Abstract
Purpose
We and others have reported that adding adefovir dipivoxil (adefovir) to lamivudine results in virological and biochemical improvement in cases of lamivudine resistance. The current study assessed the efficacy and safety of combined therapy after 104 weeks of combined treatment and analyzed the frequency of persistent lamivudine resistant HBV.
Methods
A total of 78 patients with compensated CHB (Group A) were maintained on either adefovir 10 mg daily (n = 38) or placebo (n = 40) while continuing lamivudine. An additional 38 patients with decompensated cirrhosis or post liver transplantation (Group B) received lamivudine plus adefovir. The primary endpoint was HBV DNA response at year 2.
Results
At week 104 of therapy, a significantly greater proportion of patients in Group A on combination therapy (76%) had a decline in serum HBV DNA to ≤105 copies or >2 log10 reduction from baseline compared to those receiving lamivudine alone (13%; p < 0.001). Fifty-two percent of Group A patients on combination treatment continued to have the M204V/I HBV mutation compared to 92% receiving lamivudine alone (p = 0.0013). Virologic response occurred less frequently in patients expressing persistent lamivudine resistant HBV. In Group B, 87% of patients had HBV DNA response at week 104 (median change from baseline of −5.84 log10 copies/mL).
Conclusions
The combination of lamivudine and adefovir for 2 years generally proved effective in lamivudine-resistant cases, but there was a persistently high rate of detection of lamivudine resistant mutants and impaired virologic response in compensated patients.
Keywords: Chronic hepatitis B, Resistance, Lamivudine, Adefovir dipivoxil, Combination therapy
Background
Hepatitis B virus (HBV) infection can induce significant liver disease including fulminant hepatitis, severe chronic liver disease, cirrhosis, and primary hepatocellular carcinoma. With approximately 400 million people worldwide estimated to be chronically infected, the disease remains a significant global health problem [1]. The safety and efficacy of lamivudine in the treatment of chronic hepatitis B (CHB) is well established in a variety of clinical settings [2–6]. However, long-term lamivudine monotherapy is associated with the emergence of resistance mutations. Despite the substantially higher rate of drug resistance associated with lamivudine as compared with newer nucleos(t)ide analogs, such as entecavir or tenofovir, it is still frequently used as first line therapy in Asia and other parts of the world where the newer agents are less available. Adefovir dipivoxil (adefovir) has often been added to ongoing lamivudine therapy when resistance to lamivudine occurs based upon studies demonstrating the clinical efficacy of this combination [7–10].
The clinical course of patients with lamivudine-resistant HBV is variable. In vitro studies show that the rtM204V/I mutation decreases replication fitness of HBV, but compensatory mutations, such as the rtL180M selected during continued treatment can restore replication fitness [11, 12], and this often results in virologic and biochemical breakthrough. In patients with decompensated cirrhosis and those with recurrent hepatitis B post-liver transplantation, the development of lamivudine resistance is more likely to lead to clinically significant liver disease [13].
Earlier uncontrolled studies using adefovir as an add-on to ongoing lamivudine therapy or as monotherapy have reported virological and biochemical improvements in patients with lamivudine resistance [7, 8, 14, 15]. These findings were confirmed by a randomized, placebo-controlled study to assess the benefits of adefovir added to ongoing lamivudine therapy in CHB patients with compensated or decompensated liver disease who had become resistant to lamivudine [9]. This study showed that combination treatment was well tolerated and associated with significant virological and biochemical improvement after 52 weeks of treatment compared to lamivudine monotherapy. We now report on an extension to the initial study in which treatment was continued in these patients for an additional 52 weeks to compare the efficacy and safety of 2 years of combination therapy with that of lamivudine monotherapy.
Materials and methods
Entry criteria
Eligible patients had previously completed the initial 52-week study where they had been stratified at baseline according to disease severity. Group A patients had HBeAg positive CHB with compensated liver disease, and Group B patients had CHB-associated decompensated disease or recurrent disease after liver transplantation. At screening in the initial study, Group A patients were required to be HBeAg positive while Group B patients could be either HBeAg positive or negative. Patients were required to have serum HBV DNA levels ≥106 copies/ml (Cobas Amplicor HBV Monitor assay) at screening as well as serum ALT levels >1.3 times the ULN (43 IU/L) on at least two occasions in the previous 6 months and at screening. Patients were also confirmed to have genotypic resistance to lamivudine using a restriction fragment length polymorphism (RFLP) assay as described below. Additional exclusion criteria were as previously described [9].
Study design
In the initial study, Group A patients were randomized 1:1 to either adefovir 10 mg once daily or matching placebo and Group B patients were assigned open-label adefovir 10 mg daily. Patients in both the groups continued on open-label lamivudine 100 mg once daily. In this follow-on study, patients continued the same randomized treatment for a further 52 weeks. Weeks 48 and 52 of the initial study were used as the screening and baseline timepoints for the current study and week 104 was study completion.
For Group A patients, use of open-label combination therapy was permitted if disease progression occurred or if there was other evidence of probable treatment failure. The pre-defined criteria for disease progression included an increase in Child–Pugh–Turcotte (CPT) score of ≥2 points, spontaneous bacterial peritonitis, bleeding varices, or hepatocellular carcinoma. In addition, the presence of persistently elevated serum ALT (>2 times baseline and >300 IU/L for ≥4 months) was managed as disease progression. In any patient who developed renal insufficiency (determined by calculated creatinine clearance), withdrawal from study drug or a dose reduction of lamivudine and adefovir was required. In addition, specific dose modification criteria were provided for adefovir in the event of renal laboratory abnormalities (i.e., serum creatinine elevation and/or low serum phosphorus).
