Abstract
Polyethylenimine (PEI) based polymers are efficient agents for cell transfection. However, their use has been hampered due to high cell death associated with transfection thereby resulting in low efficiency of gene delivery within the cells. To circumvent the problem of cellular toxicity, metal binding peptides were linked to PEI. Eight peptide-PEI derivatives were synthesized to improve cell survival and transfection efficiency. TAT linked PEI was used as a control polymer. Peptides linked with PEI amines formed nano gels as shown by electron microscopy and atomic force microscopic measurements. Polymers were characterized by spectroscopic methods and their ability to form complexes with plasmids was tested using electrophoretic studies. These modifications improved polymer biocompatibility as well as cell survival markedly when compared to PEI alone. A subset of the modified peptide-polymers also showed significantly higher transfection efficiency in primary human cells with respect to the widely used transfection agent, lipofectamine. Study of the underlying mechanism of the observed phenomena revealed lower levels of ‘reactive oxygen species’ (ROS) in presence of the peptide-polymers when compared to PEI alone. This was further corroborated with global gene expression analysis which showed upregulation of multiple genes and pathways involved in regulating intracellular oxidative stress.
Keywords: Polyethylenimine, nanogels, DNA vectors, metal binding peptides, primary human cells
1. INTRODUCTION
Stem cells are the precursors for embryonic development as well as adult tissue regeneration. As such, stem cell therapy holds great promise for the field of regenerative medicine. Gene therapy involves manipulation of an entire or portion of a gene in a target cell population in order to correct a disease condition [1]. In the past decade, a number of studies have shown genetic modification of stem cells can improve their therapeutic potential in vivo as well as allow for their monitoring via introduction of reporter genes [2]. The choice of vector for targeted gene therapy into stem cells, however, has been a topic of debate [3]
In order to attain optimum expression of the protein from the vector carrying the gene of interest, multiple hurdles must be overcome. These include (1) protection of the vector from degradation before entry into the cell; (2) efficient entry of the vector into the cell; (3) protection/prevention from nuclease degradation within endosomes; and (4) efficient entry into the cell nucleus [4]. Viral vectors have shown high efficiency of gene delivery, overcoming most of the hurdles listed. However, use of viral vectors has multiple disadvantages including immunogenic responses, insertional mutagenesis, and risk of tumorigenicity [5]. Hence for clinical usage, non-viral gene therapy is the preferred method of choice.
Non-viral gene delivery methods can be broadly categorized into utilizing polycationic polymers, liposomes, peptides, proteins, and organic/inorganic nanoparticles [4]. Positively charged polycationic polymers can efficiently complex with negatively charged DNA thereby increasing vector stability. Use of liposomes or micelles encapsulating the DNA enhances binding and fusion of the DNA-lipid complex to the cell surface, thereby enhancing vector entry into the cell. However, despite intensive research being conducted on non-viral gene delivery methods and existence of more than 50 commercially available kits for transfection, the majority of these methods suffer from two major drawbacks: (1) cell toxicity and (2) low transfection efficiency.
Polyethyeleneimine (PEI) is a promising candidate among polycationic polymers used for transfection [6, 7]. This is due to its relatively high and efficient capacity to complex with DNA. The ability of PEI to complex with DNA so well is attributed to its large number of protonable amino nitrogen atoms, which results in a high cationic charge density at physiological pH. This structure makes the polymer an effective ‘proton pump’ under virtually any pH, enabling osmotic swelling and rupture of endosomes and enhancing the release of DNA from the endosomal complex within cells. Due to this path, endosomes containing PEI avoid trafficking to degradative lysosomes. In addition, PEI complexes with DNA and RNA in nanometer range nanosize complexes, thereby enhancing the delivery of these nucleic acids into cells. Despite these encouraging results, PEI continues to be plagued by persistent problems such as low in vitro and in vivo transfection efficiency and strong cytotoxicity [8].
In this work, we have designed histidine-based peptide linked PEI polymers for transfection studies [9]. The pH buffering effects were achieved by addition of the co-polymers composed of histidine and lysine, which are also highly efficient carriers of plasmids. Peptide-based polymers are more advantageous than PEI-based polymers because they are easily metabolized and non-toxic to cells, making different combinations possible. In order to test the efficacy of these polymers as transfection agents, multiple cell lines and primary human cells were used. The primary human cell types used in this study were adipose stromal cells (ASCs), dermal fibroblasts, and cardiac progenitor cells (CPCs), all of which are promising candidates for autologous tissue transplantation. Because of their human origin, these three cell types have high clinical relevance. ASCs and fibroblasts in particular are plentiful and easy to obtain from patients. Both these cells types have recently been targeted for delivery of pluripotent transgenes for the generation of induced pluripotent stem cells [10, 11]. Cardiac progenitor cells, on the other hand, are an adult stem cell population that can give rise to all the cell types that recapitulate the heart [12] and therefore have a strong potential to be used for cardiac repair and regeneration following myocardial infarction. In the present study, we have tested our hypothesis that PEI based polymer hybrids can transfect primary cells at higher efficiencies than other existing non-viral methods. We have also examined ROS production, microarray based gene expression profiling and mechanistic properties that might be associated with cell survival and the transfection efficiency of these modified polymers. We report and discuss the results of these studies here, which we believe to yield significant advancements to gene delivery and in vivo stem cell therapy for clinical applications.
2. Materials and Methods
2.1. Chemicals
Polyethylenimine 25 kDa (PEI) was purchased from Sigma–Aldrich (St Louis, MO), DNase I from Qiagen (Valencia, CA), and fetal calf serum (FCS) from Invitrogen (Carlsbad, CA). All other chemicals were of reagent grade.
2.2. DNA vectors used
Two vectors were used for transfection studies. The first was a 12 kb vector containing firefly luciferase (Fluc) gene driven by CMV promoter. Transfection of this vector into cells was monitored by luciferase assay. The second vector was a 3.5 kb pMAX-GFP vector expressing green fluorescent protein under the CMV promoter. Transfection with this vector into cells was monitored by flow cytometry. Both of the vectors were amplified in the DH5α strain of E. coli and purified by using a Qiagen Maxi kit as per the manufacturer’s instructions.
2.3. Polymer synthesis
The polymers were synthesized in the BioADD facility at Stanford University. The sequences of polymers were as follows: (i) PEI-Arg; (ii) PEI-His; (iii) GHK-PEI; (iv) (t-Boc)2-GHK-PEI; (v) L-Carnosine-PEI; (vi) t-Boc-Carnosine-PEI; (vii) β-Ala-His-GHK-PEI, which is a combined peptide of L-Carnosine and GHK; and (viii) t-Boc-TAT-PEI, whereby TAT (GRKKRRQRRRPQK) is a cell permeable peptide. Details of polymer/peptide synthesis, purification and characterization are available in Supplementary Methods.
2.4. Transmission electron microscopy (TEM)
Peptide-PEI and peptide-PEI / DNA samples were adsorbed onto a Formvar-coated, carbon-stabilized copper grid (400 mesh). The grid was then rinsed briefly with water, negatively stained with 2% aqueous uranyl acetate, air-dried, and examined with a JEOL JEM 1230 transmission electron microscope at an accelerating voltage of 80 kV.
2.5. DNase protection assay
The integrity of the plasmid DNA released from the polymer/DNA complexes after exposure to DNase I was assessed by agarose gel electrophoresis as previously described [13]. Briefly, the complex was prepared by adding 2 µl DNA (500 ng/µl) to 6 µl of HBS (HEPES 10 mM and NaCl 150 mM; pH 7.3). This complex was then mixed with 50 µl polymer, incubated at room temperature for 20 min, and 6 µl of a solution containing MgCl2 (35 mM) and MnCl2 (35 mM) was added to the complex. Naked DNA samples were also prepared by replacing polymer with the same volume of water. Afterwards, 20 µl of complex or naked DNA sample was incubated with 2.2 µl DNase I (2.5 U/µl) or water at 37°C for 20 min. Degradation was stopped by adding 2.1 µl EDTA (250 mM) and placing the samples immediately in an ice bath. To release DNA from the complex for agarose gel electrophoresis, 2.2 µl heparin (200 mg/ml) was added and incubated for 10 min. Post incubation, 30 µl of the complex or naked DNA was loaded in a 1.2% agarose gel prepared in 1X TAE buffer (pH 8). Ethidium Bromide (0.5 µg/ml) was used for detection of DNA in the gel. The gel was run at 5V/cm.
