Abstract
Objective
Heightened amygdala reactivity to aversive stimuli in major depression is regarded as a core feature of the underlying physiology but individual differences in amygdala response may also arise secondary to persistent changes in limbic function during early neurodevelopment relative to stressors such as childhood trauma. We sought to determine whether heightened amygdala response is a core feature of depression or a general risk factor for psychopathology secondary to early life stress.
Method
Twenty unipolar depressed patients with and without a history of significant early life trauma and 16 healthy comparison subjects underwent functional MRI in a cross-sectional study comparing neural response to sad and neutral faces.
Results
We observed a robust positive correlation between physical abuse and right amygdala response. A much weaker relationship with other forms of abuse and neglect was also found, suggesting differences between abuse subtypes and amygdala response. Group differences in amygdala response suggest heightened reactivity was not characteristic of persons with depression in general but was true primarily in those with a significant history of abuse.
Conclusion
These findings suggest the relationship between childhood trauma and risk for depression is mediated by heightened amygdala response but varies by abuse type. Preliminary evidence for two distinct depression phenotypes based on trauma history was also supported, consistent with differential etiology.
Keywords: childhood trauma, amygdala, stress sensitization, depression
Major depressive disorder is a serious illness estimated to affect 13 to 14 million adults annually and approximately 16% of the U.S. population across the lifespan (Kessler et al., 2003). Furthermore, the considerable social, occupational and workplace related costs associated with impaired function are estimated in the tens of billions of dollars each year (Wang et al., 2006). Childhood maltreatment has emerged as a significant risk factor for the onset of adult depression with over 500,000 documented cases of physical and sexual abuse in the U.S. each year (Sedlak et al., 2010). Despite the widely appreciated magnitude of the problem, the precise mechanism by which childhood trauma may increase the risk for depression remains unclear.
Exaggerated amygdala response is regarded as a core feature of the underlying physiology of unipolar depression (Price and Drevets, 2010). Imaging studies utilizing both positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) methods have consistently demonstrated increased glucose metabolism and cerebral blood flow in response to sad stimuli (Suslow et al., 2010; Victor et al., 2010). Evidence of sustained amygdala activity post-stimulus offset and in response to masked stimuli has also been demonstrated (Dannlowski et al., 2007; Siegle et al., 2002). This increased amygdala response has subsequently been linked to the experience of momentary fluctuations in emotion in every-day life, self-induced sadness in healthy volunteers as well as negative affect in depression (Abercrombie et al., 1998; Barrett et al., 2007; Posse et al., 2003).
Rodent and non-human primate models suggest heightened amygdala response is closely linked to glucocorticoid-mediated transformations in brain morphology, neuronal hyperexcitability (Duvarci and Paré, 2007; Vyas et al., 2003) and disinhibition via medial prefrontal cortex (mPFC) atrophy (Quirk et al., 2003; Radley et al., 2006). The amygdalar complex appears to be functionally organized to play a key role in modulating the physiological and behavioral response to stress. This includes (1) evaluation of the salience of sensory input in lateral nucleus as well as (2) projections from central nucleus (CeA) to lateral hypothalamus and paraventricular nucleus (PVN) underlying autonomic (e.g., heart rate and galvanic skin response) and endocrine response (see Swanson and Petrovich, 1998, and Zald, 2003 for reviews). Thus, chronic stress, independent of depression, is likely to be associated with heightened amygdala response as the sequela of persistent glucocorticoid effects.
Evidence suggesting brain morphology and physiology are influenced by both heritable and stress induced influences has been observed in both human and animal models (Oler et al, 2010; Pennington et al 2000; Kaffman and Meaney, 2007). For instance, mild early life stress in non-human primates has been linked with later life stress inoculation, demonstrated by low plasma adrenocorticotropic hormone (ACTH), cortisol levels and decreased anxiety in response to emotional obstacles (Parker et al 2004). In contrast, investigations of more severe adverse experiences during early neurodevelopment in rodent and non-human primates, such as early maternal separation and poor postnatal care, have been linked with detrimental long-term changes in structure and function of the brain (Kaffman and Meaney, 2007; Meaney, 2001; Mirescu et al., 2004; Sanchez, 2006). Parallel findings in clinical populations suggest child abuse and neglect significantly increase risk for depression (Chapman et al., 2004; Putnam, 2003; Widom et al., 2007).
Research comparing depressed persons with and without a history of early life trauma suggests important differences on several key neurobiological features including endocrine and autonomic activity (Heim et al., 2000), as well as region-specific brain morphology differences in hippocampus and mPFC/cingulate (Vythilingham et al., 2002; Treadway et al., 2009). Based on models of stress-circuitry, the exaggerated autonomic and endocrine activity among trauma-linked depressed individuals appears initiated by increased output from CeA to lateral hypothalamus and PVN. Following this reasoning and based on our earlier morphology findings, we theorized that a history of childhood trauma (e.g., physical and sexual abuse) would be associated with potentiated amygdala activity.
To examine the role early life trauma may have in the relationship between depression and amygdala response to sad stimuli, we employed a series of regressions that modeled bilateral amygdala response in depressed participants and healthy controls. These models evaluated the influence of various forms of childhood maltreatment on amygdala response after controlling for clinical covariates unrelated to early life trauma. Next, we evaluated group differences in amygdala response. Prior investigations of stress sensitization among depressed patients with and without a significant history of trauma identified group differences in endocrine and autonomic reactivity to stress (Heim et al 2000), as well as structural differences in hippocampal volume (Vythilingham et al 2002) with increased endocrine/autonomic activity and decreased gray matter identified primarily within the trauma group. Thus, we anticipated primarily those individuals with a significant history of early life trauma and depression would demonstrate enhanced amygdala response to sad stimuli but not those with non-trauma linked depression. In addition, given the role of mPFC in inhibitory modulation of amygdala activity, we also performed a correlational analysis between early life trauma and mPFC response.
Participants
Twenty patients with current depression and 16 healthy control subjects completed this study. The Vanderbilt University Institutional Review Board approved the experimental protocol. A complete description of the study was provided to all participants, and all subjects provided written informed consent. Subjects were recruited through the Vanderbilt University Medical Center Outpatient Psychiatry Clinic and through television advertisements in the community.
All subjects were evaluated using the Structured Clinical Interview for DSM-IV (SCID; First et al., 2002). Clinical evaluations were performed by master’s level and doctoral level therapists in the department of psychiatry. Supervision and review were provided by authors MMG, RCS and SDH. Participants were between 18 and 55 years of age with no significant history of neurological disease; lifetime history of brain injury, psychosis, mania, substance dependence; or substance abuse within the previous six months. Patients were diagnosed with unipolar depression and met full criteria for one or more episodes of major depressive disorder as determined by the SCID. Patients were excluded if they met criteria for specific co-morbid Axis I disorders that included alcohol dependence, obsessive-compulsive disorder, schizophrenia and other psychotic disorders, and bipolar disorder. Participants who were currently taking antidepressants were excluded from the study, although oral contraceptive use was recorded (N=4 patients and 5 controls). A score of 16 or higher on the 17-item Hamilton Depression Rating Scale (HDRS; Hamilton, 1967) was required.
