PLA2 is responsible for this enhancement. The increased polyanionic character of LTA and WTA in the mutant facilitates the formation of Ca2+-PLA2, leading to penetration and attack of the active enzyme on bacterial membrane-phospholipids.
Insertional mutagenesis of dlt in a methicillin-resistant S. aureus (MRSA) strain increased the resistance from 16 to 128 μg/ml in the mutant (354). For expression of low-level resistance in this strain, the product of the mecA element, penicillin-binding protein 2a (PBP2a, also known as PBP2′), is essential. Thus, dltABCD is a member of a group of genes whose inactivation leads to increased resistance when the element is present. These are distinct from the more than 20 aux or fem genes, whose inactivation leads to decreased resistance (47, 48, 49, 122). Previously, O'Brien et al. (372) observed that mutagenized strains of MRSA defective for d-alanyl ester formation have increased methicillin resistance. These results correlated with the enhanced expression of methicillin resistance when MRSA is grown in either 7.5% NaCl or at pH 8, conditions that lead to reduced d-alanylation (314, 429). In studies of the mecA element in S. aureus, Jenni and Berger-Bächi (245) found that although mecA altered autolytic behavior, it had no effect on the cellular content, chain length, or d-alanine substitution of LTA and WTA. In addition, Peschel et al. (390) observed no differences in methicillin sensitivity in the DltA− PBP2′-deficient S. aureus mutant. Thus, inactivation of dltABCD enhances methicillin resistance in strains of S. aureus that contain the mec determinant. Growth of MRSA in the presence of NaCl also leads to increased resistance and thus mimics the phenotype of the inactivated dlt mutant of this strain.
Komatsuzawa et al. and Ohta et al. (276, 374) found that LTA plays an important role in the sensitivity of MRSA to oxacillin and methicillin. Growth of a variety of clinical isolates in the presence of 0.02% Triton X-100 increased both the release of LTA and the sensitivity of the strain to these β-lactams. For example, a 4.2-fold increase in the release of LTA resulted in 2,048-fold increase in the susceptibility (MIC ratio) to oxacillin. These findings strongly suggested that LTA released during growth in the presence of the detergent is associated with a reduction in resistance to the antibiotic. The d-alanyl-ester-deficient mutants of B. subtilis not only have a higher rate of autolysis but also have a higher susceptibility to methicillin (491). This was expressed by a faster loss of viability and a slower recovery in the postantibiotic phase. The addition of Mg2+ protected both the mutant and parental strains from methicillin-induced lysis. It was calculated that the net increases in negative charge for WTA and LTA in one of these mutants are 9 and 50%, respectively. The way in which the anionic methicillin triggers lysis in the Dlt− mutant is not well understood (491).
Peschel et al. (386, 389) also observed that increased d-alanylation of TAs confers resistance to cationic peptides in S. aureus. For example, complementation of S. aureus and S. xylosus Dlt− mutants with plasmid-encoded dltABCD restores the strains’ resistance to these peptides. Thus, while inhibition of d-alanylation provides an approach to enhancement of the action of innate antibacterial agents, increased d-alanylation allows the organism to resist these compounds. In another example, resistance to vancomycin in the VanB mutant of E. faecium MT9 is correlated with a two-fold increase in the d-alanyl ester content of LTA (196). The increase in the ester content of MT9 is also associated with penicillin tolerance and decreased lysis and killing by this β-lactam. In addition, it was proposed that tolerance to oxacillin (484) in S. aureus is correlated with an increase in the d-alanyl ester content of TA (312).
The cationic tear protein lactoferrin binds to the LTA of S. epidermidis, blocking the anionic sites on the cell surface (296). Studies suggest that this binding decreases the negative charge, providing the tear lysozyme with greater accessibility to the peptidoglycan of the organism. In addition, lactoferrin increases the susceptibility of this organism to vancomycin (297). Therefore, the actions of a number of antibacterial agents targeted to wall assembly, membrane disruption, and protein synthesis would appear to depend initially on the anionic binding sites of LTA and WTA for their assimilation by the cell.
Participation in Coaggregation, Biofilm Formation, and Adhesion
Bacterial coaggregation and coadhesion in biofilms is an important consequence of cell surface adhesins and other components that interact to form networks of cell-cell aggregates (105, 274). Generally, an adhesin on one cell binds specifically to a receptor on another species, strain, or substratum. The display of adhesins, as well as other wall-associated proteins, by the bacterium occurs by at least three mechanisms of tethering: (i) linkage to peptidoglycan by using sortase and the LPXTG motif, as in the case of S. aureus protein A (357, 385, 433); (ii) membrane attachment, as in β-lactamase and PrsA, via a thioether-linked diacylglyceride (334, 464, 465); and (iii) ionic binding with TAs. The importance of mechanism (iii) was recognized in 1975, when Doyle et al. (128) suggested that TAs bind hydrophilic molecules in hydrophobic areas of the cell surface.
Two well-characterized groups of LTA-binding proteins (244) are those targeted to the phosphorylcholine moieties of S. pneumoniae TAs (72, 503) and those targeted to the TA of L. monocytogenes via glycyl-tryptophan (GW) modules (81, 248). In the first group, if the phosphorylcholine is replaced with phosphorylethanolamine, the proteins do not bind. These choline-binding proteins are involved in the adherence, colonization, and immunogenicity of the organism (427, 503). In the second group, the GW module proteins are targeted to the poly (Gro-P) moiety of LTA (70, 248). For example, In1B, a surface protein required for internalization of L. monocytogenes, contains an anchor domain (Csa) made up of three tandem repeats (GW modules of approximately 80 amino acids). This example is one of several that utilize the C-terminal GW module repeat to dock cell surface proteins. Other examples include lysostaphin (a surface protein of S. simulans), LytA (a staphylococcal phage-amidase), and Atl (an autolysin targeted to the equatorial surface ring of S. aureus) (28, 29, 81).
The S-layer protein (CbsA) of Lactobacillus crispatus targets the organism for binding to epithelial cells of the intestinal and urogenital tracts. The N-terminal sequence, which is responsible for binding to host collagens and laminin, is distinct from the C-terminal sequence, which binds this protein to the LTA of the cell wall. However, the C-terminal LTA-targeting sequence is not homologous to the GW module described above (8). Another surface layer protein, the S-protein of Lactobacillus acidophilus, is also anchored to the cell wall, but in this species the anchoring is done by WTA (449). The TA-targeting sequences (26% sequence homology) differ from the GW module and thus represent a novel mechanism that functions to anchor the S-layer protein to the Lactobacillus cell wall (8).
Inactivation of dltA in S. gordonii DL1 (Challis) supports the role of d-alanyl ester residues in cell-cell aggregation between this species and its intrageneric partners, e.g., S. oralis 34 and S. oralis C104 (97). These esters are required for display of a 100-kDa adhesin that is specific for these bacteria. Coaggregation also requires divalent metal cations, especially Ca2+ (275, 338). Binding studies also strongly support the role of LTA in Ca2+-facilitated aggregation, an important factor in plaque cohesion (422, 424). The importance of metal cations is further emphasized by the inhibitory effect of chelating agents on this coaggregation process (470). Baddiley (37) proposed that the d-alanyl esters of TA are required for the proper display of metal cations. When the ester content increases, the cations on average share association with one phosphodiester anion (monodentate) and a mobile counterion. Replacement of the counterion with an anionic binding site of an adhesin defines a role for the d-alanyl ester together with that of the cation (Fig. 19). With ester-deficient LTA, the metal cation binds with two phosphodiester anionic links (bidentate) and hence is not displayed for effective binding of the adhesin. This proposal is consistent with the role of the metal cation for displaying the adhesin in coaggregation.
FIG. 19.
Role of Ca2+ in the presentation of an adhesin. With the exception of adhesin, the structure was energy minimized as described in the legend to Fig. 16B. One or more cations may be involved in the binding of adhesin.
Mutants (Dlt−) of S. aureus lose their ability to form biofilms on polystyrene or glass surfaces (193). The increased negative surface charge leading to electrostatic repulsion in the TAs of these mutants inhibits the initial step of biofilm formation. The second step requires the secretion of the intercellular adhesin poly-N-succinylglucosamine for cell-cell adhesion (110). Complementation of the DltA− mutant with a plasmid bearing a copy of dlt restored biofilm formation. Hence, the d-alanyl esters neutralize the net anionic charge, leading to a higher capacity for colonization, an important feature that facilitates the formation of a sessile, surface-oriented community of these organisms (188).
LTA is a part of the cell surface receptor that plays an important role in aggregation for the conjugal transfer of plasmid DNA in E. faecalis (137, 141). The aggregation substance of the donor cell is a protein adhesin that binds to the surface receptor of the acceptor cell. Dunny et al. (137) characterized the binding of this substance with the receptor LTA as a “molecular grappling hook” that brings the donor cell in close contact with the acceptor and allows the subsequent formation of a mating channel. It would be of interest to know whether the d-alanyl esters of LTA play a role in this cell-cell transfer mechanism. Additionally, in an endocarditis rabbit model, adherence of E. faecalis requires two similar components, aggregation substance and enterococcal binding substance (432). LTA is a component of enterococcal binding substance and hence would appear to play a role in this pathogenicity model.
Role in Virulence
A clear correlation between the d-alanyl ester content of LTA and virulence has been established for L. monocytogenes, S. agalactiae, and S. aureus (2, 103, 406). Inactivation of dlt in L. monocytogenes severely impaired the virulence of this organism in a mouse infection model (2). The Dlt− mutant showed no morphological alterations, and its growth rate was similar to that of the parental strain. However, the adherence of the mutant strain to macrophages, hepatocytes, and human epithelial cells was strongly restricted. It was suggested that the increased polyanionic surface charge might be one of the factors responsible for impaired adherence. A second factor was speculated to be the presence of adhesins possessing altered binding activities (2). Altered binding may reflect the requirement of d-alanyl esters for the proper display of the metal cation that is required for adhesin function (37). Using a similar approach, Collins et al. (103) observed that mice injected with a d-alanyl ester-deficient (Dlt−) strain of S. aureus have significantly lower rates of sepsis and septic arthritis compared with mice infected with the parental strain. The enhanced killing rate of the mutant is the result of neutrophil antimicrobial activities rather than increased phagocytosis. These examples provide the best evidence, to date, for the role of d-alanyl esters in virulence.
Attenuation of virulence in L. monocytogenes has also been achieved with a double-inactivation mutant (dal encoding alanine racemase and dat encoding d-amino acid aminotransferase) that requires d-alanine for growth (472). This mutant requires d-alanine for peptidoglycan formation and for d-alanylation of LTA. Assays for intracytosolic bacteria in a variety of cell cultures indicated that the entry of the double mutant into the cell in the absence of d-alanine is far less efficient than for the parental strain. The results are consistent with a d-alanyl-LTA requirement for entry of the bacterium into the host cell. It is also of interest that this attenuated double mutant was constructed as a vector for use in the development of an AIDS vaccine (307, 411).
LTA has been implicated in the virulence of a number of gram-positive bacteria (46, 208, 466). For example, it was proposed that adhesion of S. pyogenes (group A streptococci [GAS]) to the host substratum is a two-stage process in which the first is the interaction of the surface-oriented glycolipid moiety of LTA with fibronectin of the host cell (106, 208, 373). The ability of wall proteins to orient the glycolipid of the transient LTA is the result of electrostatic attraction of the poly(Gro-P) moiety and the positively charged Lys and Arg residues of these wall proteins. In the second stage, adhesion to the host substratum via surface-bound adhesins completes the process. At least 17 different surface components of GAS appear to play primary roles in determining the tissue tropism of the second step (106, 207). One of these, the M protein, is very selective for adherence to epithelial cells (108, 109). Another, the histone-like protein HlpA, released during limited cell lysis, would appear to act as a virulence factor complexed with LTA (459, 505). Growth of GAS in the presence of penicillin, which promotes the excretion of LTA, diminished their ability to adhere to epithelial cells (3). In studies of S. pyogenes internalization by epithelial cells (HEp-2), it was found that exogenous LTA inhibited entry of the bacterium into these cells (437). Entry is dependent on the initial LTA-mediated adhesion, and so the unique role of LTA in virulence would appear to be defined in GAS.
One of the leading causes of neonatal sepsis and meningitis is S. agalactiae (group B streptococci [GBS]). A number of virulence factors include capsular polysaccharides, CAMP factor (protein B), hemolysin, C proteins, and lipoprotein receptor antigen I (455). LTA may also play a role in mediating the adherence of this organism to host cells (325, 360, 361). However, differences in LTA content, chain lengths, and LTA release (secretion) between virulent and avirulent strains are responsible for determining a different mechanism of adherence (187, 359). The initial stage of hydrophobic binding does not involve the glycolipid fatty acyl substituents of released LTA as was described for GAS (187, 325, 360). The glycolipid moiety of GBS LTA is unavailable for hydrophobic interactions at the cell surface to bind to epithelial cells (325). It is of interest that adherence of the virulent strain did not occur if the bacterium was grown under phosphate-limiting conditions, an observation correlated with the deficiency of LTA (360). Thus, while the adherence mechanism for GBS appears to be different from that proposed for GAS (325), both are dependent on LTA. d-Alanyl ester-deficient mutants of S. agalactiae have a greatly decreased virulence in mouse and neonatal-rat models (406). This decrease correlates with the increased susceptibility of the DltA− strain to host defense peptides as well as its higher susceptibility to killing by macrophages and neutrophils. Therefore, these observations support a role for LTA and its d-alanyl esters as one of the determinants of virulence in GBS.
LTA plays a role in the adherence of S. mutans to the hydroxylapatite of the tooth surface (93). This interaction is one of the factors that govern the formation of dental plaque biofilms (220). Formation of plaque is associated with the enhanced synthesis of extracellular LTA in the presence of sucrose and increased plaque acidity (94, 203, 243). In response, the low pH induces an acid tolerance response (ATR) in S. mutans. Inactivation of dltC, which encodes the d-alanyl carrier protein, resulted in acid sensitivity and a defective ATR in the mutant (65). It was speculated that the deficiency of d-alanyl esters is linked to increased proton permeability in this strain. The involvement of LTA in the adhesion process and the role of the d-alanine esters in the ATR further emphasize the importance of d-alanyl-LTA in plaque and biofilm formation (188, 193).
S. aureus contains a variety of adhesins, including WTA as well as LTA (323). Adherence to HeLa cells results from a specific binding mediated by WTA that does not involve fibronectin. Others, however, have suggested a role for WTA in fibronectin-mediated (5, 60) binding to epithelial cells (6, 59). Additional observations have emphasized the importance of LTA from staphylococci in the adherence of various species to uroepithelial, mucosal, and mesoepithelial cells (6, 84). The role of the d-alanyl esters of LTA and WTA in these adherence mechanisms is the subject of speculation.
Although the targets of penicillin action are peptidoglycan transpeptidases (PBPs), other targets of β-lactam action may exist in the gram-positive organism. For example, the secretion of LTA is greatly stimulated during the interruption of cell wall synthesis by penicillin (3, 4, 225, 227, 299, 358, 394, 487). This stimulation is not always the result of bacteriolysis, since both penicillin-tolerant and lysis-deficient bacteria also show enhanced LTA synthesis (73, 226, 394). Penicillin greatly increased the secretion of vesicles containing LTA in the β-lactam-tolerant L. rhamnosus ATCC 7469. These vesicles ranged in size from 20 to 40 nm, and the chain lengths of the LTA were 5 to 50 Gro-P units (394). The d-alanine/P ratio (0.26) of the LTA isolated from either vesicles or membranes were the same. Enhanced secretion of d-alanyl-LTA is also accompanied by un-cross-linked peptidoglycan in many organisms (482). For example, β-lactam inhibition of S. aureus stimulated the release of LTA and peptidoglycan (4- to 9-fold and 60- to 85-fold, respectively). These observations are important in discussing the action of bacterial products in proinflammatory responses.
WTA in either intact bacteria or cell walls is a potent immunogen (77, 264, 337, 500). In contrast, acid-extracted, purified WTAs are not immunogenic unless complexed with a cationic precipitating agent (77). In the case of LTA, antibodies to the poly(Gro-P) moiety (264, 499) and glycosyl substituents (241) have all been detected. Good correlation with the type of TA and the serological specificity of lactobacilli and staphylococci provided a useful means of classifying these genera (41, 116). Antibodies in rabbits to the d-alanyl esters of TA were also detected (252, 322, 336). The inhibition of the precipitin reaction by d-alanyl methyl ester supported the conclusion that the d-alanyl esters of LTA and WTA are antigenic determinants, together with the glycosyl and Gro-P determinants. Although these inhibition experiments have been challenged (264), the number of reports describing d-alanyl esters as an antigenic determinant in LTA and WTA argues for further investigation.
The presence of antibodies to TAs in humans is widely documented and results in many cases from cariogenic streptococci or staphylococcal sepsis eliciting antibody responses. An analysis of 53 human sera showed that 17 contained antibodies to TA (318). In another study, the sera from blood donors and from a heterogeneous group of patients with verified or suspected staphylococcal infections revealed the presence of antibodies to poly(Rbo-P) WTA and LTA, as well as a variety of other cell surface antigens (494). For the d-alanyl ester epitope, about 30% of young adults have immunoglobulin G (IgG) that precipitates with d-alanyl-LTA but not with d-alanine-free LTA (303, 304, 305). Thus, the high frequency of IgG responders to the d-alanyl esters of LTA is of major interest for their immunostimulatory properties in oral biology.
LTA binds to erythrocyte membranes, rendering cells susceptible to hemagglutination (126, 362, 499, 500). With S. pyogenes LTA, agglutination requires both the d-alanyl and fatty acid acyl esters (242). The binding sensitizes erythrocytes to agglutination mediated by IgM and IgG antibodies specific for the poly(Gro-P) moiety of LTA. An analysis of these findings revealed that sheep erythrocytes contain 7 × 106 binding sites with a dissociation constant of 1.6 μM (500). The d-alanyl esters also play a role in the interaction of LTA with the classical complement pathway (311). For example, the concentration for 50% inhibition of the hemolytic activity of purified C1 (IC50) was 7.6-fold higher when the LTA was substituted with d-alanyl esters. Thus, the effectiveness of LTA binding by the first component of this pathway was compromised by the decrease in polyanionic charge.
LTA is a member of a class of macromolecules known as modulins, that induce a variety of proinflammatory mediators (56, 98, 121, 160, 180, 214, 217, 255, 410, 477). In synergy with peptidoglycan, LTA causes septic shock and multiple organ failure (121, 342, 384, 456, 471, 488). The mediators in these host responses include cytokines (e.g., interleukin-1β, interleukin-6, interleukin-8 and tumor necrosis factor alpha [TNF-α]), nitric oxide (310), and reactive oxygen (182, 306). In addition, the activation of nuclear transcription factor NF-κB (142), the induction of cyclooxygenase-2 protein (308), and the induction and secretion of macrophage inflammatory protein-1α (113) result from interaction of LTA with a variety of cell types.
