Abstract
BACKGROUND
Functional neuroimaging studies suggest that chronic cocaine use is associated with frontal lobe abnormalities. Functional connectivity (FC) alterations of cocaine dependent individuals (CD), however, are not yet clear. This is the first study to our knowledge that examines resting FC of anterior cingulate cortex (ACC) in CD. Because ACC is known to integrate inputs from different brain regions to regulate behavior, we hypothesize that CD will have connectivity abnormalities in ACC networks. In addition, we hypothesized that abnormalities would be associated with poor performance in delayed discounting and reversal learning tasks.
METHODS
Resting functional magnetic resonance imaging data were collected to look for FC differences between twenty-seven cocaine dependent individuals (CD) (5 females, age: M=39.73, SD=6.14) and twenty-four controls (5 females, age: M=39.76, SD = 7.09). Participants were assessed with delayed discounting and reversal learning tasks. Using seed-based FC measures, we examined FC in CD and controls within five ACC connectivity networks with seeds in subgenual, caudal, dorsal, rostral, and perigenual ACC.
RESULTS
CD showed increased FC within the perigenual ACC network in left middle frontal gyrus, ACC and middle temporal gyrus when compared to controls. FC abnormalities were significantly positively correlated with task performance in delayed discounting and reversal learning tasks in CD.
CONCLUSIONS
The present study shows that participants with chronic cocaine-dependency have hyperconnectivity within an ACC network known to be involved in social processing and mentalizing. In addition, FC abnormalities found in CD were associated with difficulties with delay rewards and slower adaptive learning.
Keywords: cocaine, functional connectivity, anterior cingulate, delayed discount, reversal learning, frontal
Introduction
Drug addiction is considered the end of a continuum that ranges from controlled “social” use to a disorder characterized by loss of behavioral control (1,2). Loss of behavioral control has been confirmed in cocaine dependent individuals during tasks that require self-regulation and impulse control (3–6). Deficits in self-regulation and impulse control found in cocaine dependent individuals may be mediated by abnormal neural networks. While cocaine-dependency has been often associated with abnormalities in limbic networks involved in reward processes, results from several neuroimaging studies show the importance of frontal networks involved in self-regulation and cognitive processes in cocaine dependent individuals (7–15).
Several studies have identified frontal abnormalities associated with cocaine dependency. Animal studies have shown that prefrontal cortex, particularly the medial prefrontal region, plays a role on the reinforcing effects of cocaine (16,17). Volumetric studies have found decreased gray matter tissue density in medial and lateral orbitofrontal cortex, and middle/dorsal cingulate gyrus in cocaine dependent individuals (18,19). Disruption of frontal white matter connectivity has been found in cocaine dependent individuals (7,8,10,20). Functional neuroimaging studies have found a dysfunction in anterior cingulate cortex (ACC) when cocaine dependent individuals perform tasks that involve emotion (11) and executive functioning (12,13). These findings suggest that the affective control networks involving frontal regions of the brain may be altered in cocaine dependent individuals.
While frontal abnormalities have been associated with cocaine dependency, the nature of the relationship is unclear. Previous functional magnetic resonance imaging (fMRI) studies have found both hyperactivations and hypoactivations in ACC in cocaine dependent individuals. Cocaine dependent individuals have shown increased activation in ACC when viewing films of individuals smoking cocaine than when viewing films of outdoor nature scenes or explicit sex (11,21). However, cocaine dependent individuals have also shown decreased activation in ACC when viewing drug-related words than when viewing drug-neutral words (11). Previous conflicting results may be due to type of stimuli presented. Resting functional brain connectivity allows us to investigate abnormalities in specific neural networks (i.e. ACC networks) without the possibly confounding effects of stimulus presentation or task performance to better examine the relationship between chronic cocaine use and functional connectivity disruptions (22). To our knowledge, only one study has examined resting functional connectivity in cocaine dependent individuals (23). This study used predetermined seeds in the mesocorticolimbic reward network and reported decreased strength of functional connectivity within circuits of this network in cocaine dependent individuals compared to controls (23). While the previously conducted study was important to understand intrinsic functional connectivity abnormalities specific to the bottom-up reward pathways, the resting functional connectivity pathways associated with top-down control in cocaine users were not explored. In order to better understand neural networks associated with self-regulation and control characteristic to cocaine dependent individuals, the present study examined networks centered in frontal regions known to mediate self-regulatory control.
The present study addresses the important association between impaired cognitive performance and frontal neural abnormalities in cocaine dependent individuals. Previous studies have associated frontal white matter integrity with severity of behavioral traits characteristic to cocaine dependent individuals such as impulsivity and motivation (8,10,20). Altered frontal metabolism as manifested by increased activity in orbitofrontal cortex and decreased activity in dorsolateral prefrontal cortex and medial frontal cortex has been associated with attention and executive functioning processes in abstinent cocaine dependent individuals (24). Taken together, abnormalities in specific neural networks involving frontal regions seem to be associated with impaired behavior in cocaine dependent individuals. The nature of the interaction of specific brain regions within networks involved in self-regulatory control and its relationship to behavioral measures will provide information that can be useful for developing and monitoring treatment and prevention of relapse for cocaine dependent individuals.
