Abstract
With up to 400 million affected people worldwide, chronic hepatitis B virus (HBV) infection is still a major health care problem. During the last decade, several novel therapeutic approaches have been developed and evaluated. In most regions of the world, interferon-α, and nucleos(t)ide analogues (NUCs) are currently approved. Despite major improvements, none of the existing therapies is optimal since viral clearance is rarely achieved. Recently, a better understanding of the HBV life cycle and the development of novel model systems of HBV infection have led to the development of novel antiviral strategies and drug targets. This review will focus on current and potential future drug targets in the HBV life cycle and strategies to modulate the virus–host interaction.
Keyword: HBV life cycle
Epidemiology
Infection with the HBV is a major health care problem with up to 400 million affected persons worldwide. The infection accounts annually for approximately 1 million deaths from cirrhosis, liver failure, and hepatocellular carcinoma (HCC). HBV is transmitted percutaneously, sexually, and perinatally [1]. The prevalence of HBV infection and patterns of transmission vary greatly throughout the world [1]. As a general rule, in high-prevalence countries (e.g., Asia), the source of infection is mainly through perinatal transmission from chronically infected mothers. In low-prevalence countries (e.g., western countries), infection occurs mainly by sexual contacts and percutaneously, for example, by needle sharing among injecting drug users [1, 2]. The risk of establishing a persistent, lifelong infection is very high if HBV infection is acquired perinatally (approximately 90%). By contrast, in adults about 90% spontaneously clear the infection [1]. Recently, universal HBV vaccination programs and measures to control HIV infection have led to a decrease in the incidence of acute hepatitis B. Nevertheless, HBV infection is still an important public health problem as new infections continue to occur.
Molecular virology of hepatitis B virus (HBV)
HBV is a small, enveloped DNA virus that belongs to the family Hepadnaviridae. The viral genome and its genetic organization have been studied in great detail. Currently, eight HBV genotypes (A–H) with a distinct geographic distribution have been identified: genotypes A (serotype adw) and D (serotype ayw) are most common in the US and Europe, genotypes B (serotype adw) and C (serotype adr) in China and Southeast Asia [3]. The HBV genotypes have some impact on the natural course of HBV infection, the HCC development as well as the response to antiviral therapy [4]. The virus itself is non-cytopathic. Cytotoxicity in HBV infection seems to be mainly immune mediated.
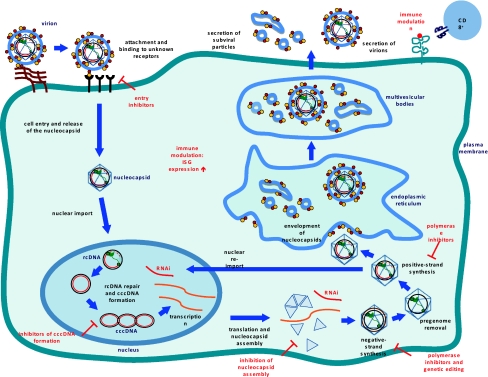
The life cycle of hepatitis B virus
After liver cell membrane attachment to most likely cell-associated heparan sulfate proteoglycans, the viral particle binds specifically to an unknown hepatocyte-specific preS1-receptor (Fig. 1) [5]. Until now, the precise mechanism of viral entry has not been elucidated. Both, endocytosis and direct fusion of the viral envelope with the plasma membrane have been proposed as potential pathways. After uncoating/release into the cytoplasm and transport of the nucleocapsid to the nucleus, the partially double-stranded viral relaxed circular DNA (rcDNA) is repaired by both viral and cellular enzymes. Specifically, the incomplete plus-strand of the rcDNA is completed by the viral polymerase and in another step the viral polymerase and RNA-primers used for DNA plus-strand synthesis are removed by cellular enzymes [6]. Eventually, covalently closed circular DNA (cccDNA) is formed by covalent ligation of both DNA strands. Lack of cccDNA formation in non-human host cells, such as hepatocytes from HBV transgenic mice suggests that host-specific factors may regulate this ligation process [5]. There is evidence that each infected cell contains 1–50 cccDNA molecules as unique episomal minichromosomes. As a replicative intermediate of the HBV life cycle, cccDNA is crucial for the persistence of HBV infection. Importantly, this seems to be true even in patients that show serologic evidence of viral clearance [7]. Viral cccDNA serves as a template for RNA synthesis. All viral RNA species are transcribed from the cccDNA using the cellular transcriptional machinery. The viral DNA contains four major open reading frames:
the precore/core gene, coding for the nucleocapsid protein and for the secreted, non-structural, precore protein, the HBeAg;
the polymerase gene coding for the reverse transcriptase, RNase H and the terminal protein domains;
the PreS1/L-, PreS2/M- and Surface/S-gene, coding for the three envelope proteins; and
the X gene, coding for the regulatory X-protein [5].
Fig. 1.
HBV life cycle
Enveloped virions bind to the host cell by an unknown preS1-receptor. The rcDNA containing nucleocapsids are released into the cytoplasm and transported to the nucleus. In the nucleus, the rcDNA is repaired to form cccDNA. The cccDNA is transcribed into subgenomic RNA (sgRNA) and pregenomic RNA (pgRNA). The pgRNA is encapsidated together with the P protein. Inside the nucleocapsid, the pgRNA is reverse transcribed into negative-strand DNA. rcDNA is generated by plus-strand synthesis from the negative-strand DNA. The nucleocapsids are either re-imported to the nucleus for cccDNA amplification or enveloped and released via the endoplasmic reticulum (ER). Drug targets within the HBV life cycle and immunomodulatory approaches are depicted in red in Fig. 1 (adapted from [5]).
After nuclear export, the pgRNA is translated into the core protein and the viral polymerase. The sgRNA is translated into the regulatory X-protein and the three envelope proteins. Self-assembly of the RNA-containing viral nucleocapsid takes place via complex formation of the pgRNA with the core protein and the polymerase. In the cytoplasm, RNA-containing nucleocapsids undergo a maturation process to DNA containing nucleocapsids by reverse transcription of the pgDNA. The reverse transcriptase lacks proofreading activity; thus, mutations of the viral genome are frequent and result in the coexistence of genetically distinct viral species in infected individuals (quasispecies).
In the HBV life cycle, the DNA containing nucleocapsids fulfil two functions. First, they can be either re-imported into the nucleus to form additional cccDNA or second, they can be enveloped for secretion via the ER. After budding into the ER lumen, the envelope proteins are secreted by the cell either as small, non-infectious subviral spherical or filamentous particles (SVPs) of 22 nm diameter or as infectious virions of 42 nm (Dane particles). Usually, the non-infectious SVPs are produced in a 1,000- to 1,000,000-fold excess over virions.
