Abstract
Background
The growing burden and morbidity of chronic kidney disease (CKD) warrant effective strategies for identifying those at increased risk. We examined the association of cystatin C and albuminuria with development of CKD stage 3.
Study Design
Prospective observational study.
Setting and Participants
5,422 participants from the Multi-Ethnic Study of Atherosclerosis with estimated glomerular filtration rate (eGFR) ≥ 60 ml/min/1.73m2.
Predictor
Participants were categorized into four mutually exclusive groups: presence or absence of microalbuminuria (albumin-creatinine ratio >17 and > 25 µg/mg in men and women, respectively) in those with or without cystatin C ≥ 1.0 mg/L.
Outcomes and Measurements
Incident CKD stage 3 was defined as eGFR < 60 ml/min/1.73m2 at the 3rd or 4th visit and an annual decline of > 1 ml/min/1.73 m2. Poisson regression was used to evaluate incident rate ratios in unadjusted and adjusted analyses that include baseline eGFR.
Results
Mean age was 61 years, 49% were men, 38% white, 11% had diabetes, 13.7% had cystatin C ≥ 1mg/L, 8.4% had microalbuminuria, and 2.7 % had cystatin C ≥ 1 mg/L with microalbuminuria. 554 (10%) participants developed CKD stage 3 over a median follow-up of 4.7 years and the adjusted incidence rate ratios (95% CI) were 1.57 (1.19–2.07), 1.37 (1.13–1.66), and 2.12 (1.61–2.80) in those with microalbuminuria, cystatin C ≥ 1 mg/L, and both, respectively, compared to those with neither.
Limitations
Relatively short follow up and absence of measured GFR.
Conclusions
Cystatin C and microalbuminuria are independent risk factors for incident CKD stage 3 and could be useful as screening tools to identify those at increased risk.
Chronic kidney disease (CKD) is a growing public health problem in the United States. Based on population data from the 2000 United States census, approximately 13.5 million adults would be expected to have CKD stages 3 or 4 (defined as an estimated glomerular filtration rate (eGFR) 15 – 60 ml/min/1.73m2).1 CKD is associated with high rates of cardiovascular disease and mortality,2–7 hospitalization,4 cognitive impairment,8, 9 frailty,10, 11 and impaired quality of life.12, 13 Risk factors for development of CKD stage 3 (eGFR 30–60 ml/min/1.73m2) include baseline level of kidney function, age and cardiovascular risk factors.14–18 Although it is well established that macroalbuminuria (>300 mg/day) is a risk factor for development and progression of established kidney disease, there are fewer data on microalbuminuria (>30 mg/day) as a risk factor for CKD stage 3, particularly in those without diabetes.19, 20
Current strategies to address the growing burden of CKD have been limited to slowing the progression of kidney disease and cardiovascular disease (CVD) risk reduction in those with established CKD stages 1–3. This is partly due to the fact that creatinine-based equations are considered unreliable when eGFR is above 60 ml/min/1.73m2. Reduction of eGFR to a level less than 60 ml/min/1.73m2 is an important outcome, given that prior studies have shown that passing beneath this threshold is associated with subsequent CVD outcomes and mortality.21, 22
Identifying kidney disease markers that precede CKD stage 3 could be an important development for prevention strategies. Serum cystatin C, a novel marker of kidney function, may be more sensitive than serum creatinine in detecting early reductions in kidney function.23, 24 Microalbuminuria is an early sign of glomerular damage in both those with diabetes25–28 and those without diabetes20 and may predict future loss of GFR.
We are not aware of studies that have evaluated both cystatin C and albuminuria as risk factors for incident CKD stage 3. We therefore, examined the association of serum cystatin C and urine albumin excretion with development of incident CKD stage 3 in the Multi-Ethnic Study of Atherosclerosis (MESA), a large and ethnically diverse population-based cohort.
Methods
Study population
MESA is a population study of community-dwelling adults aged 45 years to 84 years and was designed to determine the characteristics of subclinical cardiovascular disease and its progression. From 2000 to 2002, 6,814 adults were recruited from 6 US communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; northern Manhattan, NY; and St. Paul, MN). Sampling and recruitment procedures have been described in detail elsewhere.29 Individuals with symptoms or history of medical or surgical treatment for CVD were excluded. Each field site recruited from locally available sources, which included lists of residents, dwellings, and telephone exchanges. In the last several months of recruitment, supplemental sources (lists of Medicare beneficiaries from the Center for Medicare and Medicaid Services and referrals by participants) were used to ensure adequate numbers of minorities and elderly subjects. Institutional review board approval was obtained at all MESA sites. For this analysis, we excluded participants with baseline eGFR < 60 ml/min/1.73m2 (n=669) and those with missing baseline urine ACR (n=25), serum creatinine (n=25), or serum cystatin C (n=38). We also excluded those with missing serum creatinine at the 3rd and 4th visit (n=635).
