Non-technical summary
Action potentials generated at the level of the cell body can propagate back to neuronal dendrites, where they activate different types of voltage-sensitive calcium channels and produce massive calcium influx. Although these calcium signals may control dendritic integration, their mechanisms, dynamic properties and role in different cell types remain largely unknown. We found that in dendrites of hippocampal interneurons, an inhibitory cell type involved in control of network excitability, specific types of calcium channels are present but are recruited in an activity-dependent manner. Furthermore, their activation produces calcium rises spatially restricted to proximal dendritic sites, where they control the efficacy of transmission at inhibitory synapses. The pathway by which this happens appears to constitute a negative feedback loop – increased firing activity of interneurons potentiates the inhibitory drive that they receive, thus decreasing the activity of interneurons further. This may have a profound effect on the recruitment of interneurons and network activity.
Abstract
Abstract
In most central neurons, action potentials (APs), generated in the initial axon segment, propagate back into dendrites and trigger considerable Ca2+ entry via activation of voltage-sensitive calcium channels (VSCCs). Despite the similarity in its underlying mechanisms, however, AP-evoked dendritic Ca2+ signalling often demonstrates a cell type-specific profile that is determined by the neuron dendritic properties. Using two-photon Ca2+ imaging in combination with patch-clamp whole-cell recordings, we found that in distinct types of hippocampal inhibitory interneurons Ca2+ transients evoked by backpropagating APs not only were shaped by the interneuron-specific properties of dendritic Ca2+ handling but also involved specific Ca2+ mechanisms that were regulated dynamically by distinct activity patterns. In dendrites of regularly spiking basket cells, AP-evoked Ca2+ rises were of large amplitude and fast kinetics; however, they decreased with membrane hyperpolarization or following high-frequency firing episodes. In contrast, AP-evoked Ca2+ elevations in dendrites of Schaffer collateral-associated cells exhibited significantly smaller amplitude and slower kinetics, but increased with membrane hyperpolarization. These cell type-specific properties of AP-evoked dendritic Ca2+ signalling were determined by distinct endogenous buffer capacities of the interneurons examined and by specific types of VSCCs recruited by APs during different patterns of activity. Furthermore, AP-evoked Ca2+ transients summated efficiently during theta-like bursting and were associated with the induction of long-term potentiation at inhibitory synapses onto both types of interneurons. Therefore, the cell type-specific profile of AP-evoked dendritic Ca2+ signalling is shaped in an activity-dependent manner, such that the same pattern of hippocampal activity can be differentially translated into dendritic Ca2+ signals in different cell types. However, Cell type-specific differences in Ca2+ signals can be ‘smoothed out’ by changes in neuronal activity, providing a means for common, cell-type-independent forms of synaptic plasticity.
Introduction
When a neuron fires an action potential (AP), it does not simply transmit a signal along its axon to the postsynaptic target, but also sends it back to its own dendrites, activating specific membrane conductances and thus controlling the integrative properties of dendrites. Different types of voltage-sensitive calcium channels (VSCCs) are activated by backpropagating APs in neuronal dendrites and represent a major source of Ca2+ influx (Callaway & Ross, 1995; Yuste & Denk, 1995; Sabatini & Svoboda, 2000). The development of a two-photon Ca2+ imaging technique to monitor Ca2+ signalling in neuronal dendrites (Denk et al. 1995; Yuste & Denk, 1995; Sabatini & Svoboda, 2000; Sabatini et al. 2002) allowed the detailed investigation of the functional organization of dendritic Ca2+ and, therefore, a better understanding of its role in dendritic information processing. In inhibitory interneurons (INs), dendritic Ca2+ transients evoked by backpropagating APs (AP-CaTs) may exhibit cell type-specific properties due to specific endogenous Ca2+ binding capacities and active properties of their dendrites (Goldberg et al. 2003, 2004; Rozsa et al. 2004; Aponte et al. 2008). AP-evoked Ca2+ signalling plays a role in the induction of several forms of synaptic plasticity at both excitatory and inhibitory synapses in INs (Perez et al. 2001; Lei & McBain, 2002; Lapointe et al. 2004; Lamsa et al. 2005; Patenaude et al. 2005; Ali, 2007). These functions of AP-CaTs, however, require specific patterns of IN activity (e.g. burst firing, persistent depolarization). Thus, given that different modes of IN activity may be associated with activation of distinct Ca2+ mechanisms, it is important to elucidate their implication as well as their cell type-specific regulation by the activity itself. Despite the strong expression of different types of VSCCs in INs (Vinet & Sík, 2006), the role and regulation of VSCCs in IN dendrites remain largely unknown (Topolnik et al. 2009). Similarly, very little is currently known about the activity-dependent regulation of dendritic AP-CaTs (Yasuda et al. 2003; Scheuss et al. 2006). Activity-dependent slowing of AP-CaTs due to depression of Ca2+ extrusion via plasma membrane Ca2+ pumps and Na+/Ca2+ exchangers was reported during high-frequency firing activity in CA1 pyramidal neurons (Scheuss et al. 2006). Furthermore, following high-frequency firing episodes, AP-CaTs undergo long-term depression, which is expressed as a decrease in the open probability of R-type VSCCs (Yasuda et al. 2003). Such activity-dependent regulation of AP-evoked dendritic Ca2+ signalling remains elusive in distinct types of hippocampal inhibitory INs that are differentially involved in hippocampal rhythmic activity, associated with specific spatial and cognitive tasks in animals and humans (O'Keefe & Recce, 1993; Soltesz & Deschênes, 1993; Csicsvari et al. 1998; Klausberger et al. 2003).
In this paper, we examined the properties, activity-dependent regulation and role in synaptic plasticity of Ca2+ transients associated with backpropagating APs in dendrites of two types of hippocampal inhibitory interneurons: regularly spiking basket cells and Schaffer collateral-associated cells.
Methods
Electrophysiology
All experiments were carried out in accordance with the animal welfare guidelines of the Animal Protection Committee of Université Laval. A total of 140 C57BL/6 mice (P15–30; mean age ± SD, 22 ± 3 days; Charles River, St Laurent, Québec, Canada) were used in this study. Animals were deeply anaesthetized with isoflurane and decapitated. Transverse hippocampal slices (300 μm) were prepared in ice-cold (0–4°C) ‘cutting’ solution containing (in mm): 250 sucrose, 2 KCl, 1.25 NaH2PO4, 26 NaHCO3, 7 MgSO4, 0.5 CaCl2 and 10 glucose, saturated with 95% O2–5% CO2, pH 7.4; 320–340 mosmol l−1. Slices were transferred to a heated (35°C) and oxygenated solution containing (in mm): 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 3 MgSO4, 1 CaCl2 and 10 glucose for 30 min. After this they were kept at room temperature until use. During experiments, slices were continuously perfused (2 ml min−1) with standard artificial cerebrospinal fluid (ACSF) containing (in mm): 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 MgSO4, 2 CaCl2 and 10 glucose, saturated with 95% O2–5% CO2, pH 7.4, at near-physiological temperature (30–33°C).
Whole-cell recordings were obtained from INs in the stratum radiatum (RAD) in the CA1 area identified using a 40× water-immersion objective and Dodt infrared scanning gradient contrast (ISGC; Leica TCS SP5) optics. Recording pipettes (3.5–6 MΩ) were made from borosilicate glass capillaries (1B100F-4; World Precision Instruments Inc., Sarasota, FL, USA) pulled on a Flaming–Brown-type micropipette puller (P-97; Sutter Instrument Co., Novato, CA, USA). For whole-cell current-clamp recordings, the pipette solution contained the following (in mm): 130 KMeSO3, 2 MgCl2, 10 diNa-phosphocreatine, 10 Hepes, 2–4 ATP-Tris, 0.2 GTP-Tris and 0.15–0.2% biocytin, pH 7.25–7.35; 275–285 mosmol l−1. Patch solution also contained either 50–200 μm Oregon Green 488 BAPTA-1 (OGB-1) or 300 μm Fluo-5F, to monitor calcium elevations, and 30 μm Alexa Fluor-594, to image neuronal morphology (all from Invitrogen).
Monosynaptic inhibitory postsynaptic currents (IPSCs) were evoked at 0.1 Hz via local stimulation with an electrode (2–3 MΩ) that was filled with ACSF, connected to a constant current isolation unit (A360LA; World Precision Instruments Inc., Sarasota, FL, USA) and positioned in the RAD. IPSCs were recorded at −50 mV in the presence of the NMDA and AMPA/kainate receptor antagonists dl-2-amino-5-phosphonopentanoic acid (DL-AP5; 50 μm) and 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX; 10 μm), respectively. Synaptic plasticity at inhibitory synapses was induced by repetitive theta-burst firing (TBF) at 5 Hz for 2 s (each burst consisting of 3 APs at 100 Hz), which was delivered three times at 30 s intervals. The paired-pulse ratio (PPR) of IPSCs was calculated as the ratio between the mean peak amplitude of the second response and the mean peak amplitude of the first response. The coefficient of variation (CV) of IPSCs was calculated as the ratio between the standard deviation of current amplitude and the mean current amplitude.
Two-photon Ca2+ imaging
Intracellular Ca2+ imaging was performed using a TCS SP5 two-photon laser-scanning microscope (Leica Microsystems Inc.) based on a Ti-Sapphire laser (Chameleon Ultra II; Coherent, Santa Clara, CA, USA; >3W, 140 fs pulses, 80 Hz repetition rate) tuned to 800 nm. A long-range water-immersion objective (40×; NA, 0.8) was used and emitted photons were collected in epifluorescence mode with external photomultiplier tubes.
Neurons were filled via the patch electrode for 20–30 min before imaging. Red fluorescence from Alexa Fluor-594 was used to locate dendrites of interest 10–200 μm from the soma (Fig. 2). Backpropagating APs were evoked by somatic current injection (0.8–1 nA, 2 ms). To measure Ca2+ signals, green and red fluorescence was collected during 500 Hz line scans across the dendrite (Fig. 2). Fluorescence changes were quantified as (1) increases in green fluorescence from baseline normalized to the red fluorescence (ΔG/R) (Sabatini et al. 2002) or (2) changes in green fluorescence F(t) from baseline F0: ΔF/F(t) = (F(t) −F0)/F0. Calcium concentration ([Ca2+]) was calculated from ΔF/F or ΔG/R using a method that relies on the estimation of the fluorescence increase that would arise from a saturating Δ[Ca2+] (ΔF/Fmax or ΔG/Rmax) (Maravall et al. 2000; Sabatini et al. 2002; Yasuda et al. 2004).
Figure 2. Imaging dynamics of action potential-evoked Ca2+ transients in IN dendrites.
