Abstract
Abstract
Although the rigorous anatomical definition of the microcircuitry of the brain is essential for understanding its functions, the modulation of the physiological properties of neurons and synapses may confer an additional level of complexity. Here, I review two examples of neuromodulation within a specific microcircuit of the hippocampus, i.e. the local network of stratum lacunosum-moleculare. In particular, I will examine the actions of two different types of neuromodulators on the excitability and electrical coupling of two specific classes of cells. First, I will review the effects of noradrenaline on GABAergic networks. Particular emphasis will be placed on neurogliaform cells. Then, I will describe the chemokinergic modulation of spontaneous firing of Cajal–Retzius cells, mediated by the chemokine (C-X-C motif) ligand 12/stromal cell-derived factor-1 α (CXCL12/SDF-1) via the CXC chemokine receptor 4 (CXCR4). The complexities created by these diverse types of modulations for network activity, together with their potential implications for stratum lacunosum-moleculare processing of information in vivo, will be also presented and briefly discussed.
Gianmaria Maccaferri is an Associate Professor at the Department of Physiology, Northwestern University. He earned both his MD and his PhD at the University of Milan, Italy. Since his postdoctoral training days with Chris McBain, Peter Somogyi and Ray Dingledine, the main focus of his research and interests has been the study of the regulation of specific hippocampal microcircuits and their potential involvement in epileptiform activity. His approach is based on a combination of electrophysiological techniques, such as paired recordings from synaptically connected neurons, and anatomical methods.
 |
Synaptic input from the temporoammonic pathway is integrated by the stratum lacunosum-moleculare network
The CA1 hippocampus receives two main monosynaptic glutamatergic excitatory inputs (Ramon y Cajal, 1893). The first originates from the CA3 hippocampal subfield and reaches the dendrites of pyramidal cells in stratum oriens and stratum radiatum via the Schaffer collateral. The second is generated by neurons in layer III of the entorhinal cortex and targets the distal dendrites of pyramidal cells in stratum lacunosum-moleculare (temporoammonic pathway, see TA in Fig. 1). The fact that these incoming projections are spatially segregated has allowed the experimental study of their functions in vivo. Selective lesions of the temporoammonic pathway in rodents have been associated with disrupted spatial tuning of pyramidal cell firing and with compromised long-term memory consolidation (Brun et al. 2002, 2008; Remondes & Schuman, 2004). Therefore, excitatory input from the entorhinal cortex has been proposed to be important for hippocampal spatial information processing, which allows specific pyramidal neurons (called ‘place cells’) to fire intensely only when an animal is positioned in a specific location in an environment (called the ‘place field’, see O'Keefe & Nadel, 1978).
Figure 1. A simplified and ‘temporoammonic-centred’ view of the hippocampal circuitry and its modulation by noradrenaline (NA) and stromal cell-derived factor 1 α(SDF-1).
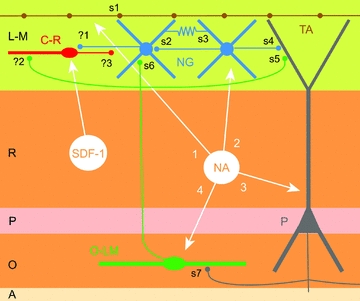
Key neuronal elements are shown: C-R, Cajal–Retzius cells (red); NG, neurogliaform interneurones (blue); TA, temporoammonic pathway (brown); P, pyramidal cell (grey); O-LM, oriens-lacunosum-moleculare interneuron (green). The different layers of the hippocampus are indicated at the left margin (L-M, stratum lacunosum-moleculare; R, stratum radiatum; P, stratum pyramidale; O, stratum oriens; A, alveus). Key synaptic (chemical and electrical) connections are shown as follows: s1, TA to NG (glutamatergic); s2, NG to NG (GABAergic); s3, NG to NG (gap junction); s4, NG to P (GABAergic); s5, O-LM to P (GABAergic); s6, OLM to NG (GABAergic); s7, P to O-LM (glutamatergic). Putative synaptic connections awaiting direct experimental confirmation are indicated by question marks: ?1, NG to C-R (GABAergic); ?2, O-LM to C-R (GABAergic); ?3, C-R to unidentified targets (unknown neurotransmitter). Notice that stromal cell-derived factor 1 α appears to modulate only Cajal–Retzius cells (white arrow), whereas at least four mechanisms are used by noradrenaline: 1, direct modulation of temporoammonic transmission; 2, modulation of intrinsic excitability and electrical coupling of interneurons; 3, modulation of pyramidal cell intrinsic excitability; and 4, modulation of firing in O-LM cells. Note that no. 4 may produce network effects in contrast to the direct modulation of interneurones in stratum lacunosum-moleculare.