Goals and end points of antiviral therapy
Clinically, the antiviral therapy should improve the quality of life and survival of chronically HBV infected patients by preventing disease progression to (1) cirrhosis, (2) decompensated cirrhosis, (3) end-stage liver disease, (4) HCC, and (5) death. Current therapies aim for a sustained suppression of viral replication that usually results in a reduced histological activity of chronic hepatitis and biochemical remission [8]. Consequently, the risk of progression to cirrhosis decreases together with the incidence of HCC in non-cirrhotic and to a lesser extent also in cirrhotic patients [8, 9].
The end point of HBV therapy differs between patient groups. The European Association for the Study of the Liver (EASL) distinguishes between three serological constellations [8]:
In HBeAg positive and HBeAg negative patients, the ideal end point is sustained HBsAg loss with or without seroconversion to anti-HBs.
In HBeAg positive patients, durable seroconversion to anti-HBe is a satisfactory end point.
In HBeAg positive patients who do not achieve an anti-HBe seroconversion, a maintained undetectable HBV DNA level on treatment with NUCs or a sustained undetectable HBV DNA level after interferon-α (IFN-α) therapy is the next most desirable end point.
In theory, the ideal goal of antiviral therapy for chronically HBV infected patients would be a complete HBV elimination including HBsAg loss and seroconversion to anti-HBs and complete eradication of cccDNA from infected hepatocytes. However, the elimination of viral cccDNA from the nucleus of infected hepatocytes cannot be achieved by any of the currently available drugs [8]. In line with this, a leading hypothesis is that the clinical resolution of HBV infection does not necessarily require the complete eradication of the virus from the liver. Rather, control of viral replication by the host’s immune system is thought to play a crucial role [10].
Current HBV therapies
In Europe and the US, seven drugs are currently approved for the treatment for chronic hepatitis B, namely conventional IFN-α, pegylated IFN-α (pegIFN-α), and the NUCs lamivudine, adefovir, entecavir, telbivudine, and tenofovir [4, 8]. We will first discuss the approved drugs and recent developments in this field. Second, we will introduce emerging antiviral strategies in HBV treatment at various stages of preclinical and clinical development.
Interferons
Interferon alpha Type I IFNs, among others including IFN alpha (IFN-α) and IFN beta (IFN-β) act through a heterodimeric receptor, called IFNAR, which seems to be expressed ubiquitously [11]. The mechanism of action of IFNs is very complex. Initially, it was believed that type I IFNs mediate their antiviral activity only by the induction of IFN-stimulated genes (ISGs) and maintenance of an antiviral state of the host cell [12]. In addition to the direct effect on the host cell, it has been shown that type I IFNs contribute to the antiviral defense by a number of other mechanisms, for example, a functional modulation of natural killer (NK) cells [12, 13] and an induction of costimulatory molecules on dendritic cells (DC) [14]. Furthermore, type I INFs have been found to enhance expression of and antigen presentation by major histocompatibility complex (MHC) class I and II [15]. In the case of HBV, it has been demonstrated that IFN-α/β eliminates HBV RNA-containing capsids from the cell in a proteasome-dependent manner [16].Type I IFNs, especially IFN-α, have been extensively studied in the treatment of chronic HBV infection. Compared with NUCs, the advantage of IFN treatment is a finite duration of treatment, the absence of resistance, and a higher rate of anti-HBe and anti-HBs seroconversion. Disadvantages are the moderate antiviral effect, need of subcutaneous injection, and significant side effects. These include, among others influenza-like symptoms (e.g., fatigue, myalgias, and fever), cytopenia, depression, anxiety, irritability, and autoimmune disorders. The use of standard IFNs that need to be administered thrice weekly has largely been replaced by pegIFN-α [1]. For pegylation, a polyethylene glycol molecule is attached to IFN-α with the advantage of a prolonged half-life amongst others due to a reduced glomerular filtration of the protein [17]. This allows pegIFN-α to be administered once weekly with a positive effects on patient compliance [17].The HBV genotype was found in some studies to affect the rate of HBeAg loss in HBeAg positive patients. Indeed, patients with HBV genotype A and B show a better treatment response than patients with genotype C and D [18].
Interferon lambda Interferon lambda (IFN-λ) belongs to the type III IFNs [19]. There are three subtypes, namely IFN-λ1, -λ2, and -λ3, also termed as IL29, IL28A, and IL28B, respectively. These cytokines have been discovered by the use of bioinformatics in 2003 and were subsequently found to resemble type I IFNs by (1) being induced by viral infections, (2) stimulating expression of ISGs by similar intracellular signaling pathways, and (3) having antiviral activity in cell culture and in mice [19–22]. Type III IFNs bind to the IL-28Rα/IL-10R2 receptor complex [19]. The distribution of this receptor in human tissues differs substantially from that of the type I IFN receptor. While the IFNARs are expressed ubiquitously, IL-28Rα has a limited expression on a very narrow range of cell types, mostly epithelial cells [23]. There is recent evidence from transgenic mice that INF-λ plays a local, rather than systemic, role in antiviral immunity [24]. Interestingly, human hepatocytes express the IL-28Rα chain and are responsive to IFN-λ [25]. Furthermore, it has been shown that IFN-λ inhibits HBV replication in vitro [26]. These recent findings increased the interest in IFN-λ based therapies for the treatment of chronic hepatitis. There are expectations that the more restricted tissue distribution of the IFN-λ receptor complex would be associated with significantly fewer side effects of IFN-λ as compared to IFN-α based therapies [27]. Whether IFN-λ is a useful antiviral to treat chronic HBV infection in humans needs further investigation. However, an early clinical phase Ib trial using pegIFN-λ1 in patients with chronic hepatitis C virus (HCV) infection showed promising results [28].
Interferon gamma Interferon gamma (IFN-γ), the single member of the type II IFNs, is synthesized only by certain cells of the immune system. These include NK cells and T cells. Physiologically, IFN-γ is secreted after mitogenic or antigenic stimulation of the respective cells. In a study published in 1991, patients with chronic HBV infection have been treated with IFN-γ or IFN-α, with the latter being superior to an IFN-γ based therapy [29]. Despite the poor antiviral effect of IFN-γ, there is evidence that this drug can be used to reduce fibrosis progression or existing fibrosis [30].