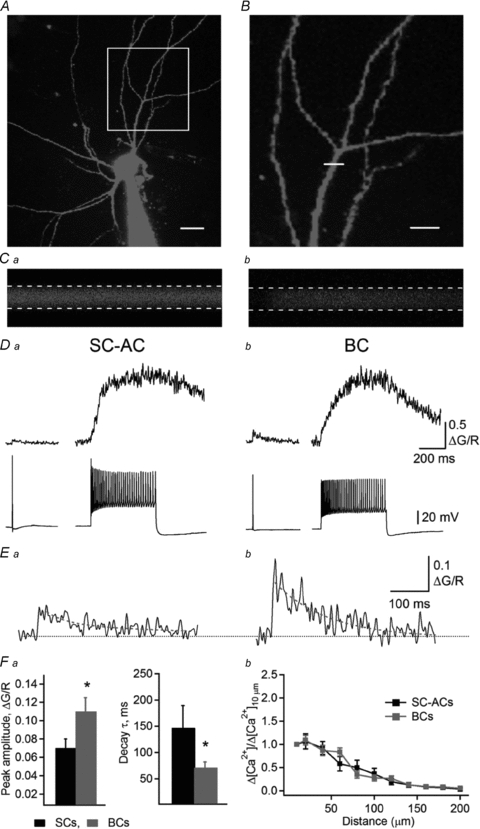
A, two-photon image (maximum projection of a Z-stack) of an IN filled with Alexa-594 and Fluo-5F. Scale bar, 20 μm. B, magnified image of a dendritic arbour (boxed region in A). The white line across the dendrite indicates the position of the linescan. Scale bar, 20 μm. C, linescan images obtained from the red (Ca) and green (Cb) channels simultaneously, in response to an 80 Hz train of backpropagating APs. Dashed lines indicate the region of interest for signal measurements. D, representative CaTs evoked by a single AP (left) or by saturating trains (right) in SC-ACs (Da) and BCs (Db). E, expanded traces of CaTs evoked by a single AP in SC-ACs (Ea) and BCs (Eb). Red lines correspond to double exponential fits. F, summary data showing the differences in the CaT peak amplitude (Fa, left) and decay time constant (Fa, right), as well as [Ca2+] changes as a function of distance (Fb) in the two cell types. Data for SC-ACs are shown in black and data for BCs are shown in red.
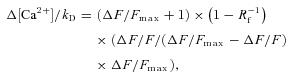 |
(1) |
or
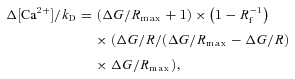 |
(2) |
where kD is the constant of dissociation and Rf is the dynamic range of the indicator. Values used were Rf= 6 and kD= 206 nm for OGB-1 (Maravall et al. 2000) and Rf= 240 and kD= 1280 nm for Fluo-5F (Woodruff et al. 2002). ΔF/Fmax or ΔG/Rmax was determined from AP trains (60–80 Hz). For calculations of resting calcium concentration ([Ca2+]0, Maravall et al. 2000), measurements with OGB-1 were used exclusively, as Fluo-5F exhibits little fluorescence under basal conditions.
| (3) |
To ensure that the high-affinity indicator OGB-1 was not saturated during the Ca2+ influx evoked by five APs, we compared the peak amplitude of CaTs evoked by five APs with that of a maximal response (ΔF/Fmax) evoked by high-frequency AP trains (60–80 Hz, 1 s). In all recorded INs, the peak amplitude of CaTs evoked by five APs was at least two times smaller than ΔF/Fmax, indicating the absence of dye saturation in response to five APs. Precautions were taken to avoid ejecting dye from the pipette into the slice, and no background correction was performed.
Buffer capacity measurement
Endogenous calcium buffer capacity (κS) was measured by competing endogenous buffers with increasing concentrations of exogenous buffer (50, 100 and 200 μm OGB-1; Fig. 3) (Helmchen et al. 1996; Maravall et al. 2000; Sabatini et al. 2002). ΔF/FAP was converted to [Ca2+]AP and the inverse peak of [Ca2+]AP was plotted as a function of the exogenous buffer capacity (κB) (Fig. 3).
Figure 3. Estimation of [Ca2+]AP dynamics and endogenous buffer capacity.
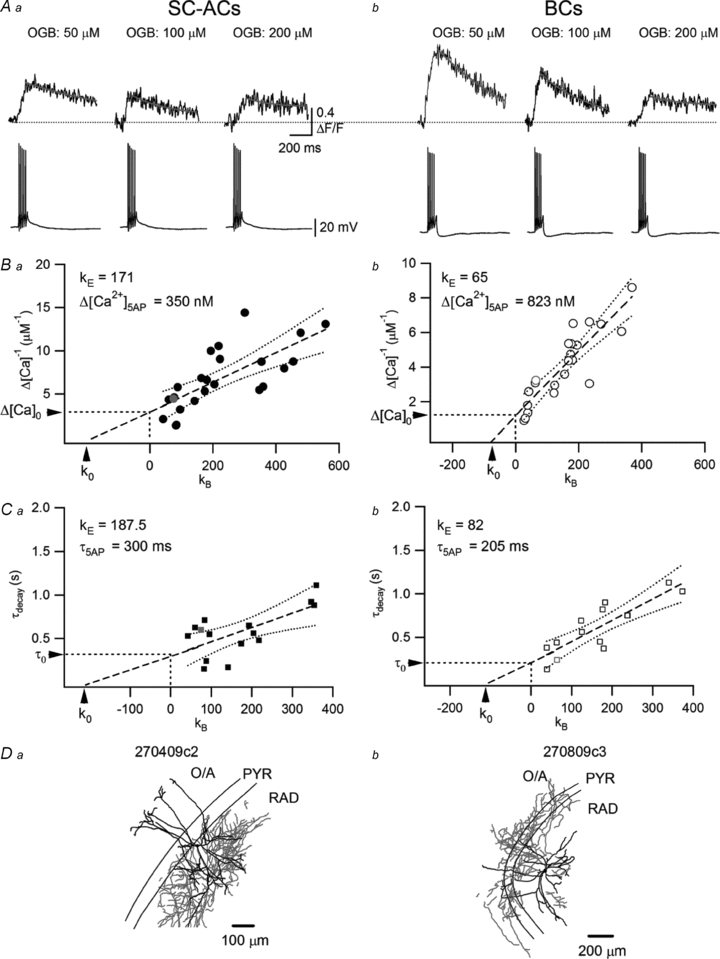
A, representative CaTs (top) recorded with different concentrations of OGB-1 in response to five APs (bottom) in SC-ACs (Aa) and BCs (Ab). B, summary plots of the inverse of the peak amplitude of AP-CaTs (1/Δ[Ca2+]5APs), as a function of added Ca2+ buffer capacity (κB) for SC-ACs (Ba) and BCs (Bb). The intercepts of linear fits (straight dashed lines) with the x-axis were used to estimate the endogenous Ca2+ binding capacity (κ0), whereas the fit extrapolation to ‘zero’ buffering conditions was used to derive the amplitude of [Ca2+]5APs in the absence of dye (Δ[Ca2+]0). C, summary plots of the decay time constant as a function of added Ca2+ buffer capacity (κB) for SC-ACs (Ca) and BCs (Cb). Arrows indicate the calculated endogenous Ca2+ binding capacity (κ0) and τdecay in the absence of added buffer (τ0). Dashed lines are the best linear fits to the data and the dotted lines are the 95% confidence bands of the fits. D, Neurolucida reconstructions of two representative biocytin-filled INs with axonal (red) and dendritic (black) arborizations in different layers.
| (4) |
where [B]t is the total concentration of the buffer (Neher & Augustine, 1992). The relationships between [Ca2+]AP−1 or τdecay and κB were fitted by linear regression. κS was determined by extrapolating the fits to the x-intercept, whereas the amplitude of Δ[Ca2+]AP in conditions of ‘zero’ added buffer (κB= 0) was found by extrapolating the fits to the y-intercept (Fig. 3) (Helmchen et al. 1996). Ninety-five per cent confidence intervals (95% CIs) were determined using curve-fitting routines and error estimation in Igor Pro (WaveMetrics Inc., Lake Oswego, OR, USA) and were reported for each value.
Data acquisition and analysis
Image acquisition was performed using the Leica LAS software (Leica Microsystems Inc.). Physiological data acquisition (filtered at 2–3 kHz and digitized at 10 kHz) was performed using a data acquisition board (Digidata 1440 with Multiclamp 700B amplifier, Molecular Devices, Sunnyvale, CA, USA) and the Clampex 10.2 software (Molecular Devices). Data were analysed using Clampfit 10.2 (Molecular Devices) and Igor Pro (WaveMetrics).
Amplitudes of AP-evoked Ca2+ transients were measured at the peak of the waveform as averages over a 10 ms time window. The decay values were determined from double exponential fits and expressed as an amplitude-weighted average decay time constant. Summary data are shown as means ± SEM and analysed using Student's paired t test. Significance between groups was assessed using the unpaired t test or one-way ANOVA.
Anatomical reconstruction and immunohistochemistry
For anatomical reconstruction, neurons were filled with biocytin (Sigma) during whole-cell recordings. Slices with recorded cells were fixed overnight with 4% paraformaldehyde at 4°C. To reveal biocytin, slices were rinsed several times in Tris-buffered saline (TBS; pH 7.4, t = 25°C), treated with hydrogen peroxide (0.3%) for 30 min and permeabilized with 3% Triton X-100 in TBS for 1 h. To reduce non-specific background staining, the slices were then incubated for 30 min in TBS containing 10% normal goat serum (NGS) and 0.5% bovine serum albumin (BSA). Finally, the slices were incubated overnight at 4°C with streptavidin-conjugated Alexa-546 (dilution, 1:200; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA, Baltimore Pike, PA, USA) in TBS containing 1% NGS and 0.5% BSA. The following day, sections were rinsed with TBS and mounted in Dako fluorescence medium (Dako Canada Inc., Mississauga, ON, Canada). Confocal images of biocytin-filled INs were obtained using a Leica TCS SP5 imaging system coupled with a 543 nm He–Ne laser. IN Z-stacks were acquired with a 1 μm step. Final stacks containing different parts of INs were merged in Neurolucida 8.26.2 (Williston, VT, USA) and selected cells were reconstructed.
For immunocytochemical analysis, whole-cell recordings were performed for a maximum of 5 min, during which neurons were filled with fluorescein dextran (0.2%; Invitrogen). Slices with recorded neurons were fixed with 4% paraformaldehyde, embedded in 2% agar and re-sectioned to 40 μm. Sections were treated with hydrogen peroxide, permeabilized with 0.2% Triton X-100 in TBS containing normal donkey serum (1%) and BSA (2%) and incubated overnight at 4°C with a rabbit cholecystokinin antibody (dilution, 1:6000; Sigma-Aldrich, St Louis, MO, USA). The following day, sections were rinsed with TBS and incubated with an anti-rabbit Texas Red secondary antibody (dilution, 1:200; Jackson ImmunoResearch) for 1.5 h. Sections were then rinsed in TBS and mounted in Dako fluorescence medium (Dako Canada Inc.). Images were captured using a Leica TCS SP5 system.
