Abstract
Male infertility caused by exposure to environmental toxicants, such as cadmium, mercury, bisphenol A (BPA) and dioxin, is a global problem, particularly in industrialized countries. Studies in the testis and other organs have illustrated the importance of environmental toxicant-induced oxidative stress in mediating disruption to cell junctions. This, in turn, is regulated by the activation of PI3K/c-Src/FAK and MAPK signaling pathways, with the involvement of polarity proteins, leading to reproductive dysfunction, such as reduced sperm count and semen quality in men. In this review, we discuss how these findings can improve understanding of the modes of action of environmental toxicants in –testicular dysfunction. Thus, specific inhibitors and/or antagonists against signaling molecules in these pathways can possibly reverse and/or block the disruptive effects of toxicant-induced damage. Additional studies comparing high level acute exposure versus low level chronic exposure to environmental toxicants are also needed to elucidate fully the underlying molecular mechanism(s) by which these toxicants disrupt male reproductive function.
Keywords: Testis, spermatogenesis, blood-testis barrier, cell adhesion, seminiferous epithelial cycle, environmental toxicants, cadmium, bisphenol A
Introduction
Infertility is defined as the inability to conceive after frequent and unprotected sexual intercourse for more than a year, a condition that currently affects 15% of couples worldwide [1, 2]. Approximately 50% of the cases are attributed to the male partner [1, 3]. Pathophysiological conditions (e.g. varicocele and urogenital infection) are directly linked to only 23% of all male infertility cases, with environmental factors, such as exposure to environmental toxicants, being one of the major causes of the remaining cases [4]. Numerous environmental toxicants have been shown to adversely affect spermatogenesis in rodents and humans (see Box 1), which can lead to low sperm count, abnormal sperm morphology and poor semen quality [5–7]. These include a vast variety of elements and different classes of compounds such as heavy metals (e.g. mercury, lead and cadmium), organic polychlorinated dibenzodioxins (e.g. 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin), dicarboximide fungicides (e.g. vinclozolin) and environmental phenols [e.g. 2, 2-bis[4-hydroxyphenyl] propane or bisphenol A (BPA)], which are often released into the environment during industrial processes. In turn, humans take up these environmental toxicants by ingestion of contaminated food and water, usage of consumer products (e.g. plasticware and cosmetics) and inhalation of polluted air [5–7].
Box 1. Spermatogenesis in mammalian testes.
Spermatogenesis is the generation of spermatozoa (haploid, 1n) from spermatogonia (diploid, 2n) via mitosis, meiosis and spermiogenesis [113, 114]. Type A spermatogonia, which are located at the basal compartment of the seminiferous tubule, undergo mitosis and differentiation to form type B spermatogonia, which are the germ cells that differentiate into primary spermatocytes. Following meiosis, primary spermatocytes develop into spermatids (haploid, 1n), which undergo extensive morphological and molecular changes to develop into spermatozoa via spermiogenesis. The entire process of spermatogenesis is complicated, and the success of producing healthy and adequate spermatozoa for fertilization with the ovum depends on at least three factors. First, similar to oogenesis, spermatogenesis is under the influence of hormones such as follicle stimulating hormone (FSH), testosterone and estrogen [111, 115]. Thus, tightly regulated hormonal levels by the pituitary and by Leydig and Sertoli cells are necessary for normal spermatogenesis. Second, the integrity of the blood-testis barrier (BTB) is essential to create a unique microenvironment for meiosis and the development of post-meiotic germ cells to isolate these events from the systemic circulation, which would otherwise develop anti-sperm antibodies. The BTB between Sertoli cells is comprised of co-existing tight junction (TJ), desmosome, gap junction and a testis-specific adherens junction (AJ) called basal ectoplasmic specialization (ES). The basal ES is typified by the presence of actin filament bundles sandwiched between the plasma membrane and the cisternae of endoplasmic reticulum in two neighboring Sertoli cells. It is believed that the presence of multiple junction types at the BTB contributes to the inherent tightness of this blood-tissue barrier, which is among the “tightest” in mammals. However, recent studies show that unique structural aspects of the BTB, such as the presence of focal adhesion protein FAK, also renders the testis highly susceptible to damage from environmental toxicants (see text for details). Third, during spermiogenesis when round spermatids differentiate into elongated spermatids, genetic material in the spermatid head condense to form the tightly packed nucleus with the formation of acrosome above the head region and the elongation of spermatid tail. During this time, spermatids migrate towards the adluminal compartment of the seminiferous tubule until elongated spermatids are released into the tubule lumen via the disassembly of another ES, the apical ES, at spermiation. The apical ES is the only anchoring device surrounding the entire head region of step 8–19 spermatids (i.e. elongating and elongated spermatids) in rats (in humans, only 6 steps of spermatids are found during spermiogenesis versus 19 and 16 in rats and mice, respectively). In contrast to the basal ES, actin filament bundles are restricted to the Sertoli cell at the Sertoli cell-spermatid interface. The apical ES anchors developing spermatids in the seminiferous epithelium until they are fully developed. Thus, disruption of the apical ES (e.g. by environmental toxicants) causes the premature release of spermatids that are structurally defective (e.g. lack of acrosome and/or tail) and are incapable of fertilizing the ovum.
According to National Health and Nutrition Examination Survey conducted by the USA Centers for Disease Control and Prevention, biologically active levels of BPA were detected in the urine of more than 90% of the general population of the USA [11, 12]. In China, where there is rapid increase in automobiles and industrial production, metabolites of polycyclic aromatic hydrocarbons were detected in 100% of test candidates in a recent study, and higher levels were associated with male infertility [13]. These results collectively indicate that environmental toxicants have infiltrated different aspects of human lives and are affecting the majority of people in both developing and developed countries.
Environmental toxicants can disrupt male reproductive function by affecting the endocrine system (i.e. acting as endocrine disruptors), by altering gene expression that is pertinent to spermatogenesis as well as steroidogenesis and by exerting epigenetic effects, which can result in abnormalities in the reproductive system of male offspring up to four generations following in utero exposure [17–19]. For example, BPA is a known endocrine disruptor, and at an environmentally relevant dose level, it is capable of mediating its biological effects (e.g. increase proliferation of testicular seminoma cells) through putative membrane estrogen receptors (ER), and possibly G-protein coupled receptor 30 (GPR30) [20]. Indeed, various common environmental toxicants (e.g. polychlorinated biphenyl and methoxychlor) are found to bind to the classical nuclear ER at an affinity 1000–2000 times lower than the endogenous 17β-estradiol [21], which suggests these toxicants can mediate their effects via non-genomic membrane ER-initiated pathways [22]. In addition, BPA was shown to cause defects in male and female reproductive systems following prenatal and neonatal exposure at less than 50 μg/kg/day, (the current “safe” dose acceptable for daily intake by humans recommended by the USA Food and Drug Administration (FDA) [23]), suggesting environmental toxicants can affect reproductive systems via multiple pathways and different mechanisms.
