Abstract
Chronic fetal anemia results in significant cardiac remodeling. The capacity to reverse these effects is unknown. We examined the effects of transfusion on cardiomyocyte adaptations following chronic anemia in fetal sheep subjected to daily hemorrhage beginning at 109d gestation age (GA; term ∼145d). Following 10 days of anemia, one group was euthanized for comparison to age-matched controls. A separate group of anemic fetuses was transfused with red blood cells at 119d GA for comparison to controls at 129d GA. Anemia significantly increased the heart-to-body weight ratio, an effect partially ameliorated following transfusion. Cardiomyocyte dimensions were similar among all groups, suggesting an absence of hypertrophy. The percentages of mono- and binucleated cardiomyocytes were similar between groups at 119d GA, though the percentage of binucleated cells was significantly less in transfused fetuses compared to controls at 129d GA. Protein levels of mitogen activated protein kinases and protein kinase B were similar between controls and their respective intervention groups, except for a significant increase in phosphorylated c-Jun N-terminal kinase 1/2 (JNK1/2) in transfused fetuses. Thus, cardiomyocyte proliferation but not hypertrophy contributes to cardiac enlargement during fetal anemia. Transfusion results in slowing but not cessation of cardiac growth following anemia.
Introduction
Chronic fetal anemia imparts a significant challenge on the cardiovascular system to maintain systemic oxygen delivery. Adaptations in the fetus include marked increases in cardiac output and cardiac mass(1, 2). The hemodynamic stress of anemia potentially leads to congestive heart failure, hydrops fetalis and fetal demise (3). In the clinical setting, transfusion of the anemic fetus with red blood cells is standard therapy (4), but treatment may fail to mitigate cardiac enlargement as determined at birth (2). Fetal cardiac growth is remarkably plastic, adapting to cardiovascular stress differently than the mature, postnatal heart. In the adult heart, cardiac myocytes are multinucleated and classically believed to be non-proliferative with cardiac growth occurring primarily by cardiomyocyte hypertrophy. In contrast, cardiomyocytes actively proliferate during normal growth in the fetal heart (5). Cellular enlargement and the transition to terminal differentiation are also important to heart growth during the perinatal period, as are coordinated expansion of vascular and connective tissues. In the near-term fetus, anemia causes cardiac enlargement by accelerating normal growth processes, including cardiomyocyte hyperplasia and hypertrophy (6, 7). The mitogen-activated protein kinase (MAPK) signaling pathways are likely important regulators of these adaptations, although there may be important differences in activation based on developmental stage (8).
Given the adaptability of the fetal heart in response to stress, it may be predicted that the immature heart would respond readily to resolution of that stress. This study was designed to test the hypothesis that in the enlarged hearts of anemic fetuses, red blood cell transfusion and restoration of hemoglobin would result in transient cessation of cardiomyocyte growth and proliferation, with normalization of heart weight and cardiomyocyte dimensions. Given their purported role in regulating cardiac growth, we also examined changes in the expression of terminal MAPK proteins and protein kinase B (Akt). We have shown previously that with induction of anemia as outlined in this study, the hearts of anemic fetuses grow to be ∼40% heavier than age-matched controls (7). According to our growth curves, the fetal sheep heart is ∼40% heavier at 129d gestational age (GA) than 119d GA (5). Therefore, this 10-day recovery period was selected in order to test the hypothesis that the heart-to-body weight ratio would normalize by cardiac growth arrest following transfusion.
Methods
Animal experiments were approved by the University of Iowa Institutional Animal Care and Use Committee, and were conducted within the regulations of the Animal Welfare Act and the National Research Council's Guide for the Care and use of Laboratory Animals. Gestation-timed ewes of mixed western breed were obtained from a local supplier and acclimatized to the laboratory. At 103d GA (term ∼145d), catheterization of the fetus was performed. After an intramuscular injection of 7.5 mg thiopental sodium (12 mg/kg; Abbott Laboratories, Chicago, IL) was used to induce anesthesia, which was maintained with isoflurane (2%) in a 30/70 mixture of oxygen/nitrous oxide. The uterus was exposed through a midline incision, and the fetal head exteriorized. Indwelling catheters were placed in a fetal carotid artery and jugular vein (0.86mm or 1.19mm internal diameter; Scientific Commodities Incorporated, Lake Havasu City, AZ). An amniotic fluid catheter (1.19mm internal diameter) was anchored to the fetal skin. Fetal, uterine and maternal flank incisions were closed in separate layers, and catheters exteriorized through a subcutaneous tunnel and stored in a cloth pouch on the ewe's flank. At the end of the surgery, and for 3 days post-operatively, ampicillin was infused into the amniotic cavity through the amniotic fluid catheter (2g) and given intramuscularly to the ewe (2g). Butorphenol (0.1 mg/kg i.v.; Torbugesic, Fort Dodge Animal Health, Fort Dodge, IA) was given for 24 hr postoperatively for analgesia. Ewes and their fetuses were allowed to recover for 6 days prior to beginning experiments.
