Abstract
γδ T cell responses are induced by various viral and bacterial infections. Different γδ T cells contribute to activation and regulation of the inflammatory response and to epithelial repair. How γδ T cells respond to rotavirus infection and how the colonization of probiotics influences the γδ T cell response were unknown. In this study, we evaluated by multicolor flow cytometry the frequencies and distribution of total γδ T cells and three major subsets (CD2−CD8−, CD2+CD8− and CD2+CD8+) in ileum, spleen and blood of gnotobiotic (Gn) pigs at early (3–5 days) and late phases (28 days) after rotavirus infection. The Gn pigs were inoculated with the virulent human rotavirus Wa strain and colonized with a mixture of two strains of probiotics Lactobacillus acidophilus and Lactobacillus reuteri. In naive pigs, the highest frequency of total γδ T cells was found in blood, followed by spleen and ileum at the early age (8–10 days old) whereas in older pigs (32 days of age) the highest frequency of total γδ T cells was found in ileum and spleen followed by blood. Rotavirus infection significantly increased frequencies of intestinal total γδ T cells and the putatively regulatory CD2+CD8+ γδ T cell subset and decreased frequencies of the putatively proinflammatory CD8− subsets in ileum, spleen and blood at post-infection days (PID) 3 or 5. The three γδ T cell subsets distributed and responded differently after rotavirus infection and/or lactobacilli colonization. The CD2+CD8+ subset contributed the most to the expansion of total γδ T cells after rotavirus infection in ileum because more than 77% of the total γδ T cells there were CD2+CD8+ cells. There was an additive effect between lactobacilli and rotavirus in inducing total γδ T cell expansion in ileum at PID 5. The overall effect of lactobacilli colonization versus rotavirus infection on frequencies of the CD2+CD8+ γδ T cell subset in ileum was similar; however, rotavirus-infected pigs maintained significantly higher frequencies of CD8− subsets in ileum than lactobacilli-colonized pigs. The dynamic γδ T cell responses suggest that γδ T cell subsets may play important roles in different stages of immune responses after rotavirus infection and probiotic colonization. The knowledge on the kinetics and distribution patterns of γδ T cell subsets in naïve pigs and after rotavirus infection or lactobacilli colonization provides the foundation for further mechanistic studies of their functions.
Keywords: gamma delta (γδ) T cells, rotaviruses, lactobacilli, gnotobiotic pigs
1. Introduction
γδ T cells represent a subset of T cells that possess a distinct T cell receptor complex (TCR) from αβ T cells. Accumulating evidence suggests that γδ T cells are components of both innate and adaptive immunity against various viral and bacterial infections (Devilder et al., 2009; Pitard et al., 2008; Wang et al., 2006) and they are especially important in early responses against infections at epithelial surfaces (Hedges et al., 2007). γδ T cell responses to several viral infections in pigs have been reported [i.e., porcine reproductive and respiratory syndrome virus (PRRSV), foot-and-mouth disease virus (FMDV) (Olin et al., 2005; Takamatsu et al., 2006)]. There is no report, however, on γδ T cell responses to rotaviruses which are one of the most important viral pathogens infecting epithelial cells of the small intestine and causing diarrhea in neonatal and newly weaned pigs (Holland, 1990), and infants and young children worldwide (Parashar et al., 2006).
In pigs, γδ T cells form a major T cell subpopulation in peripheral blood of the young (Gerner et al., 2009). It was shown that CD2+CD8+ and CD2+CD8− γδ T cells preferentially reside in lymphoid tissues, while CD2−CD8− γδ T cells dominate in blood (Saalmuller et al., 1990). The percentages and distribution of total γδ T cells and three subsets (CD2+CD8+, CD2+CD8− and CD2−CD8−) in blood and secondary lymphoid tissues (spleen, popliteal lymph nodes, mesenteric lymph nodes and tonsil) of young pigs have been reported (Stepanova et al., 2007; Yang and Parkhouse, 1996). However, an analysis of γδ T cells in the pig small intestine is still lacking and the γδ T cell responses after enteric viral infections have not been studied. Our goals in this study were to extend our knowledge of the development of γδ T cells in the small intestine and to elucidate the kinetics of total and the subset γδ T cell responses after rotavirus infection. Ileum was selected because it is the major site of rotavirus replication and the major induction site in gut-associated lymphoid tissues.
Studies of bovine and murine γδ T cells have suggested that different γδ T cell subsets may have opposite functions and can be pro-inflammatory (CD8− subsets), regulatory (CD8+ subset) (Hedges et al., 2003), or promote epithelial healing (Hayday and Tigelaar, 2003). γδ T cells demonstrated both pro-inflammatory and anti-inflammatory roles in response to infection (Tramonti et al., 2006; Ziegler, 2004). In pigs, functions of different γδ T cell subsets have not been reported.
Lactic acid bacteria (LAB), including lactobacilli, are widely evaluated as probiotics in animals and humans (Vaughan et al., 2002) and have been shown to significantly stimulate gut epithelial cell proliferation (Ichikawa et al., 1999), enhance innate and acquired immunity in young lab animals (mice, rats) and children (Herias et al., 1999; Yasui et al., 1999) and suppress intestinal inflammation (Zocco et al., 2006). Several LAB strains have been shown to reduce the severity of acute rotavirus gastroenteritis in children (Majamaa et al., 1995; Shornikova et al., 1997) and enhance the immunogenicity of rotavirus vaccines (Isolauri et al., 1995; Zhang et al., 2008b). However, the mechanisms are not fully understood. Our previous study showed that gnotobiotic (Gn) pigs infected with the virulent (Vir) Wa human rotavirus (HRV) and fed with a mixture of Lactobacillus acidophilus NCFM (LA) and L. reuteri (LR) strains developed significantly higher total intestinal IgA secreting cell responses and total intestinal IgM and IgG titers than the Gn pigs without the LAB feeding (Zhang et al., 2008a). In this study, we first elucidated γδ T cell responses to rotavirus infection in neonatal Gn pigs during the acute phase of rotavirus infection. We then compared γδ T cell responses with or without LAB colonization at the acute phase and 4 weeks after rotavirus inoculation to examine the effect of colonization of LAB on the development of γδ T cell responses to rotavirus. Our hypotheses are (1) that a robust γδ T cell response is induced by rotavirus infection; (2) different subsets of γδ T cells may respond differently in different anatomical sites to rotavirus infection; and (3) among the many immune modulating effects, LAB have stimulating or regulating effects on different γδ T cell subsets. The gnotobiotic status of Gn pigs used in this study assured that the effects of specific probiotic and rotavirus strains on γδ T cell responses were not confounded by other microbes present in conventionally reared pigs.
2. Materials and methods
2.1. Virus
The Wa strain (G1P1A[8]) VirHRV were passaged through Gn pigs and the pooled intestinal contents from the 27th passage were used for inoculation at a dose of 1 × 105 fluorescent focus-forming units (FFU). The 50 % infectious dose (ID50) of the VirHRV in Gn pigs was determined as approximately 1 FFU (Ward et al., 1996).
