Abstract
Neurologically normal subjects misperceive the midpoints of lines (PSE) as reliably leftward of veridical center, a phenomenon known as pseudoneglect. This leftward bias reflects the dominance of the right cerebral hemisphere in deploying spatial attention. Transient visual cues, delivered to either the left or right endpoints of lines, modulate PSE such that leftward biases are increased by leftward cues, and are decreased by rightward cues, relative to a no-cue control condition. We ask whether lateralized auditory cues can similarly influence PSE in a tachistoscopic visual line bisection task, and describe how visual and auditory cues, in spatially synergistic or antagonistic combinations, jointly influence PSE. Our results demonstrate that whereas auditory and visual cues both modulate PSE, visual cues are overall more potent than auditory cues. Visual and auditory cues are weighted such that visual cues are significantly more potent than auditory cues when visual cues are delivered to left hemispace. Visual and auditory cues are equipotent when visual cues are delivered to right hemispace. These results are consistent with the existence of independent lateralized networks governing the deployment of visuospatial and audiospatial attention. An analysis of the weighting of unisensory visual and auditory cues which optimally predicts PSE in multisensory cue conditions shows that cues combine additively. There was no evidence for a superadditive multisensory cue combination.
Keywords: Pseudoneglect, Visuospatial Attention, Audiospatial Attention, Line Bisection, Spatial Cueing
INTRODUCTION
Asymmetries of Spatial Attention: Hemineglect
Hemispatial neglect syndrome entails a deficiency of attention directed towards stimuli located within contralesional (typically left) hemispace which can occur in egocentric or allocentric coordinates (Arguin & Bub, 1993; Behrmann, 1999; Heilman & Valenstein, 1979; Mesulam, 1982; Bisiach, Bulgarelli, Sterzi & Vallar, 1983; Nichelli, Rinaldi & Cubelli, 1989; Driver, Baylis, Goodrich & Rafal, 1994; Driver & Halligan, 1991). Left hemispatial neglect occurs most commonly after lesions to right inferior parietal or temporoparietal cortex, but may also result from lesions to frontal or cingulate cortex, or to subcortical structures (Heilman & Valenstein, 1979; Damasio, Damasio & Chui, 1980; Watson, Valenstein & Heilman, 1981; Mesulam, 1982; Vallar, 1993; Karnath, Berger, Küver & Rorden, 2004). Line bisection is commonly employed to assay asymmetries of spatial attention, where neglect patients bisect horizontal lines of moderate length significantly rightward of veridical center (Schenkenberg, Bradford & Ajax, 1980; Robertson & Halligan, 1999; Kerkhoff, 2001).
Asymmetries of Spatial Attention: Pseudoneglect
It is well established that visuospatial attention in neurologically normal subjects is also distributed asymmetrically, resulting in a modest but systematic and significant leftward deviation of perceived line midpoint (PSE) in line bisection tasks (Bradshaw & Nettleton, 1983; Bradshaw, Nettleton, Nathan & Wilson, 1985; Bradshaw, Nathan, Nettleton, Wilson & Pierson, 1987; McCourt & Olafson, 1997; McCourt & Jewell, 1999; Jewell & McCourt, 2000; McCourt, Garlinghouse & Slater, 2000; McCourt & Garlinghouse, 2000a;b; McCourt, 2001; McCourt, Freeman, Tahmahkera-Stevens & Chaussee, 2001; McCourt, Garlinghouse & Butler, 2001; Foxe, McCourt & Javitt, 2003; McCourt, Garlinghouse & Reuter-Lorenz, 2005; McCourt, Shpaner, Javitt & Foxe, 2008; Leone & McCourt, 2010; Sosa, Teder-Sälejärvi & McCourt, 2010). It leads also to a systematic overestimation of stimulus saliency (e.g., size, brightness, and numerosity) in the left versus right visual hemifield (Luh, Rueckert & Levy, 1991; Nicholls, Bradshaw & Mattingley, 1999; Charles, Sahraie & McGeorge, 2007), to a differential ability to detect changes within the left visual half of complex visual stimulus arrays (Iyilikci, Becker, Gunturkun & Amado, 2010; Du & Abrams, 2010), and to a selective enhancement of memory for objects located within the left half of scenes (Dickson & Intraub, 2009; Della Sala, Darling & Logie, 2010). This left-biased asymmetry of normal visuospatial attention is called pseudoneglect (PN) (Bowers & Heilman, 1980; Jewell & McCourt, 2000). The phenomena of neglect and PN, as their names suggest, are theorized to be twin manifestations of the fundamental hemispheric asymmetry in the neural substrates of visuospatial attention (Heilman & Van Den Abell, 1980; Weintraub & Mesulam, 1987; McCourt & Jewell, 1999). Supporting this idea are experiments illustrating that a variety of stimulus and task-related variables modulate the magnitude and direction of both neglect and PN in a complimentary fashion (Anderson, 1996; McCourt & Jewell, 1999).
Exogenous Recruitment of Visuospatial Attention
Spatial attention can be exogenously recruited by transient visual cues (Posner, 1980). For example, in the induced line motion effect (Hikosaka, Miyauchi & Shimojo, 1993) a briefly flashed line appears to grow outward away from the spatial location of a previously presented visual cue. The visual cue recruits attention to its spatial location, with a gradient of enhanced attention radiating outward for some distance. The speed of neural processing is increased by attention, causing sensory data from the line end nearest the cued location to be centrally processed more rapidly (sooner) than data from more remote regions, leading to the percept of sequential line appearance, i.e., motion. That the induced line motion effect is attentional in origin is illustrated by the fact that the illusion can be produced by cue and line stimuli that are perceptually dissimilar in color, luminance and stereo depth (von Grünau, Saikali & Faubert, 1995), and by the ability of both auditory and somatosensory cues to elicit this visual effect (Shimojo, Miyauchi & Hikosaka, 1997).
Spatial Cueing Effects in Line Bisection
Numerous studies show that lateralized visual cues affect performance on line bisection tasks, such that PSE shifts significantly towards the cued line end. In hemineglect patients left cues ameliorate the typical rightward bias whereas right cues can exacerbate such errors (Harvey, Milner & Roberts, 1995; Halligan & Marshall, 1989; Mennemeier, Vezey, Chatterjee, Rapcsak & Heilman, 1997; Reuter-Lorenz & Posner, 1990; Ishiai, Seki, Koyama & Okiyama, 1995; Riddoch & Humphreys, 1983). Although the leftward bisection errors of normal subjects are smaller than the rightward errors of hemineglect patients, they are similarly influenced by lateralized visual cues such that PSE is drawn significantly towards the cued line end (Harvey et al., 1995; Milner, Brechmann & Pagliarini, 1992; Nichelli, Rinaldi & Cubelli, 1989; Pizzamiglio, Frasca, Guariglia, Incoccia & Antonucci, 1990; Reuter-Lorenz, Kinsbourne & Moscovitch, 1990; McCourt, Garlinghouse & Reuter-Lorenz, 2005).
Audiospatial Attention
While many properties of normal visuospatial attention are well documented, the manner whereby spatial attention is deployed toward and within the auditory environment (audiospatial attention) is less well understood. Recent evidence suggests that audiospatial attention possesses a rightward bias, in contrast to the leftward bias of visuospatial attention which gives rise to PN (Cusak, Carlyon & Robertson, 2001; Dufour, Touzalin & Candas, 2007; Corral & Escera, 2008; Ocklenburg, Hirnstein, Hausmann & Lewald, 2010; Sosa, Teder-Sälejärvi & McCourt, 2010).
