Abstract
Background
Tibial tuberosity realignment surgery is performed to improve patellofemoral alignment, but could also alter tibiofemoral kinematics.
Hypothesis
Following tuberosity realignment in the malaligned knee, the reoriented patella tendon will pull the tuberosity back toward the pre-operative position, thereby altering tibiofemoral kinematics.
Study Design
Controlled laboratory study.
Methods
Ten knees were tested at 40°, 60° and 80° of flexion in vitro. The knees were loaded with a quadriceps force of 586 N, with 200 N divided between the medial and lateral hamstrings. The position of the tuberosity was varied to represent lateral malalignment, with the tuberosity 5 mm lateral of the normal position, tuberosity medialization, with the tuberosity 5 mm medial of the normal position, and tuberosity anteromedialization, with the tuberosity 10 mm anterior of the medial position. Tibiofemoral kinematics were measured using magnetic sensors secured to the femur and tibia. A repeated measures ANOVA with a post-hoc Student-Newman-Keuls test was used to identify significant (p < 0.05) differences in the kinematic data between the tuberosity positions at each flexion angle.
Results
Medializing the tibial tuberosity primarily rotated the tibia externally, compared to the lateral malalignment condition. The largest average increase in external rotation was 13° at 40° of flexion, with the increase significant at each flexion angle. The varus orientation also increased significantly by an average of 1.5° at 40° and 80°. The tibia shifted significantly posteriorly at 40° and 60° by an average of 4 mm and 2 mm, respectively. Shifting the tuberosity from the medial to the anteromedial position translated the tibia significantly posteriorly by an average of 2 mm at 40°.
Conclusions
Following tibial tuberosity realignment in the malaligned knee, the altered orientation of the patella tendon alters tibiofemoral kinematics.
Clinical Relevance
The kinematic changes reduce the correction applied to the orientation of the patella tendon and could alter the pressure applied to tibiofemoral cartilage.
Keywords: patellofemoral malalignment, tuberosity osteotomy, tuberosity realignment, tibiofemoral kinematics
INTRODUCTION
Patellofemoral pain and instability are commonly attributed to lateral malalignment. Many surgical options are available for patellofemoral realignment, with tibial tuberosity medialization and anteromedialization being two of the more popular surgical approaches. Tibial tuberosity medialization is performed to reduce the lateral component of the force applied to the patella by the patella tendon, thereby reducing the risk of subluxation and reducing the pressure applied to lateral cartilage. Anteromedialization combines anteriorization with medialization, which increases the moment arm about the center of rotation of the knee and decreases the quadriceps force needed for knee extension. The anteriorization component of anteromedialization also decreases the posterior component of the force applied to the patella by the patella tendon, which should decrease the total compression applied to the patellofemoral joint during function. In vitro studies indicate that both medialization and anteromedialization of the tibial tuberosity tend to improve patellofemoral alignment [33] and decrease the pressure applied to lateral patellofemoral cartilage [5, 33].
What is known about the subject: By altering the orientation of the patella tendon, tibial tuberosity realignment has been shown to alter patellofemoral alignment in vitro. Reorienting the patella tendon could also influence tibiofemoral kinematics, but the tibiofemoral kinematic variations have not been previously characterized.
What this study adds to existing knowledge: This study quantifies variations in tibiofemoral translations and rotations related to tibial tuberosity medialization and anteromedialization, and shows that the changes reduce the correction in the orientation of the patella tendon applied surgically.
Altering the orientation of the patella tendon could influence tibiofemoral kinematics as well as patellofemoral kinematics. Varying the orientation of the quadriceps, and thereby altering the force transferred through the patella tendon to the tibia, has already been shown to alter tibiofemoral kinematics in vitro [25]. Moving the tibial tuberosity creates a force vector through the patella tendon acting to pull the tuberosity back toward the pre-operative position. Forces applied to the tibia by the hamstrings muscles could stabilize the tibia and minimize the influence of altering the orientation of the patella tendon on tibiofemoral kinematics. Hamstrings forces have been shown to help stabilize the tibia following an ACL injury [23, 41]. Variations in tibiofemoral kinematics [17, 20] and points of contact [21, 38, 39] related to ACL and PCL injuries have been associated with the development of tibiofemoral osteoarthritis [16, 26]. Similarly, variations in tibiofemoral kinematics that accompany tibial tuberosity medialization and anteromedialization could potentially be detrimental for tibiofemoral cartilage.
The hypothesis of the current study is that tibial tuberosity medialization and anteromedialization alter tibiofemoral kinematics. The kinematic changes are expected to be related to the tibial tuberosity moving back toward the pre-operative position, reducing the correction in the orientation of the patella tendon applied during surgery. Activation of the hamstrings is expected to limit the kinematic variations associated with tibial tuberosity realignment.