In the present study we examine functional connectivity in cocaine dependent individuals (CD). We were particularly interested in the role of ACC in cocaine dependency due to its participation in networks that affect self-regulation, self-control and attention (25–28). In addition to its role in behavior regulation, ACC has a central anatomic location with diverse cortical, limbic and paralimbic connections (28), which is useful for examining functional connectivity in CD. A previous study examined two loci of ACC in CD (caudal-dorsal and rostral-ventral ACC) during an emotionally salient task (11). To conduct a more detailed examination of ACC networks in CD, we examined resting functional connectivity of five frontal networks based in ACC. The locations of ACC seeds were selected based on previous papers that investigated the development of ACC within five domains of self-regulatory control: motor control (caudal), attention/cognitive control (dorsal), conflict monitoring (rostral), mentalizing (perigenual) and emotional regulation (subgenual) (25,28). We hypothesize that CD will have altered functional connectivity in ACC networks and that functional connectivity strength will be related with quality of performance in tasks assessing self-regulatory control. Given previous reports suggesting that the interaction between ACC and dorsolateral prefrontal cortex has an important role in top-down support and monitoring for appropriate behaviors (27,29,30), we hypothesized that ACC-DLPFC connectivity would be related to tasks assessing self-regulatory control.
Methods and Materials
Participants
Twenty-seven chronic cocaine dependent individuals (CD) and 24 healthy participants (HP) matched by age, gender and primary caregiver education level were recruited (Table 1). CD were active users and all reported to have used cocaine within the past 30 days (Table 1). Co-morbidity in CD are described in Section A of Supplement 1. CD were recruited via advertising placed in a University newspaper, a free local-area publication and fliers placed on community bulletin boards throughout the Minneapolis, MN metro area and campus. HP were recruited via fliers posted on community and bulletin boards throughout campus and the metro area and through an online employee newsletter. Inclusion and exclusion criteria are described in Section B of Supplement 1. All participants provided written informed consent and received payment for the time they spent participating. The consent process and all procedures were reviewed and approved by the institutional review board (IRB) at the University of Minnesota prior to initiating studies. HIPAA consent documentation was also obtained.
Table 1.
Demographic Characteristics of Control, Cocaine Dependent and the Group of Separate Control Subjects
| Characteristic | Control (n=24) | Cocaine Dependent (n=27) | Separate Control (n=29) | F-stats | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Mean/n | SD/% | Mean/n | SD/% | Mean/n | SD/% | |||
| Age, mean (SD) (years) | 39.76 | 7.09 | 39.73 | 6.14 | 41.1 | 10.6 | 0.40a | 0.67 |
|
| ||||||||
| Education, mean (SD) (years) | ||||||||
| Subject | 15.16 | 1.62 | 13.41 | 1.93 | 12.07 | 2.17 | 16.98a | <0.001 |
| Primary caregiver | 13.44 | 4.26 | 12.08 | 4.06 | 13.10 | 4.49 | 0.71a | 0.50 |
|
| ||||||||
| Female, No (%) | 5 | 18.52 | 5 | 20.83 | 11 | 37.93 | 3.17b | 0.21 |
|
| ||||||||
| Cocaine use, mean (SD) | ||||||||
| Days used in 30 days | -- | -- | 3.2 | 1.63 | -- | -- | -- | -- |
| Average number of days since last use | 1.6 | 0.82 | ||||||
| Years used in lifetime | -- | -- | 14.6 | 6.83 | -- | -- | -- | -- |
| Age of onset of cocaine use | -- | -- | 25.56 | 6.21 | -- | -- | -- | -- |
Values obtained by F-test.
Values obtained by χ2 test.
Diagnostic screening
All subjects underwent a structured clinical interview for DSM-IV axis I conditions (SCID) (31) administered by a trained member of the research staff. CD received the SCID I/P (patient version) and HP received the SCID I/NP (non-patient version).
Imaging Data Acquisition
Participants underwent a 6-min resting-state fMRI scan and were instructed to be as still as possible, keep their eyes closed, and stay awake. Images were collected using a Siemens Trio 3T scanner (Erlangen, Germany). Sequence parameters: gradient-echo echo-planar imaging (EPI) 180 volumes, repetition time (TR)=2s, echo time (TE)=30ms, flip angle=90°, 34 contiguous AC-PC aligned axial slices, voxel size=3.4×3.4×4.0mm. matrix=64×64×34. Participants were debriefed at the end of the scan to find out if they fell asleep. A high-resolution T1-weighted anatomical image was acquired using a magnetization prepared rapid gradient-echo sequence. A field map acquisition was collected and used to correct the fMRI data for geometric distortion caused by magnetic field inhomogeneities (TR=300ms, TE=1.91ms/4.37ms, flip angle=55°, voxel size=3.4×3.4×4.0mm).