Patients were seen at weeks 48 (Screening) and 52 (Baseline) and then monthly during the second year of treatment. Laboratory evaluations and adverse events were assessed at each visit. Sera were analyzed for ALT, HBV DNA, and HBeAg/anti-HBe at every visit. HBsAg/anti-HBs were measured at treatment weeks 48, 52, 76, 100, and 104. CPT scores were assessed at each visit for patients in Group B. Centralized laboratories (Covance Central Laboratory Services, Indianapolis, IN, USA and Geneva, Switzerland) evaluated complete blood counts, routine serum chemistries, and HBV replication markers. Serum HBeAg/anti-HBe and HBsAg/anti-HBs were assessed by commercially available enzyme immunoassays (Abbott Laboratories, Abbott Park, IL, USA and Diasorin Inc., Stillwater, MN, USA). HBV DNA testing was performed using the Cobas Amplicor HBV monitor polymerase chain reaction (PCR) assay (Roche Molecular Diagnostics, Branchburg, NJ, USA) with a lower limit of detection of 200 copies/mL. The presence of the rtM204V/I lamivudine resistance mutation was assessed at weeks 52 and 104 using a RFLP assay (GlaxoSmithKline Clinical Virology Laboratory and Quest Diagnostics Nichols Institute, San Juan Capistrano, CA, USA). This assay has been shown to be superior to DNA sequencing in detecting HBV mutations that confer resistance to lamivudine in the presence of background wild-type virus, and is sensitive to a lower limit of 1,000 copies of HBV DNA/mL [16]. Testing for the N236T and A181V/T adefovir resistance mutations was performed at week 100 using Sanger DNA sequencing (Quest Diagnostics Nichols Institute) which detected different sequences comprising approximately ≥20% of the viral population.
The study was approved by the local ethics committees of the respective institutions, and patients gave written informed consent before screening. The study was conducted in accordance with the principles of the Declaration of Helsinki (1996).
Endpoints
The primary endpoint was HBV DNA response, defined as the proportion of patients with either HBV DNA level ≤105 copies/mL or a ≥2 log10 reduction from baseline HBV DNA level at weeks 100 and 104 in patients with >105 copies/mL at baseline (week 0 of initial study). Other endpoints at weeks 100 and 104 included ALT normalization, HBeAg loss and seroconversion, undetectable HBV DNA, HBV DNA levels <104 copies/mL, change from baseline in HBV DNA, proportion of patients with disease progression, and proportion with the M204V/I resistance mutation.
Statistical analysis
The intention-to-treat (ITT) population and the as-treated (AT) population were used for the efficacy and safety analyses, respectively. The ITT population included patients with confirmed CHB randomized to treatment, regardless of whether the study drug was taken or the patient completed the study. The AT population included patients who received at least one dose of study drug. Analyses were based on the number of patients that consented to enroll in the second year of the study. Patients with missing data were considered a failure for efficacy analyses at that time point. Patients who received open-label therapy were considered a failure from that point forward for all efficacy analyses, regardless of the original randomized treatment arm. The primary analysis was a treatment comparison of HBV DNA response at weeks 100 and 104. The proportion with ALT normalization (weeks 100 and 104), HBeAg loss/seroconversion (week 104), and M204V/I mutation (week 104) were also analyzed. Treatment comparisons based on the chi-square test were provided for descriptive purposes. The median change from baseline (of the initial study) to week 104 were also provided for HBV DNA and ALT. Descriptive treatment comparisons based on the Wilcoxon Rank Sum test were provided. Safety analyses included study withdrawals, adverse events, deaths, and laboratory abnormalities. The same analyses were conducted for the Group B patients and reported separately by liver transplant status at entry into the initial study.
Results
Study population
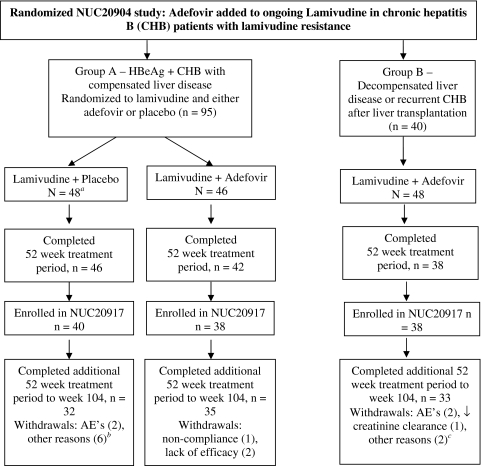
Figure 1 depicts patient disposition from the start of the initial study [9]. Of the 126 patients completing the initial study (NUC20904), 116 patients (91%) entered the follow-on study (NUC20917). Table 1 summarizes baseline characteristics (week 0, initial study) for patients entering the follow-on study. In Group A, 78 patients continued either previously randomized lamivudine and placebo (n = 40) or lamivudine and adefovir (n = 38). Within Group A, the characteristics of the two treatment groups were comparable with regard to median baseline HBV DNA and ALT levels. Of the patients who received lamivudine and placebo, 80% (32/40) completed the additional 52 week treatment period to week 104. One patient withdrew from treatment after 339 days because of increasing thrombocytopenia and a second patient discontinued after 338 days due to elevated AST/ALT values. The remaining six patients withdrew from treatment for “other” reasons (Fig. 1). Of the patients who received adefovir and lamivudine, 92% (35/38) completed treatment. One patient withdrew because of noncompliance and two patients because of lack of efficacy.
Fig. 1.
Disposition of patients from randomized study (NUC20904) through follow-on study (NUC20917)
Table 1.