MATERIALS AND METHODS
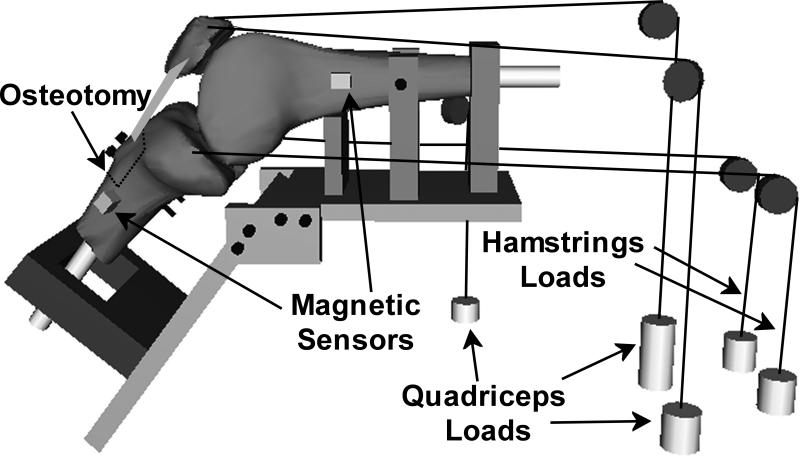
Ten cadaveric knees from ten separate donors were tested in vitro to address the hypothesis. The average age (± standard deviation) was 66 ± 12 years, and four of the knees were from female donors. Each knee was stored at -20 °C prior to dissection. The femur and tibia were transected approximately 18 cm from the joint line. The knees were dissected to remove the skin, adipose tissue and muscles other than the quadriceps muscle group, the semimembranosus, and the biceps femoris. The vastus medialis obliquus (VMO) and the vastus lateralis (VL) were separated from the combination of the vastus intermedius/vastus medialis longus/rectus femoris (VI/VML/RF). The lateral retinaculum was sectioned to simulate the lateral release that typically accompanies tibial tuberosity realignment [27, 32], which allowed inspection to ensure the ligaments were intact, with no evidence of prior surgeries or severe patellofemoral osteoarthritis. The influence of the lateral release on pre-operative joint function was considered to be minimal, since the lateral force applied to the patella was elevated and an in vitro study indicated that, for normal loading, a lateral release only increases patellofemoral medial tilt by approximately 1° from 40° to 80° of flexion [30]. A thin (0.1 mm) pressure sensor, which was coated with surgical jelly to minimize shear forces acting on the patella [13], was also inserted into the patellofemoral joint, as part of a separate study focusing on patellofemoral pressures with additional testing conditions beyond those reported for the current study. The fibula was secured to the tibia with a cable tie.
Each knee was secured to a testing frame consisting of two plates connected by a hinge, fixtures for positioning the knee, and fixtures for loading the quadriceps and hamstrings muscles (Fig. 1). Smooth rods made of Garolite were inserted into the diaphysis of the femur and tibia. The femur was secured to the testing frame by passing the femoral rod through a vertical fixture and supporting the posterior surface of the femur. The posterior condyles were aligned in a horizontal plane before fixing the rotational position of the femur at the diaphysis with set screws through another fixture. The tibial rod was passed through a nylon slotted fixture on the plate that controlled the flexion angle. Contact between the tibial rod and the fixture constrained extension, with only the relatively low friction between the two pieces of plastic constraining the other degrees of freedom. The orientation of the plate that determined the flexion angle was set to position the tibia at approximately 40°, 60° and 80° of flexion, with the tibia extending to the appropriate angle in response to loading the quadriceps muscles. Over this flexion range, the patella was sufficiently constrained within the trochlear groove to simulate lateral malalignment without the risk of lateral dislocation, and the point of contact shifted from the distal to the proximal patella [13]. The knees were kept moist with 0.9% saline solution while on the testing frame.
Figure 1.
A schematic diagram of the testing frame, without the supporting table. The cables applying the forces representing the quadriceps and hamstrings loads were secured to straps sutured into the tendons.
Each knee was tested at each flexion angle with the tibial tuberosity in a position representing pre-operative lateral malalignment, and with representation of surgical medialization and anteromedialization. The order of testing for the tibial tuberosity positions was varied between the flexion angles and between the knees. The tuberosity was osteotomized from the tibia along a plane parallel to the posterior tibia with an oscillating saw (Multi-Max, Dremel, Denver, CO) using a step-cut, creating a plane for sliding the tuberosity in the medial-lateral direction. Two pairs of holes were drilled through the tibia for fixation of the osteotomized tuberosity. The tuberosity was secured to the tibia by passing two screws through two washers, a 1 mm thick aluminum plate, the tuberosity, the tibia, a second aluminum plate and two additional washers, with nuts tightened on the end of each screw. The pre-operative condition was designed to simulate a malaligned knee with a laterally placed tibial tuberosity. The average lateral distance from the center of the trochlear groove to the tibial tuberosity (TT-TG distance), as measured by a ruler, was elevated to 19 ± 1 mm, which is approximately 5 mm greater than the average value in asymptomatic knees [2, 35]. For the medial position, the tibial tuberosity was shifted medially by 10 mm from the pre-operative position. For the anteromedial position, shims were placed between the tuberosity and the tibia to anteriorize the tuberosity by 10 mm from the medialized position.
For each combination of tibial tuberosity position and flexion angle, the knees were loaded with forces applied through the quadriceps muscles alone and also with the quadriceps and hamstrings loaded together. Straps made from cotton webbing with a nylon core were sutured into the tendons for each muscle. The straps were secured to loading cables that passed over pulleys and were connected to weights. The cable for the VI/VML/RF was aligned along the axis of the femur. The VMO cable was aligned at an angle of approximately 47° medial to the axis of the femur in the coronal plane, and the VL cable was aligned at an angle of approximately 19° lateral to the same axis [14]. The VMO and VL cables progressed posteriorly to the pulleys to represent the anatomical orientation in the sagittal plane. The loading cables for the semimembranosus and the biceps femoris proceeded proximally from the attachment points on the tibia to the pulleys. As described previously [13], loads were applied to produce physiologically realistic loading for patients with pain at each flexion angle, including a weakened VMO. Forces of 432 N, 127 N and 27 N were applied through the VI/VML/RF, the VL and the VMO, respectively. When the hamstrings forces were applied, 100 N was applied through both the semimembranosus and the biceps femoris based on a previously measured ratio of quadriceps to hamstrings forces during in vitro simulation of closed chain knee extension [12]. The decrease in quadriceps forces with anteriorization of the tibial tuberosity that would be expected in vivo due to the increased moment arm of the patella tendon about the flexion axis was not represented in order to isolate the influence of patella tendon orientation on tibiofemoral kinematics.