Physiological Studies
Fetuses were alternately allocated to one of four groups: 5 control fetuses were not bled and were euthanized after 10 days of study (Control); 6 fetuses were bled to reduce their arterial oxygen content and were euthanized after 10 days of study (Anemic); 7 control fetuses were not bled and were euthanized after 20 days of study (Control-Recovery); 7 fetuses were bled to reduce their arterial oxygen content for 10 days; on the tenth day they were transfused with autologous packed red blood cells to restore their hematocrit towards control values. These animals were euthanized after 20 days of study (Anemic-Recovery). All experiments began at 109d GA with the Control and Anemic experiments concluded at 119d GA and the Control-Recovery and Anemic-Recovery experiments concluded at 129d GA.
The experimental protocol was initiated at 109d GA (Day 0) with measurement of baseline hemodynamics, arterial blood pH, PO2, PCO2 (Gem Premier 3000, Instrumentation Laboratory, Bedford MA), hematocrit, hemoglobin, and oxygen content (IL 682 co-oximeter system, Instrumentation Laboratory). Arterial pressure was measured with Transpac pressure transducers (Abbott, Abbott Park, IL) connected to a calibrated PowerLab computerized recording system (ADInstruments, Colorado Springs, CO). Arterial pressure was referred to amniotic fluid pressure and reported as the arithmetic mean from computer tracings. Arterial pressure tracings were used to calculate fetal heart rate. Hemodynamics were recorded at baseline and on Day 10 of study. In the Anemia-Recovery and Control-Recovery groups, hemodynamics were also recorded for two days following transfusion (Days 11 and 12), and on the final experimental day (Day 20).
Fetuses assigned to an anemia group had blood removed according to a formula determined empirically during previous studies in the laboratory to rapidly induce anemia with minimal acute fetal demise, modified for the age (and lower total blood volume) of the fetuses in this study (7). In the anemic groups, an average of 69 ± 19 ml (mean ± SD) of blood was removed daily for the first three days (until arterial oxygen content was less than 4 mg/dl); thereafter 39 ± 19 ml of blood was removed daily. Blood was collected in a commercially available blood collection system, and the red blood cells separated into a RBC storage solution according to the manufacture's protocol (Optisol Triple Blood Bag system, Terumo Transfusion Solutions, Somerset, NJ). The volume of blood was replaced daily with an equivalent volume of warmed 0.9% NaCl solution. Control fetuses were not hemorrhaged. On the tenth day, fetuses in the Anemic-Recovery group were transfused with warmed, autologous packed red blood cells i after passage through an intravenous transfusion filter. If fetal arterial pressure started to rise, transfusion was terminated and resumed the following day (on average, 66% of the transfusion occurred on day 10). The total blood volume transfused to each Anemic-Recovery fetus was 56 ± 20 ml.
Postmortem
At the conclusion of the experimental period, ewes were euthanized with sodium pentobarbital (65mg/kg i.v.). Deeply anesthetized fetuses were given heparin (5000 U i.v.) and saturated potassium chloride (10ml i.v.) to arrest their hearts in diastole. Fetuses and their excised hearts were weighed.
Cardiac dissociation and cardiomyocyte analysis
A full-thickness transverse section of the left ventricular (LV) free-wall was excised and frozen in liquid nitrogen. The wound edges were sealed with cyanoacrylate. Fetal hearts were then enzymatically dissociated on a Langendorff apparatus, and the cardiomyocytes fixed for morphometric analysis as previously described (5). Myocytes were photographed at 40× on a light microscope (Zeiss Axiophot, Bartels and Stout, Bellevue, WA), and photomicrographs analyzed by calibrated software (Image Pro Plus, MediaCybernetics, Bethesda, MD). At least 50 cells of each type (mononucleated or binucleated) were measured per ventricle per fetus. Separately, at least 300 myocytes from each ventricle of each fetus were counted to determine the number of nuclei per cardiomyocyte.
Cell cycle activity
The anti-Ki-67 antibody MIB-1 (DAKO, Carpinteria, CA) was used to immunohistologically detect cell cycle activity in dissociated cardiomyocytes as previously described (9). Detection of antibody binding was carried out using an avidin-biotin system (Vectastain ABC kit; Vector Laboratories, Burlingame, CA) and staining with diaminobenzidine. No fewer than 500 myocytes were counted per ventricle per fetus for cell cycle activity analysis. Ki-67 positive myocytes are expressed as a percent of total mononucleated cardiomyocytes.
