Abstract
Purpose
To establish a method of assessing the malignant potential of hepatocellular carcinoma (HCC) using magnetic resonance imaging (MRI).
Methods
For 69 nodules [12 Edmondson (Ed)-I, 48 Ed-II, 9 Ed-III] in 54 HCC patients, signal intensity patterns and enhancement patterns of gadopentate dimeglumine (Gd-DTPA) dynamic studies were correlated with histological differentiation and serum lens culinaris agglutinin-reactive alpha-fetoprotein (AFP-L3) level, which is an indicator of poor prognosis.
Results
Hypointensity on T1-weighted imaging was seen in 17, 72, and 89% of Ed-I, Ed-II, and Ed-III HCCs, respectively (P < 0.001). Meanwhile, hyperintensity on T2-weighted imaging was seen in 42, 88, and 89% (P < 0.005). Tumor stain during the arterial phase of Gd dynamic MRI was seen in 75, 86, and 89%. Tumor stain washout during the portal phase was seen in 43% of Ed-II and 100% of Ed-III HCCs (P < 0.005). In the Ed-II and Ed-III HCCs, hypointensity on T1-weighted imaging was seen in 65% of AFP-L3-negative HCCs and 90% of AFP-L3-positive HCCs (P = 0.071). Washout of tumor stain during the portal phase was seen in 39% of AFP-L3-negative HCCs and 75% of AFP-L3-positive HCCs (P < 0.05).
Conclusions
Although hyperintensity of tumor on T2-weighted imaging and arterial hypervascularity of tumor are considered to be useful for differential diagnosis between well differentiated HCCs and moderately/poorly differentiated HCCs, hypointensity of tumor on T1-weighted imaging and tumor stain washout during the portal phase of Gd-DTPA dynamic MRI reflected poorer histological differentiation of HCCs and correlated with AFP-L3 levels.
Keywords: Hepatocellular carcinoma, Magnetic resonance imaging (MRI), Histological differentiation, Lens culinaris agglutinin-reactive alpha-fetoprotein (AFP-L3)
Introduction
Hepatocellular carcinoma (HCC) primarily develops in a multistep fashion [1]. In general, well-differentiated HCCs are fed from the portal blood flow, with tumor vascularity switching to arterial blood flow along with dedifferentiation to moderately/poorly differentiated HCC as the biological malignant potential increases [2]. The prognosis of well-differentiated HCCs is good following resection or local therapy [3–6]. However, moderately/poorly differentiated HCCs have a greater tendency for vascular invasion and metastasis with poorer histological differentiation; the prognosis for poorly differentiated HCCs following resection or local therapy is particularly poor [7–10]. Fukuda et al. [11] investigated cases of resection of HCCs ≤ 2 cm and reported that the frequency of vascular invasion increased with progressing histological differentiation grade.
Treatment for small HCCs generally aims for complete cure with resection or local therapy. Of the local therapies, radiofrequency ablation (RFA) is more curative than percutaneous ethanol injection, but prognosis-related issues, such as dissemination, recurrence accompanied by portal invasion, and aggressive recurrence after RFA, have been identified [12–15], and histologically poorly differentiated HCCs have been reported to be a risk factor for dissemination [16]. Since there is a high possibility that poorly differentiated HCCs, even if small, may be advanced, with vascular invasion or intrahepatic metastasis, complete cure cannot be obtained by local therapy alone, and there is a risk that such treatment may cause metastasis or dissemination [16–18]. Because it is extremely difficult to diagnose tiny microscopic vascular invasion or intrahepatic metastases on diagnostic imaging, it will be extremely important in the future to diagnose poorly differentiated HCCs, which have higher malignant potential, to plan the treatment of HCCs.
Histological differentiation is not the only indicator of clinical malignant potential, which reflects the prognosis; tumor markers offer another such indicator. α-fetoprotein (AFP), lens culinaris agglutinin-reactive alpha-fetoprotein (AFP-L3) fraction, and des-gamma-carboxy prothrombin (DCP), which are tumor markers for HCC, are indices that show clinical malignant potential, which reflects prognosis [19]. In particular, AFP-L3-positive HCCs have been reported to have poor prognoses [20, 21]. Tumor markers and histological differentiation are related, and it has been reported that AFP values and AFP-L3 fraction levels are significantly higher in poorly differentiated HCCs than in well or moderately differentiated HCCs [7, 20, 21].
Previous reports of the relationship between magnetic resonance imaging (MRI) signal intensity and the histological differentiation of HCCs [22–24] indicate that well differentiated and moderately/poorly differentiated HCCs can be distinguished based on MRI signal intensity. Typical moderately/poorly differentiated HCCs show hypointensity on T1-weighted imaging and hyperintensity on T2-weighted imaging [25]. Dynamic MRI can assess tumor blood flow, is useful for differential diagnosis [26], and is better than plain MRI for detecting HCC [27]. However, to the best of our knowledge, no reports have investigated the use of MRI to distinguish between moderately and poorly differentiated HCCs.