Chemicals
In some experiments, one of the following drugs was added to the ACSF: the L-type VSCC blocker nifedipine (10 μm), the R-type VSCC antagonist SNX-482 (30 nm), the selective T-type VSCC blocker NNC 55-0396 (10 μm), the P/Q-type VSCC antagonist ω-agatoxin IVA (AgTx, 250 nm), the N-type VSCC antagonist ω-conotoxin GVIA (CTx, 250 nm) or the SERCA inhibitor cyclopiazonic acid (CPA, 30 μm). A combination of all VSCC blockers and of CPA (cocktail; Fig. 4C) or of dl-AP5 (50 μm) and NBQX (10 μm) were applied when indicated. All chemicals were stored as stock solutions at −20°C, diluted on the day of the experiment and either added to the perfusion system (nifedipine, NNC 55-0396, CPA, dl-AP5 and NBQX) or applied directly into the recording chamber (toxins). In some experiments, BAPTA (10 mm) was included in the patch solution (Fig. 8B and C). Nifedipine, SNX-482, NNC 55-0396, CPA and BAPTA were purchased from Sigma, and ω-agatoxin IVA and ω-conotoxin GVIA were obtained from Alomone Labs (Jerusalem, Israel). dl-AP5 and NBQX were obtained from Ascent Scientific (Princeton, NJ, USA).
Figure 4. Differential contribution of voltage-sensitive Ca2+ channels to action potential-evoked Ca2+ transients in IN dendrites.
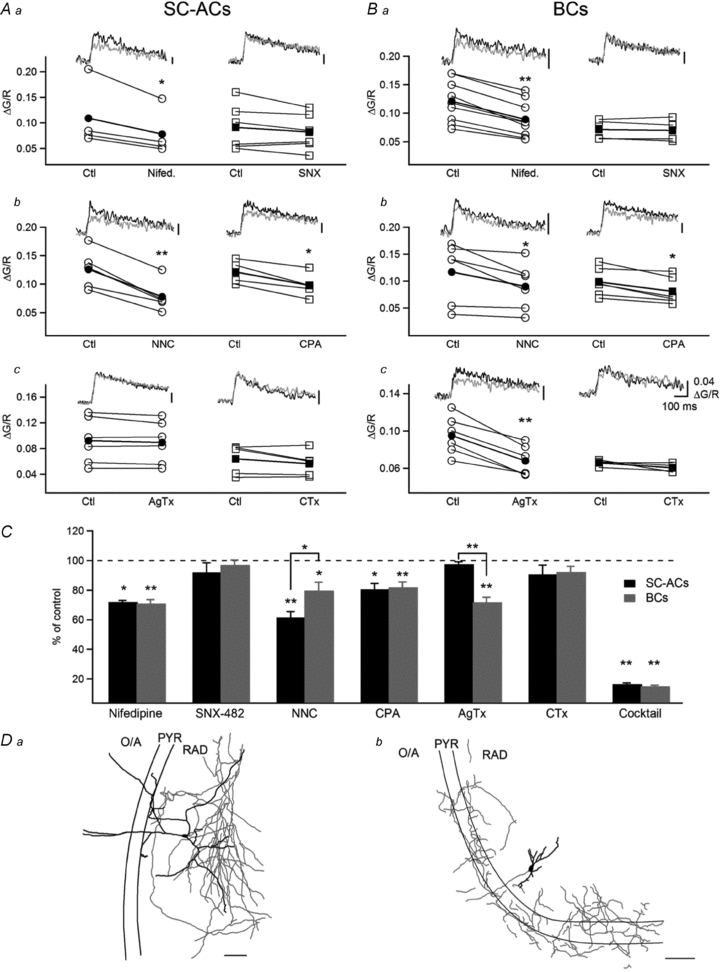
A and B, summary plots and representative AP-CaTs recorded using Fluo-5F in a control setting (black trace) and 10–15 min after the administration of blockers (green trace) of L-type (nifedipine, 10 μm; Aa, Ba, left), R-type (SNX-482, 10 μm; Aa, Ba, right), T-type (NNC 55-0396, 10 μm; Ab, Bb, left), P/Q-type (ω-agatoxin IVA (AgTx), 250 nm; Ac, Bc, left) and N-type (ω-conotoxin GVIA (CTx), 250 nm; Ac, Bc, right) VSCCs and intracellular Ca2+ stores (CPA, 30 μm; Ab, Bb, right) in SC-ACs (A) and BCs (B). C, summary bar graph of group data for AP-CaT mechanisms, showing that L- and T-type VSCCs and stores contributed to AP-CaTs in SC-ACs, whereas L-, T- and P/Q-type VSCCs and stores mediated AP-CaTs in BCs. Data are expressed as percentages of control CaTs obtained prior to drug application. D, Neurolucida reconstructions of the two representative biocytin-filled INs from which the recordings were obtained, with axonal arborization shown in red and dendrites shown in black.
Figure 8. Theta-burst firing induced LTP at inhibitory synapses onto both SC-ACs and BCs.
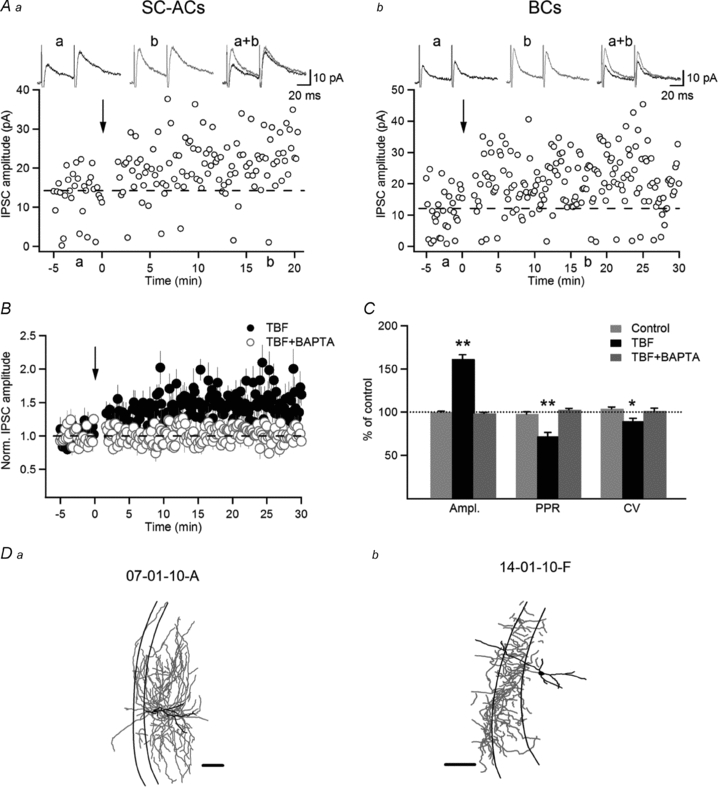
A, representative examples of IPSC amplitudes vs. time in SC-AC (Aa) and BC (Ab), showing LTP of IPSCs induced by TBF in the two cell types. The traces at the top are average IPSCs (average of 30 sweeps, failures included) in a control setting (a), 20 min after TBF (b) and their superimposition. B, normalized group data showing LTP in control (black symbols; n = 7) and its absence when BAPTA (10 mm) was included in the recording solution (red symbols; n = 4). C, summary bar graphs showing changes in the IPSC peak amplitude, PPR and CV induced by TBF. The LTP of IPSCs was associated with a significant decrease in PPR and CV, indicating a presynaptic locus for its expression. D, Neurolucida reconstructions of the two representative biocytin-filled INs that were used to obtain the recordings depicted in A. Scale bars, 100 μm. The error bars throughout represent the SEM.
Results
Identification of INs based on anatomical, neurochemical and electrophysiological criteria
In the present study, a total of 274 INs were recorded in the CA1 stratum radiatum (RAD). Of these cells, 223 neurons were labelled successfully with biocytin and reconstructed anatomically. Based on anatomical criteria, 72 recorded neurons were identified as basket cells (BCs), and 69 neurons were identified as Schaffer collateral-associated cells (SC-ACs; Fig. 1A). In particular, both cell types had extensive dendritic branching in the RAD, stratum pyramidale (PYR) and oriens/alveus (O/A), but were clearly distinguishable based on their axonal arborizations. The axons of SC-ACs ran almost entirely (∼90%) within the RAD, with a few branches entering the PYR and O/A (Fig. 1Aa, 3Da, 4Da, 5C, 6Da, 7E and 8Da). In contrast, the axons of BCs covered essentially the PYR and the adjacent part of the O/A and RAD, largely following the orientation of the pyramidal layer (Fig. 1Ab, 3Db, 4Db, 5D, 6Db, 7F and 8Db). INs that were identified anatomically were then compared in terms of their electrophysiological properties (Fig. 1B). Consistent with previous findings (Vida et al. 1998; Cope et al. 2002; Pawelzik et al. 2002), both cell types exhibited a regularly spiking firing pattern but differed in their response to hyperpolarizing current injections, with SC-ACs showing a significant hyperpolarization-activated Ih current and a rebound depolarizing potential followed by a spike (Fig. 1Ba) and the BCs demonstrating a small or no Ih and lacking the rebound spike (Fig. 1Bb). Finally, in a separate series of experiments, INs were recorded for 5 min, during which electrophysiological properties were determined and cells were filled with fluorescein dextran to allow post hoc neurochemical identification (Fig. 1C). Twelve recorded neurons proved to be cholecystokinin-immunoreactive (CCK-IR) neurons, of which five were regularly spiking neurons with a large Ih and a rebound spike (properties typical of SC-ACs; Fig. 1Ba and 1Ca), and seven were regularly spiking cells lacking Ih and a rebound spike (features of BCs; Fig. 1Bb and 1Cb). Therefore, these cells were considered as CCK-positive SC-ACs and BCs, based on the expression of CCK and the similarity of their electrophysiological properties to those of SC-ACs and BCs identified anatomically and recorded in Ca2+ imaging experiments. Given that a typical Ca2+ imaging experiment required at least 60 min of whole-cell recording followed by extensive wash-out of intracellular content (Pusch & Neher, 1988; Müller et al. 2005), we were not able to identify neurochemically INs filled with biocytin during Ca2+ imaging experiments. Accordingly, for the remainder of this study, we based the identification of INs exclusively on a combination of anatomical and electrophysiological criteria. All recorded regularly spiking INs were divided into two groups: SC-ACs if they had ∼90% of axonal arborization within the RAD and a prominent Ih and a rebound spike, and BCs if they had axons ramifying within the PYR with electrophysiological properties typical for BCs (lacking Ih and the rebound spike). Accordingly, cells that showed different morphologies (axons ramifying in all layers or directed to the stratum lacunosum-moleculare; densely packed axons) or electrophysiological properties (fast-spiking, rapidly adapting or irregularly spiking stuttering firing pattern) were excluded from the analysis (n = 82).
Figure 1. Comparison of the anatomical, electrophysiological and neurochemical properties of hippocampal CA1 basket cells and Schaffer collateral-associated cells.
