Abstract
Objective
Impairment in cardiac parasympathetic (vagal) control may confer risk for cardiac mortality in depressed populations. We evaluated the impact of acute stress and relationship-focused imagery on cardiac vagal control, as indicated by levels of respiratory sinus arrhythmia (RSA), in depressed and non-depressed women.
Methods
EKG and respiration rate were evaluated in 15 non-medicated depressed women and 15 matched controls during two laboratory conditions: (1) a relationship-focused imagery designed to elicit vagal activation, and (2) a speech stressor designed to evoke vagal withdrawal.
Results
As expected, the relationship-focused imagery increased RSA [F(3,66)=3.79, p=.02] and the speech stressor decreased RSA [F(3,66)=4.36, p=.02] across women. Depressed women exhibited lower RSA during the relationship-focused imagery, and this effect remained following control for respiratory rate and trauma history [F(1,21)=5.65, p=.027]. Depressed women with a trauma history exhibited the lowest RSA during the stress condition [F(1,22)=9.61, p=.05]. However, after controlling for respiratory rate, Trauma History × Task Order (p=.02) but not Trauma History × Depression Group (p=.12) accounted for RSA variation during the stress condition.
Conclusion
Depression in women is associated with lower RSA, particularly when women reflect on a close love relationship, a context expected to elicit vagal activation and hence increase RSA. In contrast, depression-related variation in stressor-evoked vagal activity appears to covary with women's trauma history. Associations between vagal activity and depression are complex, and should be considered in view of the experimental conditions under which vagal control is assessed, as well as physiological and behavioral factors that may affect vagal function.
Keywords: major depression, cardiac vagal control, respiratory sinus arrhythmia (RSA), trauma
INTRODUCTION
Depression is associated with multiple indicators of physiological dysregulation, including potentially diminished levels of cardiac vagal control [1]. Of clinical relevance, impaired cardiac vagal control represents one of several autonomic mechanisms that putatively increases risk for cardiac mortality among depressed populations [2–4]. Anatomically, the myocardium is dually innervated by sympathetic and parasympathetic branches of the autonomic nervous system. Functionally, parasympathetic inputs provide constant, though fluctuating, inhibitory control of heart rate via direct innervation of the heart by the vagus nerve. The vagus nerve terminates on the sinoatrial node of the heart, considered the heart’s pacemaker. Often referred to as the vagal brake, cardiac control via the vagus nerve acts to slow heart rate to favor energy conservation and parasympathetic dominance during times of rest or perceived safety [5]. Alternately, the vagal brake can be rapidly withdrawn to allow for greater sympathetic dominance during times of perceived threat or stress [6–7].
Notably, collateral branches of the vagus nerve also terminate on the soft palate, pharynx, larynx, esophagus, and facial muscles. These areas are known for their role in emotional expressiveness and social communication, which similarly tend to be dysregulated or impaired among depressed individuals. According to his Polyvagal Theory, Porges posits that the evolution of vagal pathways enabled mammals to flexibly employ behavioral responses that allowed for social affiliation via vagal modulation of sympathetic fight-or-flight responses and facilitation of emotional expression and social communication [5]. The vagal system is thought to facilitate flexible adaptation to changing environmental demands by rapidly shifting autonomic activity in response to environmental cues that elicit relaxation and social affiliation (e.g., relative parasympathetic dominance via the vagal brake) versus fight-or-flight responses to environmental threats (e.g., relative sympathetic dominance via vagal withdrawal). Thus, the vagal system may play a role in concurrent emotional, social, and cardiac-hemodynamic regulation. By extension, the common patterns of emotional, social and cardiac-hemodynamic dysregulation observed in patients with major depressive disorder (MDD) may be associated with impaired vagal control mechanisms.
Indirect indicators of cardiac vagal control such as respiratory sinus arrhythmia (RSA) can be quantified from continuous EKG signals using spectral analyses and related procedures. Because vagally-mediated parasympathetic effects on heart rate occur rapidly (in milliseconds), changes in heart rate that occur in the high-frequency range of heart rate variability (0.15–0.50 Hz) are typically used to index RSA [8]. Notably, low RSA levels have been associated with features of emotional dysregulation, cardiac risk, and social dysfunction that commonly accompany MDD. For example, lower RSA levels are associated with: (1) greater symptoms of depression and anxiety [1, 9–11]; (2) elevated risk for cardiovascular disease and mortality [3–4, 12]; and (3) diminished social functioning or being unmarried, alone or socially isolated [13–16].
Given the patterns of emotional dysregulation, cardiovascular risk, and social dysfunction exhibited among clinically depressed individuals, it is reasonable to speculate that vagal modulation may be compromised in MDD. In a meta-analysis of 13 studies including 312 depressed individuals and 374 non-depressed individuals, Rottenberg [1] found that depression covaried with a reduction in cardiac vagal control; however, the overall effect size for depression across the studies was in the small-to-medium range (d = 0.332). This modest effect size reflects the mixed nature of study findings, including reports of lower levels of RSA among depressed samples as compared with controls [17–19], as well as no differences in RSA levels between depressed and non-depressed groups [20–22].
Such mixed findings may stem from a variety of variables that can obscure detectable relationships between depression and RSA. One critical variable is the impact of antidepressant medications, which may suppress cardiac vagal control [23–24]. Given that most studies of cardiac vagal control among clinically depressed individuals include some individuals taking antidepressants, at least some of the relationship between depression and diminished RSA may be attributed not to the depressive state per se, but to antidepressant medications. Indeed, in a report evaluating CVD risk factors in 2,373 adults in Sweden, Licht et al [24] found that depressed individuals who were taking tricyclics, selective serotonin reuptake inhibitors or other antidepressants had significantly reduced RSA levels; moreover, the relationship between MDD history and reduced RSA was appreciably attenuated following control for psychotropic medication use.
Other modifying factors include gender, respiratory influences on RSA, prior experience of trauma, and the laboratory or social contexts in which RSA is measured. For instance, two studies have pointed to potential differences in RSA observed in male versus female depressed patients [10, 25]. In aggregate, however, most previous studies have included a mix of males and females, with sample sizes too modest to evaluate or control for potential gender differences or interactions that may influence RSA outcomes. In addition to gender, an ongoing controversy also persists with regard to the need to control for respiratory variables when employing RSA as a marker of cardiac vagal control. At rest, RSA magnitude inversely relates to respiration rate. Moreover, changes in respiration rate are not uncommon when individuals engage in laboratory-based tasks. Using pharmacological autonomic blockades to evaluate the degree of covariation among RSA, respiratory parameters and vagal tone, Grossman and colleagues demonstrated that changes in RSA observed in response to mental tasks may be more closely related to task-induced changes in respiration rate than to changes in cardiac vagal tone per se [26–27]. Thus, respiratory changes that occur in response to laboratory-based tasks may confound the relationship between observed RSA and cardiac vagal control. As such, Grossman and Taylor [27] noted that “it was only when respiratory variables were statistically controlled (using a within-individual regression approach) that there was a clearly improved but still imperfect association between RSA and vagal tone” (p. 266). Despite the important contributions of respiration rate, surprisingly few published RSA studies control for respiratory factors using a within-subject regression approach, particularly in depressed individuals.