Deacylation of LTA resulting in the loss of fatty acid acyl and d-alanyl substituents abolished the formation of macrophage mediators (477). Cytokine secretion in response to LTA is enhanced with cross-linking agents such as anti-poly(Gro-P) antibody (316) and the polyvalent form of poly(Gro-P)-reactive peptides (178). Pretreatment of the LTA from S. aureus with cationic peptides blocks the ability of LTA to elicit TNF-α production (436). In addition, the specific removal of the d-alanyl esters from the LTA of S. aureus, without removing the fatty acyl esters, greatly inhibits cytokine induction (350). LTA from S. aureus and L. rhamnosus are better inducers of NO in macrophages than is that from B. subtilis (258, 280, 471). While the basic structures of the LTAs are similar, the higher lipophilicity of the LTA from S. aureus (350), correlated with a higher d-alanyl ester content, may explain its enhanced activity compared with that from B. subtilis. Therefore, d-alanyl esters would appear to play an important, although so far unidentified, role in the induction of cytokines.
Signal transduction resulting in the induction of proinflammatory mediators requires the binding of LTA to CD14, a macrophage pattern recognition receptor, and Toll-like receptor 2 (TLR2) (98, 435, 468). Complex formation, facilitated by the serum lipopolysaccharide (LPS)-binding protein, is required for cellular activation and the induction of the inflammatory response (152). Peptidoglycan also binds to this recognition receptor, an interaction that is competively inhibited by LTA (140). This complex participates in host defense by facilitating the clearing of either LTA or gram-positive bacteria from the bloodstream. In addition, LTA and LPS utilize the TLR2 receptor on the antigen-presenting dendritic cells, resulting in their maturation (341). In comparing TLR2-deficient with TLR2-containing mice, Kristian et al. (281) not only found that d-alanylation of TAs contributes to the virulence of S. aureus but also found that d-alanylation protects the bacterium against the TLR2-dependent host defense. The macrophage type I scavenger receptor binds to a variety of gram-positive organisms through surface-located LTA (136). Removal of the d-alanyl esters from S. aureus LTA enhanced its binding affinity to this receptor (191). For example, the IC50 of d-alanyl-LTA is 0.84 μg/ml whereas the IC50 of alanine-free LTA is 0.23 μg/ml. Another receptor, the human mannose-binding protein, which binds LTA from E. faecalis through collagen repeats (Gly-X-Y), also is affected by the degree of d-alanylation (395). LTA activates the platelet-activating factor receptor, which is G-protein-coupled, signaling the epidermal growth factor receptor (298). This signal system results in the upregulation of mucin production in airway epithelial cells. In contrast to the signaling pathway in macrophages, this response does not require TLR2. Induction of mucin formation in cystic fibrosis patients by S. aureus greatly aggravates the condition (298). Either LPS or LTA induces cross-tolerance in murine macrophages, resulting in desensitization (295). LTA suppresses interleukin-2 function by direct binding to this T-cell autocrine growth factor (392). LTA also binds specifically to the pulmonary surfactant protein in the presence of Ca2+ (480). The LPS-binding proteins in chylomicrons induce the detoxification of LTA (486). These are just a few of the examples in which LTA plays a role in host defense against gram-positive pathogens.
A number of postinfection sequelae are dependent on the d-alanyl ester content of LTA, and the role of this ester in many other responses remains to be established. For example, Jerić et al. (246) suggested that glycation adducts of d-alanyl-LTA and host-reducing sugars could produce potential bioactive ligands or chemical messages for signaling infection. This intermolecular reaction is analogous to that involved in the formation of glycated proteins, e.g., hemoglobin A1c found in diabetic patients. However, in this example the N-terminal of the protein is analogous to the amino group of the d-alanyl ester. While this suggestion is consistent with the chemical reactivity of the d-alanyl esters, this type of adduct formation has been studied only in a series of model compounds (246).
The use of commercial LTA preparations in some of the proinflammatory experiments has been criticized (177, 206, 351, 460). It was concluded that these preparations contain, to various degrees, non-LTA immunostimulatory substances that are decomposition products. By using a modified butanol procedure, Morath et al. (350) found that the decomposition of LTA from S. aureus is inhibited. It was shown with this LTA preparation that TNF-α induction is similar to that elicited by LPS from P. aeruginosa and that good correlation between the d-alanyl-ester content and induction of TNF-α in human whole blood was demonstrated (350).
A synthetic analogue of LTA, (d-alanyl)4(α-GlcNAc)1(Gro-P)6gentiobiosyl-sn-dimyristoylglycerol, induces cytokine release with the same potency and pattern as does native LTA (120, 352). For maximal activity, both the glycolipid and the d-alanyl esters are required. Replacement of the d-alanyl esters with l-alanyl esters results in a 100-fold decrease of activity (352). The ability to synthesize LTA analogues will allow us to identify the features necessary for biological action. Together with the use of modified LTA preparations and the use of mutants Dlt−, additional approaches to testing the function of these esters in host-mediated responses are now available.
Sepsis and septic shock due to gram-positive bacteria have become increasingly common in the past couple of decades (64). Some of the clinical manifestations can be traced to LTA and peptidoglycan (180, 181). Since these wall components result mostly from bacteriolysis, LTA becomes a primary player in postinfection host responses. This review has documented the importance of the d-alanyl esters in pathogenesis and, where known, in several postinfection sequelae. Thus, the d-alanylation of LTA merits consideration as a target for defining new therapeutic strategies in addressing infections due to gram-positive bacteria.
Rationale for Designing Inhibitors of d-Alanine Incorporation
One of the goals of this review is to provide a rational basis for the design of inhibitors targeted to the synthesis of the d-alanyl esters of LTA and WTA. First, since a deficiency in these esters results in increased sensitivity to a variety of host-generated, innate antimicrobial peptides, inhibitors of d-alanine incorporation will be of interest as potential antibacterial agents. Second, since the d-alanyl esters play a role in the immunostimulatory properties of LTA, inhibition of ester formation may ameliorate some of the host responses. Two potential targets resulting in decreased d-alanylation have been defined in this review. The first is the reaction catalyzed by Dcl, the d-alanine:Dcp ligase (reaction 1 in the Overview of “Synthesis of d-alanyl-LTA”). The second is the d-alanylation of membrane-associated LTA by d-alanyl-Dcp (reaction 2).
The incorporation system has a high specificity for d-alanine (366, 368). d-Cycloserine and O-carbamoyl-d-serine, which inhibit reactions requiring d-alanine in peptidoglycan formation, have no effect on the incorporation of d-alanine into LTA (363, 368). Compounds that have only very modest inhibitory activity include d-alanine hydroxamate and d-α-amino-n-butyric acid. Other analogues that show poor inhibitory activities are β-fluoro-d-alanine and d-α-amino-n-butyric acid hydroxamate. Additional knowledge of the mechanism of d-alanine activation and ligation to Dcp catalyzed by Dc1 in reaction 1 may lead to the design of more effective analogues directed to this target.
The second target identified from our understanding of the d-alanine incorporation system is the d-alanylation of membrane-associated LTA by d-alanyl-Dcp (reaction 2). In this reaction, two possibilities are suggested: (i) modification of the conformation of membrane-associated LTA as the acceptor of the d-alanyl ester from d-alanyl-Dcp and (ii) targeting of LTA analogues to the putative binding site on Dcp. In the first, compounds that facilitate the hydrolysis of d-alanyl-Dcp are potential candidates for increasing the polyanionic charge of LTA. For example, NaCl inhibits the incorporation of d-alanine from d-alanyl-Dcp and enhances the hydrolysis of d-alanyl-Dcp. Thus, the “thioesterase” activity resulting from the binding of d-alanyl-Dcp to LTA provides a potental screening reaction. In the second, a definition of the binding site on DCP will provide the ability to target LTA analogues that may inhibit the interaction of d-alanyl-Dcp with the poly(Gro-P) moiety of LTA. The increase in the polyanionic charge of the wall matrix resulting from inhibition at each of these sites would render the organism more susceptible to innate cationic antimicrobial peptides or naturally occurring cationic antibiotics (124, 202, 387, 389). While this section does not provide significant lead compounds as inhibitors, it presents three reactions in the d-alanylation of TAs that are readily assayed for screening potential candidates. Hence, the assembly of TAs and their esterification with d-alanyl esters provide targets for the design of new antibacterial agents (163, 400).
CONCLUSION AND FUTURE DIRECTIONS
LTA and WTA, together with peptidoglycan, define the polyelectrolyte properties of the periplasm that provides the conduit—the continuum of anionic charge—between the cell membrane, wall, and glycocalyx and the environment (Fig. 20). Not only is this matrix responsible for cation homeostasis and assimilation, but also it is responsible for the trafficking of metal cations, nutrients, proteins, and antibiotics. While there is not a discrete, defined space for this periplasm, as in the case of gram-negative organisms, there is nevertheless a “compartment,” or environment, where a myriad of cellular processes occur. While this is not a compartment in the strict sense of the word, the use of a less stringent definition allows us to define a functional entity (333) where the ionic composition is regulated, enzymes and other proteins are tethered, and energy is provided by a nondiffusible intermediate, the d-alanyl ester. Within the context of this compartment or periplasm, peptidoglycan functions to protect the integrity of the cell against turgor pressure. The d-alanyl esters of LTA and WTA, the focus of this review, allow many low-G+C gram-positive organisms to modulate the polyanionic charge and surface properties of this comparment.
FIG. 20.
Continuum of ionic charge. A high-magnification, freeze-substituted image of the septal region of an exponentially growing B. subtilis 168 cell is shown. The tripartite structure of the wall shows the fibrous nature of the outer layer. The electron photomicrograph is reprinted from reference 189 with permission. (A+) represents the d-alanyl esters of TAs, ⊕ represents mobile cations and other fixed cationic functions on peptidoglycan, and ⊖ represents the phosphodiester anionic linkages of TAs and anionic groups of peptidoglycan.
In the words of Howard Rogers (419), “There may be no clear beginning and certainly there is no clear end to the cell surface. Rather, the envelope is an organ which for analytical convenience we have separated into membranes, walls, and glycocalyx, but in the living cell one shades into another and they are all interdependent in function and formation.” The d-alanyl esters of LTA and WTA described in this review are one of the constituents that define the properties of this “organ.”
Questions that address (i) the functions of d-alanyl-TAs, (ii) the mechanism of d-alanylation, and (iii) the role of d-alanyl esters in pathogenicity continue to be a focus of research in this area.
(i) How do d-alanyl esters function in the presentation of autolysins and adhesins? Is the function of the esters simply to control the anionic charge in the envelope, or are there additional roles in displaying surface proteins? Are there other cellular constituents that require d-alanyl esters for function?
(ii) What is the role of DltB, the putative transporter? Is it organized with DltD, Dcl, and the carrier protein (Dcp) into a supramolecular assembly for d-alanine incorporation? Is the carrier protein with its d-alanyl thioester transported by DltB to the periplasm? Is the carrier protein solely responsible for transacylating d-alanyl esters to membrane-associated LTA in the periplasm?
(iii) What is the mechanism(s) by which d-alanyl esters of TAs determine virulence? Can inhibitors of virulence be designed that are targeted to the incorporation of these esters?
Answers to each of these questions will provide interesting insights into the roles of the d-alanyl esters of LTA and WTA in microbial physiology as well as in host interaction and responses.
Acknowledgments
We thank our many colleagues and coworkers for their valuable contributions toward understanding the structure-function relationships of TAs from the time of their discovery to the present, almost 50 years, during which the importance of these substances to gram-positive bacteria has emerged. We are indebted to Rosemary Linzer Neuhaus, Michael P. Heaton, Brian F. Volkman, David L. Hasty, Harry S. Courtney, Frederick P. Arnold, Jr., and Thomas Gutberlet for their suggestions on and insights into this review. Preprints of papers from Martin Levine, Patrick Trieu-Cuot, and Claire Poyart and personal communications from Pascal Hols were also greatly appreciated.
The research in the laboratory of FCN was supported by AI-04615 and GM-51621.
Footnotes
Dedicated to the memory of Werner Fischer (1930-2000), whose insights and inspiration as a friend and colleague are recognized.
REFERENCES
- 1.Aasjord, P., and A. Grov. 1980. Immunoperoxidase and electron microscopy studies of staphylococcal lipoteichoic acid. Acta Pathol. Microbiol. Scand. Sect. B 88:47-52. [DOI] [PubMed] [Google Scholar]
- 2.Abachin, E., C. Poyart, E. Pellegrini, E. Milohanic, F. Fiedler, P. Berche, and P. Trieu-Cuot. 2002. Formation of d-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43:1-14. [DOI] [PubMed] [Google Scholar]
- 3.Alkan, M. L., and E. H. Beachey. 1978. Excretion of lipoteichoic acid by group A streptococci: influence of penicillin on excretion and loss of ability to adhere to human oral epithelial cells. J. Clin. Investig. 61:671-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Obeid, S., L. Gutmann, and R. Williamson. 1990. Correlation of penicillin-induced lysis of Enterococcus faecium with saturation of essential penicillin-binding proteins and release of lipoteichoic acid. Antimicrob. Agents Chemother. 34:1901-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aly, R., and S. Levit. 1987. Adherence of Staphylococcus aureus to squamous epithelium: role of fibronectin and teichoic acid. Rev. Infect. Dis. 9(Suppl. 4):S341-S350. [DOI] [PubMed] [Google Scholar]
- 6.Aly, R., H. R. Shinefield, C. Litz, and H. I. Maibach. 1980. Role of teichoic acid in the binding of Staphylococcus aureus to nasal epithelial cells. J. Infect. Dis. 141:463-465. [DOI] [PubMed] [Google Scholar]
- 7.Anderson, A. J., R. S. Green, A. J. Sturman, and A. R. Archibald. 1978. Cell wall assembly in Bacillus subtilis: location of wall material incorporated during pulsed release of phosphate limitation, its accessibility to bacteriophages and concanavalin A, and its susceptibility to turnover. J. Bacteriol. 136:886-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antikainen, J., L. Anton, J. Sillanpää, and T. K. Korhonen. 2002. Domains in the S-layer protein CbsA of Lactobacillus crispatus involved in adherence to collagens, laminin and lipoteichoic acids and in self-assembly. Mol. Microbiol. 46:381-394. [DOI] [PubMed] [Google Scholar]
- 9.Araki, Y., and E. Ito. 1989. Linkage units in cell walls of gram-positive bacteria. Crit. Rev. Microbiol. 17:121-135. [DOI] [PubMed] [Google Scholar]
- 10.Araki, Y., S. Oba, S. Araki, and E. Ito. 1980. Enzymatic deacetylation of N-acetylglucosamine residues in cell wall peptidoglycan. J. Biochem. 88:469-479. [DOI] [PubMed] [Google Scholar]
- 11.Archibald, A. R. 1974. The structure, biosynthesis and function of teichoic acid. Adv. Microb. Physiol. 11:53-95. [Google Scholar]
- 12.Archibald, A. R. 1980. Phage receptors in gram-positive bacteria, p. 7-26. In L. L Randall and L. Philipson (ed.), Receptors and recognition, series B, vol. 7. Virus receptors. Chapman & Hall, London, United Kingdom.
- 13.Archibald, A. R. 1985. Structure and assembly of the cell wall in Bacillus subtilis. Biochem. Soc. Trans. 13:990-992. [DOI] [PubMed] [Google Scholar]
- 14.Archibald, A. R. 1988. Bacterial cell wall structure and the ionic environment, p. 159-173. In R. Whittenbury, G. W. Gould, J. G. Banks, and R. G. Board (ed.), Homeostatic mechanisms in micro-organisms. Bath University Press, Bath, United Kingdom.
- 15.Archibald, A. R. 1989. The Bacillus cell envelope, p. 217-254. In C. R. Harwood (ed.), Bacillus. Plenum Press, New York, N.Y.
- 16.Archibald, A. R., J. J. Armstrong, J. Baddiley, and J. B. Hay. 1961. Teichoic acids and the structure of bacterial walls. Nature 191:570-572. [DOI] [PubMed] [Google Scholar]
- 17.Archibald, A. R., and J. Baddiley. 1966. The teichoic acids. Adv. Carbohydr. Chem. 21:323-375. [DOI] [PubMed] [Google Scholar]
- 18.Archibald, A. R., J. Baddiley, and D. Button. 1968. The membrane teichoic acid of Staphylococcus lactis I3. Biochem. J. 110:559-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Archibald, A. R., J. Baddiley, and J. E. Heckels. 1973. Molecular arrangement of teichoic acid in the cell wall of Staphylococcus lactis. Nat. New Biol. 241:29-31. [DOI] [PubMed] [Google Scholar]
- 20.Archibald, A. R., J. Baddiley, J. E. Heckels, and S. Heptinstall. 1971. Further studies on the glycerol teichoic acid of walls of Staphylococcus lactis I3: location of the phosphodiester groups and their susceptibility to hydrolysis with alkali. Biochem. J. 125:353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Archibald, A. R., J. Baddiley, and S. Heptinstall. 1973. The alanine ester content and magnesium binding capacity of walls of Staphylococcus aureus H grown at different pH values. Biochim. Biophys. Acta 291:629-634. [DOI] [PubMed] [Google Scholar]
- 22.Archibald, A. R., I. C. Hancock, and C. R. Harwood. 1993. Cell wall structure, synthesis, and turnover, p. 381-410. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 23.Arizono, T., A. Umeda, and K. Amako. 1991. Distribution of capsular materials on the cell wall surface of strain Smith diffuse of Staphylococcus aureus. J. Bacteriol. 173:4333-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong, J. J., J. Baddiley, J. G. Buchanan, B. Carss, and G. R. Greenberg. 1958. Isolation and structure of ribitol phosphate derivatives (teichoic acids) from bacterial cell walls. J. Chem. Soc. 1958:4344-4354. [Google Scholar]
- 25.Armstrong, J. J., J. Baddiley, J. G. Buchanan, A. L. Davison, M. V. Kelemen, and F. C. Neuhaus. 1959. Composition of teichoic acids from a number of bacterial walls. Nature 184:247-249. [DOI] [PubMed] [Google Scholar]
- 26.Reference deleted.