Exposure Variables
Cystatin C was measured using a BN II nephelometer (Siemens) by a particle-enhanced immunonepholometric assay (N Latex Cystatin-C) on fasting plasma specimens stored at −70°C. The intra-assay coefficient of variation for cystatin C ranged from 2.0 to 2.8%. Urine albumin-creatinine ratio was measured on a single spot sample using nephelometry and is reported as micrograms of albumin per milligram creatinine. We used sex specific cut offs (ACR >17 µg/mg in men and > 25 µg/mg in women) to define microalbuminuria because studies have shown that women have lower creatinine excretion and thus higher ACR values. 30 Serum creatinine was measured by rate reflectance spectrophotometry using thin film adaptation of the creatine amidinohydrolase method on the Vitros analyzer (www.orthoclinical.com/enus/ProductInformation/ClinicalLaboratories/Pages/default.aspx). The coefficient of variation for creatinine is 2.2%. The Vitros analyzer was previously calibrated to a CX3 machine with the Cleveland Clinic laboratory for variance between MESA and those of the MDRD Study clinical laboratories, and the results were nearly identical. On the basis of calibration, all serum creatinine values were adjusted using the following regression formula: Adjusted creatinine = 0.9954 × (serum creatinine) + 0.0208.31 Estimated GFR was calculated using 4 variable MDRD Study equation (estimated GFR = 175 × standardized serum creatinine (SCr) −1.154 × age −0.203 × 1.212 [if black] × 0.742 [if female]), in which GFR is expressed as mL/min/1.73m2 of body surface area and SCr in mg/dL.32
Participants were then categorized into four mutually exclusive groups: presence or absence of microalbuminuria in those with cystatin C < 1.0 mg/L; cystatin C level ≥ 1.0 mg/L in the absence of microalbuminuria; and both (microalbuminuria and cystatin C ≥1.0 mg/L). The cut-off of 1 mg/L for cystatin C was chosen on the basis of prior studies defining this threshold as important for development of cardiovascular disease and all-cause mortality.21, 33, 34
Outcome
Incident CKD stage 3 was defined as eGFR <60 ml/min/1.73m2 at the 3rd or 4th visit, in addition to an annual decline in eGFR > 1 ml/min/1.73m2. The latter criterion was added to avoid labeling a participant with CKD who had small changes in eGFR due to random analytic variability or who had constant serum creatinine and had lower eGFR in follow-up only because of aging. For each participant, annual change in estimated GFR was calculated as a slope using linear regression which included the frequency of measurements and the time between exams. This included estimation of GFR after the first eGFR value less than 60 ml/min/1.73m2 to avoid including participants who may have had small changes due to random variability who would otherwise be labeled as incident CKD stage 3 cases. The 1st, 3rd and 4th visits took place between July 2000 until August 2002, March 2004 until September 2005 and September 2005 through May 2007, respectively.
Covariates
All participants completed self-administered questionnaires and standardized interviews by trained research staff to collect information on demographic characteristics, medical history, medications, alcohol and tobacco use. Trained and certified clinic staff obtained blood pressure, anthropometric measurements, and assessed medication use during each visit. Hypertension was defined as self-reported treatment for hypertension or a systolic blood pressure ≥140 mmHg or diastolic BP ≥90 mmHg. Diabetes was defined as reported history of diabetes, use of insulin or oral hypoglycemic agent, or fasting glucose value 126 mg/dl. Body mass index was calculated as weight in kilograms divided by height in meters squared. C-reactive protein was measured using the BN II nephelometer.
Statistical Analyses
Descriptive analyses were used to summarize baseline characteristics of the study participants across the four groups defined previously. Continuous data were presented as mean ± SD, and categorical variables as proportions. Spearman correlation was used to assess the correlation between albuminuria and cystatin C and Pearson correlation was used to assess the correlation between eGFR and creatinine with cystatin C. A spline of cystatin C after adjusting for age, gender, and race and excluding the top and bottom 2.5% was plotted to evaluate the shape of the relationship with outcomes. Poisson regression was used to model the annual incidence of CKD and the rate in each group. Cystatin C and ACR were initially modeled as continuous variables to calculate the rate ratio of incident CKD stage 3 per SD increment of each unit change to enable a comparison between the two. Because ACR levels were skewed, ACR was log transformed and analyses were repeated per doubling of the raw ACR. Analyses were performed using sex-specific quartiles of ACR, cystatin C quartiles and in groups of microalbuminuria and cystatin C ≥ 1 mg/L. Covariates were selected for multivariate analyses based on their biologically plausible potential to confound the association between cystatin C, albuminuria, and incident CKD stage 3. Initial analyses were unadjusted, and subsequent analyses were adjusted for demographic factors, cardiovascular risk factors and further adjusted for cystatin C in the ACR analyses and for ACR in the cystatin C analyses. Estimated GFR defined by MDRD Study equation was added to the final model to evaluate whether the importance of cystatin C was independent of the baseline eGFR. Interactions were assessed between cystatin C and microalbuminuria and race/ethnicity/age and each other.
Several sensitivity analyses were performed to assess the consistency of our results. Analyses were repeated using the new CKD-EPI equation for estimating GFR, as it may be more accurate and less biased than the MDRD Study equation at estimated GFR >60 ml/min/1.73m2.1 The CKD-EPI equation is eGFR = 141 × minimum(Scr/κ, 1)α × maximum(Scr/κ, 1)−1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black], where κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males.1 Also, because an annual decline in eGFR of > 1 ml/min/1.73 m2 may be considered too low to define incident CKD stage 3, we repeated the analyses requiring an annual decline in eGFR > 3 ml/min/1.73m2. Since most studies focus on eGFR <60 ml/min/1.73m2 without requiring a certain rate of decrease of eGFR, our analysis was repeated after excluding the annual decline of > 1 ml/min/1.73m2 from our working definition. Repeat analyses were also performed excluding patients with history of diabetes at baseline. We also analyzed the data where we defined incident CKD stage 3 as eGFR < 60 ml/min/1.73 m2 at visit 4 along with annual decline in eGFR > 1 ml/min/1.73m2. Finally, we performed additional adjustment for waist-to-hip ratio, the metabolic syndrome (using National Cholesterol Education Program criteria),35 as well as blood pressure in follow up and incident heart failure or coronary disease prior to incident CKD stage 3. Analyses were performed using S-plus (release 8.0, http://spotfire.tibco.com/products/s-plus/statistical-analysis-software.aspx) and SPSS statistical software (www.spss.com). We considered two-tailed P < 0.05 as statistically significant.