Quantitative Immunoblot Analysis
Immunoblots were prepared as described previously (8). Briefly, myocardial samples were homogenized in the presence of protease inhibitors, centrifuged and total protein quantitated spectrophotometrically. Twenty mcg of protein were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were blocked with Odyssey Blocking Buffer (Li-Cor Biosciences, Lincoln, NE) for one hour at room temperature and then incubated in primary antibody overnight at 5°C. Bound primary antibody was detected by incubation with infrared-labeled secondary antibodies (IRDye 800 or IRDye 700 700DX, Li-Cor Biosciences) and quantitated with a Li-Cor Odyssey Imaging System. Primary antibodies obtained from Santa Cruz Biotechnology (Santa Cruz, CA) include: total extracellular signal regulated kinase 1/2 (ERK1/2, 1:1000 dilution, sc-93); phosphorylated ERK1/2 (1:1000, sc-7383); total c-Jun N-terminal kinase 1/2 (JNK1/2, 1:1000, sc-1648); phosphorylated JNK1/2 (1:1000, sc-6254) and total p38 (1:1000, sc-9212); and from Cell Signaling Technology (Beverly, MA): phosphorylated p38 (1:250, #9122); and Akt (1:1000, #9272).
Statistical analysis
Daily hemodynamic and blood gas data were compared within groups by one-way analysis of variance (ANOVA). If warranted by the F statistic, Dunnett's Multiple Comparison was performed as a post-test to determine what days were different than baseline. Data were also compared among groups on day 10 by one-way ANOVA and, if warranted, Bonferonni's Multiple Comparison test was performed to identify differences. On the final experimental day, hemodynamic and blood gas data were compared between groups by unpaired t-test. Body and organ weights, cardiomyocyte dimensions, terminal differentiation and cell cycle activity were compared by one-way ANOVA and, if warranted, Bonferonni's Multiple Comparison test. Protein data were compared by unpaired t-test. All data are presented as mean ± standard error of the mean. A value of P<0.05 was accepted as statistically significant.
Results
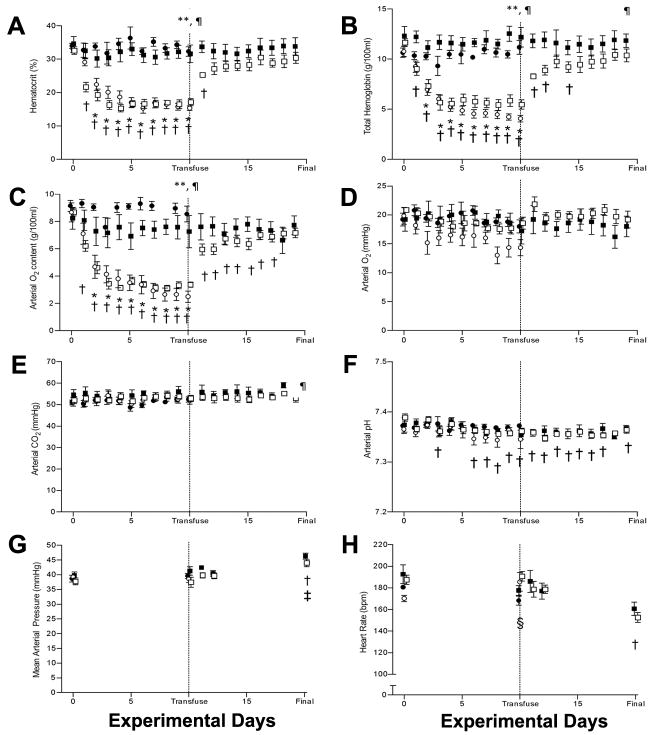
Bleeding significantly reduced fetal hematocrit below baseline values by the second (Anemic-Recovery) and third (Anemic) days (Figure 1A). In both anemic groups, hematocrit was significantly lower than the matched control group (Control, Control-Recovery) on days 3 -10. Following transfusion of the Anemia-Recovery group, hematocrit remained lower than baseline on day 11 (first day post-transfusion), but subsequently returned toward baseline (Day 0). Hematocrit also returned to values similar to those in the Control-Recovery group.
Figure 1.
Arterial blood gas and hemodynamic values. A) Hematocrit; B) Total hemoglobin; C) O2 content; D) Partial pressure of O2; E) Partial pressure of CO2; F) pH; G) Mean arterial blood pressure; H) Heart rate. Control:●; Control-Recovery: ■; Anemic: ○; Anemic-Recovery: □. Significantly different (p < 0.05) from baseline (experimental day 0) for *Anemic group; †Anemic Recovery group; ‡Control Recovery group; § Control group. **Anemic group significantly different from Control group (p<0.05); ¶Anemic-Recovery group significantly different from Control-Recovery group (p<0.05).