It is also surprising that no previous research has attempted to evaluate whether diminished RSA levels among depressed populations relates to subjects’ lifetime trauma history. Indeed, it is well established that traumatic experiences are disproportionately reported by depressed individuals. Moreover, exposure to lifetime trauma can have lasting effects on limbic brain systems that govern autonomic outflow [28]. Further, preliminary reports have shown reduced resting RSA levels in patients with post-traumatic stress disorder (PTSD) relative to controls [29–31]. Hence, because lower RSA in depressed samples may relate to elevated trauma exposure in this group, we sought to explore whether depression-related reductions in RSA could be accounted for by lifetime trauma history.
A final, yet important issue includes consideration of the social context in which RSA level or task-related RSA changes are evaluated. The Polyvagal Theory emphasizes the adaptive sensitivity of the vagal system to changing social or environmental demands, including cues related to environmental threat or stress (associated with vagal withdrawal or sympathetic dominance), as well as cues signaling safety or social engagement (associated with activation of the vagal brake or parasympathetic dominance). However, with the exception of laboratory-based stress tasks, few (if any) studies have examined or manipulated social factors when evaluating RSA levels or RSA changes observed across depressed and non-depressed groups.
Accordingly, the current study compared RSA level and RSA changes in clinically depressed women and matched controls. A secondary study aim was to address methodological issues identified in prior evaluations of RSA in depressed populations. To this end, we studied only medically healthy women free of antidepressant medications for at least 4 weeks prior to assessment. In this way we controlled for (but did not directly test) the potential impact of gender and antidepressant medication use on RSA. In addition, we assessed and tested the impact of respiratory rate and lifetime trauma history on RSA outcomes. As part of the study, women completed (1) a speech stress task designed to invoke cardiac vagal withdrawal, and (2) a relationship-focused imagery task, designed to invoke a behavioral state of social warmth or comfort and vagal activation. We hypothesized that depressed women – and particularly depressed women with a lifetime trauma history – would display lower levels of RSA during the stress task as compared with non-depressed controls. In addition, we explored depression group differences in RSA levels evaluated over the course of the relationship-focused imagery task, and evaluated whether any observed group differences in RSA would persist following control for respiration rate.
METHODS
Recruitment and Screening Procedures
Recruitment
Women aged 20–40 years were recruited to participate in a study of peripheral oxytocin release in depressed and non-depressed women [32]. Because EKG assessments were added later to the parent study, a sub-sample of 15 depressed subjects and 15 controls recruited between June 2003 and October 2005 contributed EKG data to this report. Depressed women aged 20–40 were recruited from a sample of depressed adults undergoing screening for inclusion within depression treatment protocols conducted by the Depression and Manic-Depression Prevention Program at the Western Psychiatric Institute and Clinic (WPIC). Thus, the depressed sample included in the current study was drawn from a treatment-seeking group of depressed outpatients. All depressed subjects were required to be medically healthy, and to be free of antidepressant medications for at least 4 weeks prior to assessment (patients being tapered from antidepressant treatments were not eligible). Never-depressed women were recruited from local advertisements, and matched to the depressed sample based on race and age (within 3 years). Interested women were screened by phone for basic study eligibility. Informed consent was obtained before in-clinic screening procedures and laboratory participation. All procedures were reviewed and approved by the University of Pittsburgh institutional review board.
Psychiatric Screening
Depressed women were required to meet criteria for current MDD of at least moderate severity, as documented by a Structured Clinical Interview for Axis I DSM-IV Disorders (SCID) [33] diagnosis and a 17-item Hamilton Rating Scale for Depression (HRSD) [34] score of 14 or more at initial evaluation. Women with a lifetime history of manic episodes or psychosis, those with a current diagnosis of bulimia or anorexia nervosa, and those meeting criteria for drug or alcohol abuse or dependence in the past 3 months were excluded. Non-depressed participants did not meet criteria for any current or lifetime Axis I mood or anxiety disorder.
Medical Screening
All subjects provided medical history information, as well as blood samples for complete blood count and serum pregnancy screening within one month of participation. Subjects who were anemic, pregnant, or taking anti-depressant medications were excluded, as were those who reported (or displayed evidence of) unstable medical illness. Women who had a hysterectomy/oophorectomy, took estrogen replacement therapy, were lactating or less than 6 months postpartum, or who reported significant gynecological problems were also excluded.
Laboratory Testing Procedures
Participants underwent laboratory testing procedures in the Clinical Neuroscience Research Center (CNRC) at the Western Psychiatric Institute and Clinic. Testing sessions were scheduled during the follicular phase of the menstrual cycle, based on self-reported bleeding patterns. All laboratory sessions began at approximately 2:00 pm. Participants were asked to abstain from alcohol for 48 hours prior to participation, and to abstain from eating, drinking caffeinated beverages, and smoking after 12:30 pm on the day of testing.
Participants were situated in a comfortable chair, and were instrumented with three disposable spot electrodes placed in a 3-lead configuration for assessment of electrocardiographic (EKG) signal. In addition, a strain gauge was secured around the lower chest for assessment of respiration rate. Both EKG and respiration were captured using the BioLog system (BioLog model 3992, UFI, Morro Bay CA). Following instrumentation, subjects engaged in a 25-minute habituation period followed by two, one-hour laboratory conditions: a speech stress condition and a relationship-focused imagery condition. In the speech stress condition, participants were given two minutes to prepare a speech defending themselves against a hypothetical shoplifting charge, followed by four minutes of videotaped speech delivery. The relationship-focused imagery included a guided imagery task developed to elicit feelings of love or infatuation. This task represented a modified version of Ekman’s Relived Emotions Task [35] in which subjects imagined and relived in their memory a past experience in which they felt strong feelings of love or infatuation.
Each laboratory condition took one hour and included three assessment periods: a 20-minute resting baseline period; a 10-minute task period (in which participants completed the speech stress task or guided imagery task); and a 30-minute recovery period. Participants rested quietly without distraction during both baseline and recovery periods, and were given a 20-minute break between the two hour-long tasks. Task order was counterbalanced across participants. Participants completed self-report assessments of trauma history following the laboratory task procedures.