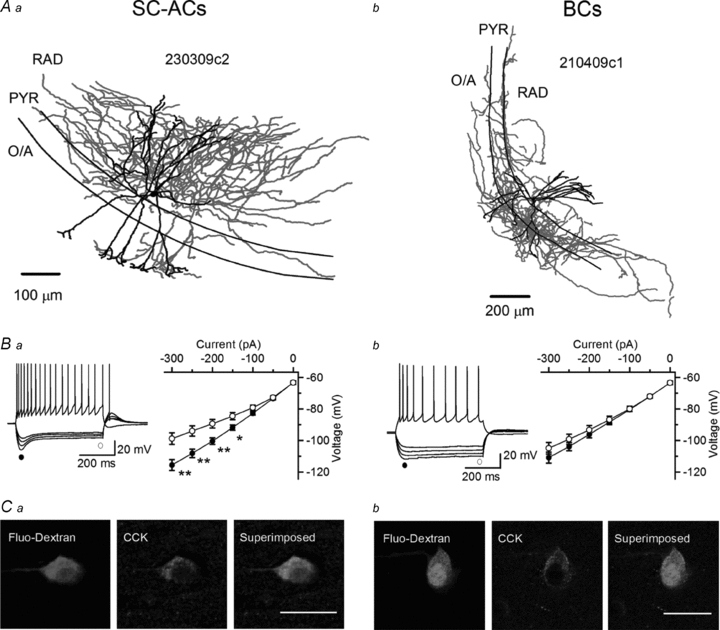
A, Neurolucida reconstructions of biocytin-filled INs with axonal (red) and dendritic (black) arborizations in different layers. B, voltage responses of an SC-AC (Ba) and a BC (Bb) to hyperpolarizing and depolarizing current injections (left) and current–voltage plots of peak (•) and steady-state (○) voltage responses to hyperpolarizing currents (right). Both SC-ACs and BCs showed a regularly spiking firing pattern, whereas only SC-ACs exhibited a significant response rectification (Ih) evoked by membrane hyperpolarization and a rebound spike. *P < 0.05, **P < 0.01, paired t test. C, confocal images showing immunoreactivity for cholecystokinin (CCK; middle) in putative SC-ACs (Ca) and BCs (Cb) that were filled with fluorescein dextran during recordings (left) and were identified based on the similarity of their electrophysiological properties to those of anatomically reconstructed INs. Scale bars, 20 μm.
Figure 5. Regulation of action potential-evoked Ca2+ transients by membrane potential.
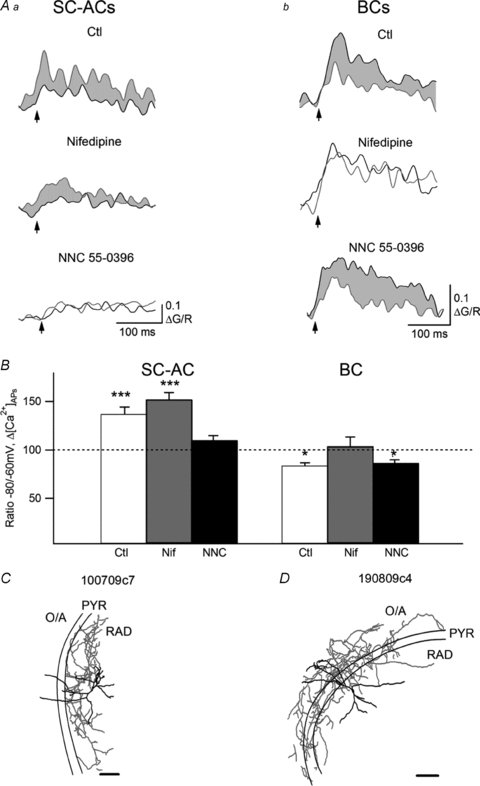
A, representative examples of AP-CaTs (average of three) evoked in SC-ACs (Aa) and BCs (Ab) by a burst of three APs applied at −60 mV (black) and at −80 mV (red) in control (top) and in the presence of the L-type VSCC blocker nifedipine (10 μm, middle) or the T-type VSCC blocker NNC 55-0396 (10 μm, bottom) tested in different experiments. Red shadowed areas show increases in CaTs in SC-ACs whereas grey shadowed areas correspond to AP-CaTs’ decreases in BCs. B, summary bar graph showing the ratio of the amplitudes of Δ[Ca2+]3APs at −80 and −60 mV, which were measured in control conditions and in the presence of nifedipine (Nif) and NNC 55-0396 (NNC). *P < 0.05, ***P < 0.001, paired t test. Data were recorded using Fluo-5F as a Ca2+ indicator. C and D, Neurolucida reconstructions of two representative INs from which the recordings were obtained. Scale bars, 200 μm.
Figure 6. Activity-dependent depression of action potential-evoked Ca2+ transients in dendrites of BCs.
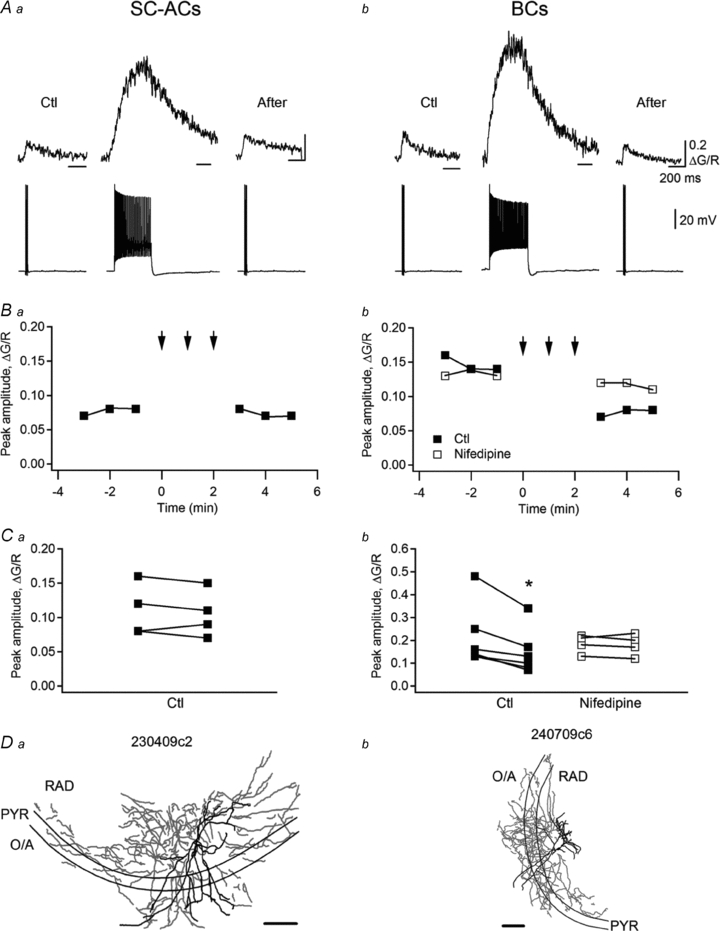
A, sample traces of AP-CaTs (average of three) evoked by a burst of three APs before (Ctl) and after (After) AP trains (65 Hz, 0.5 s) in an SC-AC (Aa) and a BC (Ab). B, representative data showing the depression of AP-CaTs selectively in BCs (Bb) and its absence in SC-ACs (Ba). Note that the depression of AP-CaT in BCs was not observed in the presence of the L-type VSCC blocker nifedipine (10 μm). Arrows indicate the periods of time during which AP trains were applied. C, summary data for a population of cells showing the effect of AP trains on AP-CaTs’ amplitude in the dendrites of SC-ACs (Ca) and BCs (Cb). Experiments were performed using Fluo-5F as a Ca2+ indicator. D, Neurolucida reconstructions of two representative INs from which the recordings were obtained. Scale bars, 200 μm.
Figure 7. Temporal summation of action potential-evoked Ca2+ transients during theta bursting.
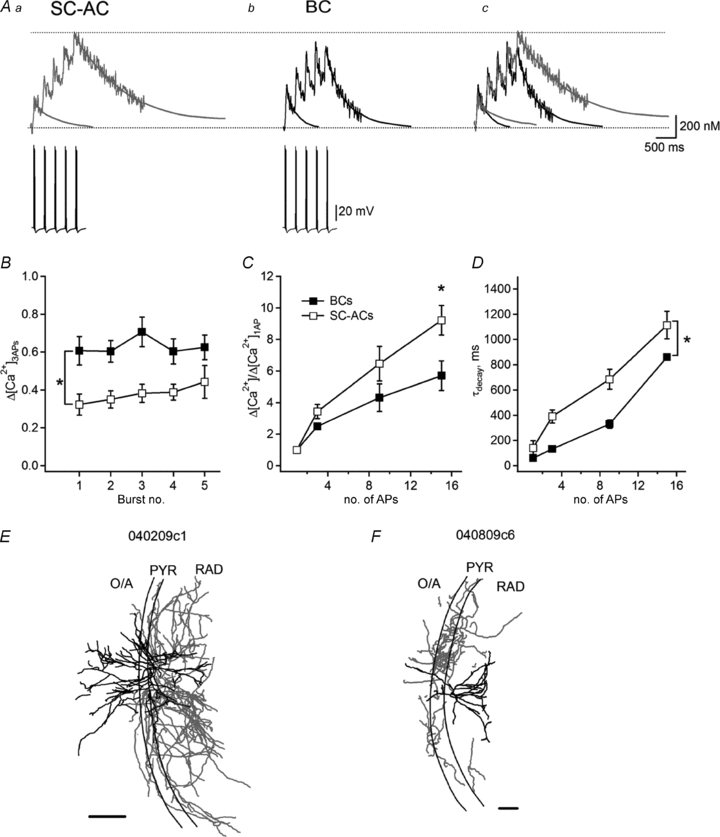
A, representative examples of AP-CaTs (average of three; top) recorded using Fluo-5F in the dendrites of SC-ACs (Aa) and BCs (Ab), as well as their superimposition (Ac) in response to five bursts of three APs applied at a 4 Hz frequency (bottom). B, summary plot showing the amplitude of Δ[Ca2+]3APs evoked by individual bursts in the two cell types. C, summary plot showing a degree of summation of Δ[Ca2+]3APs during continuous bursting in dendrites of the two cell types. Note a higher summation level in SC-ACs. D, summary plot demonstrating changes in τdecay of Δ[Ca2+]3APs during continuous bursting in the two cell types. *P < 0.05, ANOVA. E–F, Neurolucida reconstructions of two representative biocytin-filled INs from which the recordings were obtained. Scale bars, 200 μm.
Larger and faster Ca2+ transients in dendrites of BCs
Combined two-photon Ca2+ imaging and patch-clamp whole-cell recordings were performed in RAD INs at a temperature of 30–32°C. INs were filled with a combination of green medium-affinity Ca2+ indicator (Fluo-5F) and a Ca2+-insensitive red fluorophore (Alexa-594; to assess dendritic morphology, Fig. 2A–C). After the dye-loading period (20–30 min), INs were held in the current clamp mode and short depolarizing pulses (0.8–1 nA, 2 ms) were used to trigger an AP. AP-CaTs were measured in proximal dendritic branches up to 200 μm from the soma (Fig. 2). The dendritic diameter in this region was similar in both cell types (BCs, 3.7 ± 0.1 μm, n = 9; SC-ACs, 3.5 ± 0.1 μm, n = 8; P > 0.05). However, in BCs, AP-CaTs that were evoked by single APs had a large amplitude (ΔG/R: 0.11 ± 0.02, n = 11) and a relatively fast decay time constant (70.7 ± 11 ms, n = 9; Fig. 2D, E and Fa). In contrast, in SC-ACs, AP-CaTs exhibited a significantly smaller amplitude (ΔG/R: 0.07 ± 0.01, n = 9, P < 0.05) and slower decay kinetics (147 ± 42.1 ms, n = 8, P < 0.05; Fig. 2D, E and Fa). In both cell types, AP-CaTs were completely abolished by tetrodotoxin (1 μm), indicating that they were mediated by backpropagating APs.