Total hemoglobin and arterial oxygen content were also reduced on the second (Anemic-Recovery) and third (Anemic) days compared to baseline (Figure 1B and C). Hemoglobin and arterial oxygen content in both anemic groups remained significantly less than the matched control group on days 3-10. Following transfusion of the Anemic-Recovery group, total hemoglobin remained slightly but significantly less from baseline on days 11, 12, and 14, while arterial oxygen content remained different from baseline until day 18. Arterial PO2 and PCO2 were relatively unchanged by bleeding in either anemic group (Figure 1D and E). A slight, but statistically significant reduction in pH was detected in the Anemic-Recovery group on multiple study days compared to baseline, though no differences between groups were detected (Figure 1F).
Mean arterial blood pressure was similar among all groups at baseline and day 10 (Figure 1G). Arterial pressure was also unaffected by transfusion (days 11 and 12). On the final experimental day, both the Anemic-Recovery and Control-Recovery groups had elevated arterial pressures when compared to baseline, as would be expected with increasing gestation (10). Heart rate, which also tends to decline with advancing gestational age, was lower than baseline in the Control group on day 10 and in the Anemic-Recovery group on day 20 (Figure 1H).
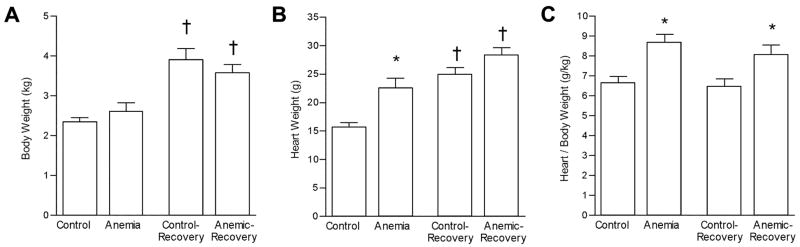
Fetal body weights were not different between anemic groups and their age-matched control groups at either 119d GA (Anemic) or 129d GA (Anemic-Recovery; Figure 2A), although as expected, body weights were increased at 129d GA compared to 119d GA. Heart weight was 45% greater in the Anemic group compared to Control group (22.58 ± 1.31 vs 15.58 ± 0.91 gm, p< 0.05) (Figure 2B). At 129d GA, mean heart weight from Anemic-Recovery fetuses (28.31 ± 1.32 gm) was still significantly greater than from Recovery-Control (24.84 ± 1.31 gm), though the percentage increase (14%) was less than that present between Anemic and Control groups at 119d GA. Heart weights were also significantly increased at 129d GA compared to 119d GA when compared within treatment. When expressed relative to body weight, heart weight in both Anemic and Anemic-Recovery groups was significantly greater than their age-matched controls (Figure 2C).
Figure 2.
Fetal body and heart weights. A) Body weight; B) Heart weight; C). Heart-to-body weight ratio. Significantly different (p < 0.05) from *aged-matched control group; †treatment-matched group.
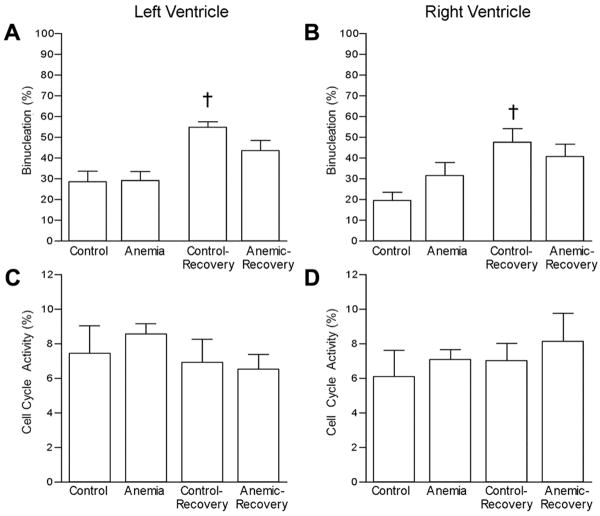
The percentage of binucleated cardiomyocytes (an index of terminal differentiation) was not different between the Anemic and Control groups in either ventricle (Figure 3A and B). Within the control groups, there was an increase in cardiomyocyte binucleation from 119d GA to 129d GA, consistent with the normal maturational process. However, this increase was not seen in the anemic groups, with binucleation similar in Anemic (119GA) and Anemic-Recovery groups (129d GA) in both ventricles. Furthermore, the percentage of binucleated cardiomyocytes was significantly greater in Control-Recovery than in Anemic-Recovery hearts in both ventricles. Cell cycle activity (as determined by Ki67 immunostaining of mononucleated cells) was similar among all groups in both the LV and right ventricle (RV) (Figure 3C and D). Evidence of cardiomyocyte hypertrophy was assessed by examination of fixed, isolated cardiomyocyte length and width. No significant differences in cardiomyocyte dimensions were detected among groups (Table 1).
Figure 3.