FMRI Imaging Analysis
Preprocessing was conducted with FEAT (FMRIB's Software Library [FSL]). The following prestatistics processing was applied for each subject: first 3 volumes deleted to account for magnetization stabilization, motion correction, B0 field map unwarping, slice-timing correction, non-brain removal, spatial smoothing (6-mm full-width half-maximum kernel), grand mean scaling, high-pass temporal filtering (100 Hz) to remove correlations associated with slow trends scanner noise, registration of all images to standard space. Probabilistic independent component analysis (PICA) was conducted for each individual to denoise individual data by removing components that represented noise. Noise components were selected by spatial and temporal characteristics detailed in MELODIC (FSL) manual (http://www.fmrib.ox.ac.uk/fslcourse/lectures/melodic.pdf) and based on Kelly et al (32) for selection criteria of noise components. While noise reduction was conducted using PICA in the present study, future studies could try using other methods (e.g. RETROICOR) for correcting physiological noise.
ROI selection and seed generation
We examined functional connectivity of five ACC connectivity maps following methods previously used by Kelly et al (25). Five spherical seeds with 3.5mm radius were placed at five ACC regions: caudal, dorsal, rostral, perigenual and subgenual with same coordinates previously described in (25) (see Section C of Supplement 1). We extracted the time series from each seed for each participant by averaging time series across all voxels within each seed.
Individual-Level Analysis
For each participant and for each ACC region of interest, we performed a multiple regression analysis (FSL-FEAT, details in Section D of Supplement 1) on the denoised data. This analysis generated a map with statistical parameter estimates (PEs) in each voxel for each individual. All voxels in the PEs maps showed the degree of positive or negative correlations with the corresponding seed time series for each ACC seed for each participant.
Generation of Resting ACC Functional Connectivity Masks from a separate Control Group
In order to limit the number of comparisons for each ACC functional connectivity map analysis, we used objectively derived spatial masks representing the five ACC connectivity maps generated from resting fMRI data of a separate set of twenty nine healthy participants (HP29) (demographics in Table 1) (33). This allowed for the generation of spatial masks from a separate control group that was not biased by the CD sample or their matched controls. Resting fMRI data were preprocessed in the same manner as described above. Each of the twenty nine individuals had five ACC functional connectivity maps. A one-sample t-test was performed for each of the five ACC functional connectivity maps. These resulting maps (see Section E of Supplement 1) served as ACC connectivity masks to limit group comparisons in the present study.
Group-Level Analysis
Group-level analyses were carried out by feeding each individual's parameter estimates (PEs) maps into a mixed-effects model (FSL-FLAME)(34). Group-level analyses produced z-score maps showing positive and negative functional connectivity networks, for each ACC seed, within groups and between groups. To protect for false positives, a threshold/cluster method derived from Monte Carlo simulations (AlphaSim AFNI) (35) was applied to all maps generated in the group-level analysis. Monte Carlo simulations (1000 iterations) accounted for the 6mm FWHM Gaussian filter with a connectivity radius of 7.1-mm. Based on these simulations, the family-wise α of .05 was preserved with an a priori voxel-wise probability of 0.025 and three-dimensional clusters with a minimum volume of 1488 μL (186 voxels).
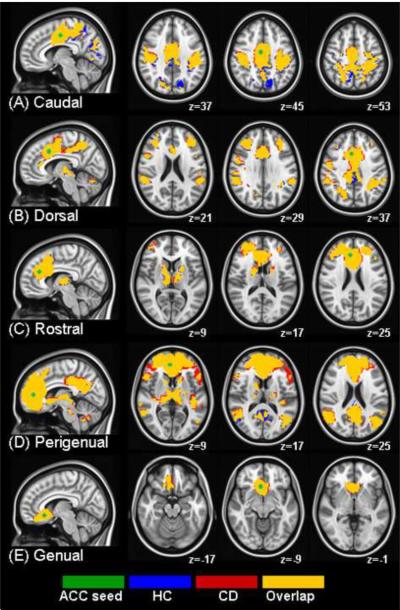
Resulting clustered and thresholded z-maps corrected for multiple comparisons showed: functional connectivity in five ROIs within the separate set of HP29 used for mask generation (see Section E of Supplement 1), functional connectivity in five ROIs within the CD group, functional connectivity in five ROIs within the HP matched to the CD group, and functional connectivity differences between the CD and HP groups (Figure 1).
Figure 1.
Resting functional connectivity maps for healthy controls (HC; blue) and cocaine-dependent (CD; red) are displayed together. Yellow: clusters in which groups overlap. Each row represents a resting connectivity map for five seeds (green) in (A) caudal, (B) dorsal, (C) rostral, (D) perigenual and (E) genual ACC. Functional maps showing a z value representing correlation with seed timecourses are overlaid on an MNI brain in radiological orientation (right is left). HC: healthy controls, CD: cocaine dependent, ACC: anterior cingulate cortex, MNI: Montreal Neurological Institute.
Behavioral Tasks
In a separate session prior to the scan, subjects completed a Reversal Learning Task and a Delay Discounting Task to assess aspects of cognitive control such as perseverative responding (36) and impulsivity (37,38) respectively. See task details in Section F of Supplement 1.