The cell-culture adapted Wa strain AttHRV, derived from the 34th passage in African green monkey kidney cells (MA104), was used as detecting antigens in the enzyme-linked immunosorbent assay (ELISA). Virus fecal shedding was detected by a cell-culture immunofluorescent (CCIF) assay and an antigen ELISA as previously described (Azevedo et al., 2005).
2.2. Bacteria
The Lactobacillus acidophilus strain NCFM™ and L. reuteri strain (ATCC 23272) (ATCC, Manassas, VA, USA) were used in this study. Both LAB strains were propagated in Lactobacilli MRS broth (Weber, Hamilton, NJ, USA). LAB inoculums were prepared and titrated as previously described (Zhang et al., 2008c). The two LAB inoculums with known titers were diluted to the specified CFU/ml in 0.1 % peptone water (BD Biosciences, Franklin Lakes, NJ, USA) and mixed in equal amounts on the day of feeding. Enumeration of LAB in fecal samples was performed as we previously described (Zhang et al., 2008c).
2.3. Inoculation of Gn pigs
Near-term pigs of Landrace and Large White cross breed were derived from pregnant sows by surgery and maintained in germ-free isolator units as described (Meyer et al., 1964). Pigs were fed with commercial ultra-high temperature (UHT)-treated sterile milk. All pigs were confirmed germ-free prior to LAB and VirHRV exposure. For the study of kinetics of early γδ T cell responses in naïve and VirHRV-infected pigs, Gn pigs (both males and females) were randomly assigned to VirHRV-inoculated group and mock-inoculated group with seven [post rotavirus-inoculation day (PID) 0 and 3] to eight (PID 5) pigs euthanized on each time point to isolate mononuclear cells (MNCs) from ileum, spleen and peripheral blood (Yuan et al., 1996). Briefly, the MNCs were extracted from the ileum by using EDTA and collagenase and enriched by discontinuous Percoll gradient, from the spleen by mechanical separation and enriched by discontinuous Percoll gradient, and from blood by using Ficoll-Paque™ plus. Inoculation of pigs with VirHRV was performed orally at 5 days of age (PID 0).
For the study of γδ T cell responses to rotavirus infection and LAB colonization, Gn pigs were assigned to four treatment groups with four to eight pigs euthanized on each time point at PID 5 (n=4–8) and 28 (n=6): (1) Mock controls (LAB−VirHRV−), (2) LAB only (LAB+VirHRV−), (3) VirHRV only (LAB−VirHRV+), or (4) LAB colonization plus VirHRV infection (LAB+VirHRV+). Pigs in LAB+ groups were orally dosed at 3, 5, 7, 9 and 11 days of age with 103, 104, 105, 106 and 106 CFU, respectively, of a 1:1 mixture of L. acidophilus and L. reuteri in 3 ml of 0.1 % peptone water. The total dose of lactobacilli received by each pig was 2.1 × 106 CFU in 5 feedings. Non-LAB-fed pigs were given an equal volume of 0.1 % peptone water. At 5 days of age, pigs in VirHRV+ groups were orally inoculated with 105 FFU virulent Wa HRV in 5 ml of Dulbecco’s Modified Eagle’s Medium (DMEM). Non-infected pigs were given an equal volume of diluent. Pigs were given 5 ml of 100 mM sodium bicarbonate to reduce gastric acidity 20 min before VirHRV inoculation. Post-VirHRV-inoculation, pigs were examined daily for clinical signs, including prevalence, duration and severity of diarrhea as described (Yuan et al., 1996). Rectal swabs were collected daily for HRV and lactobacilli shedding. All animal experimental procedures were conducted in accordance with protocols approved by Institutional Animal Care and Use Committees of The Ohio State University and Virginia Polytechnic Institute and State University.
2.4. Staining cells for flow cytometry analysis
The MNCs (2 × 106 cells/tube) were stained on the same day of MNC isolation without in vitro stimulation. MNCs were incubated for 15 min at 4°C at each step and then washed once with the staining buffer (prepared according to BD Pharmingen™ BrdU Flow Kits Instruction Manual) and centrifuged at 800 g for 5 min at 4°C. Because fluorescence-conjugated porcine Tcr1-N4 and CD2 antibodies were not commercially available, we used unconjugated primary antibodies and then fluorescence-conjugated secondary antibodies to detect porcine γδ T cells. MNCs were first stained with the mouse anti-pig Tcr1-N4 (IgG1, VMRD, PGBL22A), an antibody that defines porcine γδ T cells (Davis et al., 1998) and mouse anti-pig CD2 (IgG3, VMRD, PG168A), followed by the allophycocyanin (APC)-conjugated rat anti-mouse IgG1 (IgG1, BD pharmingen, clone X56) and fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse IgG3 (IgM, Southern Biotech, clone LO-MG3). SpectralRed™ (SPRD)-conjugated mouse anti-pig CD8α (IgG2a, Southern Biotech, clone 76-2-11) were added together with the primary antibodies. The secondary antibodies against different mouse IgG isotypes used in this study do not cross-react with each other based on the manufacturer statements. In the corresponding control tubes, only two secondary fluorescent-conjugated antibodies and SPRD mouse IgG2a isotype control (Southern Biotech, clone HOPC-1) were added to the cells. All antibodies were titrated and used at optimal concentrations. Analysis of the stained cells was performed using a FACSCalibur or a FACSAria flow cytometer (Becton Dickinson) and at least 20,000 cells were acquired. Data analysis was performed using CellQuest™ Pro (Becton Dickinson) or FlowJo 7.2.2 (Tree Star, Inc) software. Data are presented as mean frequencies of total γδ T cells among MNCs and mean frequencies of CD2+CD8+, CD2+CD8− or CD2−CD8− γδ T cells among total γδ T cells. Any non-specific staining occurring in the control tubes was subtracted from the corresponding samples.
2.5. Statistical analysis
Non-parametric Kruskal-Wallis rank sum test was performed to compare frequencies of total γδ T cells or the three subsets in ileum, spleen, and blood among groups at each time point. When differences among these groups were detected, the same test was used in a pairwise fashion to clarify the nature of the differences. Frequencies of γδ T cells were compared between time points by Analysis of Covariance (ANCOVA) using R programs. All statistical significance was assessed at p < 0.05.
3. Results
3.1. VirHRV infection and LAB colonization
HRV infection in the Gn pigs were confirmed by detection of HRV titers by CCIF and HRV antigen by ELISA in rectal swab samples and intestinal contents from all the pigs in the HRV+ groups (none in the HRV− groups) at PID 3–5. The kinetics and magnitude of virus fecal and nasal shedding and antigenemia after the Wa strain VirHRV inoculation of Gn pigs have been characterized previously (Azevedo et al., 2005; Ward et al., 1996). The VirHRV shedding titers and LAB fecal counts (confirming rotavirus infection and LAB colonization) in the Gn pigs in this study were found to be similar to those studies we described in previous reports (Wen et al., 2009; Zhang et al., 2008a).