Audiovisual Cue Combination
One strategy used by the nervous system to combine information from sensory signals (such as cues) is to weight their influence in proportion to their reliability, a process described by the rules of Bayesian inference (Alais & Burr, 2004; Ma & Pouget, 2008). There is also physiological and psychophysical evidence that multisensory signals can combine superadditively (Stein & Meredith, 1993), such that the response to the sum of two sensory inputs may exceed, in some cases by an order of magnitude or more, the algebraic sum of the separate responses to the unisensory stimuli. Conversely, multisensory integration can be subadditive, where the response to joint stimulus presentation is significantly smaller than the sum of the responses to the individual unisensory stimuli (Molholm, Ritter, Murray, Javitt, Schroeder & Foxe, 2002; Stanford & Stein, 2007). One objective of the present experiment is to quantify how exogenous auditory and visual cues combine to capture visuospatial attention and influence PSE in a line bisection task. Results reported by Cusak, Carlyon & Robertson (2001), Dufour, Touzalin & Candas (2007), Corral & Escera (2008), Ocklenburg, Hirnstein, Hausmann & Lewald (2010) and Sosa, Teder-Sälejärvi & McCourt (2010) suggest that visuospatial and audiospatial attention possess opposite spatial biases, owing perhaps to right versus left hemispheric control, respectively. If visuospatial and audiospatial attention are deployed by different hemispheres then the salience and potency of auditory and visual cues, and the nature of their combination, might critically depend on the hemifield within which they are presented.
Present Experiment
We evaluate these questions using a tachistoscopic visual line bisection task (McCourt & Olafson, 1997). Using the method of constant stimuli, perceived line midpoint (PSE: a measure of bisection accuracy) and the slope of the psychometric function (σ: a measure of bisection precision) were obtained in a no-cue condition and in conjunction with lateralized auditory and visual cues, presented alone or in spatially congruent and incongruent pairings.
METHODS
Subjects
A total of 46 (25 male, mean age = 20.1 years; 21 female, mean age = 21.3 years) right-handed subjects participated in the experiment. Handedness was assessed using a standard instrument (Oldfield, 1971) on which a combined score of −100 denotes exclusive left-handedness, and +100 denotes exclusive right-handedness. Mean handedness scores for males and females were +66.8 and +81.2, respectively. There was no significant difference in the mean age of male and female subjects (t44 = .67, p = 0.51), but female subjects were significantly more right-handed than male subjects (t44 = 3.23, p = 0.002). Despite the difference in handedness scores male and female subjects were both strongly right-handed and subsequent inferential statistical tests were performed on data collapsed across subject sex. All subjects had normal or corrected-to-normal vision and audiometric tests confirmed that all subjects had normal auditory thresholds.
The study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving human subjects. Prior to their participation in the study all subjects provided written informed consent, and all procedures were approved by the Institutional Review Board of North Dakota State University.
Instrumentation and Calibration
Visual stimuli were presented on a screen using a digital projector (Dell 5100MP) with a resolution of 640 × 480 pixels and a refresh rate of 100 Hz. Mean display luminance was 500 cd/m2. Luminance and contrast calibrations were performed using a spot photometer (Konica Minolta LS110). Auditory calibrations were made using a sound level meter (Extech instruments 407764). Mean ambient noise level was 45 dBA SPL. A microcomputer sensed and collected subject responses. Stimulus presentation and response collection were performed using Presentation (Neurobehavioral Systems, Albany, CA).
Stimuli
Line Stimuli
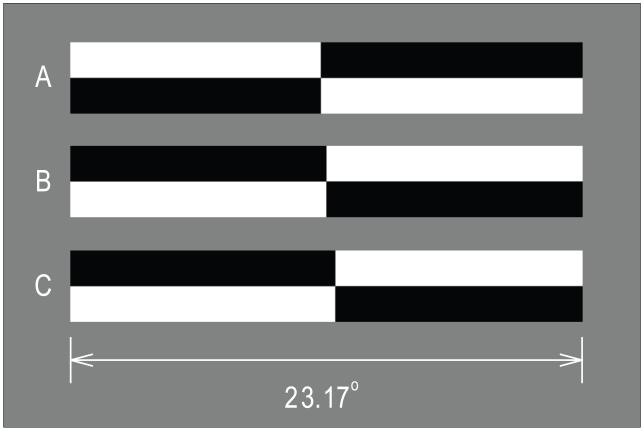
Stimuli were horizontal lines of 100% contrast presented on a homogeneous (mean luminance) background. Lines measured 23.17° × 3.22° at a viewing distance of 175 cm, and were centered with respect to the midsagittal plane of each subject and within the display. Lines were pre-transected at one of 15 locations spanning ±0.65° with respect to veridical line midpoint. This range of transector locations is sufficient to produce asymptotic “left” or “right” judgments in most subjects. Figure 1 illustrates examples of line stimuli. Line A is transected leftward (−0.14°) of center. Line B is veridically transected, and line C is transected rightward (0.27°). Lines B and C are opposite in contrast polarity to line A. Lines of opposite contrast polarity appeared with equal frequency and the order of appearance of lines with different transector locations was randomized within blocks of trials.
Figure 1.
Examples of line stimuli used in the experiments. Line A is transected to the left of veridical line midpoint by 0.14° (0.60% line length). Line C is transected rightward of veridical line midpoint by 0.27° (1.17% line length). Line B is veridically transected. Line A differs from lines B and C in contrast polarity. Lines of opposite polarity appeared with equal frequency and were counterbalanced within and across blocks of trials.
Visual Cues
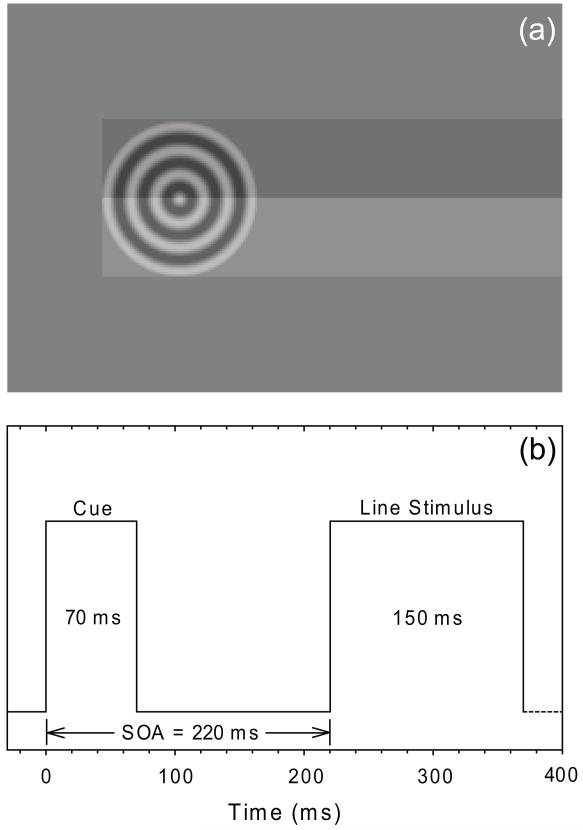
Preceding the presentation of some line stimuli was a visual cue. Cues consisted of circular cosine functions (i.e., bullseye targets; 0.5% contrast; 3.22° diameter; radial spatial frequency = 2.0 cycles/degree). Cue contrast was deliberately set to a low value in order to enhance the relative salience of the inherently less well localizable auditory cues (Alais & Burr, 2004). Cue and line stimuli are superimposed to illustrate their relative positions in Figure 2(a). The lateral extent of the visual cue coincided with the end of the line stimuli, such that line stimuli completely occluded the preceding cue. Note that cue and line stimuli were never synchronously presented, and that the line and cue contrasts in Figure 2(a) are not accurately portrayed. By positioning the cue such that it was completely occluded by the subsequent line stimulus we control for the potential confound that line stimuli might be perceptually elongated by being grouped with a more laterally positioned cue (Mattingley, Pierson, Bradshaw, Phillips & Bradshaw, 1993; Fischer, 1994; Harvey, Pool, Roberson & Olk, 2000; McCourt, Garlinghouse & Reuter-Lorenz, 2005). This procedure ensures that any visual cueing effects on perceived line midpoint are attentional rather than perceptual in origin.