2.6. Cell culture conditions
The transformed human embryonic kidney cell line HEK 293FT (Invitrogen) and the immortal cervical cancer cell line HeLa (American Type Culture Collection) were used for the standardization of optimum polymer concentration. The primary human cells used were adipose stromal cells (ASCs), human skin fibroblasts, and cardiac progenitor cells (CPCs) as described in Supplemental Methods. HEK 293FT cells, ASCs, fibroblasts, and HeLa were cultured in DMEM (Sigma Aldrich) supplemented with 10% fetal bovine serum (Invitrogen) and antibiotics (Invitrogen) under standard tissue culture conditions of 37°C and 5% CO2. All the studies were carried out in 24-well plates (Greiner Bio-One). Human CPCs were cultured in a specialized media, EGM-2, which was prepared as per a previous report [14], and supplemented with serum (Gibco) and defined factors (Lonza Biosciences). In short, the cells were thawed, resuspended in the specialized media and seeded on a 24-well plate coated with 0.1% gelatin. Media was changed every 3–4 days. Transfections were carried out 24 hours post seeding.
2.7. Transfection assays
Equal numbers of cells were seeded per well in 24-well plates 24 hours prior to transfection. Briefly, for each well, 0.8 µg of DNA encoding for firefly luciferase was added to 50 µl of DMEM + 10% FBS in a 1.5 ml tube. The calculated volume of polymer stock (resulting in 6 µg, 20 µg, 50 µg, or 100 µg final quantity of polymer) was added to the DNA-media mix. The cocktail was incubated at room temperature for 8 minutes after which 450 µl of DMEM + 10% FBS was added. The media present in the well was removed and the DNA-polymer cocktail was added drop wise to the cells. The plate was incubated for 2 hours at 37°C and 5% CO2. After 2 hours, the cells were washed with PBS followed by addition of fresh media (DMEM + 10% FBS). Analyses of cell survival and transfection efficiency were done at 24 and 48 hours post transfection. The control lipofectamine transfection was carried out exactly as per the manufacturer’s protocol (Invitrogen). Cell survival was studied using trypan blue dye exclusion analysis. For 6-well and 96-well plates, the quantity of DNA and polymers were adjusted accordingly.
2.8. Bioluminescence studies
Bioluminescence imaging of the 24-well plates containing HEK 293FT cells transfected with Fluc reporter gene was carried out on the Xenogen IVIS System (Alameda, CA) with 30 seconds of exposure and medium binning. The signal was quantified using the ROI analysis tools of Living Image software. Analysis of luciferase activity was done 48 hours post transfection using the Promega Luciferase Assay kit (E1910) as per the instructions of the manufacturer. Briefly, cells were washed in PBS 3 times, followed by cell lysis using the ‘Passive Lysis Buffer’ (Promega). 10 µl of the lysate was used to measure firefly luciferase activity. The same lysate was used to estimate total protein in each sample using the standard BCA protein assay. Bovine serum albumin (BSA) was used for the protein standard plot. Luciferase activity of each sample was normalized to the total protein content per sample. This normalized value was plotted in Excel.
2.9. Fluorescence measurements
Transfection with pMAX-GFP was done in a 6-well tissue culture plate, and immunoflorescence measurement was done using flow cytometry. 48 hours post transfection, cells in the 6-well plate were dissociated with trypsin, washed with PBS, and the pellet was resuspended in 300 µl of PBS. GFP signal intensity was measured on the FACSVantage (BD Biosciences) on FL1 channel (Excitation: 488nm; Emission: 500–520 nm). 20,000 and 50,000 events were recorded for analysis. Data analysis was performed on FlowJo software (BD Biosciences) and the ‘Mean Flourescence Intensity’ (MFI) value as obtained from the FL1 histograms was plotted on excel.
2.10. Measurement of ROS levels
ROS levels within cells were measured 24 hours post transfection with the hybrid polymers, using the fluorescence indicator dye, carboxy-DCFDA (Invitrogen). Cells were incubated with 10 µM DCFDA at 37°C for 30 mins. This was followed by two washes with phosphate buffered saline. ROS level was measured in a fluorescence plate reader by recording the emission at 510 nm.
2.11. Microarray hybridization and data analysis
Total RNA samples isolated using Qiagen mRNAeasy kit were hybridized to Affymetrix GeneChip Human Gene 1.0 ST Array, and then normalized and annotated by the Affymetrix® Expression Console™ software. The Pearson Correlation Coefficient was calculated for each pair of samples using the expression level of the top 5000 genes showing greatest variance. For hierarchical clustering, Pearson correlation for average linkage clustering was used. Genes with expression fold change greater than 2 were selected, and the significant pathways analysis was done by Ingenuity Pathway Analysis software (Ingenuity Systems; www.ingenuity.com). The enriched pathways were selected based on the p-value cutoff of <0.05.
2.12. Statistical analysis
For the firefly luciferase and GFP assays post transfection and cell survival assay, paired one-tailed t-test was used to compare the statistical significance of the differences between two transfection groups at a time. Graph Pad Prism software was used for analysis. * represents p values < 0.05; ** (p< 0.005); *** (p<0.0005).
3. RESULTS
3.1. Characterization of the primary structure of peptide-PEI polymers
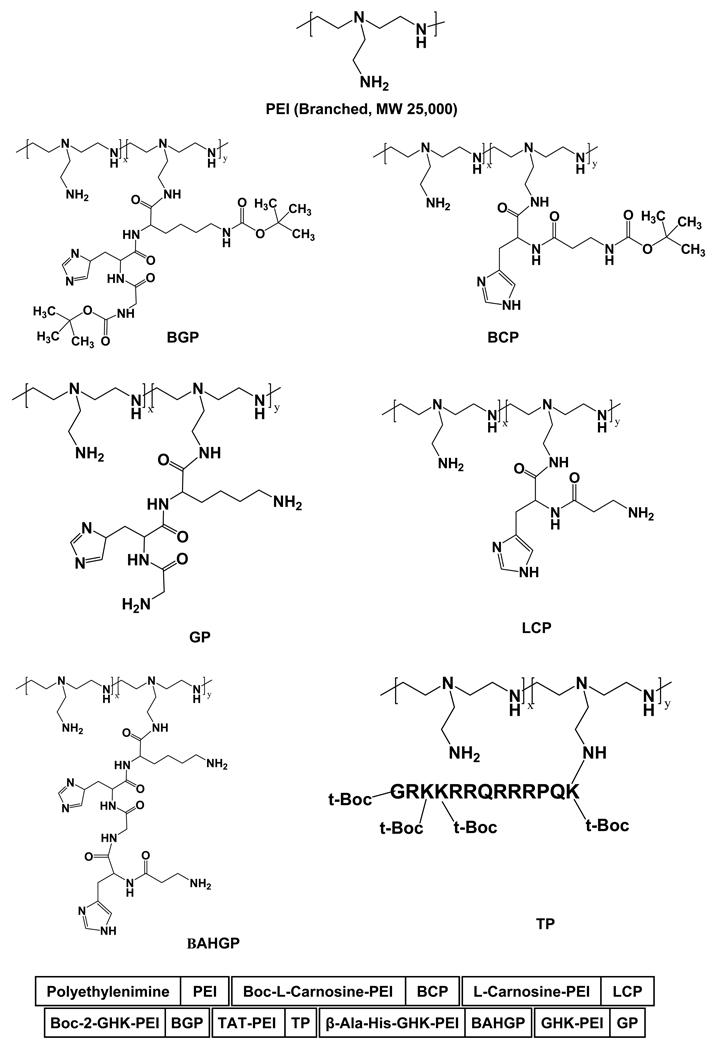
To synthesize peptide-PEI hybrid polymers, peptide moieties were linked with the primary amine groups of PEI (Fig. 1). To confirm the incorporation of the peptides in the PEI backbone, the tertiary butyl groups of the peptides were monitored using 1H-NMR spectra of the polymers (Suppl Fig. 1). Protons for Boc group are generally observed as a singlet between 1.3–1.5 ppm region and the PEI backbone is observed as multiplet between 2.3 and 2.8 ppm. From these results, the number of peptide chains grafted onto a PEI backbone was calculated based on the relative integration values between the single CH3- proton peak in Boc (s, ~1.35 ppm) and the multiple -CH2- proton peaks in PEI (m, ~2.49 ppm). According to the 1H-NMR results, the average number of conjugated peptide was 0.1 parts (~10%) per PEI backbone.