Never-depressed control subjects were free of (1) neurological disease and head injury (2) either current or past mood disorders, as well as (3) current or past history of Axis I disorders with the exception of one subject who was diagnosed with mild agoraphobia without panic disorder. Control subjects were required to have a score of six or less on the HDRS-17. Subjects who met criteria were then scheduled for a scan session within one week of admission to the study.
Imaging Protocol
Stimuli and Paradigm
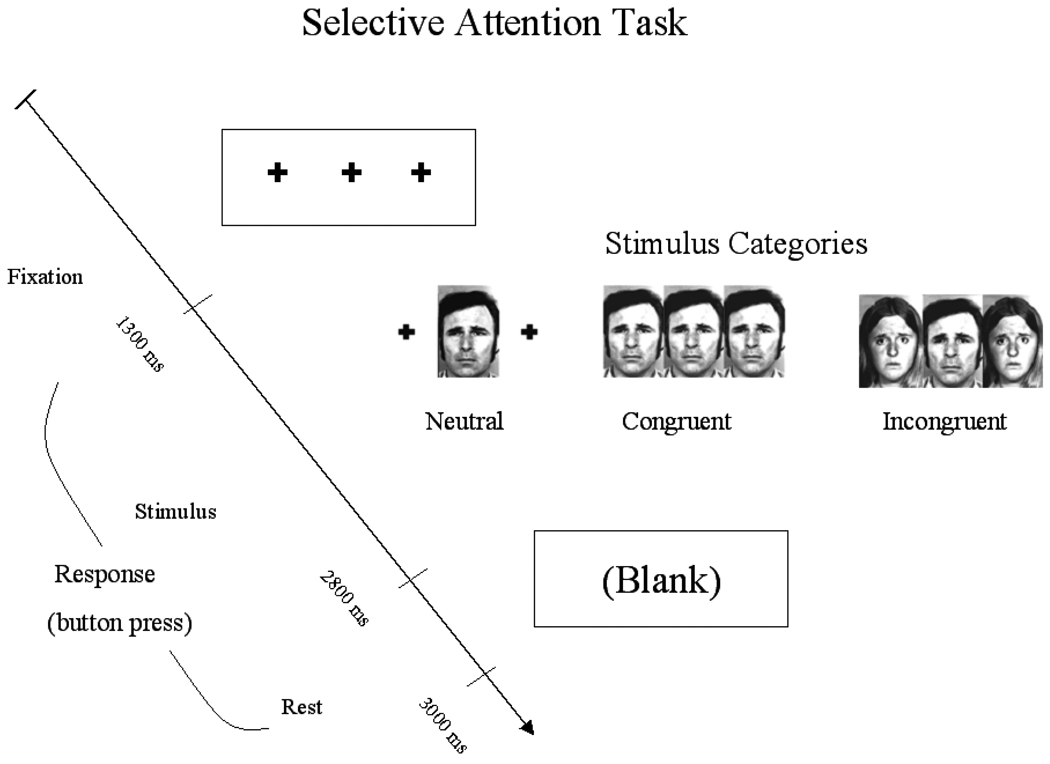
Participants performed a gender identification variant of the Eriksen flanker task of selective attention (Eriksen and Eriksen, 1974) using selected Ekman faces as stimuli (Ekman and Friesen, 1976). The task was designed to identify the influence of valence on the efficiency of selective attention by emotion (positive, sad, and neutral) and level of task difficulty (non-conflict, congruent and incongruent). To achieve the flanker effect, stimuli were arranged in arrays of three with a centralized face flanked by targets (Figure 1). Instructions were presented using E-Prime software (PST, Pittsburgh, PA). Participants were instructed to respond with a predetermined button press (index or middle key) to identify either male or female centralized target faces and asked to respond as quickly and accurately as possible. Distracter influence on performance was indicated by slowed behavioral response (i.e., reaction time). In the current study, only findings for the non-conflict level of task difficulty are presented. The more difficult levels of task difficulty, (congruent and incongruent), were not included in this analysis, as effective performance for high cognitive load processes are generally associated with dampened emotional response as a function of competition for neural resources (Lavie et al 2004).
Figure 1.
Schematic of selective attention task. Participants were instructed to respond with a predetermined button press (index or middle key) to identify either male or female centralized target faces. Each trial had a total duration of 3000 ms. Only data from the least difficult level of task difficulty (non-conflict level of task difficulty) are presented here.
Each subject performed 12 blocks of 9 trials, alternating between level of task difficulty and fixation. Each trial had a total duration of 3000 ms. A total of 108 experimental trials were presented randomly during each run. Participants completed six 3min 36s runs of the paradigm. In this design, each trial type was presented 24 times. The order of emotion presentation trials were counterbalanced across subjects and trial order randomized.
Image Acquisition
Scans were acquired on a 3T Philips Intera Achieva scanner at the Vanderbilt University Institute of Imaging Sciences (VUIIS). High-resolution structural images were acquired in the axial plane to facilitate spatial normalization using a 3D T1-weighted IR Prepped 3DFFE sequence (TR=10.1, TE=4.2, slice thickness l.2mm) and T2-weighted [TR=450ms, TE=17, FOV=24cm and slice thickness =4mm]. Twenty-eight axial interleaved 4.0 mm functional slices (with a 0.5mm skip) were acquired parallel to the AC-PC line using a gradient echo pulse sequence (EPI) providing whole brain coverage (T2*-weighted images sensitive to BOLD signal changes; TR=3000 ms, TE=28 ms, FOV= 24 cm, flip = 90 and slice thickness= 4mm).
Preprocessing and Image analysis
For each subject, six EPI runs were completed (2 runs per affective condition). Data from these runs were reconstructed and aligned with both 2-D and 3-D anatomical spatial coordinates using Brain Voyager QX. MR images were re-sliced such that horizontal slices were parallel to the AC-PC plane. Prior to co-registration, 3-D motion correction, slice-scan time correction and temporal data smoothing with a high pass filter were performed to remove linear trends. Functional image sets were then interpolated to 3mm3 voxels and aligned to the anatomical 3D. Data were translated into Talairach space (Talairach and Tournoux, 1988) for the purpose of averaging across subjects and to utilize standardized coordinates for regions of activation. Data were smoothed with an 8 mm full-width at half-maximum Gaussian kernel.
ROI Analysis
The entire amygdala has been estimated to occupy a region ranging between 17–30 mm lateral to the midline (x-axis), 1–11 mm posterior to the anterior commissure (y-axis), and 7–21mm inferior to the intercommissural line (z-axis) (Zald, 2003). However, reviews of the animal literature suggest the amygdala complex is not a functionally homogenous structure (Swanson and Petrovich, 1998). Instead, the amygdala is better defined as a cluster of structurally and functionally heterogeneous nuclei. As such, we identified a functionally defined region based on the response to sad stimuli (sad faces) using the entire sample combining patients and controls to circumvent potential differences in morphology. Subsequent analyses were based on the contrast sad > neutral, unless otherwise specified. The ROIs were defined by voxels within the search region between 21–28 mm lateral to the midline, 1–7 mm posterior to anterior commissure, and 11–19 mm inferior to the intercommissural line, significant at p<.005, uncorrected. This approach was used to assure independence of voxel selection method and the effect of interest. Mean parameter estimates of amygdala response were then extracted and employed in a series of hierarchical regression and ANOVA models in SPSS.