Correlates with strength of brain connectivity
To investigate the relationship between functional connectivity strength and cognitive control (measured by reversal learning and delayed discounting tasks), a robust regression was performed using R (39). Revisiting our hypothesis suggesting that there is a relationship between strength of ACC and DLPFC connectivity and self-regulatory control (26,29,30), average z-scores from a cluster that showed significant differences between groups between these regions were extracted. These measures of brain connectivity were correlated with demeaned scores representing the average number of trials it took for an individual to perform a reversal (reversal learning task) and the delay k measure (delay discounting task).
A previous paper on resting functional connectivity in CD reported a negative correlation between years of cocaine use and functional connectivity (23). Age, however, an important variable to consider when looking for relationships with years of cocaine use, was not controlled in the Gu et al (23) study. To investigate the relationship between functional connectivity strength in regions showing group difference and cocaine use, while controlling for the effect of age, a bivariate partial correlation was performed using R (39).
Results
Functional Connectivity Overlap
Section G of Supplement 1 lists regions functionally connected to each of the five ACC seeds in the separate group of 29 controls (see spatial map in Section E of Supplement 1). CD and controls had overlapping regions that were functionally connected to the five ACC seeds in very similar locations identified in the 29 controls (Figure 1).
Functional Connectivity Group Differences
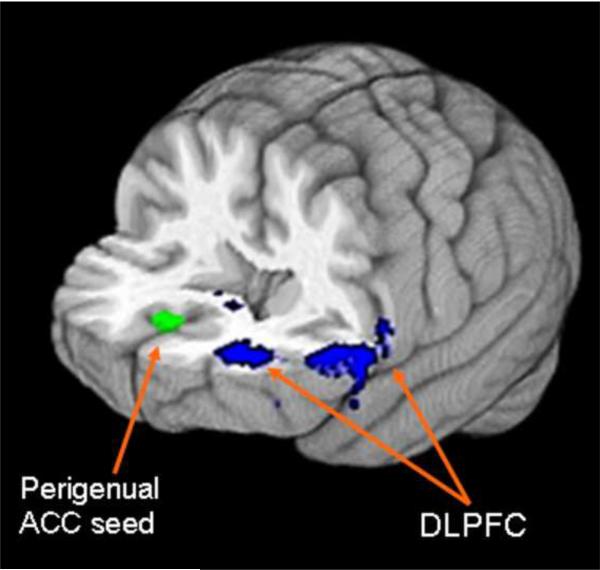
From the five ACC functional connectivity maps, only the perigenual ACC map showed significant differences between CD and their controls. Interestingly, CD had higher strength of resting functional connectivity in frontal and temporal regions within the perigenual ACC map. Resting functional connectivity was higher in CD within the perigenual ACC map in dorsolateral prefrontal cortex (DLPFC; BA 46: z=−31, y=50, z=16; BA 10: x=−53, y=29, z=14; Figure 2), middle temporal gyrus (MTG; x=57, y=33, z=11) and superior frontal gyrus (SFG; x=−12, y=12, z=55) than HP. There were no regions in which functional connectivity was higher in HP than in CD.
Figure 2.
3D brain in neurological orientation with slices cut at y=-10 and z=10. Green: Perigenual ACC seed. Blue: DLPFC clusters in which cocaine dependent individuals showed higher resting functional connectivity strength with perigenual ACC seed than controls. ACC: anterior cingulate cortex, DLPFC: dorsolateral prefrontal cortex.
Reversal Learning and Delayed Discounting Task Group Differences
The number of trials needed to perform a reversal in the reversal learning task was significantly higher (t(1,47)=3.45, p=0.001) in cocaine participants (M=31.46, SD=13.24) than controls (M=20.24, SD=9.24). The delay k value (log) in the delayed discount task was significantly higher (t(1,47)=3.08, p=0.004) in cocaine participants (M=−2.92, SD=1.77) than controls (M=−4.75, SD=2.35).
Relationship between self-regulatory control and strength of functional connectivity
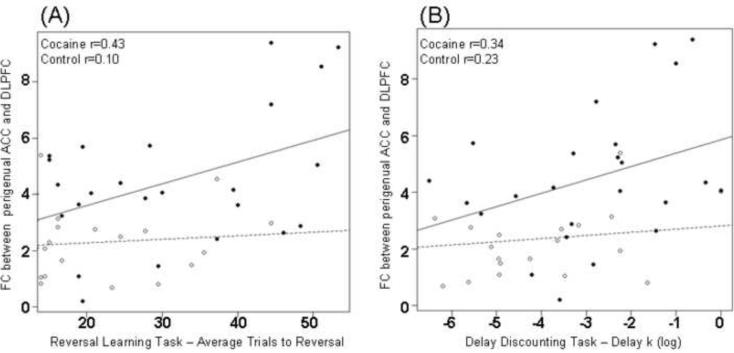
As hypothesized, there were associations between reversal and discounting scores with functional connectivity strength within the perigenual ACC network. There were significant positive correlations between reversal learning scores and FC strength in DLPFC (r=0.43, p=0.04, Figure 3A) in CD. Results revealed a similar trend in positive correlations between delayed discounting scores (log) and FC strength in DLPFC within perigenual ACC map (r=0.34, p=0.08; Figure 3B). CD with increased FC between perigenual ACC and DLPFC showed both increased compromise when learning to reverse reward contingencies (manifested with higher number of trials before a reversal) and increased levels of impulsivity (manifested with higher delay k values).