Results
Characteristics of Study Participants
Of the 6,814 MESA participants, 1,392 participants were excluded, resulting in a sample of 5,422 participants for analysis. Of these, 4,888 participants (90%) had creatinine measured at the 3rd or the 4th visit. Participants who were excluded for missing creatinine at follow-up were older, had higher baseline prevalence of diabetes and hypertension, higher triglycerides, C-reactive protein, and albumin-creatinine ratio (ACR) levels and lower eGFR compared to the participants included in the analysis (data not shown).
Included participants had a mean age of 61 years and 49% were men, 38% were Caucasian, 28% African American, 22% Hispanic, and 12% Chinese. Forty one percent had hypertension and 11% had diabetes. At baseline, 13.7% had cystatin C ≥ 1 mg/L without microalbuminuria, 8.4% had microalbuminuria with cystatin C < 1 mg/dl, and 2.7% had cystatin C ≥ 1 mg/L with microalbuminuria. Mean (SD) eGFR was 82 +/− 15 ml/min/1.73m2. Participants with cystatin C ≥ 1 mg/L and microalbuminuria were older and had higher BMI and C- reactive protein levels. They were also more likely to be men and have a higher prevalence of diabetes and hypertension (Table 1). The Spearman correlation between cystatin C and ACR was 0.041 (P=0.002). The Pearson correlation between cystatin C and creatinine was 0.381 (p<0.001) and between cystatin C and eGFR was −0.414 (p<0.001).
Table 1.
Baseline characteristics by CysC and MA groups
| Characteristics | CysC < 1.0 mg/L | CysC ≥ 1.0 mg/L |
Total | ||
|---|---|---|---|---|---|
| no MA | with MA | no MA | with MA | ||
| No. of participants | 4075 | 457 | 743 | 147 | 5422 |
| Age | 59 +/− 9 | 63 +/− 10 | 66 +/− 10 | 69 +/− 9 | 61 +/− 10 |
| Male | 1845 (45) | 270 (59) | 415 (56) | 101 (69) | 2631 (49) |
| Race | |||||
| White | 1566 (38) | 118 (26) | 340 (46) | 47 (32) | 2071 (38) |
| Chinese | 517 (13) | 65 (14) | 47 (6) | 14 (10) | 643 (12) |
| Black | 1100 (27) | 154 (34) | 207 (28) | 45 (31) | 1506 (28) |
| Hispanic | 892 (22) | 120 (26) | 149 (20) | 41 (28) | 1202 (22) |
| Former smoker | 1450 (36) | 191 (42) | 280 (38) | 64 (44) | 1985 (37) |
| Current Smoker | 498 (12) | 55 (12) | 128 (17) | 23 (16) | 704 (13) |
| Body Mass Index (kg/m2) | 27.8 +/− 5.2 | 29.2 +/− 5.8 | 29.9 +/− 5.8 | 30.7 +/− 5.8 | 28.3 +/− 5.4 |
| Diabetes | 334 (8) | 155 (34) | 86 (12) | 38 (26) | 613 (11) |
| Hypertension | 1438 (35) | 304 (67) | 382 (51) | 108 (74) | 2232 (41) |
| Systolic Blood Pressure (mm Hg) | 123 (20) | 136 (22) | 127 (20) | 139 (25) | 125 (21) |
| Diastolic Blood Pressure (mm Hg) | 72 (10) | 76 (11) | 71 (10) | 74 (11) | 72 (10) |
| Total cholesterol (mg/dl) | 195 (34) | 195 (39) | 188 (35) | 184 (35) | 194 (35) |
| LDL Cholesterol (mg/dl) | 118 (31) | 116 (33) | 114 (31) | 109 (30) | 117 (31) |
| HDL Cholesterol (mg/dl) | 52 (15) | 48 (13) | 47(13) | 45 (13) | 51 (15) |
| Triglycerides (mg/dl) | 105 [74, 152] | 121 [87, 183] | 122 [86, 172] | 135 [99, 198] | 109 [77, 158] |
| Glucose (mg/dl) | 94 +/− 25 | 118 +/− 51 | 96 +/− 22 | 112 +/− 42 | 96 +/− 29 |
| Metabolic Syndrome | 1094 (27) | 229 (50) | 344 (46) | 92 (63) | 1759 (32) |
| C-reactive Protein (mg/l) | 1.66 [0.74, 3.89] | 2.33 [0.99, 4.63] | 2.58 [1.09, 5.07] | 2.71 [1.33, 6.22] | 1.84 [0.81, 4.14] |
| Antihypertensive medications | 1150 (28) | 250 (55) | 340 (46) | 92 (63) | 1832 (34) |
| Lipid lowering medications | 579 (14) | 82 (18) | 129 (17) | 27 (18) | 817 (15) |
| eGFR (ml/min/1.73m2) | 83 +/− 14 | 89 +/− 17 | 73 +/− 12 | 75 +/− 11 | 82 +/− 15 |
| CysC (mg/L) | 0.81 +/− 0.10 | 0.81 +/− 0.11 | 1.10 +/− 0.12 | 1.13 +/− 0.11 | 0.86 +/− 0.15 |
| ACR (ug/mg) | 4.5 [3.1, 7.4] | 37.7 [26.5, 81.0] | 5.0 [3.2, 8.6] | 51.6 [27.2, 158.2] | 5.1 [3.2, 10.0] |
Note: continuous data are presented either as mean +/− standard deviation or Median [25th, 75th percentile]; categorical data as no.(%).
Conversion factors for units: cystatin C in mg/L to nmol/L, ×74.9; total, LDL and HDL cholesterol in mg/dL to mmol/L, ×0.02586; triglycerides in mg/dL to mmol/L, ×0.01129; glucose in mg/dL to mmol/L, ×0.05551; eGFR (calculated by the Modification of Diet in Renal Disease Study equation) in mL/min/1.73m2 to mL/s/1.73 m2, ×0.01667.