To determine the actual amplitude of Ca2+ rises in IN dendrites and to compare it between different cell types, we converted ΔG/R to Ca2+ concentration ([Ca2+]AP) using a method developed by the group of Svoboda (Maravall et al. 2000; Sabatini et al. 2002; Yasuda et al. 2004), which relies on the determination of the fluorescence of the Ca2+ indicator at saturating [Ca2+] (ΔG/Rmax) (eqn (2); Fig. 2D). The value of ΔG/Rmax was obtained from the plateau fluorescence reached during AP trains at 60–80 Hz (Fig. 2D). On average, [Ca2+]1AP was significantly larger in BCs compared with SC-ACs (BCs: 212 ± 34 nm, n = 11 vs. SC-ACs: 131 ± 15 nm, n = 9; P < 0.05). In both cell types, [Ca2+]APs declined significantly at distances from the cell body exceeding 60 μm and were almost undetectable in distal dendrites (>150 μm; Fig. 2Fb).
Given that the presence of exogenous Ca2+ buffer perturbs the dynamics of Ca2+ signals significantly (Helmchen et al. 1996), we next inferred [Ca2+]AP dynamics in the absence of the indicator. For this series of experiments, cells were loaded with various concentrations of a bright, high-affinity Ca2+ indicator (Oregon Green BAPTA-1: 50, 100 and 200 μm; Fig. 3) and AP-CaTs were evoked by small bursts of five APs (to obtain clearly defined fluorescence transients that allow precise measurements of the decay kinetics at different dye concentrations). Resting Ca2+ concentrations ([Ca2+]0), Δ[Ca2+]5APs and τdecay were determined for each indicator concentration. [Ca2+]0 was similar in both cell types (BCs: 64 ± 5 nm, n = 22; SC-ACs: 60 ± 4 nm, n = 23; P > 0.05; eqn (3)) and did not vary significantly with the concentration of the indicator (50 μm OGB-1, 77 ± 8 nm, n = 13; 100 μm OGB-1, 60 ± 7 nm, n = 21; 200 μm OGB-1, 77 ± 13 nm, n = 11; P > 0.05; ANOVA). As expected, Δ[Ca2+]5APs decreased and τdecay increased linearly as a function of indicator concentration and, accordingly, total buffer capacity (Fig. 3A). Extrapolation to ‘zero’ buffer concentration (the y-axis intercept of the linear fits to the data points) allowed the determination of Δ[Ca2+]5APs and τdecay under native conditions (without added Ca2+ buffer). Accordingly, in dendrites of BCs, Δ[Ca2+]5AP values were of larger amplitude and faster kinetics (Δ[Ca2+]5APs: 0.82 μm (0.5–2.8 μm); τdecay: 205 ms (30–388 ms)) compared with those recorded in dendrites of SC-ACs (Δ[Ca2+]5APs: 0.35 μm (0.21–1.0 μm); τdecay: 300 ms (85–515 ms), Fig. 3B and C).
To determine whether the differences in the amplitude and kinetics of Ca2+ rises between the two cell types arose from different endogenous Ca2+ buffering capacities in their dendrites, the endogenous binding capacity in the absence of added Ca2+ buffers (κS) was derived from the x-axis intercepts of the fits (κ0; Fig. 3B, C). From the 1/Δ[Ca2+]5APsvs.κB relationship, κS was found to be lower in the dendrites of BCs compared with those of SC-ACs (BCs: 65 (28–88); SC-ACs: 171 (100–201); Fig. 3B). Similarly, from the τdecayvs.κB relationship, κS was found to be lower in the dendrites of BCs (BCs: 82 (14–114); SC-ACs: 187 (152–189); Fig. 3C). These results suggest that the significantly larger amplitude and faster decay kinetics observed for AP-evoked Ca2+ rises in the dendrites of BCs compared with those of SC-ACs can arise from differences in the endogenous Ca2+ binding capacities between these cells.
Action potential-evoked dendritic Ca2+ transients were regulated actively by changes in IN activity
According to the results presented so far, dendritic Ca2+ transients evoked by backpropagating APs in two types of hippocampal INs differ significantly due to the cell type-specific properties of Ca2+ handling, in particular to endogenous Ca2+ buffering capacities. To examine whether these quantitatively different CaTs may also involve distinct Ca2+ mechanisms, next we assessed their sensitivity to specific blockers of VSCCs and of intracellular Ca2+ stores (Fig. 4). Experiments were performed using Fluo-5F as a Ca2+ indicator. The L-type VSCC blocker nifedipine (10 μm; Fig. 4Aa, Ba and C) decreased the AP-CaT amplitude to ∼70% of that of the control in both cell types, indicating a significant contribution of L-type VSCCs to dendritic AP-CaTs in SC-ACs and BCs. However, the effects of the T-type VSCC blocker NNC 55-0396 (10 μm; Fig. 4Ab, Bb and C), which is a modified mibefradil analogue that is exquisitely selective for T-type VSCCs when used at low concentration (Huang et al. 2004; Li et al. 2005), and of the P/Q-type VSCC blocker ω-agatoxin IVA (AgTx, 250 nm; Fig. 4Ac, Bc and C) were cell type specific. T-type VSCCs exhibited a significantly higher contribution in the dendrites of SC-ACs (SC-ACs, 39.6 ± 4.1% of AP-CaTs, n = 5; BCs, 20.5 ± 5.7% of AP-CaTs, n = 6; P < 0.05, unpaired t test), whereas P/Q-type VSCCs were recruited by APs only in the dendrites of BCs (SC-ACs, 2.5 ± 0.2% of AP-CaTs, n = 6; BCs, 28.2 ± 3.4% of AP-CaTs, n = 6; Fig. 4C). Furthermore, the inhibitors of R-type (SNX-482, 30 nm) and N-type (CTx, 250 nm) VSCCs had no effect in either cell type (Fig. 4Aa, Ba, Ac, Bc and C), suggesting that these channels do not contribute to dendritic AP-CaTs in SC-ACs and BCs. In addition, both cell types demonstrated a significant component mediated by the activation of intracellular Ca2+ stores (Fig. 4Ab, Bb and C), pointing to the Ca2+-induced Ca2+ release activated in IN dendrites by backpropagating APs.
During theta rhythm in vivo, most hippocampal INs go through active and relatively silent states (Klausberger et al. 2003) during which the IN membrane potential should fluctuate from a relatively depolarized to a hyperpolarized level, respectively. Given a cell type-specific contribution of VSCCs in the IN dendrites, we examined next whether AP-CaTs can be differentially affected by the membrane potential fluctuations in two types of INs. In SC-ACs, the transition from −60 to −80 mV significantly increased the amplitude of dendritic Ca2+ rises (to 137 ± 7% of the control, n = 5, P < 0.001; Fig. 5Aa and B). This increase in Δ[Ca2+]APs at −80 mV was not affected by the L-type VSCC blocker nifedipine (10 μm; n = 4; Fig. 5Aa and B) but was prevented in the presence of the more specific T-type VSCC blocker NNC 55-0396 (Huang et al. 2004) used at low concentration here (10 μm; n = 4; Fig. 5Aa and B), which suggests that the hyperpolarization-induced enhancement of Δ[Ca2+]APs in the dendrites of SC-ACs is mediated by enhanced recruitment of T-type VSCCs. In contrast, in dendrites of BCs, the transition from −60 to −80 mV was associated with a significant decrease in the amplitude of Ca2+ rises (to 84 ± 3% of the control, n = 6, P < 0.05; Fig. 5Ab and B), which was not affected by NNC 55-0396 (n = 5) but was prevented by nifedipine (n = 5; Fig. 5Ab and B). These results indicate that, for any phase of the theta wave, IN dendritic Δ[Ca2+]APs will fluctuate in a cell type-specific manner, which is determined by the cell type-specific contribution of distinct types of VSCCs to IN dendritic Ca2+ signalling.
In vivo, many INs fire spikes during the gamma-rhythm episodes (40–100 Hz) associated with exploratory walking and paradoxical sleep (Soltesz & Deschênes, 1993; Bragin et al. 1995). Gamma-like firing episodes (63 Hz) affect the state of dendritic VSCCs profoundly in pyramidal cells, interfering with the induction of long-term synaptic plasticity (Yasuda et al. 2003). However, whether brief episodes of high-frequency firing have an effect on AP-evoked Ca2+ dynamics in dendrites of INs remains unexplored, and was tested here. As shown in Fig. 6Aa, brief trains of high-frequency firing (60–80 Hz, 0.5 s, repeated 2–3 times) did not affect AP-CaTs in the dendrites of SC-ACs (P > 0.05, n = 4; Fig. 6Aa, Ba and Ca). In contrast, the amplitude of dendritic AP-CaTs was significantly decreased in the dendrites of BCs (to 64 ± 5% of control, n = 6, P < 0.05; Fig. 6Ab, Bb and Cb). This depression was blocked by nifedipine (n = 4; Fig. 6Bb and Cb), which suggests that it required the activation of L-type VSCCs. These data indicate that AP-evoked dendritic Ca2+ rises are differentially regulated in different types of INs that exhibit the same activity patterns. Therefore, in addition to the cell type-specific properties of dendritic Ca2+ handling, Ca2+ transients may undergo specific forms of plasticity that are likely to be controlled by the cell type-specific expression and/or availability of particular ion channels, signalling molecules and/or downstream cascades.
