Abstract
Abstract
Mammalian cortical structures are endowed with the capacity for plasticity, which emerges from a combination of the dynamics of circuit connectivity and function, and the intrinsic function of the neurons within the circuit. However, this capacity is accompanied by a significant risk: the capability to generate seizure discharges is also a property of all mammalian cortices. How do cortical circuits reconcile the requirement to maintain plasticity, but at the same time control seizure initiation? These issues come into particular focus in the hippocampus. The hippocampus is one of the main plasticity engines in the brain, and is also a structure frequently implicated in the generation of epileptic seizures, with temporal lobe epilepsy constituting the most prevalent form of epilepsy in the adult population. One aspect of hippocampal circuitry that is particularly prominent is its intimate interconnections with the entorhinal cortex. These interconnections create a number of excitatory synaptic loops within the limbic system, which, in addition to being important in cognitive function, can support reentrant activation and seizure generation. In the present review, using optical imaging approaches to elucidate circuit processing at high temporal and spatial resolution, we examine how two targets of entorhinal cortical input within the hippocampus, the dentate gyrus and area CA1, regulate these synaptic pathways in ways that can maintain functions important in generation of normal activity patterns, but that dampen the ability of these inputs to generate seizure discharges.
Douglas Coulter trained as a cellular physiologist, and received his Ph.D. from the Boston University Marine Program in Woods Hole, Massachusetts. He subsequently conducted postdoctoral training at Stanford University. With a primary research interest in the cellular and neuronal circuit mechanisms responsible for temporal lobe epilepsy, his laboratory integrates cellular physiological, molecular, pharmacological and circuit imaging approaches to elucidate the bases for seizure susceptibility in the hippocampus, using animal models of this disease. This work is conducted in the Coulter laboratory located at the Children's Hospital of Philadelphia Research Institute and the University of Pennsylvania School of Medicine, where he is a Professor of Pediatrics and Neuroscience.
 |
The hippocampus receives two primary cortical inputs: the perforant path and the temporoammonic (TA) pathway. The perforant path innervates the dentate gyrus (DG), with the lateral and medial perforant path (originating in layer II cells of the lateral and medial entorhinal cortex (EC)) synapsing on the outer and medial molecular layer of the DG, which in turn corresponds to the outer and more proximal distal dendrites of dentate granule cells, respectively. A second input to the hippocampus from the EC, the TA pathway, originates in layer III EC neurons and innervates the distal dendrites of area CA1 neurons in stratum lacunosum moleculare (Steward & Scoville, 1976). Both of these pathways also innervate the distal dendrites of area CA2. Given that the EC is in turn a target of hippocampal output fibres, three primary excitatory loops exist. A long loop is created, incorporating the canonical hippocampal trisynaptic pathway (EC to DG to CA3 to CA1, with the loop being closed via subiculum and EC); an intermediate-length loop incorporates area CA2 (EC to CA2 to CA1 to subiculum/EC); and finally, a shorter loop involves direct cortical input to area CA1 (EC to CA1 to subiculum/EC). These pathways are summarized in a circuit diagram (Fig. 1).
Figure 1. Circuit schematic diagram of entorhinal/hippocampal synaptic pathways.
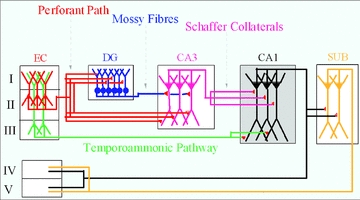
Inputs from the entorhinal cortex (EC) to hippocampus include the perforant path, originating from layer II EC neurons, and the temporoammonic pathway, originating from layer III EC neurons. These innervate the dentate gyrus (DG) and area CA1, respectively. Projections from these two structures create long (EC–DG–CA3–CA1–EC) and short (EC–CA1–EC) excitatory synaptic loops.
These excitatory synaptic loops are important in normal hippocampal function, but also are involved in creating an underlying predisposition of the hippocampus and associated limbic structures to generate seizures (Heinemann et al. 1992; Lothman et al. 1992). One hypothesis that has been posited is that, to regulate this vulnerability, both the dentate gyrus and area CA1 exhibit specialized properties to make it less likely that excitatory inputs will escape control and trigger seizures (cf. Heinemann et al. 1992; Lothman et al. 1992). Imaging the dynamics of circuit function at high temporal resolution (cf. Carlson & Coulter, 2008) facilitates characterization of these control mechanisms, allowing them to be compared and contrasted between structures, both in the normal brain and in the brains of epileptic animals at varying times during development of epilepsy.