Figure 2.
(a) Visual cue and line stimuli are superimposed to illustrate their relative positions. The most eccentric edge of the visual cue always coincided with the end of the line stimulus, such that no portion of the cue ever extended beyond the endpoint of the line. Auditory cues were delivered from speakers mounted behind the projection screen in the same position as the visual cues. Line and cue contrast are not accurately portrayed in the figure and cues and lines were never simultaneously presented. (b) A timeline illustrating cue and line presentation. Trials began with the presentation of cue stimuli for 70 ms. After an interstimulus interval of 150 ms line stimuli were presented for 150 ms. Cue-line stimulus onset asynchrony was 220 ms. Subsequent trials began 500-1000 ms after subject response.
Auditory Cues
Auditory cues were pure tones (4.4 KHz) presented from speakers mounted directly behind the visually opaque but acoustically transparent projection screen. Speaker location coincided with the spatial location of the visual cues. Auditory cues possessed an intensity of 75 dB A SPL as measured at the subjects’ ears.
Procedure
Subjects were seated in straight-backed chairs with their midsagittal plane aligned with the midpoint of the visual display and made single-interval two-alternative forced-choice judgments of transector location relative to perceived line midpoint using their right forefinger to depress the left or right mouse button as appropriate. Subjects were told that cues were uninformative with respect to transector location and were instructed to ignore them. The experiment was conducted in a single session for each subject.
As illustrated in Figure 2(b) cue stimuli were delivered at line end locations for 70 ms. Following a cue-line onset asynchrony of 220 ms line stimuli were presented for 150 ms. Responses were not speeded and subsequent trials began at random intervals between 500-1000 ms following previous responses. PSEs were assessed in a total of nine experimental conditions: auditory cue only (AL, AR), visual cue only (VL, VR), spatially congruent audiovisual cues (ALVL, ARVR), spatially incongruent audiovisual cues (ALVR, ARVL), and a no-cue control condition (NC). Subjects made 10 bisection judgments in conjunction with 15 different transector locations in each cue condition such that PSEs were based on a total of 150 (10 trials × 15 transector locations) trials. Following experimental sessions all subjects completed an auditory cue localization test in which they heard a sequence of left and right auditory cue stimuli and indicated their source location (left versus right). All subjects were able to localize the direction of the auditory cues with 100% accuracy.
Data Analysis
The dependent measure was the proportion of trials on which subjects indicated that lines were transected to the left of veridical midpoint. The method of constant stimuli was used to derive psychometric functions and nonlinear optimization was used to fit a logistic function to the psychometric data using a maximum likelihood criterion. The logistic function is described by the equation:
where x refers to transector location, μ is the point of subjective equality (PSE; a measure of bias), and σ indexes the slope of the function as it passes through the inflection point (μ) of the logistic function (a measure of sensitivity). Based on these fits transector locations corresponding to a probability of 0.5 for a “left” response (μ), and slope (σ) were extracted. Subsequent inferential statistical tests are performed on distributions of these parameter values.
RESULTS
Omnibus PSE Analysis
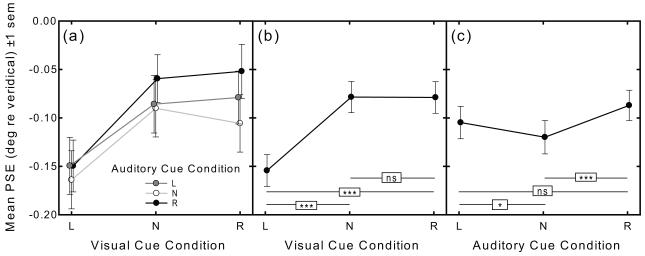
Mean PSEs (±1 sem) in all nine experimental conditions are plotted as a function of visual cue condition in Figure 3(a), with auditory cue condition indicated as a parameter. A 3×3 within-subject ANOVA performed on the PSE data revealed a highly significant main effect of visual cue condition [F1, 45 = 34.61, p < .001], and a highly significant main effect of auditory cue condition [F1, 45 = 8.36, p < .001]. The visual cue X auditory cue condition interaction was not significant [F1, 45 = 1.13, p = .35].
Figure 3.
(a) Mean PSE (degrees visual angle relative to veridical line midpoint ±1 sem) in all nine conditions of the audiovisual cued line bisection task plotted as a function of visual cue condition (abscissa: left, no-cue, right) and auditory cue condition (shown as a parameter: left, no-cue, right). There is a significant main effect of visual cue condition and a significant main effect of auditory cue condition, but no significant interaction. (b) Mean PSE as a function of visual cue condition collapsed across auditory cue condition. (c) Mean PSE as a function of auditory cue condition collapsed across visual cue condition.
To trace the source of the significant main effect of visual cueing Fig. 3(b) plots mean PSE as a function of visual cue condition collapsed across the three auditory cue conditions. Paired-samples t-tests reveal a significantly greater leftward error in the VL versus both the VN and VR cue conditions [t137 = −8.12, p < .001, and t137 = −7.92, p < .001, respectively], but no significant difference in PSE between the VN versus VR cue condition [t137 = 0.04, p = .97].
To trace the source of the significant main effect of auditory cueing Fig. 3(c) plots mean PSE as a function of auditory cue condition collapsed across the three visual cue conditions. Paired-samples t-tests reveal a significantly greater leftward error in the AN versus both the AL and AR cue conditions [t137 = −2.04, p = .043, and t137 = −3.98, p < .001, respectively]. While the difference in PSE between the AL versus AR cue conditions failed to reach significance using a two-tailed test [t137 = 1.79, p = .08], there is nevertheless a spatial orienting effect of auditory cues, as revealed by the strong trend for PSE to deviate in the direction of the auditory cue.