Fetal cardiomyocyte maturation and cell cycle activity. Cell cycle activity is represented by the %Ki-67 positive mononucleated cardiomyocytes). A) LV binucleation; B) RV binucleation; C) LV cell cycle activity; D) RV cell cycle activity. † p<0.05 compared to treatment-matched group.
Table 1. Cardiomyocyte dimensions.
| Left Ventricle | Right Ventricle | |||||||
|---|---|---|---|---|---|---|---|---|
| 119d GA | 129d GA | 119d GA | 129d GA | |||||
| Control | Anemic | Control- Recovery |
Anemic- Recovery |
Control | Anemic | Control- Recovery |
Anemic- Recovery |
|
| Mononucleated Length (μm) | 67 ± 1 | 63 ± 2 | 69 ± 2 | 68 ± 2 | 69 ± 2 | 71 ± 3 | 74 ± 2 | 70 ± 2 |
| Mononucleated Width (μm) | 12 ± 1 | 12 ± 0 | 12 ± 1 | 11 ± 0 | 13 ± 0 | 13 ± 1 | 14 ± 1 | 13 ± 1 |
| Binucleated Length (μm) | 90 ± 2 | 87 ± 1 | 90 ± 3 | 90 ± 2 | 97 ± 3 | 98 ± 5 | 98 ± 3 | 96 ± 2 |
| Binucleated Width (μm) | 13 ± 1 | 14 ± 0 | 14 ± 1 | 13 ± 1 | 16 ± 0 | 16 ± 1 | 17 ± 1 | 16 ± 1 |
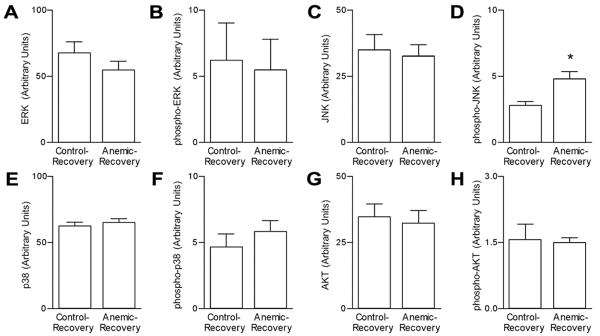
Left ventricular protein levels of MAP kinase pathway components were determined in a subset of the 129d GA groups (Figure 4). Total protein levels of, ERK, JNK, p38 and AKT were similar between the Control-Recovery and Anemic-Recovery groups. Similarly, phospho-ERK, phospho-p38 and phospho-AKT levels were not different between these groups. However, myocardial levels of phospho-JNK were significantly greater in the Anemic-Recovery compared to the Control-Recovery group.
Figure 4.
Myocardial MAPK and AKT expression. Steady-state protein levels of total (A,C,E,G) and activated (phosphorylated; B, D, F, H) extracellular signal-regulated kinase (ERK1/2), c-Jun NH2-terminal kinase (JNK), p38, and Akt. * P<0.05 compared to control levels in Anemic-Recovery hearts. Phospho-p38 tended to be higher in the Anemic-Recovery hearts (P=0.078). n = 6-7 for each group.
Discussion
Adaptive cardiac growth enables the fetus to meet the challenges of chronic anemia. Peripheral tissues respond to progressive anemia by demanding more blood flow, and cardiac enlargement in the chronically anemic fetus supports a sustained increase in cardiac output (11). While changes in cardiomyocyte morphology, metabolism and cardiac vascularity (6, 12-14) accompany cardiac growth in response to chronic fetal anemia, it is unclear whether any of these changes are reversible upon resolution of anemia. We approached this problem by examining the cardiomyocyte response following transfusion of autologous red blood cells to anemic fetuses. Our results suggest that myocardial growth continues following transfusion of the anemic fetus, albeit more slowly than normal for gestation age. Slowed growth may be mediated by a delay of the cardiomyocyte maturational process, as evidenced by the decreased percentage of binucleated cardiomyocytes and/or activation of JNK1/2, which has been shown to antagonize cardiac growth in other animal models (15). However, cardiac growth appears to continue following transfusion, indicating that mitogenic signals dominate the signaling milieu of the fetal myocardium. Thus, while transfusion obviates the need for the adaptive cardiac functional response, excess cardiac capacity does not completely inhibit continued heart growth.