- 27.Atrih, A., G. Bacher, G. Allmaier, M. P. Williamson, and S. J. Foster. 1999. Analysis of peptidoglycan structure from vegetative cells of Bacillus subtilis 168 and role of PBP 5 in peptidoglycan maturation. J. Bacteriol. 181:3956-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baba, T., and O. Schneewind. 1996. Target cell specificity of a bacteriocin molecule: a C-terminal signal directs lysostaphin to the cell wall of Staphylococcus aureus. EMBO J. 15:4789-4797. [PMC free article] [PubMed] [Google Scholar]
- 29.Baba, T., and O. Schneewind. 1998. Targeting of muralytic enzymes to the cell division site of Gram-positive bacteria: repeat domains direct autolysin to the equatorial surface ring of Staphylococcus aureus. EMBO J. 17:4639-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baddiley, J. 1962. Teichoic acids in walls and cells of gram-positive bacteria. Fed. Proc. 21:1084-1088. [PubMed] [Google Scholar]
- 31.Baddiley, J. 1968. Teichoic acids and the molecular structure of bacterial walls. Proc. R. Soc. London Ser. B 170:331-348. [DOI] [PubMed] [Google Scholar]
- 32.Baddiley, J. 1970. Structure, biosynthesis, and function of teichoic acids. Acc. Chem. Res. 3:98-105. [Google Scholar]
- 33.Baddiley, J. 1972. Teichoic acids in cell walls and membranes of bacteria. Essays Biochem. 8:35-77. [PubMed] [Google Scholar]
- 34.Baddiley, J. 1985. Trans-membrane synthesis of cell wall polymers. Biochem. Soc. Trans. 13:992-994. [DOI] [PubMed] [Google Scholar]
- 35.Baddiley, J. 1988. The function of teichoic acids in walls and membranes of bacteria, p. 223-229. In H. Kleinkauf, H. V. Döhren, and L. Jaenicke (ed.), The roots of modern biochemistry: Friz Lipmann's squiggle and its consequences. Walter de Gruyter, Berlin, Germany.
- 36.Baddiley, J. 1989. Bacterial cell walls and membranes. Discovery of the teichoic acids. Bioessays 10:207-210. [DOI] [PubMed] [Google Scholar]
- 37.Baddiley, J. 2000. Teichoic acids in bacterial coaggregation. Microbiology 146:1257-1258. [DOI] [PubMed] [Google Scholar]
- 38.Baddiley, J., J. G. Buchanan, F. E. Hardy, R. O. Martin, U. L. RajBhandary, and A. R. Sanderson. 1961. The structure of the ribitol teichoic acid of Staphylococcus aureus H. Biochim. Biophys. Acta 52:406-407. [DOI] [PubMed] [Google Scholar]
- 39.Baddiley, J., J. G. Buchanan, R. O. Martin, and U. L. RajBhandary. 1962. Teichoic acid from the walls of Staphylococcus aureus H: location of the phosphate and alanine residues. Biochem. J. 85:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baddiley, J., J. G. Buchanan, U. L. RajBhandary, and A. R. Sanderson. 1962. Teichoic acid from the walls of Staphylococcus aureus H. Structure of the N-acetylglucosaminylribitol residues. Biochem. J. 82:439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baddiley, J., and A. L. Davison. 1961. The occurrence and location of teichoic acids in Lactobacilli. J. Gen. Microbiol. 24:295-299. [DOI] [PubMed] [Google Scholar]
- 42.Baddiley, J., I. C. Hancock, and P. M. A. Sherwood. 1973. X-ray photoelectron studies of magnesium ions bound to the cell walls of gram-positive bacteria. Nature 243:43-45. [DOI] [PubMed] [Google Scholar]
- 43.Baddiley, J., and F. C. Neuhaus. 1960. The enzymic activation of d-alanine. Biochem. J. 75:579-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baltz, R. H., F. H. Norris, P. Matsushima, B. S. DeHoff, P. Rockey, G. Porter, S. Burgett, R. Peery, J. Hoskins, L. Braverman, I. Jenkins, P. Solenberg, M. Young, M. A. McHenney, P. L. Skatrud and P. R. Rosteck, Jr. 1998. DNA sequence sampling of the Streptococcus pneumoniae genome to identify novel targets for antibiotic development. Microb. Drug Resist. 4:1-9. [DOI] [PubMed] [Google Scholar]
- 45.Batley, M., J. W. Redmond, and A. J. Wicken. 1987. Nuclear magnetic resonance spectra of lipoteichoic acid. Biochim. Biophys. Acta 901:127-137. [DOI] [PubMed] [Google Scholar]
- 46.Beachey, E. H. 1981. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. J. Infect. Dis. 143:325-345. [DOI] [PubMed] [Google Scholar]
- 47.Berger-Bächi, B. 1999. Genetic basis of methicillin resistance in Staphylococcus aureus. Cell. Mol. Life Sci. 56:764-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berger-Bächi, B. 2002. Resistance mechanisms of Gram-positive bacteria. Int. J. Med. Microbiol. 292:27-35. [DOI] [PubMed] [Google Scholar]
- 49.Berger-Bächi, B., and S. Rohrer. 2002. Factors influencing methicillin resistance in staphylococci. Arch. Microbiol. 178:165-171. [DOI] [PubMed] [Google Scholar]
- 50.Bertram, K. C., I. C. Hancock, and J. Baddiley. 1981. Synthesis of teichoic acids by Bacillus subtilis protoplasts. J. Bacteriol. 148:406-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beveridge, T. J. 1995. The periplasmic space and the periplasm in gram-positive and gram-negative bacteria. ASM News 61:125-130. [Google Scholar]
- 52.Beveridge, T. J., C. W. Forsberg, and R. J. Doyle. 1982. Major sites of metal binding in Bacillus licheniformis walls. J. Bacteriol. 150:1438-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beveridge, T. J., and J. L. Kadurugamuwa. 1996. Periplasm, periplasmic spaces, and their relation to bacterial wall structure: novel secretion of selected periplasmic proteins from Pseudomonas aeruginosa. Microb. Drug Resist. 2:1-8. [DOI] [PubMed] [Google Scholar]
- 54.Beveridge, T. J., and R. G. E. Murray. 1976. Uptake and retention of metals by cell walls of Bacillus subtilis. J. Bacteriol. 127:1502-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beveridge, T. J., and R. G. E. Murray. 1980. Sites of metal deposition in the cell wall of Bacillus subtilis. J. Bacteriol. 141:876-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhakdi, S., T. Klonisch, P. Nuber, and W. Fischer. 1991. Stimulation of monokine production by lipoteichoic acids. Infect. Immun. 59:4614-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhavsar, A. P., T. J. Beveridge, and E. D. Brown. 2001. Precise deletion of tagD and controlled depletion of its product, glycerol 3-phosphate cytidylyltransferase, leads to irregular morphology and lysis of Bacillus subtilis grown at physiological temperature. J. Bacteriol. 183:6688-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhavsar, A. P., X. Zhao, and E. D. Brown. 2001. Development and characterization of a xylose-dependent system for expression of cloned genes in Bacillus subtilis: conditional complementation of a teichoic acid mutant. Appl. Environ. Microbiol. 67:403-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bibel, D. J., R. Aly, L. Lahti, H. R. Shinefield, and H. I. Maibach. 1987. Microbial adherence to vulvar epithelial cells. J. Med. Microbiol. 23:75-82. [DOI] [PubMed] [Google Scholar]
- 60.Bibel, D. J., R. Aly, H. R. Shinefield, and H. I. Maibach. 1983. The Staphylococcus aureus receptor for fibronectin. J. Investig. Dermatol. 80:494-496. [DOI] [PubMed] [Google Scholar]
- 61.Bierbaum, G., and H.-G. Sahl. 1987. Autolytic system of Staphylococcus simulans 22: influence of cationic peptides on activity of N-acetylmuramoyl-l-alanine amidase. J. Bacteriol. 169:5452-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bierbaum, G., and H.-G. Sahl. 1991. Induction of autolysis of Staphylococcus simulans 22 by Pep5 and nisin and influence of the cationic peptides on the activity of the autolytic enzymes, p. 386-396. In G. Jung and H.-G. Sahl (ed.), Nisin and novel antibiotics. ESCOM, Leiden, The Netherlands.
- 63.Birdsell, D. C., R. J. Doyle, and M. Morgenstern. 1975. Organization of teichoic acid in the cell wall of Bacillus subtilis. J. Bacteriol. 121:726-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bone, R. C. 1994. Gram-positive organisms and sepsis. Arch. Intern. Med. 154:26-34. [PubMed] [Google Scholar]
- 65.Boyd, D. A., D. G. Cvitkovitch, A. S. Bleiweis, M. Y. Kiriukhin, D. V. Debabov, F. C. Neuhaus, and I. R. Hamilton. 2000. Defects in d-alanyl-lipoteichoic acid synthesis in Streptococcus mutans results in acid sensitivity. J. Bacteriol. 182:6055-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boylan, R. J., and N. H. Mendelson. 1969. Initial characterization of a temperature-sensitive Rod mutant of Bacillus subtilis. J. Bacteriol. 100:1316-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boylan, R. J., N. H. Mendelson, D. Brooks, and F. E. Young. 1972. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J. Bacteriol. 110:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boylen, C. W., and J. C. Ensign. 1968. Ratio of teichoic acid and peptidoglycan in cell walls of Bacillus subtilis following spore germination and during vegetative growth. J. Bacteriol. 96:421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bracha, R., and L. Glaser. 1976. In vitro system for the synthesis of teichoic acid linked to peptidoglycan. J. Bacteriol. 125:872-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Braun, L., S. Dramsi, P. Dehoux, H. Bierne, G. Lindahl, and P. Cossart. 1997. In1B: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol. Microbiol. 25:285-294. [DOI] [PubMed] [Google Scholar]
- 71.Brautigan, V. M., W. C. Childs III, and F. C. Neuhaus. 1981. Biosynthesis of d-alanyl-lipoteichoic acid in Lactobacillus casei: d-alanyl-lipophilic compounds as intermediates. J. Bacteriol. 146:239-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Briese, T., and R. Hakenbeck. 1985. Interaction of the pneumococcal amidase with lipoteichoic acid and choline. Eur. J. Biochem. 146:417-427. [DOI] [PubMed] [Google Scholar]
- 73.Brissette, J. L., G. D. Shockman, and R. A. Pieringer. 1982. Effects of penicillin on synthesis and excretion of lipid and lipoteichoic acid from Streptococcus mutans BHT. J. Bacteriol. 151:838-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brock, J. H., and B. Reiter. 1976. Chemical and biological properties of extracellular slime produced by Staphylococcus aureus grown in high-carbohydrate, high-salt medium. Infect. Immun. 13:653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruice, T. C., and T. H. Fife. 1962. Hydroxyl group catalysis. III. The nature of neighboring hydroxyl group assistance in the alkaline hydrolysis of the ester bond. J. Am. Chem. Soc. 84:1973-1979. [Google Scholar]
- 76.Buckland, A. G., and D. C. Wilton. 2000. The antibacterial properties of secreted phospholipases A2. Biochim. Biophys. Acta 1488:71-82. [DOI] [PubMed] [Google Scholar]
- 77.Burger, M. M. 1966. Teichoic acids: antigenic determinants, chain separation, and their location in the cell wall. Proc. Natl. Acad. Sci. USA 56:910-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burger, M. M., and L. Glaser. 1964. The synthesis of teichoic acids. I. Polyglycerophosphate. J. Biol. Chem. 239:3168-3177. [PubMed] [Google Scholar]
- 79.Button, D., and N. L. Hemmings. 1976. Teichoic acids and lipids associated with the membrane of a Bacillus lichenifomis mutant and the membrane lipids of the parental strain. J. Bacteriol. 128:149-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cabacungan, E., and R. A. Pieringer. 1981. Mode of elongation of the glycerol phosphate polymer of membrane lipoteichoic acid of Streptococcus faecium ATCC 9790. J. Bacteriol. 147:75-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cabanes, D., P. Dehoux, O. Dussurget, L. Frangeul, and P. Cossart. 2002. Surface proteins and the pathogenic potential of Listeria monocytogenes. Trends Microbiol. 10:238-245. [DOI] [PubMed] [Google Scholar]
- 82.Calamita, H. G., and R. J. Doyle. 2002. Regulation of autolysins in teichuronic acid-containing Bacillus subtilis cells. Mol. Microbiol. 44:601-606. [DOI] [PubMed] [Google Scholar]
- 83.Calamita, H. G., W. D. Ehringer, A. L. Koch, and R. J. Doyle. 2001. Evidence that the cell wall of Bacillus subtilis is protonated during respiration. Proc. Natl. Acad. Sci. USA 98:15260-15263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carruthers, M. M., and W. J. Kabat. 1983. Mediation of staphylococcal adherence to mucosal cells by lipoteichoic acid. Infect. Immun. 40:444-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chambert, R., and M. F. Petit-Glatron. 1999. Anionic polymers of Bacillus subtilis cell wall modulate the folding rate of secreted proteins. FEMS Microbiol. Lett. 179:43-47. [DOI] [PubMed] [Google Scholar]
- 86.Chassy, B. M., and C. M. Murphy. 1993. Lactococcus and Lactobacillus, p. 65-82. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 87.Cheah, S.-C., H. Hussey, and J. Baddiley. 1981. Control of synthesis of wall teichoic acid in phosphate-starved cultures of Bacillus subtilis W23. Eur. J. Biochem. 118:497-500. [DOI] [PubMed] [Google Scholar]
- 88.Cheah, S.-C., H. Hussey, I. Hancock, and J. Baddiley. 1982. Control of synthesis of wall teichoic acid during balanced growth of Bacillus subtilis W23. J. Gen. Microbiol. 128:593-599. [DOI] [PubMed] [Google Scholar]
- 89.Chevion, M., C. Panos, R. Linzer, and F. C. Neuhaus. 1974. Incorporation of d-alanine into the membrane of Streptococcus pyogenes and its stabilized l-form. J. Bacteriol. 120:1026-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Childs, W. C., III, and F. C. Neuhaus. 1980. Biosynthesis of d-alanyl-lipoteichoic acid: characterization of ester-linked d-alanine in the in vitro-synthesized product. J. Bacteriol. 143:293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Childs, W. C., III, D. J. Taron, and F. C. Neuhaus. 1985. Biosynthesis of d-alanyl-lipoteichoic acid by Lactobacillus casei: interchain transacylation of d-alanyl ester residues. J. Bacteriol. 162:1191-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chiu, T.-H, H. Morimoto, and J. J. Baker. 1993. Biosynthesis and characterization of phosphatidylglycerophosphoglycerol, a possible intermediate in lipoteichoic acid biosynthesis in Streptococcus sanguis. Biochim. Biophys. Acta 1166:222-228. [DOI] [PubMed] [Google Scholar]
- 93.Ciardi, J. E., J. A. Reilly, R. H. Haller, W. H. Bowen, and G. Rølla. 1981. The role of lipoteichoic acid in the adherence and colonization of oral streptococci, p. 353-364. In G. D. Shockman and A. J. Wicken (ed.), Chemistry and biological activities of bacterial surface amphiphiles. Academic Press, Inc., New York, N.Y.
- 94.Ciardi, J. E., G. Rølla, W. H. Bowen, and J. A. Reilly. 1977. Adsorption of Streptococcus mutans lipoteichoic acid to hydroxyapatite. Scand. J. Dent. Res. 85:387-391. [DOI] [PubMed] [Google Scholar]
- 95.Clark, V. L., and F. E. Young. 1978. d-Alanine incorporation into macromolecules and effects of d-alanine deprivation on active transport in Bacillus subtilis. J. Bacteriol. 133:1339-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clarke-Sturman, A. J., A. R. Archibald, I. C. Hancock, C. R. Harwood, T. Merad, and J. A. Hobot. 1989. Cell wall assembly in Bacillus subtilis: partial conversion of polar wall material and the effect of growth conditions on the pattern of incorporation of new material at the polar caps. J. Gen. Microbiol. 135:657-665. [DOI] [PubMed] [Google Scholar]
- 97.Clemens, D. L., P. E. Kolenbrander, D. V. Debabov, Q. Zhang, R. D. Lunsford, H. Sakone, C. J. Whittaker, M. P. Heaton, and F. C. Neuhaus. 1999. Insertional inactivation of genes responsible for the d-alanylation of lipoteichoic acid in Streptococcus gordonii DL1 (Challis) affects intrageneric coaggregations. Infect. Immun. 67:2464-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cleveland, M. G., J. D. Gorham, T. L. Murphy, E. Tuomanen, and K. M. Murphy. 1996. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect. Immun. 64:1906-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cleveland, R. F., L. Daneo-Moore, A. J. Wicken, and G. D. Shockman. 1976. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J. Bacteriol. 127:1582-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cleveland, R. F., J.-V. Höltje, A. J. Wicken, A. Tomasz, L. Daneo-Moore, and G. D. Shockman. 1975. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem. Biophys. Res. Commun. 67:1128-1135. [DOI] [PubMed] [Google Scholar]
- 101.Cohn, E. J., and J. T. Edsall. 1943. Proteins, amino acids and peptides as ions and dipolar ions, p. 99. Reinhold Publishing Corp., New York, N.Y.
- 102.Coley, J., E. Tarelli, A. R. Archibald, and J. Baddiley. 1978. The linkage between teichoic acid and peptidoglycan in bacterial cell walls. FEBS Lett. 88:1-9. [DOI] [PubMed] [Google Scholar]
- 103.Collins, L. V., S. A. Kristian, C. Weidenmaier, M. Faigle, K. P. M. van Kessel, J. A. G. van Strijp, F. Götz, B. Neumeister, and A. Peschel. 2002. Staphylococcus aureus strains lacking d-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J. Infect. Dis. 186:214-219. [DOI] [PubMed] [Google Scholar]
- 104.Costerton, J. W., R. T. Irvin, and K.-J. Cheng. 1981. The bacterial glycocalyx in nature and disease. Annu. Rev. Microbiol. 35:299-324. [DOI] [PubMed] [Google Scholar]
- 105.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 106.Courtney, H. S., D. L. Hasty, and J. B. Dale. 2002. Molecular mechanisms of adhesion, colonization, and invasion of group A streptococci. Ann. Med. 34:77-87. [DOI] [PubMed] [Google Scholar]
- 107.Courtney, H. S., D. L. Hasty, and I. Ofek. 1990. Hydrophobicity of group A streptococci and its relationship to adhesion of streptococci to host cells, p. 361-386. In R. J. Doyle and M. Rosenberg (ed.), Microbial cell surface hydrophobicity. American Society for Microbiogy, Washington, D.C.