Nucleos(t)ide analogues (NUCs)
The nucleoside analogues lamivudine, entecavir, and telbivudine as well as the nucleotide analogues adefovir and tenofovir have been approved for HBV treatment. NUCs are competitive inhibitors of the HBV polymerase. Since their structure is similar to the natural nucleotides, these agents are integrated in the DNA during viral replication. Due to the lack of a hydroxyl group, the formation of a covalent bond with the next nucleotide is impossible. This results in a chain termination and subsequent inhibition of HBV replication. Albeit all NUCs act on the HBV polymerase, their precise mechanisms of inhibition are different (Table 1) [15]. NUCs are orally administered drugs that have a potent antiviral effect (lamivudine and adefovir < entecavir, telbivudine, and tenofovir). NUC treatment is usually well tolerated. The main drawbacks of these drugs are the indefinite duration of treatment and lower rates of anti-HBe and anti-HBs seroconversion compared to IFN-α. The risk of resistance differs with a low risk for entecavir and tenofovir, a moderate risk for adefovir, and a high risk for lamivudine and telbivudine [1]. Cross-resistance has been described, meaning that a mutation that mediates resistance to one NUC also confers resistance to another [31]. Mutations conferring resistance usually occur within the gene that encodes for the HBV polymerase. As this gene overlaps with the gene that encodes the viral envelope, resistance mutations can affect both proteins [31]. As tenofovir and entecavir have a markedly lower risk of resistance than the other approved NUCs, these drugs are preferentially used as a first-line therapy today. Since it is unlikely that newer NUCs that overcome the problem of drug resistance will be available in the near future, combination therapies of the currently available drugs need further study. Whereas first clinical trials could not show an increased antiviral effect, the use of NUCs with a complementary cross-resistance profile prevents the development of resistance [31, 32]. To date, it remains unclear whether or not there is a sustained additional effect of a combination therapy [31–34]. Further studies are therefore needed.
Table 1.
| Drug | Structure | Base priming | (−) Strand synthesis | (+) Strand synthesis |
|---|---|---|---|---|
| Lamivudine | Cytidine analogue | x | x | |
| Adefovir | Adenosine analogue | x | x | |
| Entecavir | Guanosine analogue | x | x | x |
| Telbivudine | Thymidine analogue | x | ||
| Tenofovir | Adenosine analogue | x | x |
Currently, several novel NUCs are at various stages of clinical drug development [4]. Clinical trials with clevudine, a newer pyrimidine nucleoside that was already approved in some Asian countries, have been stopped in 2009 because myopathy was reported in a number of patients [35–37]. Prodrugs that are selectively metabolized and activated in the liver, as for example, LB80380 (similar to adefovir and tenofovir) are currently being tested [38–41]. Apart from that, an acyclic pyrimidine nucleoside phosphate named PMEO-DAPym represents the prototype compound of a novel class of pyrimidine acyclic nucleoside phosphonates that are recognized as a purine nucleotide [42]. In vitro experiments showed that even multi-drug resistant HBV strains remained sensitive to this compound [43, 44]. Hence, this compound could represent a promising drug for treatment of HBV (and HIV) in the future.
Emerging antiviral approaches
Besides the aforementioned therapeutics, several emerging antiviral approaches are currently under investigation. Most of these have initially been evaluated using in vitro and in vivo models of HBV infection. Stable, inducible HBV producing HepG2 and Huh7 cell lines, the HepaRG based infection system and the HBV transgenic mice are just some of the available HBV model systems [31, 45–47]. Whether and which of these approaches will be clinically useful in the future remains to be elucidated. We will present some of the most promising approaches following the HBV life cycle starting with entry, genome processing, protein assembly, and finally immunological approaches to infection control.
Entry inhibitors As shown for HIV, specific inhibition of viral entry is a promising therapeutic concept to control both acute and chronic viral infection [48]. It has been shown that acylated peptides derived from the large HBV envelope protein block viral entry in vitro and in vivo, most likely by preventing interaction of HBV with its receptor [49, 50]. Accordingly, antiviral activity against any HBV genotype is expected. As the acylated peptides target cellular proteins, the emergence of resistant mutants during antiviral therapy seems to be less likely [50]. Clinically, entry inhibitors could be used, for example, as post-exposure prophylaxis and for prevention of vertical transmission as well as re-infection after liver transplantation [4, 50]. It might also restrain virus spread in chronically infected patients [50].
APOBEC3 cytidine deaminases DNA viruses, retroviruses, and hepadnaviruses, such as HBV, have been shown to be vulnerable to genetic editing of single stranded DNA by host cell APOBEC3 cytidine deaminases [51–53]. Mechanistically, the enzyme is thought to deaminate deoxycytidine residues to deoxyuridine in the growing minus-strand viral DNA species during reverse transcription [54]. APOBEC3 genes are up-regulated in chronic inflammatory responses to HBV infection and APOBEC3G has been shown to be a major restriction factor for HBV replication [53]. Therefore, modulation of APOBEC3G expression has been discussed as a potential antiviral strategy. However, recent ex vivo studies revealed that although the mutant spectrum resulting from APOBEC3 editing is highly deleterious, some edited genomes might help the virus to evolve and even escape the immune response of the host [53]. Further studies are needed to elucidate the clinical relevance of these findings.
Variants in the HLA-DP locus In a huge effort, a recent genome-wide association study aimed to identify risk factors for chronic HBV infection [55, 56]. It has been demonstrated that genetic variants in the HLA-DP genes are strongly associated with chronic HBV infections in the Asian population [55]. The underlying mechanism needs to be determined. In addition, it remains unclear whether or not the same is true for the non-Asian population. Nonetheless, antigen presentation on HLA-DP molecules might be critical for HBV elimination and may play an important role in the pathogenesis of chronic HBV infection [55]. These aspects may be relevant for the development of novel therapies.
Covalently closed circular DNA directed therapeutic approaches The viral cccDNA is localized in the nuclei of infected hepatocytes and has a long half-life. Since NUC based antiviral therapies cannot prevent the formation of cccDNA, it has been assumed that persistent low-level viremia during therapy leads to infection of new cells. Thus, prevention of the formation of cccDNA and elimination of cccDNA to prevent reactivation of viral replication after withdrawal of antiviral therapy is of special clinical interest [10, 31]. Despite still being in their infancy, agents that modify epigenetic regulation of cccDNA transcriptional activity as well as the modulation of duck HBV cccDNA transcription by zinc finger proteins in vitro raise hope for a therapeutic application in the future [57, 58].