Cardiomyocyte Responses
In late gestation sheep fetuses (129-138d GA), cardiac enlargement h in response to chronic anemia is accomplished by balanced growth of all components of the myocardium, including cardiomyocyte proliferation and hypertrophy (7). In these studies, the percentage of binucleated cardiomyocytes increased in the RV of anemic fetuses, presenting a picture of a more mature heart. In the present study, in which fetuses were made anemic at a much younger age (beginning 109 d GA), the mechanisms of increased cardiac mass are unclear, though cardiomyocyte proliferation was likely responsible for the majority of the substantial cardiac enlargement as we did not detect evidence of cardiomyocyte hypertrophy (by cell dimensions) or maturation (by percentage binucleation). Reasons for the differences in response likely relate to maturational differences in the heart at the initiation of the study. For example, at 109 d GA, the fetal heart is composed almost entirely of mononucleated cardiomyocytes, capable of proliferation and/or terminal differentiation into binucleated cells (5). However, by 129d GA the composition of the heart includes only about 60% mononucleated cells. The nature of the mechanisms regulating this maturational process, including terminal differentiation and its independence or interdependence on pathways stimulating cell cycle activity and proliferation remains to be explored.
Although we failed to see evidence of increased Ki-67 immunostaining, a marker of cell cycle activity, we speculate that following 10 d of chronic anemia, the cardiomyocyte proliferative response had ceased as further cardiac adaptation to anemia was not needed (7). This lack of sustained cardiomyocyte proliferation is in sharp contrast to a recent study by our group which found that after 5 d of chronic anemia and before the onset of a significant increase in myocardial mass, there were twice as many Ki-67 positive stained cardiomyocytes in anemic compared to control fetal hearts (16). Thus, we expect that at the end of the 10 d anemic period, the hearts of these fetuses contained many more cardiomyocytes than normal for their age, though the percentage of binucleated and mononucleated cells within each ventricle are similar to control.
Because we did not perform a longitudinal study and follow cardiac mass over time within each animal, it is difficult to fully assess the effect of transfusion on subsequent heart growth. There did not appear to be complete cessation of cardiac growth following transfusion, as this would have resulted in normalization of heart weights between Anemic-Recover and Control-Recovery. However, transfusion of anemic fetuses led to an apparent substantial slowing of heart growth. This reduced rate of growth was evident by the hearts from anemic fetus at 119 d GA being on average 45% greater in mass than their respective controls, while hearts from transfused fetuses were only 14% greater in mass than controls, with a rate of increase of 0.93 g/d in control animals and 0.57g/d in anemic fetuses. In addition, heart weight/body weight in anemic fetuses tended to decrease from 8.7 ± 0.4 g/kg to 8.0 ±0.5 g/kg following transfusion (p = 0.34). Taken together, these data suggest that transfusion of red cells to anemic fetuses whose hearts have undergone adaption to increase cardiac output and maintain oxygen delivery to peripheral tissues retards but does not eliminate age-dependent myocardial growth. This is consistent with the finding that intrauterine transfusion imperfectly resolved the cardiac enlargement due to fetal anemia in human pregnancy (2).
Intracellular Signaling Pathways
Mechanisms mediating the adaptive response of the fetal heart to chronic anemia have been explored. Martin et al. (6) found that the increase in cardiac mass and vascularization resulting from chronic anemia was associated with induction of hypoxia inducible factor 1 (HIF-1) and vascular endothelial growth factor (VEGF). Using this same model, we subsequently described increases in myocardial expression of VEGF receptors and a number of HIF-1 regulated glycolytic enzymes (13). The MAPK signaling pathways have also been explored in this fetal sheep model given their important roles in cardiac growth and hypertrophy (8). In genetically altered mice, concentric, compensated cardiac hypertrophy results from the overexpression of active MAPK kinase 1 (MEK1), which activates ERK, while dilated cardiomyopathy and premature death is reported in mice with over-activation of p38 or JNK (17, 18). In our sheep studies, we found the MAPK response to chronic anemia was dependent upon the gestation age of the animal, despite similar increase in cardiac mass (8). Specifically, young fetuses (∼100 d gestation) had no significant changes in myocardial expression of phosphorylated ERK, JNK or p38 in response to anemia, while older fetuses (∼130 d gestation) had a marked decrease in ERK but not JNK or p38 phosphorylation. In the present study, we again found no significant changes in myocardial MAPK or AKT activities in response to anemia. Given the nature of the chronic growth stimulus (anemia), we recognize that changes in activity of these regulatory pathways may have occurred early with the anemic stimulus but returned to baseline by the end of the study when tissue sampling occurred. Thus it remains possible that the proliferative cardiomyocyte growth in young chronically anemic fetal sheep (which necessarily occurred given the increase in mass in the absence of cellular hypertrophy) is the result of increased activity of MAPK and/or AKT pathways.
In contrast to the Control or Anemic groups, we found that JNK phosphorylation was increased in the Anemic-Recovery group in this study, consistent with its role for inhibiting myocardial growth (15). Phosphorylation of p38 also tended to be increased in the Anemic-Recovery group, though the difference was not statistically significant. These data suggest that the fetal heart responds to resolution of anemic stress by activating growth inhibitory pathways. Cardiac growth nevertheless continued during the recovery period, indicating that pro-mitogenic signals continued to dominate the signaling milieu of the fetal myocardium.