Increasing evidence suggests that an induction of oxidative stress in the testis represents another common response after exposure to environmental toxicants. Increase in oxidative stress can be seen in up to 80% of clinically proven infertile men, and exposure to environmental toxicants is a major factor contributing to such increase [24–26] Environmental toxicants that have been shown to induce oxidative stress in the testis are highly heterogeneous, with different chemical structures, and include cadmium [27, 28], bisphenol A [29] and 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin [28] [30, 31],. Interestingly, these environmental toxicants commonly increase oxidative stress by downregulating the production of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase [27, 29, 31, 32]. In turn, excessive reactive oxygen species (ROS) are produced, which damage lipids, proteins, carbohydrates and DNA in cells. Importantly, these observations were confirmed in studies illustrating that co-administration of antioxidants, such as vitamin E, with environmental toxicants could alleviate the pathophysiological effects (e.g., the reduction in sperm count) of toxicants in the testis [33, 34]. These findings thus demonstrate that oxidative stress induced by environmental toxicants is one of the major contributing factors to male infertility. In fact, oxidative stress has long been linked to male infertility; the majority of studies have focused on its roles in causing germ cell abnormalities and apoptosis [25, 35–37].
Recently, studies have shown that environmental toxicant-induced oxidative stress can cause male infertility by disrupting the cell junctions and adhesion between Sertoli-Sertoli cells and/or Sertoli-germ cells via the phosphatidylinositol 3-kinase (PI3K)/c-Src/focal adhesion kinase (FAK) signaling pathway. These findings are significant because human exposure to environmental toxicants is often below toxic dose levels that cause cell death [38]. In this review, we will examine these findings and also discuss the pathophysiological roles of mitogen-activated protein kinase (MAPK) and cytokines (which are activated during exposure to environmental toxicants) in the testis. Significantly, these signaling pathways were found to mediate their disruptive effects on intercellular junctions via polarity proteins. We will highlight recent studies that show how environmental toxicants induce male reproductive dysfunction through these signaling pathways in response to an increase in oxidative stress in the testis.. Conceptually, these studies can provide a framework to possibly develop approaches to therapeutically treat and/or manage the damaging effects of environmental toxicants to male reproductive health.
Environmental toxicant-induced oxidative damage activates the PI3K/c-Src signaling pathway
Exposure to different classes of environmental toxicants commonly increases oxidative stress in the testis [27–31]. Oxidative stress is known to increase epithelial and endothelial permeability by disrupting tight junctions (TJ) and adherens junctions (AJ) between cells [39–41]. In fact, it has been implicated in epithelial-mesenchymal transition, a process in which epithelial cells lose their junctional structures and polarity to acquire migratory capacity like mesenchymal cells [42]. Intensive research in the past decade reveals that PI3K plays a central role in mediating oxidative stress-induced junction disruption (Figure 1). Upon challenge by oxidative stress, the regulatory subunit of PI3K p85 translocates from cytosol to near the cell-cell interface [43, 44]. Such redistribution of p85 to the plasma membrane is associated with an increase in tyrosine phosphorylation of the catalytic subunit p110 [44]. Activation of PI3K in turn stimulates a non-receptor tyrosine kinase c-Src [45]. In the testis, c-Src is localized predominantly and stage-specifically at the blood-testis barrier (BTB) and apical ectoplasmic specialization (ES) (Box 1), associating with connexin43/plakofilin-2 and β1-integrin/lamininα3β3γ3 protein complexes at the corresponding cell junctions, respectively [46, 47]. Activation of the PI3K/c-Src signaling pathway in response to oxidative stress induced by environmental toxicants could be a common mechanism by which the toxicants trigger damage to the testis. Early evidence shows that the toxic effects of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin in the testis are caused by an induction in c-Src kinase activity [48]. Furthermore, significant increase in c-Src level is also detected in the testis after cadmium exposure in rodents, indicating that c-Src is activated in response to multiple environmental toxicants [49, 50].
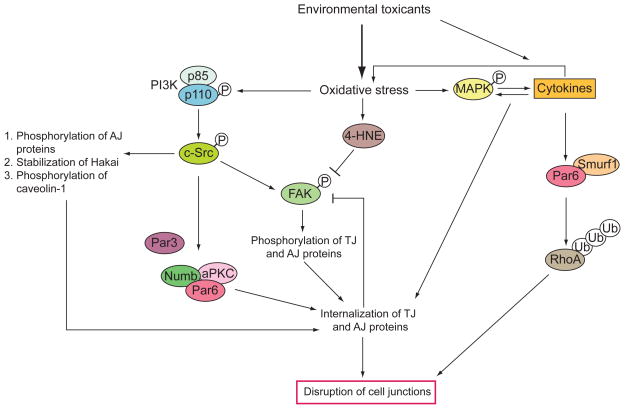
Figure 1. Signaling pathways activated by environmental toxicants via increase in oxidative stress.
Oxidative stress induced by environmental toxicants activates the PI3K/c-Src/FAK pathway, which subsequently controls the phosphorylation of caveolin-1, TJ and/or AJ proteins, the stability of E3 ubiquitin ligase Hakai and the interaction of polarity proteins with the endocytic adaptor Numb. This leads to the internalization of TJ and AJ proteins at the cell-cell interface, which imposes a feedback mechanism to dephosphorylate FAK. Aldehydes such as 4-hydroxy-2-nonenal (4-HNE) are produced during oxidative stress to inactivate FAK. In addition, environmental toxicants induce the production of cytokines which are also regulated by the activation of MAPK via oxidative stress. Cytokines stimulates production of reactive oxygen species (ROS) from leukocytes to further increase oxidative stress. Cytokines and the activation of MAPK together result in endocytic vesicle-mediated internalization of TJ and AJ proteins. Note that polarity proteins such as Par6 are also involved in mediating the action of cytokines to recruit the E3 ubiquitin ligase Smurf1 for the polyubiquitination and degradation of RhoA, which is important for the disruption of cell junctions. This illustrates that crosstalk exists between the PI3K/c-Src/FAK and cytokines/MAPK pathways via polarity proteins as their common downstream signaling mediators.
