Abstract
Accumulating data suggests that Natural Killer (NK) cells are not only involved in the innate [ET1]antiviral response following infection, but are also intimately involved in shaping the quality of the adaptive immune response by modulating the functional properties of myeloid Dendritic Cells (DC) during the acute immune response to infection. In this role, NK cells ensure that only fully maturated, immunogenic DCs gain access to inductive sites, where they might prime effective antiviral adaptive immune responses. However, increasing evidence now suggests that several aspects of this crosstalk between NK cells and DCs are compromised during HIV infection, potentially contributing to immune dysfunction.
NK-DC interactions during innate recognition of viruses
The innate immune response to infection serves as first line defense against incoming pathogens. Recent data suggests that innate immune responses might also play a vital role in shaping the quality of the ensuing adaptive immune response. This link between the innate and adaptive immune response is mediated by a unique subset of myeloid cells, dendritic cells (DC), that are innate immune sentinels centrally involved in the recognition of pathogens1,2. These include both myeloid DCs (mDCs) that act as potent antigen presenting cells and plasmacytoid DCs (DCs) that secrete copious amount of interferon-α (IFN-α) and initiate the antiviral immune response. In this capacity, tissue-resident DCs sense infection through pattern recognition receptors, rapidly take up foreign antigens, initiate the inflammatory cascade, and then traffic to inductive immune sites where they are able to present foreign antigens to cells of the adaptive immune system3,4. Mounting evidence now shows that these cells do not work in isolation, but instead interact with several other cells of the innate immune system. Among the innate immune cells involved in modulating DC activity, natural killer (NK) cells have received much attention over the past decade5–8. In addition to their role in eliminating foreign or infected cells from the body, NK cells are also involved in shaping DC function, and regulating the quality of DCs that gain access to inductive sites, thus ultimately influencing the quality of the adaptive immune response. This cross-talk is not unidirectional, and NK cells and DCs help each other acquire complete functionality to ultimately fine tune the ensuing adaptive immune response. This review will focus on the interplay between DCs and NK cells, and on how their interactions might be altered, resulting in poor antiviral control in the context of HIV infection. We suggest that the cross-talk between NK cells and DCs is impaired in HIV-1 infection, resulting in dysfunction of virus-specific adaptive immune responses.
Dendritic cells and induction immunity versus tolerance
DCs reside in tissues in an immature state, in which they are exquisitely poised to rapidly acquire and sample antigens from the extracellular milieu3,4. In this capacity, DCs persistently survey tissues for “danger signals” (Box 1), including pathogen specific antigens, through an array of germ-line encoded pattern recognition receptors, including the toll-like receptors (TLRs) that recognize conserved molecular microbial patterns9. In an immature state, DCs deliver abortive or tolerogenic signals to T cells, due to low level co-stimulatory antigen expression, resulting in suboptimal naïve T and B cell stimulation in inductive sites. Uptake of foreign/aberrant material coupled to “danger signals” (Box 1) results in the induction of a cascade of events whereupon DCs gain the capacity to present antigens due to the upregulation of major histocompatibility (MHC) class I and II molecules and a range of co-stimulatory molecules. In addition, DC motility increases during maturation allowing the cells to travel to inductive sites where they can prime adaptive immune responses. However, in the absence of “danger signals”, DCs that take up antigens or apoptotic bodies may mature incompletely, leading to the delivery of tolerogenic signals. Thus immunogenic DC maturation hinges on the delivery of a tandem signal from a foreign antigen in the presence of a danger signal for optimal antigen presenting function and priming of adaptive immunity.
Box 1. Danger Signals.
Pathogen associated signals (ex. TLR ligands)
Cytokines/Chemokines
Apoptotic Cells
Given the immune-stimulatory potency of DCs, and the fact that they heavily govern the direction of the immune response following infection, the immune system has evolved a number of checks and balances to ensure that DCs mediate their activity optimally. Among the cells that have been implicated in modulating DC function, NK cells have emerged over the past decade as key regulators of these potent antigen-presenting cells.