The position and orientation of the tibia with respect to the femur was characterized with respect to a standardized anatomical coordinate system for the knee. Sensors from a pulsed DC magnetic tracking system (trakSTAR, Ascension Technology, Burlington, VT) were secured to the femur and tibia of each knee, with the transmitter fixed near the distal end of the tibia. According to the manufacturer, the static resolutions for translations and rotations are 0.5 mm and 0.1°, respectively. Nylon sensor holders were secured to the femur and tibia with nylon screws, with the sensors secured to the holders with nylon set screws. Because of the influence of metal on the magnetic field, all components of the test frame between the sensors and the transmitter were plastic, except for the fixation piece holding the tuberosity on the tibia and the screws holding the components of the frame together. A separate sensor was fixed to a digitizing probe [34] to determine the coordinates for previously described anatomical landmarks used to establish the femoral and tibial reference axes with the knee in full extension [28, 34, 41]. The position of the probe tip relative to the coordinate system embedded in the sensor was known, allowing measurement of the position of each landmark. The transepicondylar axis was identified by digitizing the most medial and lateral points on the femoral epicondyles, with the knee center positioned midway between the two points. The long axis of the femur was established by digitizing points along the posterior femur. The mutual perpendicular of the two axes determined the anterior-posterior axis, with the proximal-distal axis formed from the cross-product of the transepicondylar axis and the anterior-posterior axis. The reference axes for the tibia were similarly identified based on the axis connecting the most medial and lateral points on the tibial epicondyles and the long axis of the tibia. Output from the sensors determined the position and orientation of the tibial and femoral reference axes during testing. The floating axis coordinate system [15] was used to characterize the translations and rotations of the tibial reference axes with respect to the femoral axes to characterize tibiofemoral kinematics.
The statistical analysis focused on kinematic changes at each flexion angle related to the position of the tibial tuberosity and application of hamstrings loading. At each flexion angle, a two level ANOVA with repeated measures on both the tibial tuberosity position and the hamstrings loading condition was used to determine if loading the hamstrings significantly (p < 0.05) influenced the kinematic parameters over the three tibial tuberosity positions. The ANOVA also determined if the output varied significantly between the tibial tuberosity positions for each flexion angle. When the tibial tuberosity position influenced the output, a repeated measures ANOVA with a post-hoc Student-Newman-Keuls test was used for comparisons between the tuberosity positions for each hamstrings loading condition at each flexion angle.
RESULTS
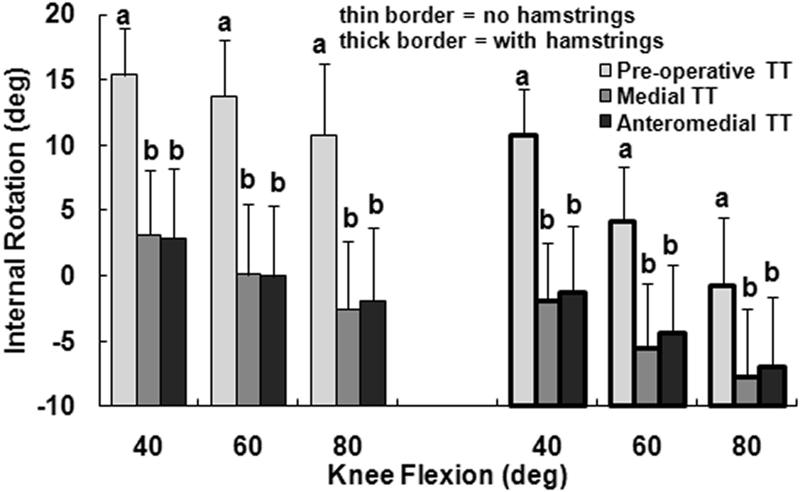
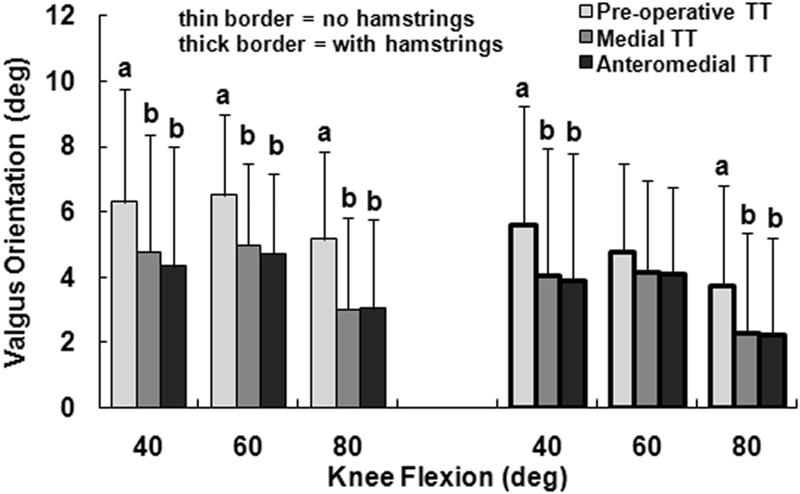
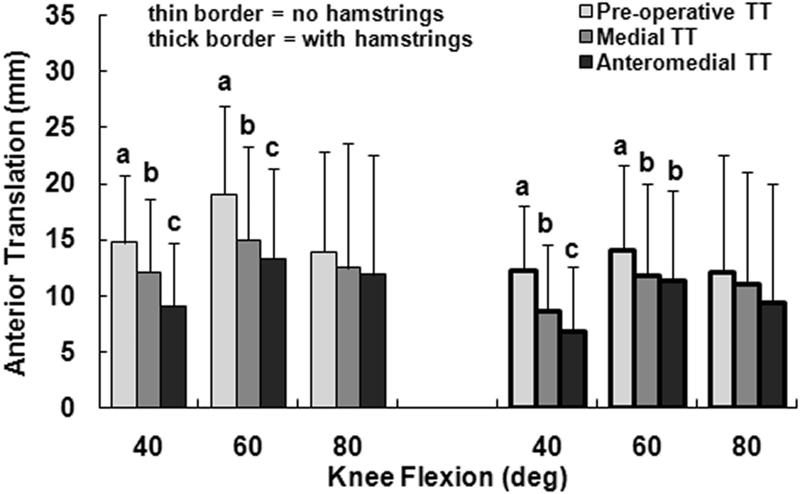
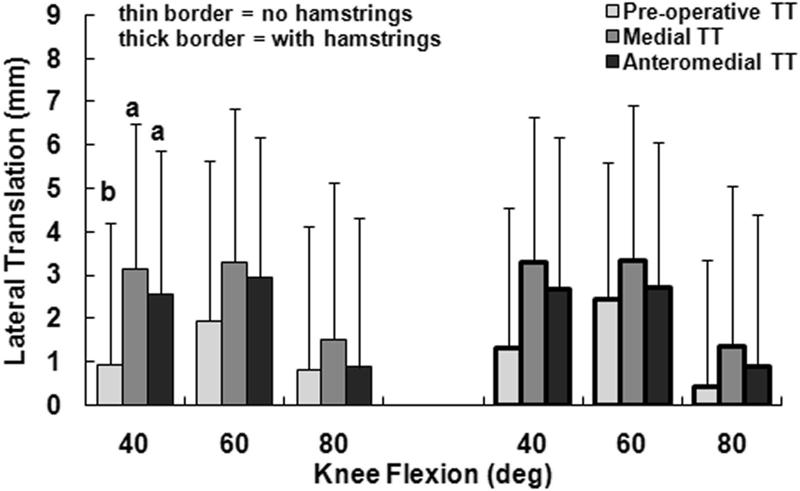
The primary kinematic change related to moving the tuberosity from the pre-operative lateral malalignment position to the medial position was external rotation of the tibia. With only the quadriceps loaded and the tuberosity in the lateral malalignment position, the tibia was internally rotated with respect to the femur, peaking at an average of 15° at 40° of flexion (Fig. 2). Medialization of the tibial tuberosity decreased the average tibial internal rotation by approximately 13° at each flexion angle, with the difference significant at each flexion angle (p < 0.001). The tibia tended to have a valgus orientation for all loading conditions, but the average valgus orientation was 1° to 2° smaller for the medial tuberosity position than for the pre-operative position (Fig. 3). The relative varus change in orientation was significant at each flexion angle with only the quadriceps loaded (p ≤ 0.02). The tibia tended to be anterior of the femur for all loading conditions, with the average anterior translation 1 to 4 mm smaller for the medial tuberosity position than for the pre-operative position (Fig. 4). The relative posterior translation was significant at 40° and 60° with only the quadriceps loaded (p < 0.001). Medializing the tibial tuberosity also shifted the tibia laterally by an average of 1 to 2 mm (Fig. 5). The increase in lateral translation was significant at 40° with only the quadriceps loaded (p = 0.007).