Whole Brain Correlation Analysis
Each affective condition was included as a separate predictor in a random effects multi-subject general linear model (GLM). Beta values derived from the contrast sad > neutral were then correlated on a voxel-wise level with measures of childhood trauma.
Childhood Trauma Questionnaire
Participants completed a modified version of a well-validated measure of child abuse and neglect before age 18, the Childhood Trauma Questionnaire – Short Form (CTQ-SF; Bernstein and Fink, 1998) that included an addendum with specific age ranges for each category of trauma (see Supplemental). The CTQ-SF was developed as a 28-item questionnaire derived from the original 70-item Childhood Trauma Questionnaire. The CTQ-SF has 25 clinical questions and three validity items. The measure has five factors comprised of five questions each that assess childhood maltreatment in the areas of emotional, physical, or sexual abuse, emotional neglect and physical neglect. Subjects rate statements about childhood experiences on a five-point scale (1=”never true” to 5=”very often true”). Scores for each factor are calculated based on the mean value of the five individual items for each subscale and range between 5 and 25.
Reliability and validity of the CTQ, including its stability over time, convergent and discriminant validity with structured trauma interviews, and corroboration using independent data, have been determined (Fink et al., 1995). The CTQ-SF has demonstrated high internal reliability, with (Cronbach’s alphas ranging from .74 to .90) and good test-retest reliability at three months (r = .80). Specifically, CTQ sexual abuse (CTQ-SA; Cronbach’s alpha= .93–.95) and physical abuse (CTQ-PA; alpha= .81–.86) subscales have robust internal consistency, while emotional abuse (CTQ-EA), emotional neglect (CTQ-EN) and physical neglect (CTQ-PN) demonstrate a range between .80 and .89. The CTQ has also demonstrated good convergent validity with both a clinician-rated interview of childhood abuse and therapists’ ratings of abuse (Fink et al., 1995).
There are no well established CTQ norms in adults, however (1) normative values have been computed in a community sample (Scher et al., 2004) and (2) exploratory cut-off scores have been investigated based on receiver operating characteristic (ROC) methods in a random sample of women members of a group health maintenance organization (Walker et al., 1999). A cut-off score of 8 on the physical abuse, physical neglect and sexual abuse subscales, 10 on the emotional abuse subscale and 15 on the emotional neglect subscale, provided very good to excellent sensitivity and specificity for confirmed abuse (≥ 0.85). These thresholds were associated with moderate to severe levels of abuse and neglect.
An addendum consisting of 15 questions that address specific time frames for the five factors of the CTQ was employed in the current study. The four time frames addressed are (a) birth to age 6 (b) 7 to 11 (c) 12 to 17 and (c) 18 or older. Consistent with the first 25 items, these statements are also rated on a five-point scale (1=”never true” to 5=”very often true”). Four additional items used as a PTSD screen are also included. These statements are rated on the same five-point scale as all other items. The inclusion of these items was exploratory and included to provide more precision in age of onset of abuse.
Additional Self-Report Measures
Measures to evaluate both anxiety and severity of depression were included in the battery and assessed as covariates in general linear models (Beck Anxiety Inventory; and Hamilton Rating Scale for Depression; HRSD-17) (Piotrowski, 1999; Hamilton, 1967). Intellectual functioning was evaluated using the Shipley-2 vocabulary test (Pollock, 1942), which is a brief measure of cognitive ability that was subsequently converted to Weschler Adult Intelligent Scale-Revised scores (WAIS-R).
Salivary Cortisol
Samples of saliva were collected using the salivette saliva collection device (Sarstedt, Newton NC). Participants collected three saliva samples per day for two consecutive days and a sample on the morning of the experimental task on the third day. The first sample was recorded within 0.5 h after awakening. Two additional samples were collected at 3:00 PM and 9:00 PM. Use of this method allowed us to adequately account for diurnal variation. Cortisol levels were determined using an enzyme immunoassay (ALPCO Diagnostics, Salem, NH). For all subsequent analyses, the average morning cortisol values were used unless otherwise specified.
Results
No significant demographic differences were observed between groups for sex or IQ (see Table 1) but a significant between-group difference in age was observed. Childhood trauma was assessed on five factors (three abuse and two neglect scales). Mean abuse and neglect scores for patients differed significantly from the control group for each of the five factors (see Table 1). In contrast, patients with and without a history of trauma differed in their report of abuse history but not neglect. Thus, all patients reported a similar level of neglect, but those categorized as depressed with trauma reported significantly more abuse (i.e., emotional, physical and sexual).
Table 1.
Demographic Data, CTQ Scores and Salivary Cortisol.
| Variable | Healthy Controls |
MDD | MDD Only | MDD+Trauma | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| Sample Size | 16 | 20 | 10 | 10 | ||||||||
| Number of female participants | 10 | 11 | 7 | 4 | ||||||||
| Age† | 16 | 31.1 | 9.2 | 20 | 34.5 | 10.7 | 10 | 29.2 | 9.3 | 10 | 39.3 | 9.5 |
| Estimated IQ (Shipley | 16 | 108.5 | 9.7 | 20 | 106.3 | 8.2 | 10 | 103.4 | 8.1 | 10 | 109.2 | 7.7 |
| Hamilton Rating Scale of Depression** | 16 | 0.9 | 1.3 | 20 | 21.4 | 4.1 | 10 | 21.4 | 4.3 | 10 | 21.4 | 4 |
| Number of previous episodes | 20 | 2.6 | 0.9 | 10 | 2.7 | 1 | 9 | 2.3 | 0.5 | |||
| Average duration of illness (years) | 20 | 11.8 | 13.2 | 10 | 5.13 | 4.4 | 10 | 17.9 | 15.6 | |||
| Past alcohol abuse | 3 | 1 | 2 | |||||||||
| Co-morbid/ anxiety disorder | 10 | 2 | 8 | |||||||||
| Past anxiety disorder | 1 | 3 | 2 | 1 | ||||||||
| CTQ Emotional Abuse Scale**† | 16 | 6.1 | 1.5 | 20 | 12.1 | 6.1 | 10 | 9.3 | 5.3 | 10 | 14.9 | 5.6 |
| CTQ Physical Abuse Scale**† | 16 | 5.4 | 0.6 | 20 | 9.9 | 4.5 | 10 | 6.7 | 1.1 | 10 | 13.1 | 4.3 |
| CTQ Sexual Abuse Scale*† | 16 | 5.6 | 2.5 | 20 | 9.8 | 6.8 | 10 | 5.5 | 1.2 | 10 | 14.0 | 7.6 |
| CTQ Emotional Neglect** | 16 | 7.2 | 2.8 | 20 | 12.6 | 4.4 | 10 | 11.0 | 4.4 | 10 | 14.9 | 3.8 |
| CTQ Physical Neglect* | 16 | 5.1 | 0.3 | 20 | 8.5 | 4 | 10 | 9.1 | 2.6 | 10 | 7.8 | 5.1 |
| Salivary Cortisol (morning samples)* | 9 | 8.1 | 2 | 15 | 11.1 | 3.6 | 7 | 10.1 | 4.5 | 8 | 12 | 2.7 |
| Salivary Cortisol (all samples) | 7 | 6.4 | 2.1 | 12 | 7.7 | 2.4 | 6 | 7.6 | 3 | 6 | 7.9 | 1.9 |
Note.