Measures
Respiratory Sinus Arrhythmia (RSA)
RSA was assessed via EKG signal sampled continuously at 1000 Hz. RSA was calculated using MindWare HRV 2.16 Software (MindWare Technologies, Ltd., Gahanna, OH). R-wave markers in the EKG signal were evaluated for artifacts by visual inspection and by the MAD/MED artifact detection algorithm [36] implemented in MindWare. Suspected artifacts were corrected manually (less than 1% of all R-waves in past work have needed correction). Spectral-power values were determined (in ms2/Hz) for 1-minute time intervals with fast Fourier transformations, and the power values in the 0.15–0.50 Hz spectral bandwidth were integrated (ms2) as the indicator of RSA for each one-minute epoch. RSA values for one-minute epochs obtained during each task period were averaged to provide mean RSA levels for each Period. Baseline RSA was evaluated over a 10-minute period (minutes 6–15 of the initial resting baseline). Because speaking may be associated with both breathing changes and movement artifacts, RSA levels for the speech task were obtained during the 2-minute speech preparation period (i.e., prior to speech initiation). RSA for the imagery task was evaluated over the 4-minute guided imagery task. Finally, RSA during the first 20 minutes of the post-task recovery period was evaluated in two 10-minute segments (defined as Recovery 1 and Recovery 2). Finally, numbered Biolog-based time markers were inserted into EKG data sets obtained during each laboratory session, to ensure accurate capture of each EKG assessment period.
Respiration Rate
Number of breaths per minute was calculated from continuous strain gauge data sample at 5 Hz using the Biolog system. Breaths per minute were calculated by summing visually-identified respiratory peaks over each of the 1-minute epochs during which RSA was evaluated.
Psychosocial Assessment
Current levels of depressive and anxiety symptoms were assessed using the observer-rated 17-item Hamilton Rating Scale for Depression (HRSD; 41), the self-reported 21-item Beck Depression Inventory (BDI) [37], and the self-reported 21-item Beck Anxiety Inventory (BAI) [38]. These instruments evaluate symptoms of depression or anxiety experienced over the past week. Subjects’ lifetime history of trauma was evaluated with the Trauma History Questionnaire [39]. This 24-item self-report questionnaire assesses the lifetime occurrence of a variety of traumatic events falling into one of three general categories: crime, general disaster/trauma, and sexual and physical assault experiences. Because of the skewed nature of the distribution in the number of lifetime traumatic events endorsed by study subjects, a median split was used to dichotomize subjects into those with low (0–2 events) versus high (> 2 events) levels of lifetime trauma.
Analysis Plan
Statistical models evaluated the effect of study Group (depressed versus non-depressed) and lifetime Trauma History (high versus low trauma history) within separate 2 (Group) X 2 (Trauma History) × 2 (Task Order) repeated measures ANOVAs for RSA outcomes obtained during the baseline, task, and recovery periods of the 1-hour speech stress condition and the 1-hour relationship imagery condition. Greenhouse-Geisser corrections were utilized for all reported ANOVA results. To investigate whether obtained Trauma History effects were driven by a history of post-traumatic stress disorder (PTSD), post-hoc analyses were conducted eliminating patients with a current or lifetime history of this Axis I anxiety disorder (n=2).
The above analyses were repeated in models controlling respiration, using methods advocated by Grossman and colleagues [26–27] to control for the impact that within-subject respiration rate fluctuations may have on RSA. Specifically, we utilized within-subject linear regression models to calculate standardized residual scores representing the variance in RSA level for each 1-minute epoch that could not be attributed to within-subject fluctuations in respiration rate for the same 1-minute epoch. We then reran study models using each subject’s standardized residual scores as an index of RSA fluctuations that are independent of the potential impact of respiratory rate changes.
RESULTS
Clinical and Demographic Characteristics
The sample consisted of predominantly young (mean age = 29.83 years, SD = 5.95), white (86.6%) women. The depressed and non-depressed groups did not significantly differ on age, race, education or body-mass index (BMI). Not surprisingly, the depressed group displayed significantly more depressive symptoms based on self-reported BDI scores and observer-rated HRSD scores. The depressed group also reported significantly greater levels of self-reported anxiety symptoms based on BAI scores (see Table 1), with the modal subject meeting criteria for at least one comorbid anxiety disorder. The most commonly observed anxiety comorbidities included: generalized anxiety disorder (GAD; 11 subjects), social phobia (5 subjects) and specific phobia (4 subjects), followed by panic disorder (2 subjects), post-traumatic stress disorder (PTSD; 2 subjects) and agoraphobia without a history of panic (1 subject). In contrast, none of the non-depressed controls met criteria for an anxiety disorder. Notably, the depressed and non-depressed groups did not differ in the proportion of subjects falling into the high lifetime trauma category (53.3% of depressed vs 40% of non-depressed women). See Table 1 for further detail.
Table 1.