Pseudoneglect Analysis
A series of Holm-Bonferroni-corrected (Holm, 1979) single-sample t-tests comparing PSE in the nine experimental conditions against veridical bisection (zero) reveal significant leftward error (PN) in eight cue conditions: No-cue (NC) [t45 = −3.05, p = .004]; VL [t45 = −5.47, p < .001]; VR [t45 = −3.58, p = .001]; AL [t45 = −2.88, p = .006]; AR [t45 = −2.41, p = .020]; VLAL [t45 = −5.09, p < .001]; VLAR [t45 = −5.60, p <.001]; and VRAL [t45 = −2.90, p = .006]. While exhibiting a trend for leftward error, mean PSE in the spatially congruent audiovisual Right-Cue condition was not significantly biased leftward: VRAR [t45 = −1.86, p = .070].
Cue Weighting Analysis
A primary motivation of the present experiment was to determine how transient lateralized A and V cues combined to jointly influence judgments of line midpoint in tachistoscopic visual line bisection. To address this question we quantified the potency of A and V cues to bias PSE as a function of their spatial location (left or right hemifield) by analyzing the difference in PSE (Δ PSE), as compared with the no-cue condition, obtained in the four multisensory cue conditions (i.e., VLAL- NC; VLAR-NC; VRAL-NC; and VRAR-NC cue conditions) with respect to PSE differences obtained in the four unisensory cue conditions (i.e., VL-NC; AL-NC; VR-NC; and AR-NC). The questions we address in this analysis include: What is the relative potency of A and V cues to bias PSE? Is relative or absolute cue potency influenced by hemifield of presentation? Is the joint effect of A and V cues additive, or do these cues combine in a super- or sub-additive fashion? Finally, does the mode of cue combination depend on the hemifield in which they are presented?
Figure 4(a-d) plots Δ PSEs obtained in the four multisensory cue conditions as a function of those obtained in the component unisensory cue conditions for the entire sample of 46 subjects. Least-squares multiple regression (Foxe, 1997) was used to fit planes to these data to derive weighting coefficients for the Δ PSEs obtained in the unisensory cueing conditions which optimally predict the Δ PSEs obtained in the audiovisual multisensory cue conditions. This analysis provided an excellent fit to the Δ PSE data and explained a significant proportion of the variance in all four multisensory cue conditions: VLAL [r44 = 0.77, p < .001]; VLAR [r44 = 0.53, p = .005]; VRAL [r44 = 0.85, p < .001]; VRAR [r44 = 0.70, p < .001].
Figure 4.
Panels (a-d) plot delta PSEs obtained in the four multisensory cue conditions as a function of those obtained in the component unisensory cue conditions for the entire sample of 46 subjects. Least-squares multiple regression was used to fit planes to these data to derive weighting coefficients for the delta PSEs obtained in the unisensory cueing conditions which optimally predict the delta PSEs obtained in the audiovisual multisensory cue conditions.
The slopes of the fitted planes (which graphically represent the values of the optimal unisensory weighting coefficients) are revealing. Irrespective of the spatial congruity of the A and V cues, when V cues are presented in left hemispace (left column: Figs. 4a and 4b), the predictive value of unisensory A cues is poor, as indicated by the low slope of the plane along the Δ AL or AR axes. However, when V cues are presented in right hemispace (right column: Figs. 4c and 4d), the predictive value of unisensory A cues is increased, as indicated by the steeper slope of the planes along the Δ AL and AR axes.
The optimal unisensory A and V weighting coefficients obtained from the regression analysis (±1 sem) appear in Figure 5, where the ordinate plots unisensory visual and auditory weights in the four multisensory cue conditions. A 4 (multisensory cue condition) × 2 (unisensory cue type) within-subjects ANOVA was conducted on the regression weights which revealed no significant main effect of multisensory cue condition [F3,37 = 0.20, p = .949], no significant main effect of unisensory cue type [F1,37 = 0.97, p = .331], but a significant interaction [F3,37 = 3.87, p = .017]. Paired-samples t-tests were conducted to reveal the source of the significant interaction. In multisensory cue conditions where visual cues are within left hemispace (i.e., the VLAL and VLAR conditions) the weighting coefficients for unisensory visual cues were significantly larger than for unisensory auditory cues [t45 = 2.38, p = .022 and t45 = 2.05, p = .046, respectively]. However, the optimal weighting coefficients for auditory and visual cues were not significantly different when visual cues were presented in right hemispace: VRAL [t45 = −0.49, p = .625]; VRAR [t45 = −0.31, p = .757].
Figure 5.
The optimal unisensory A and V weighting coefficients obtained from the multiple regression analysis (±1 sem). In multisensory cue conditions where visual cues are within left hemispace (i.e., the VLAL and VLAR conditions) the weighting coefficients for unisensory visual cues were significantly larger than for unisensory auditory cues. The optimal weighting coefficients for auditory and visual cues were not significantly different when visual cues were presented in right hemispace. Single-sample t-tests revealed that in no multisensory cue condition did the sum of unisensory weights differ significantly from 1.0.
In each multisensory cue condition the visual and auditory weighting coefficients were summed, and a one-way ANOVA was conducted to determine whether the sum of the V and A unisensory cue weights differed across the four multisensory cue conditions. The summed weights (.887, .818, .608, and .695 for the VLAL, VLAR, VRAL, and VRAR conditions, respectively) did not differ significantly from each other [F3,37 = 0.16, p = .924], and single-sample t-tests revealed that in no multisensory cue condition did the sum of unisensory weights differ significantly from 1.0: VLAL [t45 = .41, p = .683]; VLAR [t45 = .61, p = .544]; VRAL [t45 = 1.39, p = .173]; VRAR [t45 = 1.02, p = .315].
DISCUSSION
Visuospatial and Audiospatial Attention
Our results, which reveal significant leftward error in visual line midpoint estimation, are consistent with the established body of evidence that visuospatial attention in neurologically normal subjects exhibits a modest but consistent leftward bias known as pseudoneglect (Bowers & Heilman, 1980; McCourt & Olafson, 1997; Nicholls, Bradshaw & Mattingley, 1999; McCourt & Jewell, 1999; Jewell & McCourt, 2000; McCourt, 2001; McCourt et al., 2005; 2008; Nicholls & Roberts, 2002; Leone & McCourt, 2010; Sosa, Teder-Sälejärvi & McCourt, 2010). While the magnitude of the subtle leftward LB errors of normal subjects contrasts with the florid rightward biases exhibited by neglect patients, both biases are conceptualized to be manifestations of an underlying specialization of neural networks housed in the right hemisphere which deploy visuospatial attention (Heilman & Valenstein, 1979; Heilman & Van Den Abell, 1980; Kinsbourne, 1970; 1977; 1993; Nobre, Sebestyen, Gitelman, Mesulam, Frackowiak & Frith, 1997; Kastner & Ungerleider, 2000). Thus, the intact dominant (right) hemisphere projects a prepotent vector of visuospatial attention into contralateral (left) hemispace, differentially increasing the salience of left hemispace (within an egocentric reference frame), and the left halves of visual objects such as lines (within an allocentric reference frame), thereby inducing a leftward bias in the perceived midpoint of space or objects (Anderson, 1996; McCourt, Mark, Radonovich, Willison & Freeman, 1997; McCourt & Jewell, 1999), an overestimation of the relative size, brightness/darkness or numerosity (Luh, Rueckert & Levy, 1991; Nicholls, Bradshaw & Mattingley, 1999; Nicholls, Mattingley & Bradshaw, 2005; Charles, Sahraie & McGeorge, 2007), and an enhanced contingent attentional capture of stimuli in the left visual field (Du & Abrams, 2010).