Glucosidase inhibitor derivatives Glucosidases are cellular enzymes that play an important role in glycoprotein synthesis. Glucosidase inhibitors have anti-HBV properties most likely by inhibition of envelope protein glycosylation in the ER and thereby inhibiting viral morphogenesis and infectivity in vitro [59, 60]. In the case of HCV, phase II clinical trials evaluating the glucosidase inhibitor celgosivir are underway. Whether this drug can be used to treat chronic HBV infection in vivo needs further investigation.
Heteroaryldihydropyrimidines
Heteroaryldihydropyrimidines (HAPs) form a new class of antivirals that inhibit production of HBV virions in cell culture [61, 62]. HAPs have multiple effects in vivo resulting from inappropriate assembly of HBV capsid proteins. One of the most extensively studied HAP compounds, Bay 41-4109, inhibited both HBV DNA replication and HBcAg assembly in a dose-dependent manner in vitro [63–65]. Since this effect seems to be specific, Bay 41-4109 could be an effective drug in vivo.
Phenylpropenamides The phenylpropenamide derivatives AT-61 and AT-130 inhibit HBV replication in vitro. The more potent inhibitor, AT-130 inhibited HBV DNA replication in hepatoma cells without having any effect on viral DNA polymerase activity or core protein translation [66]. Phenylpropenamides have been shown to act at the level of viral encapsidation and packaging and to be active against lamivudine-resistant strains [66, 67]. Therefore, these derivatives could be valuable in combination therapies for example with NUCs [4]. No clinical trials have been conducted so far.
Helioxanthin analogues Ying et al. demonstrated that the helioxanthin analogue 8-1 has a potent antiviral effect in hepatoma cells stably producing HBV. It suppressed HBV RNA and protein expression, as well as DNA replication of both wild-type and lamivudine-resistant virus. The mechanism underlying this strong antiviral effect is a down-regulation of critical transcription factors. As a consequence, HBV promotor activity and viral gene expression as well as replication are blocked [68, 69]. Further studies are ongoing.
Nitazoxanide Nitazoxanide, belonging to the thiazolides, was licensed in the US for treatment of Cryptosporidium parvum and Giardia lamblia in 2002 [70]. A number of studies indicate that this drug might also be effective against chronic HCV and HBV infection [71]. The antiviral mechanism most likely involves the interferon-induced cellular protein kinase activated by double-stranded RNA (PKR) [72]. Nitazoxanide and its metabolite tizoxanide show potent inhibitory effects in vitro and synergistic effects with other antivirals, such as lamivudine and adefovir [71, 73]. In a small clinical trial, nitazoxanide has shown preliminary evidence of efficacy in treatment of chronic hepatitis B [74]. A formal phase II study is planned [71].
RNA-based antiviral approaches Basically, RNA-based therapies include four different approaches, namely antisense molecules, ribozymes, siRNA and aptamers [75]. In this review, we will focus on the siRNA approach.
RNA interference (RNAi) and small interfering RNA (siRNA) Currently, RNAi is one of the most progressive fields in biology. The effector RNA molecules of RNAi consist of approximately 20–30 nucleotides that are complexed with protein components of the RNA-induced silencing complex (RISC) [76]. The siRNA mediate silencing either by transcriptional or post-transcriptional gene silencing [76]. Therapeutic applications of RNAi pose challenges, like effective and specific in vivo delivery and little or no side effects [77]. As HBV replicates via an RNA intermediate, it is sensitive to RNAi [77]. Until today, several studies described an antiviral effect of RNAi against HBV in vitro and in vivo [4]. Whether or not this approach will be helpful in clinical practice needs further evaluation [78]. Currently, a phase I clinical trial with the siRNA NUC B1000 in HBV infection has been reported [76].
Immune-based therapy The weakness of current therapeutic approaches is that many patients do not establish an efficient immunological control over the virus [1]. Sustained clearance of HBV DNA and anti-HBs seroconversion are rarely achieved. This is probably related to a defective reconstitution of an efficient HBV-specific adaptive immune response. The importance of a coordinated cellular and humoral immune response in HBV control is evident [10]. Individuals with a self-limited acute HBV infection show a multi-specific CD4 and CD8 T cell response with a type I profile of cytokine production and a solid HBV-specific antibody production. Patients chronically infected with HBV usually lack adequate responses. Based on these observations, a sustained reconstitution of HBV-specific immunity in chronically infected patients could be a rational strategy for the control of chronic HBV infection. This is impressively supported by the observation that patients with chronic HBV infection can efficiently control the infection after bone marrow transplantation from donors with natural immunity to HBV [79–81].In the following, we will briefly discuss thymosin α-1 as a rather unspecific immunomodulatory approach and the pitfalls of recent efforts concerning therapeutic vaccination. Other experimental approaches to treat chronic HBV infections have been extensively reviewed elsewhere [82].
Thymosin α-1 Thymosin α-1 (thymalfasin) is a 28 amino acid protein with immunomodulatory activities. Thymosin α-1 was shown to enhance T cell function by promoting differentiation, maturation, and increased production of cytokines, like IFN-γ or IL-2. Moreover, it is thought to increase MHC class I expression and NK cell activity [83]. Several clinical studies have been conducted using thymosin α-1 [83]. Treatment with thymosin α-1 seems not to be superior to currently available antiviral therapies but might be useful in combination therapies [84]. Trials are ongoing.
Therapeutic vaccination Therapeutic vaccination aims to eliminate chronic HBV infection by boosting the patient’s HBV-specific immunity [82]. Initial approaches tried to use HBV vaccines alone or in combination with NUCs. However, these attempts have been largely unsuccessful and could not show a significant benefit [85–87]. These trials were based on the use of prophylactic HBsAg vaccines that were initially designed to stimulate anti-HBs antibody production [82]. Recent approaches using pre-S1/pre-S2/S vaccine designed to induce HBV-specific T cells also failed to achieve HBeAg or HBsAg clearance. The failure was attributed to a vaccine-induced T cell proliferation with a T helper type 2 cytokine profile [88]. A recent vaccine designed to specifically induce HBV-specific cytotoxic T lymphocyte responses has also been disappointing in terms of therapeutic efficacy [89, 90]. The role of DC vaccines in HBV infection still needs further study [91]. A recent phase IIb clinical trial using a therapeutic antigen–antibody immune complex vaccine in patients with chronic HBV infection showed some promising effects on HBeAg seroconversion [92, 93]. While the vaccines mentioned above predominantly focused on HBV envelope proteins as antigen, it might be useful to test other HBV antigens, such as core or polymerase or a combination of different cytotoxic and helper T cell epitopes in order to optimize the therapeutic vaccination [82].