Although the temporammonic pathway is glutamatergic (Colbert & Levy, 1992), its stimulation has often revealed inhibitory responses in pyramidal neurons, and its true nature has long been debated (Soltesz & Jones, 1995). This result is due to the fact that stratum lacunosum-moleculare hosts a strong feed-forward inhibitory network composed of various types of GABAergic interneurones (Freund & Buzsaki, 1996), which can be activated by the temporoammonic pathway (Dvorak-Carbone & Schuman, 1999; see s1 in Fig. 1). In addition, although the nature of their major neurotransmitter is still unclear, hippocampal Cajal–Retzius cells (see C-R in Fig. 1) are also present in large numbers in the stratum lacunosum-moleculare of young animals and persist, albeit strongly reduced in number, into adulthood (Alcántara et al. 1998; Supèr et al. 1998; Marchionni et al. 2010).
As previously mentioned, selective lesions of the temporoammonic pathway produce a more diffuse firing of place cells (Brun et al. 2008). This finding suggests the loss of GABAergic inhibition, and therefore that temporoammonic-dependent recruitment of interneurones occurs in vivo during critical physiological processes. Activation of local inhibitory networks may contribute to the selection of the cell assemblies involved in hippocampal spatial representations and of the timing of dendritic firing relative to population oscillatory activity (Kamondi et al. 1998). Furthermore, the recent observation of positively and negatively correlated place cell–interneurone pairs, independently of the presence of a putative monosynaptic excitatory connection within the pair, supports the general hypothesis that the location-specific firing of place cells is shaped, at least in part, by the activity of GABAergic networks (Hangya et al. 2010).
Thus, the temporoammonic input to the CA1 area is integrated by the activity of a local network composed of many different cell types. As a consequence, neuromodulation of the intrinsic properties and/or synaptic connections between the individual components would be predicted to play a role in the fine tuning of action potential generation in place cells. The purpose of this short article is to review two specific mechanisms of G-protein-coupled receptor-dependent neuromodulation of stratum lacunosum-moleculare microcircuits. Excellent reviews and/or research papers on the direct regulation of temporammonic input onto the distal dendrites of pyramidal neurons by various neurotransmitters are already available (see, for example: Otmakhova & Lisman, 1999, 2000; Otmakhova et al. 2005; Ito & Schuman, 2007, 2008); therefore, I will focus on the modulation of the non-pyramidal cell elements of the local network, i.e. GABAergic interneurones and Cajal–Retzius cells. Although cholinergic interneurones may also be present in this circuit, very little is known about their physiological properties, and therefore only GABAergic interneurones will be considered (Freund & Buzsaki, 1996).
Noradrenaline regulates GABAergic networks of stratum lacunosum-moleculare at multiple sites
Besides pyramidal cell dendrites, stratum lacunosum-moleculare contains a heterogeneous population of interneurons. Because of their high degree of synaptic divergence, interneurones are often the preferential synaptic targets of neuromodulation originating from subcortical nuclei (Freund & Buzsaki, 1996; Varga et al. 2009). In the case of noradrenaline, stratum lacunosum-moleculare receives, in the CA1 subfield, the highest density of noradrenergic fibres originating from the locus coeruleus (Oleskevich et al. 1989), which is the exclusive source of this neurotransmitter for the entire hippocampus.
Several cellular subtypes of stratum lacunosum- moleculare interneurones have been described: neurogliaform cells (see NG in Fig. 1 and Price et al. 2005; Zsiros & Maccaferri, 2005; and Capogna in this issue of The Journal of Physiology), perforant path and Schaffer associated interneurons, stellate cells, basket cells (Vida et al. 1998) and others (Lacaille & Schwartzkroin, 1988; Khazipov et al. 1995; Freund & Buzsaki, 1996). These interneurones may be both synaptically connected (see s2 in Fig. 1) and electrically coupled (see s3 in Fig. 1 and Price et al. 2005). Depending on the level of similarity of their excitable membrane properties, coupling may form either homologous or heterologous networks, the latter being critically dependent on neurogliaform cells (Zsiros & Maccaferri, 2005).