3.2. Detection of total γδ T cells and three subsets in ileum, spleen, and blood by flow cytometry
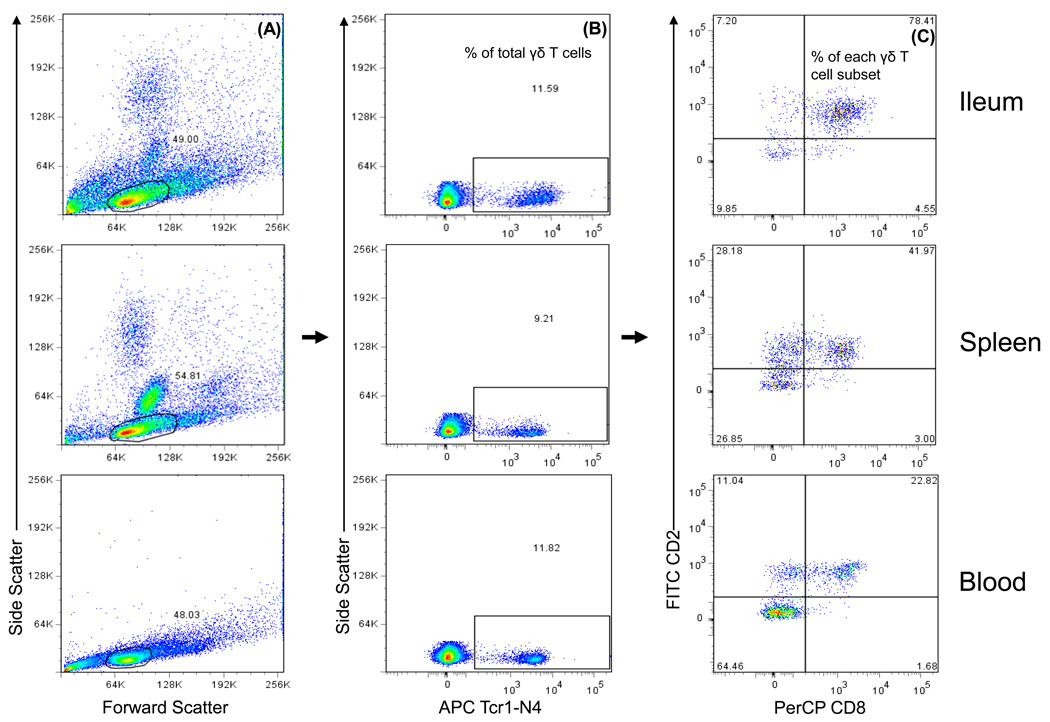
Detection of frequencies of total γδ T cells among MNCs and the subsets defined by CD2 and CD8α expression among total γδ T cells is depicted in the representative dot plots for ileum, spleen, and blood using VirHRV-inoculation pigs at PID 5 as an example (Fig. 1). Frequencies of total γδ T cells and the three major subsets CD2+CD8+, CD2−CD8− and CD2+CD8− γδ T cells differed substantially among ileum, spleen and blood. The frequencies of total γδ T cells were calculated as the percentage among total MNCs and the frequencies of each γδ T cell subset as the percentage among total γδ T cells.
Fig. 1. Detection of total γδ T cells and subsets in ileum, spleen and blood with flow cytometry.
Gn pigs were inoculated with virulent Wa HRV. The MNCs were isolated upon euthanasia at PID 5 and stained with primary antibodies to porcine γδ T cell marker (Tcr1-N4), CD2, and CD8, and secondary antibodies conjugated to APC, FITC and PerCP, respectively. The MNCs were gated based on forward scatter and side scatter profile (A) followed by gating on Tcr1-N4+ T cells (B). The frequencies (%) of total γδ T cells among MNCs are shown on the dot plots. Bivariate dot plots were drawn to define CD2+CD8−, CD2+CD8+ and CD2−CD8− γδ T cell subpopulations within Tcr1-N4+ T cells (C). The frequencies of γδ T cell subsets are labeled on the corner of each quadrant.
3.3. Kinetics of γδ T cell responses in VirHRV-inoculated Gn pigs
3.3.1. Total γδ T cell responses to VirHRV infection at PID 0, PID 3 and PID 5
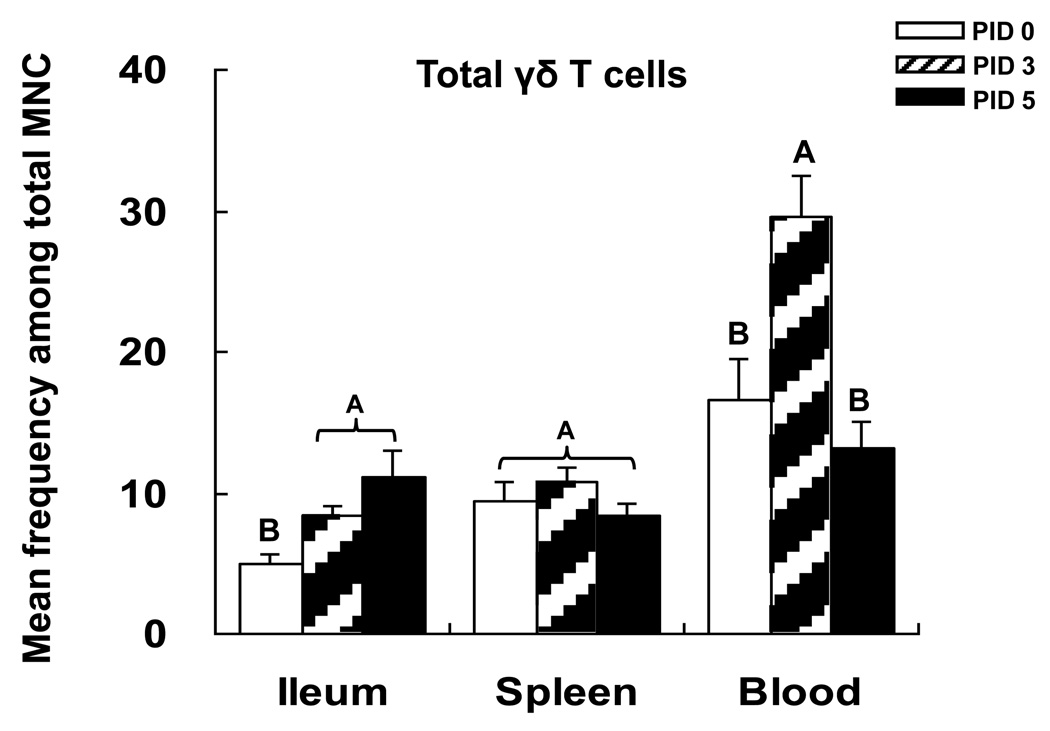
Frequencies of total and three γδ T cell subsets in each tissue of mock-infected Gn pigs of 8 (n=3) and 10 (n=4) days of age were compared using non-parametric Kruskal-Wallis rank analysis. There were no statistically significant differences in frequencies of total γδ T cells and each subset in mock-infected Gn pigs between 8 and 10 days of age, therefore the data were combined and expressed as PID 0 for the statistical analysis and summarized in Figs. 2 and 3. In the mock-infected Gn pigs, the highest mean frequencies of total γδ T cells were found in blood followed by spleen and ileum (Fig. 2). In blood of VirHRV inoculated pigs, frequencies of total γδ T cells increased significantly from PID 0 to PID 3 and then returned to pre-infection level at PID 5, demonstrating an innate immune response (Fig. 2). In ileum, the frequencies of total γδ T cells were significantly higher at both PID 3 and PID 5 compared to PID 0 (Fig. 2). Thus, the kinetics of γδ T cell responses in different tissues (blood versus intestine) at early stage after rotavirus infection are different. The data suggest activation and proliferation of γδ T cells in both the intestine and blood by PID 3 followed by the activated γδ T cells leaving blood and entering the site of rotavirus replication (ileum) by PID 5.