Baseline characteristics at time of initial study for 116 patients entering extended therapy protocol
| Group A | Group B Lamivudine + Adefovir | ||||
|---|---|---|---|---|---|
| Lamivudine + Placebo (n = 40) | Lamivudine + Adefovir (n = 38) | Liver Transplant (n = 13) | Non-Liver Transplant (n = 25) | Overall (n = 38) | |
| Median age (years), (range) | 42.5 (26, 68) | 42 (24, 67) | 55 (22, 72) | 53 (33, 73) | 53 (22, 73) |
| Duration of prior lamivudine (months), median (range) | 32.3 (4, 60) | 35.9 (10, 64) | 35.7 (9, 55) | 31.5 (1, 62) | 32.9 (1, 62) |
| Male, N (%) | 38 (95) | 37 (97) | 12 (92) | 21 (84) | 33 (87) |
| HBeAg positivea, N (%) | 34 (85) | 33 (87) | 8 (62) | 18 (72) | 26 (68) |
| HBeAb positivea, N (%) | 0 | 0 | 3 (23) | 4 (16) | 7 (18) |
| HBsAg positivea, N (%) | 40 (100) | 37 (97) | 13 (100) | 25 (100) | 38 (100)a |
| HBV DNA > 105 copies/mL | 38/40 (95) | 38/38 (100) | 13/13 (100) | 25/25 (100) | 38/38 (100) |
| Median HBV DNA log10 copies/mL (range) | 8.49 (4.2, 10.1) | 8.98 (6.7, 10.1) | 9.0 (7.2, 10.1) | 8.14 (5.4, 9.4) | 8.5 (5.4, 10.1) |
| ALT > 1.0 × ULN, N (%) | 40/40 (100) | 37/38 (97) | 13/13 (100) | 23/25 (92) | 36/38 (95) |
| Median ALT × ULN (range) | 2.78 (1.1, 40.1) | 2.19 (1.0, 18.9) | 1.72 (1.3, 11.7) | 2.02 (0.6, 16.6) | 1.87 (0.6, 16.6) |
aBaseline sera were not available for testing in all patients. All patients were HBsAg and HBeAg positive at screening as defined in the protocol
In Group B, 38 patients continued open-label adefovir and lamivudine in the second year. 25 patients had decompensated liver disease and 13 patients had recurrent CHB after liver transplantation. Of these 13 patients, 6 (46%) had a history of ascites, variceal hemorrhage, or hepatic encephalopathy after liver transplantation, and three (23%) had a CP score >8 on entering into the study. 87% (33/38) of patients completed the additional 52-week treatment period. Two patients withdrew from treatment due to disease complications (one after 355 days because of hepatocellular carcinoma and one after 262 days because of bleeding gastric varices which was fatal). One patient was withdrawn because of decrease in estimated creatinine clearance considered by the investigator to be unrelated to study drug and two patients were withdrawn for “other” reasons (Fig. 1). Thus, of the 116 patients entering the second year study, 100 (86%) continued their participation until week 104.
Biochemical and virologic responses in patients with compensated CHB (Group A)
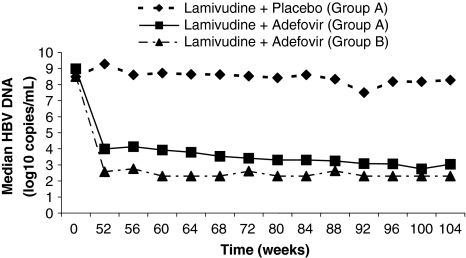
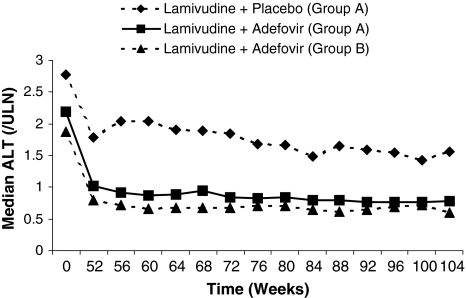
At weeks 100 and 104, significantly more patients in the combined therapy group had HBV DNA response with 76% of patients (29/38) responding compared to only 13% (5/38) receiving lamivudine monotherapy (p < 0.001) (Table 2). The median change from baseline in the initial study in the combination group at week 104 was significantly greater with −6.18 copies/mL (range −7.3, 0.6) compared to −0.11 copies/mL (range −4.6, 2.2) in patients on lamivudine monotherapy (Table 2). Treatment with lamivudine and adefovir to 104 weeks further reduced median HBV DNA levels, with an additional decline of 1.3 log10 copies/mL from week 52 to 104 (Fig. 2). In the combination group, the rate of ALT normalization (ALT ≤ 1.0 × ULN) increased over time and by the end of treatment was significantly greater, occurring in 49% of patients (18/37) compared to only 10% (4/40) on lamivudine monotherapy (p < 0.001) (Table 2; Fig. 3).
Table 2.