Figure 2.
The average (+ 95% confidence interval) internal rotation (negative values indicate external rotation) of the tibia with respect to the femur at all three flexion angles for all three positions of the tibial tuberosity (TT), for loading including and not including the hamstrings. Statistically significant differences between the tibial tuberosity positions for each loading condition at each flexion angle are indicated (a > b at p < 0.05).
Figure 3.
The average (+ 95% confidence interval) valgus orientation of the tibia with respect to the femur at all three flexion angles for all three positions of the tibial tuberosity (TT), for loading including and not including the hamstrings. Statistically significant differences between the tibial tuberosity positions for each loading condition at each flexion angle are indicated (a > b at p < 0.05).
Figure 4.
The average (+ 95% confidence interval) anterior translation of the tibia with respect to the femur at all three flexion angles for all three positions of the tibial tuberosity (TT), for loading including and not including the hamstrings. Statistically significant differences between the tibial tuberosity positions for each loading condition at each flexion angle are indicated (a > b > c at p < 0.05).
Figure 5.
The average (+ 95% confidence interval) lateral translation of the tibia with respect to the femur at all three flexion angles for all three positions of the tibial tuberosity (TT), for loading including and not including the hamstrings. Statistically significant differences between the tibial tuberosity positions for each loading condition at each flexion angle are indicated (a > b at p < 0.05).
Loading the hamstrings in addition to the quadriceps tended to reduce the influence of tuberosity medialization on tibiofemoral kinematics. Loading the hamstrings decreased the internal rotation, anterior translation and valgus orientation of the tibia with respect to the femur by averages of 4° to 12°, 1 to 5 mm, and less than 2°, respectively, with the differences significant at each flexion angle (Table 1). The lateral translation of the tibia was not significantly influenced by the hamstrings muscles. Although the relative external rotation caused by medializing the tuberosity was still significant at each flexion angle with the hamstrings loaded (p < 0.001), the average external rotation decreased from 13° to 7° at 80° of flexion. With the hamstrings loaded, the influence of tuberosity medialization on the valgus orientation was not significant at 60° (p = 0.06), as was the case with only the quadriceps loaded, and the influence of tuberosity medialization on lateral translation was not significant at any flexion angle (p > 0.07). The significant anterior translation differences related to tuberosity medialization were not influenced by loading the hamstrings.
Table 1.
Significant differences related to loading the hamstrings
| Significant Difference | Flexion Angles (p-values) | |
|---|---|---|
| Internal Rotation | No Hams > With Hams | 40° (< 0.001), 60° (< 0.001), 80° (< 0.001) |
| Valgus Orientation | No Hams > With Hams | 40° (0.03), 60° (0.004), 80° (0.004) |
| Anterior Translation | No Hams > With Hams | 40° (< 0 .001), 60° (<0.001), 80° (0.04) |
| Lateral Translation | None | 40° (0.5), 60° (0.8), 80° (0.7) |
Hams: Hamstrings Loads
The anterior shift of the tuberosity from the medial position to the anteromedial position primarily decreased tibial anterior translation. Without the hamstrings loaded, moving the tuberosity from the medial to the anteromedial position decreased the average anterior tibial translation by 1 to 3 mm, with the difference significant at 40° and 60° (p < 0.03). With the hamstrings loaded, moving the tuberosity from the medial to the anteromedial position decreased the average anterior translation by 2 mm or less, with the difference significant only at 40° (p = 0.04). No other significant differences were identified between the medial and anteromedial tuberosity positions (p > 0.15), with or without hamstrings loading.