Characteristics of the Study Sample
| Study Group | ||
|---|---|---|
| Depressed (n = 15) | Non-Depressed (n = 15) | |
| Age in years, mean (SD) | 31.77 (6.65) | 27.89 (4.58) |
| % (N) white | 86.6% (13) | 86.6% (13) |
| BMI, mean (SD) | 24.08 (2.81) | 24.69 (4.34) |
| HRSD, mean (SD) ** | 16.20 (5.43) | 3.73 (2.84) |
| BDI, mean (SD) ** | 19.36 (7.19) | 2.4 (2.87) |
| BAI, mean (SD) ** | 17.07 (10.40) | 3.07 (4.38) |
| Proportion categorized as high trauma history | 53.3% (8) | 40% (6) |
p < .001; HRSD = Hamilton Rating Scale for Depression, BDI = Beck Depression Inventory, BAI = Beck Anxiety Inventory
RSA Observed During the Speech Stress Condition
Model Predicting Non-Adjusted RSA Levels
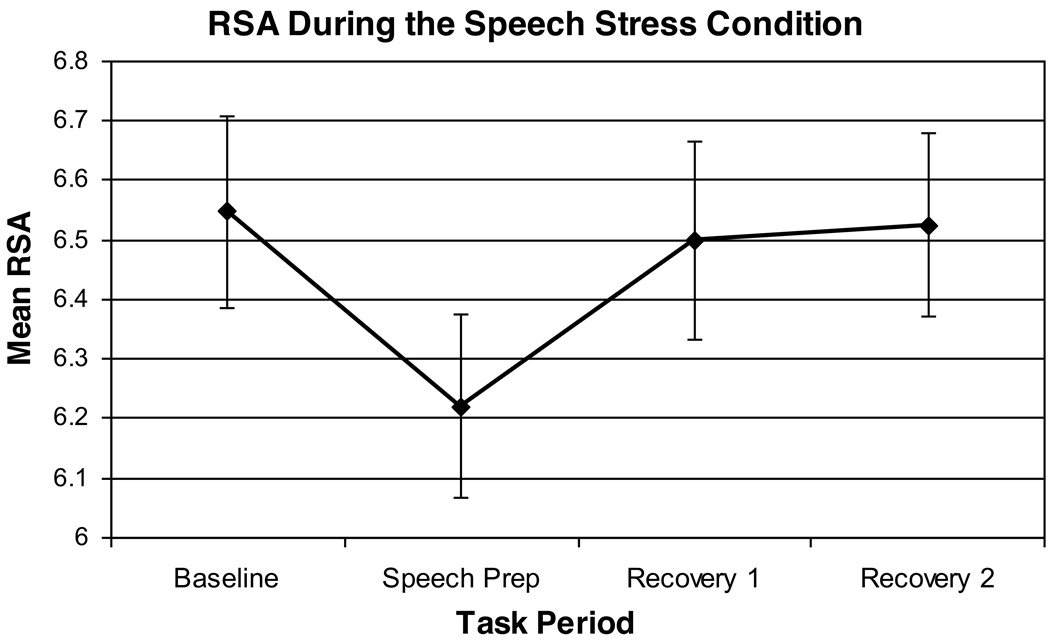
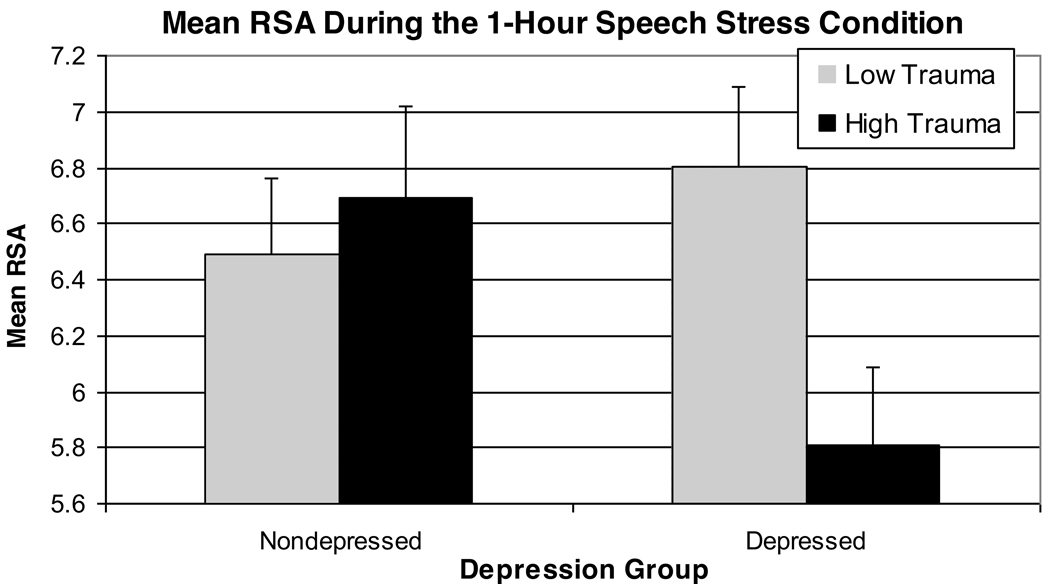
Results of a 2 (Group) × 2 (Trauma History) × 2 (Task Order) analysis indicated a significant effect of Period [F(3,66)=4.36, p=.02, partial eta squared= .17] and a marginally significant Group × Trauma History interaction [F(1,22)=9.61, p=.05, partial eta squared=.16]. The Period effect represented a withdrawal of cardiac vagal control during the stress task, followed by post-task recovery (see Figure 1). As hypothesized, depressed women with a high lifetime trauma history tended to display the lowest average RSA levels over the course of the 1-hour stress session (see Figure 2). No other within- or between-subjects effects were significant. Notably, these study findings were unchanged in post-hoc analyses eliminating the two depressed subjects with a history of PTSD [Group × Trauma History interaction, F(1,20)=5.64, p=.03].
Figure 1.
Main Effect of Period on RSA Levels Observed Over the Course of the Speech Stress Condition
Figure 2.
Average RSA Levels Observed During the 1-Hour Speech Stress Condition: Depression Group × Trauma History Interaction
Model Predicting RSA Levels Following Control for Respiration Rate
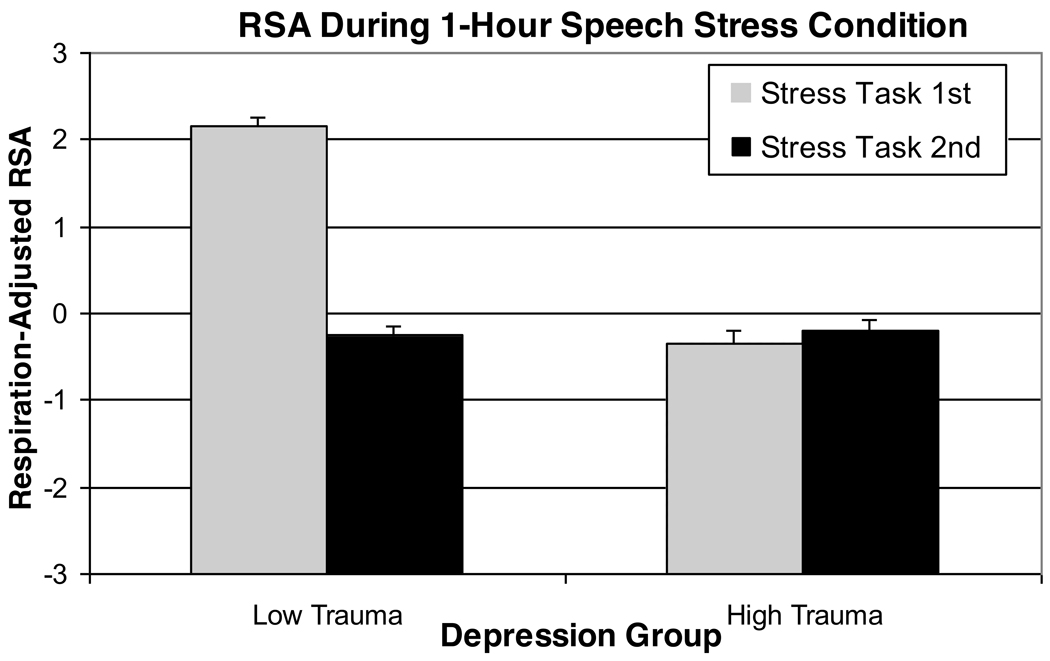
Results of a 2 (Group) × 2 (Trauma History) × 2 (Task Order) analysis predicting residualized RSA scores indicated a main effect of Period [F(3, 60)=3.79, p = .04, partial eta squared=.16], a trend-level effect of Trauma History [F(1,20)=4.05, p=.058, partial eta squared=.17], and a Trauma History X Task Order interaction [F(1,20)=6.46, p=.02, partial eta squared=.24]. Replicating findings from the above model, a reduction in residualized RSA scores was observed during the speech preparation period, followed by post-task recovery. In addition, after controlling for respiration, women with low levels of lifetime trauma exhibited significantly higher average RSA scores, but only when the Stress Task was presented first (see Figure 3). The Group × Trauma History interaction was not, however, significant in this model. Notably, the obtained effects were essentially unchanged in post-hoc analyses eliminating the two depressed subjects with a history of PTSD [main effect of Trauma History, F(1,18)=5.66, p=.03; Trauma History × Task Order interaction effect, F(1,18)=8.29, p=.01].