Summation of Ca2+ transients during theta bursting and its role in synaptic plasticity
It has been demonstrated that during hippocampal theta-rhythm activity in vivo (3–12 Hz), most of the inhibitory INs fire two to three APs (at 100 Hz) coupled in a cell type-specific manner to different phases of the theta oscillation (Klausberger et al. 2003; Klausberger, 2009). According to our findings so far, such common theta-burst firing (TBF) of different classes of hippocampal INs should be translated into cell type-specific Ca2+ signalling in their dendrites, which may play an important role in the induction of synaptic plasticity (Perez et al. 2001; Patenaude et al. 2005). This assumption was tested in the next series of experiments (Fig. 7). Experiments were performed using Fluo-5F as a Ca2+ indicator. Three APs at 100 Hz frequency were evoked by brief current injections (0.8–1 nA, 2 ms) and repeated 2 to 5 times (2–5 bursts) with a rate of 4 Hz. It was apparent that Δ[Ca2+]3APs evoked by individual bursts of three APs in dendrites of BCs were significantly larger than those evoked in SC-ACs (BCs: 0.63 ± 0.14 μm, n = 6 vs. SC-ACs: 0.38 ± 0.11 μm, n = 4; P < 0.05; Fig. 7A and B). Considering the amplitude of Ca2+ rises evoked by individual APs (BCs: 0.21 ± 0.03 nm, n = 11 vs. SC-ACs: 0.131 ± 0.015 nm, n = 9), this corresponds to a linear summation of Ca2+ transients during three APs at 100 Hz in both cell types (Fig. 7C). However, during TBF, the summation degree of Ca2+ transients appeared to be larger for SC-ACs (Fig. 7C), and reached a significantly higher level than in BCs at the fifth burst (BCs: Δ[Ca2+]5bursts= 5.65 ± 0.93 of Δ[Ca2+]1AP, n = 6 vs. SC-ACs: Δ[Ca2+]5bursts= 9.22 ± 0.94 of Δ[Ca2+]1AP, n = 4, P < 0.05; Fig. 7C). Thus, even though the amplitude of Ca2+ rises evoked by individual bursts was smaller in SC-ACs, the peak amplitude of SC-ACs’Δ[Ca2+]APs was able to reach the BCs’ level by the fifth burst due to a more efficient temporal summation (Fig. 7A). Since the previous findings of this study indicate that SC-ACs’ Ca2+ rises demonstrate slower decay kinetics compared with BCs, largely due to the differences in the endogenous Ca2+ binding capacities between the two cell types (Figs 2 and 3), these results suggest that the enhanced temporal summation of Δ[Ca2+]APs during TBF in SC-ACs could result from slowing in their Δ[Ca2+]APs decays. The plot in Fig. 7D shows a more prominent increase in the τdecay in dendrites of SC-ACs during TBF compared with that in BCs, and a significant difference in the decay kinetics of Δ[Ca2+]APs between the two cell types. Thus, the slowing of Ca2+ transient decay kinetics in SC-ACs enhanced the temporal summation of their Ca2+ signals in such a way that initially small Ca2+ signals became equal to those in BCs during TBF.
Theta-burst firing has been associated with the induction of Ca2+-dependent forms of synaptic plasticity at both excitatory and inhibitory synapses (Perez et al. 2001; Patenaude et al. 2005). In most cases, theta-burst-induced plasticity requires the pairing of postsynaptic firing with presynaptic stimulation. However, it remains unclear whether TBF alone, which is associated with the activation of multiple types of VSCCs and a massive Ca2+ influx in IN dendrites, is sufficient to support the induction of synaptic plasticity. Spatial restriction of AP-CaTs to proximal dendritic sites (Fig. 2F), together with a similar spatial distribution of inhibitory synapses onto RAD INs (Gulyas et al. 1999; Pettit & Augustine, 2000), suggests that inhibitory transmission to INs is in particular controlled by these Ca2+ signals. To test this hypothesis, next we examined whether TBF alone was sufficient to induce long-term plasticity at inhibitory synapses onto both SC-ACs and BCs. Monosynaptic IPSCs in INs held at −50 mV were evoked by local stimulation in the RAD in the presence of dl-AP5 and NBQX. In control recordings, IPSC amplitude remained stable for at least 30 min (100.9 ± 1.0% of the response recorded during the first 5 min, n = 8; P > 0.05). In contrast, long-term potentiation (LTP) of IPSCs (163.1 ± 4.9% of the control, n = 7; P < 0.05; Fig. 8B) was observed after TBF (2 s each, repeated three times at 30 s intervals) and was independent of cell type. Importantly, no significant difference was observed in the magnitude of IPSC potentiation between the two cell types (BC-LTP, 170.6 ± 4.9% of the control, n = 4; SC-AC-LTP, 153.1 ± 5.3% of the control, n = 3; P > 0.05; Fig. 8A), which suggests the presence of a similar mechanism of LTP induction at inhibitory synapses onto both SC-ACs and BCs. Accordingly, LTP was expressed presynaptically in both cell types, as it was associated with a significant decrease in the PPR (71.8 ± 4.5% of the control, n = 7; P < 0.05; Fig. 8C) and CV (89.3 ± 3.3% of the control, n = 7; P < 0.05; Fig. 8C). Moreover, in both cell types, this presynaptic LTP required the elevation of postsynaptic Ca2+, as it was blocked when BAPTA was included in the patch solution (98.7 ± 1.3% of the control, n = 4; P > 0.05; Fig. 8B and C). Together, these data indicate that significant Ca2+ elevations, which result from the efficient summation of AP-CaTs during TBF in both BCs and SC-ACs, control the induction of a presynaptic form of LTP at inhibitory synapses onto these cells.
Discussion
Cell type-specific properties of action potential-evoked dendritic Ca2+ transients in INs
Experimental approaches based on combined two-photon dendritic Ca2+ imaging and patch-clamp recordings from rigorously identified inhibitory INs are important for the clarification of the role played by cell type-specific dendritic integration in the function of INs. The majority of inhibitory INs show high endogenous Ca2+ binding capacities and, as a result, small amplitudes and slow kinetics of dendritic Ca2+ transients (Lee et al. 2000; Kaiser et al. 2001; Aponte et al. 2008). INs are also endowed with a large repertoire of active dendritic conductances (Martina et al. 2000; Goldberg et al. 2003; Vinet & Sík, 2006; Hu et al. 2010), specific signalling molecules and cascades (Liu & Jones, 1996; Sík et al. 1998; Topolnik et al. 2006) in a cell type-specific manner and are, thus, predisposed to distinct forms of activity-dependent regulation and network behaviour. The mechanisms of activity-dependent regulation of IN dendritic signalling in different cell types, as well as the dynamic profile of Ca2+ fluctuations during physiologically relevant patterns of neuronal activity, remain elusive. This study identified the activity-dependent properties of dendritic Ca2+ rises in two distinct types of CA1 RAD INs: regularly spiking BCs and SC-ACs. Our major finding is that the same patterns of activity in BCs and SC-ACs translate into differential Ca2+ signals in their dendrites. In BCs, AP-evoked Ca2+ rises exhibited a relatively large amplitude and fast kinetics. A single AP in these cells raises dendritic [Ca2+] to ∼100–500 nm, which returns to the baseline within ∼40 ms. In SC-ACs, AP-evoked Ca2+ transients are significantly smaller and slower, reaching ∼40–200 nm and returning to the baseline within ∼60 ms. These differences between the two cell types are mediated by distinct endogenous Ca2+ binding capacities: ∼70 in BCs vs.∼170 in SC-ACs.
Ca2+ binding protein calbindin expressed by both SC-ACs and BCs has been shown to undergo a rapid wash-out during whole-cell recordings (Zhou & Neher, 1993; Müller et al. 2005) and, thus, cannot contribute to distinct endogenous Ca2+ binding capacities found in these cells. Therefore, the observed difference in the endogenous Ca2+ buffering may result from differential expression of immobile Ca2+ buffers, including Ca2+ extrusion mechanisms such as plasma-membrane Ca2+ pumps, SERCA, the mitochondrial uniporter and the Na+/Ca2+ exchanger. The molecular identity of these mechanisms and their cell type-specific expression remain to be determined in the future studies.
Overall, AP-evoked Ca2+ rises in the two types of INs examined here were lower and slower than those detected in the dendrites of pyramidal cells ([Ca2+]: ∼0.4–2 μm; τdecay: ∼21 ms; Sabatini et al. 2002); however, they were larger and faster than those observed in fast-spiking (FS) BCs ([Ca2+]: ∼0.04 μm; τdecay: ∼200 ms; Aponte et al. 2008). Therefore, although the comparison between AP-CaT values obtained here for regularly spiking INs and those for FS-BCs (Aponte et al. 2008) is not entirely appropriate because of the different imaging approaches used in these studies, the data suggest that, based on the amplitude and the speed of the decay of AP-CaTs, two types of regularly spiking CA1 INs may be positioned between pyramidal neurons and fast-spiking basket cells: PYRs > RS-BCs > SC-ACs > FS-BCs. Importantly, the properties of AP-evoked Ca2+ signals differed significantly between the two types of BCs: RS (expressing CCK) and FS (expressing PV). This can be related directly to the highly specific functions played by these cells in hippocampal circuitry. FS-BCs represent fast inhibitory devices providing a very rapid and temporally precise inhibition with synchronous GABA release (Kraushaar & Jonas, 2000), whereas RS-BCs integrate multiple inputs over time and exhibit asynchronous release (Hefft & Jonas, 2005). The two release modes may exhibit differential Ca2+ dependence, which indicates the existence of two distinct types of Ca2+ sensors (Sun et al. 2007; Kerr et al. 2008; Daw et al. 2009). However, given that asynchronous release increases rapidly with increasing [Ca2+] (Daw et al. 2009), the significantly higher Ca2+ rises produced by APs in RS-BCs could account partly for the asynchronous release at RS-BC synapses. Yet, the magnitude of Ca2+ elevations in axonal terminals of RS-BCs may not be similar to those occurring in dendrites; this remains to be determined.
In dendrites of most inhibitory INs, AP-evoked Ca2+ rises attenuate significantly with distance from the soma because of a higher density of K+ channels in distal dendrites (Kaiser et al. 2001; Goldberg et al. 2003; Aponte et al. 2008; Topolnik et al. 2009; Hu et al. 2010; but see Rozsa et al. 2004). In agreement with previous studies, our data also revealed a significant decline in the amplitude of AP-evoked Ca2+ elevations along the dendritic tree in both BCs and SC-ACs of mouse hippocampus. However, these findings are in disaccord with the incremental scaling of AP-CaTs reported by Rozsa et al. (2004) in a population of CA1 RAD INs of rat hippocampus. Unfortunately, no attempt was made to identify the recorded INs in that study (Rozsa et al. 2004), and the cellular identity of the INs that demonstrated a distance-dependent increment of AP-CaTs remains unknown. In our study, the distance dependence of AP-evoked Ca2+ rises was explored in 35 INs of different subtypes (including BCs, SC-ACs, bistratified and trilaminar cells and some unidentified INs with complex axonal arborizations) in the CA1 RAD. All recorded INs demonstrated a distance-dependent decline in AP-CaT amplitude, suggesting that the interneuron-type-specific properties of dendritic organization do not explain these divergent results. Alternatively, differences in experimental conditions (rats vs. mice, room vs. physiological temperature, number of APs and/or different diameter of pipette tips (Pusch & Neher, 1988; Oliva et al. 1988) and access resistance, which are important for dye diffusion and equilibration (Yasuda et al. 2004)) may account for the discrepancy observed.