Fig. 2. Frequencies of total γδ T cells in VirHRV-infected Gn pigs.
Gn pigs were inoculated with virulent Wa HRV or mock inoculated and euthanized on PID 0, 3 and 5, respectively. The total γδ T cells were detected as shown in Fig. 1. The y-axis is the mean frequencies (%) of total γδ T cells among MNCs. The error bars represent standard error of the mean. The capital letters A, B, and C indicate the results of significance testing for differences among PID 0, 3 and 5. Unshared letters indicate significant difference among PID 0, 3 and 5 on frequencies of the total γδ T cells (Kruskal–Wallis rank sum test, p < 0.05, n=7–8), while shared letters indicate no significant difference.
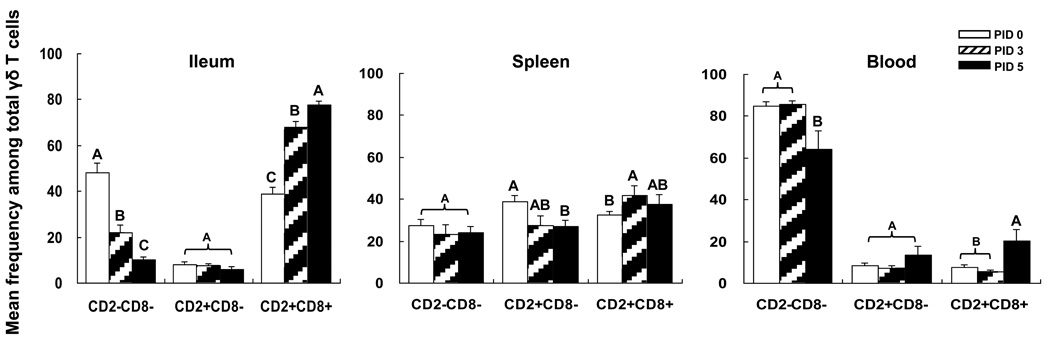
Fig. 3. Frequencies of γδ T cell subsets in VirHRV-infected Gn pigs.
The γδ T cell subsets from the VirHRV-infected pigs were detected as shown in Fig. 1. The y-axis is the mean frequencies (%) of each of three subsets among total γδ T cells. The error bars represent standard error of the mean. The capital letters A, B, and C indicate the results of significance testing for differences among PID 0, 3 and 5. Unshared letters indicate significant difference among PID 0, 3 and 5 on frequencies of the three subsets (Kruskal–Wallis rank sum test, p < 0.05, n=7–8), while shared letters indicate no significant difference.
3.3.2. γδ T cell subset responses to VirHRV infection at PID 0, PID 3 and PID 5
Each γδ T cell subset has a unique distribution pattern in different lymphoid tissues in normal neonatal pigs (Fig 3). At PID 0, the predominant γδ T cell subset in blood is the CD2−CD8− (85%) and in ileum is the CD2−CD8− (48%) followed by the CD2+CD8+ (39%). In spleen, all the three subsets have similar frequencies (27–39%). The highest mean frequencies of CD2−CD8− subset was found in blood, CD2+CD8+ in ileum, and CD2+CD8− in spleen.
Rotavirus infection significantly increased frequencies of CD2+CD8+ subset in ileum at PID 3 and PID 5 compared to PID 0, in spleen at PID 3 compared to PID 0, and in blood at PID 5 compared to PID 0 and PID 3 (Fig. 3). However, VirHRV down-regulated CD2+CD8− subset in spleen at PID 5 compared to PID 0, CD2−CD8− subset in ileum at PID 3 and PID 5 compared to PID 0 and CD2−CD8− subset in blood at PID 5 compared to PID 0 and PID 3 (Fig. 3). In summary, rotavirus infection increased frequencies of the putatively regulatory γδ T cells (CD8+ subset) and decreased frequencies of the putatively pro-inflammatory γδ T cells (CD8− subsets) in ileum, spleen and blood at 3–5 days post rotavirus infection.
3.4. Comparisons of γδ T cell responses in mock-infected, LAB-colonized, VirHRV-infected, and LAB plus VirHRV-inoculated pigs at PID 5 and PID 28
3.4.1. Total γδ T cell responses at PID 5 and PID 28
Table 1 compares the frequencies of total γδ T cells in ileum, spleen and blood at PID 5 versus PID 28 among the four treatment groups of pigs. In mock-infected pigs (LAB−VirHRV− group) at 8–10 days of age (shown under PID 5), the highest mean frequency of total γδ T cells was found in blood (16.6%), followed by spleen (9.5%) and ileum (4.9%). However, in mock-infected pigs at 32 days of age (shown under PID 28), mean frequencies of total γδ T cells in ileum and spleen were higher than in blood. At PID 28, frequencies of total γδ T cells in the mock-infected pigs increased significantly in ileum and spleen compared to PID 5.
Table 1.
Total γδ T cell responses among treatment groups at PID 5 and PID 28
| Mean frequencies of total γδ T cells among MNCs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment group | PID 5 | PID 28 | ||||||
| n | Ileum | Spleen | Blood | n | Ileum | Spleen | Blood | |
| LAB−VirHRV− | 7 | 4.9C* (0.7)** |
9.5A (1.2) |
16.6A (3.0) |
6 | 17.2A† (6.0) |
16.7A† (2.5) |
9.7A (1.5) |
| LAB+VirHRV− | 4 | 19.3AB (5.2) |
7.9A (0.9) |
11.8AB (1.6) |
6 | 6.9B† (0.9) |
16.5A† (2.5) |
8.3AB (1.5) |
| LAB−VirHRV+ | 8 | 11.2B (1.8) |
8.5A (0.8) |
13.2A (1.9) |
6 | 4.7C† (0.3) |
13.9A† (1.0) |
5.3B† (0.5) |
| LAB+VirHRV+ | 4 | 24.9 A (1.8) |
9.1A (0.6) |
6.6B (1.0) |
6 | 6.1B† (0.5) |
15.1A† (1.9) |
7.3AB (1.5) |
Means in the same column with different superscript letters (A, B, and C) differ significantly (Kruskal-Wallis rank sum test; p<0.05).
Standard error of the mean.
Indicates significant difference when compared to PID 5 for the same tissue in the same group (ANCOVA; p<0.05).
