Non-technical summary
In adult animals serotonergic neurones contribute to heat production (thermogenesis) in response to severe cold stress, as well as the respiratory response to increasing carbon dioxide. We show that in neonatal life, serotonin in the brainstem is absolutely essential for the thermogenic and heart rate responses to very mild (5°C) environmental cooling, probably by aiding the response of the sympathetic nervous system. In contrast, the respiratory response to increasing carbon dioxide is unaffected by a 90% loss of serotonin. Human infants with brainstem serotonin deficiency may be prone to a variety of homeostatic deficits owing to a reduced sympathetic response to mild cold stress.
Abstract
Abstract
Based on previous studies in adult animals, devoid of 5-HT neurones, showing altered thermoregulation in cold stress (4°C) and a reduced ventilatory response to CO2, we hypothesized that neonatal mice lacking 60–70% of their 5-HT neurones (Pet-1−/−) would have: (1) a reduced thermogenic response to a mild drop in ambient temperature (TA), (2) reduced 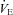 and heart rate (HR) responses to mild cooling that reflect this reduced thermogenic response, and (3) a reduced ventilatory response to CO2 after postnatal day 12 (P12), when 5-HT neurones become chemosensitive in vitro. We first determined that a 60–70% loss of 5-HT-positive neurones results in a ∼90% loss of 5-HT from the brainstems of Pet-1−/− animals. We then subjected Pet-1−/− and wild-type (WT) mice (N = 5) to mild environmental cooling (TA= 29°C) at ∼P12. TA was initially held at 34°C for ∼20 min, reduced to 29°C over 15 min and held for an additional 10 min at steady state, and then returned to 34°C. From 34°C to 29°C, there was a robust increase in
and heart rate (HR) responses to mild cooling that reflect this reduced thermogenic response, and (3) a reduced ventilatory response to CO2 after postnatal day 12 (P12), when 5-HT neurones become chemosensitive in vitro. We first determined that a 60–70% loss of 5-HT-positive neurones results in a ∼90% loss of 5-HT from the brainstems of Pet-1−/− animals. We then subjected Pet-1−/− and wild-type (WT) mice (N = 5) to mild environmental cooling (TA= 29°C) at ∼P12. TA was initially held at 34°C for ∼20 min, reduced to 29°C over 15 min and held for an additional 10 min at steady state, and then returned to 34°C. From 34°C to 29°C, there was a robust increase in 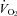 in P12 WT, but not Pet-1−/− animals (68 ± 19.9%versus−16 ± 8%, respectively; P = 0.002). On average, body temperature (TB) dropped 1.1°C more in Pet-1−/− compared to WT animals (P = 0.03). HR remained unchanged in WT but dropped 22 ± 2.3% in Pet-1−/− animals (P = 0.01). Genotype had no effect on tail temperature (TT), either at 34°C or 29°C. After cooling, values for
in P12 WT, but not Pet-1−/− animals (68 ± 19.9%versus−16 ± 8%, respectively; P = 0.002). On average, body temperature (TB) dropped 1.1°C more in Pet-1−/− compared to WT animals (P = 0.03). HR remained unchanged in WT but dropped 22 ± 2.3% in Pet-1−/− animals (P = 0.01). Genotype had no effect on tail temperature (TT), either at 34°C or 29°C. After cooling, values for 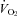 and HR of Pet-1−/− animals were no different to values predicted by Q10 effects alone, while values of WT animals were greater than predicted.