NK cells and immune surveillance
NK cells represent the body’s first line defense against incoming pathogens and some tumors10. Through a complex array of germ-line encoded activating and inhibitory receptors11,12, NK cells survey tissue and blood cells for normal expression of self-antigens, in particular major histocompatibility class (MHC) I. This provides recognition of tumor and infected cells, both of which are associated with a downregulation of MHC class I expression. NK cell recognition of aberrant cells that are “missing self”, but which have also upregulated stress ligands for activating NK receptors, results in the rapid elimination of these cells10,13. This elimination is coupled to NK cell-mediated secretion of cytokines and chemokines aimed at creating an inflammatory environment that enhances clearance and control of the pathogen or tumor.
In humans, NK cells can be divided into at least two different subsets, with unique functional properties, based on their surface density of CD5614. The first subset, CD3negCD56bright NK cells, are poorly cytolytic, do not express killer immunoglobulin receptors (KIR), Fcγ-receptors (FcγR), or perforin, but are able to secrete copious amounts of cytokines upon activation. In contrast the second subset, CD3negCD56dim NK cells, are highly cytolytic, express both KIRs involved in the recognition of MHC class I, and FcγR3a (CD16), required for antibody-mediated cellular cytotoxicity of antibody-opsonized material. Interestingly, the proportion of these two NK cell subsets diverges based on their localization within tissues and blood, where CD56dim NK cells circulate predominantly in blood (90% CD56dim: 10% CD56bright) and CD56bright NK cells form the primary resident subset in secondary lymphoid tissues such as lymph nodes (LN) (90% CD56bright: 10% CD56dim)15. This differential distribution might reflect distinct roles of these NK cell subsets in the direct antiviral response and in their unique roles in modulating DC functions during an acute immune response, as described further below.
NK/DC cross talk in tissues
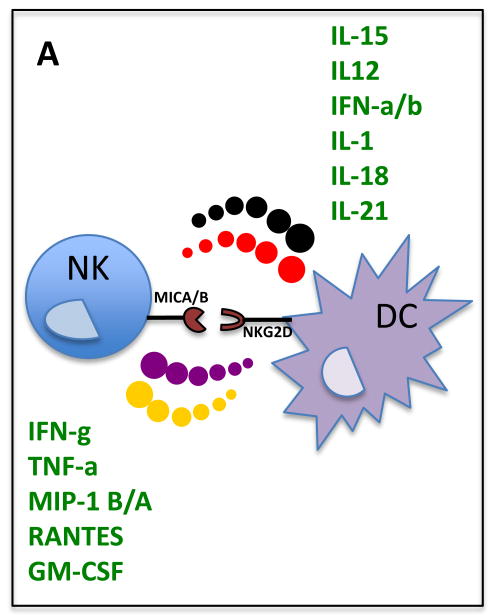
NK cells play a central role in alerting DCs to an infection, and in promoting their functional maturation6–8. NK cell recognition of infected cells results in cytokine release, aimed at recruiting innate immune cells, including DCs, to the site of the antiviral response. Furthermore, these early cytokines, such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) serve as potent signals by which NK cells ensure that DC maturation is skewed towards a Th1 response (Figure 1). NK/DC interactions seem to be most critical in the setting of improper or non-optimal DC maturation that might occur when DCs recognize a foreign/aberrant antigen in the absence of sufficient danger signals (Table 1) to undergo full maturation. In this setting of insufficient DC maturation, NK cells can play a central role in potentiating DC maturation16,17 through the generation of apoptotic bodies containing antigens and the co-secretion of cytokines (Figure 1) that are required to fully activate DCs18. This NK cell-mediated maturation of DCs is cell-contact dependent, and might involve interaction between the natural cytotoxicity receptor (NCR) NKp30 on the surface of NK cells with its still undefined cellular ligand on the surface of the immature DCs19.
Figure 1.
Bi-directional interactions between NK cells and DCs. Secretion of cytokines and receptor–ligand (NKG2D–MIC-A or –B) interactions result in the potentiation of NK and DC responses in viral infections.