Responses in Group A patients
| Lamivudine + Placebo (n = 40) | Lamivudine + Adefovir (n = 38) | |
|---|---|---|
| HBV DNA responsea | ||
| At weeks 48 and 52, N (%) | 3/38 (8) | 36/38 (95) |
| At weeks 100 and 104, N (%) | 5/38 (13)b | 29/38 (76)b |
| HBV DNA -ve | ||
| At week 52, N (%) | 0/40 | 10/38 (26) |
| At week 104, N (%) | 1/40 (3) | 13/38 (34) |
| HBV DNA change from baseline | ||
| At week 52 Median (range) | 0.11 (−3.8, 5.4) | −4.88 (−7.3, −0.9) |
| At week 104 Median (range) | −0.11 (−4.6, 2.2) | −6.18 (−7.3, 0.6) |
| HBeAg loss at week 104, N (%) | 4/34 (12) | 6/33 (18) |
| HBeAg seroconversion at week 104, N (%) | 3/34 (9) | 4/33 (12) |
| HBsAg loss at week 104, N (%) | 0/40 | 2/37 (5) |
| HBsAg seroconversion at week 104, N (%) | 0/40 | 2/37 (5) |
| ALT response (≤1.0 × ULN) | ||
| At weeks 48 and 52, N (%) | 2/40 (5) | 15/37 (41) |
| At weeks 100 and 104, N (%) | 4/40 (10)b | 18/37 (49)b |
| ALT × ULN change from baseline | ||
| At week 52 Median (range) | −0.48 (−38.2, 17.6) | −1.10 (−18.4, 1.0) |
| At week 104 Median (range) | −1.05 (−39.1, 0.5) | −1.29 (−18.4, −0.1) |
HBV DNA –ve No HBV DNA was detected using the Cobas Amplicor HBV Monitor PCR assay with a lower limit of detection of 200 copies/mL
aTwo patients, in the lamivudine and placebo group did not have HBV DNA > 105 at baseline and were excluded from the virological efficacy analysis
bp ≤ 0.001
Fig. 2.
Median values for HBV DNA over 104 weeks of treatment in Group A (both treatment groups) and Group B
Fig. 3.
Median values for ALT, expressed as multiples of upper limit of normal, over 104 weeks of treatment in Group A (both treatment groups) and Group B
In patients receiving lamivudine monotherapy, disease progression at any time during the study was reported in 7/40 (18%) of patients with all of these patients fulfilling the criteria via persistently elevated serum ALT. For patients receiving combination therapy, only 1 out of 38 (3%) had disease progression (hepatocellular carcinoma). During the treatment period, 10/40 (25%) of patients treated with monotherapy and 1/38 (3%) of the patients receiving combination therapy were given open-label combination therapy.
At week 104, HBeAg seroconversion (HBeAg negative, anti-HBe positive) had occurred in 12% (4/33) of the patients on combination therapy compared with 9% (3/34) on lamivudine monotherapy. None of the patients receiving monotherapy had HBsAg loss or seroconversion during the study whereas 5% (2/37) of patients on the combination had seroconverted (Table 2).
In Group A, all patients had the M204V/I lamivudine resistance mutation detected at baseline of the initial study. At week 104, the number of patients with detectable M204V/I resistance mutations was significantly lower in the combination group, 52% (17/33) of patients when compared to 92% (22/24) in the lamivudine monotherapy group (p = 0.0013). At weeks 100 and 104, HBV DNA response among patients with mutant HBV was 3/21 (14%) and 15/17 (88%) for patients receiving lamivudine monotherapy and combination therapy, respectively (Table 3). Conversely, for patients without the mutation, 2/2 (100%) in the monotherapy group and 13/16 (81%) in the combination group demonstrated HBV DNA response. Among patients receiving the combination, only 2/17 (12%) patients with the M204V/I mutation were PCR negative at week 104 compared to 10/16 (63%) patients without the mutation. More patients without the mutation had ALT levels <ULN at week 104 [11/16 (69%) combination treatment and 0/2 monotherapy] compared to those with the mutation [7/16 (44%) combination and 4/22 (18%) monotherapy]. At week 100, no patients in Group A tested positive for the N236T or A181V/T adefovir resistance mutations.
Table 3.
Responses by M204V/I status in Group A patients
| M204V/I Lamivudine + Placebo (n = 22)a | Non-M204V/I Lamivudine + Placebo (n = 2)a | M204V/I Lamivudine + Adefovir (n = 17) | Non-M204V/I Lamivudine + Adefovir (n = 16) | |
|---|---|---|---|---|
| HBV DNA response | ||||
| At weeks 48 and 52, N (%) | 2/21 (5) | 1/2 (50) | 16/17 (94) | 15/16 (94) |
| At weeks 100 and 104, N (%) | 3/21 (14) | 2/2 (100) | 15/17 (88) | 13/16 (81) |
| HBV DNA –ve | ||||
| At week 52, N (%) | 0/22 | 0/2 | 2/17 (12) | 6/16 (38) |
| At week 104, N (%) | 1/22 (5) | 0/2 | 2/17 (12) | 10/16 (63) |
| Median HBV DNA at baseline (log10 copies/mL), (range) | 8.61 (4.8, 10.1) | 6.76 (6.5, 7.0) | 8.97 (6.7, 10.1) | 9.02 (6.7, 9.7) |
| Median HBV DNA change from baseline at week 104 (range) | 0.10 (−4.6, 2.2) | −3.36 (−3.7, −3.1) | −4.87 (−7.0, 0.6) | −6.41 (−7.3, 0.5) |
| HBeAg loss at week 104, N (%) | 3/19 (16) | 1/2 (50) | 1/15 (7) | 5/14 (36) |
| HBeAg seroconversion at week 104, N (%) | 3/19 (16) | 0/2 | 1/15 (7) | 3/14 (21) |
| ALT response (≤1.0 × ULN) | ||||
| At weeks 48 and 52, N (%) | 1/22 (5) | 0/2 | 6/16 (38) | 7/16 (44) |
| At weeks 100 and 104, N (%) | 4/22 (18) | 0/2 | 7/16 (44) | 11/16 (69) |
HBV DNA –ve No HBV DNA was detected using the Cobas Amplicor HBV Monitor PCR assay with a lower limit of detection of 200 copies/mL
aNine patients received rescue medication and are not presented in this analysis
Biochemical and virologic responses in patients with decompensated CHB or Post-OLT recurrence (Group B)
For the Group B patients, the median baseline HBV DNA level in the initial study was 8.5 log10 copies/mL (range 5.4, 10.1). After 100 and 104 weeks of treatment with adefovir and lamivudine, respectively, 87% of patients (33/38) had HBV DNA response (Table 4). HBV DNA from baseline to week 104 was substantially decreased, with a median change of −5.84 log10 copies/mL (range: 6.9, 3.1) (Fig. 2). Ninety-five percent of patients (36/38) had ALT levels >ULN at baseline, and of these, 64% (23/36) had ALT response. Similarly, there was a reduction from baseline in bilirubin (median change −0.32 mg/dL, range −4.0, 0.6) and an increase in albumin (median change +7 g/L, range −1, 22) over the 104-week treatment period. The median baseline Child–Pugh score was 6 (range: 5, 9) and the median change from baseline was –0.5 (range: −4, 0). Two Group B patients had a ≥2 point decrease in Child-Pugh score on at least one visit during the second year of treatment.