FAK is a target of the PI3K/c-Src signaling pathway during environmental toxicant-induced junction disruption
In epithelial cells, FAK is a substrate of c-Src, and the FAK-Src dual kinase complex is known to regulate multiple cellular events in physiological (e.g., cell migration) and pathological conditions (e.g., tumor development) [51, 52]. Indeed, FAK is a downstream mediator of the PI3K/c-Src signaling pathway in oxidative-stress induced junction disruption [45, 53, 54]. It is believed that membrane translocation and activation of PI3K and c-Src by oxidative stress activates FAK via phosphorylation. This causes an increase in tyrosine phosphorylation of junction proteins (e.g., occludin, N-cadherin) by FAK to alter the adhesive function of protein complexes at TJ and/or AJ. Interestingly, depending on the cell type, the source of ROS and the duration of treatment, FAK can be dephosphorylated [55–58]. Altogether, it is likely that, at the initial phase during oxidative stress, FAK is activated by c-Src to cause unwanted phosphorylation of junction proteins at the cell-cell interface. This induces redistribution of proteins from the cell-cell interface to the cytosol and leads to the disruption of TJ and AJ [41]. In addition, cell adhesion is further compromised by the dissociation of integral membrane proteins (e.g., occludin and N-cadherin) with the corresponding cytoplasmic adaptors (e.g. ZO-1 and β-catenin) [41, 43]. Disassembly of focal adhesion contact (e.g., by decreasing and/or inactivation of integrins [56]) and the formation of biologically active aldehydes during oxidative stress (e.g., 4-hydroxy-2-nonenal, 4-HNE [59]) then inactivate FAK via dephosphorylation. Endocytosed integral membrane junction proteins eventually undergo endosome-mediated intracellular degradation which further destabilizes junctions at the cell-cell interface. Furthermore, a biphasic effect on the phosphorylation of FAK has been reported, illustrating that the duration of oxidative stress is a critical factor in determining the activation or inactivation of FAK in cell epithelium [55]. These results illustrate that FAK is a critical mediator of TJ and AJ disruption during oxidative stress. Therefore, the critical phosphorylation site of FAK (e.g., Tyr397) might provide a novel therapeutic target to protect the testis from oxidative stress.
This hypothesis is further supported by findings that illustrate FAK is a molecular target of cadmium in the testis [50]. FAK is one of the best studied non-receptor tyrosine kinases in the testis [50, 60–62]. FAK structurally interacts with the occludin-ZO-1 protein complex between Sertoli cells at the BTB in the seminiferous epithelium [50, 60]. In addition, the activated and phosphorylated forms of FAK (e.g. phospho-FAK- Tyr397 and phospho-FAK-Tyr576) are found to associate with β1-integrin between elongating/elongated spermatids and Sertoli cells at the apical ES [62]. Cadmium is known to disrupt cell junctions in the testis, such as the Sertoli cell BTB, by accelerating endocytosis of integral membrane proteins (e.g., occludin, ZO-1 and N-cadherin) (Figure 2) [60]. A knockdown of FAK by RNAi in Sertoli cell epithelium with an established functional BTB was shown to desensitize Sertoli cells to cadmium-induced TJ barrier disruption [50]. This, at least in part, is due to the loss of FAK to elicit redistribution of ZO-1 from the cell-cell interface to cytosol to maintain cell junction integrity [50]. Furthermore, due to the physical intimacy between FAK and junction proteins at the BTB, FAK mediates the disruption of the BTB (which occurs at ~ 7–16 h post-cadmium treatment at 3 mg/kg b.w., i.p.) more rapidly and efficiently than that of the endothelial TJ-barrier in the interstitial space, (which occurs at ~20–24 h) where FAK is restricted to the focal adhesion contact [49, 60] and is not an integrated component of the occludin-ZO-1 protein complex in the TJ-barrier of the microvessel [52, 63]. The unique distribution of FAK at the BTB thus explains the unusual susceptibility of the testis to cadmium-induced damage. Although it remains unknown if FAK mediates other environmental toxicant-induced testicular dysfunction at the BTB, evidence gathered from oxidative stress studies [45, 53, 57] supports the notion that FAK is likely the common target in mediating the cell junction damage caused by environmental toxicants.
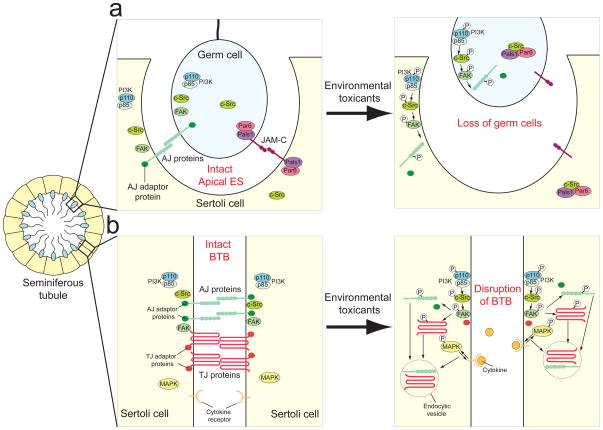
Figure 2. Pathophysiological effects of environmental toxicants in the seminiferous epithelium of mammalian testes.
a) At the apical ES, AJ proteins (e.g. N-cadherin and nectin) are present to adhere germ cells onto the Sertoli cell in the seminiferous epithelium. JAM-C, which is regarded as a TJ protein in epithelial cells, is also found at the apical ES, although no TJ ultrastructure is visible under electron microscopy between elongating spermatids and Sertoli cells. After exposure to environmental toxicants, the PI3K/c-Src/FAK pathway is activated to phosphorylate AJ proteins. This causes the internalization of AJ proteins and dissociation from their corresponding adaptors. Adhesion of germ cells in the seminiferous epithelium is further weakened by the increase in association between c-Src and Par6/Pals1 complex, which sequesters them from JAM-C, thereby destabilizing the JAM-C-based adhesion protein complexes. As a result, germ cells eventually are released from the seminiferous epithelium prematurely due to a disruption of adhesion complexes at the apical ES. b) At the BTB, activation of PI3K/c-Src/FAK pathway by environmental toxicants causes the phosphorylation of TJ and AJ proteins. Furthermore, induction of cytokines (e.g., TGF-β3) and activation of MAPK also leads to enhancement in endocytosis of junction proteins. The cell junctions at the BTB are disrupted, which destroys the microenvironment necessary for normal germ cell development. This reduces sperm count and normal sperm, leading to male infertility or subfertility.
Activation of c-Src enhances endocytosis of junction proteins
In addition to activating FAK, c-Src participates directly in the regulation of cell junctions. Elevated expression and/or activity of c-Src is widely observed in multiple types of carcinoma. In particular, activation of c-Src is linked to cancer progression, which is signified by the loss of cell-cell adhesion and cell polarity [63, 64]. In agreement with the observations in tumor cells, overexpression and/or activation of c-Src always leads to disruption of cell junctions in normal epithelial and endothelial cells [65, 66]. It is also commonly activated during environmental toxicant-induced junction disruption [45, 67]. In response to oxidative stress, Tyr418 of c-Src is known to be phosphorylated for its activation [68, 69]. Meanwhile, c-Src undergoes a conformational change that releases the auto-inhibitory C-terminal domain to allow access of substrates to the catalytic kinase domain [64].