p < .05 in the comparison between Healthy Controls and MDD,
indicates p < .001 in the comparison between Healthy Controls and MDD.
p< .05 in the comparison between MDD Only and MDD + Trauma,
indicates p < .001 in the comparison between MDD Only and MDD + Trauma.
Childhood trauma and bilateral amygdala response: ROI analysis
Sad Stimuli
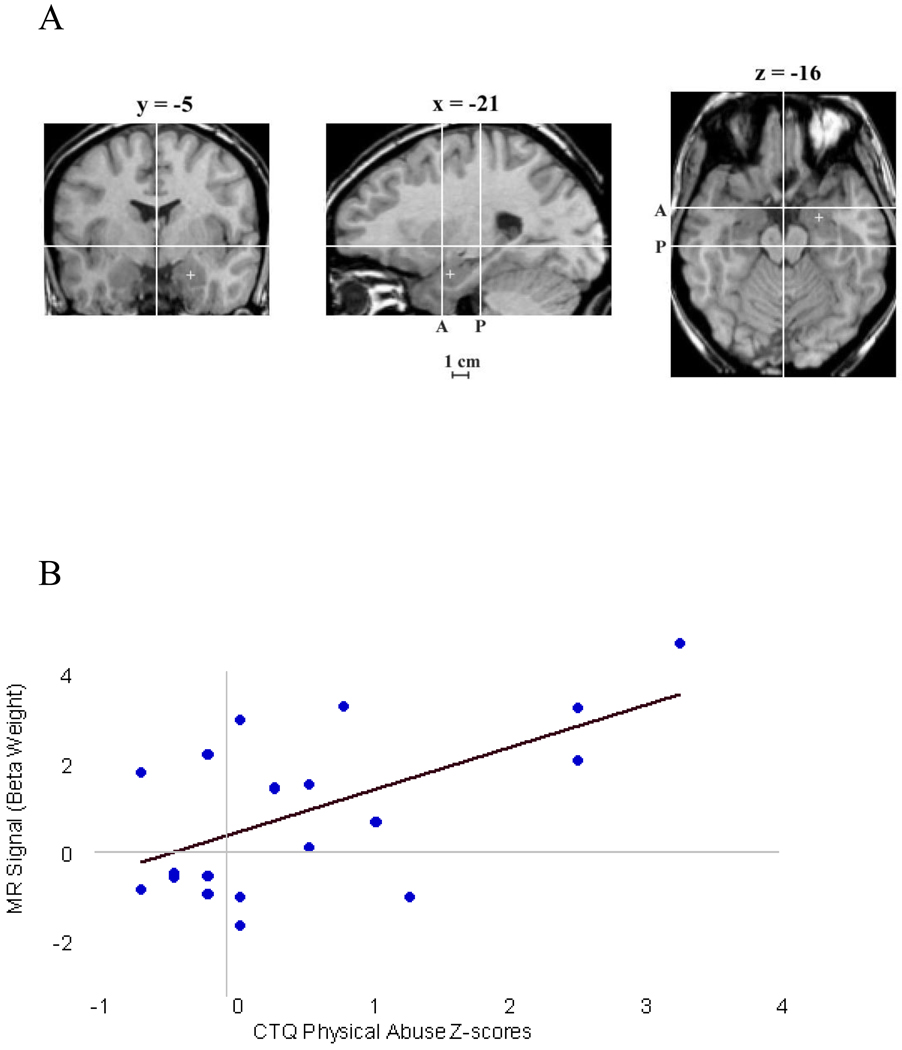
To examine the relationship between childhood trauma and bilateral amygdala activity, we performed a correlation analysis between the five factor scores of the CTQ and beta weight values extracted from the regions of interest. CTQ-PA scores correlated robustly with right amygdala activity in response to the contrast sad > neutral for the entire group (r=0.48, p=.003) and within the depressed participants alone (r=0.59, p=.005; Figure 2). A much weaker relationship was observed between CTQ factors for sexual abuse (CTQ-SA) and right amygdala response for the whole group (r=0.30, p=.07) and for the depressed participants alone (r=0.33, p=.16); emotional abuse (CTQ-EA) for whole group (r=.32. p=.056), and (r=.31 p=.19) for depressed alone; emotional neglect (CTQ-EN) whole group (r=.29, p=.09), and depressed only (r=.28, p=.23), and physical neglect (CTQ-PN) whole group (r=.22, p=.20) and (r=.18, p=.52) for depressed alone. No significant relationship was observed between any CTQ subscale and left amygdala activity utilizing this same contrast. These data suggest physical abuse during early life is associated with robust amygdala response to sad stimuli, with weaker relationships observed for other subscales such as sexual and emotional abuse and multiple forms of neglect.
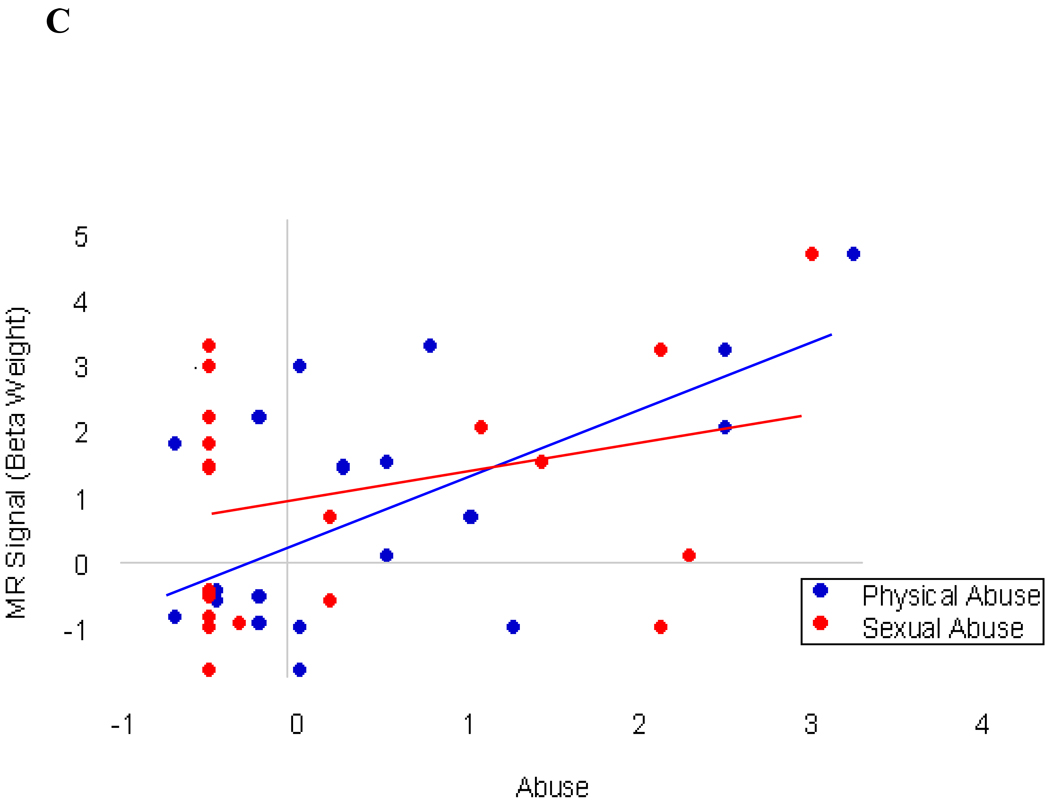
Figure 2.