To establish a method of assessing the malignant potential of HCCs using MRI, especially to differentiate between moderately and poorly differentiated HCCs, signal intensity patterns and enhancement patterns of Gd-DTPA dynamic MRI were correlated with histological differentiation and the serum AFP-L3 fraction level.
Subjects and methods
Patients
This retrospective study was approved by our institutional review board; the need for patients to give informed consent was waived.
The histological differentiation grade was compared with the MRI signal intensity in 69 nodules of 54 HCC patients who underwent MRI at our hospital and in whom the histological differentiation grade had been diagnosed by tumor biopsy between April 2001 and March 2004. For 60 of these 69 nodules, for which gadopentate dimeglumine (Gd-DTPA) dynamic MRI had also been performed, the enhancement patterns were also compared according to the histological differentiation grade.
For 45 patients with moderately or poorly differentiated HCCs, the MRI signal intensities were compared according to the AFP-L3 fraction levels. For the 41 of 45 patients who had also undergone Gd-DTPA dynamic MRI, enhancement patterns were compared according to the AFP-L3 fraction levels. For the analysis of MRI signal intensities and enhancement patterns according to AFP-L3 fraction levels, the largest nodule, which reflects the prognosis, was used in patients with multiple HCCs. Specimens from liver tumors suspected to be HCCs were obtained with an 18-gauge biopsy needle (Bard Monopty®, No 121820, Covington, GA, USA) under ultrasound guidance within one month after MRI examination. In principle, two biopsy specimens were obtained from each tumor to avoid sampling error. All specimens were fixed in 10% formalin and embedded in paraffin. The histologic slides were diagnosed by two pathologists who were blinded to the imaging information. Any discrepancies between the two pathologists were resolved by discussion to reach consensus. A diagnosis of HCC was made according to Edmondson and Steiner’s grading [28]. A total of 69 tumors were classified into three groups: Edmondson (Ed) I, 12 nodules; Ed-II, 48 nodules; and Ed-III, 9 nodules. The AFP-L3 fraction level was measured using a liquid-phase binding assay (LBA), with <10% being regarded as negative and ≥10% as positive.
MRI technique and analysis
MR scans were performed using a 1.5-T MR imager (Vision®, Siemens, Berlin, Germany). All patients underwent axial T1-weighted in-phase gradient echo (TR = 149 ms, TE = 4.1 ms, FA = 80, slice thickness 7 mm, interslice gap 7 × 0.25 mm, matrix size 140 × 256, field of view 350 mm), T2-weighted turbo spin echo (TR = 3,800 ms, TE 138 ms, slice thickness 7 mm, interslice gap 7 × 0.25 mm, matrix size 138 × 256, field of view 350 mm), and Gd-DTPA (Magnevist®, Bayer Schering Pharma, Leverkusen, Germany)-enhanced (T1-weighted imaging with fat suppression, TR 155 ms, TE 2.3 ms, FA = 80, slice thickness 7 mm, interslice gap 7 × 0.25 mm, matrix size 165 × 256, field of view 350 mm) imaging. Only axial scans were obtained in all cases. Breath holding was used as a breathing motion reduction technique. Arterial phase images were obtained at 20 s after the start of bolus administration. Portal and equilibrium phase images were obtained at 60 and 240 s, respectively. All patients received Gd-DTPA at a dose of 0.1 mmol/kg body weight; it was administered to all patients by an automated power injector at a rate of 3 mL/s followed by an injection of 20 mL saline at 3 mL/s.
The lesions’ signal intensities were visually compared with the surrounding liver and categorized as hyperintense, isointense, and hypointense. In the arterial phase of dynamic MRI, tumors for which the signal intensity increased and became hyperintense compared with their baseline before contrast study were categorized as positive for tumor stain, primarily based on visual change of relative signal intensities. Tumor stain obtained during the arterial phase of dynamic MRI that disappeared during the portal or equilibrium phase, with the tumor becoming hypointense, was categorized as positive for washout. T1-weighted images, T2-weighted images, and enhancement patterns of dynamic MRI were independently analyzed by one experienced radiologist and two experienced hepatologists who had 10–18 years of experience in liver imaging. Any disagreements of interpretation were resolved by consensus.
Statistical analyses
Values are expressed as mean ± SD. To study the relationships between the histological grade of HCC and serum AFP-L3 fraction level and MRI pattern, overall differences were analyzed by the Kruskal–Wallis test and Fisher’s exact test. P value less than 0.05 was considered significant. All analyses were performed using the SPSS 11.0 software package (SPSS, Inc., Chicago, IL, USA).
Results
Comparison of clinical features according to histological differentiation of HCC nodules (Table 1)
Table 1.