Figure 3.
Scatter plots showing correlations between resting functional connectivity strength (z-scores) perigenual ACC and DLPFC] and (A) reversal learning task and (B) delayed discounting task. Individual CD participants are represented by solid circles and normal participants by clear circles. ACC: Anterior cingulate cortex, DLPFC: dorsolateral prefrontal cortex, CD: cocaine dependent.
Relationship between years of cocaine use and functional connectivity strength
After controlling for age, partial correlation results showed that years of cocaine use were not significantly correlated with strength of functional connectivity in DLPFC (r=−0.33, p=0.12), MTG (r=−0.25, p=0.24), or SFG (r=−0.26, p=0.22).
Discussion
The present study examined resting functional connectivity in five anterior cingulate cortex (ACC) neural networks in order to identify abnormalities in frontal networks associated with poor self-regulation and impulse control in cocaine dependency. Five ACC networks involved in self-regulatory control (motor control, attentional/cognitive control, conflict monitoring, social processing/mentalizing and emotional regulation) previously reported in healthy populations were identified in CD and their controls (25). CD showed stronger strength of connectivity within the perigenual ACC social processing/mentalizing network. The present study also confirmed previous behavioral findings of delay discounting abnormalities (3–5) and failures to learn reversal reward contingencies (6) in CD. Abnormal frontal functional connectivity strength found in CD was positively correlated with behavioral measures of impulsivity and difficulty in delaying reward.
To our knowledge, this is the first resting fMRI study that examined top-down control networks in CD. A previous resting fMRI study examined bottom-up reward networks in CD (23). In general, while Gu et al (23) reported decreased resting functional connectivity within the reward networks, the present study found increased functional connectivity within top-down control networks. There are two specific differences between the present study and the Gu et al (23) study. First, Gu et al (23) found decreased connectivity between rostral ACC and limbic regions, which is not consistent with our findings. However, their center coordinates for the ACC (x=4, y=36, z=8; Talairach space), were not the same as the ones from the present study. Because ACC is structurally and functionally heterogeneous (28,40), discrepancies in findings between the present study and Gu et al (23) may be explained by the use of timeseries extracted from different ACC seeds that do not spatially overlap. Second, significant associations between years of cocaine use and resting functional connectivity reported by Gu et al (23) were not found in the present study, probably because we controlled for the potentially confounding effects of age. Despite potential methodological differences, findings from the present study and Gu et al (23) suggest that CD have functional connectivity abnormalities in both bottom-up reward pathways as well as top-down control pathways.
Our functional connectivity results showed that both control participants and CD had similar resting functional connectivity networks in ACC as the normal adult population reported by Kelly et al (25). Resting functional connectivity strength, however, was significantly higher within the perigenual ACC network in CD, manifested by increased functional connectivity between perigenual ACC and clusters in dorsolateral prefrontal cortex (DLPFC), middle temporal gyrus (MTG) and superior frontal gyrus (SFG). Interestingly, the developmental study by Kelly et al (25) found increased resting functional connectivity between perigenual ACC and DLPFC in children, which was lower in adolescents and was practically non-existent in adults. These results suggest that connectivity within these regions decreases with age, and may be “pruned” with maturation. Likewise, CD in the current study showed increased connectivity between perigenual ACC and DLPFC compared to age-matched controls, which may correspond to immature organization or interrupted synaptic pruning found in children's neural networks (25). A disruption in frontal and temporal white matter maturation in CD has been previously reported in the literature (41). Current results may indicate that CD undergo perturbations in the maturational processes of synaptic pruning and myelination related to drug use. An interesting extension of this research would be to examine both structural (white matter) and functional (gray matter) connectivity in CD as well as in adolescents with risk of drug dependency.
A recent primate study by Medalla and Barbas (30) examined the synaptic interactions of ACC and DLPFC with inhibitory neurons in rhesus monkeys, and proposed that the nature of ACC/DLPFC interactions may be contingent on the level of cognitive demand needed for cognitive control. During low cognitive demand, DLPFC involvement is sufficient to activate inhibitory neurons to suppress moderate noise and extract a relevant signal. However, during high cognitive demand when the level of noise is higher, DLPFC needs to act in synergy with ACC (which activates inhibitory neurons through large terminals) to be able to suppress excessive noise and extract a relevant signal. In addition, the authors (30) suggest the role of ACC in reversing decisions, in which previous behaviors that are now unwanted need to be reversed. In this scenario, large terminals from ACC act in synergy with shorter DLPFC terminals to inhibit unwanted behaviors and disinhibit new appropriate behaviors. A human study in which cognitive control and self-regulation was examined confirmed the nature of ACC/DLPFC interactions and reported that activity in DLPFC alone is not sufficient for behavioral regulation, and that an efficient interaction of ACC and DLPFC is needed for appropriate behavioral control (29). These proposed roles of ACC/DLPFC interaction are relevant to our findings because CD, who constantly need to regulate their thoughts and behaviors (i.e. suppress excessive noise such as craving and withdrawal symptoms), showed stronger resting functional connectivity synchrony between ACC and DLPFC. Present results suggest that due to higher demands of self-regulation, CD need to constantly increase the level of synergy between ACC and DLPFC (even during rest) due to the ongoing need of suppressing continuous and persistent noise presumably related to drug use, craving and/or withdrawal symptoms. This increased connectivity between ACC and DLPFC in the face of increased cognitive demand may also help explain our novel finding that the stronger resting functional connectivity strength between perigenual ACC and DLPFC in CD was associated with decreased ability to adapt their responses to shifting reward contingencies (measured by the reversal learning task) and the ability to suppress immediate reward (measured by the delayed discounting task).