Table 4.
Responses in Group B patients
| Liver transplant prior to entry (n = 13) | Non-liver transplant prior to entry (n = 25) | Overall (n = 38) | |
|---|---|---|---|
| HBV DNA > 105 at baseline, N (%) | 13/13 (100) | 25/25 (100) | 38/38 (100) |
| HBV DNA response, N (%) | |||
| Week 52 | 12/13 (92) | 22/25 (88) | 34/38 (89) |
| Week 104 | 12/13 (92) | 21/25 (84) | 33/38 (87) |
| Median HBV DNA (log10 copies/mL), (range) | |||
| Baseline | 9.0 (7.2, 10.1) | 8.14 (5.4, 9.4) | 8.50 (5.4, 10.1) |
| Week 52 | 3.13 (2.3, 6.4) | 2.42 (2.3, 5.9) | 2.57 (2.3, 6.4) |
| Week 104 | 2.72 (2.3, 5.7) | 2.30 (2.3, 4.8) | 2.30 (2.3, 5.7) |
| HBeAg loss, N (%) | |||
| Week 52 | 2/8 (25) | 7/18 (39) | 9/26 (35) |
| Week 104 | 3/8 (38) | 7/18 (39) | 10/26 (38) |
| HBeAg seroconversion, N (%) | |||
| Week 52 | 0/8 | 1/18 (6) | 1/26 (4) |
| Week 104 | 2/8 (25) | 2/18 (11) | 4/26 (15) |
| Median ALT × ULN change from baseline (range) | |||
| Week 52 | −0.84 (−9.9, 1.0) | −1.35 (−15.9, 1.1) | −1.02 (−15.9, 1.1) |
| Week 104 | −1.01 (−5.3, 0.9) | −1.51 (−16.1, 0.6) | −1.16 (−16.1, 0.9) |
| Median baseline bilirubin (mg/dL), (range) | 0.64 (0.2, 14.2) | 1.35 (0.4, 4.8) | 1.05 (0.2, 14.2) |
| Median bilirubin change from baseline (mg/dL), (range) | |||
| Week 52 | 0 (−13.3, 0.3) | −0.29 (−4.3, 1.8) | −0.15 (−13.3, 1.8) |
| Week 104 | −0.06 (−0.6, 0.4) | −0.47 (−4.0, 0.6) | −0.32 (−4.0, 0.6) |
| Median baseline albumin (g/L), (range) | 38 (28, 43) | 30 (21, 43) | 32 (21, 43) |
| Median albumin change from baseline (g/L), (range) | |||
| Week 52 | 5 (−3, 17) | 7 (0, 24) | 6 (−3, 24) |
| Week 104 | 5 (−1, 10) | 10 (1, 22) | 7 (−1, 22) |
| Median baseline CPT score (range) | 5.0 (5, 6) | 7 (5, 9)a | 6.0 (5, 9)a |
| Median CPT change from baseline (range) | |||
| Week 52 | 0 (−1, 1) | −1 (−3, 2)a | −1 (−3, 2)a |
| Week 104 | 0 (−1, 0) | −1 (−4, 0)a | −0.5 (−4, 0)a |
All patients were treated with lamivudine and adefovir
aTwo patients transplanted during study have been excluded for this evaluation
Disease progression was recorded by the investigator in 11% of patients (4/38) receiving lamivudine and adefovir in Group B; all of these occurred in the patients who had not undergone liver transplantation at the time of inclusion in the initial study (n = 25) with all patients having an increase of ≥2 points in CPT score. Two of these patients also had a clinical event (variceal hemorrhage at week 2 of the follow-on study and hepatocellular carcinoma at week 104) that met disease progression criteria. An additional patient, not recorded as having had disease progression by the investigator, was diagnosed with hepatocellular carcinoma at week 12. Two patients underwent transplantation during treatment. None of these patients had an increase in CPT score of ≥2 points by week 104 of treatment.
Among those patients who were HBeAg positive pre-treatment, 15% (4/26) had HBeAg seroconverted by week 104. Two patients lost HBsAg at week 104, however, both had undergone liver transplantation and had received HBIg.
In Group B, 97% (37/38) of patients had the M204V/I mutation at baseline in the initial study, but only 23% (7/31) of patients had the mutation detected at week 104. At week 100, no patients in Group B tested positive for the N236T or A181V/T adefovir resistance mutations.