Clinical features according to histological differentiation of HCC nodules
| Grading of differentiation | P value | |||
|---|---|---|---|---|
| Ed-I (n = 12) | Ed-II (n = 48) | Ed-III (n = 9) | ||
| Age (years: mean ± SD) | 65.6 ± 6.0 | 66.6 ± 7.0 | 68.3 ± 6.0 | 0.164 |
| Sex (male/female) | 11/1 | 42/6 | 8/1 | 1.000 |
| Viral marker | ||||
| HBs Ag-positive | 1/12 (8%) | 2/48 (4%) | 1/9 (11%) | 0.355 |
| HCV Ab-positive | 11/12 (92%) | 45/48 (94%) | 8/9 (89%) | 0.792 |
| HBs Ag and HCV Ab-positive | 0/12 (0%) | 1/48 (2%) | 0/9 (0%) | 1.000 |
| Tumor size (mm: mean ± SD) | 23.1 ± 12.1 | 33.3 ± 21.2 | 27.7 ± 16.8 | 0.189 |
| Serum tumor marker level (mean ± SD) | ||||
| AFP (ng/mL) | 68 ± 144 | 2,528 ± 12,460 | 12,208 ± 27,290 | <0.005 |
| DCP (mAU/mL) | 269 ± 558 | 11,453 ± 46,728 | 6,156 ± 15,904 | 0.013 |
| AFP-L3 fraction level (%) | 1.9 ± 4.6 | 16.3 ± 24.8 | 53.8 ± 36.2 | <0.001 |
NS No significant difference, HBs Ag hepatitis B surface antigen, HCV Ab hepatitis C virus antibody, AFP alpha-fetoprotein, AFP-L3 Lens culinaris agglutinin-reactive alpha-fetoprotein, DCP Des-gamma-carboxyprothrombin, Ed Edmondson
Table 1 shows the comparison of the clinical features of the three groups by their histological differentiation grade of HCC. No significant differences among the three groups were noted in age, sex, viral markers, and tumor size. There were significant differences in tumor marker levels.
Correlation between MRI signal intensity patterns and histological differentiation of HCC nodules (Table 2)
Table 2.
Correlation between signal intensity of MRI and histological differentiation of HCC nodules
| Signal intensity | Grading of differentiation | Overall P value | Ed-I versus -II P value | Ed-I versus -III P value | Ed-II versus -III P value | ||
|---|---|---|---|---|---|---|---|
| Ed-I (n = 12) | Ed-II (n = 48) | Ed-III (n = 9) | |||||
| T1-WI | |||||||
| Hyperintense (n = 15) | 8/12 (66%) | 7/48 (14%) | 0/9 (0%) | <0.001 | <0.001 | <0.005 | 0.582 |
| Isointense (n = 10) | 2/12 (17%) | 7/48 (14%) | 1/9 (11%) | 1.000 | 1.000 | 1.000 | 1.000 |
| Hypointense (n = 44) | 2/12 (17%) | 34/48 (72%) | 8/9 (89%) | <0.001 | <0.001 | <0.005 | 0.420 |
| T2-WI | |||||||
| Hyperintense (n = 55) | 5/12 (42%) | 42/48 (88%) | 8/9 (89%) | <0.005 | <0.005 | 0.067 | 1.000 |
| Isointense (n = 10) | 3/12 (25%) | 6/48 (12%) | 1/9 (11%) | 0.499 | 0.365 | 0.603 | 1.000 |
| Hypointense (n = 4) | 4/12 (33%) | 0/48 (0%) | 0/9 (0%) | <0.001 | <0.005 | 0.104 | 1.000 |
MRI Magnetic resonance imaging, T1-WI T1 weighted image, T2-WI T2 weighted image, Ed Edmondson
Table 2 shows the proportions of MRI signal intensity patterns on T1-weighted and T2-weighted MRI for each histological differentiation grade of HCC nodules. The proportion of HCC nodules showing hypointensity on T1-weighted imaging according to the histological differentiation grade was 17% for Ed-I, 72% for Ed-II, and 89% for Ed-III; the proportion of HCC nodules with hypointensity gradually increased as the histological differentiation grade progressed (P < 0.001). The proportion of HCC nodules showing hyperintensity on T2-weighted imaging was 42% for Ed-I, 88% for Ed-II, and 89% for Ed-III; the major proportion of Ed-II and Ed-III tumors showed hyperintensity (P < 0.05). However, there were no significant differences in signal intensity on both T1- and T2-weighted images between moderately and poorly differentiated HCCs.
Correlation between dynamic MRI patterns and histological differentiation of HCC nodules (Fig. 1)
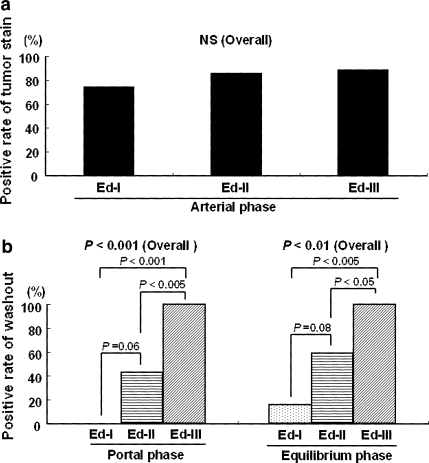
Fig. 1.