Abbreviations: CysC, cystatin C; MA, microalbuminuria; eGFR, estimated glomerular filtration rate; ACR, albumin-creatinine ratio; HDL, high density lipoprotein; LDL, low density lipoprotein
Incident CKD stage 3 rate ratios by level of cystatin C and ACR
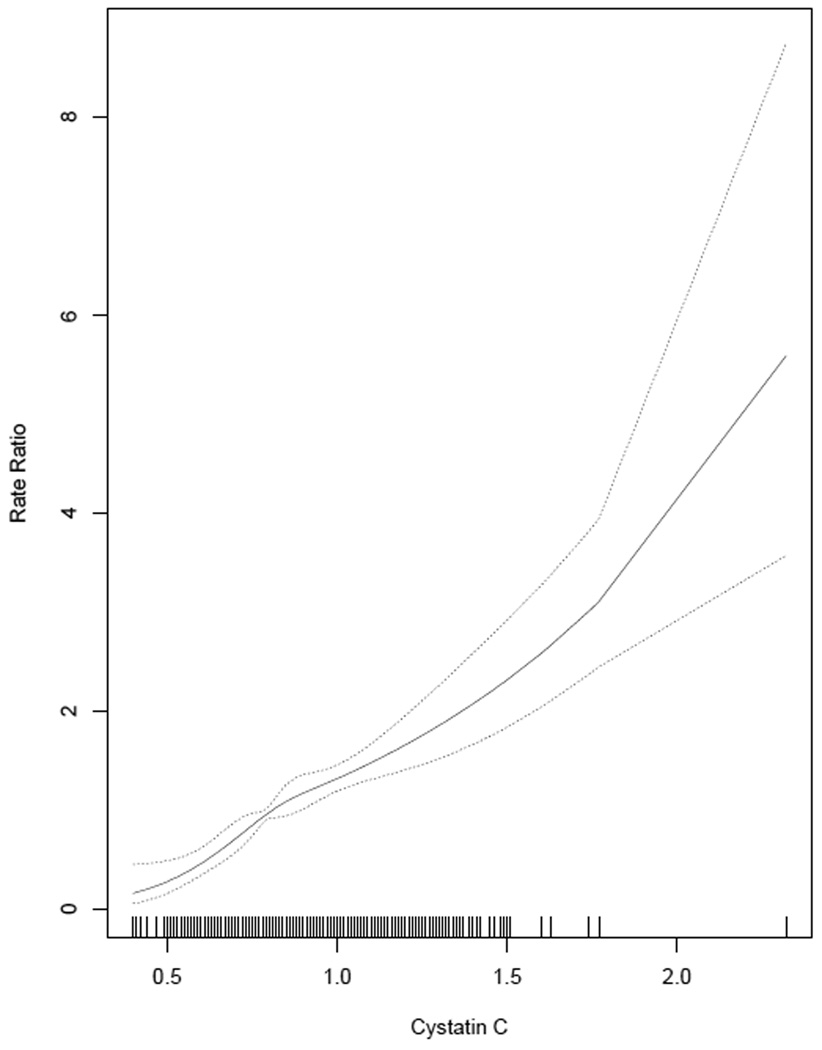
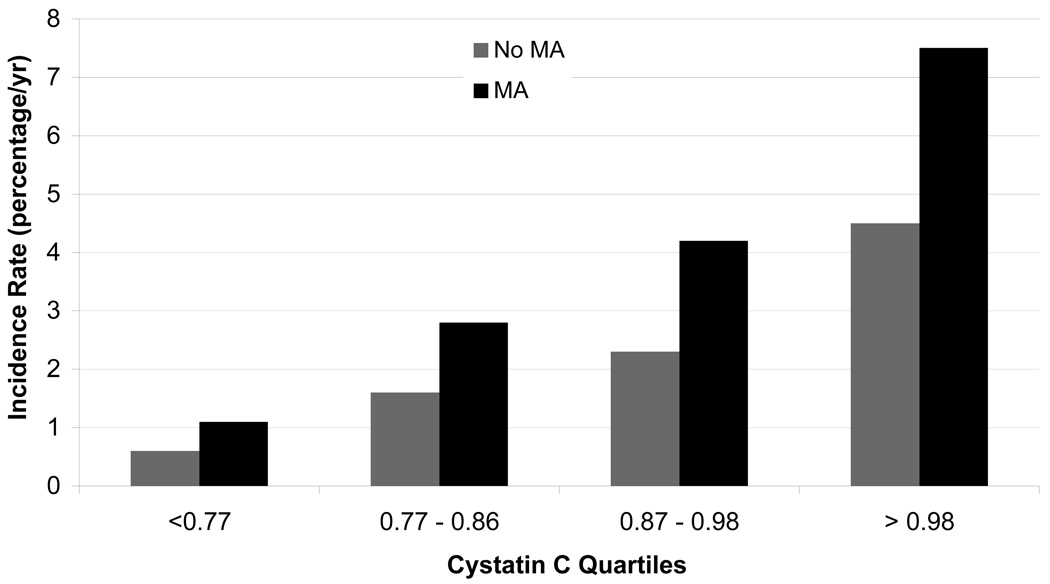
Median follow-up was 4.7 (25th-75th percentile, 4.5–4.9) years and 554 (10%) participants developed incident CKD stage 3, with a mean (SD) eGFR of 53 +/− 7 ml/min/1.73m2. The adjusted spline for cystatin C showed a linear increase in rate ratios of incident CKD stage 3 with higher levels of cystatin C (Figure 1). Annual rates of incident CKD stage 3 were 1.5%, 2.6%, 4.6%, 7.7% (p <0.001) in groups with cystatin C <1 mg/L in absence and presence of microalbuminuria, and cystatin C >1 mg/L in absence and presence of microalbuminuria, respectively. Higher levels of cystatin C were associated with greater incident CKD stage 3 rates in the presence or absence of microalbuminuria (Figure 2) and microalbuminuria was associated with increased rates at all levels of cystatin C.