Fig. 1.
Schematic diagram of the structures of the basic polymer PEI (top) and the peptide-PEI based polymers described in the current study. The peptide groups were linked to the primary amine group of PEI.
3.2. Structure and function characterization of the peptide-PEI-DNA complex
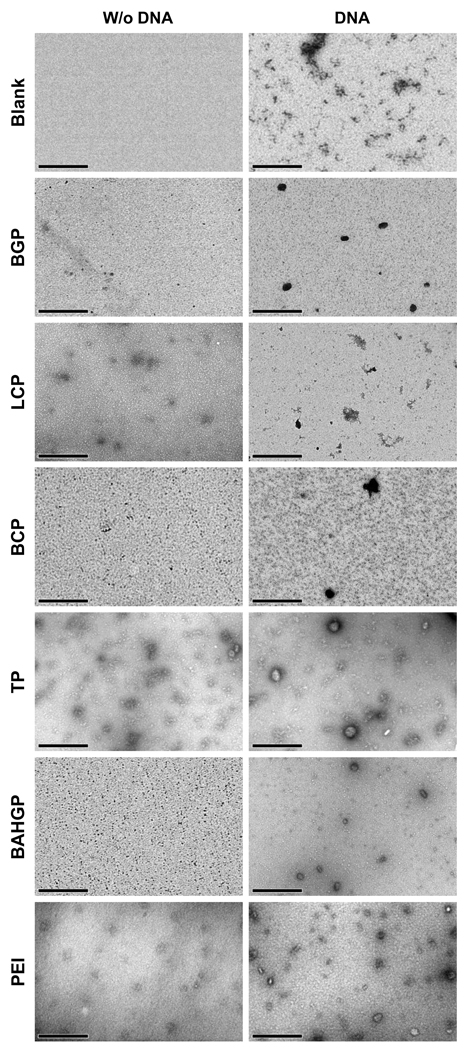
Transmission electron microscopy (TEM) was used to reveal the microstructure of the peptide-PEI hybrid polymers, and the effect of DNA conjugation on this microstructure. TEM micro graphs indicate that the PEI peptides preparations contain distinct, separated aggregates with a mean size (diameter) of roughly 10–20 nm and PEI peptide – DNA complex nanogels size range varied from 10 to 80 nm, out of which ~5–15% of the nanogels appeared to be 40 nm or more (Fig. 2). In addition, AFM imaging indicated that peptide-PEI samples form smaller aggregates compared to DNA conjugated samples (Supp Fig. 2). However, fibril formation was also seen in these samples, which can be clearly visualized in the phase images (Supp Fig. 2A and C; right panel). This fibril formation was more prevalent in Carnosine-PEI compared to the Boc2-PEI (Supp Fig. 2A and C). For the DNA-conjugated samples, the microstructure of the particles was less spherical and more plate-like (Supp Fig. 2B and D). Moreover, when compared to samples without DNA, less particle aggregation was observed in the DNA tethered samples, with absolutely no fibril formation. Such dispersion effect on polymers due to DNA binding has been observed earlier [15].
Fig. 2.
Transmission electron microscopy (TEM) images of polymer alone (left panel) and polymer-DNA complex (right panel) for all the polymers. Scale bar represents 500 nm.
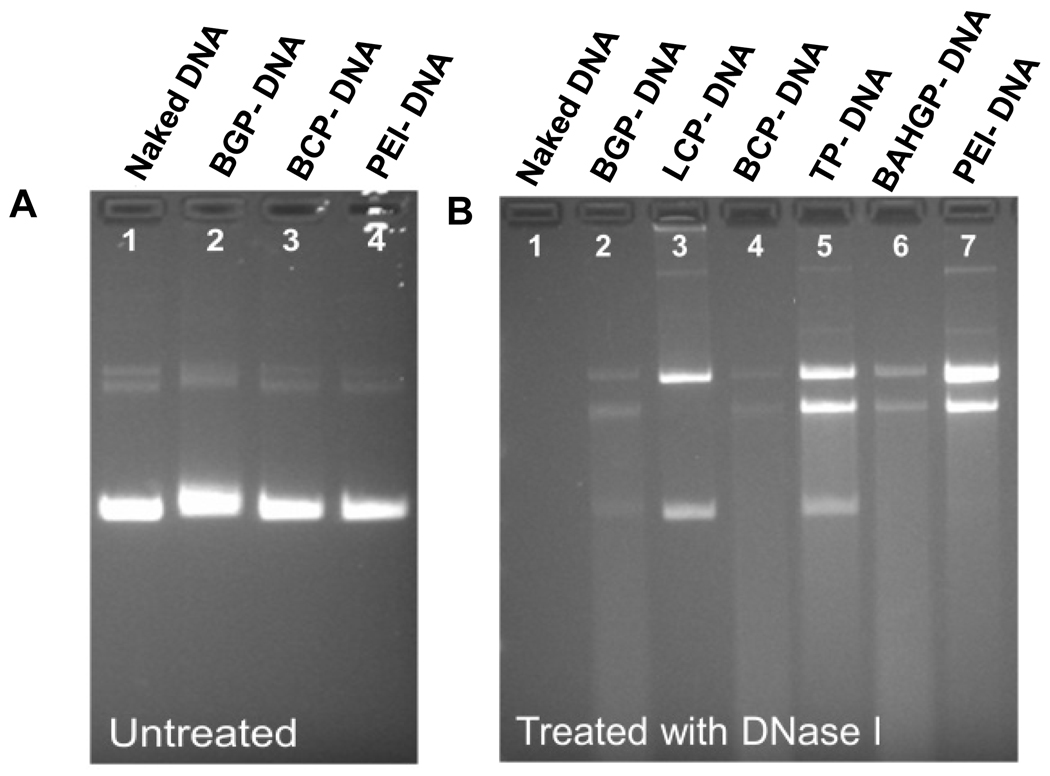
We next studied the functional significance of the peptide-polymer hybrid binding to DNA by a DNase protection assay. In our pilot study, the DNA samples mixed with polymers could not be detected by ethidium bromide staining followed by agarose gel electrophoresis, whereas naked DNA could be visualized (data not shown). This was seen in the absence of heparin. However, addition of heparin at the final concentration of 1.7% (w/v) to the polymer-DNA samples effectively released the DNA from the polymers Boc-2-GHK-PEI, Boc-L-Carnosine-PEI, and PEI, and a comparable intensity in electrophoretic bands was observed when compared to naked DNA (Fig. 3A). In order to evaluate the potential protective effects of the polymers, naked DNA and DNA-polymer complexes were incubated with DNase I for 20 mins. This treatment degraded naked DNA almost completely, as indicated by the lack of band in the agarose gel (Fig. 3B, lane 1). In contrast, complexing DNA with PEI served as ‘protection’ from DNase digestion (Fig. 3B, lane 7). Similar protection was observed in the complexes formed by TAT-PEI and carnosine-PEI. Other polymers provided relatively less potent but discernable protection (Fig. 3B).
Fig. 3.
Polymer protection of DNA from degradation. (A) Addition of heparin in the samples effectively released DNA from polymers thus enabling DNA to be visualized by Ethidium Bromide staining. (B) In the presence of DNase I, all polymers showed some degree of protection of DNA from degradation, while the naked DNA was completely digested (lane 1).