Activation of c-Src leads to a disruption of cell junctions via three pathways. First, c-Src activation is known to induce phosphorylation of AJ proteins, such as E-cadherin, N-cadherin and p120ctn [65, 70]. p120ctn is a substrate of c-Src and a peripheral adaptor of cadherins. Its association with cadherins is required to maintain the localization of cadherins on the cell surface [71]. Tyrosine phosphorylation of p120ctn by oxidative stress-induced c-Src activation triggers the translocation of p120ctn from the plasma membrane to early endosomal compartment. This leads to internalization and endosome-mediated degradation of N-cadherin and disassembly the AJ. Interestingly, phosphorylation of p120ctn by c-Src is isoform-specific which demonstrates specificity in the kinase substrate [70]. Second, c-Src functions to stabilize Hakai, an E3 ubiquitin-ligase involved in ubiquitination of E-cadherin. Activation of c-Src increases the association between E-cadherin and Hakai, which in turn causes the ubiquitination and internalization of E-cadherin [65]. Furthermore, binding of Hakai to E-cadherin competes with the interaction of p120ctn to E-cadherin which further enhances the endocytosis of E-cadherin [65]. Third, an activation of c-Src can induce the phosphorylation of the constituent protein of caveolae, caveolin-1, to interfere with the endocytosis and/or transcytosis of cell junction proteins [68]. For example, c-Src phosphorylates Tyr14 of caveolin-1 during oxidative damage to facilitate the dissociation of VE-cadherin and β–catenin [67]. Such disassembly of AJ adhesion complex is accompanied by an increase in endocytosis and transcytosis of internalized proteins. These observations were supported by findings showing that caveolin-1−/− mice were resistant to oxidative stress-induced damage in vascular permeability [68]. In this context, it is of interest to note that environmental toxicants (e.g., BPA) that can mediate their action through the non-genomic pathway can exert their effects via PI3K/c-Src and/or MAPK (see below) [20, 72, 73]. However, it remains to be determined if changes in protein ubiquitination, endocytosis, recycling and/or transcytosis are involved.
Activation of MAPKs by environmental toxicants
The MAPK pathways have emerged as a common signaling platform for multiple environmental toxicants (Figure 1). Three MAPKs, namely extracellular-signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38, have been shown to be activated in the testis after exposure to environmental toxicants (Table 1). MAPKs are involved in regulating normal reproductive functions in the testis, which include spermatogenesis (e.g., cell cycle progression, meiosis, BTB dynamics, cell adhesion dynamics and spermiogenesis), steroidogenesis, sperm hyperactivation and acrosome reaction [74, 75]. As a result, unregulated activation of MAPKs by environmental toxicants imposes an array of pathophysiological effects on Sertoli, germ and Leydig cells in the testis. These include an increase in DNA damage and apoptosis, disruption of cell junctions and steroidogenesis [76, 77]. MAPKs are activated by oxidative stress induced by environmental toxicants in cells and tissues. For example, blocking oxidative stress by free radical scavengers (e.g., N-acetyl cysteine), reverses cadmium-induced MAPK activation [78, 79]. This phenomenon is partly regulated by the inhibition of Ser/Thr protein phosphatases 2A (PP2A) and 5 (PP5) by oxidative stress, which results in an increase in phosphorylation of MAPK [79]. Unexpectedly, cadmium induces the expression of MAPK phosphatase-1 (MKP-1), [80] a major inhibitor of MAPK activation. This illustrates that the protective effect of MKP-1 to inactivate MAPK is superseded by inhibition of protein phosphatases PP2A and PP5, resulting in an overall increase in MAPK signaling after exposure to environmental toxicants. In addition, activation of ERK can lead to phosphorylation of c-Src, FAK and paxillin under oxidative stress, implying that MAPKs might be one of the upstream targets to activate these non-receptor tyrosine kinases [55].
Table 1.
Pathophysiological effects of environmental toxicants in the testis via activation of MAPK and cytokines
| Chemical group | Environmental toxicant | Animal model/Cell type | MAPK activated | Cellular responses | Effects on cytokines production |
|---|---|---|---|---|---|
| Heavy metal | Cadmium | Primary pig Sertoli cells | p38 | ↓ cell proliferation, ↑ DNA damage, ↑ apoptosis | N. D. * |
| Rat testis | p38, JNK | ↓ TJ and AJ protein levels but ↑ in p120ctn, ↑ α2-macroglobulin, damage of BTB, apical ES and endothelial TJ, ↑ premature release of germ cells, ↑ redistribution of TJ proteins from cell-cell interface to cytoplasm, ↑ association between FAK, occludin and ZO-1 | ↑TGFβ2, TGFβ3 | ||
| Rat Sertoli cell-gonocyte coculture | JNK, p38 | ↑ apoptosis, ↑ polyubiquitination of proteins, ↑ detachment of gonocytes from Sertoli cells | N. D. | ||
| Environmental phenols | Bisphenol A | Rat testis, primary rat Sertoli cells | ERK | Disruption of BTB in immature rat, ↓ occludin and N-cadherin on Sertoli cell surface, ↓ TJ, AJ and gap junction protein levels, ↑ internalization of TJ and AJ proteins, delay gap junction communication | N. D. |
| Rat testis | ERK | ↑ Raf1 and phosphotyrosine proteins, ↓ in gonocytes proliferation but transient ↑ in spermatogonia and Leydig cell number | N. D. | ||
| Rat Leydig cells (R2C cells) | ERK, JNK, p38 | ↑ aromatase, cyclooxygenase-2, prostaglandin E2, ↓ testosterone, activation of protein kinase A and B | N. D. | ||
| organic polychlorinated dibenzodioxins | 2, 3, 7, 8-tetrachlorodiben zo-p-dioxin | Mouse testis | JNK, p38, ERK | ↓ antioxidant enzymes, activation of Smad2, ↑ c-Jun and ATF3 | ↑ TGFβ1 |
| Polycyclic aromatic hydrocarbons | Rat testis | N. D. | ↓ spermatid number, testosterone and superoxide dismutase activity | ↑ IL-6 |
N.D.: not determined.
Abbreviations: AJ, adherens junction; BTB, blood-testis barrier; ERK, extracellular-signal-regulated kinase; ES, ectoplasmic specialization; FAK, focal adhesion kinase; IL-6, interleukin-6; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; TGF, transforming growth factor; TJ, tight junction.
Activation of MAPKs by environmental toxicants also upregulates the expression of pro-inflammatory cytokines such as tumor necrosis factor-α (TNFα) in macrophages and monocytes [81, 82], which can diffuse from the microvessels in the interstitial space and disrupt the BTB because they are known to perturb the Sertoli cell TJ-permeability barrier (Figure 1 and 2) [83, 84]. Similarly, cadmium and pollutants from motorcycle exhaust (e.g., polycyclic aromatic hydrocarbons) increase the expression of transforming growth factor-β (TGF-β) and interleukin-6 (IL-6) in the testis, respectively [85, 86]. TNFα, TGF-β and IL-1α are known to disrupt Sertoli-Sertoli and Sertoli-germ cell junctions via down-regulation [83, 87] and/or redistribution of junctional proteins [88–90], such as occludin, ZO-1 and N-cadherin, in the seminiferous epithelium. Consequently, the loss of integral membrane proteins at the cell-cell interface causes a disruption of the BTB and adhesion of germ cells in the seminiferous epithelium, both leading to premature release of germ cells from the epithelium and thereby infertility [83, 87, 88]. Furthermore, it is important to note that pro-inflammatory cytokines (e.g. IL-6 and TNFα) activates leukocytes to produce ROS which amplifies the deleterious effects of environmental toxicant-induced oxidative stress [25, 37]. Intriguingly, further studies in the testis found that cytokines (e.g. TGF-β3) utilize the MAPK pathways to disrupt cell junctions [91].