Figure 1.
Cystatin C spline evaluating the shape of the relationship with incident rate ratios of chronic kidney disease stage 3 after adjusting for age, gender, and race and excluding the top and bottom 2.5%. Conversion factor for units: Cystatin C in mg/L to nmol/L, ×74.9
Figure 2.
Annual unadjusted rate of incident chronic kidney disease stage 3 by quartiles of cystatin C and presence of microalbuminuria. Conversion factor for units: Cystatin C in mg/L to nmol/L, ×74.9
In adjusted continuous analysis, each standard deviation of cystatin C and ACR conferred an independent risk for incident CKD stage 3, although the risks were higher for cystatin C (Table 2). After adjustment for confounders and each other, each linear 0.15 mg/L (SD) higher baseline cystatin C was associated with 57% higher incidence of CKD stage 3 and each doubling of ACR was associated with 14% higher incidence of CKD stage 3. Further adjustment for eGFR decreased the risk per SD of cystatin C to 21%.
Table 2.
Continuous analysis for rate ratio of incident CKD stage 3
| Rate ratio (95% CI) | |
|---|---|
| Cystatin C (per SD=0.15) | |
| Unadjusted | 1.62 (1.48–1.77) |
| Adjusted* | 1.57 (1.45–1.70) |
| Additional adjustment for ACR | 1.57 (1.45–1.69) |
| Additional adjustment for ACR & eGFR | 1.21 (1.13–1.30) |
| ACR (per doubling, i.e., log transformed) | |
| Unadjusted | 1.34 (1.26–1.42) |
| Adjusted* | 1.15 (1.07–1.24) |
| Additional adjustment for CysC | 1.14 (1.06–1.22) |
| Additional adjustment for CysC & eGFR | 1.18 (1.10–1.27) |
Abbreviations and definitions: ACR, albumin-creatinine ratio; eGFR, estimated glomerular filtration rate (calculated by the MDRD Study equation); CI, confidence interval; SD, standard deviation; CysC, cystatin C; CKD, chronic kidney disease
adjusted for age, gender, race, body mass index, systolic blood pressure, diastolic blood pressure, antihypertensive medications, diabetes, low density lipoprotein cholester ol, high density lipoprotein cholesterol, smoking, education
When considered in groups, both microalbuminuria and cystatin C ≥ 1 mg/L were associated with higher adjusted rate ratios for incident CKD stage 3 (Table 3). In adjusted analysis, higher quartiles of cystatin C were associated with steadily increasing incidence of CKD stage 3 with the uppermost quartile (>0.98) having a six-fold higher risk than the lowest quartile (Table 4). The association of higher quartiles of ACR with incident CKD stage 3 did not reach statistical significance. When further adjusted for eGFR, cystatin C remained significantly associated with incident CKD stage 3, though its importance was attenuated. The results for microalbuminuria changed slightly, with the highest quartile now statistically significant.
Table 3.
Adjusted incident CKD stage 3 rate ratios in each group
| CysC and MA groups* | Rate ratio (95% CI) |
|---|---|
| CysC < 1 mg/L without MA | 1.00 (Reference) |
| MA with CysC < 1 mg/L | 1.57 (1.19–2.07) |
| CysC ≥ 1 mg/L without MA | 1.37 (1.13–1.66) |
| CysC ≥ 1 mg/L with MA | 2.12 (1.61–2.80) |
Note : Conversion factors for units : CysC mg/L to nmol/L, ×74.9
Abbreviations: MA, microalbuminuria; CI, confidence intervals ; CKD, chronic kidney disease ; CysC, cystatin C
adjusted for age, gender, race, body mass index, systolic blood pressure, diastolic blood pressure, antihypertensive medications, diabetes, low density lipoprotein cholesterol, high density lipoprotein cholesterol, smoking, education, estimated glomerular function rate
Table 4.
Rate ratio of incident CKD stage 3 by quartiles of CysC and ACR
| No. of incident cases |
Unadjusted rate ratios (95% CI) |
Adjusted* rate ratios (95% CI) |
Rate ratio (95% CI) after additional adjustment for other marker** |
Rate ratio (95% CI) after additional adjustment for eGFR and other marker |
|
|---|---|---|---|---|---|
| CysC quartiles | |||||
| <0.77 mg/L | 51 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 0.77 – 0.86 mg/L | 122 | 2.47 (1.79–3.40) | 2.46 (1.79–3.38) | 2.45 (1.78–3.37) | 1.58 (1.16–2.15) |
| 0.87 – 0.98 mg/L | 160 | 3.68 (2.71–5.00) | 3.35 (2.43–4.60) | 3.27 (2.38–4.50) | 1.63 (1.19–2.25) |
| > 0.98 mg/L | 221 | 7.28 (5.43–9.76) | 6.05 (4.40–8.33) | 5.99 (4.35–8.25) | 2.09 (1.50–2.91) |
| ACR sex-specific quartiles | |||||
| <3.6 ug/mg (♀), <3.0 ug/mg (♂) | 116 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 3.6–5.5 ug/mg (♀), 3.0–4.5 ug/mg (♂) | 103 | 0.93 (0.72–1.19) | 0.85 (0.67–1.09) | 0.80 (0.62–1.02) | 0.97 (0.76–1.23) |
| 5.5–10.4 ug/mg (♀), 4.5–9.3 ug/mg (♂) | 127 | 1.12 (0.88–1.42) | 0.84 (0.66–1.07) | 0.84 (0.67–1.07) | 1.06 (0.84–1.32) |
| >=10.5 ug/mg (♀), >=9.3 ug/mg (♂) | 208 | 1.86 (1.50–2.31) | 1.12 (0.90–1.40) | 1.11 (0.89–1.39) | 1.36 (1.10–1.69) |
Note: Conversion factor for units: CysC in mg/L to nmol/L, ×74.9
Abbreviations and definitions: CKD, chronic kidney disease; ACR, albumin-creatinine ratio; CI, confidence intervals; eGFR, estimated glomerular filtration rate (by MDRD Study equation); CysC, cystatin C
adjusted for age, gender, race, body mass index, systolic blood pressure, diastolic blood pressure, antihypertensive medications, diabetes, low density lipoprotein cholesterol, high density lipoprotein cholesterol, smoking, education.