Comparing the four treatment groups at PID 5, frequencies of total γδ T cells in LAB only (LAB+VirHRV−) and VirHRV only (LAB−VirHRV+) pigs were significantly higher in the ileum but were slightly lower in the blood than the mock-infected pigs (Table 1). Frequencies of total γδ T cells in ileum of LAB+VirHRV+ pigs were higher or significantly higher than those of the other groups, but they were lower or significantly lower in blood. There was no significant difference in frequencies of total γδ T cells in spleen among the four treatment groups at PID 5. These data suggest that there was proliferation of γδ T cells after either rotavirus infection or LAB colonization in the intestine which is the site of VirHRV infection, and LAB colonization. LAB and VirHRV had an additive effect in stimulating the proliferation of total γδ T cells in ileum of the LAB+VirHRV+ pigs. Apparently, γδ T cells in spleen are not activated by VirHRV infection or LAB colonization at PID 5.
At PID 28, frequencies of total γδ T cells in ileum of LAB+VirHRV−, LAB−VirHRV+ and LAB+VirHRV+ pigs were significantly lower compared to the mock-infected pigs. In blood, the frequencies of total γδ T cells in the LAB−VirHRV+ pigs were also significantly lower than the mock-infected pigs. There was no significant difference in frequencies of total γδ T cells in spleen among the four treatment groups at PID 28.
Frequencies of total γδ T cells differed significantly between PID 5 and PID 28 in ileum and spleen of all the pig groups, and in blood of the LAB−VirHRV+ pigs (Table 1). Frequencies of total γδ T cells in the LAB+VirHRV−, LAB−VirHRV+ and LAB+VirHRV+ pigs decreased significantly in ileum but increased significantly in spleen when compared between PID 5 and PID 28. In blood of the LAB−VirHRV+ pigs, frequencies of total γδ T cells decreased significantly from PID 5 to 28. In summary, LAB colonization and/or rotavirus infection induced significant expansion of intestinal total γδ T cells at PID 5 but then the frequencies reduced to levels significantly lower than the mock-infected control pigs by PID 28. Frequencies of total γδ T cells in spleen were not significantly influenced by LAB colonization or rotavirus infection but significantly increased with aging from 8–10 to 32 days of age.
3.4.2. γδ T cell subset responses at PID 5 and PID 28
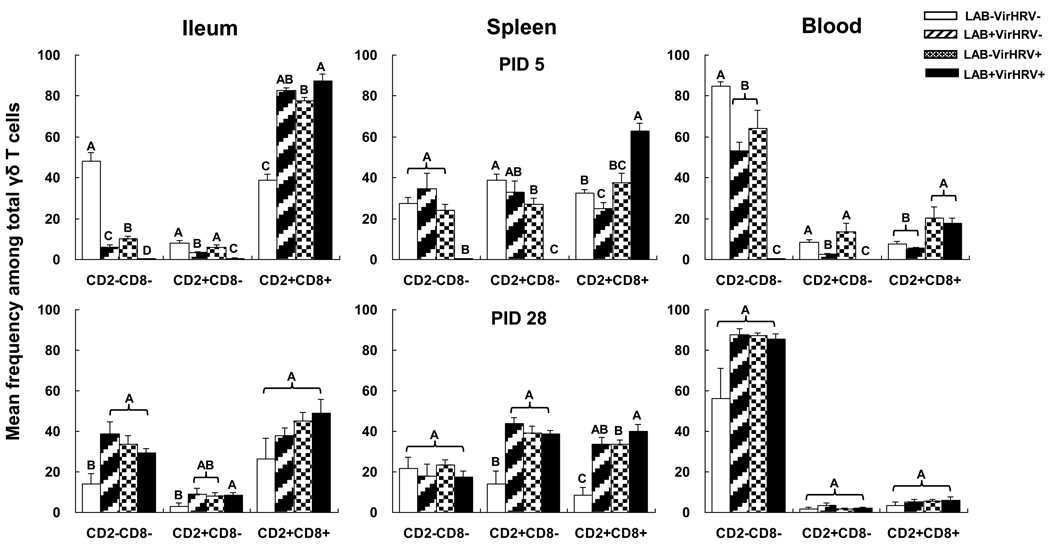
The mean frequencies of γδ T cell subsets among total γδ T cells in ileum, spleen and blood of the four treatment groups of pigs at PID 5 and PID 28 are depicted in Fig. 4. At PID 5, CD2+CD8+ subset was the predominant subset in ileum after LAB colonization and/or rotavirus infection. There were significant increases of the CD2+CD8+ γδ T cell frequencies in the ileum of LAB only, VirHRV only and LAB+VirHRV+ pigs at PID 5. CD2−CD8− subset was the predominant γδ T cell subset in blood. After LAB colonization and/or VirHRV infection, there were significant decreases of the CD2−CD8− γδ T cell frequencies in blood and also in ileum. The highest mean frequencies of CD2+CD8− subset were found in spleen of mock-infected pigs. The frequencies of CD2+CD8− γδ T cells in spleen decreased significantly in VirHRV infected pigs (LAB−VirHRV+ and LAB+VirHRV+) and also in ileum and blood of LAB colonized pigs (LAB+VirHRV− and LAB+VirHRV+) at PID 5. Important to note, in the ileum the frequencies of the two CD8− subsets were significantly higher in the VirHRV alone pigs than the LAB alone pigs whereas the CD8− subsets decreased to near undetectable levels in ileum, spleen and blood of the LAB+VirHRV+ pigs at PID 5. In summary, the overall effect of LAB colonization versus rotavirus infection on frequencies of the regulatory CD2+CD8+ γδ T cell subset was similar in ileum and spleen at PID 5, but their effects on the putatively CD8− subsets in ileum (and CD2+CD8− subset in blood) were different. Rotavirus infected pigs maintained significantly higher CD8− subsets than the LAB colonized pigs.
Fig. 4. Mean frequencies of γδ T cell subsets in Gn pigs with or without LAB and infected with VirHRV at PID 5 and 28.
Gn pigs were inoculated with LAB and virulent Wa strain HRV (LAB+VirHRV+), HRV only (LAB−VirHRV+), LAB only (LAB+VirHRV−) or mock (LAB−VirHRV−). The frequencies of γδ T cell subsets were measured on PID 5 and 28. The y-axis is the mean frequencies (%) of three subsets among total γδ T cells. The error bars represent standard error of the mean. The capital letters A, B, and C indicate the results of significance testing for differences among four treatment groups (Kruskal–Wallis rank sum test, p < 0.05, n=4–8), while shared letters indicate no significant difference.
The distribution of γδ T cell subsets at PID 28 in all tissues differs substantially from that of PID 5 (Fig. 4). The frequencies of CD2−CD8− and CD2+CD8−γδ T cell subsets in ileum and CD2+CD8− and CD2+CD8+ subsets in spleen of the LAB colonized and/or VirHRV infected pigs were significantly higher than the mock-infected pigs. There were no significant differences among the four treatment groups in any of the γδ T cell subset in blood at PID 28, although there was a trend for higher frequencies of CD2−CD8− subset in the LAB colonized and/or VirHRV infected pigs compared to the mock-infected pigs.