Thus, MAPKs are both the stimulator and mediator of cytokines induced by environmental toxicants. The involvement of MAPKs in environmental toxicant-induced signaling is further complicated by the self-protective program of the testis. This protective action is mediated by the JNK pathway to induce a non-specific protease inhibitor (α2-macroglobulin) to limit the damage caused by cadmium [92]. Although α2-macroglobulin cannot rescue germ cell loss from the seminiferous epithelium and the irreversible BTB damage, it protects the testis from unwanted proteolysis during the cascade of events that leads to germ cell loss from the testis [49, 50, 92].
Endocytosis of junction proteins via Src- and cytokine-signaling is mediated by polarity proteins
In addition to Hakai and caveolin-1, c-Src controls endocytosis of integral membrane proteins at cell junctions by regulating polarity proteins [e.g., Par3, Par6 and atypical PKC (aPKC)] via an endocytic adaptor protein, Numb (Figure 1) [93]. Studies in kidney and intestinal epithelial cells have shown that polarity proteins function as spatial cues for the establishment and maintenance of intercellular junctions. It is known that loss of polarity proteins results in either a delay in the formation or a complete loss of cell-cell junctions [94]. Hence, the precise positioning of polarity proteins in a cell epithelium is essential for the integrity of cell junctions. By contrast to the majority of epithelial cells (in which polarity proteins are restricted to the TJ), Par6 is found at the apical ES, a testis-specific anchoring junction, between Sertoli cells and elongating spermatids in the testis (where no TJ ultrastructure is present when examined by electron microscopy (Figure 2) [95]). Similarly, several polarity proteins such as Par3, aPKC and 14-3-3ζ are found either at the apical ES or expressed in germ cells [95, 96]. Nevertheless, recent studies have shown that polarity proteins also function as crucial molecules to regulate cell junction integrity in the testis.
The first piece of evidence comes from the observation that Par6 is absent from the apical ES just several hours before this ultrastructure undergoes disassembly to release mature spermatids from the seminiferous epithelium to tubule lumen at spermiation [95]. To validate that Par6 contributes to the maintenance of cell junctions at the apical ES, adjudin [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydraizde] was administered to adult rats in vivo to induce premature release of spermatids from the seminiferous epithelium[95]. In elongating spermatids that were about to deplete from the seminiferous epithelium prematurely, a loss of Par6 at the apical ES was detected. This was accompanied by a defragmentation of actin filament bundles that are necessary to maintain the apical ES integrity. These results thus indicate the pivotal role of Par6 for the adhesion of elongated spermatids by maintaining the actin filament bundles at the apical ES [95]. In this context, Par6 likely coordinates with the actin nucleation protein Arp3, which is detected predominantly at the apical ES (alongside with Par6) at stage VII to early VIII tubules. Together, they regulate the highly organized hexagonally packed actin filament bundles and facilitate endocytosis of integral membrane proteins at the apical ES prior to spermiation [97]. This idea is supported by findings showing that Arp3 is an effector downstream of the Par6/aPKC/Cdc42 complex that is necessary for the localized branching of the actin filament network and endocytosis of E-cadherin [98, 99]. Subsequently, the expression of Par6 and Arp3 diminishes rapidly to allow the disintegration of the actin filament bundles, which, in turn, allows the apical ES to facilitate spermiation at late stage VIII of the seminiferous epithelial cycle.
In v-Src-transformed MDCK cells in which Src kinase is constitutively active, Numb binds preferentially to aPKC and Par6 instead of E-cadherin and Par3 [93]. The dissociation of Numb and E-cadherin appears to be required for the internalization of E-cadherin from the cell surface to the cytosol. This conclusion is further supported by the observation that the presence of Numb is important for the proper localization of E-cadherin at the cell-cell interface [93]. Intriguingly, phosphorylation of Numb by aPKC decreases the affinity of Numb for integrin and prevents the internalization of integrin in HeLa cells [100]. This discrepancy might be explained by the specificity of Numb to link selective transmembrane proteins to α-adaptin, a subunit of the adaptor protein (AP) 2 complex that is essential for the assembly and sorting of cargoes into clathrin-coated endocytic-vesicular structures for endocytosis [101]. Furthermore, it is not clear if the dissociation of Numb from E-cadherin upon c-Src phosphorylation is actually essential to facilitate the binding of Hakai to E-cadherin for its endocytosis because Numb and Hakai bind to identical tyrosine resides of E-cadherin [65, 93]. These findings implicate that distinct mechanisms are used to regulate cadherin- and integrin-mediated adhesion by c-Src via Numb and polarity proteins.
Future investigation will generate a clearer picture as more polarity proteins are found to be substrates for c-Src [102]. Although it remains to be determined if environmental toxicant-induced c-Src activation phosphorylates polarity proteins or Numb in the testis, the interaction between Src kinase and Par6 is known to be important for the integrity of apical ES for elongating spermatids adhesion (Figure 2) [95]. This is illustrated by the increase in affinity of Src kinase for Par6 via adaptor protein Pals1 before adjudin-induced disruption of the apical ES. Such enhanced interaction among Src kinase, Par6 and Pals1 is likely for the phosphorylation of Par6 and/or Pals1 by Src kinase [95]. Nevertheless, the molecular targets (e.g., integral membrane proteins) downstream of this signaling pathway remain to be elucidated.
Not surprisingly, cytokines that are induced following exposure to environmental toxicants also regulate cell junctions via polarity proteins, similar to c-Src (Figure 1). Par6 is a substrate of receptor tyrosine kinase TGFβ receptor II(TβRII) [103]. TGFβ stimulation recruits TβRII to phosphorylate Par6 at the cell surface which constantly interacts with endogenous TβRI. Non-phosphorylatable Par6 protects epithelial cells from TGFβ-induced junction disruption, illustrating that Par6 is essentially the mediator in this signaling pathway [103]. Ser-phosphorylated Par6 by TβRII promotes the assembly of E3 ubiquitin ligase Smurf1 to cell-cell interface [103]. Currently, it is not clear if Smurf1 directly facilitates the ubiquitination of cell junction proteins, although the TJ protein occludin associates with the TβRI/II/Par6 complex. Nevertheless, ubiquitination of Ras homolog gene family, member A (RhoA) by Smurf1 certainly plays a pivotal role in this process [103]. Cytokines (e.g., TGFβ3 and TNFα) have recently shown to regulate junction dynamics in the testis via endocytic vesicle-mediated pathway [89, 90, 104, 105]. Significantly, TGFβ3-enhanced endocytosis is mediated by the activation of Cdc42 in the Sertoli cell epithelium [89]. Activated Cdc42 is part of the Par6 complex, and both have been shown to be required for endocytic vesicle-mediated protein trafficking in epithelial cells [106]. Together, these data strongly suggest that polarity proteins are involved in cytokine-mediated cell junction dynamics in the testis, and they are critical components of the environmental toxicant-mediated damage to cells and tissues via oxidative stress.