Correlation between dynamic MRI patterns and histological differentiation of HCC nodules. a Rate of HCC nodules positive for tumor stain during the arterial phase of the 60 nodules for which gadolinium-DTPA dynamic MRI was performed (8 Ed-I, 43 Ed-II, 9 Ed-III). The positive rates of tumor stain according to the tumor histological differentiation grade are 75% for Ed-I, 86% for Ed-II, and 89% for Ed-III, with no significant difference among the three groups; b Rates of tumor stain washout according to the histological differentiation grade in the portal and equilibrium phases of the 51 nodules that were stained in the arterial phase of dynamic MRI. The washout rate is 0% for Ed-I, 43% for Ed-II, and 100% for Ed-III (P < 0.001) during the portal phase, and 16% for Ed-I, 59% for Ed-II, and 100% for Ed-III (P < 0.01) during the equilibrium phase; washout of tumor stain occurs at an earlier phase the lower the histological differentiation grade
For the 60 HCC nodules for which dynamic MRI was performed, there were 8 Ed-I, 43 Ed-II, and 9 Ed-III nodules. The positive rate of tumor stain during the arterial phase of dynamic MRI was 75% for Ed-I, 86% for Ed-II, and 89% for Ed-III; there was no significant difference among the rates for different histological differentiation grades (Fig. 1a). Of the 51 nodules that were hyperintense during the arterial phase, the positive rate of washout was 0% for Ed-I, 43% for Ed-II, and 100% for Ed-III during the portal phase and 16% for Ed-I, 59% for Ed-II, and 100% for Ed-III during the equilibrium phase. The positive rate of washout was significantly higher for poorer histological differentiation grade in both phases (Fig. 1b). The sensitivity, specificity, and accuracy for diagnosis of Ed-III HCC using tumor stain washout during the portal phase were 100, 63, and 69%, respectively. Furthermore, the sensitivity, specificity, and accuracy for diagnosis of Ed-III HCC using combined MRI features of hypointensity of tumor on T1-weighted imaging and tumor stain washout during the portal phase were 88, 67, and 71%, respectively. The positive rates of tumor stain washout during the portal phase in hyperintensity, isointensity, and hypointensity of the tumor on pre-contrast T1-weighted imaging were 0, 33, and 66%, respectively (P < 0.001). Figure 2 shows a typical case of an Ed-I HCC showing no washout of tumor stain during the portal phase. Figure 3 shows a typical case of an Ed-III HCC that showed washout of tumor stain during the portal phase.
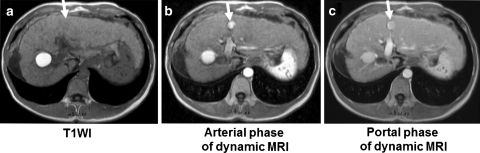
Fig. 2.
52-year-old man with hepatitis C, cirrhosis, and an 18-mm Edmondson-I, HCC in segment III of the liver. a The tumor is isointense on T1-weighted imaging and cannot be identified; b The tumor is strongly stained in the arterial phase of dynamic MRI; c Tumor stain is less but maintained during the portal phase of dynamic MRI, without complete washout
Fig. 3.
a 72-year-old man with hepatitis C, cirrhosis, and a 20-mm Edmondson-III, HCC in segment VIII of the liver. a The tumor is hypointense on T1-weighted imaging; b The tumor is strongly hyperintense during the arterial phase of dynamic MRI; c Tumor stain undergoes washout during the portal phase of dynamic MRI and hypointensity is seen
Comparison of clinical features according to serum AFP-L3 fraction levels (Table 3)
Table 3.
Clinical features according to AFP-L3 fraction levels
| AFP-L3 fraction level | P value | ||
|---|---|---|---|
| Negative (<10%) (n = 23) | Positive (≥10%) (n = 22) | ||
| Age (years: mean ± SD) | 67.0 ± 6.5 | 66.8 ± 7.9 | 0.949 |
| Sex (Male/Female) | 20/3 | 18/4 | 0.699 |
| Viral marker | |||
| HBs Ag-positive | 2/23(9%) | 1/22(5%) | 1.000 |
| HCV Ab-positive | 21/23(91%) | 20/22(91%) | 1.000 |
| HBs Ag and HCV Ab-positive | 0/23(0%) | 1/22 (5%) | 0.489 |
| Tumor size (mm: mean ± SD) | 30.6 ± 15.2 | 42.1 ± 25.0 | 0.068 |
| Serum tumor marker level (mean ± SD) | |||
| AFP (ng/mL) | 78 ± 107 | 10,093 ± 24,524 | 0.057 |
| DCP (mAU/mL) | 896 ± 1,782 | 25,937 ± 67,726 | 0.083 |
| AFP-L3 fraction level (%) | 0.9 ± 1.5 | 46.7 ± 25.6 | <0.001 |
NS No significant difference, HBs Ag hepatitis B surface antigen, HCV Ab hepatitis C virus antibody, AFP alpha-fetoprotein, AFP-L3 lens culinaris agglutinin-reactive alpha-fetoprotein, DCP des-gamma-carboxyprothrombin
Table 3 exhibits the comparison of the clinical features of the two groups by their serum AFP-L3 fraction levels. Although mean serum AFP levels and tumor size of AFP-L3-positive HCCs tended to be higher than those of AFP-L3-negative HCCs, no significant differences between the two groups were noted in age, sex, viral markers, and DCP levels.