NK/DC interactions are not unidirectional, and DCs can secrete a variety of cytokines that potentiate the proliferative and cytolytic capacity of NK cells (Figure 1). pDC production of type 1 IFNs represents one of the strongest early indirect activators of NK cells through mDCs20. In fact, IFN-α promotes IL-15-independent proliferation of CD56bright NK cells via DCs using a yet unknown, potentially contact dependent mechanism21,22,23. Similarly, IP-10, IL-8, , IFN-α and IL-18 augment NK cytolytic capacity and IFN-γ secretion16,24,25, and receptor-bound IL-15 promotes proliferation of CD56dim NK cells24 and NK cell priming and survival26,27. NK cell activation by DCs requires direct cell-to-cell contact, including NKG2D recognition of MHC class I –related (MIC) A and B stress ligands upregulated on IFN-α-stimulated mDCs22. This interaction results in the formation of a stimulatory synapse28 allowing for the polarized secretion of IL-12 directly towards the NK cell. In response, NK cells secrete a number of cytokines that further promote DC activation. Taken together, NK/DC cross-talk is bidirectional and is critical for the optimal activation of NK cells and DCs.
Editing of DCs by NK cells
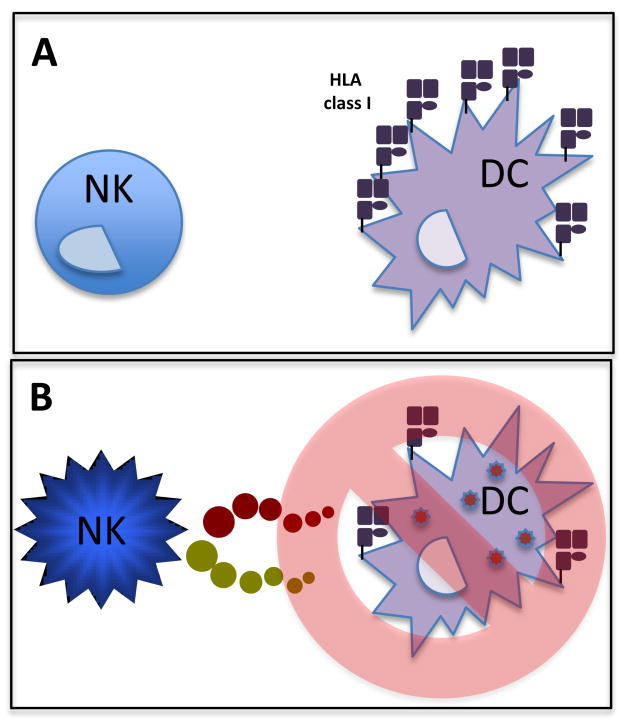
Given the potent immunomodulatory role of DCs, aberrant or improperly matured DCs could potentially induce autoimmunity or compromise the immune response to infection. Thus, in addition to their role played in DC-activation and maturation, NK cells also edit DC populations by eliminating inadequately matured DCs. This NK-mediated editing of DCs was first reported in mice, where NK cells were shown to inhibit myelopoesis in autologous hosts29,30. NK cell depletion resulted in increased numbers of myeloid precursors in the spleen, but not in the bone marrow, suggesting that NK cells eliminate DCs in the periphery31. The DC-editing function of NK cells is regulated by the phenotypic differences in immature and mature DCs, mainly related to differential MHC expression (Figure 2). As mentioned above, DC maturation results in an upregulation of MHC and co-stimulatory molecules, and DCs gain migratory capacity. In contrast, immature DCs that aberrantly gain the capacity to traffic to secondary lymphoid sites can deliver tolerogenic signals due to incomplete upregulation of co-stimulatory molecules. To ensure that only fully mature DCs gain access to inductive sites, NK cells can eliminate immature DCs that gain aberrant access to the peripheral circulation (Figure 2). NK cell-mediated elimination of immature DCs32, which is prompted by low levels of MHC class I and consequent low levels of HLA-E expression, is mediated by KIR-NKG2A+ NK cells32 through the activating receptor NKp3033.