DISCUSSION
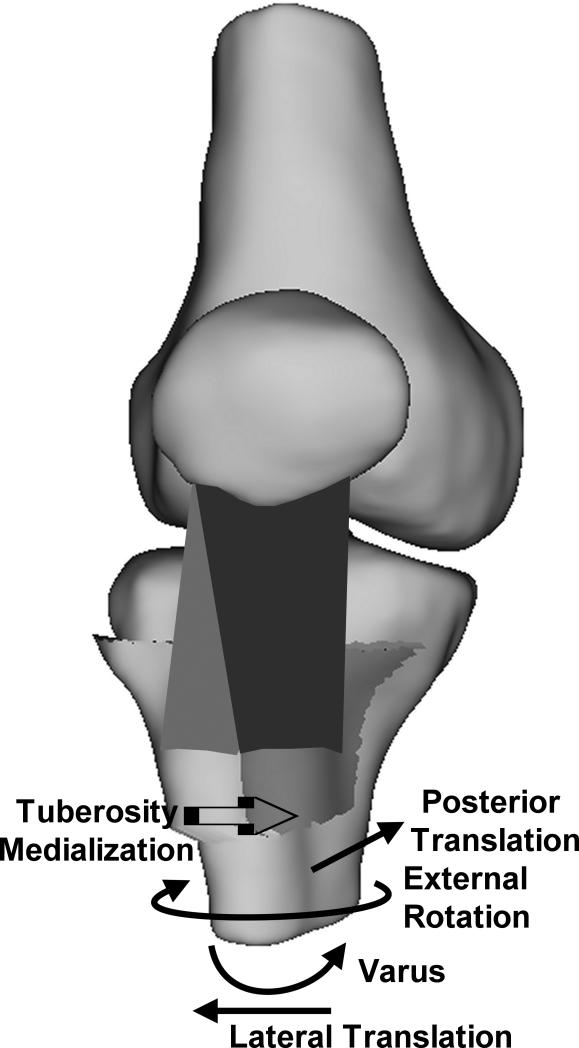
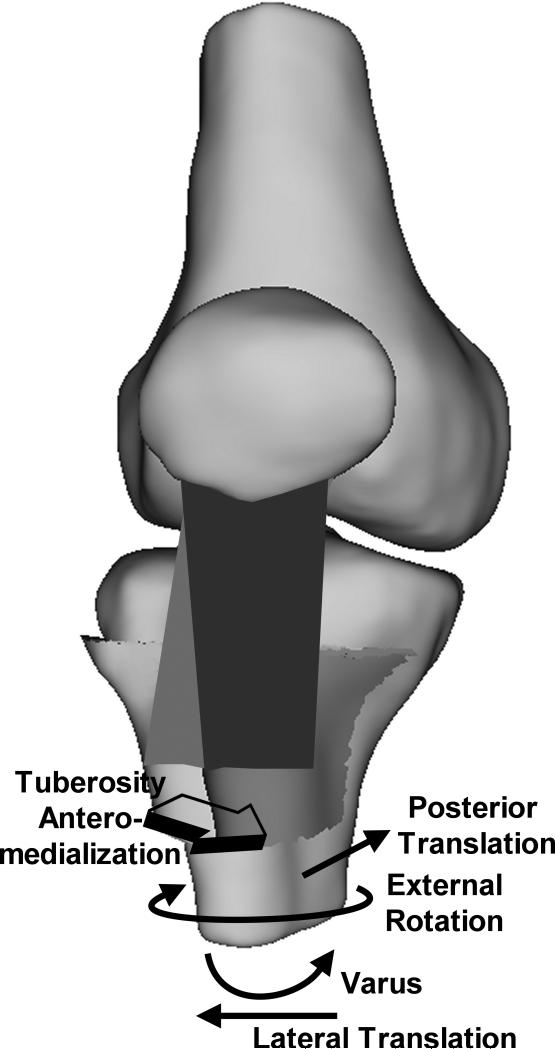
The current results indicate that, in response to applied quadriceps forces, the patella tendon moves toward a preferred orientation, influencing tibiofemoral kinematics (Fig. 6). Over the flexion range considered for the current study, previous in vitro studies have indicated that, with only the quadriceps loaded, the tibia typically has an anterior position [20, 23, 24, 41] and valgus orientation [22] with respect to the femur. The tibia has also been shown to be internally rotated at low flexion angles, approaching external rotation with increased flexion [19, 20, 23, 40, 41]. All of these trends were noted for the current study. The most consistent change from these trends was a relative external rotation of the tibia due to tuberosity medialization. The tibia also shifted laterally following tuberosity medialization, although the shift was only significant at 40°. External rotation and lateral translation of the tibia both decrease the medial correction applied to the orientation of the patella tendon surgically. The decrease in anterior translation associated with tuberosity medialization may be related to the rotation. With the large TT-TG distance for the pre-operative condition, the anterior component of the force applied by the patella tendon has a large moment arm for internal rotation about the medial plateau of the tibia. With the center of rotation moving toward the medial plateau, internal rotation would anteriorize the tibia. With the tuberosity medialized, the moment arm with respect to the medial plateau decreases, as does the internal rotation, which could contribute to the decreased anterior translation. The decreased anterior translation related to medialization reverses some of the anterior correction applied to the patella tendon during tibial tuberosity anteromedialization. Moving the tuberosity from the medial to the anteromedial position tended to further decrease the anterior translation, likely due to the decreased anterior component of the force applied to the tibia by the patella tendon.
Fig. 6.
Schematic diagram illustrating the directions of the changes in the translations and rotations of the tibia with respect to the femur following medialization (A) and anteromedialization (B) of the tibial tuberosity. The trends were similar for both types of realignment, although the posterior translation tended to be larger for anteromedialization than medialization. The tuberosity is shown in the pre-operative position on the tibia with an elevated TT-TG distance, with the direction of tuberosity realignment indicated.