Traditionally, the effects of noradrenaline in the brain may be mediated by α1-, α2-, or β-adrenergic receptors, which are usually associated with the G protein isotypes Gq, Gi and Gs, respectively (Raymond, 1995). Activation of adrenergic receptors has been shown to increase the excitability of interneurones of stratum lacunosum-moleculare and of other CA1 layers (Bergles et al. 1996; Parra et al. 1998). This is reflected by the increased number of spontaneous GABAergic inhibitory postsynaptic currents/potentials (IPSP/Cs) recorded from pyramidal cells (Madison & Nicoll, 1988; Bergles et al. 1996). However, it is important to highlight that kinetically slow postsynaptic events are the most sensitive to noradrenergic modulation (Banks et al. 2002). These GABAergic events (GABAA,slow IPSCs, see Pearce, 1993) have been proposed to originate from neurogliaform cells of which stratum lacunosum-moleculare is particularly rich (see article by Capogna, this issue). The axonal arborization of these cells is dense and provides a local GABAergic input to the distal regions of pyramidal cell dendrites (see s4 in Fig. 1). Although the direct effect of noradrenaline on the membrane potential of identified hippocampal neurogliaform cells remains untested, depolarizations have been recorded in an analogous class of cell in layer II/III of the neocortex (Kawaguchi & Shindou, 1998). Furthermore, several additional types of interneurones in other hippocampal layers are also sensitive to noradrenaline, and evidence for noradrenaline-induced firing has been directly provided by recordings from interneurones (Bergles et al. 1996; Parra et al. 1998). Overall, depolarization was the most common response, although interneurones that failed to respond to the neurotransmitter were also observed; in a very small percentage of cases hyperpolarizing responses were recorded (Parra et al. 1998). Two main distinct mechanisms may account for the reported increase in interneurone excitability. The first one is the closure of a potassium conductance following activation of α1-adrenergic receptors (Bergles et al. 1996; Hillman et al. 2009) and the second is the β-adrenergic receptor-dependent shift towards more positive potentials of the activation curve of the hyperpolarization-activated current (Maccaferri & McBain, 1996). This last mechanism appears to be especially prominent in oriens lacunosum-moleculare (O-LM) interneurones (see O-LM, Fig. 1), which express somatostatin (Maccaferri et al. 2000) and whose soma is located in stratum oriens, but whose axon selectively targets stratum lacunosum-moleculare. Consistently, a very high proportion of somatostatin-expressing interneurones of stratum oriens are immunoreactive for the β1-subtype of adrenergic receptor (Cox et al. 2008). O-LM interneurones provide monosynaptic GABAergic input to both pyramidal cells (see s5 in Fig. 1, and Maccaferri et al. 2000) and other interneurons, including neurogliaform cells (see s6 in Fig. 1, and Elfant et al. 2008).
GABAA,slow-mediated inhibition evoked by direct stimulation of stratum lacunosum-moleculare not only impacts the distal dendrites of pyramidal neurons, but also reduces the activity of other GABAergic interneurones that presumably target pyramidal cells at more proximal locations (Banks et al. 2000). Therefore, the overall effects of noradrenergic modulation of the microcircuitry of stratum lacunosum-moleculare may be more complicated than it appears at first glance. At low levels, noradrenaline might increase firing in neurogliaform cells because of a direct, selective effect, which would result in increased GABAergic inhibition to the distal dendrites of principal cells.