Figure 3.
Standardized Residual RSA Scores During the 1-Hour Speech Stress Condition Following Adjustment for Respiration: Trauma History X Task Order Interaction
RSA Observed During the Relationship Imagery Condition
Model Predicting Non-Adjusted RSA Levels
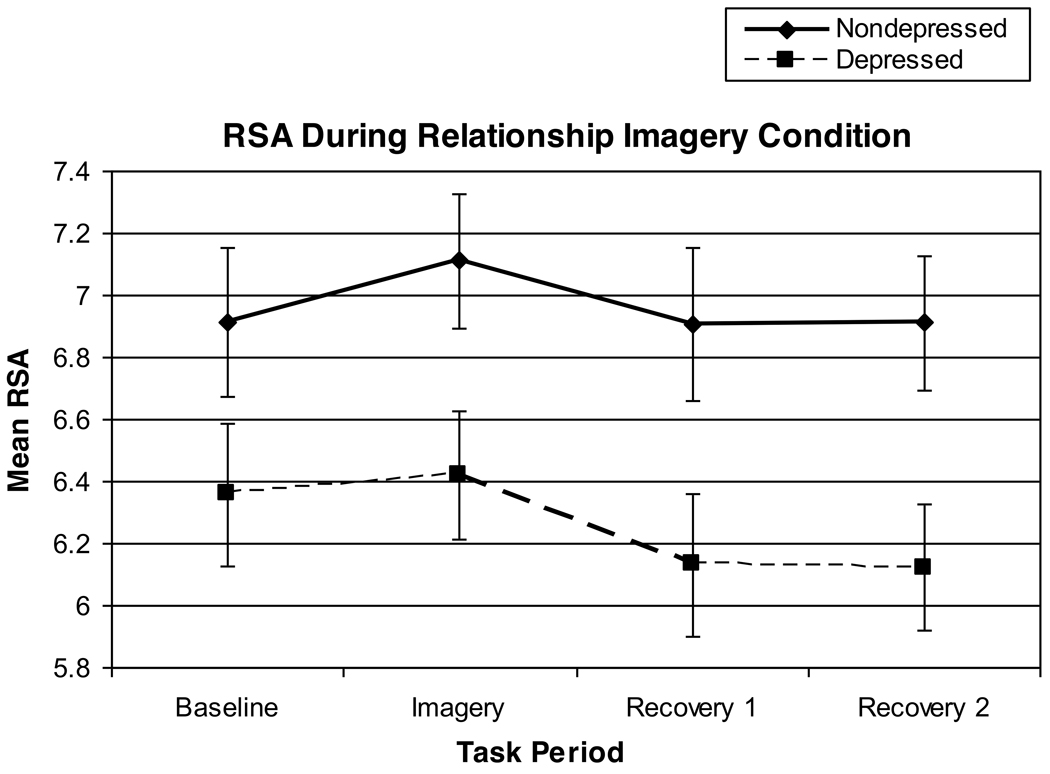
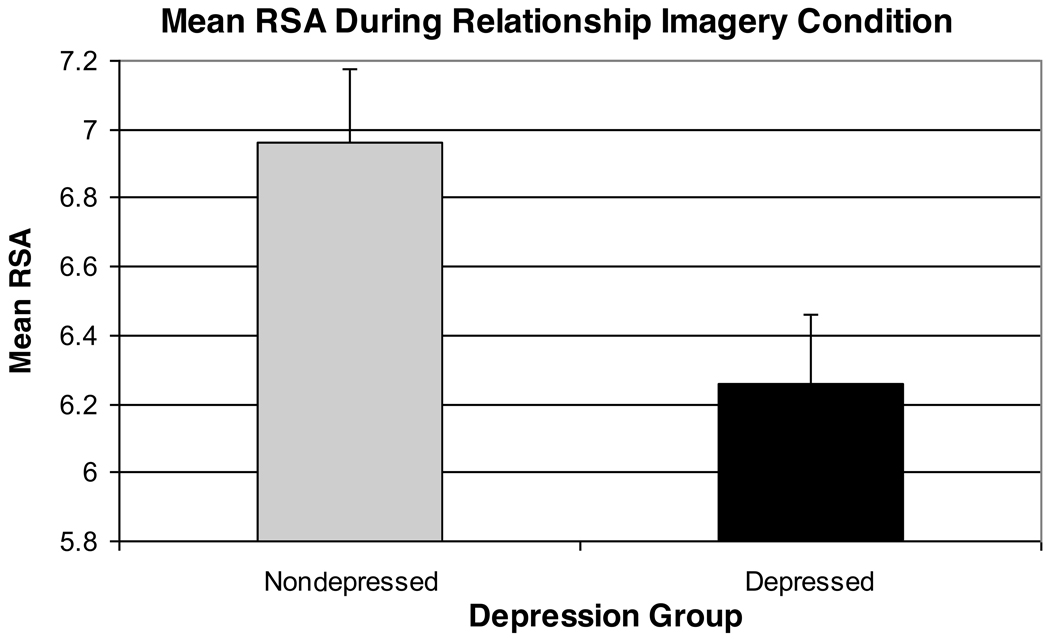
Results of a 2 (Group) × 2 (Trauma History) × 2 (Task Order) analysis indicated a significant effect of Period [F(3,66)=3.79, p=.02, partial eta squared=.15] and a main effect of Group [F(1,22)=5.54, p=.028, partial eta squared=.20]. The Period effect reflected an increase in RSA from the baseline to the imagery task, followed by a post-task RSA reduction. In addition, the depressed group displayed significantly lower RSA levels during the 1-hour imagery task as compared with the non-depressed group (see Figures 4 and 5).