Loading the medial and lateral hamstrings provided some stability for the tibia, but did not alter the basic trends relating the position of the tibial tuberosity to tibiofemoral kinematics. Previous in vitro studies have indicated that loading the hamstrings shifts the tibia posteriorly by up to 6 mm [23, 41], rotates the tibia externally by up to 10° [19, 23, 24, 40, 41], and re-orients the tibia toward varus by less than 2° [22]. Similar trends were noted for the current study. The kinematic changes caused by medializing the tibial tuberosity tended to decrease when hamstrings loading was added to quadriceps loading. The magnitude of the change in rotation tended to decrease as the flexion angle increased when the hamstrings were loaded. The internal/external rotation moment applied by each hamstrings muscle increased with the flexion angle as the forces became nearly perpendicular to the tibia, which likely provided some rotational stability for the tibia. The number of significant differences in the valgus orientation, anterior translation and the lateral translation of the tibia related to tuberosity medialization and anteromedialization also decreased with the hamstrings loaded. Rehabilitation including hamstrings activity and strengthening is expected to help maintain the surgical correction following tuberosity realignment, although the benefit may be small.
Even though kinematic changes tend to reduce the surgical correction applied to the orientation of the patella tendon, tibial tuberosity realignment does improve patellofemoral alignment and reduces the pressure applied to cartilage. Previous in vitro studies have indicated that medialization of the tibial tuberosity medializes the patella [33] and tilts the patella medially [31, 33]. Medialization and anteromedialization of the tibial tuberosity have also been shown to reduce the pressure applied to lateral cartilage within the patellofemoral joint [5, 33]. The previous studies included no hamstrings forces [5, 33] to stabilize the tibia or lower hamstrings force than used for the current study [31], indicating that a relatively small change in the orientation of the patella tendon can alter patellofemoral alignment and pressures. The kinematic variations related to tuberosity medialization and anteromedialization may help prevent overcorrection of patellofemoral alignment. Overcorrection, with an increase in the pressure applied to the cartilage on the medial facet of the patella, has been demonstrated as a concern for medial patellofemoral ligament reconstruction [4, 11] and medial capsulorraphy [29] performed for lateral patellofemoral instability. For tibial tuberosity realignment, overcorrecting the tuberosity position to 15 mm medial of the normal attachment site has not been shown to elevate the peak patellofemoral pressure at 45° of flexion [18].
The variations in tibiofemoral kinematics related to tibial tuberosity realignment have implications for computational studies focused on patellofemoral pressures. Several computational studies have explored the influence of tibial tuberosity medialization and anteromedialization on the patellofemoral pressure distribution without accounting for variations in tibiofemoral kinematics [7 - 10]. In the future, computational models should include tibiofemoral degrees of freedom or at least use the present data to estimate kinematic changes related to tibial tuberosity realignment.
Altered tibiofemoral kinematics following tibial tuberosity realignment could have implications for the tibiofemoral joint. Kinematic variations related to ACL and PCL injuries are not similar, but both have been shown to increase tibiofemoral cartilage deformation during functional activities [38, 39]. The increased cartilage deformation following ACL and PCL injuries is believed to cause the development of tibiofemoral osteoarthritis associated with these injuries [16, 26]. Altered kinematics and cartilage deformation could be a source of concern following tuberosity realignment. Medial tibiofemoral arthrosis has been mentioned as a complication of tibial tuberosity medialization [36], which could be related to the noted change in varus orientation shifting compression to the medial compartment of the tibiofemoral joint. Follow-up studies focused on tuberosity realignment typically utilize average follow up times of only two to four years, so tibiofemoral arthrosis has not been reported [6, 32, 37]. For one study, however, mild tibiofemoral pain was reported in five of forty-five knees at a mean of 161 months following tibial tuberosity medialization [27]. Tibiofemoral arthrosis has also been identified as a common complication following tuberosity medialization combined with distalization [3].
An alternative to the speculation that kinematic changes caused by tuberosity medialization could induce arthrosis is that the elevated TT-TG distance produces abnormal kinematics that are corrected following tuberosity medialization. The proper interpretation is complicated by the nature of in vitro studies. The tuberosity was shifted laterally to create a TT-TG distance that would simulate the elevated distance in a patient who is a candidate for surgical realignment. Shifting the tuberosity laterally does not fully reproduce the conditions that are present in a knee with developmental malalignment, although this may be the best approximation short of locating cadaver knees with naturally occurring malalignment. Based on the current results, the shift likely altered tibiofemoral kinematics from the normal condition for the knees tested. When treating patients with patellofemoral malalignment but asymptomatic tibiofemoral joints, the pre-operative tibiofemoral kinematics with the elevated TT-TG distance would be considered physiologic for that patient. For this study, kinematics were not characterized for the native tuberosity position for the tested knees to prevent the interpretation that the kinematics are closer to “normal” for the medial or pre-operative tuberosity position, since the normal condition for the properly aligned specimens does not correlate with the in vivo condition present in malaligned knees. This limitation will have to be considered when planning additional studies focused on the influence of tuberosity realignment on the tibiofemoral pressure distribution.
Tibiofemoral kinematics were characterized at individual flexion angles, similar to several previous studies [1, 18, 19, 22, 23, 41]. The testing protocol provided some advantages. The applied quadriceps forces were physiologically realistic in magnitude and distribution among the muscles of the quadriceps for patients with patellofemoral pain [13]. In vitro studies are typically performed with lower quadriceps forces [4, 5, 22, 23, 34, 41] and/or with the quadriceps group represented by a single force [4, 5, 12, 17, 20 - 25, 29, 31, 33, 40, 41]. Hamstrings forces were added to the quadriceps without unloading the knee, reducing experimental error, and the level of co-contraction was similar to a weight-bearing activity [12], with the load evenly divided between the medial and lateral hamstrings [23, 24, 41]. Additional force applied by components of the medial hamstrings, such as the semitendinosus, could possibly reduce the external rotation caused by medializing the tibial tuberosity, although a previous study applied additional force to the semitendinosus and still found a trend for external rotation caused by the hamstrings similar to the current study [19], while another study indicated that the medial hamstrings has a small influence on tibial rotation compared to the lateral hamstrings [40]. Because testing was performed at individual flexion angles, and due to the additional tests focused on patellofemoral pressures being reported separately, testing was performed at only three flexion angles. Because of the simulated lateral malalignment and VMO weakness and the large quadriceps forces, flexion angles less than 40° were not tested to avoid patellar instability. The three flexion angles were sufficient to establish the primary trends relating tuberosity realignment to kinematics.