Voltage sensitive dye imaging as a tool to characterize circuit dynamics
Optical recording of circuit activity is a powerful tool to explore circuit dynamics. One such technique that has been utilized successfully is optical recording using signals derived from voltage sensitive dyes as an output measure. These dyes integrate into cell membranes, and their fluorescence properties change (for many dyes, in a linear manner) with varying transmembrane voltages. Use of these dyes coupled with fast cameras can provide information on voltage fluctuations in several thousand compartments simultaneously, at high temporal resolution (1–2 kHz frame rates) (Salzberg et al. 1983; Contreras & Llinas, 2001; technical approach reviewed in Carlson & Coulter, 2008). Use of this recording mode provides information about integration in many cellular compartments inaccessible to somatic patch recording techniques, and also can visualize IPSP dynamics, which are not apparent in field potential recordings. A limitation of this form of recording is frequently a low signal to noise ratio, which necessitates averaging. This can compromise the ability to visualize action potential firing, due to jitter in these events. Additional limitations of a voltage sensitive dye imaging approach in brain slices in characterization of neuronal circuit dynamics include (a) the use of in vitro slice techniques, which sever pathways, complicating interpretation; and (b) toxicity introduced both by fluorescent light exposure and the innate toxicity of the dyes (Carlson & Coulter, 2008). Using voltage sensitive dye recording techniques, we have explored the dynamics of activation of both the dentate gyrus and area CA1 circuits, in response to stimulation of EC afferents to these structures. The focus of these experiments was to determine how these diverse circuits process and regulate EC inputs, and how these dynamics may regulate the initiation and propagation of both normal and pathological events, such as seizures.
Regulation of cortical inputs by the dentate gyrus
The principal cells of the dentate gyrus, dentate granule cells, receive excitatory perforant path input on the outer and medial portions of their dendritic trees, and also are innervated by axons from excitatory hilar mossy cells, on proximal dendrites. Several GABAergic interneuron variants provide robust feedforward and feedback inhibition within this circuit (cf. Han et al. 1993). Despite the powerful, multisynaptic nature of perforant path activation of dentate granule cells, which exhibit robust EPSPs in response to EC input (Fig. 2), these neurons rarely fire action potentials in response to perforant path activation at reasonable stimulation strengths (3–5 times threshold for minimal activation; Coulter & Carlson, 2007), but can be forced to fire in higher numbers by supramaximal stimulation (10–20 times threshold). Firing of single action potentials results in very little relay of excitation to downstream targets of the dentate gyrus, principally area CA3 pyramidal neurons, which in turn dampens the possibility for excitatory loop reentrant activation and seizure initiation, at least for isolated activation of the circuit at low frequencies (Fig. 2). What mechanisms mediate this dentate granule cell property of being reluctant to fire, together with the corresponding circuit property of filtering synchronous inputs characteristic of the dentate gyrus?
Figure 2. Dentate gyrus activation by perforant path stimulation, and filtering defined as lack of relay to CA3.
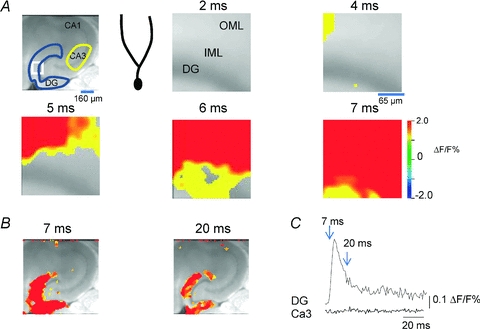
A, left, greyscale image of a hippocampal entorhinal cortical slice, together with a region of interest (ROI) delineation for recordings in A (white box), and in B (blue and yellow ROIs for dentate and CA3 traces in B). The cartoon dentate granule cell depicts the composition of the various layers in the voltage sensitive dye (VSD) imaging depictions in A, corresponding to the white box. The molecular layer is depicted, onto which the VSD recordings at varying time points post-stimulation (2–7 ms) are projected. At early time points (4 and 5 ms), the outer molecular layer (OML) activates, and this activation rapidly propagates down the dendrites (inner molecular layer, IML) to the cell body layer (DG) (note complete activation of the dentate gyrus by 7 ms post-stimulus). Perforant path activation rapidly activates the entire dendritic tree of dentate granule cells. B, larger area image of activation of the dentate gyrus and area CA3 by perforant path activation. Note that, both at the peak of the DG response (7 ms) and at later time points, little or no CA3 activation is evident. This is also illustrated in the VSD traces in C, where the integrated response in the DG and CA3 ROIs depicted in the top left panel of A are plotted vs. time. Note the robust activation of the dentate gyrus, which generates little or no response in CA3. Perforant path inputs, although robust, are filtered by the dentate gyrus and are ineffective in activating area CA3. Unpublished data of Yue and Coulter.