Correlation between physical abuse and right amygdala. (A). Coronal, sagittal and axial schematic of neuroanatomical boundaries of amygdala adapted with permission from Zald (2003). (B) X-axis; Fisher's z-score transformation of CTQ-PA scores for patients, N=20. Y-axis; MR signal beta weight values extracted from right amygdala, (r=0.59, p<.005); (C) X-axis: Fisher’s z-score transformation of CTQ-PA and CTQ-SA scores for patients, N=20. Y-axis: MR signal beta weight values extracted from right amygdala, CTQ-PA (r=0.59, p<.005) and CTQ-SA (r=0.33, p=.16).
Positive Stimuli
Only sexual abuse correlated with amygdala response for the contrast of positive > neutral (See Supplemental Materials).
Regression Analysis
Our correlational analysis suggests a history of childhood physical abuse is robustly associated with heightened amygdala response to sad stimuli. However, a number of factors other than childhood trauma are also linked to amygdala response including sex, (Killgore and Yurgelun-Todd, 2001; Schneider et al., 2000) severity of illness, (Drevets, 2003) anxiety, (Phan et al., 2006; Etkin and Wager, 2007) adult onset trauma (Shin et al., 2005) and basal glucocorticoid activity (Drevets et al., 2002).
We implemented a series of hierarchical regression analyses that included sex, the HRSD-17, the Beck Anxiety Inventory, duration of illness, average morning cortisol and traumatic experiences reported after age 18 (using a modified version of the childhood trauma questionnaire (CTQ-M), that included an addendum with specific ages for each adverse event), as covariates to address potential confound or mediating effects in the relationship between abuse and amygdala response. To retain the maximum number of participants in the analysis, we initially excluded morning cortisol from the analysis since data were available for only 26 participants due to a combination of participant failure to collect morning samples and insufficient volume in some participants (n=5 patients and n=5 controls), as well as CTQ adult onset items, since this version of the measure was added after the initiation of the study and data were available for only 18 participants. Duration of illness was only included for the patient model as this variable was not assessed in healthy controls. The adjusted model significantly predicted right amygdala response to sad stimuli among all participants, (F=3.74, df=4,31, p=.01, f2=.57; excluding cortisol and adult onset of trauma), as well as CTQ-PA (t=3.55, p=.001). Replacing clinician rated depression with a self-report measure of depression (BDI-2; see Table 2) did not significantly alter the significance of physical abuse in this model (t=3.16, p=.003). However, while the HRSD-17 trended toward significance, the BDI-2 did not (see Table 2). As such, subsequent analysis included the HRSD-17 but not the BDI-2.
Table 2.
Regression Model Physical Abuse-All participants
| Unstandardized Estimates B |
Standard Error SE |
Standardized Estimates B |
Degrees of Freedom df |
t |
p-level |
|
|---|---|---|---|---|---|---|
| Sex | .82 | .53 | .25 | 4, 31 | 1.56 | .12 |
| BAI | .00 | .03 | .01 | 4, 31 | .25 | .81 |
| BDI-2 | −.02 | .02 | −.16 | 4, 31 | −.80 | .49 |
| HRSD-17 | −.05 | .03 | −.28 | 4, 31 | −1.63 | .17 |
| Duration | −.03 | .05 | .21 | 5,11 | .67 | .51 |
| Morning Cortisol | −.04 | .06 | −.13 | 5,20 | −.72 | .48 |
| Adult Onset Trauma | .08 | .97 | .02 | 5,17 | .08 | .94 |
| CTQ-PA | .29 | .08 | .69 | 4,31 | 3.55 | .001 |
Note. All participants included, N=34 total. Degrees of freedom vary due to missing data. Adult onset trauma is derived from a modified version of the childhood trauma questionnaire (CTQ-M) that includes an addendum to the CTQ that specifies age ranges of abuse and neglect. Beck Anxiety Inventory, (BAI); Beck Depression Inventory, (BDI-II); Hamilton Rating Scale for Depression-17 item, (HRSD-17); Duration is the average duration of depression in years; physical abuse (CTQ-PA).
The regression model including all of the aforementioned covariates and morning cortisol, again found the fully adjusted model to be significant (F=3.19, p<.05) as well as the CTQ-PA (t=2.93, p=.008) but not morning cortisol. Similarly, the addition of other covariates such as duration of illness or adult onset trauma into separate models did not negate the significance of physical abuse in predicting amygdala response to sad stimuli (significance of CTQ-PA when adding these covariates: morning cortisol, p=.006; duration of illness, p<.01; adult onset trauma, p<05). Other than childhood physical abuse, no other variable was significant in any model, although the HRSD-17 did trend toward significance in each model (see Table 2).
We subsequently evaluated this relationship among the depressed participants only, narrowing the focus to those individuals with the greatest risk for early life trauma (Table 3). Again the adjusted model with all of the covariates except morning cortisol or adult onset trauma was significant at (F=4.50, df=4,15, p< .01) and CTQ-PA remained significant (t=3.52, p= .003). Among the covariates, only the HRSD-17 trended toward significance in these models with p-values ranging between .07 and .16. Again the addition of other covariates such as duration of illness or adult onset trauma into separate models did not negate the significance of physical abuse in predicting amygdala response to sad stimuli (significance of CTQ-PA when adding these covariates: morning cortisol, p=.01; duration of illness, p<.01; adult onset trauma, p<05). The models for bilateral amygdala and all other forms of abuse were non-significant (ps>.10). Collectively, these findings indicate early life trauma is not merely a proxy for severity of illness, anxiety or sex differences in relation to amygdala response.
Table 3.