- 108.Courtney, H. S., I. Ofek, and D. L. Hasty. 1997. M protein mediated adhesion of M type 24 Streptococcus pyogenes stimulates release of interleukin-6 by HEp-2 tissue culture cells. FEMS Microbiol. Lett. 151:65-70. [DOI] [PubMed] [Google Scholar]
- 109.Courtney, H. S., C. vonHunolstein, J. B. Dale, M. S. Bronze, E. H. Beachey, and D. L. Hasty. 1992. Lipoteichoic acid and M protein: dual adhesins of group A streptococci. Microb. Pathol. 12:199-208. [DOI] [PubMed] [Google Scholar]
- 110.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Götz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crandall, A. D., and T. J. Montville. 1998. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl. Environ. Microbiol. 64:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Craynest, M., S. Jørgensen, M. Sarvas, and V. P. Kontinen. 2003. Enhanced secretion of heterologous cyclodextrin glycosyltransferase by a mutant of Bacillus licheniformis defective in the d-alanylation of teichoic acids. Lett. Appl. Microbiol. 37:75-80. [DOI] [PubMed] [Google Scholar]
- 113.Danforth, J. M., R. M. Strieter, S. L. Kunkel, D. A. Arenberg, G. M. VanOtteren, and T. J. Standiford. 1995. Macrophage inflammatory protein-1α expression in vivo and in vitro: the role of lipoteichoic acid. Clin. Immunol. Immunopathol. 74:77-83. [DOI] [PubMed] [Google Scholar]
- 114.Davie, J. M., and T. D. Brock. 1966. Effect of teichoic acid on resistance to the membrane-lytic agent of Streptococcus zymogenes. J. Bacteriol. 92:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davies, E. A., M. B. Falahee, and M. R. Adams. 1996. Involvement of the cell envelope of Listeria monocytogenes in the acquisition of nisin resistance. J. Appl. Bacteriol. 81:139-146. [DOI] [PubMed] [Google Scholar]
- 116.Davison, A. L., J. Baddiley, T. Hofstad, N. Losnegard, and P. Oeding. 1964. Teichoic acids in the walls of staphylococci; serological investigations on teichoic acids from the walls of staphylococci. Nature 202:872-874. [DOI] [PubMed] [Google Scholar]
- 117.Debabov, D. V., M. P. Heaton, Q. Zhang, K. D. Stewart, R. H. Lambalot, and F. C. Neuhaus. 1996. The d-alanyl carrier protein in Lactobacillus casei: cloning, sequencing, and expression of dltC. J. Bacteriol. 178:3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Debabov, D. V., M. Y. Kiriukhin, and F. C. Neuhaus. 2000. Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in d-alanylation. J. Bacteriol. 182:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Chastellier, C., R. Hellio, and A. Ryter. 1975. Study of cell wall growth in Bacillus megaterium by high-resolution autoradiography. J. Bacteriol. 123:1184-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deininger, S., A. Stadelmaier, S. von Aulock, S. Morath, R. R. Schmidt, and T. Hartung. 2003. Definition of structural prerequisites for lipoteichoic acid-inducible cytokine induction by synthetic derivatives. J. Immunol. 170:4134-4138. [DOI] [PubMed] [Google Scholar]
- 121.De Kimpe, S. J., M. Kengatharan, C. Thiemermann, and J. R. Vane. 1995. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. USA 92:10359-10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Lencastre, H., and A. Tomasz. 1994. Reassessment of the number of auxiliary genes essential for expression of high-level methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 38:2590-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122a.Delcour, J., T. Ferain, M. Deghorain, E. Palumbo, and P. Hols. 1999. The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie Leuwenhoek 76:159-184. [PubMed] [Google Scholar]
- 123.Demchick, P., and A. L. Koch. 1996. The permeability of the wall fabric of Escherichia coli and Bacillus subtilis. J. Bacteriol. 178:768-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Devine, D. A., and R. E. W. Hancock. 2002. Cationic peptides: distribution and mechanisms of resistance. Curr. Pharm. Des. 8:703-714. [DOI] [PubMed] [Google Scholar]
- 125.Dijkstra, A. J., and W. Keck. 1996. Peptidoglycan as a barrier to transenvelope transport. J. Bacteriol. 178:5555-5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dishon, T., R. Finkel, Z. Marcus, and I. Ginsburg. 1967. Cell-sensitizing products of streptococci. Immunology 13:555-564. [PMC free article] [PubMed] [Google Scholar]
- 127.Doyle, R. J. 1989. How cell walls of gram-positive bacteria interact with metal ions. p. 275-293. In T. J. Beveridge and R. J. Doyle (ed.), Metal ions and bacteria. John Wiley & Sons, Inc., New York, N.Y.
- 128.Doyle, R. J., A. N. Chatterjee, U. N. Streips, and F. E. Young. 1975. Soluble macromolecular complexes involving bacterial teichoic acids. J. Bacteriol. 124:341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Doyle, R. J., and A. L. Koch. 1987. The functions of autolysins in the growth and division of Bacillus subtilis. Crit. Rev. Microbiol. 15:169-222. [DOI] [PubMed] [Google Scholar]
- 130.Doyle, R. J., and R. E. Marquis. 1994. Elastic, flexible peptidoglycan and bacterial cell wall properties. Trends Microbiol. 2:57-60. [DOI] [PubMed] [Google Scholar]
- 131.Doyle, R. J., T. H. Matthews, and U. N. Streips. 1980. Chemical basis for selectivity of metal ions by the Bacillus subtilis cell wall. J. Bacteriol. 143:471-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Doyle, R. J., M. L. McDannel, J. R. Helman, and U. N. Streips. 1975. Distribution of teichoic acid in the cell wall of Bacillus subtilis. J. Bacteriol. 122:152-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Doyle, R. J., M. L. McDannel, U. N. Streips, D. C. Birdsell, and F. E. Young. 1974. Polyelectrolyte nature of bacterial teichoic acids. J. Bacteriol. 118:606-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Duckworth, M. 1977. Teichoic acids, p. 177-208. In I. Sutherland (ed.) Surface carbohydrates of the prokaryotic cell. Academic Press, Ltd., London, United Kingdom.
- 135.Duckworth, M., A. R. Archibald, and J. Baddiley. 1975. Lipoteichoic acid and lipoteichoic acid carrier in Staphylococcus aureus H. FEBS Lett. 53:176-179. [DOI] [PubMed] [Google Scholar]
- 136.Dunne, D. W., D. Resnick, J. Greenberg, M. Krieger, and K. A. Joiner. 1994. The type I macrophage scavenger receptor binds to gram-positive bacteria and recognizes lipoteichoic acid. Proc. Natl. Acad. Sci. USA 91:1863-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dunny, G. M., B. A. B. Leonard, and P. J. Hedberg. 1995. Pheromone-inducible conjugation in Enterococcus faecalis: interbacterial and host-parasite chemical communication. J. Bacteriol. 177:871-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dunman, P. M., E. Murphy, S. Haney, D. Palacios, G. Tucker-Kellogg, S. Wu, E. L. Brown, R. J. Zagursky, D. Shlaes, and S. J. Projan. 2001. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 183:7341-7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Duwat, P., A. Cochu, S. D. Ehrlich, and A. Gruss. 1997. Characterization of Lactococcus lactis UV-sensitive mutants obtained by IS S1 transposition. J. Bacteriol. 179:4473-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dziarski, R., R. I. Tapping, and P. S. Tobias. 1998. Binding of bacterial peptidoglycan to CD14. J. Biol. Chem. 273:8680-8690. [DOI] [PubMed] [Google Scholar]
- 141.Ehrenfeld, E. E., R. E. Kessler, and D. B. Clewell. 1986. Identification of pheromone-induced surface proteins in Streptococcus faecalis and evidence of a role for lipoteichoic acid in formation of mating aggregates. J. Bacteriol. 168:6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Elgavish, A. 2000. NF-κB activation mediates the response of a subpopulation of basal uroepithelial cells to a cell wall component of Enterococcus faecalis. J. Cell. Physiol. 182:232-238. [DOI] [PubMed] [Google Scholar]
- 143.Ellwood, D. C. 1970. The wall content and composition of Bacillus subtilis var. niger grown in a chemostat. Biochem. J. 118:367-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ellwood, D. C. 1971. The anionic polymers in the cell wall of Bacillus subtilis var. niger grown in phosphorus-limiting environments supplemented with increasing concentrations of sodium chloride. Biochem. J. 121:349-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ellwood, D. C., and D. W. Tempest. 1972. Effects of environment on bacterial wall content and composition. Adv. Microb. Physiol. 7:83-116. [Google Scholar]
- 146.Ellwood, D. C., and D. W. Tempest. 1972. Influence of culture pH on the content and composition of teichoic acids in the walls of Bacillus subtilis. J. Gen. Microbiol. 73:395-402. [DOI] [PubMed] [Google Scholar]
- 147.Emdur, L., and T.-H. Chiu. 1975. The role of phosphatidylglycerol in the in vitro biosynthesis of teichoic acid and lipoteichoic acid. FEBS Lett. 55:216-219. [DOI] [PubMed] [Google Scholar]
- 148.Reference deleted.
- 149.Endl, J., H. P. Seidl, F. Fiedler, and K. H. Schleifer. 1983. Chemical composition and structure of cell wall teichoic acids of staphylococci. Arch. Microbiol. 135:215-223. [DOI] [PubMed] [Google Scholar]
- 150.Evans, R. P., C. L. Nelson, W. R. Bowen, M. G. Kleve, and S. G. Hickmon. 1998. Visualization of bacterial glycocalyx with a scanning electron microscope. Clin. Orthop. Relat. Res. 347:243-249. [PubMed] [Google Scholar]
- 151.Fan, D. P., B. E. Beckman, and H. L. Gardner-Eckstrom. 1975. Mode of cell wall synthesis in gram-positive bacilli. J. Bacteriol. 123:1157-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fan, X., F. Stelter, R. Menzel, R. Jack, I. Spreitzer, T. Hartung, and C. Schütt. 1999. Structures in Bacillus subtilis are recognized by CD14 in a lipopolysaccharide binding protein-dependent reaction. Infect. Immun. 67:2964-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ferrari, E., D. J. Henner, and M. Y. Yang. 1985. Isolation of an alanine racemase gene from Bacillus subtilis and its use for plasmid maintenance in B. subtilis Bio/Technology 3:1003-1007. [Google Scholar]
- 154.Fiedler, F., and L. Glaser. 1974. The synthesis of polyribitol phosphate. I. Purification of polyribitol phosphate polymerase and lipoteichoic acid carrier. J. Biol. Chem. 249:2684-2689. [PubMed] [Google Scholar]
- 155.Fischer, W. 1982. d-Alanine ester-containing glycerophosphoglycolipids in the membrane of gram-positive bacteria. Biochim. Biophys. Acta 711:372-375. [DOI] [PubMed] [Google Scholar]
- 156.Fischer, W. 1987. ‘Lipoteichoic acid’ of Bifidobacterium bifidum subspecies pennsylvanicum DSM 20239. Eur. J. Biochem. 165:639-646. [DOI] [PubMed] [Google Scholar]
- 157.Fischer, W. 1988. Physiology of lipoteichoic acids in bacteria. Adv. Microb. Physiol. 29:233-302. [DOI] [PubMed] [Google Scholar]
- 158.Fischer, W. 1990. Bacterial phosphoglycolipids and lipoteichoic acids, p. 123-234. In M. Kates (ed.) Handbook of lipid research, vol VI. Glycolipids, phosphoglycolipids and sulfoglycolipids. Plenum Press, Inc., New York, N.Y.
- 159.Fischer, W. 1993. Molecular analysis of lipid macroamphiphiles by hydrophobic interaction chromatography, exemplified with lipoteichoic acids. Anal. Biochem. 208:49-56. [DOI] [PubMed] [Google Scholar]
- 160.Fischer, W. 1994. Lipoteichoic acids and lipoglycans. New Compr. Biochem. 27:199-215. [Google Scholar]
- 161.Fischer, W. 1994. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med. Microbiol. Immunol. 183:61-76. [DOI] [PubMed] [Google Scholar]
- 162.Fischer, W. 1996. Molecular analysis of lipid macroamphiphiles by hydrophobic interaction chromatography. J. Microbiol. Methods 25:129-144. [Google Scholar]
- 163.Fischer, W. 1997. Lipoteichoic acid and teichoic acid biosynthesis. Targets of new antibiotics. Biospektrum 1997:47-50.
- 164.Fischer, W. 2000. Pneumococcal lipoteichoic and teichoic acid, p. 155-177. In A. Tomasz (ed.), Streptococcus pneumoniae: molecular biology and mechanisms of disease. Mary Ann Liebert, Inc., Larchmont, N.Y.
- 165.Fischer, W. and H. U. Koch. 1981. Alanine ester substitution and its effect on the biological properties of lipoteichoic acids, p. 181-194. In G. D. Shockman and A. J. Wicken (ed.), Chemistry and biological activities of bacterial surface amphiphiles: Academic Press, Inc., New York, N.Y.
- 166.Fischer, W., and H. U. Koch. 1985. Alanyl lipoteichoic acid of Staphylococccus aureus: functional and dynamic aspects. Biochem. Soc. Trans. 13:984-986. [DOI] [PubMed] [Google Scholar]
- 167.Fischer, W., H. U. Koch, and R. Haas. 1983. Improved preparation of lipoteichoic acids. Eur. J. Biochem. 133:523-530. [DOI] [PubMed] [Google Scholar]
- 168.Fischer, W., H. U. Koch, P. Rösel and F. Fiedler. 1980. Alanine ester-containing native lipoteichoic acids do not act as lipoteichoic acid carrier: isolation, structural and functional characterization. J. Biol. Chem. 255:4557-4562. [PubMed] [Google Scholar]
- 169.Fischer, W., H. U. Koch, P. Rösel, F. Fiedler, and L. Schmuck. 1980. Structural requirements of lipoteichoic acid carrier for recognition by the poly(ribitol phosphate) polymerase from Staphylococcus aureus H: a study of various lipoteichoic acids, derivatives, and related compounds. J. Biol. Chem. 255:4550-4556. [PubMed] [Google Scholar]
- 170.Fischer, W., T. Mannsfeld, and G. Hagen. 1990. On the basic structure of poly(glycerophosphate) lipoteichoic acids. Biochem. Cell Biol. 68:33-43. [DOI] [PubMed] [Google Scholar]
- 171.Fischer, W., and P. Rösel. 1980. The alanine ester substitution of lipoteichoic acid (LTA) in Staphylococcus aureus. FEBS Lett. 119:224-226. [DOI] [PubMed] [Google Scholar]
- 172.Fischer, W., P. Rösel, and H. U. Koch. 1981. Effect of alanine ester substitution and other structural features of lipoteichoic acids on their inhibitory activity against autolysins of Staphylococcus aureus. J. Bacteriol. 146:467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Foster, S. J., and D. L. Popham. 2002. Structure and synthesis of cell wall, spore cortex, teichoic acids, S-layers, and capsules, p. 21-41. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 174.Franklin, M. J., and D. E. Ohman. 1996. Identification of algI and algJ in the Pseudomonas aeruginosa alginate biosynthetic gene cluster which are required for alginate O acetylation. J. Bacteriol. 178:2186-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Franklin, M. J., and D. E. Ohman. 2002. Mutant analysis and cellular localization of the AlgI, AlgJ, and AlgF proteins required for O acetylation of alginate in Pseudomonas aeruginosa. J. Bacteriol. 184:3000-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ganfield, M.-C. W., and R. A. Pierginger. 1980. The biosynthesis of nascent membrane lipoteichoic acid of Streptococcus faecium (S. faecalis ATCC 9790) from phosphatidylkojibiosyldiacylglycerol and phosphatidylglycerol. J. Biol. Chem. 255:5164-5169. [PubMed] [Google Scholar]
- 177.Gao, J. J., Q. Xue, E. G. Zuvanich, K. R. Haghi, and D. C. Morrison. 2001. Commercial preparations of lipoteichoic acid contain endotoxin that contributes to activation of mouse macrophages in vitro. Infect. Immun. 69:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gargir, A., I. Ofek, D. Hasty, S. Meron-Sudai, H. Tsubery, Y. Keisari, and A. Nissim. 2001. Inhibition of antibody-dependent stimulation of lipoteichoic acid-treated human monocytes and macrophages by polyglycerolphosphate-reactive peptides. J. Leukoc. Biol. 70:537-542. [PubMed] [Google Scholar]
- 179.Gilbert, P., T. Maira-Litran, A. J. McBain, A. H. Rickard, and F. W. Whyte. 2002. The physiology and collective recalcitrance of microbial biofilm communities. Adv. Microb. Physiol. 46:203-256. [PubMed] [Google Scholar]
- 180.Ginsburg, I. 2002. Role of lipoteichoic acid in infection and inflammation. Lancet Infect. Dis. 2:171-179. [DOI] [PubMed] [Google Scholar]
- 181.Ginsburg, I. 2002. The role of bacteriolysis in the pathophysiology of inflammation, infection and post-infectious sequelae. APMIS 110:753-770. [DOI] [PubMed] [Google Scholar]
- 182.Ginsburg, I., S. E. G. Fligiel, P. A. Ward, and J. Varani. 1988. Lipoteichoic acid-antilipoteichoic acid complexes induce superoxide generation by human neutrophils. Inflammation 12:525-548. [DOI] [PubMed] [Google Scholar]
- 183.Glaser, L. 1964. The synthesis of teichoic acids. II. Polyribitol phosphate. J. Biol. Chem. 239:3178-3186. [PubMed] [Google Scholar]
- 184.Glaser, L., and M. M. Burger. 1964. The synthesis of teichoic acids. III. Glucosylation of polyglycerophosphate. J. Biol. Chem. 239:3187-3191. [PubMed] [Google Scholar]
- 185.Glaser, L., and B. Lindsay. 1974. The synthesis of lipoteichoic acid carrier. Biochem. Biophys. Res. Commun. 59:1131-1136. [DOI] [PubMed] [Google Scholar]
- 186.Glaser, P., F. Kunst, M. Arnaud, M.-P. Coudart, W. Gonzales, M.-F. Hullo, M. Ionescu, B. Lubochinsky, L. Marcelino, I. Moszer, E. Presecan, M. Santana, E. Schneider, J. Schweizer, A. Vertes, G. Rapoport, and A. Danchin. 1993. Bacillus subtilis genome project: cloning and sequencing of the 97 kb region from 325° to 333°. Mol. Microbiol. 10:371-384. [DOI] [PubMed] [Google Scholar]
- 187.Goldschmidt, J. C., Jr., and C. Panos. 1984. Teichoic acids of Streptococcus agalactiae: chemistry, cytotoxicity, and effect on bacterial adherence to human cells in tissue culture. Infect. Immun. 43:670-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Götz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 189.Graham, L. L., and T. J. Beveridge. 1994. Structural differentiation of the Bacillus subtilis 168 cell wall. J. Bacteriol. 176:1413-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Grant, W. D. 1979. Cell wall teichoic acid as a reserve phosphate source in Bacillus subtilis. J. Bacteriol. 137:35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Greenberg, J. W., W. Fischer, and K. A. Joiner. 1996. Influence of lipoteichoic acid structure on recognition by the macrophage scavenger receptor. Infect. Immun. 64:3318-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Griffin, B. E., M. Jarman, C. B. Reese, J. E. Sulston, and D. R. Trentham. 1966. Some observations relating to acyl mobility in aminoacyl soluble ribonucleic acids. Biochemistry 5:3638-3649. [Google Scholar]
- 193.Gross, M., S. E. Cramton, F. Götz, and A. Peschel. 2001. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 69:3423-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reference deleted.