Although the detailed reasons underlying the proposed special sensitivity of neurogliaform cells to noradrenaline remain unknown, it is interesting to note that, in contrast to what was found in other hippocampal layers, only a very small percentage of stratum lacunosum-moleculare interneurones are immunoreactive for either the β1 or β2 adrenergic receptor subtype, with most cells showing no expression (Cox et al. 2008). This may suggest that modulation of excitability in neurogliaform cells, of which stratum lacunosum-moleculare is particularly rich, may be predominantly mediated by α-adrenergic receptors, which have a higher affinity for noradrenaline than the β-subtypes (Ramos & Arnsten, 2007). In contrast, almost 100% of somatostain-expressing interneurones of stratum oriens, which presumably contain a high proportion of O-LM cells, expressed β1-type adrenergic receptors (Cox et al. 2008). During temporoammonic excitation (Price et al. 2005), populations of neurogliaform cells would be expected to suppress GABAergic input to more proximal postsynaptic locations provided by other interneurone subtypes (Banks et al. 2000). However, at increased noradrenergic levels, the situation could be reversed. Indeed, enhanced firing of O-LM interneurones and pyramidal cells would be expected to reduce the activity of neurogliaform cells. The anatomical and functional substrate of this switch from feed-forward to feedback inhibition (provided by neurogliaform and O-LM cells, respectively) was recently shown by Elfant et al. (2008) with the demonstration of GABAergic connections from O-LM interneurones to neurogliaform cells. The direct actions of noradrenaline enhancing the excitability of pyramidal neurons (Madison & Nicoll, 1982, 1986) would further strengthen the excitatory drive at the CA1 pyramidal cell–O-LM interneurone synapse, which is endowed with facilitatory short-term plasticity (see s7 in Fig. 1 and Ali & Thomson, 1998; Pouille & Scanziani, 2004).
Thus, an attractive hypothesis is that the degree of activity of the locus coeruleus-hippocampal projection could bi-directionally modulate the strength of competing GABAergic networks based on neurogliaform cells vs. O-LM interneurons, and affect the flow of incoming information from the entorhinal cortex to the CA1 subfield.
Additionally, recent work has suggested that membrane excitability may not be the exclusive target of noradrenergic modulation of lacunosum-moleculare GABAergic networks. In fact, noradrenaline application to hippocampal slices has been shown to reduce electrical coupling between interneurones via a cAMP–cAMP-dependent protein kinase (PKA) intracellular signalling cascade depending on β-receptor activation (Zsiros & Maccaferri, 2008). Because of the low proportion of interneurones in stratum lacunosum-moleculare expressing β1- or β2-adrenergic receptors (Cox et al. 2008), this result suggests either that the effect on coupling may be mediated by a different receptor subtype (β3?) or that expression of β1- and/or β2-receptors below detection levels may be sufficient to modulate coupling if receptors and their target adenylyl cyclase were, for example, strategically placed in close proximity of the gap junction site. Although yet untested for stratum lacunosum-moleculare interneurons, electrical coupling in other cortical GABAergic networks has been shown to depend on the expression of connexin36 (Venance et al. 2000; Deans et al. 2001), which is a substrate of PKA in vitro (Urschel et al. 2006). Therefore, it is tempting to speculate that the direct phosphorylation of interneuronal gap junction channels may affect their functional properties, or alternatively, their trafficking/localization at gap junction sites (see Flores et al. 2010 for connexin35, the fish orthologue of connexin36). Decreased coupling of GABAergic networks may enhance the separation and functional competition of GABAA,slowvs. other GABAergic networks by preventing the spread of GABAergic inhibitory potentials (Zsiros et al. 2007) across the two microcircuits.
What can be the consequence of noradrenergic modulation of this complex microcircuitry for temporoammonic signalling leading to the firing of place cells in vivo? Despite the richness of studies on the effects mediated by noradrenaline on the membrane/synaptic properties of various hippocampal neurons in slices, little is known about its effect(s) on hippocampal-dependent spatial representation by place cells in vivo. Pharmacological blockade of presynaptic α2-inhibitory noradrenergic receptors has been used as a tool to increase noradrenaline release. Under these conditions, instability of place fields was observed, concomitant with increased firing rates of place cells, but in a spatially non-selective manner (Tanila, 2001). In general, firing of putative hippocampal interneurones was also found to be decreased (Tanila, 2001), which may suggest, once again, that GABAergic inhibition is important for the integration of temporoammonic signalling. The anatomical identity of these interneurones, however, remains unknown, and probably several distinct subtypes were involved, suggesting the possibility that most of the recorded unit activity did not originate from neurogliaform cells. If this is the case, then it is tempting to speculate on the possibility of an effect due to GABAA,slow mediated suppression of competing inhibitory networks, as this would be predicted by in vitro results (Banks et al. 2000).
This interpretation, however, remains highly speculative. An unequivocal microcircuit-based explanation of this result is made problematic by the multiple direct actions of noradrenaline on pyramidal cell excitability (Madison & Nicoll, 1982, 1986) as well as on the temporoammonic synaptic input (Otmakhova & Lisman, 2000). The coordinated advance in knowledge coming from work in vitro and in vivo, together with a unifying modelling approach, is still required for a firmer interpretation.