Figure 2.
NK cell-mediated editing of DC populations. a) NK cells perform quality control on all DCs in the circulatory system to ensure that only properly matured DCs, that express high levels of MHC class I are able to traffic to inductive sites. b) NK cells that come in contact with DCs that have been incompletely matured or infected (express low MHC class 1) are rapidly eliminated by NK cells.
How can NKp30+ NK cells promote both DC activation and elimination of immature DCs? After an inflammatory response, innate immune cells gradually accumulate in inflamed tissues, and the ratio of NK:DC appears to dictate whether an NK cell will activate or kill emigrating DCs. At low NK:DC ratios, NK cells secrete cytokines, and potently activate DCs, in an incompletely defined manner, although the receptors NKp30 and/or NKG2A may be involved18. At higher NK:DC ratios, however, NK cells eliminate DCs in an HLA-E-dependent manner32. This differential interaction between NK cells and DCs based on NK frequencies might represent an elegant mechanism aimed at limiting the window of opportunity in which DCs are able to leave tissues to prime adaptive immune responses, but the precise mechanisms underlying this differential effect need further investigation.
Dysregulation of NK/DC cross talk in HIV
Accumulating evidence suggests that the NK/DC axis is significantly impaired during HIV infection, related to significant alterations in phenotype and function of both NK cells and DCs. As early as the acute phase of infection, significant redistributions occur within the NK cell compartment, resulting in an early loss of CD56bright NK cells, followed by a loss of cytolytic CD56dim NK cells 34,35. The loss of CD56pos NK cells occurs in concert with the accumulation of CD56neg NK cells that express a number of NK cell receptors, but fail respond upon stimulation 34,36. Furthermore, progressive HIV infection results in an accumulation NKp30low expressing NK cells with aberrant TNF-related apoptosis-inducing ligand (TRAIL) activity, resulting in a reduced capacity to eliminate immature DCs 37,38. Conversely, mDCs generated from untreated individuals with progressive HIV-1 infection exhibited an impaired capacity to secrete the cytokines needed to activate NK cells, resulting in insufficient levels of IFN-γ required to further mature the mDCs. Successful viral suppression under antiretroviral therapy can lead to the reconstitution of cytokine-secretion by mDCs, suggesting that active viral replication can reversibly impair myeloid cells and render them poor activators of NK cells37. Given that these impairments of NK/DC interactions occur early in HIV-1 infection, they can potentially contribute to poor initial viral control and compromised induction of protective adaptive immunity.
Recent data have highlighted novel mechanisms by which viruses may manipulate NK/DC cross-talk to evade protective immunity. Both chronic lymphocytic choriomeningitis virus and HIV infection are associated with high-level secretion of the anti-inflammatory cytokine interleukin (IL-10), which plays a central role in compromising the quality of the adaptive immune response in both infections39,40. Interestingly, IL-10 can profoundly modulate DC maturation, resulting in the differentiation of tolerogenic DCs that result in poor T cell induction41–44. Additionally, exposure of DCs to IL-10 can significantly change the expression of MHC class I and NKG2D-ligands on DCs, rendering mature DCs susceptible and immature DCs resistant to killing by NK cells45. Thus IL-10, which is normally secreted transiently following acute viral infections, might limit the window in which DCs traffic to inductive sites rapidly through the generation of aberrant DC subsets that may be more vulnerable to NK-editing. However, in the setting of chronic persistent viral infections such as HIV-1, protracted IL-10 secretion can result in altered NK/DC editing, leading to an accumulation of tolerogenic DCs potentially responsible for the induction of dysfunctional adaptive immune responses. Immunotherapeutic modulation of the NK/DC crosstalk in chronic infection might therefore represent an attractive approach to enhance protective immunity to HIV-1.