Limitations
The red cell volume transfused back to fetuses was insufficient to return hematocrit, total hemoglobin, and arterial oxygen content entirely to pre-hemorrhage levels. This may be due to destruction of some red blood cells during handling, as well as the increase in total circulating blood volume that accompanies fetal growth. It is undetermined if this small difference in oxygen content was the stimulus for continued heart growth following transfusion, or if other mitogenic signals were responsible.
Changes in extracellular matrix and vascularity were not examined and could contribute to the increased cardiac mass in anemic animals. However, Jonker et al. previously demonstrated in chronically anemic fetuses that the ventricular tissue fraction of collagen, other connective tissue and vascular space did not differ between anemic and control fetuses (19). On the other hand, fetal cardiac vascular adaptations to chronic anemia, including increased capillary volume density and diameter, as well as decreased intercapillary distance has been described (6, 13). More detailed examination of these cardiac components and their adaption to anemia and transfusion is warranted.
While our discussion of cardiomyocyte maturation has focused on nucleation (ie, mononucleated cells being immature), it must be noted that mononucleated cells may become polyploid, and thus terminally differentiated, particular in response to hemodynamic stress (20). Although we did not assess cellular ploidy, such changes, if present, may suggest chronic anemia alters the maturation of the fetal heart and account for the lack of observed effect on cardiomyocyte proliferation.
Perspective
Anemia is a powerful stimulus for accelerated growth of the immature fetal heart. Systemic oxygen demand drives the need for increased cardiac output, which is achieved by an increase in stroke volume resulting from expanding the ventricular chamber volume. This cardiac remodeling includes increased ventricular wall thickness to reduce wall stress, according to the Law of Laplace. While restoration of hematocrit by transfusion reduces the demand for cardiac output, correction of anemia by red blood cell transfusion does not arrest growth of the enlarged fetal heart. Activation of anti-growth MAPK pathways (JNK) suggests that the fetal heart responded to changing hemodynamic conditions, but even in the context of this recovery period, the signaling milieu of the fetal myocardium remained pro-growth. Thus, factors other than demand for cardiac output likely drive basal heart growth in the growing fetus.
The ultrastructure of the heart is likely a primary factor in maintaining cardiac health through the lifespan. Cumulative loss of cardiomyocytes contributes to progression of cardiac disease, thus an increased cardiomyocyte endowment at birth may be beneficial. Fetal anemia compromises pregnancy and may lead to fetal demise, but the long-term cardiac risks for surviving infants are not clear. Adult sheep made anemic in utero and transfused to normal hematocrit before birth have an enhanced contractile response during hypoxia (21), but increased susceptibility to ischemia-reperfusion injury (22). The extent to which these differences are the result of altered coronary anatomy and physiology, or augmented cardiomyocyte endowment is unknown. Nevertheless, disruption of the anatomical and physiological relationships between vasculature and cardiomyocyte may have serious consequences. Further research is indicated in order to determine therapies additional to transfusion to mitigate the long-term cardiac outcomes of fetal anemia.
Acknowledgments
This study was supported by grant R01HL080657 [to J.L.S.], and grants F32HL088787, L40HL097627-01, and Oregon BIRCWH K12HD043488 [S.S.J.]. S.S.J. is currently at Oregon Health & Science University.
Abbreviations
- AKT
protein kinase B
- ERK
extracellular signal regulated kinase
- JNK
c-Jun N-terminal kinase
- LV
left ventricle
- MAPK
mitogen activated protein kinase
- RV
right ventricle
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sivasankaran S, Sharland GK, Simpson JM. Dilated cardiomyopathy presenting during fetal life. Cardiol Young. 2005;15:409–416. doi: 10.1017/S1047951105000855. [DOI] [PubMed] [Google Scholar]
- 2.Carter BS, DiGiacomo JE, Balderston SM, Wiggins JW, Merenstein GB. Disproportionate septal hypertrophy associated with erythroblastosis fetalis. Am J Dis Child. 1990;144:1225–1228. doi: 10.1001/archpedi.1990.02150350057024. [DOI] [PubMed] [Google Scholar]
- 3.Huhta JC. Guidelines for the evaluation of heart failure in the fetus with or without hydrops. Pediatr Cardiol. 2004;25:274–286. doi: 10.1007/s00246-003-0591-3. [DOI] [PubMed] [Google Scholar]