3.2. Determination of optimum concentration for activity of polymers
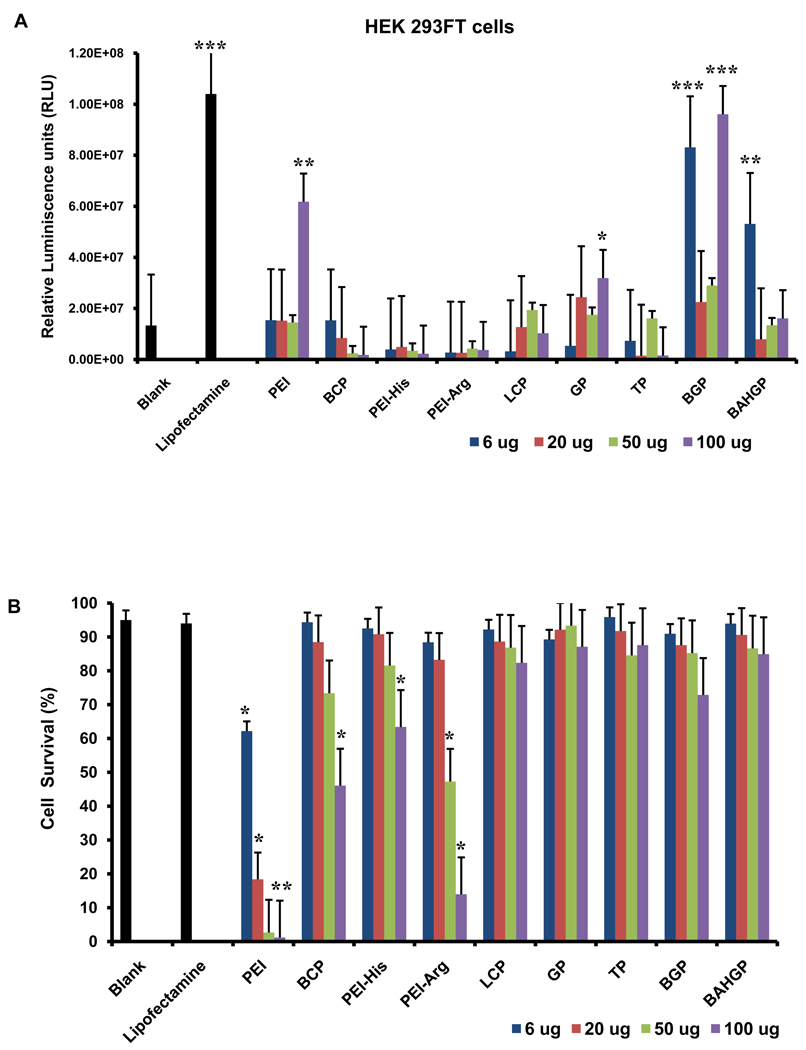
To test the efficacy of the polymers for transfection, synthesized DNA complexes were first used to transfect the human embryonic kidney cell line (HEK 293FT), a standard cell type regularly used for transfection studies. A plasmid DNA containing the firefly luciferase reporter gene was used. Transfection of cells with the plasmid-polymer complex was carried out as described in the Materials and Methods section. In order to determine the optimum concentration to be used for the polymers, four different concentrations were tested for all polymers using a constant quantity of plasmid DNA (0.8 µg). A commercially available transfection reagent, Lipofectamine, was used as a positive control. Bioluminiscence imaging was used to assess transfection efficiency. A significant number of polymers showed positive bioluminescence signal at one or more of the different concentrations (Fig. 4A). PEI-Histidine and PEI-Arginine failed to show positive signals at any concentrations.
Fig. 4.
Determination of the optimum concentration of the polymers to be used for transfection. (A) Transformed human embryonic kidney (HEK 293FT) cells were transfected with a luciferase-encoding vector in the presence of four different concentrations of the polymers such that luminescence intensity was used as a readout of transfection. Lipofectamine was used as the positive control. (B) Cell death was assessed for each concentration for all the polymers using trypan blue assay and cell survival was plotted as a percentage. Each group was compared to the blank to analyze the statistical differences observed in the relative luminescence and/or cell survival. Error bars represent triplicates with standard deviation (* p<0.05; ** p<0.005; *** p<0.0005).
Cell viability was used as a second parameter to determine the optimum concentration to be used for each polymer. Trypan blue based analysis of cell death indicated that all the modified hybrid polymers demonstrated high cell survival when compared to the parent polymer, PEI (Fig. 4B). The cell survival in presence of the modified polymers was similar to the positive control. This study also indicated that cell survival was minimal at 100 µg (the highest concentration) for almost all polymers (Fig. 4B). Based on the bioluminescence and cell survival data, six polymers were selected (GHK-PEI, Boc-2-GHK-PEI, L-Carnosine-PEI, Boc-L-Carnosine-PEI, β-Ala-His-GHK-PEI, TAT-PEI) at the concentration of minimum cell death and maximum transfection efficiency. Based on these experiments, the concentrations of polymers to be used for 0.8 µg of DNA which was finalized for all further experiments are as follows: GHK-PEI(20 µg); Boc-2-GHK-PEI (6 µg); L-Carnosine-PEI (50 µg); Boc-L-Carnosine-PEI (6 µg); β-Ala-His-PEI (6 µg), and TAT-PEI (50 µg).
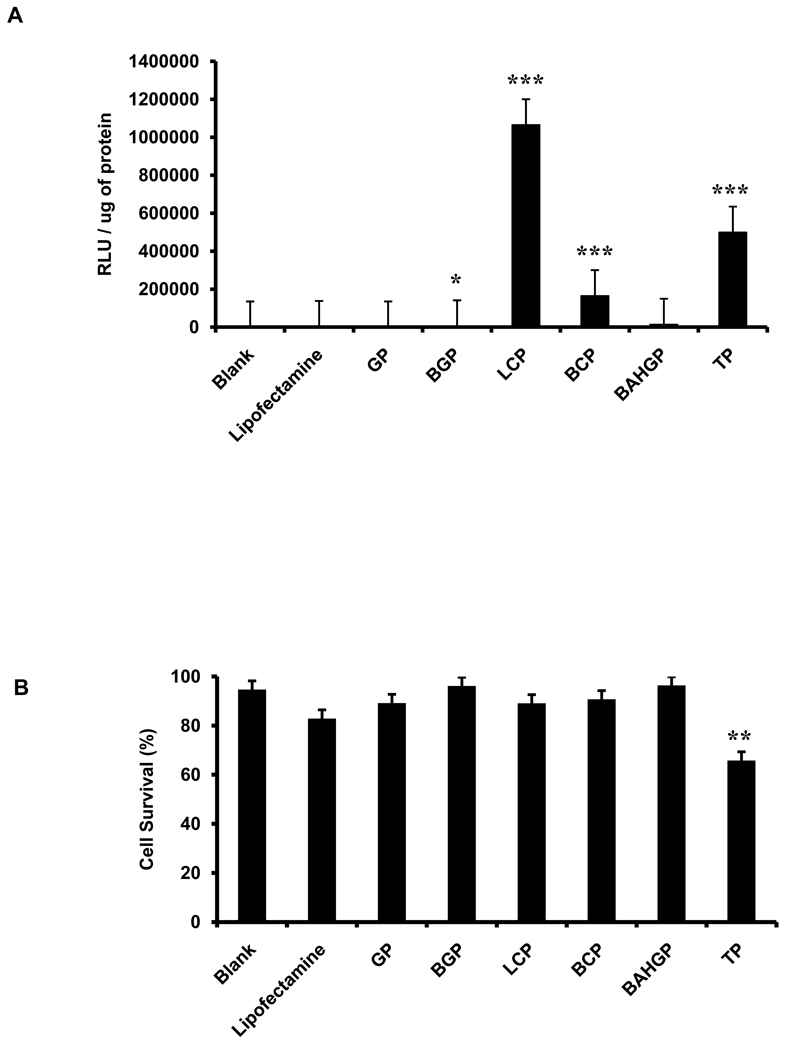
3.3. Transfection of primary human adipose stromal cells (ASCs) using screened polymers
The selected polymers were used at their optimum concentrations to transfect primary ASCs. Primary cells have high clinical relevance for both cell and gene therapy. However, these cells are highly resistant to transfection when compared to transformed cell lines such as HEK 293FT [16]. An additional problem with primary cells is high cell death associated with transfection. The same plasmid used to transfect the HEK 293FT cells was used to transfect the ASCs. Lipofectamine was used as the positive control. 24 hours post transfection with the polymers, cell morphology and any associated cell death were studied under a phase contrast microscope (Supp Fig. 3A). Luciferase assay of the cells conducted 24 hours post transfection demonstrated higher transfection efficiency in cells incubated with the polymers as compared with lipofectamine (Fig. 5A). Trypan blue analysis of cell survival was carried out at 48 hours post transfection. This demonstrated that in presence of five polymers, namely GHK-PEI, Boc-2-GHK-PEI, L-Carnosine-PEI, Boc-L-Carnosine-PEI and β-Ala-His-GHK-PEI, cell survival was comparable to the positive control (lipofectamine), and there was no significant difference in cell survival among the different groups except TAT-PEI (Fig. 5B).