For the cysC analyses this is additional adjustment for ACR, while for the ACR analyses this is additional adjustment for cysC
There was no interaction noted between cystatin C and microalbuminuria on the multiplicative scale (p = 0.2) or on the additive scale with a synergy index of 1.51 (95% CI 0.87–2.14). Also there were no evidence of interactions of cystatin C or microalbuminuria with diabetes and race (p > 0.3 for all) or with age (p>0.1).
Sensitivity Analyses
In sensitivity analysis using the new CKD-EPI equation, 5239 participants had eGFR ≥ 60 ml/min/1.73m2 at baseline, of which 652 participants (12%) developed CKD stage 3 and the incidence rates in the four groups were slightly higher than using the MDRD Study equation, at 1.8%, 3.6%, 5.9%, 8.3% (p <0.001) in groups with cystatin C <1 mg/L in absence and presence of microalbuminuria, cystatin C ≥1 mg/L without microalbuminuria, and cystatin C ≥1 mg/L with microalbuminuria, respectively. Nearly identical incidence rate ratios were obtained using continuous analyses; each linear 0.15 mg/L increase in cystatin C was associated with 50% higher incidence of CKD stage 3 (95% CI, 1.40–1.61) and each doubling of ACR was associated with 9% (95% CI, 1.02–1.16) higher incidence of CKD stage 3. When a more stringent definition of incident CKD stage 3 was used (i.e. annual decline in eGFR > 3 ml/min/1.73m2), 406 (7.5%) participants developed CKD stage 3. In the adjusted analysis (which includes additional adjustment for ACR and cystatin C in the respective analyses, but not additional adjustment for eGFR), each linear 0.15 mg/L increase in cystatin C was associated with 57% higher incidence of CKD stage 3 (95% CI, 1.43–1.71) and each doubling of ACR was associated with 20% (95% CI, 1.10–1.29) higher incidence of CKD stage 3. When incident CKD stage 3 was defined only as eGFR < 60 ml/min/1.73m2, 575 participants (10.6%) developed incident CKD stage 3, with nearly identical incidence rate ratios (data not shown). After excluding participants with diabetes, 442 (9.2%) developed incident CKD stage 3, with almost identical rate ratios obtained using continuous analysis (data not shown). When incident CKD stage 3 was defined as eGFR < 60 ml/min/1.73m2 at visit 4 along with annual decline in eGFR > 1 ml/min/1.73m2, 5397 participants were included in the analysis with 529 participants (9.8%) developing incident CKD stage 3. Both microalbuminuria and cystatin C ≥ 1 mg/L were associated with higher adjusted rate ratios for incident CKD stage 3 and the results were essentially unchanged (data not shown). Finally, additional adjustment for either waist-to-hip ratio, the metabolic syndrome, follow-up blood pressure or incident cardiovascular events did not significantly change the results (data not shown).
Discussion
In this analysis we demonstrate that higher serum cystatin C and microalbuminuria are independently associated with incident CKD stage 3 in a large representative multi-ethnic cohort. The risks of incident CKD stage 3 associated with increased cystatin C and microalbuminuria were independent of each other and baseline eGFR, and there was no statistically significant interaction between the two variables. Furthermore, there was no overt difference in these relationships among groups based on diabetes or race/ethnicity.
Cystatin C is likely associated with incident CKD stage 3 because it is a sensitive measure of GFR. Interestingly, we noted that cystatin C was a risk factor for incident CKD stage 3 and added information beyond that provided by baseline kidney function. This result is consistent with a sub-analysis from the Cardiovascular Health Study among participants with eGFR > 60 ml/min/1.73m2, in which cystatin C ≥ 1 mg/L was associated with incident CKD stage 3 independent of baseline creatinine.21 In our study cystatin C was able to distinguish a gradient of risk across quartiles among those with eGFR > 60 ml/min/1.73m2 including those without microalbuminuria. The exact reason why cystatin C adds to eGFR as a risk factor for incident CKD stage 3 is not clear and cannot be evaluated by this study. The two possibilities include that cystatin C is a better estimate of measured GFR in those with eGFR >60 ml/min/1.73m2,23, 36, 37 the range in which the MDRD Study and CKD EPI equations are known to be less accurate.38, 39 Alternatively, cystatin C reflects other factors independent of measured GFR that are associated with kidney disease progression.40, 41
Several studies have assessed microalbuminuria as risk factor for ESRD; 42–44 however, there are fewer studies that have evaluated microalbuminuria as a risk factor for incident CKD stage 3 in patients without diabetes. There are several reasons why microalbuminuria may be associated with incident CKD stage 3. In patients with diabetes it is well recognized that microalbuminuria may reflect incipient glomerular damage.45 In patients without diabetes, microalbuminuria may also reflect more widespread vascular and endothelial dysfunction,46 which are risk factors for progression of CKD.47–51 Our results are consistent with prior data in patients with Type 152 or Type 2 diabetes53 as well as in those without diabetes.54, 55 In the HOPE (Heart Outcomes Prevention Evaluation) Study, albuminuria below the level of microalbuminuria was associated with development of macroalbuminuria in patients with and without diabetes.56 Similarly, in the PREVEND (Prevention of Renal and Vascular End-stage Disease) Study in individuals with eGFR > 60ml/min/1.73m2, higher albumin excretion even in the normal range was associated with development of reduced kidney function (ie, <60ml/min/1.73m2) in the general population.20 The latter is consistent with CVD and all-cause mortality endpoint studies, which have shown a graded increase in risk with incremental increase in ACR, even below the microalbuminuria range.57, 58 In our study, microalbuminuria was associated with incident CKD stage 3, as was ACR on a linear and log linear scale.