4. Discussion
To understand how γδ T cells respond to rotavirus infection, including the responses of each γδ T cell subset, and how colonization of probiotic bacteria modulates the γδ T responses, we studied the magnitude and kinetics of total γδ T cell responses and the distribution of the three subsets in ileum, spleen and blood in Gn pigs of early age (8 or 10 days) and older age (32 days) with or without LAB colonization and rotavirus infection. It is known that γδ T cells have the highest abundance in intraepithelial lymphocytes (IELs) in humans and mice (Thielke et al., 2003); however, IELs were not examined in this study due to low cell yield.
In Gn pigs, γδ T cells were most abundant in blood followed by spleen and intestine at PID 0–3 (8–10 days of age), which is in agreement with the previous report by Stepanova et al (Stepanova et al., 2007). Rotavirus infection significantly expanded total γδ T cells in blood and ileum at PID3 and γδ T frequencies continued to increase in ileum but decreased in blood from PID 3 to PID 5, suggesting proliferation of γδ T cells in ileum and blood, and potential recruitment of γδ T cells from blood to ileum. Because we did not track the trafficking of γδ T cells from blood to ileum or collect the data of absolute numbers of γδ T cells in ileum, spleen and blood at each time point, we could not know for sure if there were changes in absolute γδ T cell numbers. Proliferation of total γδ T cells in ileum and recruitment from blood to ileum (the site of rotavirus infection) were again suggested in the LAB colonized pigs with or without rotavirus infection at PID 5 because significant increases in the frequencies of total γδ T cells in ileum and decreases in blood were also observed in LAB+VirHRV− and LAB+VirHRV+ pigs. There was a clear additive effect between LAB and rotavirus in inducing total γδ T cell expansion in ileum and in recruiting γδ T cells from blood as the frequencies in LAB+VirHRV+ pigs increased the most (5-fold) in ileum and decreased the most (2.5-fold) in blood by PID 5 compared to LAB only and VirHRV only pigs. Worliczek et al (Worliczek et al.) also reported that Isospora suis infection in neonatal pigs reduced the numbers of γδ T cells in blood, spleen and mesenteric lymph nodes and strongly increased the numbers in jejunum at PID 6–17, suggesting migration of γδ T cells from blood and secondary lymphoid organs to the site of infection.
Frequencies of total γδ T cells in spleen of all the pig groups and in ileum of the control pigs (LAB−VirHRV−) increased significantly from PID 5 to PID 28, suggesting age-dependent development (Stepanova et al., 2007). However, frequencies of total γδ T cells in ileum of all the rotavirus infected and/or LAB colonized pigs (LAB−VirHRV+, LAB+VirHRV−, and LAB+VirHRV+) and in blood of the VirHRV-only pigs decreased significantly by PID 28. Reduction of total CD4+ and CD8+ T cells (lymphopenia) after rotavirus infection in pigs and humans has been reported previously (Wang et al., 2007; Yuan et al., 2008). But it is interesting to see that LAB colonization alone also significantly reduced total γδ T cell frequencies in ileum. The mechanism and immunologic significance of the observation require further studies. It was reported that the anti-inflammatory probiotic Escherichia coli strain Nissle 1017, but not Lactobacillus and Bifidobacterium strains contained in the SymbioLact® Comp., induced apoptosis and necrosis in activated γδ T cells in vitro (Guzy et al., 2008). Another study reported that γδ T cells were significantly reduced from the colonic epithelial layer in Brachyspira hyodysenteriae (causes swine dysentery) infected pigs at PID 15 (Hontecillas et al., 2005). It is possible that after the innate immune response phase, the activated γδ T cells migrated from gut to other secondary lymphoid organs, such as spleen, or underwent apoptosis. Consistent with our observation that frequencies of total γδ T cells in blood decreased from PID 5 to PID 28 in the control pigs, γδ T cell amounts in blood of human, cattle and goats decrease with age (Caro et al., 1998; Davis et al., 1998; Wilson et al., 1996). On the contrary, Stepanova et al reported decreases of γδ T cells in pig blood between 1 day to 2 weeks of age and then elevations from 2 weeks to 6 months. The authors suggested that the elevation of γδ T cells in blood observed in the study was caused by a factor other than age alone (Stepanova et al., 2007).
Three γδ T cell subsets, CD2+CD8+, CD2+CD8−, and CD2−CD8− distributed differently in intestinal and systemic lymphoid tissues and responded differently after rotavirus infection and/or LAB colonization in Gn pigs during the innate immune response phase. Among the three subsets, CD2+CD8+ subset likely contributed the most to the expansion of total γδ T cells after rotavirus infection in ileum because more than 77% of the total γδ T cells in ileum were CD2+CD8+ cells at PID 5. This subset has been suggested to have anti-viral and anti-inflammatory functions. In studies of PRRSV and FMDV infection and vaccination in pigs, CD8+ γδ T cells were shown to have antigen-specific cytolysis activity and expressed IFN-γ (Lopez Fuertes et al., 1999; Takamatsu et al., 2006; Thielke et al., 2003). On the other hand, gene expression studies showed that bovine CD8+ γδ T cells were involved in promoting quiescence whereas CD8− γδ T cells expressed pro-inflammatory genes (Hedges et al., 2003). In the present study, we focused on evaluating the kinetics and magnitude of γδ T cell subset responses. Our subsequent study (manuscript in preparation) showed that CD8− γδ T cells enhanced CD4+ T cell proliferation and IFN-γ production whereas CD8+ γδ T cells increased IL-10 and TGF-β production in the co-culture with CD4+ T cells (Wen and Yuan, unpublished data). Apparently, γδ T cells may have diverse immune functions and should be further subdivided into different functional γδ T cell subsets.
Over the time course of rotavirus infection in Gn pigs, the increase of CD2+CD8+ γδ T cells in ileum at PID 3–5 corresponded to the beginning of clearance of the virus infection and the recovery of the villous atrophy of the small intestine (Ward et al., 1996). After VirHRV inoculation, the virus shedding peaked at PID 2, started to decline by PID 3 and was cleared by PID 7. The villous atrophy was most severe at PID 2–3 and completely recovered by PID 7. It is likely that CD2+CD8+ γδ T cells in ileum played a major role in the epithelial cell repair and also contributed to the virus clearance because production of both TGF-β and IFN-γ by CD2+CD8+ γδ T cells have been detected in Gn pigs after rotavirus infection (Wen and Yuan, unpublished data).
The CD2−CD8− γδ T cell is the most abundant subset in Gn pig blood at both PID 5 and PID 28 in all the four treatment groups, except for the LAB+VirHRV+ pigs at PID 5. The CD8− γδ T cells have been suggested to be highly activated, proliferative, and inflammatory in cattle (Hedges et al., 2003). However, after rotavirus infection (and/or LAB colonization), frequencies of CD2−CD8− subset significantly reduced at PID 5 in both blood and ileum. Thus this γδ T cell subset is not likely to play the role as anti-viral effector cells. The effect of LAB alone on the CD2−CD8− γδ T cell subset response in ileum and blood was slightly more pronounced than rotavirus alone. The function of this subset in the immune responses to rotavirus infection or LAB colonization needs further study. The observed additive effect of rotavirus infection and LAB colonization on the severe depletion of CD2−CD8− and CD2+CD8− γδ T cells in ileum, spleen and blood of LAB+VirHRV+ pigs at PID 5 is intriguing. Because CD2+CD8+ γδ T cell frequencies in ileum and spleen and the total γδ T cells in ileum increased significantly in the LAB+VirHRV+ pigs compared to VirHRV only pigs, it is tempting to postulate that the CD8− γδ T cells acquired CD8 marker upon activation and differentiated into CD2+CD8+ γδ T cells and migrated to the site of infection, the ileum. In addition, LAB plus VirHRV may have provided the most suitable stimulations for the differentiation and migration.