Fig. 5.
Testing the transfection efficiency of selected polymers at their optimum concentration on human adipose stromal cells (ASCs). (A) Transfection efficiency was assessed by quantitative luciferase assay, with the signal intensity normalized to the total protein (* p<0.05; ** p<0.005; *** p<0.0005). (B) Cell death was assessed by trypan blue staining and represented as % cell survival. Each polymer group was individually compared to the blank to analyze the statistical differences observed in the relative luminescence as well as cell survival. Error bars represent triplicates with standard deviation.
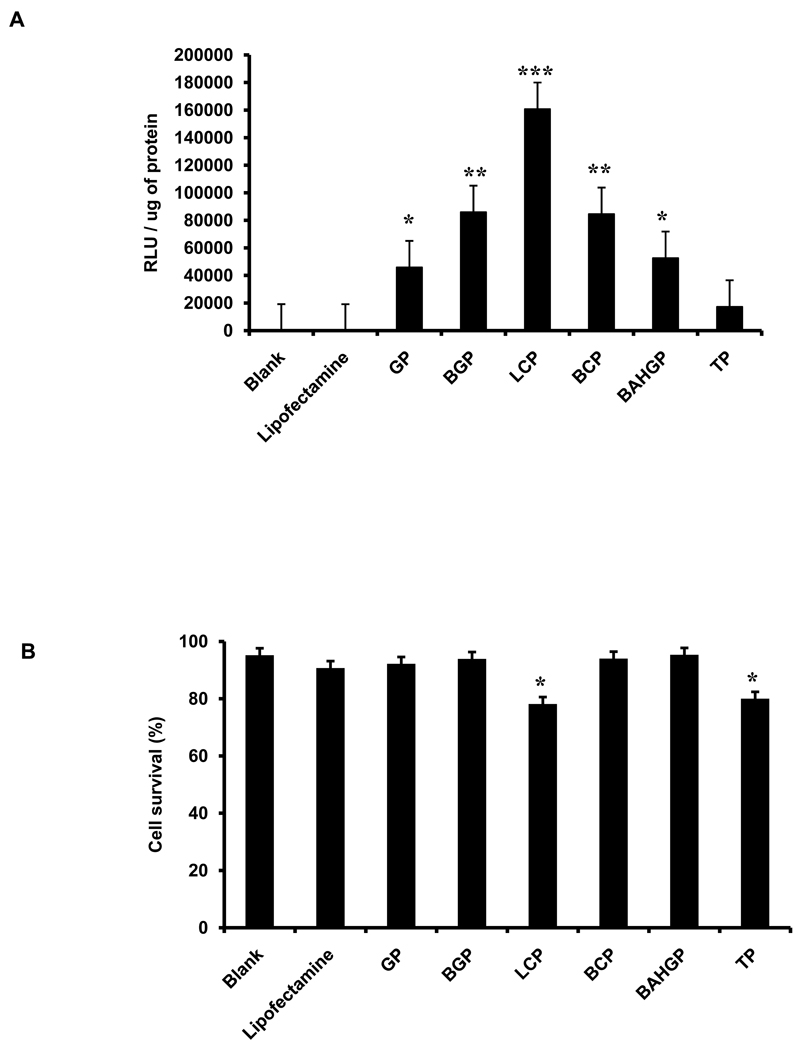
3.4. Transfection of human dermal fibroblasts with the screened polymers
In order to determine the efficiency of the polymers in a wide range of cell types, we next tested the same set of six polymers upon primary human dermal fibroblasts isolated from patients as described in the Supplemental Methods section. Photomicrographs of the transfected cells 24 hours post transfection as shown in Supp Fig. 3B. Similar to ASCs, the polymers showed significantly higher transfection efficiency in fibroblasts when compared to lipofectamine as assessed by luciferase assay of the cells 24 hours post transfection (Fig. 6A). Cell survival in presence of the polymers was also similar to that of the positive control lipofectamine (Fig. 6B), except for L-Carnosine-PEI, which demonstrated lower cell survival when compared to the other polymers for this specific cell type.
Fig. 6.
Testing the transfection efficiency of selected polymers at their optimum concentration on human dermal fibroblasts. (A) Transfection efficiency was assessed by quantitative luciferase assay, with the signal intensity normalized to the total protein (* p<0.05; ** p<0.005; *** p<0.0005). (B) Cell death was assessed by trypan blue staining and represented as % cell survival. Each polymer group was individually compared to the blank to analyze the statistical differences observed in the relative luminescence as well as cell survival. Error bars represent triplicates with standard deviation.
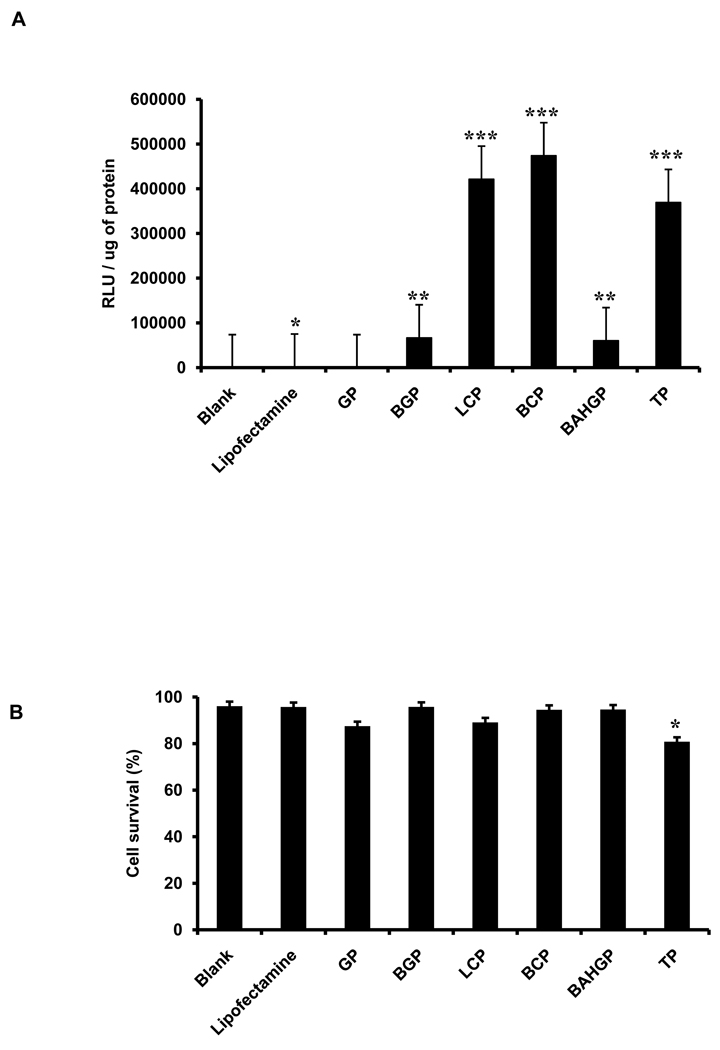
3.5. Transfection of human cardiac progenitor cells (CPCs) with screened polymers
Human CPCs were used as a third human cell type to validate the efficiency of the PEI-derived polymers. Human CPCs give rise to all the functional cell types of the heart and therefore have very high clinical relevance. One of the paramount goals in clinical cardiology is to successfully perform gene therapy of cardiac cells in order to correct genetic mutations that underlie multiple intractable cardiac diseases such as dilated and hypertrophic cardiomyopathies [17]. Polymer-based transfections using the firefly luciferase reporter gene demonstrated significantly higher transfection efficiency in these cells when compared to positive control (lipofectamine) as assessed by a quantitative luciferase assay (Fig. 7A). Importantly, cell survival in presence of the polymers was similar to lipofectamine (Fig. 7B).