Among the mechanisms intrinsic to the dentate gyrus that contribute to reluctant firing by dentate granule cells, two predominate. These are the inexcitable intrinsic properties of granule cells, and the powerful and diverse forms of feedforward, feedback, and tonic GABAergic inhibition which dampen responses to excitatory synaptic input. The principal cells of the dentate gyrus, dentate granule cells, exhibit very hyperpolarized resting potentials (between −80 and −90 mV, Spruston & Johnston, 1992; Staley et al. 1992), a depolarized action potential threshold (Spruston & Johnston, 1992; Staley et al. 1992), and a lack of regenerative calcium conductances (Fricke & Prince, 1984), making them difficult to depolarize sufficiently to fire action potentials. These intrinsic properties contribute to dentate gyrus filter function. However, the majority of this filtering property is mediated by the inhibition resulting from the strong and multifaceted inhibitory neuron populations, which function to only allow a very short, high threshold window for action potential firing in response to perforant path activation. Like dentate granule cells, these diverse dentate gyrus and hilar interneuron populations receive perforant path input (including both basket cells and somatostatin positive interneurons, cf. Lerant et al. 1990; Freund & Buzsaki, 1996). However, unlike granule cells, these interneurons activate in response to cortical inputs at much lower threshold, providing powerful feedforward inhibition with one synaptic delay, significantly constraining granule cell activation (Ewell & Jones, 2010).
Regulation of cortical inputs by hippocampal area CA1
The dynamic properties regulating cortical inputs to dentate gyrus contrast with those controlling entorhinal cortical inputs onto area CA1. Similar to inputs to dentate granule cells, entorhinal cortical input to CA1 only innervates a very constrained portion of the dendritic tree, the distal apical tuft (Desmond et al. 1994). However, unlike in dentate gyrus, the EPSP generated by TA input to CA1 is also restricted to the apical tuft (Ang et al. 2005) and does not propagate to the cell soma (Soltesz, 1995; Ang et al. 2005), and so usually does not elicit action potential firing in the cell (Fig. 3; Soltesz, 1995; Ang et al. 2005). Again, as with perforant path inputs to dentate gyrus discussed above, this constraint to the distal tuft is evident in response to moderate, physiologically relevant levels of pathway stimulation (3–5 times threshold to induce an EPSP). Driving this pathway using very strong stimulation (10-20 times threshold) can activate more of the dendritic tree, but the relevance and specificity of these strong stimuli is unclear (strong stimulation may also activate the Schaffer collateral pathway, which innervates more proximal dendritic compartments). What mediates the constraint of TA inputs to the distal dendritic tree?
Figure 3. Feedforward GABAA-mediated inhibition activated via TA pathway stimulation spatially restricts evoked EPSPs to the distal dendrites of the CA1 pyramidal neurons.
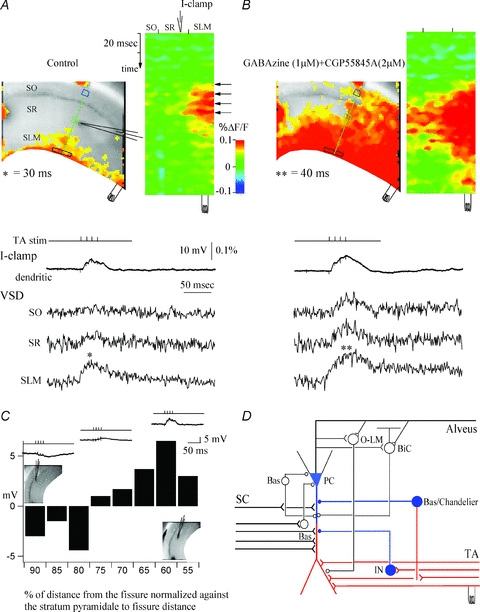
A, control. A snapshot of activation at 30 ms of the VSD responses of evoked EPSPs (denoted by asterisk) to stimulation in stratum lacunosum moleculare (left) and the activation profile (right) generated from the raster line scan along the path of interest (green line). The location of the patch recording electrode is denoted by the electrode graphic. Top trace (I-clamp, current clamp recording), whole-cell recording trace from the apical dendrite of a CA1 pyramidal cell in stratum radiatum. VSD SO, SR, and SLM are the local VSD signals quantified from regions of interest in stratum oriens (blue box), stratum radiatum (green box), and stratum lacunosum moleculare (black box). Note that the TA-evoked EPSP is spatially restricted to the extreme distal dendrites of CA1 pyramidal neurons. B, effects of the GABAA and GABAB antagonists gabazine (1 μm) and CGP 55845A (2 μm). Left, snapshot at 40 ms. Note that GABAergic inhibition blockade results in loss of spatial segregation of the TA EPSPs in stratum lacunosum moleculare and significant propagation of TA EPSPs to stratum radiatum and stratum oriens. C, plot of dendritic whole-cell recording responses along the dendrites of the CA1 pyramidal neuron, as a function of the normalized distance of the patch electrode from the hippocampal fissure to stratum pyramidale. Insets show the relative current-clamp dendritic specimen records and locations of the patch electrode. Note that TA-evoked IPSPs are prevalent in recordings close to the cell somata, whereas EPSPs are recorded from more distal dendritic sites. D, CA1 schematic showing the response to TA stimulation. Red represents excitation and blue represents inhibition. SC, Schaffer collateral; TA, temporoammonic pathway; PC, pyramidal cell; O-LM, oriens–lacunosum moleculare interneuron; BiC, bistratified cell; Bas, basket cell; Chandelier, chandelier cell; IN, interneuron. From Ang et al. (2005), with permission from the Society for Neuroscience.