Although tibial tuberosity realignment is performed to improve patellofemoral alignment, reorienting the patella tendon also alters tibiofemoral kinematics. The patella tendon tends to have a preferred orientation when loaded, reducing the correction applied surgically. The most consistent change is a relative external rotation of the tibia caused by medialization of the tuberosity. Variations in tibial lateral translation and anterior translation following tuberosity realignment also reflect the loss of correction. Loading the hamstrings tends to reduce the tibiofemoral kinematic changes caused by tuberosity realignment.
ACKNOWLEDGEMENT
The study was supported by Award Number R03AR054910 from the National Institute Of Arthritis And Musculoskeletal And Skin Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Arthritis And Musculoskeletal And Skin Diseases or the National Institutes of Health.
REFERENCES
- 1.Ahmad CS, Kwak SD, Ateshian GA, Warden WH, Steadman JR, Mow VC. Effects of patellar tendon adhesion to the anterior tibia on knee mechanics. Am J Sports Med. 1998;26:715–24. doi: 10.1177/03635465980260051901. [DOI] [PubMed] [Google Scholar]
- 2.Alemparte J, Ekdahl M, Burnier L, Hernández R, Cardemil A, Cielo R, Danilla S. Patellofemoral evaluation with radiographs and computed tomography scans in 60 knees of asymptomatic subjects. Arthroscopy. 2007;23:170–7. doi: 10.1016/j.arthro.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Barbari S, Raugstad TS, Lichtenberg N, Refvem D. The Hauser operation for patellar dislocation. 3-32-year results in 63 knees. Acta Orthop Scand. 1990;61:32–5. doi: 10.3109/17453679008993061. [DOI] [PubMed] [Google Scholar]
- 4.Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35:1557–63. doi: 10.1177/0363546507300872. [DOI] [PubMed] [Google Scholar]
- 5.Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33:1710–5. doi: 10.1177/0363546505278300. [DOI] [PubMed] [Google Scholar]
- 6.Bellemans J, Cauwenberghs F, Witvrouw E, Brys P, Victor J. Anteromedial tibial tubercle transfer in patients with chronic anterior knee pain and a subluxation-type patellar malalignment. Am J Sports Med. 1997;25:375–81. doi: 10.1177/036354659702500318. [DOI] [PubMed] [Google Scholar]
- 7.Benvenuti JF, Rakotomanana L, Leyvraz PF, Pioletti DP, Heegaard JH, Genton MG. Displacements of the tibial tuberosity. Effects of the surgical parameters. Clin Orthop Relat Res. 1997;343:224–34. [PubMed] [Google Scholar]
- 8.Cohen ZA, Henry JH, McCarthy DM, Mow VC, Ateshian GA. Computer simulations of patellofemoral joint surgery. Patient-specific models for tuberosity transfer. Am J Sports Med. 2003;31:87–98. doi: 10.1177/03635465030310012701. [DOI] [PubMed] [Google Scholar]
- 9.Elias JJ, Bratton DR, Weinstein DM, Cosgarea AJ. Comparing two estimations of the quadriceps force distribution for use during patellofemoral simulation. J Biomech. 2006;39:865–72. doi: 10.1016/j.jbiomech.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Elias JJ, Cech JA, Weinstein DM, Cosgrea AJ. Reducing the lateral force acting on the patella does not consistently decrease patellofemoral pressures. Am J Sports Med. 2004;32:1202–8. doi: 10.1177/0363546503262167. [DOI] [PubMed] [Google Scholar]
- 11.Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34:1478–85. doi: 10.1177/0363546506287486. [DOI] [PubMed] [Google Scholar]
- 12.Elias JJ, Faust AF, Chu YH, Chao EY, Cosgarea AJ. The soleus muscle acts as an agonist for the anterior cruciate ligament. An in vitro experimental study. Am J Sports Med. 2003;31:241–6. doi: 10.1177/03635465030310021401. [DOI] [PubMed] [Google Scholar]
- 13.Elias JJ, Kilambi S, Goerke DR, Cosgarea AJ. Improving vastus medialis obliquus function reduces pressure applied to lateral patellofemoral cartilage. J Orthop Res. 2009;27:578–83. doi: 10.1002/jor.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farahmand F, Senavongse W, Amis AA. Quantitative study of the quadriceps muscles and trochlear groove geometry related to instability of the patellofemoral joint. J Orthop Res. 1998;16:136–43. doi: 10.1002/jor.1100160123. [DOI] [PubMed] [Google Scholar]
- 15.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 16.Keller PM, Shelbourne KD, McCarroll JR, Rettig AC. Nonoperatively treated isolated posterior cruciate ligament injuries. Am J Sports Med. 1993;21:132–6. doi: 10.1177/036354659302100122. [DOI] [PubMed] [Google Scholar]
- 17.Kumagai M, Mizuno Y, Mattessich SM, Elias JJ, Cosgarea AJ, Chao EY. Posterior cruciate ligament rupture alters in vitro knee kinematics. Clin Orthop Relat Res. 2002;395:241–8. doi: 10.1097/00003086-200202000-00029. [DOI] [PubMed] [Google Scholar]
- 18.Kuroda R, Kambic H, Valdevit A, Andrish JT. Articular cartilage contact pressure after tibial tuberosity transfer. A cadaveric study. Am J Sports Med. 2001;29:403–9. doi: 10.1177/03635465010290040301. [DOI] [PubMed] [Google Scholar]
- 19.Kwak SD, Ahmad CS, Gardner TR, Grelsamer RP, Henry JH, Blankevoort L, Ateshian GA, Mow VC. Hamstrings and iliotibial band forces affect knee kinematics and contact pattern. J Orthop Res. 2000;18:101–8. doi: 10.1002/jor.1100180115. [DOI] [PubMed] [Google Scholar]