Fig. 7.
Testing the transfection efficiency of selected polymers at their optimum concentration on human cardiac progenitor cells (CPCs). (A) Transfection efficiency was assessed by quantitative luciferase assay, with the signal intensity normalized to the total protein (* p<0.05; ** p<0.005; *** p<0.0005). (B) Cell death was assessed by trypan blue staining and represented as % cell survival. Each polymer group was individually compared to the blank to analyze the statistical differences observed in the relative luminescence as well as cell survival. Error bars represent triplicates with standard deviation.
3.6. Transfection of primary human ASCs and CPCs with GFP-expressing vector using L-Carnosine and Boc-L-Carnosine
Based upon the transfection efficiencies of all 6 polymers tested for human ASCs, dermal fibroblasts, and CPCs, the two polymers with the highest overall transfection efficiencies were determined to be L-Carnosine-PEI and its Boc derivative. To confirm the potential of these two polymers to transfect primary stem cells at high efficiencies, we applied L-Carnosine-PEI (50 µg for 0.8 µg DNA) and its Boc derivative (6 µg for 0.8 µg DNA) upon human ASCs and CPCs using a different vector encoding for GFP only. Flow cytometry based fluorescence intensity measurements were used as a readout of transfection efficiency using this vector. Fluorescence intensity values of the samples confirmed the high transfection efficiencies observed in the luciferase assay measurements conducted previously (Supp Fig. 4B and E). In both cell types, GFP signal was significantly higher for L-Carnosine-PEI and Boc-L-Carnosine-PEI than for the commercially available agent lipofectamine. These results validate our earlier observation that the modified-PEI polymers can serve as highly efficient gene delivery agents for primary human cell types, which are otherwise difficult to transfect. Finally, we compared transfection efficiency of the GFP reporter gene in the HeLa cell line using L-Carnosine-PEI, Boc-L-Carnosine-PEI, and lipofectamine. Similarly, we found that L-Carnosine-PEI and Boc-L-Carnosine-PEI were characterized by higher transfection efficiencies as compared to lipofectamine (Supp Fig. 5B).
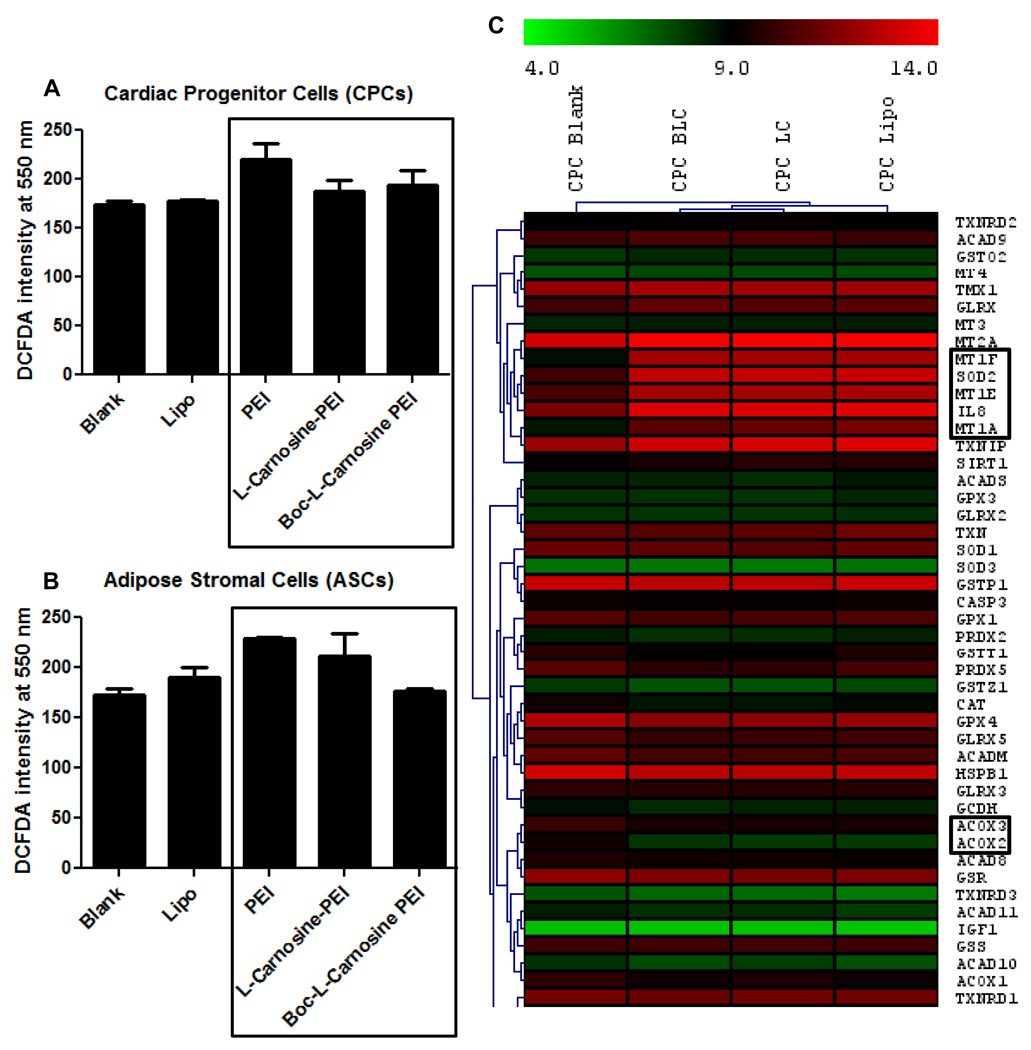
3.7. Analysis of oxidative stress in cells transfected with the modified polymers
One of the major problems with PEI-based transfections has been the high cell toxicity resulting in cell death and low efficiency of gene delivery. Modification of PEI by peptide conjugation might reduce the oxidative damage within the cells, thereby resulting in higher cell survival and high transfection efficiency. In order to address this, we compared the level of ‘reactive oxygen species’ (ROS) within cells transfected with PEI and the hybrid polymers. DCFDA based fluorescence measurements indicated that ROS levels were significantly lower in cells transfected with the hybrid polymers as compared to PEI (Fig. 8A and B). This was confirmed independently in two primary cell types, adipose stromal cells and cardiac progenitor cells.
Fig. 8.
Quantification of intracellular ROS levels by DCFDA staining 24 hours post transfection with PEI and the modified, hybrid-PEI polymers. ROS level was assessed for (A) adipose stromal cells (ASCs) and (B) cardiac progenitor cells (CPCs). Lipofectamine was used as the positive control. Boxed region indicates the comparison of ROS levels in PEI versus the modified polymers. Error bars represent triplicates with standard deviation (* p<0.05). (C) Hierarchical clustering of microarray based gene expression data of cardiac progenitor cells transfected with L-Carnosine-PEI and Boc-L-Carnosine-PEI. Genes showing differential regulation with respect to the control (‘Blank’) have been highlighted in boxes.
We next carried out microarray based global gene expression profiling of cardiac progenitor cells transfected with L-Carnosine-PEI and Boc-L-carnosine-PEI to study the genes and pathways involved in regulating oxidative stress under these conditions when compared to untreated CPCs (‘Blank’) and the positive control (‘Lipofectamine’). A set of genes known to be involved in mitochondrial functions, glucose and fatty acid metabolism, and those regulating oxidative stress were shortlisted and their expression profile was analyzed by hierarchical clustering of the gene sets (Fig. 8C). Genes encoding enzymes which are typically involved in reducing oxidative stress like superoxide dismutase and metallothioneins were found to be upregulated in cells transfected with L-Carnosine-PEI and Boc-L-carnosine-PEI when compared to the blank, shown in boxes in the cluster diagram (Fig. 8C). Some of the interleukins, which are released by cells in response to external stimuli, were also found to be upregulated. In addition, some of the pathways activated to enhance survival under stress were found to be upregulated in the two transfected groups as determined by Ingenuity Pathway Analysis (Supplementary Tables 1 and 2).