Cell type-specific mechanisms and regulation of Ca2+ transients
The results of the present study also revealed the cell type-specific mechanisms of AP-CaTs in RAD INs. T-type VSCCs mediated 39.6 ± 4.1% of AP-CaTs in SC-ACs and only 20.5 ± 5.7% of AP-CaTs in BCs. In contrast, P/Q-type VSCCs contributed to 28.2 ± 3.4% of AP-CaTs in BCs and did not participate in AP-CaTs in SC-ACs. Accordingly, the AP-CaT amplitude in IN dendrites was differentially affected by the level of membrane potential from which the APs were generated. In SC-ACs, AP-CaTs were significantly increased by membrane hyperpolarization due to enhanced contribution of T-type VSCCs, which could result from the channel recovery from the inactivation state at −80 mV (McRory et al. 2001; Carter & Sabatini, 2004). T-type VSCCs contribute to dendritic Ca2+ signals in pyramidal cells (Magee et al. 1995), granule cells of the olfactory bulb (Egger et al. 2003), Purkinje cells (Mouginot et al. 1997) and cortical and hippocampal INs (Goldberg et al. 2004; Topolnik et al. 2009). Consistent with previous findings regarding the expression of the Cav3.1 subunit of T-type VSCCs in CCK-IR CA1 INs (Vinet & Sík, 2006), the results of the present study indicate that these channels contribute to AP-CaTs in both BCs and SC-ACs. However, the higher contribution of T-type VSCCs in dendrites of SC-ACs together with their membrane-potential-dependent recruitment suggests that these channels may preferentially shape the dynamics of AP-CaTs in SC-ACs. In contrast, in BCs, AP-CaTs were decreased with membrane hyperpolarization. In this case, the membrane-potential-induced regulation of AP-CaTs progressed independently of the T-type VSCCs, but was associated with a decreased availability of L-type VSCCs and, possibly, of P/Q-type VSCCs at −80 mV. L-type VSCCs contribute to AP-CaTs in hippocampal INs (Topolnik et al. 2009) and both Cav1.2 and Cav1.3 subunits are expressed in CCK-IR cells (although the Cav1.2 was detected only in half the CCK-IR cells; Vinet & Sík, 2006). Given the channel activation profile, it is unlikely that it contributes significantly to AP-CaTs at −80 mV, as was apparent from present results in BCs. These findings are in agreement with previous results obtained in striatal medium spiny neurons (Carter & Sabatini, 2004), which also indicate the presence of a membrane-potential-dependent regulation of AP-CaTs in relation to different states of network (up state vs. down state).
From the experiments presented here, it appears that following high-frequency firing episodes in the gamma band AP-CaTs are significantly depressed selectively in BCs. A similar activity-dependent depression of AP-CaTs after relatively brief firing episodes (63 Hz, 0.5 s) occurs in dendritic spines of pyramidal cells and interferes with the induction of long-term synaptic plasticity (Yasuda et al. 2003). Induction of AP-CaT depression in pyramidal neurons is Ca2+ dependent and requires Ca2+ influx via L-type VSCCs, activation of the Ca2+/calmodulin-dependent kinase II (CaMKII) and a cAMP-dependent pathway. AP-CaT depression is expressed as a decrease in the open probability of R-type VSCCs. While L-type VSCCs are expressed at high density in CCK-IR GABAergic INs, the Cav1.2 exhibits a cell type-specific distribution (Vinet & Sík, 2006). Furthermore, αCaMKII, found in pyramidal cells, is not detected in inhibitory INs (Liu & Jones, 1996; Sík et al. 1998) and appears to be replaced by a different isoform (Lamsa et al. 2007). Thus, it was unclear whether L-type VSCC/CaMKII/cAMP-dependent depression of AP-CaTs may be induced in IN dendrites by brief episodes of high-frequency firing. The results of the present study revealed a cell type-specific depression of AP-CaTs controlled by L-type VSCCs, pointing to a fulfilment of all required conditions for its specific induction in BCs. The lack of AP-CaT depression in SC-ACs, which express L-type VSCCs, could be due to a different organization of L-type/Ca2+-dependent signalling cascade, thereby interfering with the induction of this form of plasticity. In this study, no attempt was made to determine possible cell type-specific mechanisms of this form of dendritic plasticity and its consequences in synaptic plasticity, which will have to be addressed in the future.
Role of action potential-evoked dendritic Ca2+ transients in plasticity at IN inhibitory synapses
Our data also indicate that the smaller and slower AP-CaTs found in the dendrites of SC-ACs compared with those detected in BCs were amplified significantly during theta-like activity as a result of a more efficient temporal summation of AP-CaTs in SC-ACs; this suggests that the quantitatively different Ca2+ rises recorded in the two cell types under basal conditions can be essentially normalized by changes in neuron activity. Overall efficient temporal summation of AP-CaTs during theta-burst firing in both SC-ACs and BCs resulted in the induction of LTP at inhibitory synapses onto these cells. These data suggest that under natural conditions theta episodes can be associated with an increase in the inhibition of CCK-expressing INs and a subsequent disinhibition of principal cells. This downregulation of the inhibition may be necessary for the efficient integration of excitatory inputs by place cells supporting their firing and resulting in the induction of a short-term depression of CCK inhibitory inputs via release of endocannabinoids (Katona et al. 1999). Accordingly, theta oscillations may be associated with an overall decrease in inhibition originating from the CCK-expressing INs, which can be particularly important in sparse coding by place cells (Klausberger et al. 2005).
LTP at inhibitory synapses onto both SC-ACs and BCs was induced postsynaptically, as it was triggered by postsynaptic firing alone and required postsynaptic Ca2+ elevation. However, the expression locus of this LTP was located at the presynaptic site, suggesting the implication of a retrograde signal. Although no attempt was made in the current study to investigate the mechanisms of LTP induction, nitric oxide (NO) or brain-derived neurotrophic factor (BDNF) may be involved in this presynaptic LTP. The Ca2+-dependent neuronal NO synthase is expressed in calbindin-positive INs (Jinno & Kosaka, 2002). NO synthesis is tightly controlled by intracellular Ca2+ elevation, so that as little as 250–350 nm of Ca2+ is sufficient to trigger the production of NO (Bredt & Snyder, 1990; Pollock et al. 1991). Furthermore, an NO-dependent form of LTP was identified at inhibitory synapses onto dopaminergic neurons of the ventral tegmental area (Nugent et al. 2007). Whether this form of presynaptic plasticity represents a common mechanism of regulation of inhibitory synapses remains to be determined. As for BDNF, a role for this molecule in the LTP expressed presynaptically was reported at inhibitory synapses onto CA3 pyramidal cells early during development, although dendritic synthesis of BDNF required a significantly higher stimulation protocol (depolarizing pulses of 500 ms) and, accordingly, higher Ca2+ elevations (Caillard et al. 1999; Kuczewski et al. 2008). Remarkably, in our study, BCs and SC-ACs (as well as one bistratified cell; S. Chamberland & L. Topolnik, unpublished data) exhibited this form of inhibitory synapse plasticity, demonstrating the presence of a fairly universal form of LTP at inhibitory synapses of RAD INs.
In conclusion, the results presented here favour a model in which AP-evoked dendritic Ca2+ rises fluctuate continuously and are dependent on IN state and activity pattern. These dynamic [Ca2+] fluctuations are controlled by the basic Ca2+ handling properties of INs and by the distribution and availability of particular Ca2+ sources and/or Ca2+ extrusion mechanisms in different cell types. Given that the state and/or the availability of Ca2+ mechanisms control the induction of synaptic plasticity, cell type-specific dynamics of Ca2+ signalling will determine the rules of the induction of cell type-specific forms of plasticity. Conversely, the interneuron-specific differences in AP-evoked dendritic Ca2+ signals can be essentially ‘smoothed out’ by changes in neuronal activity, so that Ca2+ signals of similar profile will be produced in different cell types, leading to the induction of common, cell-type-independent forms of synaptic plasticity. Therefore, given that dendritic Ca2+ mechanisms represent a highly dynamic entity (Yasuda et al. 2003; Topolnik et al. 2009; present study), the history of neuron activity can be stored in dendrites via dynamically regulated Ca2+ signals, thus having a profound effect on the integration of synaptic inputs.
Acknowledgments
We thank Dimitry Topolnik for excellent technical assistance and cells’ reconstruction. This work was supported by the Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada (NSERC Discovery Grant) and Savoy Foundation. L.T. is a recipient of the University Faculty Award from NSERC. S.C. was supported by a fellowship from the Fonds de la Recherche en Santé du Québec.
Glossary
Abbreviations
- AP
action potential
- AP-CaTs
action potential-evoked Ca2+ transients
- BC
basket cell
- CaMKII
calmodulin-dependent kinase II
- CCK-IR
cholecystokinin-immunoreactive
- CV
coefficient of variation
- FS-BC
fast spiking basket cell
- IN
interneuron
- LTP
long-term potentiation
- NGS
normal goat serum
- O/A
stratum oriens/alveus
- OGB-1
Oregon Green 488 BAPTA-1
- PPR
paired-pulse ratio
- PYR
stratum pyramidale
- RAD
stratum radiatum
- SC-AC
Schaffer collateral-associated cell
- TBF
theta-burst firing
- VSCC
voltage-sensitive calcium channel
Author contributions
A.E. and S.C. collected the data and took part in the analysis of results and preparation of Figs 1, 4 and 8. L.T. designed the experiments, analysed the data and wrote the manuscript. All authors approved the final version of the manuscript for publication.