Kinetics of DC-NK interactions modulate the quality of adaptive immunity
Recent data suggest that the duration and potency of the early NK-DC interaction can profoundly modulate the quality of the ensuing adaptive immune response. In the MCMV model, a single gene for an activating NK cell receptor (Ly49H) that recognizes a virally encoded MHC-class I homologue accounts for protection from MCMV infection46,47. Following MCMV infection, Ly49H+ NK cells expand specifically and contain infection48. Interestingly, recent studies suggest that qualitative rather than quantitative features of the acute NK cell response in Ly49H+ mice have a profound impact on the induction of more effective antiviral T cell responses49,50. These data from the MCMV model might provide some clues for the potential mechanisms that underlie the described epidemiologic associations between particular combined KIR and HLA class I genotypes and slower HIV disease progression, including the activating KIR3DS1 allele and particular KIR3DL1 alleles, when co-expressed with HLA class I Bw4 alleles51,52.
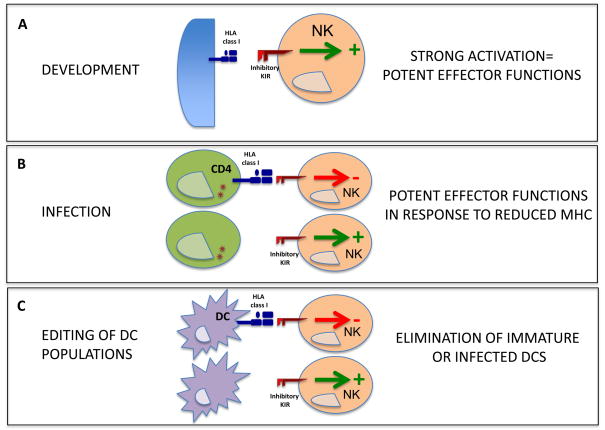
Like the Ly49H+ NK cells in MCMV infection, KIR3DS1+ and KIR3DL1+ NK cell populations preferentially expand during acute HIV-1 infection in the blood of individuals also encoding for HLA-B Bw4 alleles53. This expansion of specific NK cell populations may have a direct impact on modulating the quality of the ensuing adaptive immune responses both through direct control of HIV viral replication, but also potentially by fine-tuning the DC populations that are able to gain access to inductive sites. While mounting evidence suggests that the KIR3DS1+ NK cells have a strong direct anti-HIV-1 activity in Bw4+ individuals54, the mechanisms by which KIR3DL1+ NK cells might mediate their protective activity in HIV-1 infection is still unclear. The rules that determine the function of NK cells during development might provide some clues for the observed antiviral activity of NK cells from individuals encoding for KIR3DL1 and HLA-Bw4.
According to the “licensing” model of NK cell development, engagement of an inhibitory NK cell receptor to self MHC class I is required during NK cell development to render that particular NK cell fully competent55,56. In the absence of such an interaction between an inhibitory receptor and its MHC class I ligand that prevent auto-reactivity, NK cells remain unlicensed and are unable to respond to diverse stimuli56. Recent evidence furthermore suggests that licensing is a quantitative process, and that stronger inhibitory signals, conferred by higher frequency interactions between inhibitory KIRs and self-MHC, may lead to more functional NK cells that respond more strongly to MHC class I-deficient target cells 57. Thus in addition to a potential direct antiviral role, KIR3DL1high NK cells may also become better licensed in the presence of their HLA class I ligand HLA-B Bw4 during development55,58, allowing them to more potently eliminate immature DCs that express lower levels of HLA class I, and limit production of excessive amounts of IFN-α50. Furthermore, KIR3DL1high NK cells might be strongly inhibited by mature DCs expressing high levels of HLA class I. This capacity of licensed KIR3DL1high NK cells to fine tune the balance between mature and immature DCs might have direct consequences for the quality of the antiviral T cell response, as suggested by recent data in the MCMC model49,50, and its ability to effectively control viral replication.