The perigenual ACC network, involved in social processing and mentalizing, might mediate particular aspects of reversal learning and delayed discounting performance. The processes measured by the reversal learning task may directly contribute to an individual's social processing and mentalizing. One of the main cognitive constructs needed to perform the reversal learning task is flexibility or an ability to shift strategies. Substance dependent individuals are often exposed to complex social contexts with higher demands of behavioral flexibility (to successfully adapt to changing environments and come up with alternative solutions to social problems they encounter)(42). Lack of behavioral flexibility may be related to poor social processing, traits that have been identified in substance abuse populations (43,44). In addition performance in both the reversal learning and delayed discounting tasks is related to mentalizing. Mentalizing is the ability to understand thoughts and intentions of oneself and others. Both tasks involve the need to properly understand thought and intentions of the self and others (experimenter/task) through contingency management (reversal learning) and delayed gratification (delayed discounting).
Previous fMRI studies in which healthy participants performed delayed discounting and reversal learning tasks in the scanner have found that signal intensity in ACC and DLPFC is positively correlated with delayed discounting task performance (45,46) and reversal learning task performance (47,48). These studies, however did not investigate the interaction between these regions during self-regulation or control tasks. To further investigate the nature of our findings and explore whether local intensity of neural firing or larger local variability in BOLD (blood-oxygen-level dependence) signal accounted for increased functional connectivity between ACC and DLPFC in CD, we compared BOLD signal within each region separately between groups. A paired t-test was performed to compare the mean BOLD signal within ACC and within DLPFC between CD and their controls. Results showed that BOLD signal intensity within ACC (t(1,23)=1.22, p=0.23) and DLPFC (t(1,23)=6.31, p=0.53) was not significantly different between groups (see Section H of Supplement 1). This confirms that CD have an abnormality in the regulation of the strength of connectivity between these regions, and not an abnormality in the level of signal intensity in individual regions during rest. Although previous studies have suggested a local abnormality in either ACC or DLPFC (11–13), this difference does not appear to be present at rest.
There are two caveats to bear in mind when considering these results. First, resting functional connectivity abnormalities found in the present study may be a preexisting abnormality that renders an individual at risk of developing an addiction, an alteration resulting from chronic drug abuse or both. Studies involving younger populations at risk of developing an addiction need to be conducted to better understand the relationship between functional connectivity abnormalities and drug abuse. Second, while participants were debriefed at the end of the resting fMRI scan to find out if they remained awake, they were not monitored with a periodic response or eye-tracking. There is a risk that they were not truthful in their report and that this tendency may have differed across groups.
Future directions should include characterizing the timing and directionality of the functional connectivity using data with higher temporal resolution than fMRI, such as time frequency event related potentials or single electrode implantation in non-human samples (49,50). Further, to allow for communication between cortical regions within specific networks there must be a white matter fiber path connecting them. Future studies using both fMRI and diffusion tensor imaging techniques to look for a relationship between functional and anatomical connectivity measures (51) would provide important cross validation to these results. Finally, it would be interesting to investigate whether these abnormalities present in current CD can be reversed after rehabilitation.
Present results suggest that impulsivity or difficulty to delay immediate reward characteristic to cocaine dependent individuals may be associated with ongoing abnormal neural connectivity in frontal regions. This information is potentially useful for designing prevention and treatment strategies for cocaine dependent individuals by suggesting both the need to regulate behavior (i.e. enhance self-control, cognitive flexibility and ability to delay rewards) as well as the need to regulate functional connectivity within frontal regions.