Developmental aspects of stratum lacunosum-moleculare signalling: Cajal–Retzius cells
Recent work performed on young rat pups has shown that hippocampal cells have place fields at the onset of navigational experience (Langston et al. 2010; Wills et al. 2010), when Cajal–Retzius cells are still present in high numbers in the stratum lacunosum-moleculare microcircuit (Supèr et al. 1998). Thus, neuromodulation of both GABAergic networks and Cajal–Retzius cells has the potential to affect the integrative processes that follow activity of the temporoammonic pathway and that may be implicated in physiological functions.
Although Cajal–Retzius cells have been the subject of intense study as a major source of the glycoprotein reelin (Tissir & Goffinet, 2003), their ‘conventional’ role in the fast regulation of network activity in the hippocampus remains mysterious. Interestingly, these cells do not possess a resting potential, but are spontaneously active and appear to receive predominant, if not exclusive, GABAergic input (Marchionni et al. 2010). GABAergic input remains excitatory at developmental stages associated with inhibition in other cell types (Marchionni et al. 2010), most likely because of the lack of expression of the KCC2 transporter (Pozas et al. 2008). Thus, it would appear that Cajal–Retzius cells are the only neuronal type in stratum lacunosum-moleculare that does not require a direct, monosynaptic glutamatergic input from the temporoammonic pathway, but, in addition to their intrinsic firing, may be driven polysynaptically by GABA (see ?1 in Fig. 1). As a consequence, one prediction would be that increased firing rates due to temporoammonic signalling would occur in a different temporal window compared to temporoammonic monosynaptic excitation of pyramidal cells or stratum lacunosum-moleculare interneurons. Furthermore, activation of place cells could directly promote firing of O-LM cells during theta cycles (Klausberger et al. 2003) and generate excitatory GABAergic input to selected populations of Cajal–Retzius cells (see ?2 in Fig. 1), while simultaneously silencing stratum neurogliaform cells (Elfant et al. 2008). Although evidence in vitro suggests that neurogliaform and Cajal–Retzius cells share GABAergic input under certain conditions (Marchionni et al. 2010) the nature of the common presynaptic cell has not been clearly defined. The idea that O-LM interneurones contact Cajal–Retzius cells remains appealing, but speculative.
The apparent lack of GABAA receptor-mediated synaptic inhibition suggests that, in contrast to other neuronal types, different neuromodulatory mechanisms may be required to down-regulate the activity of Cajal–Retzius cells. Recent work suggests that chemokines may be, indeed, involved (Marchionni et al. 2010). In addition to their classical role as mediators of inflammatory responses, chemokines have rapidly attracted the attention of neuroscientists as novel physiological modulators of neuronal functions in the developing and adult brain, following the demonstration that several molecules belonging to this family and their receptors are expressed by neurons, endothelial cells and glia (Rostène et al. 2007). Interestingly, hippocampal Cajal–Retzius cells of young/adult animals express the G-protein coupled chemokine receptor CXCR4 (Stumm et al. 2002; Marchionni et al. 2010). The natural ligand of the CXCR4 receptor is the chemokine (C-X-C motif) ligand 12/stromal cell-derived factor-1 α (CXCL12/SDF-1), which is known to produce a specific modulatory effect in various regions of the brain (Guyon & Nahon, 2007). Application of SDF-1 in vitro powerfully reduces the spontaneous firing frequency of Cajal–Retzius cells, most likely by activation of a potassium conductance (Marchionni et al. 2010). Although the evidence is purely correlative, SDF-1 also reduces the strength of local field potentials evoked by stimulation of the temporoammonic input to the CA1 region (Marchionni et al. 2010). It is therefore very tempting to speculate that constant firing of Cajal–Retzius cells may play a role in stratum lacunosum-moleculare processing and impact its physiological functions by regulating the strength of temporoammonic synapses. However, the exact mechanism involved is difficult to identify because the nature of the major neurotransmitter used by Cajal–Retzius cells has not been unequivocally established (see ?3 in Fig. 1). Although growing evidence points to glutamate (del Rio et al. 1995; Hevner et al. 2003; Ina et al. 2007), further studies establishing directly the postsynaptic effects of Cajal–Retzius cells on their cellular targets will shed light on the role of these neurons in the stratum lacunosum-moleculare network.