Correlation between MRI signal intensity patterns and serum AFP-L3 fraction levels (Table 4)
Table 4.
Correlation between MRI signal intensity of HCC nodules and AFP-L3 fraction levels
| Signal intensity | AFP-L3 fraction level | P value | |
|---|---|---|---|
| Negative (<10%) (n = 23) | Positive (≥10%) (n = 22) | ||
| T1-WI | |||
| Hyperintense (n = 5) | 4/23 (17%) | 1/22 (5%) | 0.346 |
| Isointense (n = 5) | 4/23 (17%) | 1/22 (5%) | 0.346 |
| Hypointense (n = 35) | 15/23 (65%) | 20/22 (90%) | 0.071 |
| T2-WI | |||
| Hyperintense (n = 40) | 21/23 (91%) | 19/22 (86%) | 0.665 |
| Isointense (n = 5) | 2/23 (9%) | 3/22 (14%) | 0.665 |
| Hypointense (n = 0) | 0/23 (0%) | 0/22 (0%) | 1.000 |
MRI Magnetic resonance imaging, AFP-L3 lens culinaris agglutinin-reactive alpha-fetoprotein, T1-WI T1 weighted image, T2-WI T2 weighted image
Of the 45 HCCs with Ed-II or Ed-III histological differentiation, 23 were AFP-L3-negative, and 22 were AFP-L3-positive. Table 4 shows the proportions of T1-weighted and T2-weighted MRI signal intensity patterns by serum AFP-L3 fraction levels. There was no significant difference between the proportions of signal-intensity patterns in the two groups, but the proportion of AFP-L3-negative HCCs showing hypointensity on T1-weighting was 65%, whereas that of AFP-L3-positive HCCs was 90%; there was a trend for a higher proportion of AFP-L3-positive HCCs to show hypointensity (P = 0.071).
Dynamic MRI patterns according to serum AFP-L3 fraction levels (Fig. 4a, b)
Fig. 4.
Dynamic MRI patterns according to serum AFP-L3 fraction. a Positive rates of tumor stain in the arterial phase of 41 Ed-II and Ed-III HCCs (21 AFP-L3-negative, 20 AFP-L3-positive) for which dynamic MRI was performed. The positive rates of tumor stain are 86% for AFP-L3-negative tumors and 80% for AFP-L3-positive tumors, with no significant difference in the positive rate of tumor stain between the two groups; b Washout rates for tumor stain by AFP-L3 fraction in the portal and equilibrium phases of 34 Ed-II and Ed-III HCCs that were stained during the arterial phase of dynamic MRI. The washout rate during the portal phase is 39% for the AFP-L3-negative group and 75% for the positive group; the washout rate is significantly higher in the AFP-L3-positive group (P < 0.05). There is no difference in the washout rate between the two groups during the equilibrium phase (P = 0.062)
Of the 41 Ed-II and Ed-III HCCs for which Gd dynamic MRI was performed, 21 were AFP-L3-negative and 20 were AFP-L3-positive. A comparison of enhancement patterns between the two groups revealed that tumor stain was seen in 18 (86%) tumors in the AFP-L3-negative group and 16 (80%) in the AFP-L3-positive group; there was no significant difference between the two groups (Fig. 4a). Tumor stain washout during the portal phase and in the equilibrium phase tended to be observed more frequently in the AFP-L3-positive group than in the negative group, and the difference between the two groups was significant in the portal phase (P < 0.05) (Fig. 4b).
Discussion
In the present study, the proportion of HCCs showing hypointensity on T1-weighted imaging increased in a stepwise manner with progression in the degree of histological differentiation grade. On the other hand, the major proportion of Ed-II and Ed-III HCCs showed hyperintensity on T2-weighted imaging. From these results, it appears that, when the histological differentiation grade progresses from Ed-I to Ed-II, the T2-weighted images first change to hyperintensity, and then with further progression of the differentiation grade, the T1-weighted images change to hypointensity. Muramatsu et al. [22] reported that further the histological differentiation progresses from early HCC to HCC with early components and overt HCC, the greater the proportion of tumors exhibiting hyperintensity on T2-weighted imaging, and that T2-weighted hyperintensity is useful for evaluating the progress of differentiation in histologically well-differentiated HCCs. The results of the present study are consistent with that study. In 42% of Ed-I HCCs in the present study, hyperintensity was seen on T2-weighted imaging, and 75% were hyperintense in the arterial phase on dynamic MRI. Although these tumors were still histologically categorized as Ed-I, they had arterial hypervascularity. Therefore, they are considered to have higher malignant potential than hypovascular Ed-I HCCs. Honda et al. [29] reported that there is a correlation between high T2-weighted signal intensity and arterial vascularity29, and high T2-weighted signal intensity may be a good reflection of increased arterial tumor blood supply in histologically well-differentiated HCCs. On the other hand, histologically well-differentiated HCCs may show hyperintensity on T1-weighted imaging because they contain more fat and copper than the surrounding hepatic parenchyma. For Ed-I HCCs, 83% did not exhibit low signal intensity on T1-weighted imaging; they showed high or isointensity. Thus, T1-weighted signals may provide a better reflection of the histological characteristics of well-differentiated HCCs than T2-weighted signals.