- 4.Moise KJ., Jr Intrauterine transfusion with red cells and platelets. West J Med. 1993;159:318–324. [PMC free article] [PubMed] [Google Scholar]
- 5.Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol. 2007;102:1130–1142. doi: 10.1152/japplphysiol.00937.2006. [DOI] [PubMed] [Google Scholar]
- 6.Martin C, Yu AY, Jiang BH, Davis L, Kimberly D, Hohimer AR, Semenza GL. Cardiac hypertrophy in chronically anemic fetal sheep: Increased vascularization is associated with increased myocardial expression of vascular endothelial growth factor and hypoxia-inducible factor 1. Am J Obstet Gynecol. 1998;178:527–534. doi: 10.1016/s0002-9378(98)70433-8. [DOI] [PubMed] [Google Scholar]
- 7.Jonker SS, Giraud MK, Giraud GD, Chattergoon NN, Louey S, Davis LE, Faber JJ, Thornburg KL. Cardiomyocyte enlargement, proliferation, and maturation during chronic fetal anemia in sheep. Exp Physiol. 2010;95:131–139. doi: 10.1113/expphysiol.2009.049379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson AK, Protheroe KN, Scholz TD, Segar JL. The mitogen-activated protein kinases and Akt are developmentally regulated in the chronically anemic fetal sheep heart. J Soc Gynecol Investig. 2006;13:157–165. doi: 10.1016/j.jsgi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Jonker SS, Faber JJ, Anderson DF, Thornburg KL, Louey S, Giraud GD. Sequential growth of fetal sheep cardiac myocytes in response to simultaneous arterial and venous hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;292:R913–R919. doi: 10.1152/ajpregu.00484.2006. [DOI] [PubMed] [Google Scholar]
- 10.Unno N, Wong CH, Jenkins SL, Wentworth RA, Ding XY, Li C, Robertson SS, Smotherman WP, Nathanielsz PW. Blood pressure and heart rate in the ovine fetus: ontogenic changes and effects of fetal adrenalectomy. Am J Physiol. 1999;276:H248–H256. doi: 10.1152/ajpheart.1999.276.1.H248. [DOI] [PubMed] [Google Scholar]
- 11.Davis LE, Hohimer AR. Hemodynamics and organ blood flow in fetal sheep subjected to chronic anemia. Am J Physiol. 1991;261:R1542–R1548. doi: 10.1152/ajpregu.1991.261.6.R1542. [DOI] [PubMed] [Google Scholar]
- 12.Davis LE, Hohimer AR, Morton MJ. Myocardial blood flow and coronary reserve in chronically anemic fetal lambs. Am J Physiol. 1999;277:R306–R313. doi: 10.1152/ajpregu.1999.277.1.R306. [DOI] [PubMed] [Google Scholar]
- 13.Mascio CE, Olison AK, Ralphe JC, Tomanek RJ, Scholz TD, Segar JL. Myocardial vascular and metabolic adaptations in chronically anemic fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1736–R1745. doi: 10.1152/ajpregu.00278.2005. [DOI] [PubMed] [Google Scholar]
- 14.Ralphe JC, Nau PN, Mascio CE, Segar JL, Scholz TD. Regulation of myocardial glucose transporters GLUT1 and GLUT4 in chronically anemic fetal lambs. Pediatr Res. 2005;58:713–718. doi: 10.1203/01.PDR.0000180546.42475.69. [DOI] [PubMed] [Google Scholar]
- 15.Liang Q, Bueno OF, Wilkins BJ, Kuan CY, Xia Y, Molkentin JD. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 2003;22:5079–5089. doi: 10.1093/emboj/cdg474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonker SS, Scholz TD, Segar JL. The effect of adrenalectomy on the cardiac response to subacute fetal anemia. Can J Physiol Pharmacol. 2011;89:79–88. doi: 10.1139/y10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, Saffitz J, Chien K, Xiao RP, Kass DA, Wang Y. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci USA. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonker SS, Giraud MK, Giraud GD, Chattergoon NN, Louey S, Davis LE, Faber JJ, Thornburg KL. Cardiomyocyte enlargement, proliferation and maturation during chronic fetal anaemia in sheep. Exp Physiol. 2010;95:131–139. doi: 10.1113/expphysiol.2009.049379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bensley JG, Stacy VK, De Matteo R, Harding R, Black MJ. Cardiac remodelling as a result of pre-term birth: implications for future cardiovascular disease. Eur Heart J. 2010;31:2058–2066. doi: 10.1093/eurheartj/ehq104. [DOI] [PubMed] [Google Scholar]
- 21.Broberg CS, Giraud GD, Schultz JM, Thornburg KL, Hohimer AR, Davis LE. Fetal anemia leads to augmented contractile response to hypoxic stress in adulthood. Am J Physiol Regul Integr Comp Physiol. 2003;285:R649–R655. doi: 10.1152/ajpregu.00656.2002. [DOI] [PubMed] [Google Scholar]
- 22.Yang Q, Hohimer AR, Giraud GD, Van Winkle DM, Underwood MJ, He GW, Davis LE. Effect of fetal anaemia on myocardial ischaemia-reperfusion injury and coronary vasoreactivity in adult sheep. Acta Physiol (Oxf) 2008;194:325–334. doi: 10.1111/j.1748-1716.2008.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]