As with factors encountered in dentate granule cells, TA constraint is mediated through a combination of the intrinsic properties of CA1 pyramidal neurons, and the dynamic function of the complex inhibitory circuitry within area CA1. Among the intrinsic properties that regulate TA constraint, the high density dendritic expression of HCN channels is a significant contributor (Magee, 1999; Tsay et al. 2007). These channels are hyperpolarization activated, and so may be active at cell resting potential, introducing a leak conductance which alters the passive properties of CA1 dendrites, constraining EPSP propagation. Blockade of these channels facilitates TA EPSP propagation (Ang et al. 2005; Tsay et al. 2007).
Inhibitory regulation is also a prime contributor to constraint of TA EPSP propagation. Possible feedforward interneurons mediating this regulation include basket, bistratified and neurogliaform cells, which have dendrites in anatomical juxtaposition with EC inputs, and which innervate CA1 neurons on more proximal dendritic and/or somatic compartments (Klausberger & Somogyi, 2008). Activation of these feedforward interneurons leaves the powerful TA EPSP relatively unaffected in the distal dendritic compartment. Instead, these interneurons target proximal neuronal membrane, shunt the propagating EPSP, constraining it to the distal dendritic tuft (Fig. 3). Because of this, the soma primarily responds to TA activation with an IPSP, which reflects the proximally targeted inhibition evident as an IPSP in stratum radiatum in voltage-senstive dye imaging studies (Fig. 3). The distal EPSP is not evident in somatic recordings under normal conditions (Soltesz, 1995), using traditional whole-cell patch techniques.
Feedback inhibition further regulates cortical inputs to area CA1
The TA pathway is further regulated when CA1 pyramidal neurons fire. This action potential is relayed to subiculum and EC, but also activates local circuit feedback interneurons in stratum oriens. These feedback interneurons include principally oriens–lacunosum moleculare (O-LM) and axoaxonic, basket and bistratified cells, the last three of which receive inputs allowing them to function in both feedforward and feedback roles (Klausberger & Somogyi, 2008). Using the distinct properties of excitatory recurrent collateral synapses from CA1 pyramidal neurons onto basket and O-LM neurons to selectively activate these two populations of feedback neurons (Pouille & Scanziani, 2004) we examined effects of activation of these distinct interneurons on regulation of TA inputs (Fig. 4). We found that single alvear stimuli, which preferentially activate basket cells, did not affect the amplitude of TA-mediated EPSPs in the distal tuft, but did constrain the proximal propagation of these events down the dendrite. In contrast, alvear burst stimuli, which activate both basket and O-LM interneurons, effectively shunted the distal, TA mediated EPSP, powerfully preventing any measurable postsynaptic depolarization (Fig. 4). In addition to activating feedback inhibitory neurons, alvear stimulation would also be expected to directly activate CA1 pyramidal neurons, and potentially EC and Schaffer collateral axons as well. Only minor contributions of these additional effects of alvear stimulation were evident (Fig. 4B). These data clearly support a significant role for O-LM interneurons in feedback regulation of TA inputs. This also effectively prevents reentrant activation of the CA1–subiculum–EC synaptic loop, which might result in inappropriate potentiation of this circuit, resulting in generation of seizure discharges.
Figure 4. Feedback inhibition further constrains TA EPSP activation.
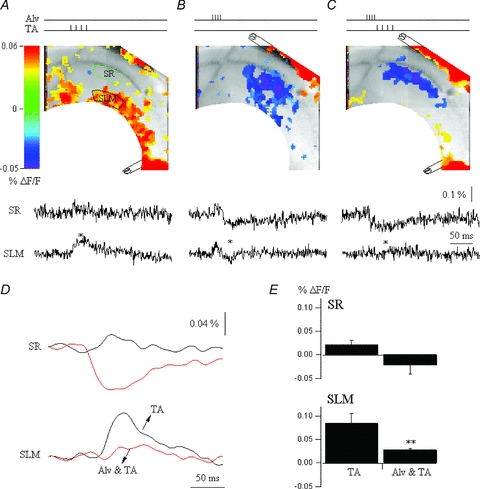
A, CA1 VSD snapshot at the peak of the EPSP response (red, 30 ms) to burst stimulus to the TA input pathway. VSD SR and SLM are the local VSD signals quantified from regions of interest in stratum radiatum (green box) and stratum lacunosum moleculare (black box), respectively. B, snapshot at the peak (35 ms) of the alvear stimulation-evoked response, an IPSP (blue). The alveus was activated with a burst stimulation to recruit O-LM feedback interneurons. C, alvear and TA stimulation. The alvear and TA activation are paired so the alvear burst stimulus precedes the TA stimulus by 5 ms, corresponding to recruitment of feedback inhibition. The snapshot depicts activation at 35 ms. Note that the TA EPSP in stratum lacunosum moleculare is completely suppressed. D, comparison of the VSD signals quantified from stratum radiatum (top) and stratum lacunosum moleculare (bottom) for TA stimulation, and alvear and TA stimulation. E, summary data on the suppression of TA EPSPs by distally targeted inhibition. Note the suppression of TA EPSPs in SLM (ANOVA, **P≤ 0.05, n = 4). Unpublished data of Ang and Coulter.