Regression Model Physical Abuse-Depressed Patients
| Unstandardized Estimates B |
Standard Error SE |
Standardized Estimates B |
Degrees of Freedom df |
t |
p-level |
|
|---|---|---|---|---|---|---|
| Sex | 1.07 | .70 | .23 | 4,15 | 1.57 | .13 |
| BAI | −.01 | .04 | −.03 | 4,15 | −.01 | .86 |
| BDI-2 | −.01 | .04 | −.04 | 4,15 | −.21 | .84 |
| HRSD-17 | −.15 | .08 | −.31 | 4,15 | −1.92 | .07 |
| Duration | .03 | .05 | .21 | 5,11 | .67 | .52 |
| Cortisol | −.01 | .11 | −.02 | 5,9 | −.08 | .94 |
| Adult Onset Trauma | .41 | .91 | .13 | 5,6 | .45 | .67 |
| CTQ-PA | .27 | .07 | .86 | 4,15 | 3.52 | .003 |
Note. MDD without a history of trauma, N=20. Degrees of freedom vary due to missing data. Adult onset trauma is derived from a modified version of the childhood trauma questionnaire (CTQ-M) that includes an addendum to the CTQ that specifies age ranges of abuse and neglect. Beck Anxiety Inventory, (BAI); Beck Depression Inventory, (BDI-II); Hamilton Rating Scale for Depression-17 item, (HRSD-17); Duration is the average duration of depression in years; physical abuse (CTQ-PA).
A similar series of hierarchical regression models were performed among the control participants alone. No significant omnibus test, effect for physical abuse or any covariate was observed (see Table 4).
Table 4.
Regression Model Physical Abuse -Controls
| Unstandardized Estimates B |
Standard Error SE |
Standardized Estimates B |
Degrees of Freedom df |
t |
p-level |
|
|---|---|---|---|---|---|---|
| Sex | .18 | 1.03 | .06 | 4,11 | .18 | .86 |
| BAI | −.25 | .21 | −.36 | 4,11 | −1.21 | .25 |
| BDI-2 | −.10 | .40 | .08 | 4, 11 | .25 | .80 |
| HRSD-17 | −.00 | .43 | −.00 | 4,11 | −.00 | .99 |
| Cortisol | −.06 | .10 | −.26 | 5,5 | −.62 | .56 |
| CTQ-PA | .14 | .89 | .06 | 4,11 | .16 | .87 |
Note. Controls alone, N=16. Degrees of freedom vary due to missing data. Beck Anxiety Inventory, (BAI); Beck Depression Inventory, (BDI-II); Hamilton Rating Scale for Depression-17 item, (HRSD-17); physical abuse (CTQ-PA).
Behavioral Response (Reaction Time; RT)
We subsequently compared reaction time (RT) between groups for the non-conflict level of task difficulty as a behavioral validation of attention during the task. A one-way ANOVA comparing group RTs found no significant differences for the three groups (F=1.17, df= 2, 36, p=.32).
Group Differences: ANOVA
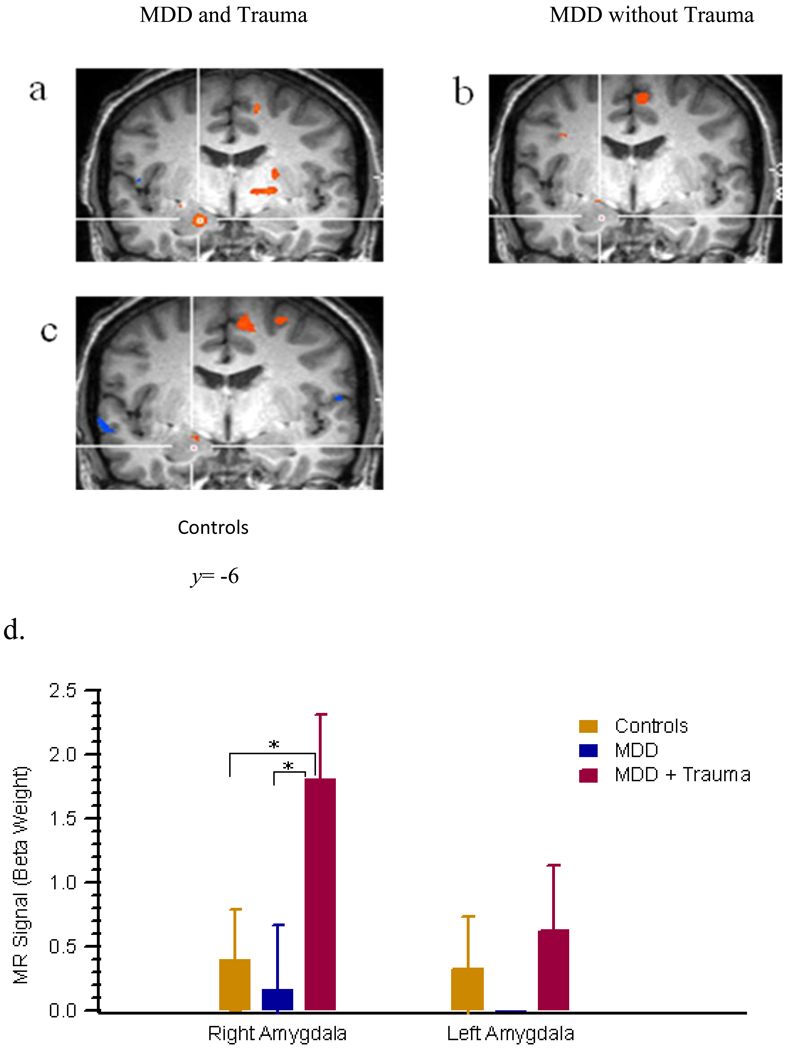
Potential group differences in neurophysiology have yet to be addressed between depressed individuals with and without a significant history of trauma as such we investigated amygdala response to sad stimuli based on abuse status. A one-way analysis of variance (ANOVA) based on the Walker et al. (1999) criteria for cut-off scores ≥8 on the CTQ-PA were performed. The omnibus test of group was significant (F=3.27, df=2, 33, p=.05 (see Fig 3). Post-hoc analysis based on Tukey’s test demonstrated only marginal significance between depressed with and without a significant history of abuse (p=.06) and depressed with a significant history of trauma and healthy controls (p=.09). No differences were noted between depressed without a significant history of trauma and healthy controls (p=.93).
Figure 3.
Group differences in bilateral amygdala. (A) MDD with a significant history of childhood trauma and right amygdala activity [x=18,y=−7,z=13], (B) MDD without a significant history of childhood trauma and (C) Healthy Controls. (D) One-way ANOVA comparison of right amygdala between three groups. Significant right amygdala activity among MDD with trauma relative to MDD alone, p<.05 and healthy controls, p<.05.