Conclusions
In conclusion, with the aforementioned caveats, stratum lacunosum moleculare interneurones and Cajal–Retzius cells could be thought of as two elements of the local network playing different integrative functions during temporoammonic signalling driving place cells in vivo. Direct temporoammonic input would be predicted to increase activity in both types of cells, possibly in different temporal windows. However, firing of place cells would activate O-LM interneurons, which could powerfully inhibit specific assemblies of stratum lacunosum-moleculare cells such as neurogliaform interneurons, but possibly increase spontaneous firing of Cajal–Retzius cells. Thus, the spatiotemporal dynamic balance of the activity of these different cell types could be the selective target of neuromodulation mediated by either noradrenaline or SDF-1.
Considering that only two neuromodulators and just a few cellular types were examined, the emerging picture appears to be that neuromodulation by noradrenaline and SDF-1 endows specific hippocampal microcircuits with the necessary complexity to adapt to various roles. This flexibility may be related to the diverse and still unknown functions of the processing performed by this network during development and/or different brain states in vivo.
Acknowledgments
I would like to thank Dr Ivan Marchionni for his comments during the preparation of this manuscript. This work was supported by the NIH (grants NS057445 and NS064135).
Glossary
Abbreviations
- CXCL12
chemokine (C-X-C motif) ligand 12
- CXCR4
CXC chemokine receptor 4
- KCC2
potassium chloride cotransporter 2
- O-LM
oriens–lacunosum-moleculare
- SDF-1
stromal cell derived factor-1 α
References
- Alcántara S, Ruiz M, D'Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB, Thomson AM. Facilitating pyramid to horizontal oriens-alveus interneurone inputs: dual intracellular recordings in slices of rat hippocampus. J Physiol. 1998;507:185–199. doi: 10.1111/j.1469-7793.1998.185bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, White JA, Pearce RA. Interactions between distinct GABAA circuits in hippocampus. Neuron. 2000;25:449–457. doi: 10.1016/s0896-6273(00)80907-1. [DOI] [PubMed] [Google Scholar]
- Banks MI, Hardie JB, Pearce RA. Development of GABAA receptor-mediated inhibitory postsynaptic currents in hippocampus. J Neurophysiol. 2002;88:3097–3107. doi: 10.1152/jn.00026.2002. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Doze VA, Madison DV, Smith SJ. Excitatory actions of norepinephrine on multiple classes of hippocampal CA1 interneurons. J Neurosci. 1996;16:572–85. doi: 10.1523/JNEUROSCI.16-02-00572.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Brun VH, Leutgeb S, Wu HQ, Schwarcz R, Witter MP, Moser EI, Moser MB. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008;57:290–302. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Levy WB. Electrophysiological and pharmacological characterization of perforant path synapses in CA1: mediation by glutamate receptors. J Neurophysiol. 1992;68:1–8. doi: 10.1152/jn.1992.68.1.1. [DOI] [PubMed] [Google Scholar]
- Cox DJ, Racca C, LeBeau FE. β-Adrenergic receptors are differentially expressed in distinct interneurone subtypes in the rat hippocampus. J Comp Neurol. 2008;509:551–565. doi: 10.1002/cne.21758. [DOI] [PubMed] [Google Scholar]
- Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin 36. Neuron. 2001;31:477–485. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- del Río JA, Martínez A, Fonseca M, Auladell C, Soriano E. Glutamate-like immunoreactivity and fate of Cajal-Retzius cells in the murine cortex as identified with calretinin antibody. Cereb Cortex. 1995;5:13–21. doi: 10.1093/cercor/5.1.13. [DOI] [PubMed] [Google Scholar]
- Dvorak-Carbone H, Schuman EM. Patterned activity in stratum lacunosum moleculare inhibits CA1 pyramidal neuron firing. J Neurophysiol. 1999;82:3213–3222. doi: 10.1152/jn.1999.82.6.3213. [DOI] [PubMed] [Google Scholar]
- Elfant D, Pál BZ, Emptage N, Capogna M. Specific inhibitory synapses shift the balance from feedforward to feedback inhibition of hippocampal CA1 pyramidal cells. Eur J Neurosci. 2008;27:104–113. doi: 10.1111/j.1460-9568.2007.06001.x. [DOI] [PubMed] [Google Scholar]