Aberrant NK/DC interactions at the sites of HIV infection
The vast majority of HIV infections worldwide occur through mucosal tissues of the female genital tract59, and following infection, the mucosal tissues of the gastrointestinal tract form the primary site of viral replication and dissemination60. However, these mucosal tissues harbor unique populations of NK cells that might modulate DCs in a disparate fashion to NK cells in the blood and LN. In fact, uterine and gut NK cells express high levels of CD5661,62, but also express KIR, FcγRs, and perforin granules63–65, more characteristic of CD56dim NK cells in the peripheral blood. Additionally, these mucosal NK cells secrete anti-inflammatory or regulatory cytokines such as IL-1061,62, IL-2266, and/or IL-1767 rather than pro-inflammatory cytokines. The debate is still ongoing whether these IL-22-secreting cells are NK cells or lymphoid tissue inducer cells. Yet, it is clear that if mucosal NK cells secrete a remarkably different set of anti-inflammatory cytokines than the conventional/pro-inflammatory NK cells, they may have a profoundly different impact on DCs that encounter antigens within these sites. Thus DCs exposed to antigen at mucosal sites of initial HIV-1 transmission/replication in the presence of non-inflammatory NK cells may receive predominantly anti-inflammatory -less immunogenic signals, rendering them tolerogenic. Further studies of the NK/DC interactions at mucosal sites are required to gain a better understanding on how HIV may take advantage of the anti-inflammatory mucosal environment to compromise the induction of potent antiviral immunity.
Concluding remarks
NK cell-mediated activation and editing of DCs is now emerging as a novel axis that modulates the adaptive immune response to viral infection. Important questions that need to be addressed by future research are how HIV-1 precisely interferes with the cross-talk between NK cells and DCs, whether this cross-talk can be modulated, and whether the innate cross-talk between NK cells and DCs can be harnessed by vaccines and/or immunotherapeutic interventions to enhance the quality of adaptive immune responses. Novel approaches aimed at modulating the interactions between NK cells and DCs will provide exciting new avenues by which to potentiate the efficacy of vaccine approaches to fight various infections as well as other disease conditions.
Figure 3.
Impact of NK cell licensing on the elimination of HIV-1-infected DCs. a) During NK cell development in the bone marrow, inhibitory KIRs expressed on NK cells that interact with a self-MHC class I ligand mediate a potent licensing signal that allows NK cells to emerge as cytolytic killers. b) Licensed NK cells expressing inhibitory KIRs might be more responsive to HIV-1-infected cells that have reduced expression of MHC class I. c) Similarly, licensed NK cells may be more easily inhibited by mature DCs expressing high levels of MHC class I, while licensed NK cells can effectively eliminate immature or infected DCs that have reduced MHC class I expression. (Red arrows indicate an inhibitory signal. Green arrows indicate an activating signal.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinbrink K, Mahnke K, Grabbe S, Enk AH, Jonuleit H. Myeloid dendritic cell: From sentinel of immunity to key player of peripheral tolerance? Hum Immunol. 2009;70:289–293. doi: 10.1016/j.humimm.2009.02.003. S0198-8859(09)00043-3 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37(Suppl 1):S53–60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM. Linking innate to adaptive immunity through dendritic cells. Novartis Found Symp. 2006;279:101–109. discussion 109–113, 216–109. [PubMed] [Google Scholar]
- 5.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. 2005-03-1154 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Walzer T, Dalod M, Vivier E, Zitvogel L. Natural killer cell-dendritic cell crosstalk in the initiation of immune responses. Expert Opin Biol Ther. 2005;5(Suppl 1):S49–59. doi: 10.1517/14712598.5.1.S49. [DOI] [PubMed] [Google Scholar]
- 7.Ferlazzo G. Natural killer and dendritic cell liaison: recent insights and open questions. Immunology letters. 2005;101:12–17. doi: 10.1016/j.imlet.2005.04.015. S0165-2478(05)00123-9 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Moretta A, et al. Early liaisons between cells of the innate immune system in inflamed peripheral tissues. Trends Immunol. 2005;26:668–675. doi: 10.1016/j.it.2005.09.008. S1471-4906(05)00243-7 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Inaba K, Metlay JP, Crowley MT, Witmer-Pack M, Steinman RM. Dendritic cells as antigen presenting cells in vivo. Int Rev Immunol. 1990;6:197–206. doi: 10.3109/08830189009056630. [DOI] [PubMed] [Google Scholar]