- 195.Gutberlet, T., J. Frank, H. Bradaczek, and W. Fischer. 1997. Effect of lipoteichoic acid on thermotropic membrane properties. J. Bacteriol. 179:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gutman, L., S. Al-Obeid, D. Billot-Klein, E. Ebnet, and W. Fischer. 1996. Penicillin tolerance and modification of lipoteichoic acid associated with expression of vancomycin resistance in vanB-type Enterococcus faecium D366. Antimicrob. Agents Chemother. 40:257-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Haas, R., H. U. Koch, and W. Fischer. 1984. Alanyl turnover from lipoteichoic acid to teichoic acid in Staphylococcus aureus. FEMS Microbiol. Lett. 21:27-31. [Google Scholar]
- 198.Hancock, I. C. 1981. The biosynthesis of wall teichoic acid by toluenised cells of Bacillus subtilis W23. Eur. J. Biochem. 119:85-90. [DOI] [PubMed] [Google Scholar]
- 199.Hancock, I. C. 1991. Microbial cell surface architecture, p. 23-59. In N. Mozes, P. S. Handley, H. J. Busscher, and P. G. Rouxhet (ed.), Microbial cell surface analysis: structural and physicochemical methods. VCH Publishers, Inc., New York, N.Y.
- 200.Hancock, I. C., and J. Baddiley. 1976. In vitro synthesis of the unit that links teichoic acid to peptidoglycan. J. Bacteriol. 125:880-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hancock, I. C., and J. Baddiley. 1985. Biosynthesis of the bacterial envelope polymers teichoic acid and teichuronic acid, p. 279-307. In A. Martonosi (ed.), The enzymes of biological membranes, vol. 2, 2nd ed., Plenum Press, New York, N.Y.
- 202.Hancock, R. E. W. 1997. Peptide antibiotics. Lancet 349:418-422. [DOI] [PubMed] [Google Scholar]
- 203.Hardy, L., N. A. Jacques, H. Forester, L. K. Campbell, K. W. Knox, and A. J. Wicken. 1981. Effect of fructose and other carbohydrates on the surface properties, lipoteichoic acid production, and extracellular proteins of Streptococcus mutans Ingbritt grown in continuous culture. Infect. Immun. 31:78-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Harrington, C. R., and J. Baddiley. 1984. Synthesis of peptidoglycan and teichoic acid in Bacillus subtilis: role of the electrochemical proton gradient. J. Bacteriol. 159:925-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Harrington, C. R., and J. Baddiley. 1985. Biosynthesis of wall teichoic acids in Staphylocccus aureus H, Micrococcus varians and Bacillus subtilis W23: involvement of lipid intermediates containing the disaccharide N-acetylmannosaminyl N-acetylglucosamine. Eur. J. Biochem. 153:639-645. [DOI] [PubMed] [Google Scholar]
- 206.Hashimoto, M., J. Yasuoka, Y. Suda, H. Takada, T. Yoshida, S. Kotani, and S. Kusumoto. 1997. Structural features of the major but not cytokine-inducing molecular species of lipoteichoic acid. J. Biochem. 121:779-786. [DOI] [PubMed] [Google Scholar]
- 207.Hasty, D., and H. S. Courtney. 1996. Group A streptococcal adhesion: all of the theories are correct, p. 81-94. In I. Kahane and I. Ofek (ed.), Advances in Experimental Medicine, vol. 408. Toward anti-adhesion therapy for microbial diseases. Plenum Press, New York, N.Y. [PubMed]
- 208.Hasty, D. L., I. Ofek, H. S. Courtney, and R. J. Doyle. 1992. Multiple adhesins of streptococci. Infect. Immun. 60:2147-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Heaton, M. P., and F. C. Neuhaus. 1992. Biosynthesis d-alanyl-lipoteichoic acid: cloning, nucleotide sequence, and expression of the Lactobacillus casei gene for the d-alanine-activating enzyme. J. Bacteriol. 174:4707-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Heaton, M. P., and F. C. Neuhaus. 1993. The significance of secondary cell wall polymers in gram-positive organisms: Lactobacillus casei as a model system for the study of d-alanyl-lipoteichoic acid biosynthesis and function, p. 89-98. In E. L. Foo, H. G. Griffin, R. Mollby, and C. G. Heden (ed.), The lactic acid bacteria. Horizon Scientific Press, Wymondham, United Kingdom.
- 211.Heaton, M. P., and F. C. Neuhaus. 1994. Role of the d-alanyl carrier protein in the biosynthesis of d-alanyl-lipoteichoic acid. J. Bacteriol. 176:681-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Heckels, J. E., P. A. Lambert, and J. Baddiley. 1977. Binding of magnesium ions to cell walls of Bacillus subtilis W23 containing teichoic acid or teichuronic acid. Biochem. J. 162:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Helmann, J. D. 2002. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 46:47-110. [DOI] [PubMed] [Google Scholar]
- 214.Henderson, B., S. Poole, and M. Wilson. 1996. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol. Rev. 60:316-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Heptinstall, S., A. R. Archibald, and J. Baddiley. 1970. Teichoic acids and membrane function in bacteria. Nature 225:519-521. [DOI] [PubMed] [Google Scholar]
- 216.Herbold, D. R., and L. Glaser. 1975. Bacillus subtilis N-acetylmuramic acid l-alanine amidase. J. Biol. Chem. 250:1676-1682. [PubMed] [Google Scholar]
- 217.Hermann, C., I. Spreitzer, N. W. J. Schröder, S. Morath, M. D. Lehner, W. Fischer, C. Schütt, R. R. Schumann, and T. Hartung. 2002. Cytokine induction by purified lipoteichoic acids from various bacterial species-role of LBP, sCD14, CD14 and failure to induce IL-12 and subsequent IFN-γ release. Eur. J. Immunol. 32:541-551. [DOI] [PubMed] [Google Scholar]
- 218.Higgins, M. L., L. C. Parks, and L. Daneo-Moore. 1981. The mesosome, p. 75-94. In B. K. Ghosh (ed.), Organization of prokaryotic cell membranes, vol. 2. CRC Press, Inc., Boca Raton, Fla.
- 219.Hofmann, K. 2000. A superfamily of membrane-bound O-acyltransferases with implications for Wnt signaling. Trends Biochem. Sci. 25:111-112. [DOI] [PubMed] [Google Scholar]
- 220.Hogg, S. D., and I. Lightfoot. 1989. Interaction of streptococcal lipoteichoic acid with artificial tooth pellicle. Arch. Oral Biol. 34:615-620. [DOI] [PubMed] [Google Scholar]
- 221.Hogg, S. D., R. A. Whiley, and J. J. De Soet. 1997. Occurrence of lipoteichoic acid in oral streptococci. Int. J. Syst. Bacteriol. 47:62-66. [DOI] [PubMed] [Google Scholar]
- 222.Hols, P., C. Defrenne, T. Ferain, S. Derzelle, B. Deplace, and J. Delcour. 1997. The alanine racemase gene is essential for growth of Lactobacillus plantarum. J. Bacteriol. 179:3804-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Höltje, J.-V., and A. Tomasz. 1975. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc. Natl. Acad. Sci. USA 72:1690-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Honeyman, A. L., and G. C. Stewart. 1989. The nucleotide sequence of the rodC operon of Bacillus subtilis. Mol. Microbiol. 3:1257-1268. [DOI] [PubMed] [Google Scholar]
- 225.Horne, D., R. Hakenbeck, and A. Tomasz. 1977. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J. Bacteriol. 132:704-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Horne, D., and A. Tomasz. 1977. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob. Agents Chemother. 11:888-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Horne, D., and A. Tomasz. 1979. Release of lipoteichoic acid from Streptococcus sanguis: stimulation of release during penicillin treatment. J. Bacteriol. 137:1180-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P.-M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Houtsmuller, U. M. T., and L. L. M. van Deenen. 1965. On the amino acid esters of phosphatidylglycerol from bacteria. Biochim. Biophys. Acta 106:564-576. [DOI] [PubMed] [Google Scholar]
- 230.Hughes, A. H., I. C. Hancock, and J. Baddiley. 1973. The function of teichoic acids in cation control in bacterial membranes. Biochem. J. 132:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hulett, F. M. 1993. Regulation of phosphorus metabolism, p. 229-235. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 232.Hummell, D. S., and J. A. Winkelstein. 1986. Bacterial lipoteichoic acid sensitizes host cells for destruction by autologous complement. J. Clin. Investig. 77:1533-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hurst, A., A. Hughes, M. Duckworth, and J. Baddiley. 1975. Loss of d-alanine during sublethal heating of Staphylococcus aureus s6 and magnesium binding during repair. J. Gen. Microbiol. 89:277-284. [DOI] [PubMed] [Google Scholar]
- 234.Hussain, M., M. H. Wilcox, and P. J. White. 1993. The slime of coagulase-negative staphylococci: biochemistry and relation to adherence. FEMS Microbiol. Rev. 104:191-208. [DOI] [PubMed] [Google Scholar]
- 235.Hussey, H., D. Brooks, and J. Baddiley. 1969. Direction of chain extension during the biosynthesis of teichoic acids in bacterial cell walls. Nature 221:665-666. [DOI] [PubMed] [Google Scholar]
- 236.Hyyryläinen, H.-L., M. Vitikainen, J. Thwaite, H. Wu., M. Sarvas, C. R. Harwood, V. P. Kontinen, and K. Stephenson. 2000. d-Alanine substitution of teichoic acids as a modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis. J. Biol. Chem. 275:26696-26703. [DOI] [PubMed] [Google Scholar]
- 237.Ike, Y., D. B. Clewell, R. A. Segarra, and M. S. Gilmore. 1990. Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis: Tn 917 insertional mutagenesis and cloning. J. Bacteriol. 172:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Iwasaki, H., Y. Araki, E. Ito, M. Nagaoka, and T. Yokokura. 1990. Structure of macroamphiphiles from several Bifidobacterium strains. J. Bacteriol. 172:845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Iwasaki, H., A. Shimada, and E. Ito. 1986. Comparative studies of lipoteichoic acids from several Bacillus strains. J. Bacteriol. 167:508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Iwasaki, H., A. Shimada, K. Yokoyama, and E. Ito. 1989. Structure and glycosylation of lipoteichoic acids in Bacillus strains. J. Bacteriol. 171:424-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jackson, D. E., W. Wong, M. T. Largen, and G. D. Shockman. 1984. Monoclonal antibodies to immunodeterminants of lipoteichoic acids. Infect. Immun. 43:800-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jackson, R. W., and M. Moskowitz. 1966. Nature of a red cell sensitizing substance from streptococci. J. Bacteriol. 91:2205-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jacques, N. A., L. Hardy, L. K. Campbell, K. W. Knox, J. D. Evans, and A. J. Wicken. 1979. Effect of carbohydrate source and growth conditions on the production of lipoteichoic acid by Streptococcus mutans Ingbritt. Infect. Immun. 26:1079-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jenkinson, H. F. 2000. Polypeptide linkage to bacterial cell envelope glycopolymers, p. 67-91. In R. J. Doyle (ed.), Glycomicrobiology. Kluwer Academic/Plenum, New York, N.Y.
- 245.Jenni, R., and B. Berger-Bächi. 1998. Teichoic acid content in different lineages of Staphylococcus aureus NCTC8325. Arch. Microbiol. 170:171-178. [DOI] [PubMed] [Google Scholar]
- 246.Jerić, I., L. Šimičić, M. Stipetić, and S. Horvat. 2000. Synthesis and reactivity of the monosaccharide esters of amino acids as models of teichoic acid fragment. Glycoconj. J. 17:273-282. [DOI] [PubMed] [Google Scholar]
- 247.Jolliffe, L. K., R. J. Doyle, and U. N. Streips. 1981. The energized membrane and cellular autolysis in Bacillus subtilis. Cell 25:753-763. [DOI] [PubMed] [Google Scholar]
- 248.Jonquières, R., H. Bierne, F. Fiedler, P. Gounon, and P. Cossart. 1999. Interaction between the protein InlB of Listeria monocytogenes and lipoteichoic acid: a novel mechanism of protein association at the surface of Gram-positive bacteria. Mol. Microbiol. 34:902-914. [DOI] [PubMed] [Google Scholar]
- 249.Jorasch, P., D. C. Warnecke, B. Lindner, U. Zähringer, and E. Heinz. 2000. Novel processive and nonprocessive glycosyltransferases from Staphylococcus aureus and Arabidopsis thaliana synthesize glycoglycerolipids, glycophospholipids, glycosphingolipids and glycosylsterols. Eur. J. Biochem. 267:3770-3783. [DOI] [PubMed] [Google Scholar]
- 250.Jorasch, P., F. P. Wolter, U. Zähringer, and E. Heinz. 1998. A UDP-glucosyltransferase from Bacillus subtilis successively transfers up to four glucose residues to 1,2-diacylglycerol: expression of ypfP in Escherichia coli and structural analysis of its reaction products. Mol. Microbiol. 29:419-430. [DOI] [PubMed] [Google Scholar]
- 251.Kalomiris, E., C. Bardin, and F. C. Neuhaus. 1982. Biosynthesis of peptidoglycan in Gaffkya homari: reactivation of membranes by freeze-thawing in the presence and absence of walls. J. Bacteriol. 150:535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Karakawa, W. W., and J. A. Kane. 1971. Immunochemical analysis of a galactosamine-rich teichoic acid of Staphylococcus aureus, phage type 187. J. Immunol. 106:900-906. [PubMed] [Google Scholar]
- 253.Kehoe, M. A. 1994. Cell-wall-associated proteins in Gram-positive bacteria. New Compr. Biochem. 27:217-261. [Google Scholar]
- 254.Kelemen, M. V., and J. Baddiley. 1961. Structure of the intracellular glycerol teichoic acid from Lactobacillus casei ATCC 7469. Biochem. J. 80:246-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Keller, R., W. Fischer, R. Keist, and S. Bassetti. 1992. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect. Immun. 60:3664-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kemper, M. A., and R. J. Doyle. 1993. The cell wall of Bacillus subtilis is protonated during growth, p. 245-252. In M. A. de Pedro, J.-V. Höltje, and W. Löffelhardt (ed.), Bacterial growth and lysis: metabolism and structure of the bacterial sacculus. Plenum Press, London, United Kingdom.
- 257.Kemper, M. A., M. M. Urrutia, T. J. Beveridge, A. L. Koch, and R. J. Doyle. 1993. Proton motive force may regulate cell wall-associated enzymes of Bacillus subtilis. J. Bacteriol. 175:5690-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kengatharan, K. M., S. De Kimpe, C. Robson, S. J. Foster, and C. Thiemermann. 1998. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J. Exp. Med. 188:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kennedy, L. D., and D. R. D. Shaw. 1968. Direction of polyglycerolphosphate chain growth in Bacillus subtilis. Biochem. Biophys. Res. Commun. 32:861-865. [Google Scholar]
- 260.Kiriukhin, M. Y., D. V. Debabov, D. L. Shinabarger, and F. C. Neuhaus. 2001. Biosynthesis of the glycolipid anchor in lipoteichoic acid of Staphylococcus aureus RN4220: role of YpfP, the diglucosyldiacylglycerol synthase. J. Bacteriol. 183:3506-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kiriukhin, M. Y., and F. C. Neuhaus. 2001. d-Alanylation of lipoteichoic acid: role of the d-alanyl carrier protein in acylation. J. Bacteriol. 183:2051-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kleinkauf, H., and H. von Döhren. 1996. A nonribosomal system of peptide biosynthesis. Eur. J. Biochem. 236:335-351. [DOI] [PubMed] [Google Scholar]
- 263.Kleyman, T. R., and E. J. Cragoe, Jr. 1988. Amiloride and its analogs as tools in the study of ion transport. J. Membr. Biol. 105:1-21. [DOI] [PubMed] [Google Scholar]
- 264.Knox, K. W., and A. J. Wicken. 1973. Immunological properties of teichoic acids. Bacteriol. Rev. 37:215-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kobayashi, K., S. D. Ehrlich, A. Albertini, G. Amati, K. K. Andersen, M. Arnaud, K. Asai, S. Ashikaga, S. Aymerich, P. Bessieres, F. Boland, S. C. Brignell, S. Bron, K. Bunai, J. Chapuis, L. C. Christiansen, A. Danchin, M. Débarbouillé, E. Dervyn, E. Deuerling, K. Devine, S. K. Devine, O. Dreesen, J. Errington, S. Fillinger, S. J. Foster, Y. Fujita, A. Galizzi, R. Gardan, C. Eschevins, T. Fukushima, K. Haga, C. R. Harwood, M. Hecker, D. Hosoya, M. F. Hullo, H. Kakeshita, D. Karamata, Y. Kasahara, F. Kawamura, K. Koga, P. Koski, R. Kuwana, D. Imamura, M. Ishimaru, S. Ishikawa, I. Ishio, D. Le Coq, A. Masson, C. Mauël, R. Meima, R. P. Mellado, A. Moir, S. Moriya, E. Nagakawa, H. Nanamiya, S. Nakai, P. Nygaard, M. Ogura, T. Ohanan, M. O'Reilly, M. O'Rourke, Z. Pragai, H. M. Pooley, G. Rapoport, J. P. Rawlins, L. A. Rivas, C. Rivolta, A. Sadaie, Y. Sadaie, M. Sarvas, T. Sato, H. H. Saxild, E. Scanlan, W. Schumann, J. F. M. L. Seegers, J. Sekiguchi, A. Sekowska, S. J. Séror, M. Simon, P. Stragier, R. Studer, H. Takamatsu, T. Tanaka, M. Takeuchi, H. B. Thomaides, V. Vagner, J. M. van Dijl, K. Watabe, A. Wipat, H. Yamamoto, M. Yamamoto, Y. Yamamoto, K. Yamane, K. Yata, K. Yoshida, H. Yoshikawa, U. Zuber, and N. Ogasawara. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA 100:4678-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Koch, A. L. 1986. The pH in the neighborhood of membranes generating a protonmotive force. J. Theor. Biol. 120:73-84. [DOI] [PubMed] [Google Scholar]
- 267.Koch, A. L. 1988. Biophysics of bacterial walls viewed as stress-bearing fabric. Microbiol. Rev. 52:337-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Koch, A. L. 2001. Bacterial growth and form, 2nd ed., p. 102-134. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 269.Koch, A. L., and R. J. Doyle. 1985. Inside-to-outside growth and turnover of the wall of gram-positive rods. J. Theor. Biol. 117:137-157. [DOI] [PubMed] [Google Scholar]
- 270.Koch, H. U., R. Döker, and W. Fischer. 1985. Maintenance of d-alanine ester substitution of lipoteichoic acid by reesterification in Staphylococcus aureus. J. Bacteriol. 164:1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Koch, H. U., W. Fischer, and F. Fiedler. 1982. Influence of alanine ester and glycosyl substitution on the lipoteichoic acid carrier activity of lipoteichoic acids. J. Biol. Chem. 257:9473-9479. [PubMed] [Google Scholar]
- 272.Koch, H. U., R. Haas, and W. Fischer. 1984. The role of lipoteichoic acid biosynthesis in membrane lipid metabolism of growing Staphylococcus aureus. Eur. J. Biochem. 138:357-363. [DOI] [PubMed] [Google Scholar]
- 273.Kojima, N., Y. Araki, and E. Ito. 1985. Structure of the linkage units between ribitol teichoic acids and peptidoglycan. J. Bacteriol. 161:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kolenbrander, P. E., R. N. Andersen, K. M. Kazmerzak, and R. J. Palmer, Jr. 2000. Coaggregation and coadhesion in oral biofilms, p. 65-85. In D. Allison, P. Gilbert, H. Lappin-Scott, and M. Wilson (ed.), Community structure and co-operation in biofilms. Cambridge University Press, Cambridge, United Kingdom.