- Flores CE, Cachope R, Nannapaneni S, Ene S, Nairn AC, Pereda AE. Variability of distribution of Ca2+/calmodulin-dependent kinase II at mixed synapses on the mauthner cell: colocalization and association with connexin 35. J Neurosci. 2010;30:9488–9499. doi: 10.1523/JNEUROSCI.4466-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurones of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Guyon A, Nahon JL. Multiple actions of the chemokine stromal cell-derived factor-1α on neuronal activity. J Mol Endocrinol. 2007;38:365–376. doi: 10.1677/JME-06-0013. [DOI] [PubMed] [Google Scholar]
- Hangya B, Li Y, Muller RU, Czurkó A. Complementary spatial firing in place cell-interneurone pairs. J Physiol. 2010;588:4165–4175. doi: 10.1113/jphysiol.2010.194274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Neogi T, Englund C, Daza RA, Fink A. Cajal-Retzius cells in the mouse: transcription factors, neurotransmitters, and birthdays suggest a pallial origin. Brain Res Dev Brain Res. 2003;141:39–53. doi: 10.1016/s0165-3806(02)00641-7. [DOI] [PubMed] [Google Scholar]
- Hillman KL, Lei S, Doze VA, Porter JE. Alpha-1A adrenergic receptor activation increases inhibitory tone in CA1 hippocampus. Epilepsy Res. 2009;84:97–109. doi: 10.1016/j.eplepsyres.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ina A, Sugiyama M, Konno J, Yoshida S, Ohmomo H, Nogami H, Shutoh F, Hisano S. Cajal-Retzius cells and subplate neurons differentially express vesicular glutamate transporters 1 and 2 during development of mouse cortex. Eur J Neurosci. 2007;26:615–623. doi: 10.1111/j.1460-9568.2007.05703.x. [DOI] [PubMed] [Google Scholar]
- Ito HT, Schuman EM. Frequency-dependent gating of synaptic transmission and plasticity by dopamine. Front Neural Circuits. 2007;1:1–13. doi: 10.3389/neuro.04.001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Schuman EM. Frequency-dependent signal transmission and modulation by neuromodulators. Front Neurosci. 2008;2:138–144. doi: 10.3389/neuro.01.027.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamondi A, Acsády L, Wang XJ, Buzsáki G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus. 1998;8:244–261. doi: 10.1002/(SICI)1098-1063(1998)8:3<244::AID-HIPO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Shindou T. Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J Neurosci. 1998;18:6963–6976. doi: 10.1523/JNEUROSCI.18-17-06963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Congar P, Ben-Ari Y. Hippocampal CA1 lacunosum-moleculare interneurons: comparison of effects of anoxia on excitatory and inhibitory postsynaptic currents. J Neurophysiol. 1995;74:2138–2149. doi: 10.1152/jn.1995.74.5.2138. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Márton LF, Roberts JD, Cobden PM, Buzsáki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurones in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Schwartzkroin PA. Stratum lacunosum-moleculare interneurones of hippocampal CA1 region. II Intrasomatic and intradendritic recordings of local circuit synaptic interactions. J Neurosci. 1988;8:1411–1424. doi: 10.1523/JNEUROSCI.08-04-01411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston RF, Ainge JA, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser EI, Moser MB. Development of the spatial representation system in the rat. Science. 2010;328:1576–1580. doi: 10.1126/science.1188210. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol. 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol. 2000;524:91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature. 1982;299:636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. J Physiol. 1986;372:221–244. doi: 10.1113/jphysiol.1986.sp016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Norepinephrine decreases synaptic inhibition in the rat hippocampus. Brain Res. 1988;442:131–138. doi: 10.1016/0006-8993(88)91440-0. [DOI] [PubMed] [Google Scholar]
- Marchionni I, Takács VT, Nunzi MG, Mugnaini E, Miller RJ, Maccaferri G. Distinctive properties of CXC chemokine receptor 4-expressing Cajal–Retzius cells versus GABAergic interneurones of the postnatal hippocampus. J Physiol. 2010;588:2859–2878. doi: 10.1113/jphysiol.2010.190868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Oxford University Press; 1978. [Google Scholar]