Voxel-Wise Correlational Analysis: Ventral mPFC
We next investigated the relationship between ventral mPFC function and physical abuse based upon the specific role of mPFC in restraint of amygdala response. We limited our analysis to physical abuse since this was the only factor associated with significant amygdala response. This was accomplished by performing a whole brain voxel-wise analysis based upon the contrast sad > neutral. A positive correlation between physical abuse and left BA 32 [CTQ-PA; r=.54, p<.01, uncorrected, x=−5, y=35, z=−5] and BA10 [CTQ-PA; r=.54, p<.01, uncorrected, x=−18, y=45, z=−1] was observed for patients. Similarly, a positive correlation was observed among controls between CTQ-PA and left BA11 (r=.56, p<.01, uncorrected, x=−3, y=28, z=−13). None of the correlations between ventral mPFC and physical abuse retained significance for either group after correcting for multiple comparisons using the False Discovery Rate (FDR). Additional areas of activation in whole brain in response to the contrast sad > neutral stimuli are included in the Supplemental (Table 1–2).
Discussion
The current study identified a robust relationship between a history of childhood abuse and amygdala response to sad stimuli. To our knowledge, this is the first investigation of the influence of early life trauma on amygdala response among individuals with major depression. The significance of this finding is twofold: (1) there appears to be a dose-response relationship whereby increased severity of abuse is associated with elevated amygdala response to sad stimuli and (2) the relationship between abuse and amygdala response differs by abuse dimension. Our secondary analysis of stress sensitization demonstrated modest evidence for group differences in amygdala response suggesting heightened reactivity was not characteristic of persons with depression in general but was true primarily in those with a significant history of abuse. This is consistent with a growing literature suggesting several of the theorized features of depression may in fact be secondary to sensitization of the limbic system ensuing from early life stress (Sanchez, 2006; Heim et al., 2008).
Exaggerated amygdala response is regarded as a core feature of the underlying physiology of major depression (Price and Drevets, 2010) with increased amygdala reactivity to sad stimuli demonstrated consistently (Suslow et al., 2010; Victor et al ., 2010). As such, the dampening of amygdala response is considered an essential component of symptom remission (Sheline et al., 2001; Fu et al., 2004), while heightened amygdala activity is considered a significant risk factor for relapse (Ramel et al., 2007) and increased amygdala response has been linked to negative affect in depression (Abercrombie et al., 1998). Together these findings indicate the importance of identifying the specific causal mechanisms underlying the perturbations in amygdala response that influence risk for psychopathology.
We propose that this potentiated amygdala response which is hypothesized to be linked to depression may instead be driven primarily by sensitization of amygdala secondary to persistent exposure to elevated glucocorticoid levels following early life adversity. Our premise is consistent with the group differences observed in the current study between depressed with and without a history of significant early life trauma, as well as animal and human models of glucocorticoid effects on amygdala response (Duvarci and Pare, 2007; Kukolja et al. 2008). Consistent with this hypothesis, Kukolja et al. (2008) demonstrated a comparable exaggerated amygdala response to aversive stimuli in healthy volunteers using pharmacological manipulation of noradrenergic-glucocorticoid interactions in comparison to placebo to simulate the stress response. This suggests acute exposure to glucocorticoids is linked with at least temporary increases in amygdala response. Conceivably, the more persistent exposure to glucocorticoids thought to be associated with early life trauma may drive long term alterations in structure and function.
While significant stress is generally linked to increased risk for psychopathology across age ranges, adverse experiences occurring during early neurodevelopment in rodent and non-human primates, such as early maternal separation and poor postnatal care, has been linked specifically with long-term changes in structure and function of the brain (Kaffman and Meaney, 2007; Meaney, 2001; Mirescu et al., 2004; Sanchez, 2006). Consistent with this finding, adult onset trauma was not a significant predictor in the current study, whereas childhood trauma was significant and remained so with the inclusion of multiple covariates in the model. In particular, given the relationship between PTSD and amygdala response (Shin et al 2005), our findings are notable in that the exaggerated amygdala response observed was not linked with comorbid PTSD. Analysis with and without the two participants diagnosed with PTSD did not alter our findings. It is possible these differences are attributable to discrepancies in brain maturation in relation to the timing of the stressor that may influence susceptibility to persistent changes in brain structure and function following trauma (Andersen and Teicher, 2004; Buss et al., 2009), but we cannot rule out insufficient power in the current study based on sample size.
A number of clinical and non-clinical factors are predictive of amygdala response other than childhood trauma. As such we included these as covariates in our models of amygdala response to sad stimuli. Although severity of illness did trend toward significance within the depressed group alone and in the group as a whole, no covariate actually reached significance in any model. This was true despite our use of hierarchical regression in which all covariates were entered into the model prior to abuse in order to observe the effects of abuse above and beyond other variables. Inclusion of covariates did result in a slightly less robust relationship between abuse and amygdala response but it remained significant in both the depressed only and whole group models. Thus, amygdala response was not better explained by sex differences, severity of illness or anxiety.
It is also important to note the strength of the relationship between amygdala response to sad stimuli and early life trauma differed by type of abuse, with a more robust relationship observed with physical abuse than other forms of abuse or neglect. This is consistent with maltreatment as a multidimensional construct and suggests the relationship may not generalize to all forms of abuse. This finding may have implications for the relationship between abuse and depression in general as it parallels the findings from recent investigations of childhood trauma and depression in which physical and emotional abuse have demonstrated strong relationships with depression but not sexual abuse (Lumley & Harkness, 2007; Widom et al., 2007). On the other hand, the factor scores of the CTQ-SF are not orthogonal, nor is the experience of abuse and this may explain the differences between factors. In this study for instance, most of those who reported physical abuse did not report experiencing sexual abuse; however the majority of those who reported sexual abuse did report concurrent physical abuse, thus possibly confounding the contributions of sexual abuse on long-term amygdala response.
Our secondary analysis of the stress sensitization hypothesis yielded support for the observation that depressed persons with a significant history of trauma demonstrated increased amygdala response, while those with no history of only mild histories of trauma did not differ from controls. This was true in spite of the fact that there were no differences in severity between the depressed groups. This finding is consistent with rodent and non-human primate studies that suggest persistent stress may modify the structure and function of limbic regions in such a way as to subsequently alter future responsiveness (Abercrombie et al., 1998; Radley et al., 2006). Our finding is tempered however by a consistent limitation within this literature (Heim et al 2000; Vythilingham et al 2002), which is that our groups were based on a comparison of a moderate to severely abused group with those reporting only mild abuse and not the absolute presence or absence of abuse. This approach was used for two reasons a) the criteria for scoring the CTQ were based on an ROC analysis that did not support cut-off scores consistent with absolute presence/absence of abuse and b) in the current study, almost the entire patient sample reported some history of abuse or neglect. Again, future investigations that oversample for individuals with MDD with no history of abuse will be needed to address the question of whether there are group differences in brain physiology based on the absolute presence or absence of abuse.
Finally, medial PFC in humans is hypothesized to underlie inhibition of amygdala via GABAergic modulation in a manner analogous to prelimbic/infralimbic cortex in non-human primates and rodents (Amaral, 1992; Quirk et al 2003). We have previously observed a robust relationship between cingulate volume (BA 32) and abuse history among individuals with unipolar depression (Treadway et al., 2009), however the relationship between mPFC and childhood trauma history did not survive correction for multiple comparisons in the current study. Thus, while reductions in gray matter in this area relative to early life trauma may contribute to the subsequent perturbations in amygdala associated with stress and depression, current findings did not support this explanation.