- 275.Kolenbrander, P. E., R. N. Andersen, and L. V. H. Moore. 1990. Intrageneric coaggregation among strains of human oral bacteria: potential role in primary colonization of the tooth surface. Appl. Environ. Microbiol. 56:3890-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Komatsuzawa, H., J. Suzuki, M. Sugai, Y. Miyake, and H. Suginaka. 1994. The effect of Triton X-100 on the in vitro susceptibility of methicillin-resistant Staphylococcus aureus to oxacillin. J. Antimicrob. Chemother. 34:885-897. [DOI] [PubMed] [Google Scholar]
- 277.Kontinen, V. P., and M. Sarvas. 1993. The PrsA lipoprotein is essential for protein secretion in Bacillus subtilis and sets a limit for high-level secretion. Mol. Microbiol. 8:727-737. [DOI] [PubMed] [Google Scholar]
- 278.Konz, D., and M. A. Marahiel. 1999. How do peptide synthetases generate structural diversity? Chem. Biol. 6:R39-R48. [DOI] [PubMed] [Google Scholar]
- 279.Koprivnjak, T., A. Peschel, M. H. Gelb, N. S. Liang, and J. P. Weiss. 2002. Role of charge properties of bacterial envelope in bactericidal action of human group IIA phospholipase A2 against Staphylococcus aureus. J. Biol. Chem. 277:47636-47644. [DOI] [PubMed] [Google Scholar]
- 280.Korhonen, R., R. Korpela, M. Saxelin, M. Mäki, H. Kankaanranta, and E. Moilanen. 2001. Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macrophages and human T84 intestinal epithelial cells. Inflammation 25:223-232. [DOI] [PubMed] [Google Scholar]
- 281.Kristian, S. A., X. Lauth, V. Nizet, F. Goetz, B. Neumeister, A. Peschel, and R. Landmann. 2003. Alanylation of teichoic acids protects Staphylococcus aureus against toll-like receptor 2-dependent host defense in a mouse tissue cage infection model. J. Infect. Dis. 188:414-423. [DOI] [PubMed] [Google Scholar]
- 282.Kullik, I., R. Jenni, and B. Berger-Bächi. 1998. Sequence of the putative alanine racemase operon in Staphylococcus aureus: insertional interruption of this operon reduces D-alanine substitution of lipoteichoic acid and autolysis. Gene 219:9-17. [DOI] [PubMed] [Google Scholar]
- 283.Reference deleted.
- 284.Labischinski, H., and H. Maidhof. 1994. Bacterial peptidoglycan: overview and evolving concepts. New Compr. Biochem. 27:23-38. [Google Scholar]
- 285.Labischinski, H., D. Naumann, and W. Fischer. 1991. Small- and medium-angle X-ray analysis of bacterial lipoteichoic acid phase structure. Eur. J. Biochem. 202:1269-1274. [DOI] [PubMed] [Google Scholar]
- 286.Lahooti, M., and C. R. Harwood. 1999. Transcriptional analysis of the Bacillus subtilis teichuronic acid operon. Microbiology 145:3409-3417. [DOI] [PubMed] [Google Scholar]
- 287.Laine, R. A., and W. Fischer. 1978. On the relationship between glycerophosphoglycolipids and lipoteichoic acids of gram-positive bacteria. III. Di(glycerophospho)-acylkojibiosyldiacylglycerol and related compounds from Streptococcus lactis NCDO 712. Biochim. Biophys. Acta 529:250-262. [DOI] [PubMed] [Google Scholar]
- 288.Lambe, D. W., Jr., C. Jeffery, K. P. Ferguson, and M. D. Cooper. 1994. Examination of the glycocalyx of four species of Staphylococcus by transmission electron microscopy and image analysis. Microbios 78:133-143. [PubMed] [Google Scholar]
- 289.Lambert, P. A., I. C. Hancock, and J. Baddiley. 1975. The interaction of magnesium ions with teichoic acid. Biochem. J. 149:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lambert, P. A., I. C. Hancock, and J. Baddiley. 1975. Influence of alanyl ester residues on the binding of magnesium ions to teichoic acids. Biochem. J. 151:671-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lambert, P. A., I. C. Hancock, and J. Baddiley. 1977. Occurrence and function of membrane teichoic acids. Biochim. Biophys. Acta 472:1-12. [DOI] [PubMed] [Google Scholar]
- 292.Lazarevic, V., F.-X. Abellan, S. B. Möller, D. Karamata, and C. Maül. 2002. Comparison of ribitol and glycerol teichoic acid genes in Bacillus subtilis W23 and 168: identical function, similar divergent organization, but different regulation. Microbiology 148:815-824. [DOI] [PubMed] [Google Scholar]
- 293.Lazarevic, V., and D. Karamata. 1995. The tagGH operon of Bacillus subtilis 168 encodes a two-component ABC transporter involved in the metabolism of two wall teichoic acids. Mol. Microbiol. 16:345-355. [DOI] [PubMed] [Google Scholar]
- 293a.Lazarevic, V., H. M. Pooley, C. Mauël, and D. Karamata. 2002. Teichoic and teichuronic acids from gram-positive bacteria. p. 465-492. In E. J. Vandamme, S. De Baets, and A. Steinbüchel (ed.), Biopolymers, vol. 5. Polysaccharides from prokaryotes. Wiley-VCH Verlag, Weinheim, Germany.
- 294.Leaver, J., I. C. Hancock, and J. Baddiley. 1981. Fractionation studies of the enzyme complex involved in teichoic acid synthesis. J. Bacteriol. 146:847-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lehner, M. D., S. Morath, K. S. Michelsen, R. R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highy purified lipoteichoic acid via different toll-like receptors independent of paracrine mediators. J. Immunol. 166:5161-5167. [DOI] [PubMed] [Google Scholar]
- 296.Leitch, E. C., and M. D. P. Willcox. 1999. Elucidation of the antistaphylococcal action of lactoferrin and lysozyme. J. Med. Microbiol. 48:867-871. [DOI] [PubMed] [Google Scholar]
- 297.Leitch, E. C., and M. D. P. Willcox. 1999. Lactoferrin increases the susceptibility of S. epidermidis biofilms to lysozyme and vancomycin. Cur. Eye Res. 19:12-19. [DOI] [PubMed] [Google Scholar]
- 298.Lemjabbar, H., and C. Basbaum. 2002. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 8:41-46. [DOI] [PubMed] [Google Scholar]
- 299.Leon, O., and C. Panos. 1990. Streptococcus pyogenes clinical isolates and lipoteichoic acid. Infect. Immun. 58:3779-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Leopold, K., and W. Fischer. 1991. Separation of the poly(glycerophosphate) lipoteichoic acids of Enterococcus faecalis Kiel 27738, Enterococcus hirae ATCC 9790 and Leuconostoc mesenteroides DSM 20343 into molecular species by affinity chromatography on concanavalin A. Eur. J. Biochem. 196:475-482. [DOI] [PubMed] [Google Scholar]
- 301.Leopold, K., and W. Fischer. 1992. Hydrophobic interaction chromatography fractionates lipoteichoic acid according to the size of the hydrophilic chain: a comparative study with anion-exchange and affinity chromatography for suitability in species analysis. Anal. Biochem. 201:350-355. [DOI] [PubMed] [Google Scholar]
- 302.Leopold, K., and W. Fischer. 1992. Heterogeneity of lipoteichoic acid detected by anion exchange chromatography. Arch. Microbiol. 157:446-450. [DOI] [PubMed] [Google Scholar]
- 303.Levine, M. 1982. Naturally occurring human serum precipitins specific for d-alanyl esters of glycerol teichoic acid. Mol. Immunol. 19:133-142. [DOI] [PubMed] [Google Scholar]
- 304.Levine, M., R. L. Brumley, K. T. Avery, W. L. Owen, and D. E. Parker. 2002. Elevated antibody to d-alanyl lipoteichoic acid indicates caries experience associated with fluoride and gingival health. BMC Oral Health 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Levine, M., and B. F. Movafagh. 1984. d-Alanyl-substituted glycerol lipoteichoic acid in culture fluids of Streptococcus mutans GS-5 and BHT. Infect. Immun. 46:870-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Levy, R., M. Kotb, O. Nagauker, G. Majumdar, M. Alkan, I. Ofek, and E. H. Beachey. 1990. Stimulation of oxidative burst in human monocytes by lipoteichoic acids. Infect. Immun. 58:566-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lieberman, J., and F. R. Frankel. 2002. Engineered Listeria monocytogenes as an AIDs vaccine. Vaccine 20:2007-2010. [DOI] [PubMed] [Google Scholar]
- 308.Lin, C.-H., I.-H. Kuan, H.-M. Lee, W.-S. Lee, J.-R. Sheu, Y.-S. Ho, C.-H. Wang, and H.-P. Kuo. 2001. Induction of cyclooxygenase-2 protein by lipoteichoic acid from Staphylococcus aureus in human pulmonary epithelial cells: involvement of a nuclear factor-κB-dependent pathway. Br. J. Pharmacol. 134:543-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Linzer, R., and F. C. Neuhaus. 1973. Biosynthesis of membrane teichoic acid: a role for the d-alanine activating enzyme. J. Biol. Chem. 248:3196-3201. [PubMed] [Google Scholar]
- 310.Lonchampt, M. O., M. Auguet, S. Delaflotte, J. Goulin-Schulz, P. E. Chabrier, and P. Braquet. 1992. Lipoteichoic acid: a new inducer of nitric oxide synthase. J. Cardiovasc. Pharmacol. 20(Suppl. 12):S145-S147. [DOI] [PubMed] [Google Scholar]
- 311.Loos, M., F. Clas, and W. Fischer. 1986. Interaction of purified lipoteichoic acid with the classical complement pathway. Infect. Immun. 53:595-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.MacArthur, A. E., and A. R. Archibald. 1984. Effect of culture pH on the d-alanine ester content of lipoteichoic acid in Staphylococcus aureus. J. Bacteriol. 160:792-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Madigan, M. T., J. M. Martinko, and J. Parker. 2000. Brock: biology of microorganisms, 8th ed., p. 786-787. Prentice-Hall, Upper Saddle River, N.J.
- 314.Madiraju, M. V. V. S., D. P. Brunner, and B. J. Wilkinson. 1987. Effects of temperature, NaCl, and methicillin on penicillin-binding proteins, growth, peptidoglycan synthesis, and autolysis in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 31:1727-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Mancuso, D. J., D. D. Junker, S. C. Hsu, and T.-H. Chiu. 1979. Biosynthesis of glycosylated glycerolphosphate polymers in Streptococcus sanguis. J. Bacteriol. 140:547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Mancuso, G., F. Tomasello, I. Ofek, and G. Teti. 1994. Anti-lipoteichoic acid antibodies enhance release of cytokines by monocytes sensitized with lipoteichoic acid. Infect. Immun. 62:1470-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Mantovani, H. C., and J. B. Russell. 2001. Nisin resistance of Streptococcus bovis. Appl. Environ. Microbiol. 67:808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Markham, J. L., K. W. Knox, and R. G. Schamschula. 1973. Antibodies to teichoic acids in man. Arch. Oral Biol. 18:313-319. [DOI] [PubMed] [Google Scholar]
- 319.Marquis, R. E. 1988. Turgor presure, sporulation, and the physical properties of cell walls, p. 21-32. In P. Actor, L. Daneo-Moore, M. L. Higgins, M. R. J. Salton, and G. D. Shockman (ed.), Antibiotic inhibition of bacterial cell surface assembly and function. American Society for Microbiology, Washington, D.C.
- 320.Marquis, R. E., K. Mayzel, and E. L. Carstensen. 1976. Cation exchange in cell walls of gram-positive bacteria. Can. J. Microbiol. 22:975-982. [DOI] [PubMed] [Google Scholar]
- 321.Mater, Y., and S. Karaçali. 2001. Fine structural demonstration of anionic sites on streptococcal and staphylococcal envelopes by cationic dyes. Turk. J. Med. Sci 31:291-295. [Google Scholar]
- 322.Matsuno, T., and H. D. Slade. 1971. Group A streptococcal polysaccharide antigens. Infect. Immun. 3:385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Matsuura, T., Y. Miyake, S. Nakashima, H. Komatsuzawa, Y. Akagawa, and H. Suginaka. 1996. Isolation and characterization of teichoic acid-like substance as an adhesin of Staphylococcus aureus to HeLa cells. Microbiol. Immunol. 40:247-254. [DOI] [PubMed] [Google Scholar]
- 324.Matthews, T. H., R. J. Doyle, and U. N. Streips. 1979. Contribution of peptidoglycan to the binding of metal ions by the cell wall of Bacillus subtilis. Curr. Microbiol. 3:51-53. [Google Scholar]
- 325.Mattingly, S. J., and B. P. Johnston. 1987. Comparative analysis of the localization of lipoteichoic acid in Streptococcus agalactiae and Streptococcus pyogenes. Infect. Immun. 55:2383-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Mauck, J., and L. Glaser. 1972. An acceptor-dependent polyglycerolphosphate polymerase. Proc. Natl. Acad. Sci. USA 69:2386-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Mauck, J., and L. Glaser. 1972. On the mode of in vivo assembly of the cell wall of Bacillus subtilis. J. Biol. Chem. 247:1180-1187. [PubMed] [Google Scholar]
- 328.Mauël, C., A. Bauduret, C. Chervet, S. Beggah, and D. Karamata. 1995. In Bacillus subtilis 168, teichoic acid of the cross-wall may be different from that of the cylinder: a hypothesis based on transcription analysis of tag genes. Microbiology 141:2379-2389. [DOI] [PubMed] [Google Scholar]
- 329.Mauël, C., M. Young, and D. Karamata. 1991. Genes concerned with synthesis of poly(glycerol phophate), the essential teichoic acid in Bacillus subtilis strain 168, are organized in two divergent transcription units. J. Gen. Microbiol. 137:929-941. [DOI] [PubMed] [Google Scholar]
- 330.Mauël, C., M. Young, P. Margot, and D. Karamata. 1989. The essential nature of teichoic acids in Bacillus subtilis as revealed by insertional mutagenesis. Mol. Gen. Genet. 215:388-394. [DOI] [PubMed] [Google Scholar]
- 331.Maurer, J. J., and S. J. Mattingly. 1991. Molecular analysis of lipoteichoic acid from Streptococcus agalactiae. J. Bacteriol. 173:487-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Reference deleted.