- Oleskevich S, Descarries L, Lacaille JC. Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. J Neurosci. 1989;9:3803–3815. doi: 10.1523/JNEUROSCI.09-11-03803.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. Dopamine selectively inhibits the direct cortical pathway to the CA1 hippocampal region. J Neurosci. 1999;19:1437–1445. doi: 10.1523/JNEUROSCI.19-04-01437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. Dopamine, serotonin, and noradrenaline strongly inhibit the direct perforant path-CA1 synaptic input, but have little effect on the Schaffer collateral input. Ann N Y Acad Sci. 2000;911:462–464. doi: 10.1111/j.1749-6632.2000.tb06746.x. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Lewey J, Asrican B, Lisman JE. Inhibition of perforant path input to the CA1 region by serotonin and noradrenaline. J Neurophysiol. 2005;94:1413–1422. doi: 10.1152/jn.00217.2005. [DOI] [PubMed] [Google Scholar]
- Parra P, Gulyás AI, Miles R. How many subtypes of inhibitory cells in the hippocampus? Neuron. 1998;20:983–993. doi: 10.1016/s0896-6273(00)80479-1. [DOI] [PubMed] [Google Scholar]
- Pearce RA. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- Pozas E, Paco S, Soriano E, Aguado F. Cajal-Retzius cells fail to trigger the developmental expression of the Cl− extruding co-transporter KCC2. Brain Res. 2008;1239:85–91. doi: 10.1016/j.brainres.2008.08.058. [DOI] [PubMed] [Google Scholar]
- Price CJ, Cauli B, Kovacs ER, Kulik A, Lambolez B, Shigemoto R, Capogna M. Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci. 2005;25:6775–6786. doi: 10.1523/JNEUROSCI.1135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. Estructura del asta de Ammon y fascia dentate. Ann Soc Esp Hist Nat. 1893;22:53–114. [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JR. Multiple mechanisms of receptor-G protein signaling specificity. Am J Physiol Renal Physiol. 1995;269:F141–158. doi: 10.1152/ajprenal.1995.269.2.F141. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Rostène W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. 2007;8:895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Jones RS. The direct perforant path input to CA1: excitatory or inhibitory? Hippocampus. 1995;5:101–103. doi: 10.1002/hipo.450050202. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Höllt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supèr H, Martínez A, Del Río JA, Soriano E. Involvement of distinct pioneer neurons in the formation of layer-specific connections in the hippocampus. J Neurosci. 1998;18:4616–4626. doi: 10.1523/JNEUROSCI.18-12-04616.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanila H. Noradrenergic regulation of hippocampal place cells. Hippocampus. 2001;11:793–808. doi: 10.1002/hipo.1095. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Urschel S, Honer T, Schubert T, Alev C, Sohl G, Worsdorfer P, Asahara T, Dermietzel R, Weiler R, Willecke K. Protein kinase A-mediated phosphorylation of Connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem. 2006;281:33163–33171. doi: 10.1074/jbc.M606396200. [DOI] [PubMed] [Google Scholar]
- Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- Venance L, Rozov A, Blatow M, Burnashev N, Feldmayer D, Monyer H. Connexin expression in electrically coupled postnatal rat brain neurons. Proc Natl Acad Sci U S A. 2000;97:10260–10265. doi: 10.1073/pnas.160037097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH. Unitary IPSPs evoked by interneurones at the stratum radiatum–stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol. 1998;506:755–773. doi: 10.1111/j.1469-7793.1998.755bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TJ, Cacucci F, Burgess N, O'Keefe J. Development of the hippocampal cognitive map in preweanling rats. Science. 2010;328:1573–1576. doi: 10.1126/science.1188224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsiros V, Maccaferri G. Electrical coupling between interneurones with different excitable properties in stratum lacunosum-moleculare of the juvenile CA1 rat hippocampus. J Neurosci. 2005;25:8686–8695. doi: 10.1523/JNEUROSCI.2810-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsiros V, Aradi I, Maccaferri G. Propagation of postsynaptic currents and potentials via gap junctions in GABAergic networks of the rat hippocampus. J Physiol. 2007;578:527–544. doi: 10.1113/jphysiol.2006.123463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsiros V, Maccaferri G. Noradrenergic modulation of electrical coupling in GABAergic networks of the hippocampus. J Neurosci. 2008;28:1804–1815. doi: 10.1523/JNEUROSCI.4616-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