There are several potential implications of these results. Cystatin C and microalbuminuria should be evaluated as screening tools to identify individuals at highest risk for development of CKD stage 3. Also, consideration should be given to evaluating interventions that are currently recommended for patients with established CKD, for example, tighter control of blood pressure, use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers, in those without CKD but with high levels of cystatin C and albuminuria.
The strengths of our study include the use of data from a community-based cohort that is large, racially and ethnically diverse, with detailed ascertainment of risk factors, covariates and outcomes. To avoid misclassification of participants due to imprecision of the MDRD Study equation in the range of near normal, we defined incident CKD stage 3 as the presence of both an eGFR < 60 ml/min/1.73m2 at the 3rd or 4th visit and an annual decline in eGFR > 1 ml/min/1.73m2. Furthermore, our results were consistent despite several sensitivity analyses.
There are also several limitations that need to be considered. We examined risk factors for incident CKD stage 3 during only 4.7 years of follow-up; however, we studied a large cohort and noted a 10–12% cumulative incidence of CKD stage 3, so there was adequate power to evaluate the characteristics of interest. The statistical power to detect interactions, particularly between microalbuminuria and diabetes, but also between microalbuminuria and cystatin C, may have been limited. There was only one sample of urine for ACR estimation and ACR is known to vary considerably on a day-to-day basis. Random fluctuations in ACR would have biased our results towards the null and thus we may have underestimated the importance of albuminuria. Our estimate of kidney function is limited, as we do not have actual measurement of GFR. Those with creatinine values in follow up were slightly healthier than those without follow up creatinine values but this most likely would have biased our results to the null. Furthermore, we acknowledge that cystatin C may be influenced somewhat by factors other than GFR such as age, gender, body fat, smoking, and inflammation; despite adjustment for these variables, residual confounding may have remained.40, 41
In summary, serum cystatin C and microalbuminuria were independent risk factors for development of incident CKD stage 3 in this multiethnic cohort. Future studies should evaluate whether these subclinical markers may be useful for screening strategies and whether intervention based on these screening strategies can improve patient outcomes.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of MESA for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Support: The study was funded through contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI), which played a role in study design and data collection and by National Institute of Diabetes and Digestive Disease and Kidney Disease (NIDDK) grant K24 078204.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
N section : Because a quorum could not be reached after those editors with potential conflicts recused themselves from consideration of this manuscript, the peer-review and decision-making processes were handled entirely by an Associate Editor (Mark M. Mitsnefes, MD, Cincinnati Children's Hospital Medical Center) who served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
References
- 1.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12) Suppl:S16–S23. [PubMed] [Google Scholar]
- 3.Fried LF, Shlipak MG, Crump C, et al. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003;41(8):1364–1372. doi: 10.1016/s0735-1097(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Eng J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63(3):1121–1129. doi: 10.1046/j.1523-1755.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- 6.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42(5):1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 7.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 8.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16(7):2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 9.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004;15(7):1904–1911. doi: 10.1097/01.asn.0000131529.60019.fa. [DOI] [PubMed] [Google Scholar]
- 10.Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43(5):861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm-Leen ER, Hall YN, Tamura MK, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122(7):664–671. doi: 10.1016/j.amjmed.2009.01.026. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow FY, Briganti EM, Kerr PG, Chadban SJ, Zimmet PZ, Atkins RC. Health-related quality of life in Australian adults with renal insufficiency: a population-based study. Am J Kidney Dis. 2003;41(3):596–604. doi: 10.1053/ajkd.2003.50121. [DOI] [PubMed] [Google Scholar]
- 13.Gorodetskaya I, Zenios S, McCulloch CE, et al. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005;68(6):2801–2808. doi: 10.1111/j.1523-1755.2005.00752.x. [DOI] [PubMed] [Google Scholar]
- 14.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. Jama. 2004;291(7):844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 15.Taal MW, Brenner BM. Renal risk scores: progress and prospects. Kidney Int. 2008;73(11):1216–1219. doi: 10.1038/ki.2008.36. [DOI] [PubMed] [Google Scholar]
- 16.Gelber RP, Kurth T, Kausz AT, et al. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46(5):871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Elsayed EF, Sarnak MJ, Tighiouart H, et al. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis. 2008;52(1):29–38. doi: 10.1053/j.ajkd.2008.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16(7):2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 19.Mogensen CE, Keane WF, Bennett PH, et al. Prevention of diabetic renal disease with special reference to microalbuminuria. Lancet. 1995;346(8982):1080–1084. doi: 10.1016/s0140-6736(95)91747-0. [DOI] [PubMed] [Google Scholar]
- 20.Verhave JC, Gansevoort RT, Hillege HL, Bakker SJ, De Zeeuw D, de Jong PE. An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int Suppl. 2004;(92):S18–S21. doi: 10.1111/j.1523-1755.2004.09205.x. [DOI] [PubMed] [Google Scholar]