Conclusions
Successful treatment of chronically HBV infected patients had first been published in the late 1970s. Although antiviral therapy of chronic HBV infection has improved dramatically during the last decades and novel drugs have been approved recently, a 100% effective treatment is still not available [4]. There is a strong need for treatment regimens that result in a sustained response, have a short duration, result in low resistance rates, lack major side effects, and are affordable. On the one hand, a combination therapy of already approved drugs might help to achieve this goal. On the other hand, some of the experimental approaches discussed above are very promising and will hopefully enter clinical practice soon.
Conflict of interest
None.
References
- 1.Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359(14):1486–1500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- 2.Thimme R, Blum HE. (Therapy of hepatitis B). Praxis (Bern 1994) 2006;95(36):1383–1388 [DOI] [PubMed]
- 3.ZF SeegerC, Mason WS. Hepadnaviruses. 5. Philadelphia: Lippincott, Williams and Wilkins; 2007. [Google Scholar]
- 4.Stein LL, Loomba R. Drug targets in hepatitis B virus infection. Infect Disord Drug Targets. 2009;9(2):105–116. doi: 10.2174/187152609787847677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urban S, Schulze A, Dandri M, Petersen J. The replication cycle of hepatitis B virus. J Hepatol. 2010;52(2):282–284. doi: 10.1016/j.jhep.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Nassal M. Hepatitis B viruses: reverse transcription a different way. Virus Res. 2008;134(1–2):235–249. doi: 10.1016/j.virusres.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Balsano C, Alisi A. Viral hepatitis B: established and emerging therapies. Curr Med Chem. 2008;15(9):930–939. doi: 10.2174/092986708783955383. [DOI] [PubMed] [Google Scholar]
- 8.European Association for the Study of the Liver (EASL) EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50(2):227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351(15):1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 10.Levrero M, Pollicino T, Petersen J, Belloni L, Raimondo G, Dandri M. Control of cccDNA function in hepatitis B virus infection. J Hepatol. 2009;51(3):581–592. doi: 10.1016/j.jhep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89(Pt 1):1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 12.Ank N, West H, Paludan SR. IFN-lambda: novel antiviral cytokines. J Interferon Cytokine Res. 2006;26(6):373–379. doi: 10.1089/jir.2006.26.373. [DOI] [PubMed] [Google Scholar]
- 13.Stegmann KA, Bjorkstrom NK, Veber H, Ciesek S, Riese P, Wiegand J, Hadem J, Suneetha PV, Jaroszewicz J, Wang C, Schlaphoff V, Fytili P, Cornberg M, Manns MP, Geffers R, Pietschmann T, Guzman CA, Ljunggren HG, Wedemeyer H. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138(5):1885–1897. doi: 10.1053/j.gastro.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 14.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 15.Dusheiko G, Antonakopoulos N. Current treatment of hepatitis B. Gut. 2008;57(1):105–124. doi: 10.1136/gut.2005.077891. [DOI] [PubMed] [Google Scholar]
- 16.Robek MD, Boyd BS, Wieland SF, Chisari FV. Signal transduction pathways that inhibit hepatitis B virus replication. Proc Natl Acad Sci USA. 2004;101(6):1743–1747. doi: 10.1073/pnas.0308340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung WJ, Porter JE, Ehrlich GK, Pan W, Xu ZX, Modi MW, Farid A, Berthold W, Graves M. Rational design of a potent, long-lasting form of interferon: A 40 kDa branched polyethylene glycol-conjugated interferon alpha-2a for the treatment of hepatitis C. Bioconjug Chem. 2001;12(2):195–202. doi: 10.1021/bc000082g. [DOI] [PubMed] [Google Scholar]
- 18.Janssen HL, Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, Simon C, So TM, Gerken G, Man RA, Niesters HG, Zondervan P, Hansen B, Schalm SW. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365(9454):123–129. doi: 10.1016/S0140-6736(05)17701-0. [DOI] [PubMed] [Google Scholar]
- 19.Ank N, Paludan SR. Type III IFNs: new layers of complexity in innate antiviral immunity. Biofactors. 2009;35(1):82–87. doi: 10.1002/biof.19. [DOI] [PubMed] [Google Scholar]
- 20.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4(1):69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 21.Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, Sorgeloos F, Ehl S, Mayer D, Kochs G, Schwemmle M, Gunther S, Drosten C, Michiels T, Staeheli P. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010;84(11):5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4(1):63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 23.Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4(3):e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagliaccetti NE, Chu EN, Bolen CR, Kleinstein SH, Robek MD. Lambda and alpha interferons inhibit hepatitis B virus replication through a common molecular mechanism but with different in vivo activities. Virology. 2010;401(2):197–206. doi: 10.1016/j.virol.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle CM, Han J, Weigert MG, Prak ET. Consequences of receptor editing at the lambda locus: multireactivity and light chain secretion. Proc Natl Acad Sci USA. 2006;103(30):11264–11269. doi: 10.1073/pnas.0604053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong SH, Cho O, Kim K, Shin HJ, Kotenko SV, Park S. Effect of interferon-lambda on replication of hepatitis B virus in human hepatoma cells. Virus Res. 2007;126(1–2):245–249. doi: 10.1016/j.virusres.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien TR. Interferon-alfa, interferon-lambda and hepatitis C. Nat Genet. 2009;41(10):1048–1050. doi: 10.1038/ng.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muir AJ, Shiffman ML, Zaman A, Yoffe B, de la Torre A, Flamm S, Gordon SC, Marotta P, Vierling JM, Carlos Lopez-Talavera J, Byrnes-Blake K, Fontana D, Freeman J, Gray T, Hausman D, Hunder NN, Lawitz E. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology 2010 (Epub ahead of print) [DOI] [PubMed]