Concluding remarks
The male reproductive system has emerged as one of the major toxicity targets of environmental toxicants. Although acute exposure of toxicants contributes to apoptosis and necrosis of testicular cells, chronic and sub-lethal exposure is prevailing in the general public [38]. Due to the unusual long half-lives of some of these toxicants in mammalian body (e.g. cadmium has a mean half-life of 15 years [107], chronic and low level exposure to humans could cause long-term unwanted health effects. We highlighted the disruptive effects of environmental toxicants on cell junctions mediated by non-receptor tyrosine kinases (e.g. c-Src and FAK) and cytokines through oxidative stress because these damages are often observed in low level exposure before apoptosis takes place. [108] Significantly, these signaling pathways converge to utilize polarity proteins to regulate intercellular junctions. Polarity proteins, which are known to control cell adhesion in the testis, thus emerge as novel targets for therapeutic intervention to limit environmental toxicant-induced infertility. Although it is equally important to study the epigenetic (e.g., vinclozolin) and endocrine disruptive (e.g. BPA, dioxin, cadmium) effects of environmental toxicants, it is increasingly clear that these toxicants are imposing immediate deleterious effect in the testis via disruption of cell junctions between testicular cells due to increase in oxidative stress. In addition, endocrine-disrupting toxicants that affect estrogen levels might cause a disturbed balance of ROS and oxidative stress as estrogen is an important free radical scavenger in human [109, 110], besides being essential for spermatogenesis [111].
Recently, various studies have emphasized on the importance of assessing the effects of a mixture of environmental toxicants on male reproductive functions because humans are exposed to an array of chemicals which might antagonize or agonize each other [38, 108]. Although this kind of study is inherently difficult to perform, it is crucial for a full understanding of the impact of environmental toxicants on the reproductive system. Studies showing antioxidant (e.g. vitamin E and selenium) supplementation therapy has some success in term of improving sperm qualities (e.g. sperm motility) [25, 112] offer the potential for treatment. However, much work is needed to understand the precise molecular events and mechanism(s) regulated by environmental toxicants to target c-Src, FAK, MAPK and polarity proteins in the testis, in the hope of identifying specific phosphorylation targets or isoforms so that small molecule agonists and/or antagonists can be designed to limit systemic toxicity in vivo.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NICHD U54 HD029990 Project 3, R01 HD056034, and R01 HD056034-02S1 to CYC). EWPW was supported by a postdoctoral fellowship from the University of Hong Kong (Hong Kong, China) and the Noopolis Foundation (Rome, Italy).
Footnotes
Disclosure
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharlip ID, et al. Best practice policies for male infertility. Fertil Steril. 2002;77:873–882. doi: 10.1016/s0015-0282(02)03105-9. [DOI] [PubMed] [Google Scholar]
- 2.Juul S, et al. Regional differences in waiting time to pregnancy: pregnancy-based surveys from Denmark, France, Germany, Italy and Sweden. The European Infertility and Subfecundity Study Group. Hum Reprod. 1999;14:1250–1254. doi: 10.1093/humrep/14.5.1250. [DOI] [PubMed] [Google Scholar]
- 3.Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod. 1998;13(Suppl 1):33–44. doi: 10.1093/humrep/13.suppl_1.33. [DOI] [PubMed] [Google Scholar]
- 4.Kashir J, et al. Oocyte activation, phospholipase C zeta and human infertility. Hum Reprod Update. 2010;16:690–703. doi: 10.1093/humupd/dmq018. [DOI] [PubMed] [Google Scholar]
- 5.Aitken RJ, et al. Seeds of concern. Nature. 2004;432:48–52. doi: 10.1038/432048a. [DOI] [PubMed] [Google Scholar]
- 6.Wong EW, et al. Cell junctions in the testis as targets for toxicants. In: Richburg JH, Hoyer P, editors. Comprehesive toxicology. Academic Press; 2010. pp. 167–188. [Google Scholar]
- 7.Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1697–1712. doi: 10.1098/rstb.2009.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siu ER, et al. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 2009;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong EW, et al. Cell junctions in the testis as targets for toxicants. In: McQueen CA, Hoyer PB, Richburg JH, editors. Comprehensive Toxicology. 2. Vol. 11. Oxford: Academic Press, Elseiver; 2010. pp. 167–188. Reproductive and Endocrine Toxicology. [Google Scholar]
- 10.Wirth JJ, Mijal RS. Adverse effects of low level heavy metal exposure on male reproductive function. Syst Biol Reprod Med. 56:147–167. doi: 10.3109/19396360903582216. [DOI] [PubMed] [Google Scholar]
- 11.Calafat AM, et al. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welshons WV, et al. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 13.Xia Y, et al. Urinary metabolites of polycyclic aromatic hydrocarbons in relation to idiopathic male infertility. Hum Reprod. 2009;24:1067–1074. doi: 10.1093/humrep/dep006. [DOI] [PubMed] [Google Scholar]
- 14.Anway MD, et al. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerrero-Bosagna CM, Skinner MK. Epigenetic transgenerational effects of endocrine disruptors on male reproduction. Semin Reprod Med. 2009;27:403–408. doi: 10.1055/s-0029-1237428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skinner MK, et al. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skinner MK, et al. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diamanti-Kandarakis E, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutts SM, et al. Environmental toxicant-induced germ cell apoptosis in the human fetal testis. Hum Reprod. 2007;22:2912–2918. doi: 10.1093/humrep/dem300. [DOI] [PubMed] [Google Scholar]
- 20.Bouskine A, et al. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ Health Perspect. 2009;117:1053–1058. doi: 10.1289/ehp.0800367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuiper GG, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 22.Nadal A, et al. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci U S A. 2000;97:11603–11608. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt PA, et al. The bisphenol A experience: a primer for the analysis of environmental effects on mammalian reproduction. Biol Reprod. 2009;81:807–813. doi: 10.1095/biolreprod.109.077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma RK, et al. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 1999;14:2801–2807. doi: 10.1093/humrep/14.11.2801. [DOI] [PubMed] [Google Scholar]
- 25.Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal A, et al. Prevention of oxidative stress injury to sperm. J Androl. 2005;26:654–660. doi: 10.2164/jandrol.05016. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, et al. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol. 2009;238:209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mates JM, et al. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic Biol Med. doi: 10.1016/j.freeradbiomed.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 29.Kabuto H, et al. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 2004;74:2931–2940. doi: 10.1016/j.lfs.2003.07.060. [DOI] [PubMed] [Google Scholar]