Next, Gd dynamic MRI demonstrated arterial hypervascularity in the major proportion of Ed-II and Ed-III HCCs. This was a good reflection of the arterial hypervascularity that is a characteristic of classical HCCs. However, a comparison of Ed-II and Ed-III HCCs revealed no significant difference in the proportion of hyperintensity, making it difficult to distinguish between Ed-II and Ed-III HCCs based on enhancement patterns during the arterial phase alone. Although tumor stain washout was frequently seen in hypointensity HCCs on pre-contrast T1-weighted imaging, it occurred more swiftly as the histological differentiation grade became poorer. Yamashita et al. [30] reported that the sinusoid-like vascular space was significantly larger within moderately/poorly differentiated HCCs than within well-differentiated HCCs, and the tumor peak contrast enhancement ratio in the arterial phase of dynamic MRI increased the poorer histological differentiation grade30. It is assumed that the poorer is the differentiation grade, the faster the speed of the tumor blood flow becomes, and the washout of tumor stain occurs earlier.
In the present study, the MRI findings for moderately/poorly differentiated HCCs were hypointensity on T1-weighted imaging and tumor stain washout during the portal phase. Although the sensitivity of diagnosis for Ed-III using tumor stain washout was very high, the specificity and accuracy were not very high. However, combining the MRI features of hypointensity on T1-weighted imaging and tumor stain washout during the portal phase, they improved slightly over those using tumor stain washout alone. The characteristics of these MRI findings are consistent with the MRI findings showing progression from moderate to poor histological differentiation obtained in the present study, and they may be seen as extremely important findings with respect to the assessment of the malignant potential of moderately/poorly differentiated HCCs.
In the light of the above, hyperintensity on T2-weighted imaging and tumor stain during the arterial phase are useful for assessing the progression of malignant potential in histologically well-differentiated HCCs, while hypointensity on T1-weighted imaging and tumor stain washout during the portal phase are useful for assessing the progression of malignant potential in histologically moderately differentiated HCCs. If T1-weighted hypointensity and tumor stain washout during the portal phase are observed in a classical HCC with arterial hypervascularity, this would suggest that it is a poorly differentiated HCC with a more advanced differentiation grade, and that the possibility of microscopic vascular invasion exists even in a small HCC, meaning that its malignant potential must be fully taken into account when selecting treatment. If resectable, consideration should be given to systematic resection of the liver as the treatment of choice for a possible poorly differentiated HCC that may have microscopic vascular invasion. Conversely, intervention that involves only minor invasiveness should be considered when tumors are unresectable, and careful planning is required to prevent blood-borne cancer cell dissemination. In particular, RFA should only be applied after tumor blood flow has been totally stopped by performing transcatheter arterial chemo-embolization.
The main drawbacks of the present study are the use of needle biopsy results as proof of diagnosis. This clearly is not ideal because of heterogeneity of differentiation within the HCC. Moreover, the data are relatively limited, since the authors stopped collecting histopathological data after 2005, because it was stated that tumor biopsy should not be performed in HCC diagnosed on imaging in the evidence-based Japanese clinical practice guideline for HCC of 2005 [31]. This is a relatively small-scale retrospective study, and the distribution of cases among the histological groups was uneven. However, the present results strongly indicate that the clinical malignant potential of HCC can be assessed using the MRI signal intensity pattern together with the washout patterns of Gd-DTPA dynamic MRI.
In conclusion, hypointensity of tumor on T1-weighted imaging and tumor stain washout during the portal phase of Gd-DTPA dynamic MRI reflected poorer histological differentiation of HCCs and correlated with AFP-L3 levels. The combination of these MRI features is useful for the diagnosis of poorly differentiated HCC. A further large-scale cohort study is required to investigate whether these findings will contribute greatly to the selection of treatment for each HCC nodule and, eventually, to a better prognosis for each patient.
Acknowledgments
The authors would like to thank Dr. Takuya Iwamoto and Dr. Motoki Nakai for their technical advice.
This study was presented in part at the Digestive Disease Week meeting held on 15–20 May, 2004, in New Orleans, LA, USA.
Conflict of interest The authors have no conflicts of interest.