- 21.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145(4):237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Segura J, Campo C, Gil P, et al. Development of chronic kidney disease and cardiovascular prognosis in essential hypertensive patients. J Am Soc Nephrol. 2004;15(6):1616–1622. doi: 10.1097/01.asn.0000127045.14709.75. [DOI] [PubMed] [Google Scholar]
- 23.Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36(1):29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 24.Newman DJ, Thakkar H, Edwards RG, et al. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47(1):312–318. doi: 10.1038/ki.1995.40. [DOI] [PubMed] [Google Scholar]
- 25.Fioretto P, Mauer M, Brocco E, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996;39(12):1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- 26.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Eng J Med. 1984;310(6):356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 27.Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32 Suppl 2:64–78. doi: 10.2337/diab.32.2.s64. [DOI] [PubMed] [Google Scholar]
- 28.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1(8287):1430–1432. doi: 10.1016/s0140-6736(82)92450-3. [DOI] [PubMed] [Google Scholar]
- 29.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 30.Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996;7(6):930–937. doi: 10.1681/ASN.V76930. [DOI] [PubMed] [Google Scholar]
- 31.Kramer H, Palmas W, Kestenbaum B, et al. Chronic kidney disease prevalence estimates among racial/ethnic groups: the Multi-Ethnic Study of Atherosclerosis. Clin J Am Soc Nephrol. 2008;3(5):1391–1397. doi: 10.2215/CJN.04160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 33.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Eng J Med. 2005;352(20):2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 34.Shlipak MG, Wassel Fyr CL, Chertow GM, et al. Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2006;17(1):254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 35.Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 36.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37(1):79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 37.Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399–405. doi: 10.1038/sj.ki.5000073. [DOI] [PubMed] [Google Scholar]
- 38.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16(2):459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 39.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Eng J Med. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 40.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65(4):1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 41.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20(5):1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 44.Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003;63(4):1468–1474. doi: 10.1046/j.1523-1755.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- 45.Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18(4):1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 46.Paisley KE, Beaman M, Tooke JE, Mohamed-Ali V, Lowe GD, Shore AC. Endothelial dysfunction and inflammation in asymptomatic proteinuria. Kidney Int. 2003;63(2):624–633. doi: 10.1046/j.1523-1755.2003.00768.x. [DOI] [PubMed] [Google Scholar]
- 47.Elsayed EF, Tighiouart H, Griffith J, et al. Cardiovascular disease and subsequent kidney disease. Arch Intern Med. 2007;167(11):1130–1136. doi: 10.1001/archinte.167.11.1130. [DOI] [PubMed] [Google Scholar]
- 48.Fliser D, Kronenberg F, Kielstein JT, et al. Asymmetric dimethylarginine and progression of chronic kidney disease: the mild to moderate kidney disease study. J Am Soc Nephrol. 2005;16(8):2456–2461. doi: 10.1681/ASN.2005020179. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto Y, Ueda S, Yamagishi S, et al. Dimethylarginine dimethylaminohydrolase prevents progression of renal dysfunction by inhibiting loss of peritubular capillaries and tubulointerstitial fibrosis in a rat model of chronic kidney disease. J Am Soc Nephrol. 2007;18(5):1525–1533. doi: 10.1681/ASN.2006070696. [DOI] [PubMed] [Google Scholar]
- 50.Peralta CA, Katz R, Madero M, et al. The differential association of kidney dysfunction with small and large arterial elasticity: the multiethnic study of atherosclerosis. Am J Epidemiol. 2009;169(6):740–748. doi: 10.1093/aje/kwn392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueda S, Yamagishi S, Matsumoto Y, Fukami K, Okuda S. Asymmetric dimethylarginine (ADMA) is a novel emerging risk factor for cardiovascular disease and the development of renal injury in chronic kidney disease. Clin Exp Nephrol. 2007;11(2):115–121. doi: 10.1007/s10157-007-0471-x. [DOI] [PubMed] [Google Scholar]
- 52.Mogensen CE. Prediction of clinical diabetic nephropathy in IDDM patients. Alternatives to microalbuminuria? Diabetes. 1990;39(7):761–767. doi: 10.2337/diab.39.7.761. [DOI] [PubMed] [Google Scholar]
- 53.Nelson RG, Bennett PH, Beck GJ, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Eng J Med. 1996;335(22):1636–1642. doi: 10.1056/NEJM199611283352203. [DOI] [PubMed] [Google Scholar]
- 54.Pinto-Sietsma SJ, Janssen WM, Hillege HL, Navis G, De Zeeuw D, De Jong PE. Urinary albumin excretion is associated with renal functional abnormalities in a nondiabetic population. J Am Soc Nephrol. 2000;11(10):1882–1888. doi: 10.1681/ASN.V11101882. [DOI] [PubMed] [Google Scholar]
- 55.de Jong PE, Brenner BM. From secondary to primary prevention of progressive renal disease: the case for screening for albuminuria. Kidney Int. 2004;66(6):2109–2118. doi: 10.1111/j.1523-1755.2004.66001.x. [DOI] [PubMed] [Google Scholar]
- 56.Mann JF, Gerstein HC, Yi QL, et al. Development of renal disease in people at high cardiovascular risk: results of the HOPE randomized study. J Am Soc Nephrol. 2003;14(3):641–647. doi: 10.1097/01.asn.0000051594.21922.99. [DOI] [PubMed] [Google Scholar]
- 57.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112(7):969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 58.Weir MR. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol. 2007;2(3):581–590. doi: 10.2215/CJN.03190906. [DOI] [PubMed] [Google Scholar]