Safety
In Group A, the proportion of patients with adverse events during treatment was similar across treatment groups. Serious adverse events (SAEs) were reported in 2/22 (9%) of patients on monotherapy compared to 3/38 (8%) of patients on the combination. None of the serious events in either treatment group was fatal and none was attributable to treatment by the investigator. No patients who received lamivudine plus placebo had increases in serum creatinine confirmed at two consecutive study visits. Confirmed increases in serum creatinine >0.5 mg/dL above baseline were only observed in one (3%) Group A patient receiving lamivudine + adefovir. Following dose reduction of adefovir, the patient continued on therapy and serum creatinine levels stabilized. During treatment, ALT elevations according to predefined categories were only seen in the lamivudine monotherapy group (6/22, 27%), mainly due to ALT elevations ≥2× baseline and ≥3× baseline. One (1/22, 5%) subject in the monotherapy group had an ALT value that was both ≥2× the baseline value and >500 IU/L.
In Group B, 33/38 of patients (87%) treated with lamivudine + adefovir had least one adverse event during treatment and 11/38 (29%) reported SAEs including cholangitis, bacteremia, gastrointestinal bleeding, abdominal pain, chest pain, and prostate cancer. All but one of the serious events was non-fatal and all were deemed by the investigator to be unrelated to treatment. Four patients had a confirmed serum creatinine elevation >0.5 mg/dL above baseline. In three of these patients, serum creatinine remained stable despite continuation of combination therapy without dose reduction; the dose of both lamivudine and adefovir was reduced in the fourth patient who then continued in the study. Confirmed changes in serum phosphate <1.5 mg/dL below baseline were observed in one patient at week 64. The patient temporarily stopped adefovir therapy and following improvement in serum phosphate levels resumed therapy approximately 3 months later. However, this patient experienced a fatal SAE (variceal hemorrhage) approximately 4 months following resumption of adefovir. A second patient in Group B was diagnosed with hepatocellular carcinoma at week 12 and subsequently expired. Neither death was attributed to study treatment by the investigator. During treatment, ALT elevations occurred in three of the Group B patients (3/38, 8%), mainly due to ALT elevations ≥2× baseline and ≥3× baseline. One of these patients (1/22, 3%) had an ALT elevation ≥2× baseline in association with an elevation of bilirubin (bilirubin > 2× ULN and ≥2× baseline value).
Discussion
Nucleoside analogue therapy can achieve sustained suppression of HBV replication and remission of liver disease and has been shown to forestall disease progression as well as prevent long-term complications, such as hepatocellular carcinoma [6]. However, a concern with long-term antiviral treatment is the selection of antiviral-resistant mutations. This in turn has been shown to lead to reduced clinical benefit and may be associated with flares of the disease and even frank hepatic decompensation [17]. This has been particularly well described with lamivudine [18]. Thus, alternative treatment strategies, such as add-on therapies with another agent which lacks cross resistance is most often necessary in order to suppress viral replication and prevent further worsening of disease.
Adefovir was the first nucleoside analog to be used in cases of lamivudine resistance [7]. A number of studies have clearly demonstrated that switching to adefovir in both HBeAg positive and HBeAg negative patients with lamivudine-resistant HBV was associated with an earlier and higher risk of adefovir-resistance compared to adding adefovir to ongoing lamivudine [10, 19–21]. Thus, current guidelines now recommend that when adefovir is used to treat patients with lamivudine-resistant HBV, lamivudine should be continued indefinitely to decrease the risk of hepatitis flares during the transition period and to reduce the risk of subsequent adefovir resistance [22].
A randomized, placebo-controlled study previously demonstrated that the addition of adefovir to lamivudine in patients with either compensated or decompensated liver disease who had become refractory to lamivudine was well tolerated and associated with substantial virological (median change of −4.6 log10 copies/mL at week 52) and biochemical benefit after 52 weeks of treatment [9]. The current follow-on study offered extended treatment for a further 52 weeks to assess the longer-term safety and efficacy of lamivudine and adefovir combination therapy.
In the compensated patients (Group A) who received lamivudine and adefovir for a further 52 weeks, there was continued improvement over the second year in HBV DNA levels, with an additional decline in median HBV DNA levels of 1.38 log10 copies/mL from week 52 to 104 with the overall median change from pre-treatment baseline of −6.18 log10 copies/mL in the combination patients compared to −0.11 log10 copies/mL in patients receiving monotherapy. The rate of ALT normalization also continued to increase in the combination group so that by the end of the second year, one-half of the patients had normal ALT levels compared to only 10% of patients receiving lamivudine monotherapy. Although the rate of HBeAg seroconversion at week 104 was similar among patients receiving either the combination or the lamivudine monotherapy (12 vs. 9%), it is of interest that none of the patients receiving monotherapy had surface antigen seroconversion to anti-HBs as compared with 5% of patients receiving the combination. One must be cautious of overinterpreting this finding, however, since the number of treated patients was small, and spontaneous seroconversion could have accounted for this finding. Moreover, the patient population was lamivudine refractory to begin with which should have made them less likely to clear HBsAg. However, we feel that the second year efficacy data clearly indicates continued clinical benefit in the Group A patients receiving combination therapy.
The study also evaluated the virological suppression capability of extended treatment with adefovir added to ongoing lamivudine in patients with lamivudine resistance and decompensated liver disease (Group B). The results in this population also indicate that the additional 52 weeks of treatment resulted in a continued reduction in viral replication, with a median change from pre-treatment baseline in HBV DNA of –5.84 log10 copies/mL at week 104. The majority of the patients (64%) also achieved normal ALT levels by the end of treatment. In addition, in the majority of the non-transplanted patients, a one-point or greater improvement in CPT score was observed, significant improvements in bilirubin and albumin levels occurred and most patients appeared to clinically benefit from treatment.