γδ T cells are also suggested to be involved in memory response (Blumerman et al., 2007; Gerner et al., 2009). At PID 28, CD2+CD8− and CD2+CD8+ subsets in spleen and CD2−CD8− subset in ileum were significantly higher in the LAB colonized and/or rotavirus infection pigs than the controls, thus these γδ T cells are potential memory γδ T cells. A re-infection/challenge study is needed to identify the role of γδ T cells in memory T cell responses.
In summary, the present study in Gn pigs demonstrated that γδ T cells responded vigorously to rotavirus infection and the majority of responding γδ T cells was the CD2+CD8+ subset in ileum. This finding is consistent with the studies using BoWC1+ lymphocyte depletion in gnotobiotic calves (Oldham et al., 1993) and γδ T cell knockout mice (Franco and Greenberg, 1997) showing that γδ T cells were not important in limiting primary rotavirus infection, because CD2+CD8+ γδ T cells are known to be regulatory (Hedges et al., 2003) or promote epithelial healing (Hayday and Tigelaar, 2003). Although the exact function of each γδ T cell subset in innate and adaptive immunity remains to be determined in more in-depth studies, this present study illustrated for the first time the γδ T cell subset responses to rotavirus infection and LAB colonization. The dynamic γδ T cell responses we observed indicate that γδ T cells are important components in responses to rotavirus infection and LAB colonization. The knowledge on the kinetics and distribution patterns of total and each γδ T cell subset in the intestinal and systemic lymphoid tissues in Gn pigs provides the foundation for further mechanistic studies of the functional characteristics of γδ T cells in their role in the development or regulation of immune responses to rotavirus infection and in the immune modulating effect of LAB on rotavirus infection and vaccines.
5. Acknowledgements
We thank Dr. Juliette Hanson and Rich McCormick from The Ohio State University and Dr. Marlice Vonck, Dr. Kevin Pelzer, Pete Jobst, and Andrea Aman from TRACSS, Virginia Tech for animal care. We thank Ana Gonzalez and Melissa Makris for technical advice and assistance in flow cytometry and Dr. Inyoung Kim for assistance in statistic analysis. This work was supported in part by grants from the National Institutes of Health (R21AT002524 and R01AT004789 to LY and R01AI033561 to LJS), an internal grant from Ohio Agricultural Research and Development Center, The Ohio State University (OHOA1208) to LY, and the start-up fund from Virginia Tech to LY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azevedo MS, Yuan L, Jeong KI, Gonzalez A, Nguyen TV, Pouly S, Gochnauer M, Zhang W, Azevedo A, Saif LJ. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J Virol. 2005;79:5428–5436. doi: 10.1128/JVI.79.9.5428-5436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumerman SL, Herzig CT, Baldwin CL. WC1+ gammadelta T cell memory population is induced by killed bacterial vaccine. Eur J Immunol. 2007;37:1204–1216. doi: 10.1002/eji.200636216. [DOI] [PubMed] [Google Scholar]
- Caro MR, Gallego MC, Buendia AJ, Navarro E, Navarro JA. Postnatal evolution of lymphocyte subpopulations in peripheral blood and lymphoid organs in the goat. Res Vet Sci. 1998;65:145–148. doi: 10.1016/s0034-5288(98)90166-7. [DOI] [PubMed] [Google Scholar]
- Davis WC, Zuckermann FA, Hamilton MJ, Barbosa JI, Saalmuller A, Binns RM, Licence ST. Analysis of monoclonal antibodies that recognize gamma delta T/null cells. Vet Immunol Immunopathol. 1998;60:305–316. doi: 10.1016/s0165-2427(97)00107-4. [DOI] [PubMed] [Google Scholar]
- Devilder MC, Allain S, Dousset C, Bonneville M, Scotet E. Early triggering of exclusive IFN-gamma responses of human Vgamma9Vdelta2 T cells by TLR-activated myeloid and plasmacytoid dendritic cells. J Immunol. 2009;183:3625–3633. doi: 10.4049/jimmunol.0901571. [DOI] [PubMed] [Google Scholar]
- Franco MA, Greenberg HB. Immunity to rotavirus in T cell deficient mice. Virology. 1997;238:169–179. doi: 10.1006/viro.1997.8843. [DOI] [PubMed] [Google Scholar]
- Gerner W, Kaser T, Saalmuller A. Porcine T lymphocytes and NK cells--an update. Dev Comp Immunol. 2009;33:310–320. doi: 10.1016/j.dci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Guzy C, Paclik D, Schirbel A, Sonnenborn U, Wiedenmann B, Sturm A. The probiotic Escherichia coli strain Nissle 1917 induces gammadelta T cell apoptosis via caspase- and FasL-dependent pathways. Int Immunol. 2008;20:829–840. doi: 10.1093/intimm/dxn041. [DOI] [PubMed] [Google Scholar]
- Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- Hedges JF, Buckner DL, Rask KM, Kerns HM, Jackiw LO, Trunkle TC, Pascual DW, Jutila MA. Mucosal lymphatic-derived gammadelta T cells respond early to experimental Salmonella enterocolitis by increasing expression of IL-2R alpha. Cell Immunol. 2007;246:8–16. doi: 10.1016/j.cellimm.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges JF, Cockrell D, Jackiw L, Meissner N, Jutila MA. Differential mRNA expression in circulating gammadelta T lymphocyte subsets defines unique tissue-specific functions. J Leukoc Biol. 2003;73:306–314. doi: 10.1189/jlb.0902453. [DOI] [PubMed] [Google Scholar]
- Herias MV, Hessle C, Telemo E, Midtvedt T, Hanson LA, Wold AE. Immunomodulatory effects of Lactobacillus plantarum colonizing the intestine of gnotobiotic rats. Clin Exp Immunol. 1999;116:283–290. doi: 10.1046/j.1365-2249.1999.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland RE. Some infectious causes of diarrhea in young farm animals. Clin Microbiol Rev. 1990;3:345–375. doi: 10.1128/cmr.3.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hontecillas R, Bassaganya-Riera J, Wilson J, Hutto DL, Wannemuehler MJ. CD4+ T-cell responses and distribution at the colonic mucosa during Brachyspira hyodysenteriae-induced colitis in pigs. Immunology. 2005;115:127–135. doi: 10.1111/j.1365-2567.2005.02124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa H, Kuroiwa T, Inagaki A, Shineha R, Nishihira T, Satomi S, Sakata T. Probiotic bacteria stimulate gut epithelial cell proliferation in rat. Dig Dis Sci. 1999;44:2119–2123. doi: 10.1023/a:1026647024077. [DOI] [PubMed] [Google Scholar]
- Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. Improved immunogenicity of oral D × RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine. 1995;13:310–312. doi: 10.1016/0264-410x(95)93319-5. [DOI] [PubMed] [Google Scholar]