- 30.Jin MH, et al. Enhanced TGF-beta1 is involved in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induced oxidative stress in C57BL/6 mouse testis. Toxicol Lett. 2008;178:202–209. doi: 10.1016/j.toxlet.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Dhanabalan S, Mathur PP. Low dose of 2,3,7,8 tetrachlorodibenzo-p-dioxin induces testicular oxidative stress in adult rats under the influence of corticosterone. Exp Toxicol Pathol. 2009;61:415–423. doi: 10.1016/j.etp.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Mates JM, et al. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic Biol Med. 2010;49:1328–1341. doi: 10.1016/j.freeradbiomed.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Latchoumycandane C, Mathur PP. Effects of vitamin E on reactive oxygen species-mediated 2,3,7,8-tetrachlorodi-benzo-p-dioxin toxicity in rat testis. J Appl Toxicol. 2002;22:345–351. doi: 10.1002/jat.866. [DOI] [PubMed] [Google Scholar]
- 34.Acharya UR, et al. Effect of vitamins C and E on spermatogenesis in mice exposed to cadmium. Reprod Toxicol. 2008;25:84–88. doi: 10.1016/j.reprotox.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Turner TT, Lysiak JJ. Oxidative stress: a common factor in testicular dysfunction. J Androl. 2008;29:488–498. doi: 10.2164/jandrol.108.005132. [DOI] [PubMed] [Google Scholar]
- 36.Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr Med Chem. 2001;8:851–862. doi: 10.2174/0929867013373039. [DOI] [PubMed] [Google Scholar]
- 37.Fraczek M, Kurpisz M. Inflammatory mediators exert toxic effects of oxidative stress on human spermatozoa. J Androl. 2007;28:325–333. doi: 10.2164/jandrol.106.001149. [DOI] [PubMed] [Google Scholar]
- 38.Hauser R, Sokol R. Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult male. Fertil Steril. 2008;89:e59–65. doi: 10.1016/j.fertnstert.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 39.Lucas R, et al. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol. 2009;77:1763–1772. doi: 10.1016/j.bcp.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Rao RK, et al. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cannito S, et al. Epithelial-mesenchymal transition: from molecular mechanisms, redox regulation to implications in human health and disease. Antioxid Redox Signal. 2010;12:1383–1430. doi: 10.1089/ars.2009.2737. [DOI] [PubMed] [Google Scholar]
- 43.Sheth P, et al. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem. 2003;278:49239–49245. doi: 10.1074/jbc.M305654200. [DOI] [PubMed] [Google Scholar]
- 44.Qin S, Chock PB. Implication of phosphatidylinositol 3-kinase membrane recruitment in hydrogen peroxide-induced activation of PI3K and Akt. Biochemistry. 2003;42:2995–3003. doi: 10.1021/bi0205911. [DOI] [PubMed] [Google Scholar]
- 45.Basuroy S, et al. Hydrogen peroxide activates focal adhesion kinase and c-Src by a phosphatidylinositol 3 kinase-dependent mechanism and promotes cell migration in Caco-2 cell monolayers. Am J Physiol Gastrointest Liver Physiol. 2010;299:G186–195. doi: 10.1152/ajpgi.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li MW, et al. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci U S A. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan HH, Cheng CY. Laminin alpha 3 forms a complex with beta3 and gamma3 chains that serves as the ligand for alpha 6beta1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- 48.el-Sabeawy F, et al. Treatment of rats during pubertal development with 2,3,7,8-tetrachlorodibenzo-p-dioxin alters both signaling kinase activities and epidermal growth factor receptor binding in the testis and the motility and acrosomal reaction of sperm. Toxicol Appl Pharmacol. 1998;150:427–442. doi: 10.1006/taap.1998.8426. [DOI] [PubMed] [Google Scholar]
- 49.Wong CH, et al. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- 50.Siu ER, et al. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci U S A. 2009;106:9298–9303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brunton VG, Frame MC. Src and focal adhesion kinase as therapeutic targets in cancer. Curr Opin Pharmacol. 2008;8:427–432. doi: 10.1016/j.coph.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Bolos V, et al. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco Targets Ther. 2010;3:83–97. doi: 10.2147/ott.s6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sen U, et al. Homocysteine-induced biochemical stress predisposes to cytoskeletal remodeling in stretched endothelial cells. Mol Cell Biochem. 2007;302:133–143. doi: 10.1007/s11010-007-9435-4. [DOI] [PubMed] [Google Scholar]
- 54.Sen U, et al. Homocysteine-induced myofibroblast differentiation in mouse aortic endothelial cells. J Cell Physiol. 2006;209:767–774. doi: 10.1002/jcp.20752. [DOI] [PubMed] [Google Scholar]
- 55.Alderliesten M, et al. Extracellular signal-regulated kinase activation during renal ischemia/reperfusion mediates focal adhesion dissolution and renal injury. Am J Pathol. 2007;171:452–462. doi: 10.2353/ajpath.2007.060805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molina A, et al. Renal ischemia/reperfusion injury: functional tissue preservation by anti-activated {beta}1 integrin therapy. J Am Soc Nephrol. 2005;16:374–382. doi: 10.1681/ASN.2004070528. [DOI] [PubMed] [Google Scholar]
- 57.Saenz-Morales D, et al. Requirements for proximal tubule epithelial cell detachment in response to ischemia: role of oxidative stress. Exp Cell Res. 2006;312:3711–3727. doi: 10.1016/j.yexcr.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 58.Weinberg JM, et al. Energetic determinants of tyrosine phosphorylation of focal adhesion proteins during hypoxia/reoxygenation of kidney proximal tubules. Am J Pathol. 2001;158:2153–2164. doi: 10.1016/S0002-9440(10)64687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Usatyuk PV, et al. Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J Biol Chem. 2006;281:35554–35566. doi: 10.1074/jbc.M607305200. [DOI] [PubMed] [Google Scholar]
- 60.Siu ER, et al. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009;150:3336–3344. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beardsley A, et al. A complex containing alpha6beta1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J Endocrinol. 2006;190:759–770. doi: 10.1677/joe.1.06867. [DOI] [PubMed] [Google Scholar]
- 62.Siu MK, et al. Adhering junction dynamics in the testis are regulated by an interplay of beta 1-integrin and focal adhesion complex-associated proteins. Endocrinology. 2003;144:2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- 63.Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol. 2005;17:542–547. doi: 10.1016/j.ceb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Kim LC, et al. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 65.Fujita Y, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 66.Gong P, et al. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem. 2008;283:13437–13449. doi: 10.1074/jbc.M707986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park S, et al. Rapid activation of c-Src kinase by dioxin is mediated by the Cdc37-HSP90 complex as part of Ah receptor signaling in MCF10A cells. Biochemistry. 2007;46:899–908. doi: 10.1021/bi061925f. [DOI] [PubMed] [Google Scholar]