In contrast to the leftward bias of normal visuospatial attention, audiospatial attention in neurologically normal subjects has been reported to exhibit a modest but significant rightward bias (Cusak, Carlyon & Robertson, 2001; Dufour, Touzalin & Candas, 2007; Corral & Escera, 2008; Sosa, Teder-Sälejärvi & McCourt, 2010). Sosa et al. (2010) interpreted the rightward bias of audiospatial attention to suggest that the left hemisphere, which plays a prominent role in auditory spatial localization generally (Schonwiesner, Krumbholz, Rubsamen, Fink & von Cramon, 2007) and in speech perception in particular, might also play a dominant role in the deployment of spatial attention toward the auditory environment.
Spatial Cueing Effects in Line Bisection
Visual Cues
Numerous studies in the clinical literature describe the effects of visual cues on line bisection performance in patients with neglect. Almost universally, cueing the left or right side of a line shifts perceived line midpoint toward the cued line end. Hence, left cues ameliorate rightward bisection error whereas right cues can exacerbate it (Harvey, Milner & Roberts, 1995; Halligan & Marshall, 1989; Mennemeier, Vezey, Chatterjee, Rapcsak & Heilman, 1997; Reuter-Lorenz & Posner, 1990; Ishiai, Seki, Koyama & Okiyama, 1995; Riddoch & Humphreys, 1983). Neurologically normal subjects are similarly influenced by lateral cues such that perceived line midpoint is drawn significantly towards the cued line end (Harvey et al., 1995; Milner, Brechmann & Pagliarini, 1992; Nichelli, Rinaldi & Cubelli, 1989; Pizzamiglio, Frasca, Guariglia, Incoccia & Antonucci, 1990; Reuter-Lorenz, Kinsbourne & Moscovitch, 1990; McCourt, Garlinghouse & Reuter-Lorenz, 2005). Visual cueing effects are frequently explained by attentional recruitment (Yantis & Jonides, 1990), such that cues delivered in the vicinity of a line endpoint recruit attention toward the cued line end, thus increasing the salience of this segment of the line and leading to an overestimation of its size or extent. The perceived midpoint of the line is thus drawn into the overestimated (cued) segment, although this phasic cueing effect is superimposed on the tonic leftward or rightward bias exhibited by normal observers or neglect patients, respectively.
We report a significant effect of unisensory visual cues, such that left visual cues significantly shifted PSE leftward relative to the no-cue and right-cue conditions, whereas PSE in the right-cue condition did not differ significantly from the no-cue condition. The greater potency of left visual cues is consistent with findings that stimuli delivered to the left hemifield possess greater saliency than those delivered to the right hemifield. For example, stimuli in the left hemifield enjoy an enhancement of perceived luminance, as well as exaggerated numerosity and size relative to those in the right hemifield (Nicholls et al., 1999; Charles et al., 2007), and in feature search tasks left hemifield distractors are significantly more potent in capturing attention than those in the right hemifield (Du & Abrams, 2010; Burnham, Rozell, Kasper, Bianco & Delliturri, 2011). The fact that perceived line midpoint deviates leftward of veridical center is thought to result from an excess of attention toward, and consequent magnification of, the left line half (McCourt et al., 2005; Toba, Cavanagh & Bartolomeo, 2011). The greater potency of left cues that we observe does, however, differ from results reported by Michel, Cavezian, d’Amato, Dalery, Rode, Saoud & Rossetti (2007), Michel, Bidot, Bonnetblanc & Quercia (2011), and Toba et al. (2011), who report that right and left cues are roughly equipotent, and from those of Nicholls & Roberts (2002) and McCourt et al. (2005), who report a bias favoring right hemifield cues. In comparing these results it should be kept in mind, however, that there are numerous differences in stimuli, methodology, and even subject populations across these studies which could explain the discrepant findings.
Auditory Cues
While the difference in PSE between left and right auditory cue conditions is not significant, it nevertheless shows a strong trend for PSE to deviate in the cued direction [t137 = 1.79, p = .08]. Thus, while the potency of auditory cueing to modulate spatial orienting is relatively weak compared to visual cuing, it is not entirely absent.
We also find that both leftward and rightward auditory cues cause significant rightward shifts of PSE relative to the no-cue condition. According to activation-orientation theory (Kinsbourne, 1970; 1977; 1993) the two cerebral hemispheres compete for control of various functions through mutual inhibition. One interpretation of the rightward shift of PSE in response to auditory cues begins by recognizing that many aspects of the auditory cues (e.g., their frequency, timbre, onset, offset, spatial location, loudness) are preferentially processed by networks in the left hemisphere. Therefore, relative to no-cue trials, auditory cue-related activation of the left hemisphere, particularly of mechanisms responsible for the involuntary recruitment and deployment of audiospatial attention, might subsequently inhibit homologous areas of the right hemisphere, thus decreasing the normal tonic leftward bias of visuospatial attention.
We further suggest that the weaker potency of right versus left visual cues could be due to an auditory-cue-related ceiling effect. Auditory cues were presented on two-thirds of all trials. If auditory stimuli are preferentially processed in the left hemisphere then one effect of this frequent auditory cueing may have been to elevate activity in left hemisphere attentional networks, thereby antagonizing right hemisphere attentional deployment and inducing a tonic rightward bias in PSE relative to visual line bisection experiments lacking auditory cues. This auditory-cue-induced tonic rightward bias would, in turn, provide less “headroom” for right visual cues to induce any additional rightward deviation in PSE. This explanation, which hypothesizes a compressive transducer function for exogenous attention, was suggested previously by McCourt et al. (2005) to account for the weaker effect of rightward cues on PSE when line geometry itself induced rightward bisection error, and vice versa for leftward cues.
Robertson et al. (1998) and Chica et al. (2011) found that phasic auditory cues delivered several hundred milliseconds prior to a visual stimulus ameliorated the tonic rightward bias of neglect patients. The explanation offered for this effect is that the auditory alerting system involves networks housed within the right hemisphere, and that the activation of this alerting system by phasic auditory cues serves, in a Kinsbournian manner, to co-activate the right hemisphere spatial orienting system, thus lessening the rightward bias of neglect patients. Similarly, Manly et al. (2005) and Fimm et al. (2006) report that low arousal (the opposite of phasic alertness) induced by sleep-deprivation causes a rightward shift in the distribution of spatial attention. These results, and their explanation, does not explain our results since we find that phasic auditory cues cause, in addition to a directional effect produced via the spatial orienting system, a global rightward shift of spatial attention relative to the no-cue condition. If the results of Robertson et al. (1998), Chica et al. (2011), Manly et al. (2005) and Fimm et al. (2006) are a guide, then any phasic alerting effect of the auditory cues should have shifted PSE leftward.
Multisensory Cue Integration
We inferred the potency of transient visual and auditory stimuli to capture visuospatial attention and so alter perceived line midpoint under conditions of multisensory audiovisual cueing and find that their influence sums linearly. Surprisingly, the most significant factor moderating relative cue potency is not spatial congruity (where, for example, one might have expected a superadditive combination for spatially congruent A and V cues, and a subadditive combination for spatially incongruent cues), but is instead the location of the visual cue. Thus, visual cues in the left visual field are simply significantly more potent than auditory cues, whereas visual cues in the right visual field are equipotent with auditory cues with respect to influencing perceived line midpoint.