What circuit dynamics allow cortical inputs to activate the hippocampus?
As described above for EC inputs to both the dentate gyrus and CA1, the circuit filter constraint is so strong, it solicits the question: how does cortical input ever activate the hippocampus? Clearly, both of these pathways subserve important functions during normal hippocampal operations. The reluctance of granule cells to fire in response to EC inputs is also seen in vivo, where only a small percentage of granule cells (2%) exhibit activation during spatial navigation (Chawla et al. 2005). However, this sparse firing behaviour is functionally important. Dentate granule cells act as a sparsely activating population of neurons encoding spatial aspects of the environment (i.e. exhibit place cell behaviour), and are important in pattern separation (Leutgeb et al. 2007), in modulation of CA3 place cell behaviour (Leutgeb et al. 2007), and in cognitive processes such as learning and memory (McHugh et al. 2007; Clelland et al. 2009). Individual granule cells can also activate the CA3 network in vivo when stimulated at higher frequencies, which correspond to firing behaviour evident during place cell responses (Henze et al. 2002). Similarly, although apparently a very weak pathway, direct cortical inputs to CA1 are necessary for CA1 neuron place cell behaviour and place recognition in vivo (Brun et al. 2002), and for consolidation of long-term memory (Remondes & Schuman, 2004). How can these important functions of cortical inputs to the hippocampus be reconciled with the tight regulation of these inputs constraining circuit activation?
Despite their innate reluctance to fire, dentate granule cells do exhibit place cell behaviour in vivo (although this is sparse). Part of their activation may depend both on gamma frequency oscillations present during spatial navigation behaviour, and competition between granule cells, mediated through feedback inhibition (de Almeida et al. 2009). Once granule cells fire, they do so in patterned, high frequency bursts. This may facilitate the activation of the CA3 network (Henze et al. 2002), despite the fact that 75% of the downstream targets of granule cell axons are feedforward inhibitory interneurons in area CA3 (Acsady et al. 1998). This may be an emergent property of the distinct properties of mossy fibre bouton terminals onto CA3 pyramidal neurons and mossy fibre filopodial synapses onto interneurons, which show remarkable differences in frequency facilitation. So, despite their intrinsic reluctance to fire, specific, dynamic circuit properties within the dentate gyrus allow this firing to occur in precise patterns, which can facilitate activation of downstream hippocampal structures.
How might the apparently very weak TA inputs to area CA1 participate in generation of place cell behaviour in these neurons (e.g. Brun et al. 2002)? One interesting possibility is that TA inputs might propagate more powerfully to the CA1 cell soma when activated in precise temporal relationship with CA3 inputs. In vivo, during theta oscillations and periods of place cell firing, these two inputs arrive onto CA1 dendrites a half theta cycle apart (Kocsis et al. 1999; Buzsaki, 2002). When we mimicked this temporal relationship between pathways in voltage sensitive dye recording studies in vitro, we found that this type of Schaffer collateral/TA activation resulted in TA EPSP propagation to the soma, mediated in part through an NMDA receptor-dependent process (Fig. 5, Ang et al. 2005).
Figure 5. Circuit integration: paired activation of Schaffer collaterals at a half-theta interval gates TA EPSPs.