- 68.Sun Y, et al. Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ Res. 2009;105:676–685. 615. doi: 10.1161/CIRCRESAHA.109.201673. following 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basuroy S, et al. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem. 2003;278:11916–11924. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- 70.Inumaru J, et al. Molecular mechanisms regulating dissociation of cell-cell junction of epithelial cells by oxidative stress. Genes Cells. 2009;14:703–716. doi: 10.1111/j.1365-2443.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 71.Reynolds AB. Exposing p120 catenin’s most intimate affair. Cell. 141:20–22. doi: 10.1016/j.cell.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 72.Kim KH, Bender JR. Membrane-initiated actions of estrogen on the endothelium. Mol Cell Endocrinol. 2009;308:3–8. doi: 10.1016/j.mce.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watson CS, et al. Nongenomic signaling pathways of estrogen toxicity. Toxicol Sci. 2010;115:1–11. doi: 10.1093/toxsci/kfp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Almog T, Naor Z. The role of Mitogen activated protein kinase (MAPK) in sperm functions. Mol Cell Endocrinol. 314:239–243. doi: 10.1016/j.mce.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 75.Li MW, et al. Mitogen-activated protein kinases in male reproductive function. Trends Mol Med. 2009;15:159–168. doi: 10.1016/j.molmed.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang M, et al. Cadmium suppresses the proliferation of piglet Sertoli cells and causes their DNA damage, cell apoptosis and aberrant ultrastructure. Reprod Biol Endocrinol. 2010;8:97. doi: 10.1186/1477-7827-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim JY, et al. Bisphenol A-induced aromatase activation is mediated by cyclooxygenase-2 up-regulation in rat testicular Leydig cells. Toxicol Lett. 2010;193:200–208. doi: 10.1016/j.toxlet.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 78.Xu J, et al. Mediation of cadmium-induced oxidative damage and glucose-6-phosphate dehydrogenase expression through glutathione depletion. J Biochem Mol Toxicol. 2003;17:67–75. doi: 10.1002/jbt.10062. [DOI] [PubMed] [Google Scholar]
- 79.Chen L, et al. Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic Biol Med. 2008;45:1035–1044. doi: 10.1016/j.freeradbiomed.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 80.Kim SM, et al. Cadmium specifically induces MKP-1 expression via the glutathione depletion-mediated p38 MAPK activation in C6 glioma cells. Neurosci Lett. 2008;440:289–293. doi: 10.1016/j.neulet.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 81.Haase H, et al. Cadmium ions induce monocytic production of tumor necrosis factor-alpha by inhibiting mitogen activated protein kinase dephosphorylation. Toxicol Lett. 2010;198:152–158. doi: 10.1016/j.toxlet.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Lecureur V, et al. ERK-dependent induction of TNFalpha expression by the environmental contaminant benzo(a)pyrene in primary human macrophages. FEBS Lett. 2005;579:1904–1910. doi: 10.1016/j.febslet.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 83.Li MW, et al. Tumor necrosis factor {alpha} reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 84.Siu MK, et al. The interplay of collagen IV, tumor necrosis factor-alpha, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 85.Huang JY, et al. Motorcycle exhaust induces reproductive toxicity and testicular interleukin-6 in male rats. Toxicol Sci. 2008;103:137–148. doi: 10.1093/toxsci/kfn020. [DOI] [PubMed] [Google Scholar]
- 86.Lui WY, et al. TGF-beta3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- 87.Xia W, et al. Differential interactions between transforming growth factor-beta3/TbetaR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem. 2006;281:16799–16813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- 88.Sarkar O, et al. Interleukin 1 alpha (IL1A) is a novel regulator of the blood-testis barrier in the rat. Biol Reprod. 2008;78:445–454. doi: 10.1095/biolreprod.107.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong EW, et al. Regulation of blood-testis barrier dynamics by TGF-beta3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci U S A. 2010;107:11399–11404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xia W, et al. TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lui WY, et al. Transforming growth factor beta3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003;68:1597–1612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- 92.Wong CH, et al. Blood-testis barrier dynamics are regulated by {alpha}2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- 93.Wang Z, et al. Numb regulates cell-cell adhesion and polarity in response to tyrosine kinase signalling. EMBO J. 2009;28:2360–2373. doi: 10.1038/emboj.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Assemat E, et al. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 95.Wong EW, et al. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci U S A. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wong EW, et al. 14-3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009 doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lie PP, et al. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci U S A. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leibfried A, et al. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol. 2008;18:1639–1648. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 99.Georgiou M, Baum B. Polarity proteins and Rho GTPases cooperate to spatially organise epithelial actin-based protrusions. J Cell Sci. 2010;123:1089–1098. doi: 10.1242/jcs.060772. [DOI] [PubMed] [Google Scholar]
- 100.Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 101.Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol. 2008;9:355–366. doi: 10.1038/nrm2388. [DOI] [PubMed] [Google Scholar]
- 102.Wang Y, et al. Tyrosine phosphorylated Par3 regulates epithelial tight junction assembly promoted by EGFR signaling. EMBO J. 2006;25:5058–5070. doi: 10.1038/sj.emboj.7601384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ozdamar B, et al. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 104.Yan HH, et al. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Su L, et al. Differential effects of testosterone and TGF-beta3 on endocytic vesicle-mediated protein trafficking events at the blood-testis barrier. Exp Cell Res. 316:2945–2960. doi: 10.1016/j.yexcr.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Balklava Z, et al. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol. 2007;9:1066–1073. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- 107.Kjellstrom T, Nordberg GF. A kinetic model of cadmium metabolism in the human being. Environ Res. 1978;16:248–269. doi: 10.1016/0013-9351(78)90160-3. [DOI] [PubMed] [Google Scholar]
- 108.Moline JM, et al. Exposure to hazardous substances and male reproductive health: a research framework. Environ Health Perspect. 2000;108:803–813. doi: 10.1289/ehp.00108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prokai L, et al. Mechanistic insights into the direct antioxidant effects of estrogens. Drug Develop Res. 2005;66:118–125. [Google Scholar]
- 110.Borras C, et al. Direct antioxidant and protective effect of estradiol on isolated mitochondria. Biochim Biophys Acta. 2010;1802:205–211. doi: 10.1016/j.bbadis.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 111.Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kefer JC, et al. Role of antioxidants in the treatment of male infertility. Int J Urol. 2009;16:449–457. doi: 10.1111/j.1442-2042.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 113.Wong EW, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol. 2009;278:309–353. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neil JD, editors. The Physiology of Reproduction. Raven Press; 1994. pp. 1363–1434. [Google Scholar]
- 116.Yu X, et al. Cadmium-induced activation of stress signaling pathways, disruption of ubiquitin-dependent protein degradation and apoptosis in primary rat Sertoli cell-gonocyte cocultures. Toxicol Sci. 2008;104:385–396. doi: 10.1093/toxsci/kfn087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li MW, et al. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41:2302–2314. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li MW, et al. Connexin 43 is critical to maintain the homeostasis of the blood-testis barrier via its effects on tight junction reassembly. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1007047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thuillier R, et al. Changes in MAPK pathway in neonatal and adult testis following fetal estrogen exposure and effects on rat testicular cells. Microsc Res Tech. 2009;72:773–786. doi: 10.1002/jemt.20756. [DOI] [PubMed] [Google Scholar]