Figure 4.
RSA Levels Obtained Over the Course of the Relationship Imagery Condition
Figure 5.
Depression Group Effect: Average RSA Levels Observed During the 1-Hour Relationship Imagery Condition
Model Predicting RSA Levels Following Control for Respiration Rate
Results of a 2 (Group) × 2 (Trauma History) × 2 (Task Order) analysis predicting residualized RSA scores indicated a trend-level effect of Period [F(3,63)=2.75, p=.059, partial eta squared=.12] and a significant effect of Group [F(1,21)=5.65, p=.027, partial eta squared=.21]. In line with the above, after control for within-subject respiratory rate changes, residualized RSA increased while women thought about a love relationship, followed by a post-task RSA decrease. Moreover, even after control for respiratory changes, the depressed women continued to exhibit lower average RSA over the 1-hour imagery task, as compared with their non-depressed counterparts.
DISCUSSION
Relative to non-depressed controls, physically healthy, unmedicated depressed women displayed diminished cardiac vagal control, as indicated by reduced RSA. These study findings are based on a carefully selected, yet relatively small group of depressed and non-depressed women, and thus should be replicated in future research. Nevertheless, the current study design highlights the importance of taking a nuanced approach to the assessment of RSA in depressed samples that carefully considers both the heterogeneity of MDD, as well as the social and experimental context in which RSA is assessed.
Exposure to adversity, trauma or stress can have lasting effects on brain systems that play a dual role in mood and physiological regulation, particularly limbic brain systems that govern autonomic outflow [28]. Early life trauma increases risk for major depressive disorder, and some have argued that depressed individuals with a trauma history make up a biologically distinguishable subtype of depression [40], which shows a differential treatment response [41]. Thus, it is notable that during the speech stress condition, depressed women with an elevated trauma history showed appreciably lower levels of RSA, as compared with the non-depressed controls and depressed women with little or no trauma history (see Figure 2). Moreover, these findings could not be accounted for by current or lifetime PTSD diagnoses within the depressed group.
These findings further highlight the importance of subtyping depressed groups based on trauma history, particularly in research paradigms that examine dysregulation of stress-related autonomic responses. Notably, these results are based on a simple summary measure of lifetime traumatic events which assessed neither the intensity nor the precise timing of these life events. Thus, it is possible that more comprehensive trauma assessments would better capture additional subgroup differences related to either the intensity or childhood occurrence of traumatic events, factors that may play a significant role in the pathogenesis of both mood and stress dysregulation along the autonomic and neuroendocrine axes [40].
As would be expected, the speech stressor evoked a withdrawal of vagal cardiac control (or withdrawal of the vagal brake on heart rate), followed by post-stress vagal recovery (see Figure 1). In contrast, we found an opposite effect in the relationship-focused imagery task. Specifically, thinking about a close love relationship elicited a small increase in vagal control across the groups (see Figure 4). While several previous studies have documented vagal withdrawal during acute stress tasks [42–44], relatively few have reported laboratory manipulations that increase vagal activation or control [45–47]. In this respect, it is notable that the most robust depression group differences in RSA (that held following control for both trauma history and respiratory parameters) were obtained in the experimental condition designed to enhance feelings of social comfort and thus elicit vagal activation.
This latter finding is novel, and calls for greater attention to the particular conditions in which RSA is assessed in depressed subjects and non-depressed controls – as such attention would inform inferences regarding vagal function in depression. Porges’ polyvagal theory posits that positive social affiliation should relate to increases in cardiac vagal control. However, depressed individuals have long been shown to display deficits in social or interpersonal function, including elevated levels of social isolation, decreased levels of social support, and greater levels of relationship distress [48–50]. For these reasons, depressed individuals may be less likely to mount enhanced cardiac vagal control during daily social interactions, and thus may not reap the protective cardiovascular benefits of supportive social relationships documented in healthy samples without clinical depression.
A second advantage of evaluating RSA in the context of the relationship-imagery condition is that within-subject respiratory fluctuations had less impact on RSA outcomes in this laboratory condition. While we refrained from assessing RSA while subjects actively gave their speech, respiratory fluctuations across the speech stress condition did affect RSA outcomes. Indeed, the Trauma History × Task Order interaction obtained for respiration-controlled RSA outcomes during the stress condition was unexpected, and may reflect potential spill-over effects from the preceding imagery condition. Other intriguing possibilities are that such interactions may have been driven by trauma-induced alterations in neuroendocrine function or inflammatory activity, which have been related to both depressive symptomatology and impaired cardiac vagal control [51]. Lacking measures of neuroendocrine function (e.g., cortisol) and inflammation (e.g. interleukin-6), further research including such measures and reporting both standard and respiration-controlled RSA levels is needed to clarify this finding. In contrast, control for respiratory variables using the same within-subject regression approach advocated by Grossman and Taylor [27] had little to no impact on study outcomes obtained during the relationship-focused imagery task.
It is noteworthy that we observed a high level of anxiety comorbidity in the depressed women included in this report. Anxiety disorders represent the most common psychiatric comorbidity observed among depressed individuals [52]. Community-based epidemiological research indicates that nearly 60% of individuals reporting MDD in the past year also meet criteria for one or more past-year anxiety disorders [53], and anxiety comorbidity rates are particularly high among treatment-seeking depressed patients [54–56]. The elevated anxiety comorbidity obtained in the current study also reflects the fact that we limited our study to women, who report higher anxiety disorder prevalence rates relative to males. In particular, 11 of the 15 depressed women met current criteria for GAD. This finding may reflect the often-cited overlap across MDD and GAD disorders, as well are our research group’s decision to ignore hierarchical SCID diagnostic rules for GAD (which instructs raters not to code GAD that occurs exclusively during MDD episodes). The level of co-occurring GAD reported in the current sample may therefore represent an accurate reflection of the high levels of worry and anxious rumination observed within many depressed females. Both experimentally induced and naturally occurring worry episodes, as well as dispositional measures of worry and generalized anxiety associated with GAD are associated with lower levels of RSA [9, 57–59]. Unfortunately, given the small sample size and study design, the current study was unable to disentangle whether RSA outcomes were driven by depression per se, or by concomitant levels of anxiety or worry. Future trials that include sufficient numbers of depressed (but non-anxious) and anxious (but non-depressed) individuals will be needed to address this important study question.