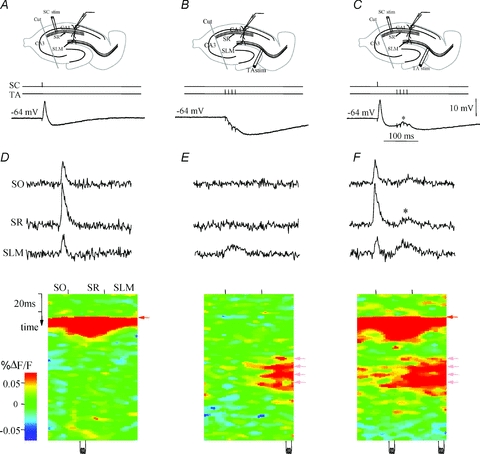
A–C, current-clamp (I-clamp) dendritic recording traces derived from whole-cell recordings from the apical dendrite of a CA1 pyramidal cell in response to a single stimulus applied in stratum radiatum Schaffer (SC) pathway (A), burst stimulation of the TA pathway (B), and sequential activation of the SC and TA pathways at a 40 ms interval (C). Note the de novo appearance of a TA-mediated EPSP during sequential stimulation. D–F, top traces: VSD SO, SR and SLM are the local VSD signals quantified from regions of interest in stratum oriens, stratum radiatum, and stratum lacunosum moleculare, respectively. Bottom plot, activation profile, generated from a raster line scan along the dendritic axis of CA1, which depicts the spatiotemporal response to a Schaffer collateral stimulus (D). Note the short, powerful EPSP (red) followed by an IPSP (blue; see current clamp response in A). E, raster plot of response to TA pathway stimulation. Note that the VSD response is spatially restricted to the apical tuft in stratum lacunosum moleculare, and the current-clamp dendritic recording of a CA1 pyramidal neuron apical dendrite in stratum radiatum shows an inhibitory response (B). F, sequential Schaffer–TA (SC&TA) pathway stimulation. The Schaffer and TA stimuli are paired so that a single Schaffer stimulus precedes the TA burst stimulus by 40 ms. With the previous Schaffer stimulus, the TA inputs integrate synergistically and propagate to stratum radiatum and stratum oriens (note red TA activation extending to SR and SO, also note EPSP appearance in C). The asterisk denotes gating of temporoammonic EPSPs to stratum radiatum and stratum oriens. Modified from Ang et al. 2005, with permission from the Society for Neuroscience.
What happens to normal regulatory mechanisms in temporal lobe epilepsy?
In animal models of temporal lobe epilepsy, it is likely that the normal hippocampal circuit control mechanisms controlling pathological activity have been damaged or lost. How are the circuit dynamics of EC input regulation preserved or lost in the dentate gyrus and area CA1 of animals with epilepsy? In these animals, it is known that the properties of inhibition are significantly altered. Both inhibitory synaptic function and GABAA receptor expression and function are altered in the dentate gyrus (Gibbs et al. 1997; Schwarzer et al. 1997; Brooks-Kayal et al. 1998; Cohen et al. 2003; Pathak et al. 2007; Sun et al. 2007; Zhang et al. 2007) and area CA1 (Gibbs et al. 1997; Schwarzer et al. 1997; Cossart et al. 2001). This compromise in inhibition is manifest at the level of GABA receptors, transmembrane chloride gradients (Huberfeld et al. 2007; Pathak et al. 2007), and GABA neurotransmitter replenishment (Liang et al. 2006; Ortinski et al. 2010). Since inhibition is a powerful regulator of EC input processing in both these circuits, this would lead to the prediction that the nature of EC input processing by both circuits should be significantly compromised. However, we found that only the CA1 processing of EC input was affected. There was a dramatic loss of CA1 neurons’ ability to appropriately regulate EC inputs, with a 10- to 20-fold increase in TA pathway response measures (Wozny et al. 2005; Ang et al. 2006, Fig. 6). However, regulation of EC inputs by dentate granule cells was largely unchanged (Wu & Leong, 2001; Ang et al. 2006). This preserved gating function of the dentate gyrus may be due, at least in part, to the overexpression of synaptic GABAA receptors in dentate granule cells of epileptic animals, a possible compensatory cellular response to hyperactivity in the hippocampus (Gibbs et al. 1997; Brooks-Kayal et al. 1998; Cohen et al. 2003).
Figure 6. Excitation evoked by activation of the TA pathway is increased by an order of magnitude in area CA1 of epileptic animals.