- 20.Li G, Gill TJ, DeFrate LE, Zayontz S, Glatt V, Zarins B. Biomechanical consequences of PCL deficiency in the knee under simulated muscle loads--an in vitro experimental study. J Orthop Res. 2002;20:887–92. doi: 10.1016/S0736-0266(01)00184-X. [DOI] [PubMed] [Google Scholar]
- 21.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88:1826–34. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Papannagari R, DeFrate LE, Yoo JD, Park SE, Gill TJ. The effects of ACL deficiency on mediolateral translation and varus-valgus rotation. Acta Orthop. 2007;78:355–60. doi: 10.1080/17453670710013924. [DOI] [PubMed] [Google Scholar]
- 23.Li G, Rudy TW, Sakane M, Kanamori A, Ma CB, Woo SL. The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. J Biomech. 1999;32:395–400. doi: 10.1016/s0021-9290(98)00181-x. [DOI] [PubMed] [Google Scholar]
- 24.MacWilliams BA, Wilson DR, DesJardins JD, Romero J, Chao EY. Hamstrings cocontraction reduces internal rotation, anterior translation, and anterior cruciate ligament load in weight-bearing flexion. J Orthop Res. 1999;17:817–22. doi: 10.1002/jor.1100170605. [DOI] [PubMed] [Google Scholar]
- 25.Mizuno Y, Kumagai M, Mattessich SM, Elias JJ, Ramrattan N, Cosgarea AJ, Chao EY. Q-angle influences tibiofemoral and patellofemoral kinematics. J Orthop Res. 2001;19:834–40. doi: 10.1016/S0736-0266(01)00008-0. [DOI] [PubMed] [Google Scholar]
- 26.Murrell GA, Maddali S, Horovitz L, Oakley SP, Warren RF. The effects of time course after anterior cruciate ligament injury in correlation with meniscal and cartilage loss. Am J Sports Med. 2001;29:9–14. doi: 10.1177/03635465010290012001. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa K, Wada Y, Minamide M, Tsuchiya A, Moriya H. Deterioration of long-term clinical results after the Elmslie-Trillat procedure for dislocation of the patella. J Bone Joint Surg Br. 2002;84:861–4. doi: 10.1302/0301-620x.84b6.12804. [DOI] [PubMed] [Google Scholar]
- 28.Nha KW, Papannagari R, Gill TJ, Van de Velde SK, Freiberg AA, Rubash HE, Li G. In vivo patellar tracking: clinical motions and patellofemoral indices. J Orthop Res. 2008;26:1067–74. doi: 10.1002/jor.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostermeier S, Holst M, Bohnsack M, Hurschler C, Stukenborg-Colsman C, Wirth CJ. Dynamic measurement of patellofemoral contact pressure following reconstruction of the medial patellofemoral ligament: an in vitro study. Clin Biomech. 2007;22:327–35. doi: 10.1016/j.clinbiomech.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sports Traumatol Arthrosc. 2007;15:547–54. doi: 10.1007/s00167-006-0261-0. [DOI] [PubMed] [Google Scholar]
- 31.Ostermeier S, Stukenborg-Colsman C, Hurschler C, Wirth CJ. In vitro investigation of the effect of medial patellofemoral ligament reconstruction and medial tibial tuberosity transfer on lateral patellar stability. Arthroscopy. 2006;22:308–19. doi: 10.1016/j.arthro.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25:533–7. doi: 10.1177/036354659702500417. [DOI] [PubMed] [Google Scholar]
- 33.Ramappa AJ, Apreleva M, Harrold FR, Fitzgibbons PG, Wilson DR, Gill TJ. The effects of medialization and anteromedialization of the tibial tubercle on patellofemoral mechanics and kinematics. Am J Sports Med. 2006;34:749–56. doi: 10.1177/0363546505283460. [DOI] [PubMed] [Google Scholar]
- 34.Sakai N, Luo ZP, Rand JA, An KN. The influence of weakness in the vastus medialis oblique muscle on the patellofemoral joint: an in vitro biomechanical study. Clin Biomech. 2000;15:335–9. doi: 10.1016/s0268-0033(99)00089-3. [DOI] [PubMed] [Google Scholar]
- 35.Schoettle PB, Zanetti M, Seifert B, Pfirrmann CW, Fucentese SF, Romero J. The tibial tuberosity-trochlear groove distance; a comparative study between CT and MRI scanning. Knee. 2006;13:26–31. doi: 10.1016/j.knee.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Teitge RA. Patellofemoral syndrome a paradigm for current surgical strategies. Orthop Clin North Am. 2008;39:287–311. doi: 10.1016/j.ocl.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Tjoumakaris FP, Forsythe B, Bradley JP. Patellofemoral instability in athletes: Treatment via modified Fulkerson osteotomy and lateral release. Am J Sports Med. 2010;38:992–9. doi: 10.1177/0363546509357682. [DOI] [PubMed] [Google Scholar]
- 38.Van de Velde SK, Bingham JT, Gill TJ, Li G. Analysis of tibiofemoral cartilage deformation in the posterior cruciate ligament-deficient knee. J Bone Joint Surg Am. 2009;91:167–75. doi: 10.2106/JBJS.H.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van De Velde SK, Bingham JT, Hosseini A, Kozanek M, DeFrate LE, Gill TJ, Li G. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis Rheum. 2009;60:3693–702. doi: 10.1002/art.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Victor J, Labey L, Wong P, Innocenti B, Bellemans J. The influence of muscle load on tibiofemoral knee kinematics. J Orthop Res. 2010;28:419–28. doi: 10.1002/jor.21019. [DOI] [PubMed] [Google Scholar]
- 41.Yoo JD, Papannagari R, Park SE, DeFrate LE, Gill TJ, Li G. The effect of anterior cruciate ligament reconstruction on knee joint kinematics under simulated muscle loads. Am J Sports Med. 2005;33:240–6. doi: 10.1177/0363546504267806. [DOI] [PubMed] [Google Scholar]