Additional study limitations included the fact that we controlled for the potential impact of gender and antidepressant medication use by restricting the study sample to nonmedicated women. Thus, the current study cannot speak to potential gender differences in RSA, nor to potential RSA differences between depressed and non-depressed men. In addition, given recent epidemiologic data to suggest associations between antidepressant medication use and diminished RSA [24], future experimentally-controlled research is needed to test the impact of antidepressant medication use on RSA levels within subjects over time. Finally, we would note that the relationship-focused imagery condition did not include an actual social interaction, and thus does not address the direct impact of social engagement or affiliation on RSA levels across the depressed and non-depressed groups.
Strengths of the current work include the fact that the depressive sample was well-characterized and relatively homogeneous in nature. The depressed group was assessed to exclude any frank medical or cardiovascular disease, and was free of antidepressant medications or other medications that may impact outcomes of interest. The group was limited in both gender and age, and both RSA and respiratory parameters were rigorously assessed across two separate laboratory conditions deigned to evaluate both vagal withdrawal and vagal activation. By design, this investigation included a high level of experimental control and careful sample selection. Not surprisingly (given the small sample size), all of reported study findings were associated with large effect sizes (i.e., partial eta squared > .14). While such effect sizes speak to the strength of our study findings, the extent to which such findings will generalize to larger samples of depressed outpatients needs to be tested within future research protocols. Specifically, these results should be replicated in future research paradigms that evaluate RSA outcomes across larger and more heterogeneous samples of trauma-relevant depressive subgroups, and in social contexts that would be expected to promote both activation and withdrawal of cardiac vagal control.
ACKNOWLEDGMENTS
The current study was supported by NIH grants MH64144 (Dr. Cyranowski), the WPIC Mental Health Intervention Research Center (MH30915), the WPIC Clinical Neuroscience Research Center (RR0000056), and the Pittsburgh Mind-Body Center (NIH grants HL076852/076858). Dr. Swartz serves as a consultant for Servier, and receives CME honorarium from Bristol Myers Squibb.
RESEARCH SUPPORT: The current work was supported by the National Institutes of Health. Dr. Swartz serves as a consultant for Servier, and receives CME honorarium from Bristol Myers Squibb.
Glossary
- MDD
major depressive disorder
- RSA
respiratory sinus arrhythmia
- EKG
electrocardiogram
- BMI
body mass index
- HRSD
Hamilton Rating Scale for Depression
- BDI
Beck Depression Inventory
- BAI
Beck Anxiety Inventory
- GAD
generalized anxiety disorder
- PTSD
post-traumatic stress disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no other potential conflicts of interest to report with respect to the research presented in the current report. The authors would like to acknowledge the work of the clinical staff at the WPIC Depression and Manic-Depression Prevention Program, and to thank all of the women who participated in this research.
REFERENCES
- 1.Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;67:S29–S33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 3.Thayer JF, Lane RD. The role of vagal function in the risk of cardiovascular disease and mortality. Biol Psychology. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Tsuji H, Larson MJ, Venditti FJJ, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reducted heart rate variability on risk for cardiac evants: The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 5.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-aged children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 7.George DT, Nutt DJ, Walker WV, Porges SW, Adinoff B, Linnoila M. Lactate and hyperventilation substantially attenuate vagal tone in normal volunteers. A possible mechanism of panic provocation? Arch Gen Psychiatry. 1989;46(2):153–156. doi: 10.1001/archpsyc.1989.01810020055009. [DOI] [PubMed] [Google Scholar]
- 8.Berntson GG, Bigger TJ, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Magaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 9.Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 10.Thayer JF, Smith M, Rossy LA, Sollers JJ, Friedman BH. Heart period variability and depressive symptoms: Gender differences. Biol Psychiatry. 1998;44:304–306. doi: 10.1016/s0006-3223(98)00008-0. [DOI] [PubMed] [Google Scholar]
- 11.Rottenberg J, Clift A, Bolden A, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44:450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 12.Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-min rhythm strip predicts risk of coronary heart disease and mortality from several causes: The ARIC study. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 13.Egizio VB, Jennings JR, Christie IC, Sheu LK, Matthews KA, Gianaros PJ. Cardiac vagal activity during psychological stress varies with social functioning in older women. Psychophysiology. 2008;45:1046–1054. doi: 10.1111/j.1469-8986.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenberg N, Fabes R, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children’s social functioning: A longitudinal study. Child Dev. 1995;66:1360–1384. [PubMed] [Google Scholar]
- 15.Fabes RA, Eisenberg N. Regulatory control and adults stress-related responses to daily life events. J Pers Soc Psychology. 1997;73:1107–1117. doi: 10.1037//0022-3514.73.5.1107. [DOI] [PubMed] [Google Scholar]
- 16.Randall G, Bhattacharyya MR, Steptoe A. Marital status and heart rate variability in patients with suspected coronary artery disease. Ann Behav Med. 2009;38:115–123. doi: 10.1007/s12160-009-9137-0. [DOI] [PubMed] [Google Scholar]
- 17.Dalack GW, Roose SP. Perspectives on the relationship between cardiovascular disease and affective disorders. J Clin Psychiatry. 1990;51:409. [PubMed] [Google Scholar]
- 18.Lehofer M, Moser M, Hoehn-Saric R, McLeod D, Hildebrandt G, Egner S, Steinbrenner B, Liebmann P, Zapotoczky H. Influence of age on the parasympathetic property of tricyclic antidepressants. Psychiatry Res. 1999;85:199–207. doi: 10.1016/s0165-1781(99)00005-0. [DOI] [PubMed] [Google Scholar]
- 19.Rechlin T, Weis M, Spitzer A, Kaschka WP. Are affective disorders associated with alterations of heart rate variability? J Affect Disord. 1994;32:271–275. doi: 10.1016/0165-0327(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 20.Lehofer M, Moser M, Hoehn-Saric R, McLeod D, Liebmann P, Drnovsek B, Egner S, Hildebrandt G, Zapotoczky H. Major depression and cardiac autonomic control. Biol Psychiatry. 1997;42:914–919. doi: 10.1016/S0006-3223(96)00494-5. [DOI] [PubMed] [Google Scholar]