References
- 1.Theise ND. Macroregenerative (dysplastic) nodules and hepatocarcinogenesis: theoretical and clinical considerations. Semin Liver Dis. 1995;15:360–371. doi: 10.1055/s-2007-1007287. [DOI] [PubMed] [Google Scholar]
- 2.Kudo M. Multistep human hepatocarcinogenesis: correlation of imaging with pathology. J Gastroenterol. 2009;44(Suppl 19):112–118. doi: 10.1007/s00535-008-2274-6. [DOI] [PubMed] [Google Scholar]
- 3.Sato M, Watanabe Y, Lee T, Kito K, Kimura S, Itoh Y, Akamatsu K, Ueda N. Well-differentiated hepatocellular carcinoma: clinicopathological features and results of hepatic resection. Am J Gastroenterol. 1995;90:112–116. [PubMed] [Google Scholar]
- 4.Yamamoto M, Takasaki K, Otsubo T, Katsuragawa H, Katagiri S, Yoshitoshi K, Ariizumi S, Saito A, Nakano M. Favorable surgical outcomes in patients with early hepatocellular carcinoma. Ann Surg. 2004;239:395–399. doi: 10.1097/01.sla.0000114215.03112.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda T, Shimada M, Harimoto N, Tsujita E, Aishima S, Tanaka S, Shirabe K, Maehara Y. Prognosis of early hepatocellular carcinoma after hepatic resection. Hepatogastroenterology. 2008;55:1428–1432. [PubMed] [Google Scholar]
- 6.Kim SH, Lim HK, Kim MJ, Choi D, Rhim H, Park CK. Radiofrequency ablation of high-grade dysplastic nodules in chronic liver disease: comparison with well-differentiated hepatocellular carcinoma based on long-term results. Eur Radiol. 2008;18:814–821. doi: 10.1007/s00330-007-0823-7. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki Y, Imaoka S, Ishiguro S, Nakano H, Kasugai H, Fujita M, Inoue E, Ishikawa O, Furukawa H, Nakamori S, Kuroda C, Iwanaga T. Clinical features of small hepatocellular carcinomas as assessed by histologic grades. Surgery. 1996;119:252–260. doi: 10.1016/S0039-6060(96)80110-3. [DOI] [PubMed] [Google Scholar]
- 8.Oishi K, Itamoto T, Amano H, Fukuda S, Ohdan H, Tashiro H, Shimamoto F, Asahara T. Clinicopathologic features of poorly differentiated hepatocellular carcinoma. J Surg Oncol. 2007;95:311–316. doi: 10.1002/jso.20661. [DOI] [PubMed] [Google Scholar]
- 9.Akamatsu M, Ishikawa T, Shiratori Y, Koike Y, Shiina S, Teratani T, Hamamura K, Obi S, Sato S, Tateishi R, Fujishima T, Imai Y, Yoshida H, Omata M. Factors predisposing to poorly differentiated hepatocellular carcinoma and its recurrence. Hepatogastroenterology. 2005;52:391–397. [PubMed] [Google Scholar]
- 10.Kim SH, Lim HK, Choi D, Lee WJ, Kim MJ, Kim CK, Jeon YH, Lee JM, Rhim H. Percutaneous radiofrequency ablation of hepatocellular carcinoma: effect of histologic grade on therapeutic results. AJR Am J Roentgenol. 2006;186:S327–S333. doi: 10.2214/AJR.05.0350. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda S, Itamoto T, Nakahara H, Kohashi T, Ohdan H, Hino H, Ochi M, Tashiro H, Asahara T. Clinicopathologic features and prognostic factors of resected solitary small-sized hepatocellular carcinoma. Hepatogastroenterology. 2005;52:1163–1167. [PubMed] [Google Scholar]
- 12.Portolani N, Tiberio GA, Ronconi M, Coniglio A, Ghidoni S, Gaverini G, Giulini SM. Aggressive recurrence after radiofrequency ablation of liver neoplasms. Hepatogastroenterology. 2003;50:2179–2184. [PubMed] [Google Scholar]
- 13.Takada Y, Kurata M, Ohkohchi N. Rapid and aggressive recurrence accompanied by portal tumor thrombus after radiofrequency ablation for hepatocellular carcinoma. Int J Clin Oncol. 2003;8:332–335. doi: 10.1007/s10147-003-0328-6. [DOI] [PubMed] [Google Scholar]
- 14.Nicoli N, Casaril A, Hilal MA, Mangiante G, Marchiori L, Ciola M, Invernizzi L, Campagnaro T, Mansueto G. A case of rapid intrahepatic dissemination of hepatocellular carcinoma after radiofrequency thermal ablation. Am J Surg. 2004;188:165–167. doi: 10.1016/j.amjsurg.2003.12.061. [DOI] [PubMed] [Google Scholar]