- 333.Mayer, F. 1993. Principles of functional and structural organization in the bacterial cell: ‘compartments’ and their enzymes. FEMS Microbiol. Rev. 104:327-346. [DOI] [PubMed] [Google Scholar]
- 334.Mazmanian, S. K., and O. Schneewind. 2002. Cell wall-anchored surface proteins and lipoproteins of gram-positive bacteria, p. 57-70. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 335.McArthur, H. A. I., I. C. Hancock, and J. Baddiley. 1981. Attachment of the main chain to the linkage unit in biosynthesis of teichoic acids. J. Bacteriol. 145:1222-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.McCarty, M. 1964. The role of d-alanine in the serological specificity of group A streptococcal glycerol teichoic acid. Proc. Natl. Acad. Sci. USA 52:259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.McCarty, M., and S. I. Morse. 1964. Cell wall antigens of gram-positive bacteria. Adv. Immunol. 4:249-286. [DOI] [PubMed] [Google Scholar]
- 338.McIntire, F. C., A. E. Vatter, J. Baros, and J. Arnold. 1978. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect. Immun. 21:978-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Merad, T., A. R. Archibald, I. C. Hancock, C. R. Harwood, and J. A. Hobot. 1989. Cell wall assembly in Bacillus subtilis: visualization of old and new wall material by electron microscopic examination of samples stained selectively for teichoic acid and teichuronic acid. J. Gen. Microbiol. 135:645-655. [DOI] [PubMed] [Google Scholar]
- 340.Merchante, R., H. M. Pooley, and D. Karamata. 1995. A periplasm in Bacillus subtilis. J. Bacteriol. 177:6176-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Michelsen, K. S., A. Aicher, M. Mohaupt, T. Hartung, S. Dimmeler, C. J. Kirschning, and R. R. Schumann. 2001. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCs): peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J. Biol. Chem. 276:25680-25686. [DOI] [PubMed] [Google Scholar]
- 342.Middelveld, R. J. M., and K. Alving. 2000. Synergistic septicemic action of the Gram-positive bacterial cell wall components peptidoglycan and lipoteichoic acid in the pig in vivo. Shock 13:297-306. [DOI] [PubMed] [Google Scholar]
- 343.Millward, G. R., and D. A. Reaveley. 1974. Electron microscope observations on the cell walls of some gram-positive bacteria. J. Ultrastruct. Res. 46:309-326. [DOI] [PubMed] [Google Scholar]
- 344.Miörner, H., G. Johansson, and G. Kronvall. 1983. Lipoteichoic acid is the major cell wall compnent responsible for surface hydrophobicity of group A streptococci. Infect. Immun. 39:336-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Mirelman, D., and N. Sharon. 1972. Biosynthesis of peptidoglycan by a cell wall preparation of Staphylococcus aureus and its inhibition by penicillin. Biochem. Biophys. Res. Commun. 46:1909-1917. [DOI] [PubMed] [Google Scholar]
- 346.Mirelman, D., B. D. Beck, and D. R. D. Shaw. 1970. The location of the d-alanyl ester in the ribitol teichoic acid of Staphylococcus aureus. Biochem. Biophys. Res. Commun. 39:712-717. [DOI] [PubMed] [Google Scholar]
- 347.Mirelman, D., R. Bracha, and N. Sharon. 1972. Role of the penicillin-sensitive transpeptidation reaction in attachment of newly synthesized peptidoglycan to cell walls of Micrococcus luteus. Proc. Natl. Acad. Sci. USA 69:3355-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Moncrief, M. B. C., and M. E. Maguire. 1999. Magnesium transport in prokaryotes. J. Biol. Inorg. Chem. 4:523-527. [DOI] [PubMed] [Google Scholar]
- 349.Mootz, H. D., R. Finking, and M. A. Marahiel. 2001. 4′-Phosphopantetheine transfer in primary and secondary metabolism of Bacillus subtilis. J. Biol. Chem. 276:37289-37298. [DOI] [PubMed] [Google Scholar]
- 350.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Morath, S., A. Geyer, I. Spreitzer, C. Hermann, and T. Hartung. 2002. Structural decomposition and heterogeneity of commercial lipoteichoic acid preparations. Infect. Immun. 70:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Morath, S., A. Stadelmaier, A. Geyer, R. R. Schmidt, and T. Hartung. 2002. Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. J. Exp. Med. 195:1635-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Nakano, M., and W. Fischer. 1978. Trihexosyldiacylglycerol and acyltrihexosyldiacylglycerol as lipid anchors of the lipoteichoic acid of Lactotobacillus casei DSM 20021. Hoppe-Seyler's Z. Physiol. Chem. 359:1-11. [DOI] [PubMed] [Google Scholar]
- 354.Nakao, A., S.-I. Imai, and T. Takano. 2000. Transposon-mediated insertional mutagenesis of the d-alanyl-lipoteichoic acid (dlt) operon raises methicillin resistance in Staphylococcus aureus. Res. Microbiol. 151:823-829. [DOI] [PubMed] [Google Scholar]
- 355.Naumova, I. B., and A. S. Shashkov. 1997. Anionic polymers in cell walls of gram-positive bacteria. Biochemistry (Moscow) 62:809-840. [PubMed] [Google Scholar]
- 356.Naumova, I. B., A. S. Shashkov, N. K. Scoblilova, N. S. Agre, and V. V. Romanov. 1982. Lysylteichoic acid of the cell-wall of Streptomyces roseoflavus var. roseofungini-1128. Bioorg. Khim. 8:848-856. [Google Scholar]
- 357.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Nealon, T. J., E. H. Beachey, H. S. Courtney, and W. A. Simpson. 1986. Release of fibronectin-lipoteichoic acid complexes from group A streptococci with penicillin. Infect. Immun. 51:529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Nealon, T. J., and S. J. Mattingly. 1983. Association of elevated levels of cellular lipoteichoic acids of group B streptococci with human neonatal disease. Infect. Immun. 39:1243-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Nealon, T. J., and S. J. Mattingly. 1984. Role of cellular lipoteichoic acids in mediating adherence of serotype III strains of group B streptococci to human embryonic, fetal, and adult epithelial cells. Infect. Immun. 43:523-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Nealon, T. J., and S. J. Mattingly. 1985. Kinetic and chemical analyses of the biologic significance of lipoteichoic acids in mediating adherence of serotype III group B streptococci. Infect. Immun. 50:107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Ne'eman, N., and I. Ginsburg. 1972. Red cell-sensitizing antigen of group A streptococci. II. Immunological and immunopathological properties. Isr. J. Med. Sci. 8:1807-1816. [PubMed] [Google Scholar]
- 363.Neuhaus, F. C. 1967. d-Cycloserine and O-carbamyl-d-serine, p. 40-83. In D. Gottlieb and P. D. Shaw (ed.) Antibiotics: mechanism of action, vol 1. Springer-Verlag KG, Berlin, Germany.
- 364.Reference deleted.
- 365.Neuhaus, F. C. 1985. Inter-chain transacylation of d-alanine ester residues of lipoteichoic acid: a unique mechanism of membrane communication. Biochem. Soc. Trans. 13:987-989. [DOI] [PubMed] [Google Scholar]
- 366.Neuhaus, F. C., and W. P. Hammes. 1981. Inhibition of cell wall biosynthesis by analogues of alanine. Pharm. Ther. 14:265-319. [DOI] [PubMed] [Google Scholar]
- 367.Neuhaus, F. C., M. P. Heaton, D. V. Debabov, and Q. Zhang. 1996. The dlt operon in the biosynthesis of d-alanyl-lipoteichoic acid in Lactobacillus casei. Microb. Drug Resist. 2:77-84. [DOI] [PubMed] [Google Scholar]
- 368.Neuhaus, F. C., R. Linzer, and V. M. Reusch, Jr. 1974. Biosynthesis of membrane teichoic acid: role of the d-alanine activating enzyme and d-alanine: membrane acceptor ligase. Ann. N. Y. Acad. Sci. 235:502-518. [DOI] [PubMed] [Google Scholar]
- 369.Neuhaus, F. C., C. E. Tobin, and J. A. Ahlgren. 1980. Membrane-wall interrelationship in Gaffkya homari: sulfhydryl sensitivity and heat lability of nascent peptidoglycan incorporation into walls. J. Bacteriol. 143:112-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Novitsky, T. J., M. Chan, R. H. Himes, and J. M. Akagi. 1974. Effect of temperature on the growth and cell wall chemistry of a facultative thermophilic Bacillus. J. Bacteriol. 117:858-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Ntamere, A. S., D. J. Taron, and F. C. Neuhaus. 1987. Assembly of d-alanyl-lipoteichoic acid in Lactobacillus casei: mutants deficient in the d-alanyl ester content of this amphiphile. J. Bacteriol. 169:1702-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.O'Brien, M. J., S. A. Kuhl, and M. J. Starzyk. 1995. Correlation of teichoic acid d-alanyl esterification with the expression of methicillin resistance in Staphylococcus aureus. Microbios 83:119-137. [PubMed] [Google Scholar]
- 373.Ofek, I., W. A. Simpson, and E. H. Beachey. 1982. Formation of molecular complexes between a structurally defined M protein and acylated or deacylated lipoteichoic acid of Streptococcus pyogenes. J. Bacteriol. 149:426-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ohta, K., H. Komatsuzawa, M. Sugai, and H. Suginaka. 2000. Triton X-100-induced lipoteichoic acid release is correlated with the methicillin resistance in Staphylococcus aureus. FEMS Microbiol. Lett. 182:77-79. [DOI] [PubMed] [Google Scholar]
- 375.Oppenheim, D. S., and C. Yanofsky. 1980. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics 95:785-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Orefici, G., A. Molinari, G. Donelli, S. Paradisi, G. Teti, and G. Arancia. 1986. Immunolocation of lipoteichoic acid on group B streptococcal surface. FEMS Microbiol. Lett. 34:111-115. [Google Scholar]
- 377.Ou, L.-T., A. N. Chatterjee, F. E. Young, and R. E. Marquis. 1973. The physiology of teichoic acid deficient staphylococci. Can. J Microbiol. 19:1393-1399. [DOI] [PubMed] [Google Scholar]
- 378.Ou, L.-T., and R. E. Marquis. 1970. Electromechanical interactions in cell walls of gram-positive cocci. J. Bacteriol. 101:92-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Owen, P., and M. R. J. Salton. 1975. Isolation and characterization of a mannan from mesosomal membrane vesicles of Micrococcus lysodeikticus. Biochim. Biophys. Acta 406:214-234. [DOI] [PubMed] [Google Scholar]
- 380.Pal, M. K., T. C. Ghosh, and J. K. Ghosh. 1990. Studies on the conformation of and metal ion binding by teichoic acid of Staphylococcus aureus. Biopolymers 30:273-277. [DOI] [PubMed] [Google Scholar]
- 381.Park, J. T., D. R. D. Shaw, A. N. Chatterjee, D. Mirelman, and T. Wu. 1974. Mutants of staphylococci with altered cell walls. Ann. N. Y. Acad. Sci. 236:54-62. [DOI] [PubMed] [Google Scholar]
- 382.Park, Y. S., T. D. Sweitzer, J. E. Dixon, and C. Kent. 1993. Expression, purification, and characterization of CTP:glycerol-3-phosphate cytidylyltransferase from Bacillus subtilis. J. Biol. Chem. 268:16648-16654. [PubMed] [Google Scholar]
- 383.Perego, M., P. Glaser, A. Minutello, M. A. Strauch, K. Leopold, and W. Fischer. 1995. Incorporation of d-alanine into lipoteichoic and wall teichoic acid in Bacillus subtilis: identification of genes and regulation. J. Biol. Chem. 270:15598-15606. [DOI] [PubMed] [Google Scholar]
- 384.Periti, P. and T. Mazzei. 1998. Antibiotic-induced release of bacterial cell wall components in the pathogenesis of sepsis and septic shock: a review. J. Chemother. 10:427-448. [DOI] [PubMed] [Google Scholar]
- 385.Perry, A. M., H. Ton-That, S. K. Mazmanian, and O. Schneewind. 2002. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. III. Lipid II is an in vivo peptidoglycan substrate for sortase-catalyzed surface protein anchoring. J. Biol. Chem. 277:16241-16248. [DOI] [PubMed] [Google Scholar]
- 386.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179-186. [DOI] [PubMed] [Google Scholar]
- 387.Peschel, A., and L. V. Collins. 2001. Staphylococcal resistance to antimicrobial peptides of mammalian and bacterial origin. Peptides 22:1651-1659. [DOI] [PubMed] [Google Scholar]
- 388.Peschel, A., R. W. Jack, M. Otto, L. V. Collins, P. Staubitz, G. Nicholson, H. Kalbacher, W. F. Nieuwenhuizen, G. Jung, A. Tarkowski, K. P. M. van Kessel, and J. A. G. van Strijp. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Götz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 390.Peschel, A., C. Vuong, M. Otto, and F. Götz. 2000. The d-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob. Agents Chemother. 44:2845-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Petit-Glatron, M.-F., L. Grajcar, A. Munz, and R. Chambert. 1993. The contribution of the cell wall to a transmembrane calcium gradient could play a key role in Bacillus subtilis protein secretion. Mol. Microbiol. 9:1097-1106. [DOI] [PubMed] [Google Scholar]
- 392.Plitnick, L. M., R. A. Jordan, J. A. Banas, D. M. Jelley-Gibbs, M. C. Walsh, M. T. Preissler, and E. J. Gosselin. 2001. Lipoteichoic acid inhibits interleukin-2 (IL-2) function by direct binding to IL-2. Clin. Diagn. Lab. Immunol. 8:972-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Pollack, J. H., and F. C. Neuhaus. 1994. Changes in wall teichoic acid during the rod-sphere transition of Bacillus subtilis 168. J. Bacteriol. 176:7252-7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Pollack, J. H., A. S. Ntamere, and F. C. Neuhaus. 1992. d-Alanyl-lipoteichoic acid in Lactobacillus casei: secretion of vesicles in response to benzylpenicillin. J. Gen. Microbiol. 138:849-859. [DOI] [PubMed] [Google Scholar]
- 395.Polotsky, V. Y., W. Fischer, R. A. B. Ezekowitz, and K. A. Joiner. 1996. Interactions of human mannose-binding protein with lipoteichoic acids. Infect. Immun. 64:380-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Pooley, H. M. 1976. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J. Bacteriol. 125:1127-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Pooley, H. M. 1976. Layered distribution, according to age, within the cell wall of Bacillus subtilis. J. Bacteriol. 125:1139-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Pooley, H. M., F.-X. Abellan, and D. Karamata. 1991. A conditional-lethal mutant of Bacillus subtilis 168 with a thermosensitive glycerol-3-phosphate cytidylyltransferase, an enzyme specific for the synthesis of the major wall teichoic acid. J. Gen. Microbiol. 137:921-928. [DOI] [PubMed] [Google Scholar]
- 399.Pooley, H. M., F.-X. Abellan, and D. Karamata. 1992. CDP-glycerol:poly(glycerophosphate) glycerophosphotransferase, which is involved in the synthesis of the major wall teichoic acid in Bacillus subtilis 168, is encoded by tagF (rodC). J. Bacteriol. 174:646-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Pooley, H. M., and D. Karamata. 1988. Can synthesis of cell wall anionic polymers in Bacillus subtilis be a target for antibiotics? p. 591-594. In P. Actor, L. Daneo-Moore, M. L. Higgins, M. R. J. Salton, and G. D. Shockman (ed.), Antibiotic inhibition of bacterial cell surface assembly and function. American Society for Microbiology, Washington, D.C.
- 401.Pooley, H. M., and D. Karamata. 1994. Teichoic acid synthesis in Bacillus subtilis: genetic organization and biological roles. New Compr. Biochem. 27:187-198. [Google Scholar]
- 402.Pooley, H. M., and D. Karamata. 2000. Incorporation of [2-3H]glycerol into cell surface componenets of Bacillus subtilis 168 and thermosensitive mutants affected in wall teichoic acid synthesis: effect of tunicamycin. Microbiology 146:797-805. [DOI] [PubMed] [Google Scholar]
- 403.Potekhina, N. V., E. M. Tul'skaya, I. B. Naumova, A. S. Shashkov, and L. I. Evtushenko. 1993. Erythritolteichoic acid in the cell wall of Glycomyces tenuis VKM Ac-1250. Eur. J. Biochem. 218:371-375. [DOI] [PubMed] [Google Scholar]
- 404.Powell, D. A., M. Duckworth, and J. Baddiley. 1975. A membrane-associated lipomannan in micrococci. Biochem. J. 151:387-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Poyart, C., M.-C. Lamy, C. Boumaila, F. Fiedler, and P. Trieu-Cuot. 2001. Regulation of D-alanyl-lipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J. Bacteriol. 183:6324-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Poyart, C., E. Pellegrini, M. Marceau, M. Baptista, F. Jaubert, M.-C. Lamy, and P. Trieu-Cuot. 2003. Attenuated virulence of Streptococcus agalactiae deficient in d-alanyl-lipoteichoic acid is due to an increase susceptibility to defensins and phagocytic cells. Mol. Microbiol. 49:1615-1625. [DOI] [PubMed] [Google Scholar]
- 407.Qi, Y., and F. M. Hulett. 1998. Role of PhoP∼P in transcriptional regulation of genes involved in cell wall anionic polymer biosynthesis in Bacillus subtilis. J. Bacteriol. 180:4007-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Rallu, F., A. Gruss, S. D. Ehrlich, and E. Maguin. 2000. Acid- and multistress-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol. Microbiol. 35:517-528. [DOI] [PubMed] [Google Scholar]
- 409.Reference deleted.
- 410.Räsänen, L., and H. Arvilommi. 1981. Cell walls, peptidoglycans, and teichoic acids of gram-positive bacteria as polyclonal inducers and immunomodulators of proliferative and lymphokine responses of human B and T lymphocytes. Infect. Immun. 34:712-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Rayevskaya, M. V., and F. R. Frankel. 2001. Systemic immunity and mucosal immunity are induced against human immunodeficiency virus Gag protein in mice by a new hyperattenuated strain of Listeria monocytogenes. J. Virol. 75:2786-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Resing, H. A., and R. A. Neihof. 1970. Nuclear magnetic resonance relaxation of water adsorbed on bacterial cell walls. J. Colloid Interface Sci. 34:480-487. [DOI] [PubMed] [Google Scholar]
- 413.Reusch, V. M., Jr. 1984. Lipopolymers, isoprenoids, and the assembly of the gram-positive cell wall. Crit. Rev. Microbiol. 11:129-155. [DOI] [PubMed] [Google Scholar]
- 414.Reusch, V. M., Jr., and F. C. Neuhaus. 1971. d-Alanine: membrane acceptor ligase from Lactobacillus casei. J. Biol. Chem. 246:6136-6143. [PubMed] [Google Scholar]
- 415.Riske, K. A., H.-G. Döbereiner, and M. T. Lamy-Freund. 2002. Gel-fluid transition in dilute versus concentrated DMPG aqueous dispersions. J. Phys. Chem. Ser. B 106:239-246. [Google Scholar]
- 416.Roberts, M. F., G. R. Jacobson, P. J. Scott, C. S. Mimura, and M. W. Stinson. 1985. 31P-NMR studies of the oral pathogen Streptococcus mutans: observation of lipoteichoic acid. Biochim. Biophys. Acta 845:242-248. [DOI] [PubMed] [Google Scholar]
- 417.Roethlisberger, P., N. Iida-Tanaka, K. Hollemeyer, E. Heinzle, I. Ishizuka, and W. Fischer. 2000. Unique poly(glycerophosphate) lipoteichoic acid and the glycolipids of Streptococcus sp closely related to Streptococcus pneumoniae. Eur. J. Biochem. 267:5520-5530. [DOI] [PubMed] [Google Scholar]
- 418.Rogers, H. J. 1970. Bacterial growth and the cell envelope. Bacteriol. Rev. 34:194-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Rogers, H. J. 1988. The bacterial surface—where does it begin and end? p. 639-648. In P. Actor, L. Daneo-Moore, M. L. Higgins, M. R. J. Salton, and G. D. Shockman (ed.), Antibiotic inhibition of bacterial cell surface assembly and function. American Society for Microbiology, Washington, D.C.
- 420.Rogers, H. J., M. McConnell, and I. D. J. Burdett. 1968. Cell wall or membrane mutants of Bacillus subtilis and Bacillus licheniformis with grossly deformed morphology. Nature (London) 219:285-288. [DOI] [PubMed] [Google Scholar]
- 421.Rogers, H. J., M. McConnell, and R. C. Hughes. 1971. The chemistry of the cell walls of rod mutants of Bacillus subtilis. J. Gen. Microbiol. 66:297-308. [DOI] [PubMed] [Google Scholar]
- 422.Rose, R. K., and S. D. Hogg. 1995. Competitive binding of calcium and magnesium to streptococcal lipoteichoic acid. Biochim. Biophys. Acta 1245:94-98. [DOI] [PubMed] [Google Scholar]
- 423.Rose, R. K., S. D. Hogg, and R. P. Shellis. 1994. A quantitative study of calcium binding by isolated streptococcal cell walls and lipoteichoic acid: comparison with whole cells. J. Dent. Res. 73:1742-1747. [DOI] [PubMed] [Google Scholar]
- 424.Rose, R. K., S. P. Matthews, and R. C. Hall. 1997. Investigation of calcium-binding sites on the surfaces of selected gram-positive oral organisms. Arch. Oral Biol. 42:595-599. [DOI] [PubMed] [Google Scholar]
- 425.Rose, R. K., R. P. Shellis, and A. R. Lee. 1996. The role of cation bridging in microbial fluoride binding. Caries Res. 30:458-464. [DOI] [PubMed] [Google Scholar]
- 426.Rosenberg, M., and R. J. Doyle. 1990. Microbial cell surface hydrophobicity: history, measurement, and significance, p. 1-37. In R. J. Doyle and M. Rosenberg (ed.) Microbial cell surface hydrophobicity. American Society for Microbiology, Washington, D.C.