4. DISCUSSION
The present study focused on the development of a set of transfection agents for efficient gene delivery into cells, which is necessary for successful genetic modification of stem cells to take place. The polymers described here have been derived by chemical modification of the 25kDa basic cationic molecule, polyethylenimine (PEI), with distinct side groups, namely L-Carnosine, GHK, Boc, β-Ala-His and TAT. Transfection of the human embryonic kidney cell line (HEK 293FT) with the modified polymers demonstrated high transfection efficiency as well as enhanced cell survival when compared to PEI. When further tested on three human primary cell types (fibroblasts, ASCs, and CPCs), which are known to be highly resistant to transfection, the modified PEI polymers could transfect these cells with high efficiency. These polymers include GHK-PEI, Boc-2-GHK-PEI, L-Carnosine-PEI, Boc-L-Carnosine-PEI, β-Ala-His-GHK-PEI, and TAT-PEI. An additional advantage of using these polymers is that all transfections are carried out in the same medium in which cells are grown in the presence of serum and antibiotics. This eliminates the need to change medium before, during, or post transfection and therefore highly increases the ease of transfection.
Transfection with the basic molecule, PEI, resulted in very high cell death as assessed by trypan blue based cell viability assays (Fig. 4B) even at the lowest concentrations tested. These results support findings by other studies that show high cytotoxicity is one of the biggest drawbacks of PEI-based transfections [7]. In contrast, most of the modified PEI compounds demonstrated very low cell toxicity (Fig. 4B). For some of the polymers such as Boc-L-Carnosine-PEI, PEI, and PEI-Arg, higher levels of cell death was observed with increasing concentration levels, whereas for others such as GHK-PEI, L-Carnosine-PEI, and β-Ala-His-GHK-PEI, differences in cell death at different concentrations were minimal. Overall, trypan blue based cell viability analysis clearly indicated that chemical modification of PEI significantly reduces cellular toxicity.
In our study, a subset of the eight polymers was chosen (in addition to PEI as control) as especially promising for clinical applications based on two parameters: (1) transfection efficiency and (2) cell survival in the presence of these polymers. Based on these two criteria, two of the polymers (PEI-Histidine and PEI-Arginine) were excluded for further analysis as they demonstrated negligible luciferase activity at all concentrations. The six remaining polymers demonstrated significantly strong luciferase activity at one or more concentrations. The control PEI was excluded due to its high cell toxicity even when used at low concentrations (Fig. 4B). Overall, our findings are concordant to the report of Hashemi et al. [18] who elegantly demonstrated the effectiveness of KH peptides-linked PEI complexes as gene carriers and the significant enhancement of gene delivery by these modified polymers when compared to PEI alone.
The ultimate goal of all gene and cell therapies is application in a clinical setting. We therefore tested the efficiency of these polymers to deliver plasmid DNA into primary human cells, which are known to be significantly more difficult to transfect than immortalized cell lines. Lipofectamine, a widely used transfection agent was used as the positive control. To this end, we selected three cell types relevant to regenerative medicine and clinical research; namely adipose stromal cells [19], fibroblasts [20], and cardiac progenitor cells [21]. Because these cells are known to be difficult to transfect, a sensitive luciferase assay with normalization to total protein content was used as readout of transfection efficiency. Cell death was measured by trypan blue staining. Similar to the results seen in the HEK 293FT cells, the six shortlisted polymers (GHKPEI, Boc2-GHK-PEI, L-Carnosine-PEI, Boc-L-Carnosine-PEI, β-Ala-His-GHK-PEI and TAT-PEI) demonstrated robust cell survival, which was comparable to the positive control (lipofectamine). Morphologically, most of the hybrid polymer transfected groups were similar to the blank control (Supp Fig. 3). Exceptions were TAT-PEI and L-Carnosine-PEI, where clustering and shrinkage of cells was seen. One of the reasons for this could be the high concentration of the two polymers that was used for transfection (50 µg for 0.8 µg DNA).
Analysis of luciferase activity showed significantly high transfection efficiency in the hybrid polymer-transfected primary cells as compared to lipofectamine. Different polymers demonstrated selective propensities to transfect certain cell types. For example, TAT-PEI showed higher transfection efficiency in ASCs compared to fibroblasts, whereas β-Ala-His-GHK-PEI showed higher transfection in fibroblasts compared to ASCs. This may reflect the differences in cell surface receptors on different cell types (which is exploited by the TAT-PEI system) or differences in intracellular metabolism of chemical moieties (which might affect the release of DNA from the β-Ala-His-GHK-PEI complex within different cells).
Comparison of the six polymers chosen for further analysis revealed two polymers to be the most promising as demonstrated by consistently high transfection efficiencies in all cell types: L-Carnosine-PEI and Boc-L-Carnosine-PEI. L-Carnosine is a dipeptide of β-Alanine and histidine, and is an endogenous peptide present in excitable tissues like the brain and muscles where it has been shown to reduce endoplasmic reticulum (ER) stress [22]. L-carnosine acts as an antioxidant by scavenging hydroxyl radicals, binding divalent metals, upregulating enzymes that metabolize free radicals, and by promoting antiglycation [23, 24]. As a result, carnosine has been used as a protective agent in stroke models [25], liver injuries [26], and ophthalmologic disorders [27].
We hypothesized that one of the major reasons of the toxicity of PEI as a transfection agent could be due to PEI-mediated oxidation of the Cu2+ and Fe2+ moieties of intracellular enzymes, which results in production of hydroxyl radicals, consequently leading to enzymatic inactivation. Analysis of oxidative stress in transfected cells by DCFDA staining demonstrated reduced levels of ROS in the presence of hybrid polymers when compared to PEI (Fig. 8A and B). Global gene expression profiling studies indicated the upregulation of multiple enzymes involved in regulating ROS and reducing oxidative stress, like superoxide dismutase and metallothioneins (Fig. 8C). Downregulation of Acyl Co-A oxidase in cells transfected with L-Carnosine-PEI and Boc-L-Carnosine-PEI might also contribute to the lowering of oxidative stress since this enzyme produces H2O2 as one of the end products. In addition, several pathways involved in mitochondrial functions and glucose and fatty acid metabolism were also found to be activated (Supplementary Tables 1 and 2). Activation of the PPAR pathway especially indicated activity of peroxisomes, the cellular organelles involved in scavenging ROS and reducing intracellular oxidative stress. Taken together, these mechanistic studies suggest that L-Carnosine-PEI and Boc-L-Carnosine-PEI are able to reduce oxidative damage in cells when compared to PEI. They do so by upregulation of multiple enzymes and pathways involved in scavenging free radicals and ROS and downregulating those which generate ROS and free radicals.
The altered structure of PEI might underlie the reduction in ROS levels. Introduction of the H-β-Ala-His (L-Carnosine) as a side chain in the PEI backbone might reduce the toxic effect of the large number of free available −NH2 groups in PEI. Interestingly, ROS levels reduce even further in the presence of Boc-L-Carnosine-PEI. This might be explained by the introduction of the tri-methyl containing ‘Boc’ group of Boc-L-Carnosine-PEI, which protects all the reactive amine groups of L-Carnosine-PEI. This further reduces the toxicity of PEI by blocking some and making the other −NH2 groups inaccessible for reaction with intracellular components. Protection of L-Carnosine-PEI with the Boc group significantly increased the effectiveness of this polymer in two ways. First, Boc-L-Carnosine-PEI potentiated transfection efficiency such that 8-fold less concentration of the polymer was required to induce maximal plasmid expression as compared to L-Carnosine-PEI alone (50 µg of L-Carnosine-PEI vs 6 µg of Boc-L-Carnosine-PEI for the same quantity of DNA). Second, cell death was much lower at this concentration of polymer. The combination of higher transfection efficiency with lower cell death results in a much higher number of live, positively transfected cells from Boc-L-Carnosine-PEI transfection compared to other agents.