- 15.Ruzzenente A, Manzoni GD, Molfetta M, Pachera S, Genco B, Donataccio M, Guglielmi A. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol. 2004;10:1137–1140. doi: 10.3748/wjg.v10.i8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imamura J, Tateishi R, Shiina S, Goto E, Sato T, Ohki T, Masuzaki R, Goto T, Yoshida H, Kanai F, Hamamura K, Obi S, Omata M. Neoplastic seeding after radiofrequency ablation for hepatocellular carcinoma. Am J Gastroenterol. 2008;103:3057–3062. doi: 10.1111/j.1572-0241.2008.02153.x. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Vilana R, Bru C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pages M, Ayuso C, Sole M, Rodes J, Bruix J. Increased risk of tumour seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 18.Mori Y, Tamai H, Shingaki N, Moribata K, Shiraki T, Deguchi H, Ueda K, Enomoto S, Magari H, Inoue I, Maekita T, Iguchi M, Yanaoka K, Oka M, Ichinose M. Diffuse intrahepatic recurrence after percutaneous radiofrequency ablation for solitary and small hepatocellular carcinoma. Hepatol Int. 2009;3:509–515. doi: 10.1007/s12072-009-9131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyaaki H, Nakashima O, Kurogi M, Eguchi K, Kojiro M. Lens culinaris agglutinin-reactive alpha-fetoprotein and protein induced by vitamin K absence II are potential indicators of a poor prognosis: a histopathological study of surgically resected hepatocellular carcinoma. J Gastroenterol. 2007;42:962–968. doi: 10.1007/s00535-007-2117-x. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita F, Tanaka M, Satomura S, Tanikawa K. Prognostic significance of Lens culinaris agglutinin A-reactive alpha-fetoprotein in small hepatocellular carcinomas. Gastroenterology. 1996;111:996–1001. doi: 10.1016/S0016-5085(96)70067-7. [DOI] [PubMed] [Google Scholar]
- 21.Tada T, Kumada T, Toyoda H, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Kitabatake S, Kuzuya T, Nonogaki K, Shimizu J, Yamaguchi A, Isogai M, Kaneoka Y, Washizu J, Satomura S. Relationship between Lens culinaris agglutinin-reactive alpha-fetoprotein and pathologic features of hepatocellular carcinoma. Liver Int. 2005;25:848–853. doi: 10.1111/j.1478-3231.2005.01111.x. [DOI] [PubMed] [Google Scholar]
- 22.Muramatsu Y, Nawano S, Takayasu K, Moriyama N, Yamada T, Yamasaki S, Hirohashi S. Early hepatocellular carcinoma: MR imaging. Radiology. 1991;181:209–213. doi: 10.1148/radiology.181.1.1653443. [DOI] [PubMed] [Google Scholar]
- 23.Kadoya M, Matsui O, Takashima T, Nonomura A. Hepatocellular carcinoma: correlation of MR imaging and histopathologic findings. Radiology. 1992;183:819–825. doi: 10.1148/radiology.183.3.1316622. [DOI] [PubMed] [Google Scholar]
- 24.Ebara M, Fukuda H, Kojima Y, Morimoto N, Yoshikawa M, Sugiura N, Satoh T, Kondo F, Yukawa M, Matsumoto T, Saisho H. Small hepatocellular carcinoma: relationship of signal intensity to histopathologic findings and metal content of the tumour and surrounding hepatic parenchyma. Radiology. 1999;210:81–88. doi: 10.1148/radiology.210.1.r99ja4181. [DOI] [PubMed] [Google Scholar]
- 25.Kelekis NL, Semelka RC, Worawattanakul S, Lange EE, Ascher SM, Ahn IO, Reinhold C, Remer EM, Brown JJ, Bis KG, Woosley JT, Mitchell DG. Hepatocellular carcinoma in North America: a multiinstitutional study of appearance on T1-weighted, T2-weighted, and serial gadolinium-enhanced gradient-echo images. AJR Am J Roentgenol. 1998;170:1005–1013. doi: 10.2214/ajr.170.4.9530051. [DOI] [PubMed] [Google Scholar]
- 26.Beers B, Demeure R, Pringot J, Defalque D, Geubel A, Gigot JF, Jamart J. Dynamic spin-echo imaging with Gd-DTPA: value in the differentiation of hepatic tumours. AJR Am J Roentgenol. 1990;154:515–519. doi: 10.2214/ajr.154.3.2154913. [DOI] [PubMed] [Google Scholar]
- 27.Peterson MS, Baron RL, Murakami T. Hepatic malignancies: usefulness of acquisition of multiple arterial and portal venous phase images at dynamic gadolinium-enhanced MR imaging. Radiology. 1996;201:337–345. doi: 10.1148/radiology.201.2.8888220. [DOI] [PubMed] [Google Scholar]
- 28.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::AID-CNCR2820070308>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 29.Honda H, Kaneko K, Maeda T, Kuroiwa T, Fukuya T, Yoshimitsu K, Irie H, Aibe H, Takenaka K, Masuda K. Small hepatocellular carcinoma on magnetic resonance imaging. Relation of signal intensity to angiographic and clinicopathologic findings. Invest Radiol. 1997;32:161–168. doi: 10.1097/00004424-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita Y, Fan ZM, Yamamoto H, Matsukawa T, Yoshimatsu S, Miyazaki T, Sumi M, Harada M, Takahashi M. Spin-echo and dynamic gadolinium-enhanced FLASH MR imaging of hepatocellular carcinoma: correlation with histopathologic findings. J Magn Reson Imaging. 1994;4:83–90. doi: 10.1002/jmri.1880040117. [DOI] [PubMed] [Google Scholar]
- 31.Kudo M, Okanoue T. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice manual proposed by the Japan Society of Hepatology. Oncology. 2007;72(Suppl 1):2–15. doi: 10.1159/000111702. [DOI] [PubMed] [Google Scholar]