- 21.Moser M, Lehofer M, Hoehn-Saric R, McLeod DR, Hildebrandt G, Steinbrenner B, Voica M, Liebmann P, Zapotoczky H. Increased heart rate in depressed subjects in spite of unchanged autonomic balance? J Affect Disord. 1998;48:115–124. doi: 10.1016/s0165-0327(97)00164-x. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor MF, Allen JJ, Kaszniak AW. Autonomic and emotion regulation in bereavement and depression. J Psychosomatic Res. 2002;52:183–185. doi: 10.1016/s0022-3999(02)00292-1. [DOI] [PubMed] [Google Scholar]
- 23.Bar KJ, Greiner W, Jochum T, Friedrich M, Wagner G, Sauer H. The influence of major depression and its treatment on heart rate variability and papillary light reflex parameters. J Affect Disord. 2004;82:245–252. doi: 10.1016/j.jad.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Licht CMM, de Geus EJC, Zitman FG, Hoogendijk WJG, van Dyck R, Penninx BWJH. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA) Arch Gen Psychiatry. 2008;65:1358–1367. doi: 10.1001/archpsyc.65.12.1358. [DOI] [PubMed] [Google Scholar]
- 25.Chambers AS, Allen JJB. Sex differences in cardiac vagal control in a depressed sample: Implications for differential cardiovascular mortality. Biol Psychol. 2007;75:32–36. doi: 10.1016/j.biopsycho.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology. 1991;28(2):201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 27.Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol. 2007;74(2):263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 29.Cohen H, Matar MA, Kaplan Z, Kotler M. Power spectral analysis of heart rate variability in psychiatry. Psychother Psychosom. 1999;68:59–66. doi: 10.1159/000012314. [DOI] [PubMed] [Google Scholar]
- 30.Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: Application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Res. 2000;95:1–13. doi: 10.1016/s0165-1781(00)00195-5. [DOI] [PubMed] [Google Scholar]
- 31.Cohen H, Kotler M, Matar MA, Kaplan Z, Miodownik H, Cassuto Y. Power spectrul analysis of heart rate variability in posttraumatic stress disorder patients. Biol Psychiatry. 1997;41:627–629. doi: 10.1016/s0006-3223(96)00525-2. [DOI] [PubMed] [Google Scholar]
- 32.Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai H, Amico J. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom Med. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders. New York: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- 34.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekman P, Levenson R, Friesen W. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221:1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- 36.Berntson GG, Quigley KS, Jang J, Boysen ST. An approach to artifact identification: Application to heart period data. Psychophysiology. 1990;27:586–598. doi: 10.1111/j.1469-8986.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 37.Beck AT, Steer R. Manual for the revised beck depression inventory. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 38.Beck AT, Steer RA. Manual for the revised beck anxiety inventory. San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- 39.Green BL. Trauma History Questionnaire. In: Stamm BH, Varra EM, editors. Measurement of stress, trauma and adaptation. Lutherville MD: Sidran; 1995. [Google Scholar]
- 40.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullought JP, Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. PNAS. 2003;24:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology. 1994;31:412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- 43.Gianaros PJ, Salomon K, Zhou F, Owens JF, Edmundowicz D, Kuller LH, Matthews KA. A greater reduction in high-frequency heart rate variability to a psychological stressor is associated with subclinical coronary and aortic calcification in postmenopausal women. Psychosom Med. 2005;67:553–560. doi: 10.1097/01.psy.0000170335.92770.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawkley LC, Burleson MH, Poehlmann KM, Berntson GG, Malarkey WB, Cacioppo JT. Cardiovascular and endocrine reactivity in older females: Intertask consistency. Psychophysiology. 2001;38:863–872. doi: 10.1111/1469-8986.3860863. [DOI] [PubMed] [Google Scholar]
- 45.Berntson GG, Cacioppo JT, Fieldstone A. Illusions, arithmetic, and the bidirectional modulation of vagal control of the heart. Biol Psychol. 1996;44:1–17. doi: 10.1016/s0301-0511(96)05197-6. [DOI] [PubMed] [Google Scholar]
- 46.Gianaros PJ, Quigley KS. Autonomic origins of a nonsignal stimulus-elicited bradycardia and it habituation in humans. Psychophysiology. 2001;38:540–547. doi: 10.1017/s004857720100004x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiz JM, Uchino BN, Smith TW. Hostility and sex differences in the magnitude, duration, and determinants of heart rate response to forehead cold pressor: parasympathetic aspects of risk. Int J Psychophysiology. 2006;60:274–283. doi: 10.1016/j.ijpsycho.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Gotlib IH, Whiffen VE. Depression and marital functioning: an examination of specificity and gender differences. J Abnorm Psychol. 1989;98(1):23–30. [PubMed] [Google Scholar]
- 49.Hammen C. Interpersonal stress and depression in women. J Affect Disord. 2003;74(1):49–57. doi: 10.1016/s0165-0327(02)00430-5. [DOI] [PubMed] [Google Scholar]
- 50.Hammen C, Brennen PA. Interpersonal dysfunction in depressed women. Impairments independent of depressive symptoms. J Affect Disord. 2002;72:145–156. doi: 10.1016/s0165-0327(01)00455-4. [DOI] [PubMed] [Google Scholar]
- 51.Marsland AL, Gianaros PJ, Prather AA, Jennings JR, Neumann SA, Manuck SB. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosom Med. 2007;69:709–716. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- 52.Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depress Anxiety. 2000;12:69–76. doi: 10.1002/1520-6394(2000)12:1+<69::AID-DA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 53.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 54.Fava M, Rankin MA, Wright EC, Alpert JE, Nierenberg AA, Pava J, Rosenbaum JF. Anxiety disorders in major depression. Compr Psychiatry. 2000;41:97–102. doi: 10.1016/s0010-440x(00)90140-8. [DOI] [PubMed] [Google Scholar]
- 55.Melartin TK, Rytsala HJ, Leskela US, Lestela-Mielonen PA, Sokero TP, Isometsa ET. Current comorbidity of psychiatric disorders among DSM-IV major depressive disorder patients in psychiatric care in the Vantaa Depression Study. J Clin Psychiatry. 2002;63:126–134. [PubMed] [Google Scholar]
- 56.Zimmerman M, Chelminski I, McDermut W. Major depressive disorder and Axis I diagnostic comorbidity. J Clin Psychiatry. 2002;63:187–193. doi: 10.4088/jcp.v63n0303. [DOI] [PubMed] [Google Scholar]
- 57.Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- 58.Lyonfields JD, Borkovec TD, Thayer JF. Vagal tone in generalized anxiety disorder and the effects of aversive imagery and worrisome thinking. Behav Ther. 1995;25:457–466. [Google Scholar]
- 59.Pieper S, Brosschot JF, van der Leeden R, Thayer JF. Prolonged cardiac effects of momentary assessed stressful events and worry episodes. Psychosom Med. 2007;75:570–577. doi: 10.1097/PSY.0b013e3181dbc0e9. [DOI] [PubMed] [Google Scholar]