- 29.Kakumu S, Ishikawa T, Mizokami M, Orido E, Yoshioka K, Wakita T, Yamamoto M. Treatment with human gamma interferon of chronic hepatitis B: comparative study with alpha interferon. J Med Virol. 1991;35(1):32–37. doi: 10.1002/jmv.1890350108. [DOI] [PubMed] [Google Scholar]
- 30.Weng HL, Wang BE, Jia JD, Wu WF, Xian JZ, Mertens PR, Cai WM, Dooley S. Effect of interferon-gamma on hepatic fibrosis in chronic hepatitis B virus infection: a randomized controlled study. Clin Gastroenterol Hepatol. 2005;3(8):819–828. doi: 10.1016/S1542-3565(05)00404-0. [DOI] [PubMed] [Google Scholar]
- 31.Zoulim F, Locarnini S. 2009. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology 137(5):1593–1608, e1591–e1592 [DOI] [PubMed]
- 32.Sung JJ, Lai JY, Zeuzem S, Chow WC, Heathcote EJ, Perrillo RP, Brosgart CL, Woessner MA, Scott SA, Gray DF, Gardner SD. Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. J Hepatol. 2008;48(5):728–735. doi: 10.1016/j.jhep.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 33.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW, Chang WY, Berg T, Flisiak R, McCloud P, Pluck N. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352(26):2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 34.Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C, Diago M, Gurel S, Lai MY, Button P, Pluck N. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351(12):1206–1217. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 35.Fleischer RD, Lok AS. Myopathy and neuropathy associated with nucleos(t)ide analog therapy for hepatitis B. J Hepatol. 2009;51(4):787–791. doi: 10.1016/j.jhep.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Jang JH, Kim JW, Jeong SH, Myung HJ, Kim HS, Park YS, Lee SH, Hwang JH, Kim N, Lee DH. Clevudine for chronic hepatitis B: antiviral response, predictors of response, and development of myopathy. J Viral Hepat (Epub ahead of print) [DOI] [PubMed]
- 37.Kim BK, Oh J, Kwon SY, Choe WH, Ko SY, Rhee KH, Seo TH, Lim SD, Lee CH. Clevudine myopathy in patients with chronic hepatitis B. J Hepatol. 2009;51(4):829–834. doi: 10.1016/j.jhep.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Yuen MF, Han KH, Um SH, Yoon SK, Kim HR, Kim J, Kim CR, Lai CL. Antiviral activity and safety of LB80380 in hepatitis B e antigen-positive chronic hepatitis B patients with lamivudine-resistant disease. Hepatology. 2010;51(3):767–776. doi: 10.1002/hep.23462. [DOI] [PubMed] [Google Scholar]
- 39.Yuen MF, Kim J, Kim CR, Ngai V, Yuen JC, Min C, Kang HM, Shin BS, Yoo SD, Lai CL. A randomized placebo-controlled, dose-finding study of oral LB80380 in HBeAg-positive patients with chronic hepatitis B. Antivir Ther. 2006;11(8):977–983. [PubMed] [Google Scholar]
- 40.Yuen MF, Lee SH, Kang HM, Kim CR, Kim J, Ngai V, Lai CL. Pharmacokinetics of LB80331 and LB80317 following oral administration of LB80380, a new antiviral agent for chronic hepatitis B (CHB), in healthy adult subjects, CHB patients, and mice. Antimicrob Agents Chemother. 2009;53(5):1779–1785. doi: 10.1128/AAC.01290-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erion MD, Poelje PD, Mackenna DA, Colby TJ, Montag AC, Fujitaki JM, Linemeyer DL, Bullough DA. Liver-targeted drug delivery using HepDirect prodrugs. J Pharmacol Exp Ther. 2005;312(2):554–560. doi: 10.1124/jpet.104.075903. [DOI] [PubMed] [Google Scholar]
- 42.Herman BD, Votruba I, Holy A, Sluis-Cremer N, Balzarini J. The acyclic 2, 4-diaminopyrimidine nucleoside phosphonate acts as a purine mimetic in HIV-1 reverse transcriptase DNA polymerization. J Biol Chem. 2010;285(16):12101–12108. doi: 10.1074/jbc.M109.096529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brunelle MN, Lucifora J, Neyts J, Villet S, Holy A, Trepo C, Zoulim F. In vitro activity of 2, 4-diamino-6-[2-(phosphonomethoxy)ethoxy]-pyrimidine against multidrug-resistant hepatitis B virus mutants. Antimicrob Agents Chemother. 2007;51(6):2240–2243. doi: 10.1128/AAC.01440-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balzarini J, Pannecouque C, Naesens L, Andrei G, Snoeck R, Clercq E, Hockova D, Holy A. 6-[2-phosphonomethoxy)alkoxy]-2,4-diaminopyrimidines: a new class of acyclic pyrimidine nucleoside phosphonates with antiviral activity. Nucleosides Nucleotides Nucleic Acids. 2004;23(8–9):1321–1327. doi: 10.1081/NCN-200027573. [DOI] [PubMed] [Google Scholar]
- 45.Babinet C, Farza H, Morello D, Hadchouel M, Pourcel C. Specific expression of hepatitis B surface antigen (HBsAg) in transgenic mice. Science. 1985;230(4730):1160–1163. doi: 10.1126/science.3865370. [DOI] [PubMed] [Google Scholar]
- 46.Chisari FV, Pinkert CA, Milich DR, Filippi P, McLachlan A, Palmiter RD, Brinster RL. A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science. 1985;230(4730):1157–1160. doi: 10.1126/science.3865369. [DOI] [PubMed] [Google Scholar]
- 47.Sun D, Nassal M. Stable HepG2- and Huh7-based human hepatoma cell lines for efficient regulated expression of infectious hepatitis B virus. J Hepatol. 2006;45(5):636–645. doi: 10.1016/j.jhep.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Root MJ, Steger HK. HIV-1 gp41 as a target for viral entry inhibition. Curr Pharm Des. 2004;10(15):1805–1825. doi: 10.2174/1381612043384448. [DOI] [PubMed] [Google Scholar]
- 49.Gripon P, Cannie I, Urban S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J Virol. 2005;79(3):1613–1622. doi: 10.1128/JVI.79.3.1613-1622.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersen J, Dandri M, Mier W, Lutgehetmann M, Volz T, Weizsacker F, Haberkorn U, Fischer L, Pollok JM, Erbes B, Seitz S, Urban S. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol. 2008;26(3):335–341. doi: 10.1038/nbt1389. [DOI] [PubMed] [Google Scholar]