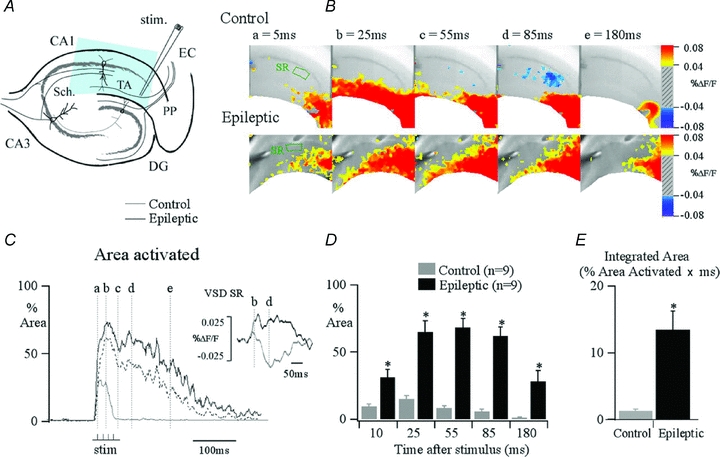
A, hippocampal schematic diagram illustrating the major afferent pathways to the hippocampus and the position of the stimulation electrode that is used to activate the TA pathway (stim.). The blue area indicates typical area imaged with our camera. B, VSD recording snapshots of control and epileptic responses at varying time points during the response to a burst stimulation applied to the TA pathway at the angular bundle. This stimulus produces spatially restricted TA activity in control. In contrast, in epileptic tissue, excitation is propagated throughout the stratum radiatum and pyramidale. This is demonstrated in C by comparing the percentage of the total CA1 area activated by TA stimulation in control (grey) and epileptic (black) plotted against time. Pixels showing depolarizations of ≥0.05%ΔF/F for control and ≥0.035%ΔF/F for epileptic are counted, normalized to the total CA1 area imaged, and computed as percentage area activated. Different ΔF/F scales were used in the two conditions because of the higher background fluorescence evident in epileptic tissue, probably due to gliosis. Computing percentage area activated using identical ΔF/F scales did not alter the findings (dotted line in C corresponds to data from epileptic slice computed at ≥0.05%ΔF/F). This reveals a greater area and prolonged period of activation in epileptic tissue compared with controls; inset traces depicting percentage change in fluorescence shows that this is caused in part by loss of inhibition, with an IPSP present in the stratum radiatum (SR) in control animals, which is transformed into an EPSP in epileptic animals. The dashed lines correspond to the time points illustrated in B. D, summary data illustrate the differences in both peak amplitude and time course of area activated for control (grey) compared against epileptic (black) for varying time points. An asterisk indicates that epileptic animals demonstrate a significant increase in the maximum area activated by temporoammonic stimulation over control for the selected time points. E, these results can be summarized as a simple comparison by integrating the area under the percentage pixel activated traces, capturing both the number and duration of pixels activated, which illustrates a 10-fold increase in activation in slices from epileptic animals compared with controls (ANOVA, *P≤ 0.05; n = 12). PP, perforant path; DG, dentate gyrus; TA, temporoammonic pathway; EC, entorhinal cortex. From Ang et al. 2006, with permission from the Society for Neuroscience.
Summary and conclusions
Both the dentate gyrus and CA1 circuits exert powerful regulation of cortical afferent input to the hippocampus, each using a differing combination of the intrinsic cellular properties of the principal neurons, and the dynamics of inhibitory circuit function in the two microcircuits. Although the precise mechanisms mediating this afferent regulation differ between circuits, the net outcome is the same: they strongly regulate potential loop interactions which, when unchecked, could predispose the hippocampus to seizures.
Identifying mechanisms regulating these nested EC–hippocampal loops was aided by use of voltage sensitive dye imaging techniques, which facilitate direct measures of membrane voltage activity in dendritic compartments. These compartments are the predominant cellular regions receiving the afferent cortical input into the hippocampus, as well as being downstream targets of hippocampal network activity. This focus on membrane voltage emphasizes the dynamics of movement of activity through a circuit, and reveals the mix of excitation and inhibition generated by local circuitry in response to afferent input. Such information is absent in field potential recordings, and is often obscured in traditional single cell intracellular approaches. The findings summarized above now pose additional questions about how these complex spatial and temporal dynamics modulate normal or abnormal cell firing; this is difficult to characterize with voltage sensitive dyes as the signals are often averaged in both space and time. Development of additional multicellular imaging techniques focusing on capturing ensemble firing activity promises to link our understanding of circuit level dendritic integration of afferent and local circuit activity to local circuit firing and patterned principal cell output.
References
- Acsady L, Kamondi A, Sik A, Freund TF, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang CW, Carlson G, Coulter DA. Hippocampal CA1 circuitry dynamically gates direct cortical inputs preferentially at theta frequencies. J Neurosci. 2005;25:9567–9580. doi: 10.1523/JNEUROSCI.2992-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci. 2006;6:11850–11856. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Hong Y, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression correlate with altered function in epileptic hippocampus. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Brun VH, Otnaess MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Carlson GC, Coulter DA. In vitro functional imaging in brain slices using fast voltage-sensitive dye imaging combined with whole-cell patch recording. Nat Prot. 2008;3:249–255. doi: 10.1038/nprot.2007.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla MK, Guzowski JF, McNaughton BL, Barnes CA. Sparse, environmentally selective expresssion of Arc RNA in the upper blade of teh rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragneire A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Quirk GL, Coulter DA. Dentate granule cell GABAA receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci. 2003;17:1607–1616. doi: 10.1046/j.1460-9568.2003.02597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Llinas R. Voltage-sensitive dye imaging of neocortical spatiotemporal dynamics to afferent activation frequency. J Neurosci. 2001;21:9403–9413. doi: 10.1523/JNEUROSCI.21-23-09403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Carlson GC. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog Brain Res. 2007;163:235–243. doi: 10.1016/S0079-6123(07)63014-3. [DOI] [PubMed] [Google Scholar]