Ventriloquism
A celebrated interaction between visual and auditory stimuli is the phenomenon of ventriloquism (Howard & Templeton, 1966; Welch & Warren, 1980; Alais & Burr, 2004). Here, the perceived location of an auditory stimulus is “captured” by a simultaneously presented visual stimulus. We did not ask subjects to indicate the perceived location of auditory cues on multisensory trials but we can indirectly assess visual capture since if the perceived spatial location (and cueing effect) of auditory cues was captured by concurrent visual cues, then PSEs in the ARVR and ALVR conditions should be identical, since they would be determined only by the V cue. A similar logic applies to the ARVL and ALVL conditions, since the spatially incongruent auditory cues would be assigned to the spatial location of the visual cue. To test this hypothesis we compared PSEs in the unisensory auditory cue conditions (AR and AL) against those obtained in the multisensory cue conditions (ARVL and ALVL) and (ARVR and ALVR), using two-way repeated-measures ANOVAs, where the independent variables were the number of cues (A only versus AV) and auditory cue location (AL versus AR). Comparing the effect of unisensory auditory cues (AL and AR) with their multisensory effect when combined with VR cues revealed no significant effect of the number of cues [F1,45 = 0.49, p = .484], but a highly significant effect of A cue location [F1,45 = 6.07, p = .018], where leftward A cues led to significantly greater leftward shifts of PSE. The interaction was not significant [F1,45 = 0.01, p = .972]. The same comparison of the effect of unisensory auditory cues with their multisensory effect when combined with VL cues, however, revealed a significant effect of the number of cues [F1,45 = 55.30, p < .001], no significant effect of A cue location [F1,45 = 1.85, p = .181], and no significant interaction [F1,45 = 1.15, p = .288].
Thus, V cues presented in the right hemifield appear not to capture the spatial location of A cues, since the location of A cues significan tly modulates PSE in this condition. However, when V cues are presented in the left hemifield there is no modulating influence of A cue location on PSE. These results indicate a greater potency of visual capture (ventriloquism) in the left versus right visual hemifield. Aside from a report by Slutsky & Recanzone (2001), who found a greater mislocalization of A stimuli in the left versus right hemifield (at 8° eccentricity) in the presence of a concurrently presented foveal V stimulus, the issue of whether there is differential hemifield susceptibility ventriloquism has not received systematic investigation.
ACKNOWLEDGMENTS
This work was supported by a grant to MEM: NIH P20 RR020151. The National Center for Research Resources (NCRR) is a component of the National Institutes of Health (NIH). The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the NIH or NCRR. Author YS conducted this research in partial fulfillment of the requirements for the M.S. degree in psychology at North Dakota State University, and was supported by a Fulbright Fellowship. The authors thank Dan Gu for assistance with programming.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alais D, Burr D. The ventriloquist effect results from near-optimal bimodal integration. Current Biology. 2004;14:257–262. doi: 10.1016/j.cub.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Anderson B. A mathematical model of line bisection behavior in neglect. Brain. 1996;119:841–850. doi: 10.1093/brain/119.3.841. [DOI] [PubMed] [Google Scholar]
- Arguin M, Bub DN. Evidence for an independent stimulus-centered reference frame from a case of visual hemineglect. Cortex. 1993;29:349–357. doi: 10.1016/s0010-9452(13)80188-8. [DOI] [PubMed] [Google Scholar]
- Behrmann M. Spatial reference frames and hemispatial neglect. In: Gazzaniga MS, editor. The New Cognitive Neurosciences. 2nd Edition MIT Press; Cambridge, MA: 1999. pp. 651–666. [Google Scholar]
- Bisiach E, Bulgarelli C, Sterzi R, Vallar G. Line bisection and cognitive plasticity of unilateral neglect of space. Brain and Cognition. 1983;2:32–38. doi: 10.1016/0278-2626(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Bowers D, Heilman KM. Pseudoneglect: effects of hemispace on a tactile line bisection task. Neuropsychologia. 1980;18:491–498. doi: 10.1016/0028-3932(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Nettleton NC. Human cerebral asymmetry. Prentice Hall; Englewood Cliffs, NJ: 1983. [Google Scholar]
- Bradshaw JL, Nathan G, Nettleton NC, Wilson L, Pierson J. Why is there a left side underestimation in rod bisection? Neuropsychologia. 1987;25:735–738. doi: 10.1016/0028-3932(87)90067-4. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Nettleton NC, Nathan G, Wilson LE. Bisecting rods and lines: Effects of horizontal and vertical posture on left-side underestimation by normal subjects. Neuropsychologia. 1985;23:421–426. doi: 10.1016/0028-3932(85)90029-6. [DOI] [PubMed] [Google Scholar]
- Bultitude JH, Aimola Davies AM. Putting attention on the line: Investigating the activation-orientation hypothesis of pseudoneglect. Neuropsychologia. 2006;44:1849–1858. doi: 10.1016/j.neuropsychologia.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Burnham BR, Rozell CA, Kasper A, Bianco NE, Delliturri A. The visual hemifield asymmetry in the spatial blink during singleton search and feature search. Brain and Cognition. 2011 doi: 10.1016/j.bandc.2011.01.003. (in press), doi:10.1016/j.bandc.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Charles J, Sahraie A, McGeorge P. Hemispatial asymmetries in judgment of stimulus size. Perception & Psychophysics. 2007;69:687–698. doi: 10.3758/bf03193771. [DOI] [PubMed] [Google Scholar]
- Chica AB, de Schotten MT, Toba M, Malhotra P, Lupianez J, Bartolomeo P. Attention networks and their interactions after right hemisphere damage. Cortex. 2011 doi: 10.1016/j.cortex.2011.01.009. (in press), doi:10.1016/j.cortex.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Corral M-J, Escera C. Effects of sound location on visual task performance and electrophysiological measures of distraction. NeuroReport. 2008;19:1535–1539. doi: 10.1097/WNR.0b013e3283110416. [DOI] [PubMed] [Google Scholar]
- Cusak R, Carlyon RP, Robertson IH. Auditory midline and spatial discrimination in patients with unilateral neglect. Cortex. 2001;37:706–709. doi: 10.1016/s0010-9452(08)70620-8. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H, Chui HC. Neglect following damage to frontal lobe or basal ganglia. Neuropsychologia. 1980;18:123–132. doi: 10.1016/0028-3932(80)90058-5. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Darling S, Logie RH. Items on the left are better remembered. The Quarterly Journal of Experimental Psychology. 2010;63:848–855. doi: 10.1080/17470211003690672. [DOI] [PubMed] [Google Scholar]
- Dickinson CA, Intraub H. Spatial asymmetries in viewing and remembering scenes: Consequences of an attentional bias? Attention, Perception, & Psychophysics. 2009;71:1251–1262. doi: 10.3758/APP.71.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Baylis GC, Goodrich SJ, Rafal R. Axis-based neglect of visual shapes. Neuropsychologia. 1994;32:1353–1365. doi: 10.1016/0028-3932(94)00068-9. [DOI] [PubMed] [Google Scholar]