- 51.Kock J, Blum HE. Hypermutation of hepatitis B virus genomes by APOBEC3G, APOBEC3C and APOBEC3H. J Gen Virol. 2008;89(Pt 5):1184–1191. doi: 10.1099/vir.0.83507-0. [DOI] [PubMed] [Google Scholar]
- 52.Rosler C, Kock J, Kann M, Malim MH, Blum HE, Baumert TF, Weizsacker F. APOBEC-mediated interference with hepadnavirus production. Hepatology. 2005;42(2):301–309. doi: 10.1002/hep.20801. [DOI] [PubMed] [Google Scholar]
- 53.Vartanian JP, Henry M, Marchio A, Suspene R, Aynaud MM, Guetard D, Cervantes-Gonzalez M, Battiston C, Mazzaferro V, Pineau P, Dejean A, Wain-Hobson S. Massive APOBEC3 editing of hepatitis B viral DNA in cirrhosis. PLoS Pathog. 2010;6(5):e1000928. doi: 10.1371/journal.ppat.1000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turelli P, Mangeat B, Jost S, Vianin S, Trono D. Inhibition of hepatitis B virus replication by APOBEC3G. Science. 2004;303(5665):1829. doi: 10.1126/science.1092066. [DOI] [PubMed] [Google Scholar]
- 55.Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, Hosono N, Kubo M, Tsunoda T, Kamatani N, Kumada H, Puseenam A, Sura T, Daigo Y, Chayama K, Chantratita W, Nakamura Y, Matsuda K. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet. 2009;41(5):591–595. doi: 10.1038/ng.348. [DOI] [PubMed] [Google Scholar]
- 56.Lau GK, Ye D. Novel insights into the association between HLA-DP variants and persistent hepatitis B virus infection: a genome-wide association study. Gastroenterology. 2010;138(1):394–396. doi: 10.1053/j.gastro.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 57.Pollicino T, Belloni L, Raffa G, Pediconi N, Squadrito G, Raimondo G, Levrero M. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology. 2006;130(3):823–837. doi: 10.1053/j.gastro.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Zimmerman KA, Fischer KP, Joyce MA, Tyrrell DL. Zinc finger proteins designed to specifically target duck hepatitis B virus covalently closed circular DNA inhibit viral transcription in tissue culture. J Virol. 2008;82(16):8013–8021. doi: 10.1128/JVI.00366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Durantel D, Alotte C, Zoulim F. Glucosidase inhibitors as antiviral agents for hepatitis B and C. Curr Opin Investig Drugs. 2007;8(2):125–129. [PubMed] [Google Scholar]
- 60.Mehta A, Carrouee S, Conyers B, Jordan R, Butters T, Dwek RA, Block TM. Inhibition of hepatitis B virus DNA replication by imino sugars without the inhibition of the DNA polymerase: therapeutic implications. Hepatology. 2001;33(6):1488–1495. doi: 10.1053/jhep.2001.25103. [DOI] [PubMed] [Google Scholar]
- 61.Stray SJ, Bourne CR, Punna S, Lewis WG, Finn MG, Zlotnick A. A heteroaryldihydropyrimidine activates and can misdirect hepatitis B virus capsid assembly. Proc Natl Acad Sci USA. 2005;102(23):8138–8143. doi: 10.1073/pnas.0409732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bourne C, Lee S, Venkataiah B, Lee A, Korba B, Finn MG, Zlotnick A. Small-molecule effectors of hepatitis B virus capsid assembly give insight into virus life cycle. J Virol. 2008;82(20):10262–10270. doi: 10.1128/JVI.01360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu X, Zhao G, Zhou X, Xu X, Xia G, Zheng Z, Wang L, Yang X, Li S. 2,4-Diaryl-4,6,7,8-tetrahydroquinazolin-5(1H)-one derivatives as anti-HBV agents targeting at capsid assembly. Bioorg Med Chem Lett. 2010;20(1):299–301. doi: 10.1016/j.bmcl.2009.10.119. [DOI] [PubMed] [Google Scholar]
- 64.Wu GY, Zheng XJ, Yin CC, Jiang D, Zhu L, Liu Y, Wei L, Wang Y, Chen HS. Inhibition of hepatitis B virus replication by Bay 41–4109 and its association with nucleocapsid disassembly. J Chemother. 2008;20(4):458–467. doi: 10.1179/joc.2008.20.4.458. [DOI] [PubMed] [Google Scholar]
- 65.Stray SJ, Zlotnick A. BAY 41–4109 has multiple effects on hepatitis B virus capsid assembly. J Mol Recognit. 2006;19(6):542–548. doi: 10.1002/jmr.801. [DOI] [PubMed] [Google Scholar]
- 66.Feld JJ, Colledge D, Sozzi V, Edwards R, Littlejohn M, Locarnini SA. The phenylpropenamide derivative AT-130 blocks HBV replication at the level of viral RNA packaging. Antiviral Res. 2007;76(2):168–177. doi: 10.1016/j.antiviral.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 67.Delaney WE, Edwards R, Colledge D, Shaw T, Furman P, Painter G, Locarnini S. Phenylpropenamide derivatives AT-61 and AT-130 inhibit replication of wild-type and lamivudine-resistant strains of hepatitis B virus in vitro. Antimicrob Agents Chemother. 2002;46(9):3057–3060. doi: 10.1128/AAC.46.9.3057-3060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tseng YP, Kuo YH, Hu CP, Jeng KS, Janmanchi D, Lin CH, Chou CK, Yeh SF. The role of helioxanthin in inhibiting human hepatitis B viral replication and gene expression by interfering with the host transcriptional machinery of viral promoters. Antiviral Res. 2008;77(3):206–214. doi: 10.1016/j.antiviral.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Ying C, Li Y, Leung CH, Robek MD, Cheng YC. Unique antiviral mechanism discovered in anti-hepatitis B virus research with a natural product analogue. Proc Natl Acad Sci USA. 2007;104(20):8526–8531. doi: 10.1073/pnas.0609883104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson VR, Curran MP. Nitazoxanide: a review of its use in the treatment of gastrointestinal infections. Drugs. 2007;67(13):1947–1967. doi: 10.2165/00003495-200767130-00015. [DOI] [PubMed] [Google Scholar]