In general, findings from the current study provide support for the premise that the heightened amygdala response and sensitivity to aversive stimuli observed during a depressive episode is a component of the long-term effects of stress that increase risk for psychopathology and is not better explained by comorbid anxiety symptoms, severity of illness or sex differences.
Limitations
The current study is the first to examine whether exaggerated amygdala response is a principal characteristic of unipolar depression or a consequence of persistent changes in limbic system secondary to early life stress. The primary analysis involved a dose-response relationship between severity of childhood trauma and amygdala response that yielded significant findings consistent with stress sensitization of limbic function but would benefit from a larger sample size to further explore group differences in amygdala response. A separate limitation of the current findings was the use of a self-report measure to evaluate childhood trauma given the known relationship between current mood and memory. It should be noted however that the CTQ-SF has very good to excellent sensitivity and specificity for confirmed abuse. Yet, future studies would benefit from a prospective analysis of the relationship between childhood trauma and subsequent changes in limbic function based on an interview based measure of childhood maltreatment or documented cases of abuse.
Supplementary Material
Acknowledgments
The authors thank David Zald for his helpful comments on the manuscript and Morgan Shields and Rob Hilton for their technical assistance with data collection and management in the completion of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie HC, Schafer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, Perlman SB, Turski PA, Krahn DO, Benca RM, Davidson RJ. Metabolic rate in the right amygdala predicts negative affect in depressed patients. NeuroReport. 1998;9:3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- Amaral DG. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Bliss-Moreau E, Duncan SL, Rauch SL, Wright CI. The amygdala and the experience of affect. Social Cognitive and Affective Neuroscience. 2007;2:73–83. doi: 10.1093/scan/nsl042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. The Childhood Trauma Questionnaire: A retrospective self-report [Google Scholar]
- Bogdan R, Pizzagalli D. Acute stress reduces reward responsiveness. Implications for Depression. Biol Psychiatry. 2006;60:1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis E, Muftuler L, Head K, Sandman C. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoendocrinology. 2009;35:141–153. doi: 10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affective Disorders. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Clark L, Watson D. Tripartite model of anxiety and depression: Psychometric and Taxonomic implications. J Abnormal Psych. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, Kersting A, Baune BT, Suslow T. Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3T fMRI study. J Psychiatry Neurosci. 2007;32:423–429. [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: Relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann NY Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Parè D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and social phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink LA, Bernstein D, Handelsman L, Foote J, Lovejoy M. Initial reliability and validity of the childhood trauma interview: A new multidimensional measure of childhood interpersonal trauma. Am J Psychiatry. 1995;152:1329–1335. doi: 10.1176/ajp.152.9.1329. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. Nov, [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore E. Attenuation of the neural response to sad faces in major depression by antidepressant treatment. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport D, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:593–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder. JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Sex differences in amygdala activation during the perception of facial affect. NeuroReport. 2001;12:2543–2547. doi: 10.1097/00001756-200108080-00050. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Schlapfer TE, Keysers C, Klingmuller D, Maier W, Funk GR, Hurlemann R. Modeling a negative response bias in the human amygdala by noradrenergic-glucocorticoid interactions. J Neurosci. 2008;28:12868–12976. doi: 10.1523/JNEUROSCI.3592-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert WW, Viding E. Load theory of selective attention and cognitive control. J Exp Psychol Gen. 2004 Sep;133:33–54. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lumley MN, Harkness KL. Specificity in the relations among childhood adversity, early maladaptive schemas and symptom profiles in adolescent depression. Cognitive Therapy and Research. 2007;31:63–657. [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Ann Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Oler JA, Fox A, Shelton S, Rogers J, Dyer T, Davidson RJ, Shelledy W, Oakes TR, Blangero J, Kalin NH. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Arch Gen Psychiatry. 2004;61:933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Filipek P, Lefly D, Chhabildas N, Kennedy D, Simon J, Filley C, Galaburda A, DeFries JC. A twin MRI study of size variations in the human brain. J Cognitve Neurosci. 2000;12:223–232. doi: 10.1162/089892900561850. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety. Biol Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Piotrowski C. The status of the Beck Anxiety Inventory in contemporary research. Psychol Rep. 1999;85:261–262. doi: 10.2466/pr0.1999.85.1.261. [DOI] [PubMed] [Google Scholar]
- Posse S, Fitzgerald D, Gao K, Habel U, Rosenberg D, Moore GJ, Schneider F. Real-time fMRI of temporolimbic regions detects amygdala activation during single-trial self-induced sadness. NeuroImage. 2003;18:760–768. doi: 10.1016/s1053-8119(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam FW. Ten-Year research update review: Child sexual abuse. J Am Acad Child Adolesc Psychiatry. 2003;42:269–278. doi: 10.1097/00004583-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Parè D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR. Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biol Psychiatry. 2007;61:231–239. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Sanchez M. The impact of early adverse care on HPA axis development: Nonhuman primate models. Horm Behav. 2006;50(4):623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Scher CD, Forde DR, McQuaid JR, Stein MB. Prevalence and demographic correlates of childhood maltreatment in an adult community sample. Child Abuse Negl. 2004;28:167–180. doi: 10.1016/j.chiabu.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Kessler C, Salloum JB, Posse S. Gender differences in regional cerebral activity during sadness. Human Brain Mapping. 2000;9:226–238. doi: 10.1002/(SICI)1097-0193(200004)9:4<226::AID-HBM4>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, Greene A, Li S. Fourth National Incidence Study of Child Abuse and Neglect (NIS–4): Report to Congress. Washington, DC: U.S. Department of Health and Human Services, Administration for Children and Families; 2010
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schöning S, Ohrmann P, Bauer J, Pyka M, Kersting A, Arolt V, Heindel W, Dannlowski U. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry. 2010;67(2):155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 3-dimensional Proportional Systems: An Approach to Cerebral Imaging. New York: Thieme Medical Publishers; 1988. Co-planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Treadway MT, Grant MM, Ding Z, Hollon SD, Gore JC, Shelton RC. Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PloS ONE. 2009;4:e4887. doi: 10.1371/journal.pone.0004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T, Furey M, Fromm S, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010;67(11):1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vythilingham M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Stabib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Gelfand A, Katon WJ, Koss MP, Von Kroff M, Bernsteiin D, Russo J. Adult health status of women HMO members with histories of childhood abuse and neglect. Am J Med. 1999;107:332–339. doi: 10.1016/s0002-9343(99)00235-1. [DOI] [PubMed] [Google Scholar]
- Wang PS, Patrick A, Avorn J, Azocar F, Ludman E, McCulloch J, Simon G, Kessler R. The costs and benefits of enhanced depression care to employers. Arch of Gen Psychiatry. 2006;63:1345–1353. doi: 10.1001/archpsyc.63.12.1345. [DOI] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- Zald D. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.