- 10.Moretta A, Bottino C, Mingari MC, Biassoni R, Moretta L. What is a natural killer cell? Nat Immunol. 2002;3:6–8. doi: 10.1038/ni0102-6. [DOI] [PubMed] [Google Scholar]
- 11.Moretta L, Biassoni R, Bottino C, Mingari MC, Moretta A. Human NK-cell receptors. Immunol Today. 2000;21:420–422. doi: 10.1016/s0167-5699(00)01673-x. S016756990001673X [pii] [DOI] [PubMed] [Google Scholar]
- 12.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 13.Karre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol. 2002;55:221–228. doi: 10.1046/j.1365-3083.2002.01053.x. 1053 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 15.Fehniger TA, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 16.Gerosa F, et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 17.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc Natl Acad Sci U S A. 2004;101:7669–7674. doi: 10.1073/pnas.0402431101 0402431101. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vitale M, et al. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–571. doi: 10.1182/blood-2004-10-4035. 2004-10-4035 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Ferlazzo G, et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. ni1141 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Romagnani C, et al. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+ CD25hi T regulatory cells. Eur J Immunol. 2005;35:2452–2458. doi: 10.1002/eji.200526069. [DOI] [PubMed] [Google Scholar]
- 22.Jinushi M, et al. Critical role of MHC class I-related chain A and B expression on IFN-alpha-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J Immunol. 2003;170:1249–1256. doi: 10.4049/jimmunol.170.3.1249. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Fontecha A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. ni1138 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Ferlazzo G, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. 0407522101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granucci F, et al. A contribution of mouse dendritic cell-derived IL-2 for NK cell activation. J Exp Med. 2004;200:287–295. doi: 10.1084/jem.20040370 jem.20040370. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koka R, et al. Cutting edge: murine dendritic cells require IL-15R alpha to prime NK cells. J Immunol. 2004;173:3594–3598. doi: 10.4049/jimmunol.173.6.3594. 173/6/3594 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. S1074-7613(07)00186-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borg C, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–3275. doi: 10.1182/blood-2004-01-0380 2004-01-0380. [pii] [DOI] [PubMed] [Google Scholar]
- 29.Hansson M, Petersson M, Koo GC, Wigzell H, Kiessling R. In vivo function of natural killer cells as regulators of myeloid precursor cells in the spleen. Eur J Immunol. 1988;18:485–488. doi: 10.1002/eji.1830180326. [DOI] [PubMed] [Google Scholar]
- 30.Trinchieri G. Natural killer cells wear different hats: effector cells of innate resistance and regulatory cells of adaptive immunity and of hematopoiesis. Semin Immunol. 1995;7:83–88. doi: 10.1006/smim.1995.0012. S1044-5323(85)70012-7 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Pisa P, Sitnicka E, Hansson M. Activated natural killer cells suppress myelopoiesis in mice with severe combined immunodeficiency. Scand J Immunol. 1993;37:529–532. doi: 10.1111/j.1365-3083.1993.tb03330.x. [DOI] [PubMed] [Google Scholar]
- 32.Della Chiesa M, et al. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol. 2003;33:1657–1666. doi: 10.1002/eji.200323986. [DOI] [PubMed] [Google Scholar]
- 33.Castriconi R, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A. 2003;100:4120–4125. doi: 10.1073/pnas.0730640100 0730640100. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alter G, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005 doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 35.Alter G, et al. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J Infect Dis. 2007;195:1452–1460. doi: 10.1086/513878. [DOI] [PubMed] [Google Scholar]
- 36.Mavilio D, et al. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mavilio D, et al. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J Exp Med. 2006;203 doi: 10.1084/jem.20060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tasca S, et al. Escape of monocyte-derived dendritic cells of HIV-1 infected individuals from natural killer cell-mediated lysis. AIDS. 2003;17:2291–2298. doi: 10.1097/00002030-200311070-00003. [DOI] [PubMed] [Google Scholar]