- Lopez Fuertes L, Domenech N, Alvarez B, Ezquerra A, Dominguez J, Castro JM, Alonso F. Analysis of cellular immune response in pigs recovered from porcine respiratory and reproductive syndrome infection. Virus Res. 1999;64:33–42. doi: 10.1016/s0168-1702(99)00073-8. [DOI] [PubMed] [Google Scholar]
- Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1995;20:333–338. doi: 10.1097/00005176-199504000-00012. [DOI] [PubMed] [Google Scholar]
- Meyer RC, Bohl EH, Kohler EM. Procurement and Maintenance of Germ-Free Seine for Microbiological Investigations. Appl Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham G, Bridger JC, Howard CJ, Parsons KR. In vivo role of lymphocyte subpopulations in the control of virus excretion and mucosal antibody responses of cattle infected with rotavirus. J Virol. 1993;67:5012–5019. doi: 10.1128/jvi.67.8.5012-5019.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olin MR, Batista L, Xiao Z, Dee SA, Murtaugh MP, Pijoan CC, Molitor TW. Gammadelta lymphocyte response to porcine reproductive and respiratory syndrome virus. Viral Immunol. 2005;18:490–499. doi: 10.1089/vim.2005.18.490. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon ME, Merville P, Moreau JF, Dechanet-Merville J. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood. 2008;112:1317–1324. doi: 10.1182/blood-2008-01-136713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmuller A, Hirt W, Reddehase MJ. Porcine gamma/delta T lymphocyte subsets differing in their propensity to home to lymphoid tissue. Eur J Immunol. 1990;20:2343–2346. doi: 10.1002/eji.1830201026. [DOI] [PubMed] [Google Scholar]
- Shornikova AV, Casas IA, Mykkanen H, Salo E, Vesikari T. Bacteriotherapy with Lactobacillus reuteri in rotavirus gastroenteritis. Pediatr Infect Dis J. 1997;16:1103–1107. doi: 10.1097/00006454-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Stepanova H, Samankova P, Leva L, Sinkora J, Faldyna M. Early postnatal development of the immune system in piglets: the redistribution of T lymphocyte subsets. Cell Immunol. 2007;249:73–79. doi: 10.1016/j.cellimm.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Takamatsu HH, Denyer MS, Stirling C, Cox S, Aggarwal N, Dash P, Wileman TE, Barnett PV. Porcine gammadelta T cells: possible roles on the innate and adaptive immune responses following virus infection. Vet Immunol Immunopathol. 2006;112:49–61. doi: 10.1016/j.vetimm.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Thielke KH, Hoffmann-Moujahid A, Weisser C, Waldkirch E, Pabst R, Holtmeier W, Rothkotter HJ. Proliferating intestinal gamma/delta T cells recirculate rapidly and are a major source of the gamma/delta T cell pool in the peripheral blood. Eur J Immunol. 2003;33:1649–1656. doi: 10.1002/eji.200323442. [DOI] [PubMed] [Google Scholar]
- Tramonti D, Andrew EM, Rhodes K, Newton DJ, Carding SR. Evidence for the opposing roles of different gamma delta T cell subsets in macrophage homeostasis. Eur J Immunol. 2006;36:1729–1738. doi: 10.1002/eji.200635959. [DOI] [PubMed] [Google Scholar]
- Vaughan EE, de Vries MC, Zoetendal EG, Ben-Amor K, Akkermans AD, de Vos WM. The intestinal LABs. Antonie Van Leeuwenhoek. 2002;82:341–352. [PubMed] [Google Scholar]
- Wang T, Gao Y, Scully E, Davis CT, Anderson JF, Welte T, Ledizet M, Koski R, Madri JA, Barrett A, Yin Z, Craft J, Fikrig E. Gamma delta T cells facilitate adaptive immunity against West Nile virus infection in mice. J Immunol. 2006;177:1825–1832. doi: 10.4049/jimmunol.177.3.1825. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dennehy PH, Keyserling HL, Tang K, Gentsch JR, Glass RI, Jiang B. Rotavirus infection alters peripheral T-cell homeostasis in children with acute diarrhea. J Virol. 2007;81:3904–3912. doi: 10.1128/JVI.01887-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77(Pt 7):1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- Wen K, Azevedo MS, Gonzalez A, Zhang W, Saif LJ, Li G, Yousef A, Yuan L. Toll-like receptor and innate cytokine responses induced by lactobacilli colonization and human rotavirus infection in gnotobiotic pigs. Vet Immunol Immunopathol. 2009;127:304–315. doi: 10.1016/j.vetimm.2008.10.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RA, Zolnai A, Rudas P, Frenyo LV. T-cell subsets in blood and lymphoid tissues obtained from fetal calves, maturing calves, and adult bovine. Vet Immunol Immunopathol. 1996;53:49–60. doi: 10.1016/0165-2427(95)05543-6. [DOI] [PubMed] [Google Scholar]
- Worliczek HL, Buggelsheim M, Alexandrowicz R, Witter K, Schmidt P, Gerner W, Saalmuller A, Joachim A. Changes in lymphocyte populations in suckling piglets during primary infections with Isospora suis. Parasite Immunol. 32:232–244. doi: 10.1111/j.1365-3024.2009.01184.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Parkhouse RM. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunology. 1996;89:76–83. doi: 10.1046/j.1365-2567.1996.d01-705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui H, Shida K, Matsuzaki T, Yokokura T. Immunomodulatory function of lactic acid bacteria. Antonie Van Leeuwenhoek. 1999;76:383–389. [PubMed] [Google Scholar]
- Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Wen K, Azevedo MS, Gonzalez AM, Zhang W, Saif LJ. Virus-specific intestinal IFN-gamma producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine. 2008;26:3322–3331. doi: 10.1016/j.vaccine.2008.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Azevedo MS, Gonzalez AM, Saif LJ, Van Nguyen T, Wen K, Yousef AE, Yuan L. Influence of probiotic Lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Vet Immunol Immunopathol. 2008a;122:175–181. doi: 10.1016/j.vetimm.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Azevedo MS, Wen K, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008b;26:3655–3661. doi: 10.1016/j.vaccine.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wen K, Azevedo MS, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet Immunol Immunopathol. 2008c;121:222–231. doi: 10.1016/j.vetimm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler HK. The role of gamma/delta T cells in immunity to infection and regulation of inflammation. Immunol Res. 2004;29:293–302. doi: 10.1385/ir:29:1-3:293. [DOI] [PubMed] [Google Scholar]
- Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, Novi M, Rigante D, Cazzato IA, Ojetti V, Armuzzi A, Gasbarrini G, Gasbarrini A. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1567–1574. doi: 10.1111/j.1365-2036.2006.02927.x. [DOI] [PubMed] [Google Scholar]