- 427.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819-829. [DOI] [PubMed] [Google Scholar]
- 428.Ruhland, G. J., and F. Fiedler. 1990. Occurrence and structure of lipoteichoic acids in the genus Staphylococcus. Arch. Microbiol. 154:375-379. [DOI] [PubMed] [Google Scholar]
- 429.Sabath, L. D. 1977. Chemical and physical factors influencing methicillin resistance of Staphylococcus aureus and Staphylococcus epidermidis. J. Antimicrob. Chemother. 3(Suppl. C):47-51. [DOI] [PubMed] [Google Scholar]
- 430.Sanderson, A. R., J. L. Strominger, and S. G. Nathenson. 1962. Chemical structure of teichoic acid from Staphylococcus aureus, strain Copenhagen. J. Biol. Chem. 237:3603-3613. [PubMed] [Google Scholar]
- 431.Schertzer, J. W., and E. D. Brown. 2003. Purified, recombinant TagF protein from Bacillus subtilis 168 catalyzes the polymerization of glycerol phosphate onto a membrane acceptor in vitro. J. Biol. Chem. 278:18002-18007. [DOI] [PubMed] [Google Scholar]
- 432.Schlievert, P. M., P. J. Gahr, A. P. Assimacopoulos, M. M. Dinges, J. A. Stoehr, J. W. Harmala, H. Hirt, and G. M. Dunny. 1998. Aggregation and binding substances enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect. Immun. 66:218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Schneewind, O., D. Mihaylova-Petkov, and P. Model. 1993. Cell wall sorting signals in surface proteins of Gram-positive bacteria. EMBO J. 12:4803-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Schurek, J., and W. Fischer. 1989. Distribution analyses of chain substituents of lipoteichoic acids by chemical degradation. Eur. J. Biochem. 186:649-655. [DOI] [PubMed] [Google Scholar]
- 435.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 436.Scott, M. G., M. R. Gold, and R. E. W. Hancock. 1999. Interaction of cationic peptides with lipoteichoic acid and gram-positive bacteria. Infect. Immun. 67:6445-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Sela, S., M. J. Marouni, R. Perry, and A. Barzilai. 2000. Effect of lipoteichoic acid on the uptake of Streptococcus pyogenes by HEp-2 cells. FEMS Microbiol. Lett. 193:187-193. [DOI] [PubMed] [Google Scholar]
- 438.Reference deleted.
- 439.Shabarova, Z. A., N. A. Hughes, and J. Baddiley. 1962. The influence of adjacent phosphate and hydroxyl groups on amino acid esters. Biochem. J. 83:216-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Shashkov, A. S., G. M. Streshinskaya, V. A. Gnilozub, L. I. Evtushenko, and I. B. Naumova. 1995. Poly(arabitol phosphate) teichoic acid in the cell wall of Agromyces cerinus subsp. cerinus VKM Ac-1340. FEBS Lett. 371:163-166. [DOI] [PubMed] [Google Scholar]
- 441.Shibaev, V. N., M. Duckworth, A. R. Archibald, and J. Baddiley. 1973. The structure of a polymer containing galactosamine from walls of Bacillus subtilis 168. Biochem. J. 135:383-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Shimada, A., and E. Ito. 1986. Studies on glucosyltransferase and endogenous glucosyl acceptor in Bacillus cereus AHU 1030 membranes. J. Biochem. 100:1507-1521. [DOI] [PubMed] [Google Scholar]
- 443.Shimada, A., M. Ohta, H. Iwasaki, and E. Ito. 1988. The function of β-N-acetyl-d-glucosaminyl monophosphorylundecaprenol in biosynthesis of lipoteichoic acids in a group of Bacillus strains. Eur. J. Biochem. 176:559-565. [DOI] [PubMed] [Google Scholar]
- 444.Shirtliff, M. E., J. T. Mader, and A. K. Camper. 2002. Molecular interactions in biofilms. Chem. Biol. 9:859-871. [DOI] [PubMed] [Google Scholar]
- 445.Shockman, G. D., and J.-V. Höltje. 1994. Microbial peptidoglycan (murein) hydrolases. New Compr. Biochem. 27:131-166. [Google Scholar]
- 446.Shockman, G. D., L. Daneo-Moore, R. Kariyama, and O. Massidda. 1996. Bacterial walls, peptidoglycan hydrolases, autolysins, and autolysis. Microb. Drug Resist. 2:95-98. [DOI] [PubMed] [Google Scholar]
- 447.Sinha, R. K., and F. C. Neuhaus. 1991. Biosynthesis of peptidoglycan in Gaffkya homari: on the target(s) of benzylpenicillin. Antimicrob. Agents Chemother. 35:1753-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Slabyj, B. M., and C. Panos. 1973. Teichoic acid of a stabilized L-form of Streptococcus pyogenes. J. Bacteriol. 114:934-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Smit, E., and P. H. Pouwels. 2002. One repeat of the cell wall binding domain is sufficient for anchoring the Lactobacillus acidophilus surface layer protein. J. Bacteriol. 184:4617-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Smith, T. J., S. A. Blackman, and S. J. Foster. 2000. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology 146:249-262. [DOI] [PubMed] [Google Scholar]
- 451.Soldo, B., V. Lazarevic, and D. Karamata. 2002. tagO is involved in the synthesis of all anionic cell-wall polymers in Bacillus subtilis 168. Microbiology 148:2079-2087. [DOI] [PubMed] [Google Scholar]
- 452.Soldo, B., V. Lazarevic, M. Pagni, and D. Karamata. 1999. Teichuronic acid operon of Bacillus subtilis 168. Mol. Microbiol. 31:795-805. [DOI] [PubMed] [Google Scholar]
- 453.Sonnenfeld, E. M., T. J. Beveridge, A. L. Koch, and R. J. Doyle. 1985. Asymmetric distribution of charge on the cell wall of Bacillus subtilis. J. Bacteriol. 163:1167-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Spatafora, G. A., M. Sheets, R. June, D. Luyimbazi, K. Howard, R. Hulbert, D. Barnard, M. E. Janne, and M. C. Hudson. 1999. Regulated expression of the Streptococcus mutans dlt genes correlates with intracellular polysaccharide accumulation. J. Bacteriol. 181:2363-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Spellerberg, B., E. Rozdzinski, S. Martin, J. Weber-Heynemann, N. Schnitzler, R. Lütticken, and A. Podbielski. 1999. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 67:871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Sriskandan, S., and J. Cohen. 1999. Gram-positive sepsis: mechanisms and differences from Gram-negative sepsis. Infect. Dis. Clin. North Am. 13:397-412. [DOI] [PubMed] [Google Scholar]
- 457.Stewart, J. J. P. 1989. Optimization of parameters for semiempirical methods. I. Method. J. Comput. Chem. 10:209-220. [Google Scholar]
- 458.Stewart, J. J. P. 1989. Optimization of parameters for semiempirical methods. II. Applications. J. Comput. Chem. 10:221-264. [Google Scholar]
- 459.Stinson, M. W., R. McLaughlin, S. H. Choi, Z. E. Juarez, and J. Barnard. 1998. Streptococcal histone-like protein primary structure of hlpA and protein binding to lipoteichoic acid and epithelial cells. Infect. Immun. 66:259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Suda, Y., H. Tochio, K. Kawano, H. Takada, T. Yoshida, S. Kotani, and S. Kusumoto. 1995. Cytokine-inducing glycolipids in the lipoteichoic acid fraction from Enterococcus hirae ATCC 9790. FEMS Immunol. Med. Microbiol. 12:97-112. [DOI] [PubMed] [Google Scholar]
- 461.Suginaka, H., M. Shimatani, Y. Ohno, and I. Yano. 1979. Effects of bacterial lipids and lipoteichoic acid on extracellular autolysin activity from Staphylococcus aureus. FEMS Microbiol. Lett. 5:353-355. [Google Scholar]
- 462.Surana, U., A. J. Wolfe, and N. H. Mendelson. 1988. Regulation of Bacillus subtilis macrofiber twist development by d-alanine. J. Bacteriol. 170:2328-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Sutcliffe, I. C. 1994. The lipoteichoic acids and lipoglycans of gram-positive bacteria: a chemotaxonomic perspective. Syst. Appl. Microbiol. 17:467-480. [Google Scholar]
- 464.Sutcliffe, I. C., and D. J. Harrington. 2002. Pattern searches for the identification of putative lipoprotein genes in Gram-positive bacterial genomes. Microbiology 148:2065-2077. [DOI] [PubMed] [Google Scholar]
- 465.Sutcliffe, I. C., and R. R. B. Russell. 1995. Lipoproteins of gram-positive bacteria. J. Bacteriol. 177:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Sutcliffe, I. C., and N. Shaw. 1991. Atypical lipoteichoic acids of gram-positive bacteria. J. Bacteriol. 173:7065-7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Sutherland, I. W. 1999. Biofilm expolysaccharides, p. 73-92. In J. Wingender, T. R. Neu, and H.-C. Flemming (ed.), Microbial extracellular polymeric substances. Springer Verlag KG, Berlin, Germany.
- 468.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 469.Taron, D. J., W. C. Childs III, and F. C. Neuhaus. 1983. Biosynthesis of d-alanyl-lipoteichoic acid: role of diglyceride kinase in the synthesis of phosphatidylglycerol for chain elongation. J. Bacteriol. 154:1110-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Taweechaisupapong, S., and R. J. Doyle. 2000. Sensitivity of bacterial coaggregation to chelating agents. FEMS Immunol. Med. Microbiol. 28:343-346. [DOI] [PubMed] [Google Scholar]
- 471.Thiemermann, C. 2002. Interactions between lipoteichoic acid and peptidoglycan from Staphylococcus aureus: a structural and functional analysis. Microbes Infect. 4:927-935. [DOI] [PubMed] [Google Scholar]
- 472.Thompson, R. J., H. G. A. Bouwer, D. A. Portnoy, and F. R. Frankel. 1998. Pathogenicity and immunogenicity of a Listeria monocytogenes strain that requires d-alanine for growth. Infect. Immun. 66:3552-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Thwaite, J. E., L. W. J. Baillie, N. M. Carter, K. Stephenson, M. Rees, C. R. Harwood, and P. T. Emmerson. 2002. Optimization of the cell wall microenvironment allows increased production of recombinant Bacillus anthracis protective antigen from B. Subtilis. Appl. Environ. Microbiol. 68:227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Thwaites, J. J., and N. H. Mendelson. 1991. Mechanical behaviour of bacterial cell walls. Adv. Microb. Physiol. 32:173-221. [DOI] [PubMed] [Google Scholar]
- 475.Tipper, D. J., W. Katz, J. L. Strominger, and J.-M. Ghuysen. 1967. Substituents on the α-carboxyl group of d-glutamic acid in the peptidoglycan of several bacterial cell walls. Biochemistry. 6:921-929. [DOI] [PubMed] [Google Scholar]
- 476.Toon, P., P. E. Brown, and J. Baddiley. 1972. The lipid-teichoic acid complex in the cytoplasmic membrane of Streptococcus faecalis NCIB 8191. Biochem. J. 127:399-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Tsutsui, O., S. Kokeguchi, T. Matsumura, and K. Kato. 1991. Relationship of the chemical structure and immunobiological activities of lipoteichoic acid from Streptococcus faecalis (Enterococcus hirae) ATCC 9790. FEMS Microbiol. Immun. 76:211-218. [DOI] [PubMed] [Google Scholar]
- 478.Umeda, A., S. Yokoyama, T. Arizono, and K. Amako. 1992. Location of peptidoglycan and teichoic acid on the cell wall surface of Staphylococcus aureus as determined by immunoelectron microscopy. J. Electron Microsc. 41:46-52. [PubMed] [Google Scholar]
- 479.Urrutia Mera, M., M. Kemper, R. Doyle, and T. J. Beveridge. 1992. The membrane-induced proton motive force influences the metal binding ability of Bacillus subtilis cell walls. App. Environ. Microbiol. 58:3837-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.van de Wetering, J. K., M. van Eijk, L. M. G van Golde, T. Hartung, J. A. G. van Strijp, and J. J. Batenburg. 2001. Characteristics of surfactant protein A and D binding to lipoteichoic acid and peptidoglycan, 2 major cell wall components of gram-positive bacteria. J. Infect. Dis. 184:1143-1151. [DOI] [PubMed] [Google Scholar]
- 481.van Driel, D., A. J. Wicken, M. R. Dickson, and K. W. Knox. 1973. Cellular location of the lipoteichoic acids of Lactobacillus fermenti NCTC 6991 and Lactobacillus casei NCTC 6375. J. Ultrastruct. Res. 43:483-497. [DOI] [PubMed] [Google Scholar]
- 482.van Langevelde, P., J. T. van Dissel, E. Ravensbergen, B. J. Appelmelk, I. A. Schrijver, and P. H. P. Groeneveld. 1998. Antibiotic-induced release of lipoteichoic acid and peptidoglycan from Staphylococcus aureus: quantitative measurements and biological reactivities. Antimicrob. Agents Chemother. 42:3073-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.van Wely, K. H. M., J. Swaving, R. Freudl, and A. J. M. Driessen. 2001. Translocation of proteins across the cell envelope of Gram-positive bacteria. FEMS Microbiol. Rev. 25:437-454. [DOI] [PubMed] [Google Scholar]
- 484.Venglarcik, J. S., III, L. L. Blair, and L. M. Dunkle. 1983. pH-dependent oxacillin tolerance of Staphylococcus aureus. Antimicrob. Agents Chemother. 23:232-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Volkman, B. F., Q. Zhang, D. V. Debabov, E. Rivera, G. C. Kresheck, and F. C. Neuhaus. 2001. Biosynthesis of d-alanyl-lipoteichoic acid: the tertiary structure of apo-d-alanyl carrier protein. Biochemistry 40:7964-7972. [PubMed] [Google Scholar]
- 486.Vreugdenhil, A. C. E., C. H. Rousseau, T. Hartung, J. W. M. Greve, C. van't Veer, and W. A. Buurman. 2003. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J. Immun. 170:1399-1405. [DOI] [PubMed] [Google Scholar]
- 487.Waks, S., and A. Tomasz. 1978. Secretion of cell wall polymers into the growth medium of lysis-defective pneumococci during treatment with penicillin and other inhibitors of cell wall synthesis. Antimicrob. Agents Chemother. 13:293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Wang, J. E., P. F. Jorgensen, M. Almlöf, C. Thiemermann, S. J. Foster, A. O. Aasen, and R. Solberg. 2000. Peptidoglycan and lipoteichoic acid from Staphylococcus aureus induce tumor necrosis factor alpha, interleukin 6 (IL-6), and IL-10 production in both T cells and monocytes in a human whole blood model. Infect. Immun. 68:3965-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Ward, J. B. 1981. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiol. Rev. 45:211-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Watkinson, R. J., H. Hussey, and J. Baddiley. 1971. Shared lipid phosphate carrier in the biosynthesis of teichoic acid and peptidoglycan. Nat. New Biol. 229:57-59. [DOI] [PubMed] [Google Scholar]
- 491.Wecke, J., K. Madela, and W. Fischer. 1997. The absence of d-alanine from lipoteichoic acid and wall teichoic acid alters surface charge, enhances autolysis and increases susceptibility to methicillin in Bacillus subtilis. Microbiology 143:2953-2960. [DOI] [PubMed] [Google Scholar]
- 492.Wecke, J., M. Perego, and W. Fischer. 1996. d-Alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects the autolytic activity. Microb. Drug Resist. 2:123-129. [DOI] [PubMed] [Google Scholar]
- 493.Weidel, W., and H. Pelzer. 1964. Bagshaped macromolecules—a new outlook on bacterial cell walls. Adv. Enzymol. 26:193-232. [DOI] [PubMed] [Google Scholar]
- 494.Wergeland, H. I., L. R. Haaheim, O. B. Natås, F. Wesenberg, and P. Oeding. 1989. Antibodies to staphylococcal peptidoglycan and its peptide epitopes, teichoic acid, and lipoteichoic acid in sera from blood donors and patients with staphylococcal infections. J. Clin. Microbiol. 27:1286-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Wiberg, K. B., and K. E. Laidig. 1987. Barriers to rotation adjacent to double bonds. 3. The C-O barrier in formic acid, methyl formate, acetic acid, and methyl acetate. the origin of ester and amide “resonance.” J. Am Chem. Soc. 109:5935-5943. [Google Scholar]
- 496.Wicken, A. J., and J. Baddiley. 1963. Structure of intracellular teichoic acids from group D streptococci. Biochem. J. 87:54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Wicken, A. J., J. K. Evans, and K. W. Knox. 1986. Critical micelle concentrations of lipoteichoic acids. J. Bacteriol. 166:72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Wicken, A. J., and K. W. Knox. 1970. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J. Gen. Microbiol. 60:293-301. [DOI] [PubMed] [Google Scholar]
- 499.Wicken, A. J., and K. W. Knox. 1975. Lipoteichoic acids: a new class of bacterial antigen. Science 187:1161-1167. [DOI] [PubMed] [Google Scholar]
- 500.Wicken, A. J., and K. W. Knox. 1980. Bacterial cell surface amphiphiles. Biochim. Biophys. Acta 604:1-26. [DOI] [PubMed] [Google Scholar]
- 501.Wicken, A. J., and K. W. Knox. 1984. Variable nature of the bacterial cell surface. Aust. J. Biol. Sci. 37:315-322. [DOI] [PubMed] [Google Scholar]
- 502.Yokoyama, K., H. Mizuguchi, Y. Araki, S. Kaya, and E. Ito. 1989. Biosynthesis of linkage units for teichoic acids in gram-positive bacteria: distribution of related enzymes and their specificities for UDP-sugars and lipid-linked intermediates. J. Bacteriol. 171:940-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Yother, J., and J. M. White. 1994. Novel surface attachment mechanism of the Streptococcus pneumoniae protein PspA. J. Bacteriol. 176:2976-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Zachau, H. G., and W. Karau. 1960. Reaktionsfähige Aminosäureester als Modelle der Aminoacyl-Ribonucleinsäure. II. Chem. Ber. 93:1830-1839. [Google Scholar]
- 505.Zhang, L., T. A. Ignatowski, R. N. Spengler, B. Noble, and M. W. Stinson. 1999. Streptococcal histone induces murine macrophages to produce interleukin-1 and tumor necrosis factor alpha. Infect. Immun. 67:6473-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Zipperle, G. F., Jr., J. W. Ezzell Jr., and R. J. Doyle. 1984. Glucosamine substitution and muramidase susceptibility in Bacillus anthracis. Can. J. Microbiol. 30:553-559. [DOI] [PubMed] [Google Scholar]