Lamivudine and adefovir combination therapy, extended by a further 52 to 104 weeks, continued to be well tolerated. In Group B, four patients had a serum creatinine elevation >0.5 mg/dL above baseline; in three of these patients, serum creatinine remained stable following continuation of combination therapy without dose reduction. However, it is well accepted that patients with advanced liver disease or those in the liver transplant setting should be monitored closely and may require dose modification for changes in renal function.
As previously reported, after 1 year of treatment with combination therapy, the M204V/I mutation was still detectable in 62% and 57% of patients in Groups A and B, respectively [9]. In the second year, mutant HBV remained detectable in a smaller proportion of patients, being detectable in 52% and 23% of Group A and B patients, respectively, in those receiving combination therapy as compared with 92% receiving lamivudine alone. However, a considerable number of patients, particularly in Group A continued to harbor the M204V/I mutation after receiving 2 years of combination therapy suggesting that adefovir was not sufficiently potent to suppress the lamivudine-associated mutants in a subgroup of patients. This is likely to have been due to compensatory secondary mutations which improved the replication fitness of the M204V/I mutant [17]. Similar observations were recently observed in treatment naïve patients who were given a combination of lamivudine and adefovir and followed for 2 years [23].
The mechanism for this persistent mutant HBV is unclear, but the data are consistent with the in vitro observation that adefovir does not control viral replication of the M204I and L180M mutants as effectively as it does wild type HBV [24]. To validate this explanation would require a comparison of treatment response in patients having the dual substitutions at positions 180 and 204 to that observed in patients with the 204 mutation alone. Unfortunately, the number of patients with single point mutations (n = 4 in each treatment arm, data not shown) was too small in our patient population to allow confirmation of this hypothesis.
Although the virological and biochemical responses in the combination patients with the mutation were substantially better than patients receiving monotherapy, they were inferior to patients on combination therapy without the mutant. For example, only 12% of the Group A patients with the M204 V/I mutation treated with combination therapy were PCR negative at week 104 compared to 63% of patients without the mutation in this treatment group and ALT normalization was less (44 vs. 69%). An unexplained finding is the markedly lower detection rate of the M204V/I mutation in the Group B patients, first noted in the first year data and seen to a greater extent in the second year of treatment. The reasons for this finding remain unclear, but did not appear to be due to differences in compliance, as measured by patient-reported missed doses. Importantly, no adefovir resistance mutations (N236T and A181V/T) were detected in the first or second years of combination therapy in either patient group.
Due to the long lead-time in the use of lamivudine worldwide, there are currently a large number of patients with lamivudine resistance who are undergoing treatment with the combination of lamivudine and adefovir. The current study has shown that a surprising number of these patients still harbor detectable nucleotide substitutions associated with lamivudine resistance. These data have several potentially important implications. First, continuance of therapy may ultimately result in the development of viral breakthrough and clinical progression due to a relatively inability of adefovir to control replication of lamivudine resistant HBV. Second, the use of entecavir either in these cases used alone or in combination with adefovir may ultimately facilitate selection of HBV mutant virus that is resistant to entecavir [25]. Third, the continued presence of the substitutions at the 180 and 204 codons suggest that a more potent agent like tenofovir might be a better choice to be added to lamivudine than adefovir. Future studies need to be done to address this issue.
In summary, the current study assessed the clinical benefits of extending adefovir and lamivudine combination therapy from 52 to 104 weeks in CHB patients with lamivudine resistance. Patients receiving combination therapy in the second year continued to have progressive improvements in virological and biochemical responses over those observed during the first year of treatment and this finding was observed in patients with both compensated and decompensated liver disease. However, clinically compensated patients tended to have a high rate of persistent lamivudine resistant HBV and were less apt to reach a virologic response. Even given the high sensitivity of the molecular assay (RFLP) we used to detect these mutants, the data suggest that there may be an unacceptably high rate of virologic breakthroughs as mutant HBV replication fitness is further restored with continuation of combination therapy. The data also support the need for more studies of rescue therapy with lamivudine combined with more potent drugs such as tenofovir.
Acknowledgements
The authors gratefully acknowledge and thank both the NUC20917 investigators and patients who participated in this study. Study (Protocol NUC20917) supported by GlaxoSmithKline Research and Development, Research Triangle Park, NC, USA. Adefovir dipivoxil was provided for the study by Gilead Sciences, Foster City, CA, USA.