References
- Ali AB. Presynaptic inhibition of GABAA receptor-mediated unitary IPSPs by cannabinoid receptors at synapses between CCK-positive interneurons in rat hippocampus. J Neurophysiol. 2007;98:861–869. doi: 10.1152/jn.00156.2007. [DOI] [PubMed] [Google Scholar]
- Aponte Y, Bischofberger J, Jonas P. Efficient Ca2+ buffering in fast-spiking basket cells of rat hippocampus. J Physiol. 2008;586:2061–2075. doi: 10.1113/jphysiol.2007.147298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Jandó G, Nádasdy Z, van Landeghem M, Buzsáki G. Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J Neurophysiol. 1995;73:1691–1705. doi: 10.1152/jn.1995.73.4.1691. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillard O, Ben-Ari Y, Gaiarsa JL. Long-term potentiation of GABAergic synaptic transmission in neonatal rat hippocampus. J Physiol. 1999;518:109–119. doi: 10.1111/j.1469-7793.1999.0109r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway JC, Ross WN. Frequency-dependent propagation of sodium action potentials in dendrites of hippocampal CA1 pyramidal neurons. J Neurophysiol. 1995;74:1395–1403. doi: 10.1152/jn.1995.74.4.1395. [DOI] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Cope DW, Maccaferri G, Márton LF, Roberts JD, Cobden PM, Somogyi P. Cholecystokinin-immunopositive basket and Schaffer collateral-associated interneurones target different domains of pyramidal cells in the CA1 area of the rat hippocampus. Neuroscience. 2002;109:63–80. doi: 10.1016/s0306-4522(01)00440-7. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Buzsáki G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron. 1998;21:179–189. doi: 10.1016/s0896-6273(00)80525-5. [DOI] [PubMed] [Google Scholar]
- Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J Neurosci. 2009;29:11112–11122. doi: 10.1523/JNEUROSCI.5760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Sugimori M, Llinás R. Two types of calcium response limited to single spines in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1995;92:8279–8282. doi: 10.1073/pnas.92.18.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger V, Svoboda K, Mainen ZF. Mechanisms of lateral inhibition in the olfactory bulb: efficiency and modulation of spike-evoked calcium influx into granule cells. J Neurosci. 2003;23:7551–7558. doi: 10.1523/JNEUROSCI.23-20-07551.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Yuste R, Tamas G. Ca2+ imaging of mouse neocortical interneuron dendrites: Ia type K+ channels control action potential backpropagation. J Physiol. 2003;551:49–65. doi: 10.1113/jphysiol.2003.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Lacefield CO, Yuste R. Global dendritic calcium spikes in mouse layer 5 low threshold spiking interneurones: implications for control of pyramidal cell bursting. J Physiol. 2004;558:465–478. doi: 10.1113/jphysiol.2004.064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Helmchen F, Imoto K, Sakmann B. Ca2+ buffering and action potential-evoked Ca2+ signaling in dendrites of pyramidal neurons. Biophys J. 1996;70:1069–1081. doi: 10.1016/S0006-3495(96)79653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Martina M, Jonas P. Dendritic mechanisms underlying rapid synaptic activation of fast-spiking hippocampal interneurons. Science. 2010;327:52–58. doi: 10.1126/science.1177876. [DOI] [PubMed] [Google Scholar]
- Huang L, Keyser BM, Tagmose TM, Hansen JB, Taylor JT, Zhuang H, Zhang M, Ragsdale DS, Li M. NNC 55-0396 [(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride]: a new selective inhibitor of T-type calcium channels. J Pharmacol Exp Ther. 2004;309:193–199. doi: 10.1124/jpet.103.060814. [DOI] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Patterns of expression of calcium binding proteins and neuronal nitric oxide synthase in different populations of hippocampal GABAergic neurons in mice. J Comp Neurol. 2002;449:1–25. doi: 10.1002/cne.10251. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sík A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser KM, Zilberter Y, Sakmann B. Back-propagating action potentials mediate calcium signalling in dendrites of bitufted interneurons in layer 2/3 of rat somatosensory cortex. J Physiol. 2001;535:17–31. doi: 10.1111/j.1469-7793.2001.t01-1-00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr AM, Reisinger E, Jonas P. Differential dependence of phasic transmitter release on synaptotagmin 1 at GABAergic and glutamatergic hippocampal synapses. Proc Natl Acad Sci U S A. 2008;105:15581–15586. doi: 10.1073/pnas.0800621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill P, Márton LF, Roberts JD, Cobden PM, Buzsáki G, Somogyi P. Brain-state- and Cell type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O'Neill J, Huck JHJ, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P. Complementary roles of cholecystokinin- and parvalbumin- expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci. 2009;30:947–957. doi: 10.1111/j.1460-9568.2009.06913.x. [DOI] [PubMed] [Google Scholar]
- Kraushaar U, Jonas P. Efficacy and stability of quantal GABA release at a hippocampal interneuron-principal neuron synapse. J Neurosci. 2000;20:5594–5607. doi: 10.1523/JNEUROSCI.20-15-05594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczewski N, Porcher C, Ferrand N, Fiorentino H, Pellegrino C, Kolarow R, Lessmann V, Medina I, Gaiarsa JL. Backpropagating action potentials trigger dendritic release of BDNF during spontaneous network activity. J Neurosci. 2008;28:7013–7023. doi: 10.1523/JNEUROSCI.1673-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa KP, Heeroma JH, Kullmann DM. Hebbian LTP in feed-forward inhibitory interneurons and the temporal fidelity of input discrimination. Nat Neurosci. 2005;8:916–924. doi: 10.1038/nn1486. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Irvine EE, Giese KP, Kullmann DM. NMDA receptor-dependent long-term potentiation in mouse hippocampal interneurons shows a unique dependence on Ca2+/calmodulin-dependent kinases. J Physiol. 2007;584:885–894. doi: 10.1113/jphysiol.2007.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe V, Morin F, Ratte S, Croce A, Conquet F, Lacaille J-C. Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. J Physiol. 2004;15:125–135. doi: 10.1113/jphysiol.2003.053603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Rosenmund C, Schwaller B, Neher E. Differences in Ca2+ buffering properties between excitatory and inhibitory hippocampal neurons from the rat. J Physiol. 2000;525:405–418. doi: 10.1111/j.1469-7793.2000.t01-3-00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Distinct NMDA receptors provide differential modes of transmission at mossy fiber-interneuron synapses. Neuron. 2002;33:921–933. doi: 10.1016/s0896-6273(02)00608-6. [DOI] [PubMed] [Google Scholar]
- Li M, Hansen JB, Huang L, Keyser BM, Taylor JT. Towards selective antagonists of T-type calcium channels: design, characterization and potential applications of NNC 55-0396. Cardiovasc Drug Rev. 2005;23:173–196. doi: 10.1111/j.1527-3466.2005.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Liu XB, Jones EG. Localization of α type II calcium calmodulin-dependent protein kinase at glutamatergic but not γ-aminobutyric acid (GABAergic) synapses in thalamus and cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:7332–7336. doi: 10.1073/pnas.93.14.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Christofi G, Miyakawa H, Christie B, Lasser-Ross N, Johnston D. Subthreshold synaptic activation of voltage-gated Ca2+ channels mediates a localized Ca2+ influx into the dendrites of hippocampal pyramidal neurons. J Neurophysiol. 1995;74:1335–1342. doi: 10.1152/jn.1995.74.3.1335. [DOI] [PubMed] [Google Scholar]
- Maravall M, Mainen ZF, Sabatini BL, Svoboda K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys J. 2000;78:2655–2667. doi: 10.1016/S0006-3495(00)76809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Vida I, Jonas P. Distal initiation and active propagation of action potentials in interneuron dendrites. Science. 2000;287:295–300. doi: 10.1126/science.287.5451.295. [DOI] [PubMed] [Google Scholar]
- McRory JE, Santi CM, Hamming KS, Mezeyova J, Sutton KG, Baillie DL, Stea A, Snutch TP. Molecular and functional characterization of a family of rat brain T-type calcium channels. J Biol Chem. 2001;276:3999–4011. doi: 10.1074/jbc.M008215200. [DOI] [PubMed] [Google Scholar]
- Mouginot D, Bossu JL, Gähwiler BH. Low-threshold Ca2+ currents in dendritic recordings from Purkinje cells in rat cerebellar slice cultures. J Neurosci. 1997;17:160–170. doi: 10.1523/JNEUROSCI.17-01-00160.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Kukley M, Stausberg P, Beck H, Müller W, Dietrich D. Endogenous Ca2+ buffer concentration and Ca2+ microdomains in hippocampal neurons. J Neurosci. 2005;25:558–565. doi: 10.1523/JNEUROSCI.3799-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Augustine GJ. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Oliva C, Cohen IS, Mathias RT. Calculation of time constants for intracellular diffusion in whole cell patch clamp configuration. Biophys J. 1988;54:791–799. doi: 10.1016/S0006-3495(88)83017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude C, Massicotte G, Lacaille JC. Cell-type specific GABA synaptic transmission and activity-dependent plasticity in rat hippocampal stratum radiatum interneurons. Eur J Neurosci. 2005;22:179–188. doi: 10.1111/j.1460-9568.2005.04207.x. [DOI] [PubMed] [Google Scholar]
- Pawelzik H, Hughes DI, Thomson AM. Physiological and morphological diversity of immunocytochemically defined parvalbumin- and cholecystokini-positive interneurons in CA1 of the adult rat hippocampus. J Comp Neurol. 2002;443:346–367. doi: 10.1002/cne.10118. [DOI] [PubMed] [Google Scholar]
- Perez Y, Morin F, Lacaille JC. A hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci U S A. 2001;98:9401–9406. doi: 10.1073/pnas.161493498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit DL, Augustine GJ. Distribution of functional glutamate and GABA receptors on hippocampal pyramidal cells and interneurons. J Neurophysiol. 2000;84:28–38. doi: 10.1152/jn.2000.84.1.28. [DOI] [PubMed] [Google Scholar]
- Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Rozsa B, Zelles T, Vizi ES, Lendvai B. Distance-dependent scaling of calcium transients evoked by backpropagating spikes and synaptic activity in dendrites of hippocampal interneurons. J Neurosci. 2004;24:661–670. doi: 10.1523/JNEUROSCI.3906-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini B, Svoboda K. Analysis of calcium channels in single spines using optical fluctuation analysis. Nature. 2000;408:589–593. doi: 10.1038/35046076. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Oertner TG, Svoboda K. The life cycle of Ca2+ ions in dendritic spines. Neuron. 2002;33:439–452. doi: 10.1016/s0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- Scheuss V, Yasuda R, Sobczyk A, Svoboda K. Nonlinear [Ca2+] signaling in dendrites and spines caused by activity-dependent depression of Ca2+ extrusion. J Neurosci. 2006;26:8183–8194. doi: 10.1523/JNEUROSCI.1962-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock JS, Förstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sík A, Hájos N, Gulácsi A, Mody I, Freund TF. The absence of a major Ca2+ signaling pathway in GABAergic neurons of the hippocampus. Proc Natl Acad Sci U S A. 1998;95:3245–3250. doi: 10.1073/pnas.95.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Deschênes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J Neurophysiol. 1993;70:97–116. doi: 10.1152/jn.1993.70.1.97. [DOI] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Südhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topolnik L, Azzi M, Morin F, Kougiuomoutzakis A, Lacaille JC. mGluR1/5 subunit-specific Ca2+ signalling and long-term potentiation in rat hippocampal oriens/alveus interneurons. J Physiol. 2006;575:115–131. doi: 10.1113/jphysiol.2006.112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topolnik L, Chamberland S, Pelletier JG, Ran I, Lacaille JC. Activity-dependent compartmentalized regulation of dendritic Ca2+ signaling in hippocampal interneurons. J Neurosci. 2009;29:4658–4663. doi: 10.1523/JNEUROSCI.0493-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH. Unitary IPSPs evoked by interneurons at the stratum radiatum–stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol. 1998;506:755–773. doi: 10.1111/j.1469-7793.1998.755bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinet J, Sík A. Expression pattern of voltage-dependent calcium channel subunits in hippocampal inhibitory neurons in mice. Neuroscience. 2006;143:189–212. doi: 10.1016/j.neuroscience.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Woodruff ML, Sampath AP, Matthews HR, Krasnoperova NV, Lem J, Fain GL. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J Physiol. 2002;542:843–854. doi: 10.1113/jphysiol.2001.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda R, Sabatini BL, Svoboda K. Plasticity of calcium channels in dendritic spines. Nat Neurosci. 2003;6:948–955. doi: 10.1038/nn1112. [DOI] [PubMed] [Google Scholar]
- Yasuda R, Nimchinsky EA, Scheuss V, Pologruto TA, Oertner TG, Sabatini BL, Svoboda K. Imaging calcium concentration dynamics in small neuronal compartments. Sci STKE. 2004;219:l5. doi: 10.1126/stke.2192004pl5. [DOI] [PubMed] [Google Scholar]
- Yuste R, Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol. 1993;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]