- 39.Brooks DG, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. nm1492 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205:533–541. doi: 10.1084/jem.20071948. jem.20071948 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiorentino DF, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 42.de Waal Malefyt R, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willems F, et al. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol. 1994;24:1007–1009. doi: 10.1002/eji.1830240435. [DOI] [PubMed] [Google Scholar]
- 44.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 45.Alter G, et al. IL-10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. J Clin Invest. 2010;120:1905–1913. doi: 10.1172/JCI40913. 40913 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scalzo AA, et al. Genetic mapping of Cmv1 in the region of mouse chromosome 6 encoding the NK gene complex-associated loci Ly49 and musNKR-P1. Genomics. 1995;27:435–441. doi: 10.1006/geno.1995.1074. S0888-7543(85)71074-9 [pii] [DOI] [PubMed] [Google Scholar]
- 47.Webb JR, Lee SH, Vidal SM. Genetic control of innate immune responses against cytomegalovirus: MCMV meets its match. Genes Immun. 2002;3:250–262. doi: 10.1038/sj.gene.6363876. [DOI] [PubMed] [Google Scholar]
- 48.Dokun AO, et al. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 49.Andrews DM, et al. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med. 2010;207:1333–1343. doi: 10.1084/jem.20091193. jem.20091193 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robbins SH, et al. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog. 2007;3:e123. doi: 10.1371/journal.ppat.0030123. 07-PLPA-RA-0192 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin MP, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nature genetics. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 52.Martin MP, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nature genetics. 2007;39:733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alter G, et al. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J Virol. 2009;83:6798–6805. doi: 10.1128/JVI.00256-09. JVI.00256-09 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alter G, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204:3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 56.Anfossi N, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. S1074-7613(06)00348-7 [pii] [DOI] [PubMed] [Google Scholar]
- 57.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2434–2441. doi: 10.1182/blood-2008-05-156836. blood-2008-05-156836 [pii] [DOI] [PubMed] [Google Scholar]
- 58.Kim S, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A. 2008;105:3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon V, Ho DD, Abdool Karim Q. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet. 2006;368:489–504. doi: 10.1016/S0140-6736(06)69157-5. S0140-6736(06)69157-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brenchley JM, Douek DC. The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS. 2008;3:356–361. doi: 10.1097/COH.0b013e3282f9ae9c 01222929-200805000-00025. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manaster I, Mandelboim O. The unique properties of human NK cells in the uterine mucosa. Placenta. 2008;29(Suppl A):S60–66. doi: 10.1016/j.placenta.2007.10.006. S0143-4004(07)00255-X [pii] [DOI] [PubMed] [Google Scholar]
- 62.Hanna J, Mandelboim O. When killers become helpers. Trends Immunol. 2007;28:201–206. doi: 10.1016/j.it.2007.03.005. S1471-4906(07)00073-7 [pii] [DOI] [PubMed] [Google Scholar]
- 63.Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. 170 [pii] [DOI] [PubMed] [Google Scholar]
- 64.Eiras P, et al. Intestinal intraepithelial lymphocytes contain a CD3- CD7+ subset expressing natural killer markers and a singular pattern of adhesion molecules. Scand J Immunol. 2000;52:1–6. doi: 10.1046/j.1365-3083.2000.00761.x. sji761 [pii] [DOI] [PubMed] [Google Scholar]
- 65.Eiras P, et al. Flow cytometry description of a novel CD3−/CD7+ intraepithelial lymphocyte subset in human duodenal biopsies: potential diagnostic value in coeliac disease. Cytometry. 1998;34:95–102. doi: 10.1002/(SICI)1097-0320(19980415)34:2<95::AID-CYTO6>3.0.CO;2-B. [pii] [DOI] [PubMed] [Google Scholar]
- 66.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010;107:10961–10966. doi: 10.1073/pnas.1005641107. 1005641107 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]