Supplementary Material
Acknowledgements
This research was supported by the National Institute on Drug Abuse (NIDA: P20DA024196) and the National Center for Research Resources (NCRR: P41 RR008079) from the National Institutes of Health, the General Clinical Research Center (M01 RR00400), NCC grant P30 NS057091, the MIND Institute, R21MH79262, the PharmacoNeuroImmunology Program at the University of Minnesota (NIDA/NIH T32 DA007097) and the Minnesota Supercomputing Institute. The authors would like to thank the subjects who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, et al. Severity of Neuropsychological Impairment in Cocaine and Alcohol Addiction: Association with Metabolism in the Prefrontal Cortex. Neuropsychologia. 2004;42:1447–58. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 2.van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive Control Deficits in Substance-Dependent Individuals: A Comparison of Alcohol, Cocaine, and Methamphetamine and of Men and Women. J Clin Exp Neuropsychol. 2009;31:706–19. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirby KN, Petry NM. Heroin and Cocaine Abusers Have Higher Discount Rates for Delayed Rewards Than Alcoholics or Non-Drug-Using Controls. Addiction. 2004;99:461–71. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- 4.Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay Discounting in Currently Using and Currently Abstinent Cocaine-Dependent Outpatients and Non-Drug-Using Matched Controls. Addict Behav. 2006;31:1290–4. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez CW. Differences in Impulsivity and Risk-Taking Propensity between Primary Users of Crack Cocaine and Primary Users of Heroin in a Residential Substance-Use Program. Exp Clin Psychopharmacol. 2005;13:311–8. doi: 10.1037/1064-1297.13.4.311. [DOI] [PubMed] [Google Scholar]
- 6.Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic Cocaine but Not Chronic Amphetamine Use Is Associated with Perseverative Responding in Humans. Psychopharmacology (Berl) 2008;197:421–31. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced Frontal White Matter Integrity in Cocaine Dependence: A Controlled Diffusion Tensor Imaging Study. Biol Psychiatry. 2002;51:890–5. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- 8.Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, et al. Brain Macrostructural and Microstructural Abnormalities in Cocaine Dependence. Drug Alcohol Depend. 2008;92:164–72. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, et al. Working Memory Fmri Activation in Cocaine-Dependent Subjects: Association with Treatment Response. Psychiatry Res. 2010;181:174–82. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero MJ, Asensio S, Palau C, Sanchez A, Romero FJ. Cocaine Addiction: Diffusion Tensor Imaging Study of the Inferior Frontal and Anterior Cingulate White Matter. Psychiatry Res. 2010;181:57–63. doi: 10.1016/j.pscychresns.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA, et al. Anterior Cingulate Cortex Hypoactivations to an Emotionally Salient Task in Cocaine Addiction. Proc Natl Acad Sci U S A. 2009;106:9453–8. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hester R, Garavan H. Executive Dysfunction in Cocaine Addiction: Evidence for Discordant Frontal, Cingulate, and Cerebellar Activity. J Neurosci. 2004;24:11017–22. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, et al. Prefrontal Cortical Dysfunction in Abstinent Cocaine Abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–64. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, et al. Long-Term Frontal Brain Metabolic Changes in Cocaine Abusers. Synapse. 1992;11:184–90. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein RZ, Volkow ND. Drug Addiction and Its Underlying Neurobiological Basis: Neuroimaging Evidence for the Involvement of the Frontal Cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schenk S, Horger BA, Peltier R, Shelton K. Supersensitivity to the Reinforcing Effects of Cocaine Following 6-Hydroxydopamine Lesions to the Medial Prefrontal Cortex in Rats. Brain Res. 1991;543:227–35. doi: 10.1016/0006-8993(91)90032-q. [DOI] [PubMed] [Google Scholar]
- 17.Goeders NE, Smith JE. Reinforcing Properties of Cocaine in the Medical Prefrontal Cortex: Primary Action on Presynaptic Dopaminergic Terminals. Pharmacol Biochem Behav. 1986;25:191–9. doi: 10.1016/0091-3057(86)90252-2. [DOI] [PubMed] [Google Scholar]
- 18.Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal Cortical Tissue Composition in Abstinent Cocaine Abusers: A Magnetic Resonance Imaging Study. Neuroimage. 2003;19:1095–102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- 19.Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased Gray Matter Concentration in the Insular, Orbitofrontal, Cingulate, and Temporal Cortices of Cocaine Patients. Biol Psychiatry. 2002;51:134–42. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 20.Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, et al. Reduced Anterior Corpus Callosum White Matter Integrity Is Related to Increased Impulsivity and Reduced Discriminability in Cocaine-Dependent Subjects: Diffusion Tensor Imaging. Neuropsychopharmacology. 2005;30:610–7. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- 21.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-Induced Cocaine Craving: Neuroanatomical Specificity for Drug Users and Drug Stimuli. Am J Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 22.Calhoun VD, Kiehl KA, Pearlson GD. Modulation of Temporally Coherent Brain Networks Estimated Using Ica at Rest and During Cognitive Tasks. Hum Brain Mapp. 2008;29:828–38. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, et al. Mesocorticolimbic Circuits Are Impaired in Chronic Cocaine Users as Demonstrated by Resting-State Functional Connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, et al. Orbitofrontal Cortex Dysfunction in Abstinent Cocaine Abusers Performing a Decision-Making Task. Neuroimage. 2003;19:1085–94. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of Anterior Cingulate Functional Connectivity from Late Childhood to Early Adulthood. Cereb Cortex. 2009;19:640–57. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the Role of the Dorsolateral Prefrontal and Anterior Cingulate Cortex in Cognitive Control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 27.Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior Cingulate Conflict Monitoring and Adjustments in Control. Science. 2004;303:1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 28.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the Functional Connectivity of Anterior Cingulate Cortex. Neuroimage. 2007;37:579–88. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Morishima Y, Okuda J, Sakai K. Reactive Mechanism of Cognitive Control System. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq013. doi: 10.1093/cercor/bhq013. [DOI] [PubMed] [Google Scholar]
- 30.Medalla M, Barbas H. Synapses with Inhibitory Neurons Differentiate Anterior Cingulate from Dorsolateral Prefrontal Pathways Associated with Cognitive Control. Neuron. 2009;61:609–20. doi: 10.1016/j.neuron.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I Dsm-Iv Disorders. Patient Edition (Scid-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- 32.Kelly RE, Jr., Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, et al. Visual Inspection of Independent Components: Defining a Procedure for Artifact Removal from Fmri Data. J Neurosci Methods. 2010;189:233–45. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camchong J, Macdonald AW, 3rd, Bell C, Mueller BA, Lim KO. Altered Functional and Anatomical Connectivity in Schizophrenia. Schizophr Bull. 2009 doi: 10.1093/schbul/sbp131. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in Functional and Structural Mr Image Analysis and Implementation as Fsl. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 35.Ward B. Simultaneous inference for fMRI data. Medical College of Wisconsin; Milwaukee: 2000. [Accessed August 4, 2010]. Alphasim Program Documentation for Afni. Available at http://afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html. [Google Scholar]
- 36.Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW. Probabilistic Learning and Reversal Deficits in Patients with Parkinson's Disease or Frontal or Temporal Lobe Lesions: Possible Adverse Effects of Dopaminergic Medication. Neuropsychologia. 2000;38:596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 37.Monterosso J, Ainslie G. Beyond Discounting: Possible Experimental Models of Impulse Control. Psychopharmacology (Berl) 1999;146:339–47. doi: 10.1007/pl00005480. [DOI] [PubMed] [Google Scholar]
- 38.Kirby KN, Petry NM, Bickel WK. Heroin Addicts Have Higher Discount Rates for Delayed Rewards Than Non-Drug-Using Controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- 39.Ihaka R, Gentleman R. R: A Language for Data Analysis and Graphics. Journal of Computational and Graphical Statistics. 1996;5:299–314. [Google Scholar]
- 40.Ongur D, Ferry AT, Price JL. Architectonic Subdivision of the Human Orbital and Medial Prefrontal Cortex. J Comp Neurol. 2003;460:425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 41.Bartzokis G, Beckson M, Lu PH, Edwards N, Bridge P, Mintz J. Brain Maturation May Be Arrested in Chronic Cocaine Addicts. Biol Psychiatry. 2002;51:605–11. doi: 10.1016/s0006-3223(02)01315-x. [DOI] [PubMed] [Google Scholar]
- 42.Bond AB, Kamil AC, Balda RP. Serial Reversal Learning and the Evolution of Behavioral Flexibility in Three Species of North American Corvids (Gymnorhinus Cyanocephalus, Nucifraga Columbiana, Aphelocoma Californica) J Comp Psychol. 2007;121:372–9. doi: 10.1037/0735-7036.121.4.372. [DOI] [PubMed] [Google Scholar]
- 43.Simkin DR. Adolescent Substance Use Disorders and Comorbidity. Pediatr Clin North Am. 2002;49:463–77. doi: 10.1016/s0031-3955(01)00014-1. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Serrano MJ, Perez-Garcia M, Schmidt Rio-Valle J, Verdejo-Garcia A. Neuropsychological Consequences of Alcohol and Drug Abuse on Different Components of Executive Functions. J Psychopharmacol. 2010;24:1317–32. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- 45.Marco-Pallares J, Mohammadi B, Samii A, Munte TF. Brain Activations Reflect Individual Discount Rates in Intertemporal Choice. Brain Res. 2010;1320:123–9. doi: 10.1016/j.brainres.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 46.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate Neural Systems Value Immediate and Delayed Monetary Rewards. Science. 2004;306:503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 47.Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA. Neural Components Underlying Behavioral Flexibility in Human Reversal Learning. Cereb Cortex. 2010;20:1843–52. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freyer T, Valerius G, Kuelz AK, Speck O, Glauche V, Hull M, et al. Test-Retest Reliability of Event-Related Functional Mri in a Probabilistic Reversal Learning Task. Psychiatry Res. 2009;174:40–6. doi: 10.1016/j.pscychresns.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Adhikari A, Sigurdsson T, Topiwala MA, Gordon JA. Cross-Correlation of Instantaneous Amplitudes of Field Potential Oscillations: A Straightforward Method to Estimate the Directionality and Lag between Brain Areas. J Neurosci Methods. 2010;191:191–200. doi: 10.1016/j.jneumeth.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silchenko AN, Adamchic I, Pawelczyk N, Hauptmann C, Maarouf M, Sturm V, et al. Data-Driven Approach to the Estimation of Connectivity and Time Delays in the Coupling of Interacting Neuronal Subsystems. J Neurosci Methods. 2010;191:32–44. doi: 10.1016/j.jneumeth.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G. Measuring Brain Connectivity: Diffusion Tensor Imaging Validates Resting State Temporal Correlations. Neuroimage. 2008;43:554–61. doi: 10.1016/j.neuroimage.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.