- 71.Keeffe EB, Rossignol JF. Treatment of chronic viral hepatitis with nitazoxanide and second generation thiazolides. World J Gastroenterol. 2009;15(15):1805–1808. doi: 10.3748/wjg.15.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elazar M, Liu M, McKenna SA, Liu P, Gehrig EA, Puglisi JD, Rossignol JF, Glenn JS. The anti-hepatitis C agent nitazoxanide induces phosphorylation of eukaryotic initiation factor 2alpha via protein kinase activated by double-stranded RNA activation. Gastroenterology. 2009;137(5):1827–1835. doi: 10.1053/j.gastro.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 73.Korba BE, Montero AB, Farrar K, Gaye K, Mukerjee S, Ayers MS, Rossignol JF. Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antiviral Res. 2008;77(1):56–63. doi: 10.1016/j.antiviral.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 74.Rossignol JF, Keeffe EB. Thiazolides: a new class of drugs for the treatment of chronic hepatitis B and C. Future Microbiol. 2008;3:539–545. doi: 10.2217/17460913.3.5.539. [DOI] [PubMed] [Google Scholar]
- 75.Zundorf I, Dingermann T. siRNAs—your friend and helper? Pharm Unserer Zeit. 2009;38(1):9–11. doi: 10.1002/pauz.200890127. [DOI] [PubMed] [Google Scholar]
- 76.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun D, Rosler C, Kidd-Ljunggren K, Nassal M. Quantitative assessment of the antiviral potencies of 21 shRNA vectors targeting conserved, including structured, hepatitis B virus sites. J Hepatol. 2010;52(6):817–826. doi: 10.1016/j.jhep.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 78.Locarnini S. Therapies for hepatitis B: where to from here? Gastroenterology. 2005;128(3):789–792. doi: 10.1053/j.gastro.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 79.Ilan Y, Nagler A, Adler R, Tur-Kaspa R, Slavin S, Shouval D. Ablation of persistent hepatitis B by bone marrow transplantation from a hepatitis B-immune donor. Gastroenterology. 1993;104(6):1818–1821. doi: 10.1016/0016-5085(93)90664-x. [DOI] [PubMed] [Google Scholar]
- 80.Lau GK, Lok AS, Liang RH, Lai CL, Chiu EK, Lau YL, Lam SK. Clearance of hepatitis B surface antigen after bone marrow transplantation: role of adoptive immunity transfer. Hepatology. 1997;25(6):1497–1501. doi: 10.1002/hep.510250631. [DOI] [PubMed] [Google Scholar]
- 81.Loggi E, Bihl F, Chisholm JV, 3rd, Biselli M, Bontadini A, Vitale G, Ercolani G, Grazi GL, Pinna AD, Bernardi M, Brander C, Andreone P. Anti-HBs re-seroconversion after liver transplantation in a patient with past HBV infection receiving a HBsAg positive graft. J Hepatol. 2009;50(3):625–630. doi: 10.1016/j.jhep.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 82.Bertoletti A, Gehring A. Therapeutic vaccination and novel strategies to treat chronic infection. Expert Rev Gastroenterol Hepatol. 2009;3(5):561–569. doi: 10.1586/egh.09.48. [DOI] [PubMed] [Google Scholar]
- 83.Ciancio A, Rizzetto M. Thymalfasin in the treatment of hepatitis B and C. Ann NY Acad Sci. 2010;1194:141–146. doi: 10.1111/j.1749-6632.2010.05487.x. [DOI] [PubMed] [Google Scholar]
- 84.Zhang YY, Chen EQ, Yang J, Duan YR, Tang H. Treatment with lamivudine versus lamivudine and thymosin alpha-1 for e antigen-positive chronic hepatitis B patients: a meta-analysis. Virol J. 2009;6:63. doi: 10.1186/1743-422X-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pol S, Nalpas B, Driss F, Michel ML, Tiollais P, Denis J, Brecho C. Efficacy and limitations of a specific immunotherapy in chronic hepatitis B. J Hepatol. 2001;34(6):917–921. doi: 10.1016/S0168-8278(01)00028-9. [DOI] [PubMed] [Google Scholar]
- 86.Vandepapeliere P, Lau GK, Leroux-Roels G, Horsmans Y, Gane E, Tawandee T, Merican MI, Win KM, Trepo C, Cooksley G, Wettendorff M, Ferrari C. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: a randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine. 2007;25(51):8585–8597. doi: 10.1016/j.vaccine.2007.09.072. [DOI] [PubMed] [Google Scholar]
- 87.Rahman F, Dahmen A, Herzog-Hauff S, Bocher WO, Galle PR, Lohr HF. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology. 2000;31(2):521–527. doi: 10.1002/hep.510310237. [DOI] [PubMed] [Google Scholar]
- 88.Jung MC, Gruner N, Zachoval R, Schraut W, Gerlach T, Diepolder H, Schirren CA, Page M, Bailey J, Birtles E, Whitehead E, Trojan J, Zeuzem S, Pape GR. Immunological monitoring during therapeutic vaccination as a prerequisite for the design of new effective therapies: Induction of a vaccine-specific CD4+ T-cell proliferative response in chronic hepatitis B carriers. Vaccine. 2002;20(29–30):3598–3612. doi: 10.1016/S0264-410X(02)00309-2. [DOI] [PubMed] [Google Scholar]
- 89.Heathcote J, McHutchison J, Lee S, Tong M, Benner K, Minuk G, Wright T, Fikes J, Livingston B, Sette A, Chestnut R. A pilot study of the CY-1899 T-cell vaccine in subjects chronically infected with hepatitis B virus. The CY1899 T Cell Vaccine Study Group. Hepatology. 1999;30(2):531–536. doi: 10.1002/hep.510300208. [DOI] [PubMed] [Google Scholar]
- 90.Vitiello A, Ishioka G, Grey HM, Rose R, Farness P, LaFond R, Yuan L, Chisari FV, Furze J, Bartholomeuz R, et al. Development of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. I. Induction of a primary cytotoxic T lymphocyte response in humans. J Clin Invest. 1995;95(1):341–349. doi: 10.1172/JCI117662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Akbar SM, Murakami H, Horiike N, Onji M. Dendritic cell-based therapies in the bench and the bedsides. Curr Drug Targets Inflamm Allergy. 2004;3(3):305–310. doi: 10.2174/1568010043343787. [DOI] [PubMed] [Google Scholar]
- 92.Xu DZ, Zhao K, Guo LM, Li LJ, Xie Q, Ren H, Zhang JM, Xu M, Wang HF, Huang WX, Bai XF, Niu JQ, Liu P, Chen XY, Shen XL, Yuan ZH, Wang XY, Wen YM. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PLoS One. 2008;3(7):e2565. doi: 10.1371/journal.pone.0002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yao X, Zheng B, Zhou J, Xu DZ, Zhao K, Sun SH, Yuan ZH, Wen YM. Therapeutic effect of hepatitis B surface antigen-antibody complex is associated with cytolytic and non-cytolytic immune responses in hepatitis B patients. Vaccine. 2007;25(10):1771–1779. doi: 10.1016/j.vaccine.2006.11.019. [DOI] [PubMed] [Google Scholar]