References
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepatol. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 2.Dienstag J, Schiff ER, Wright TL, Perrillo RP, Hann HWL, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M, Brown NA. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 3.Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J, Stephenson SL, Gray F. A one-year trial of lamivudine for chronic hepatitis B. New Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 4.Schalm SW, Heathcote J, Cianciara J, Farrell G, Sherman M, Willems B, Dhillon A, Moorat A, Barber J, Gray DF. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomized trial. Gut. 2000;46:562–568. doi: 10.1136/gut.46.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonas M, Kelley D, Mizerski J, Badia IB, Areias JA, Schwarz KB, Little NR, Greensmith MJ, Gardner SD, Bell MS, Sokal EM. Clinical trial of lamivudine in children with chronic hepatitis B. N Engl J Med. 2002;346:1706–1713. doi: 10.1056/NEJMoa012452. [DOI] [PubMed] [Google Scholar]
- 6.Liaw Y-F, Sung JJY, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, D Gray F, Sabbat, J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521–1531, 1587 [DOI] [PubMed]
- 7.Perrillo R, Schiff E, Yoshida E, Statler A, Hirsch K, Wright T, Gutfreund K, Lamy P, Murray A. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology. 2000;32:129–134. doi: 10.1053/jhep.2000.8626. [DOI] [PubMed] [Google Scholar]
- 8.Peters MG, Hann HW, Martin P, Heathcote J, Buggisch P, Rubin R, Bourliere M, Kowdley K, Trepo C, Gray DF, Sullivan M, Kleber K, Ebrahimi R, Xiong S, Brosgart CL. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:91–101. doi: 10.1053/j.gastro.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 9.Perrillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, Moorat A, Gardner S, Woessner M, Bourne E, Brosgart CL, Schiff E. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004;126:81–90. doi: 10.1053/j.gastro.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 10.Lampertico P, Viganò M, Manenti E, Iavarone M, Lunghi G, Colombo M. Adefovir rapidly suppresses hepatitis B in HBeAg-negative patients developing genotypic resistance to lamivudine. Hepatology. 2005;42:1414–1419. doi: 10.1002/hep.20939. [DOI] [PubMed] [Google Scholar]
- 11.Ono-Nita SK, Kato N, Shiratori Y, Masaki T, Lan KH, Carrilho FJ, Omata M. YMDD motif in hepatitis B virus DNA polymerase influences on replication and lamivudine resistance: a study by in vitro full-length viral DNA transfection. Hepatology. 1999;29:939–945. doi: 10.1002/hep.510290340. [DOI] [PubMed] [Google Scholar]
- 12.Pallier C, Castera L, Soulier A, Hézode C, Nordmann P, Dhumeaux D, Pawlotsky JM. Dynamics of hepatitis B virus resistance to lamivudine. J Virol. 2006;80:643–653. doi: 10.1128/JVI.80.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutimer D, Pillay D, Shields P, Cane P, Ratcliffe D, Martin B, Buchan S, Boxall L, O’Donnell K, Shaw J, Hubscher S, Elias E. Outcome of lamivudine resistant hepatitis B virus infection in the liver transplant recipient. Gut. 2000;46:107–113. doi: 10.1136/gut.46.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benhamou Y, Bochet M, Thibault V, Calvez M, Fievet H, Vig P, Gibbs CS, Brosgart C, Fry J, Namini H, Katlama C, Poynard T. Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivudine-resistant hepatitis B virus: an open label pilot study. Lancet. 2001;358:718–723. doi: 10.1016/S0140-6736(01)05840-8. [DOI] [PubMed] [Google Scholar]
- 15.Schiff ER, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, Tillmann HL, Samuel D, Zeuzem S, Lilly L, Rendina M, Villeneuve JP, Lama N, James C, Wulfsohn MS, Namini H, Westland C, Xiong S, Choy GS, Doren S, Fry J, Brosgart CL. Adefovir dipivoxil therapy for lamivudine-resistant hepatitis B in pre- and post-liver transplantation patients. Hepatology. 2003;38:1419–1427. doi: 10.1016/j.hep.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Allen MI, Gauthier J, DesLauriers M, Bourne EJ, Carrick KM, Baldanti F, Ross LL, Lutz MW, Condreay LD. Two sensitive PCR-based methods for detection of hepatitis B virus variants associated with reduced susceptibility to lamivudine. J Clin Microbiol. 1999;37:3338–3347. doi: 10.1128/jcm.37.10.3338-3347.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw T, Bartholomeusz A, Locarnini S. HBV drug resistance: mechanism, detection and interpretation. J Hepatol. 2006;44:593–606. doi: 10.1016/j.jhep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Lok ASF, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA, Gardner SD, Castiglia M. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714–1722. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 19.Fabien Z, Perrillo R. Hepatitis B: reflections on the current approach to antiviral therapy. J Hepatol. 2008;48:S2–S19. doi: 10.1016/j.jhep.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Snow A, Thibault V, Qi X, Zhu Y, Westland CE, Arterbrun S. Combination of adefovir dipivoxil (ADV) and lamivudine (LAM) prevented emergence of ADV resistance mutations in chronic hepatitis B (CHB) patients with LAM-Resistant HBV. Gastroenterology. 2005;128:M945. [Google Scholar]
- 21.Fung SK, Chae HB, Fontana RJ, Conjeevaram H, Marrero J, Oberhelman K, Hussain M, Lok AS. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006;44:283–290. doi: 10.1016/j.jhep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Lok ASF, McMahon BJ. AASLD practice guidelines, chronic hepatitis B. Hepatology. 2007;47:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 23.Sung JJY, Lai JY, Zeuzem S, Chow WC, Heathcote EJ, Perrillo RP, Brosgart CL, Woessner MA, Scott SA, Gray DF, Gardner SD. Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. J Hepatology. 2008;48:728–735. doi: 10.1016/j.jhep.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Xiong S, Flores C, Yang H, Toole JJ, Gibbs CS. Mutations in hepatitis B DNA polymerase associated with resistance to lamivudine do not confer resistance to adefovir in vitro. Hepatology. 1998;28:1669–1673. doi: 10.1002/hep.510280629. [DOI] [PubMed] [Google Scholar]
- 25.Tenney DJ, Levine SM, Rose RE, Walsh AW, Weinheimer SP, Discotto L, Plym M, Pokornowski K, Yu CF, Angus P, Ayres A, Bartholomeusz A, Sievert W, Thompson G, Warner N, Locarnini S, Colonno RJ. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to lamivudine. Antimicrob Agents Chemother. 2004;48:3498–3507. doi: 10.1128/AAC.48.9.3498-3507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]