- de Almeida L, Idiart M, Lisman JE. The input-output transformation of the hippocampal granule cells: from grid cells to place fields. J Neurosci. 2009;29:7504–7512. doi: 10.1523/JNEUROSCI.6048-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond NL, Scott CA, Jane JA, Jr, Levy WB. Ultrastructural identification of entorhinal cortical synapses in CA1 stratum lacunosum-moleculare of the rat. Hippocampus. 1994;4:594–600. doi: 10.1002/hipo.450040509. [DOI] [PubMed] [Google Scholar]
- Ewell LA, Jones MV. Frequency-tuned distribution of inhibition in the dentate gyrus. J Neurosci. 2010;30:12597–12607. doi: 10.1523/JNEUROSCI.1854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fricke RA, Prince DA. Electrophysiology of dentate gyrus granule cells. J Neurophysiol. 1984;51:195–209. doi: 10.1152/jn.1984.51.2.195. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, III, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABAA receptor function in dentate granule and CA1 neurons. J Neurophysiol. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- Han ZS, Buhl EH, Lorinczi Z, Somogyi P. A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local-circuit neurons in the dentate gyrus of the rat hippocampus. Eur J Neurosci. 1993;5:395–410. doi: 10.1111/j.1460-9568.1993.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res. 1992;(Suppl 7):273–280. [PubMed] [Google Scholar]
- Henze DA, Wittner L, Buzsaki G. Single granule cells reliably discharge targets in the hippocampal CA3 network. Nat Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Bragin A, Buzsaki G. Interdependence of multiple theta generators in the hippocampus: a partial coherence analysis. J Neurosci. 1999;19:6200–6212. doi: 10.1523/JNEUROSCI.19-14-06200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerant C, Malcolm AJ, Frotscher M. Afferent and efferent synaptic connections of somatostatin-immunoreactive neurons in the rat fascia dentate. J Comp Neurol. 1990;295:111–122. doi: 10.1002/cne.902950110. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Liang SL, Carlson GC, Coulter DA. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J Neurosci. 2006;26:8537–8548. doi: 10.1523/JNEUROSCI.0329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothman EW, Stringer JL, Bertram EH. The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res. 1992;(Suppl 7):301–313. [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Toneqawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2:508–514. doi: 10.1038/12229. [DOI] [PubMed] [Google Scholar]
- Ortinski PI, Dong J, Mungenast A, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak H, Weissinger F, Terunuma M, Carlson GC, Hsu F-C, Moss SJ, Coulter DA. Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci. 2007;27:14012–14022. doi: 10.1523/JNEUROSCI.4390-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- Remondes M, Shuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Salzberg BM, Obaid AL, Senseman DM, Gainer H. Optical recording of action potentials from vertebrate nerve terminals using potentiometric probes provides evidence for sodium and calcium components. Nature. 1983;306:36–40. doi: 10.1038/306036a0. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Tsunashima K, Wanzenbock C, Fuchs K, Sieghart W, Sperk G. GABAA receptor subunits in the rat hippocampus II: altered distribution in kainic acid-induced epilepsy. Neuroscience. 1997;80:1001–1017. doi: 10.1016/s0306-4522(97)00145-0. [DOI] [PubMed] [Google Scholar]
- Soltesz I. A brief history of cortico-hippocampal time with a special reference to the direct entorhinal input to CA1. Hippocampus. 1995;6:149–173. doi: 10.1002/hipo.450050206. [DOI] [PubMed] [Google Scholar]
- Spruston N, Johnston D. Perforated patch clamp analysis of the passive membrane properties of three classes of hippocampal neurons. J Neurophysiol. 1992;70:508–529. doi: 10.1152/jn.1992.67.3.508. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Otis TS, Mody I. Membrane properties of dentate granule cells: comparison of sharp microelectrode and whole cell recordings. J Neurophysiol. 1992;67:1346–1358. doi: 10.1152/jn.1992.67.5.1346. [DOI] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentate of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Sun C, Mtchedishvili Z, Erisir A, Kapur J. Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the α4 subunit of GABAA receptors in an animal model of temporal lobe epilepsy. J Neurosci. 2007;27:12461–12450. doi: 10.1523/JNEUROSCI.4141-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay D, Dudman JT, Siegelbaum SA. HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron. 2007;56:1076–1089. doi: 10.1016/j.neuron.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozny C, Gabriel S, Jandova K, Schulze K, Heinemann U, Behr J. Entorhinal cortex entrains epileptiform activity in CA1 in pilocarpine-treated rats. Neurobiol Dis. 2005;19:451–460. doi: 10.1016/j.nbd.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Wu K, Leong LS. Enhanced but fragile inhibition in the dentate gyrus in vivo in the kainic acid model of temporal lobe epilepsy: a study using current source density analysis. Neuroscience. 2001;104:1067–1077. doi: 10.1016/s0306-4522(01)00043-4. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of temporal lobe epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