- Driver J, Halligan PW. Can visual neglect operate in object-centered coordinates? An affirmative single-case study. Cognitive Neuropsychology. 1991;8:475–496. [Google Scholar]
- Du F, Abrams RA. Visual field asymmetry in attentional capture. Brain and Cognition. 2010;72:310–316. doi: 10.1016/j.bandc.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Dufour A, Touzalin P, Candas V. Rightward shift of the auditory subjective straight ahead in right- and left-handed subjects. Neuropsychologia. 2007;45:447–453. doi: 10.1016/j.neuropsychologia.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Fimm B, Willmes K, Spijkers W. The effect of low arousal on visuo-spatial attention. Neuropsychologia. 2006;44:1261–1268. doi: 10.1016/j.neuropsychologia.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Fischer MH. Less attention and more perception in cued line bisection. Brain and Cognition. 1994;25:24–33. doi: 10.1006/brcg.1994.1020. [DOI] [PubMed] [Google Scholar]
- Foxe J. Applied Regression Analysis, Linear Models, and Related Methods. SAGE Publications, Inc.; Thousand Oaks, CA: 1997. [Google Scholar]
- Foxe JJ, McCourt ME, Javitt DC. Right hemisphere control of visuospatial attention: line bisection judgments evaluated with high-density electrical mapping and source analysis. NeuroImage. 2003;19:710–726. doi: 10.1016/s1053-8119(03)00057-0. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Marshall JC. Perceptual cueing and perceptuo-motor compatibility in visuo-spatial neglect: A single case study. Cognitive Neuropsychology. 1989;6:423–435. [Google Scholar]
- Harvey M, Milner AD, Roberts RC. An investigation of hemispatial neglect using the landmark task. Brain and Cognition. 1995;27:59–78. doi: 10.1006/brcg.1995.1004. [DOI] [PubMed] [Google Scholar]
- Harvey M, Pool TD, Roberson MJ, Olk B. Effects of visible and invisible cueing procedures on perceptual judgments in young and elderly subjects. Neuropsychologia. 2000;38:22–31. doi: 10.1016/s0028-3932(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E. Mechanisms underlying hemispatial neglect. Annals of Neurology. 1979;5:166–170. doi: 10.1002/ana.410050210. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Van Den Abell T. Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect) Neurology. 1980;30:327–330. doi: 10.1212/wnl.30.3.327. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Miyauchi S, Shimojo S. Voluntary and stimulus-induced attention detected as motion sensation. Perception. 1993;22:517–526. doi: 10.1068/p220517. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Howard IP, Templeton WB. Human Spatial Orientation. Wiley; New York: 1966. [Google Scholar]
- Ishiai S, Seki K, Koyama Y, Okiyama R. Effects of cueing on visuospatial processing in unilateral spatial neglect. Journal of Neurology. 1995;242:367–373. doi: 10.1007/BF00868391. [DOI] [PubMed] [Google Scholar]
- Iyilikci O, Becker C, Gunturkun O, Amado S. Visual processing asymmetries in change detection. Perception. 2010;39:761–769. doi: 10.1068/p6623. [DOI] [PubMed] [Google Scholar]
- Jewell G, McCourt ME. Pseudoneglect: a review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38:93–110. doi: 10.1016/s0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Karnath H, Berger M, Küver W, Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: A study of 140 patients. Cerebral Cortex. 2004;14:1164–1172. doi: 10.1093/cercor/bhh076. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kerkhoff G. Spatial hemineglect in humans. Prog. Neurobiol. 2001;63:1–27. doi: 10.1016/s0301-0082(00)00028-9. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. The cerebral basis of lateral asymmetries in attention. Acta Psychologica. 1970;33:193–201. doi: 10.1016/0001-6918(70)90132-0. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. Hemi-neglect and hemisphere rivalry. Adv. Neurol. 1977;18:41–49. [PubMed] [Google Scholar]
- Kinsbourne M. Orientational bias model of unilateral neglect: Evidence from attentional gradients within hemispace. In: Robertson IH, Marshall JC, editors. Unilateral neglect: Clinical and experimental studies. Lawrence Erlbaum Associates; Hove, U.K.: 1993. pp. 63–86. [Google Scholar]
- Leone L, McCourt ME. The effect of acute alcohol challenge on global visuospatial attention: Exaggeration of leftward bias in line bisection. Laterality: Asymmetries of Body, Brain and Cognition. 2010;15:327–342. doi: 10.1080/13576500902781745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luh KE, Rueckert LM, Levy J. Perceptual asymmetries for free viewing of several types of chimeric stimuli. Brain and Cognition. 1991;16:83–103. doi: 10.1016/0278-2626(91)90087-o. [DOI] [PubMed] [Google Scholar]
- Ma WJ, Pouget A. Linking neurons to behavior in multisensory perception: A computational review. Brain Research. 2008;1242:4–12. doi: 10.1016/j.brainres.2008.04.082. [DOI] [PubMed] [Google Scholar]
- Manly T, Dobler VB, Dodds CM, George MA. Rightward shift in spatial awareness with declining alertness. Neuropsychologia. 2005;43:1721–1728. doi: 10.1016/j.neuropsychologia.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Mattingley JB, Pierson JM, Bradshaw JL, Phillips JG, Bradshaw JA. To see or not to see: The effects of visible and invisible cues on line bisection judgments in unilateral neglect. Neuropsychologia. 1993;31:1201–1215. doi: 10.1016/0028-3932(93)90068-b. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Freeman P, Tahmahkera-Stevens C, Chaussee M. The influence of unimanual response on pseudoneglect magnitude. Brain and Cognition. 2001;45:52–63. doi: 10.1006/brcg.2000.1255. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M. Stimulus modulation of pseudoneglect: Effect of line geometry. Neuropsychologia. 2000a;38:520–524. doi: 10.1016/s0028-3932(99)00085-8. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M. Asymmetries of visuospatial attention are modulated by viewing distance and visual field elevation: Pseudoneglect in peripersonal and extrapersonal space. Cortex. 2000b;36:715–732. doi: 10.1016/s0010-9452(08)70548-3. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Jewell G. Visuospatial attention in line bisection: Stimulus modulation of pseudoneglect. Neuropsychologia. 1999;37:843–855. doi: 10.1016/s0028-3932(98)00140-7. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Olafson C. Cognitive and perceptual influences on visual line bisection: psychophysical and chronometric analyses of pseudoneglect. Neuropsychologia. 1997;35:369–380. doi: 10.1016/s0028-3932(96)00143-1. [DOI] [PubMed] [Google Scholar]