In summary, our studies demonstrate that L- Carnosine (H-β-Ala-His) derivatives of PEI significantly enhance transfection efficiency of primary human cells, which are otherwise known to be highly resistant to transfection. Furthermore, modification of PEI with carnosine appears to reduce the cytotoxic effect of PEI by lowering the formation of ROS within cells by modulating the activity of multiple enzymes and pathways involved in metabolism of free radicals and reactive oxygen species. Protection of L-Carnosine-PEI with the ‘Boc’ group further enhances the antioxidant property of the hybrid polymer. For future studies, the concentration of the present formulation of L-Carnosine-PEI needs to be optimized so that this polymer can achieve the same efficiency at lower concentrations which will result in even higher cell survival.
5. CONCLUSIONS
In this study, a panel of polymers has been described which can improve gene delivery to primary human stem cells. We have demonstrated that peptide-linked PEI complexes can efficiently condense DNA into nano-aggregates. These polyplexes significantly reduce the toxic effects of the 25kDa PEI and demonstrate marked improvement in cell survival and transfection efficiency when compared to the polyplexes of linear PEI polymers. PEI linked with the dipeptide, L-Carnosine, and its derivative, Boc-L-Carnosine, appear to be the most promising gene delivery agents among all the complexes based on their reduced production of reactive oxygen species and markedly high transfection efficiency. Future research should focus on other important applications of these polymers such as potential for in vivo gene delivery in diseased patients, with the goal of a safe and effective clinical translation in the near future.
Supplementary Material
Acknowledgements
This research was supported by the Bio-X Program at Stanford (ASL), NIH HL095571, HL093172, and EB009689 (JCW). The authors would like to thank the Imaging Facility Center and the Flow Cytometry Facility at Stanford.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Persons DA. Gene therapy: Targeting beta-thalassaemia. Nature. 2010;467:277–278. doi: 10.1038/467277a. [DOI] [PubMed] [Google Scholar]
- 2.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 3.Glover DJ, Lipps HJ, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nat Rev Genet. 2005;6:299–310. doi: 10.1038/nrg1577. [DOI] [PubMed] [Google Scholar]
- 4.van der Woude I, Wagenaar A, Meekel AA, ter Beest MB, Ruiters MH, Engberts JB, et al. Novel pyridinium surfactants for efficient, nontoxic in vitro gene delivery. Proc Natl Acad Sci U S A. 1997;94:1160–1165. doi: 10.1073/pnas.94.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeifer A, Verma IM. Gene therapy: promises and problems. Annu Rev Genomics Hum Genet. 2001;2:177–211. doi: 10.1146/annurev.genom.2.1.177. [DOI] [PubMed] [Google Scholar]
- 6.Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schafer J, Hobel S, Bakowsky U, Aigner A. Liposome-polyethylenimine complexes for enhanced DNA and siRNA delivery. Biomaterials. 2010;31:6892–6900. doi: 10.1016/j.biomaterials.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 8.Brownlie A, Uchegbu IF, Schatzlein AG. PEI-based vesicle-polymer hybrid gene delivery system with improved biocompatibility. Int J Pharm. 2004;274:41–52. doi: 10.1016/j.ijpharm.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Beretta G, Artali R, Regazzoni L, Panigati M, Facino RM. Glycyl-histidyl-lysine (GHK) is a quencher of alpha,beta-4-hydroxy-trans-2-nonenal: a comparison with carnosine. insights into the mechanism of reaction by electrospray ionization mass spectrometry, 1H NMR, and computational techniques. Chem Res Toxicol. 2007;20:1309–1314. doi: 10.1021/tx700185s. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci U S A. 2009;106:15720–15725. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu SM, Chien KR, Mummery C. Origins and fates of cardiovascular progenitor cells. Cell. 2008;132:537–543. doi: 10.1016/j.cell.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moret I, Esteban Peris J, Guillem VM, Benet M, Revert F, Dasi F, et al. Stability of PEI-DNA and DOTAP-DNA complexes: effect of alkaline pH, heparin and serum. J Control Release. 2001;76:169–181. doi: 10.1016/s0168-3659(01)00415-1. [DOI] [PubMed] [Google Scholar]
- 14.Smits AM, van Vliet P, Metz CH, Korfage T, Sluijter JP, Doevendans PA, et al. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc. 2009;4:232–243. doi: 10.1038/nprot.2008.229. [DOI] [PubMed] [Google Scholar]
- 15.Zheng M, Jagota A, Semke ED, Diner BA, McLean RS, Lustig SR, et al. DNA-assisted dispersion and separation of carbon nanotubes. Nat Mater. 2003;2:338–342. doi: 10.1038/nmat877. [DOI] [PubMed] [Google Scholar]
- 16.Partridge KA, Oreffo ROC. Gene delivery in bone tissue engineering: progress and prospects using viral and nonviral strategies. Tissue Eng. 2004;10:295–307. doi: 10.1089/107632704322791934. [DOI] [PubMed] [Google Scholar]
- 17.Barry SP, Townsend PA. What causes a broken heart--molecular insights into heart failure. Int Rev Cell Mol Biol. 2010;284:113–179. doi: 10.1016/S1937-6448(10)84003-1. [DOI] [PubMed] [Google Scholar]
- 18.Hashemi M, Parhiz BH, Hatefi A, Ramezani M. Modified polyethyleneimine with histidinelysine short peptides as gene carrier. Cancer Gene Ther. 2011;18:12–19. doi: 10.1038/cgt.2010.57. [DOI] [PubMed] [Google Scholar]
- 19.Kim WS, Park BS, Sung JH. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin Biol Ther. 2009;9:879–887. doi: 10.1517/14712590903039684. [DOI] [PubMed] [Google Scholar]
- 20.Mathiasen AB, Haack-Sorensen M, Kastrup J. Mesenchymal stromal cells for cardiovascular repair: current status and future challenges. Future Cardiol. 2009;5:605–617. doi: 10.2217/fca.09.42. [DOI] [PubMed] [Google Scholar]
- 21.Gersh BJ, Simari RD, Behfar A, Terzic CM, Terzic A. Cardiac cell repair therapy: a clinical perspective. Mayo Clin Proc. 2009;84:876–892. doi: 10.4065/84.10.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh YM, Jang EH, Ko JH, Kang JH, Park CS, Han SB, et al. Inhibition of 6-hydroxydopamine-induced endoplasmic reticulum stress by l-carnosine in SH-SY5Y cells. Neurosci Lett. 2009;459:7–10. doi: 10.1016/j.neulet.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 23.Gariballa SE, Sinclair AJ. Carnosine: physiological properties and therapeutic potential. Age Ageing. 2000;29:207–210. doi: 10.1093/ageing/29.3.207. [DOI] [PubMed] [Google Scholar]
- 24.Aydin AF, Kusku-Kiraz Z, Dogru-Abbasoglu S, Uysal M. Effect of carnosine treatment on oxidative stress in serum, apoB-containing lipoproteins fraction and erythrocytes of aged rats. Pharmacol Rep. 2010;62:733–739. doi: 10.1016/s1734-1140(10)70331-5. [DOI] [PubMed] [Google Scholar]
- 25.Cheng J, Wang F, Yu DF, Wu PF, Chen JG. The cytotoxic mechanism of malondialdehyde and protective effect of carnosine via protein cross-linking/mitochondrial dysfunction/reactive oxygen species/MAPK pathway in neurons. Eur J Pharmacol. 2010 doi: 10.1016/j.ejphar.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 26.Artun BC, Kusku-Kiraz Z, Gulluoglu M, Cevikbas U, Kocak-Toker N, Uysal M. The effect of carnosine pretreatment on oxidative stress and hepatotoxicity in binge ethanol administered rats. Hum Exp Toxicol. 2010;29:659–665. doi: 10.1177/0960327109359460. [DOI] [PubMed] [Google Scholar]
- 27.Babizhayev MA, Yegorov YE. Telomere attrition in lens epithelial cells - a target for N-acetylcarnosine therapy. Front Biosci. 2010;15:934–956. doi: 10.2741/3655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.