- McCourt ME. Performance consistency of normal observers in forced-choice tachistoscopic visual line bisection. Neuropsychologia. 2001;39:1065–1076. doi: 10.1016/s0028-3932(01)00044-6. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Butler J. The influence of viewing eye on pseudoneglect magnitude. Journal of the International Neuropsychological Society. 2001;7:391–395. doi: 10.1017/s1355617701003137. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Reuter-Lorenz P. Unilateral visual cueing and asymmetric line geometry share a common attentional origin in the modulation of pseudoneglect. Cortex. 2005;41:499–511. doi: 10.1016/s0010-9452(08)70190-4. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Slater J. Centripetal versus centrifugal bias in visual line bisection: Focusing attention on two hypotheses. Frontiers in Bioscience. 2000;5:d58–71. doi: 10.2741/a496. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Mark VW, Radonovich KJ, Willison S, Freeman P. The effects of gender, practice and menstrual phase on the perceived location of the midsagittal plane. Neuropsychologia. 1997;35:717–724. doi: 10.1016/s0028-3932(96)00115-7. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Shpaner M, Javitt DC, Foxe JJ. Hemispheric asymmetry and callosal integration of visuospatial attention in schizophrenia: A tachistoscopic line bisection study. Schizophrenia Research. 2008;102:189–196. doi: 10.1016/j.schres.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennemeier M, Vezey E, Chatterjee A, Rapcsak SZ, Heilman KM. Contributions of the left and right cerebral hemispheres to line bisection. Neuropsychologia. 1997;35:703–715. doi: 10.1016/s0028-3932(96)00114-5. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1982;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Michel C, Bidot S, Bonnetblanc F, Quercia P. Left minineglect or inverse pseudoneglect in children with dyslexia. NeuroReport. 2011;22:93–96. doi: 10.1097/WNR.0b013e328342d2df. [DOI] [PubMed] [Google Scholar]
- Michel C, Cavezian C, d’Amato T, Dalery J, Rode G, Saoud M, Rossetti Y. Pseudoneglect in schizophrenia: A line bisection study with cueing. Cognitive Neuropsychiatry. 2007;12:222–234. doi: 10.1080/13546800601033266. [DOI] [PubMed] [Google Scholar]
- Milner AD, Brechmann M, Pagliarini L. To halve and halve not: An analysis of line bisection judgments in normal subjects. Neuropsychologia. 1992;30:515–526. doi: 10.1016/0028-3932(92)90055-q. [DOI] [PubMed] [Google Scholar]
- Molholm S, Ritter W, Murray M, Javitt DC, Schroeder CE, Foxe JJ. Multisensory auditory-visual interactions during early sensory processing in humans: a high-density electrical mapping study. Cognitive Brain Research. 2002;14:115–129. doi: 10.1016/s0926-6410(02)00066-6. [DOI] [PubMed] [Google Scholar]
- Nichelli P, Rinaldi M, Cubelli R. Selective spatial attention and length representation in normal subjects and in patients with unilateral spatial neglect. Brain and Cognition. 1989;9:57–70. doi: 10.1016/0278-2626(89)90044-4. [DOI] [PubMed] [Google Scholar]
- Nicholls MER, Roberts GR. Can free-viewing perceptual asymmetries be explained by scanning, pre-motor or attentional biases? Cortex. 2002;38:113–136. doi: 10.1016/s0010-9452(08)70645-2. [DOI] [PubMed] [Google Scholar]
- Nicholls MER, Bradshaw JL, Mattingley JB. Free-viewing perceptual asymmetries for the judgement of shade, numerosity and size. Neuropsychologia. 1999;37:307–314. doi: 10.1016/s0028-3932(98)00074-8. [DOI] [PubMed] [Google Scholar]
- Nicholls MER, Mattingley JB, Bradshaw JL. The effect of strategy on pseudoneglect for luminance judgments. Cognitive Brain Research. 2005;25:71–77. doi: 10.1016/j.cogbrainres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120:515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- Ocklenburg S, Hirnstein M, Hausmann M, Lewald J. Auditory space perception in left- and right-handers. Brain and Cognition. 2010;72:210–217. doi: 10.1016/j.bandc.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pizzamiglio L, Frasca R, Guariglia C, Incoccia C, Antonucci C. Effect of optokinetic stimulation in patients with visual neglect. Cortex. 1990;26:535–540. doi: 10.1016/s0010-9452(13)80303-6. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Kinsbourne M, Moscovitch M. Hemispheric control of spatial attention. Brain and Cognition. 1990;12:240–266. doi: 10.1016/0278-2626(90)90018-j. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Posner MI. Components of neglect from right-hemisphere damage: An analysis of line bisection. Neuropsychologia. 1990;28:327–333. doi: 10.1016/0028-3932(90)90059-w. [DOI] [PubMed] [Google Scholar]
- Riddoch MJ, Humphreys GW. The effect of cueing on unilateral neglect. Neuropsychologia. 1983;21:589–599. doi: 10.1016/0028-3932(83)90056-8. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Halligan PW. Spatial Neglect: A Clinical Handbook for Diagnosis and Treatment. Psychology Press; 1999. [Google Scholar]
- Robertson IH, Mattingley JB, Rorden C, Driver J. Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature. 1998;395:169–172. doi: 10.1038/25993. [DOI] [PubMed] [Google Scholar]
- Schenkenberg T, Bradford DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 1980;30:509–517. doi: 10.1212/wnl.30.5.509. [DOI] [PubMed] [Google Scholar]
- Schonwiesner M, Krumbholz K, Rubsamen R, Fink GR, von Cramon DY. Hemispheric asymmetry for auditory processing in the human auditory brain stem, thalamus, and cortex. Cerebral Cortex. 2007;17:492–499. doi: 10.1093/cercor/bhj165. [DOI] [PubMed] [Google Scholar]
- Shimojo S, Miyauchi S, Hikosaka O. Visual motion sensation yielded by non-visually driven attention. Vision Research. 1997;37:1575–1580. doi: 10.1016/s0042-6989(96)00313-6. [DOI] [PubMed] [Google Scholar]
- Slutsky DA, Recanzone GH. Temporal and spatial dependency of the ventriloquism effect. NeuroReport. 2001;12:7–10. doi: 10.1097/00001756-200101220-00009. [DOI] [PubMed] [Google Scholar]
- Sosa Y, Teder-Sälejärvi WA, McCourt ME. Biases of spatial attention in vision and audition. Brain and Cognition. 2010;73:229–235. doi: 10.1016/j.bandc.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford TR, Stein BE. Superadditivity in multisensory integration: Putting the computation in context. NeuroReport. 2007;18:787–792. doi: 10.1097/WNR.0b013e3280c1e315. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The merging of the senses. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Toba M-N, Cavanagh P, Bartolomeo P. Attention biases the perceived midpoint of horizontal lines. Neuropsychologia. 2011;49:238–246. doi: 10.1016/j.neuropsychologia.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Vallar G. The anatomical basis of spatial neglect in humans. In: Robertson IH, Marshall JC, editors. Unilateral Neglect: Clinical and Experimental Studies. Lawrence Erlbaum Associates; Hove, U.K.: 1993. pp. 27–59. [Google Scholar]
- von Grünau M, Saikali Z, Faubert J. Processing speed in the motion-induction effect. Perception. 1995;24:477–490. doi: 10.1068/p240477. [DOI] [PubMed] [Google Scholar]
- Watson RT, Valenstein E, Heilman KM. Thalamic neglect. Possible role of the medial thalamus and nucleus reticularis in behavior. Archives of Neurology. 1981;38:501–506. doi: 10.1001/archneur.1981.00510080063009. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Mesulam MM. Right cerebral dominance in spatial attention. Further evidence based on ipsilateral neglect. Archives of Neurology. 1987;44:621–625. doi: 10.1001/archneur.1987.00520180043014. [DOI] [PubMed] [Google Scholar]
- Welch RB, Warren DH. Immediate perceptual response to inter-sensory discrepancy. Psychological Bulletin. 1980;88:638–667. [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: Voluntary versus automatic allocation. Journal of Experimental Psychology: Human Perception and Performance. 1990;16:121–134. doi: 10.1037//0096-1523.16.1.121. [DOI] [PubMed] [Google Scholar]