and HR of Pet-1−/− animals were no different to values predicted by Q10 effects alone, while values of WT animals were greater than predicted. 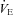 increased in WT with cooling, while it decreased in Pet-1−/− animals (P = 0.002). Still, Pet-1−/− animals hyperventilated relative to WT (increased
increased in WT with cooling, while it decreased in Pet-1−/− animals (P = 0.002). Still, Pet-1−/− animals hyperventilated relative to WT (increased 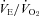 ) irrespective of TA (P = 0.002). As tested in a separate group of pups, there was no difference in the ventilatory response to CO2 between WT and Pet-1−/− animals, either at P5 or P15. We conclude that during neonatal life in mouse pups: (1) brainstem 5-HT is critical for the thermogenic response to a mild drop in environmental temperature probably via a sympathetically-mediated increase in brown fat metabolism; (2) reduced thermogenesis probably contributes to the reduced HR and
) irrespective of TA (P = 0.002). As tested in a separate group of pups, there was no difference in the ventilatory response to CO2 between WT and Pet-1−/− animals, either at P5 or P15. We conclude that during neonatal life in mouse pups: (1) brainstem 5-HT is critical for the thermogenic response to a mild drop in environmental temperature probably via a sympathetically-mediated increase in brown fat metabolism; (2) reduced thermogenesis probably contributes to the reduced HR and 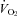 observed with 5-HT deficiency; and (3) the presence of some brainstem 5-HT is sufficient for an appropriate ventilatory response to hypercapnia up until P15. Infants with reduced brainstem 5-HT could be prone to cardiovascular and respiratory abnormalities resulting from compromised thermogenesis.
observed with 5-HT deficiency; and (3) the presence of some brainstem 5-HT is sufficient for an appropriate ventilatory response to hypercapnia up until P15. Infants with reduced brainstem 5-HT could be prone to cardiovascular and respiratory abnormalities resulting from compromised thermogenesis.
Introduction
In homeotherms, an acute drop in environmental temperature induces an increase in sympathetic outflow to the brown fat and heart, which increases 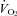 and HR (Morrison et al. 2008). The physiological mechanism in adults involves serotonergic neurones in the raphe pallidus that are activated by cold stress via descending pathways from the preoptic and dorsomedial hypothalamus (Martin-Cora et al. 2000; Rathner et al. 2001). These in turn activate presympathetic neurones in the intermediolateral cell column that project to post-sympathetic neurones innervating the brown fat and heart (Madden & Morrison, 2006, 2010; Ootsuka & Blessing, 2006; Nakamura & Morrison, 2007; Morrison et al. 2008). A complete loss of central 5-HT in adult Lmx1b−/− and Tph2−/− mice compromises thermoregulation during severe cold challenge (Hodges et al. 2008; Alenina et al. 2009) and the reduced
and HR (Morrison et al. 2008). The physiological mechanism in adults involves serotonergic neurones in the raphe pallidus that are activated by cold stress via descending pathways from the preoptic and dorsomedial hypothalamus (Martin-Cora et al. 2000; Rathner et al. 2001). These in turn activate presympathetic neurones in the intermediolateral cell column that project to post-sympathetic neurones innervating the brown fat and heart (Madden & Morrison, 2006, 2010; Ootsuka & Blessing, 2006; Nakamura & Morrison, 2007; Morrison et al. 2008). A complete loss of central 5-HT in adult Lmx1b−/− and Tph2−/− mice compromises thermoregulation during severe cold challenge (Hodges et al. 2008; Alenina et al. 2009) and the reduced 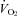 could explain the reduced
could explain the reduced 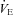 of Lmx1b−/− neonates compared to WT at reduced TA (Hodges et al. 2009). The extent to which brainstem 5-HT neurones contribute to thermogenesis in neonatal life during a more modest, physiologically relevant drop in environmental temperature is unknown. In this study, we measure the thermogenic response of neonatal mice, previously shown to be lacking ∼60–70% of their 5-HT-positive neurones (Pet-1−/−; Hendricks et al. 2003), testing the hypothesis that reduced brainstem 5-HT compromises the thermogenic response to a mild cold stress. Given that
of Lmx1b−/− neonates compared to WT at reduced TA (Hodges et al. 2009). The extent to which brainstem 5-HT neurones contribute to thermogenesis in neonatal life during a more modest, physiologically relevant drop in environmental temperature is unknown. In this study, we measure the thermogenic response of neonatal mice, previously shown to be lacking ∼60–70% of their 5-HT-positive neurones (Pet-1−/−; Hendricks et al. 2003), testing the hypothesis that reduced brainstem 5-HT compromises the thermogenic response to a mild cold stress. Given that 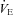 and
and 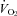 are normally tightly coupled (Frappell et al. 1992), and that sympathetic neurones innervating the brown fat and heart can be activated in parallel during cold stress, we also hypothesized that the
are normally tightly coupled (Frappell et al. 1992), and that sympathetic neurones innervating the brown fat and heart can be activated in parallel during cold stress, we also hypothesized that the 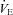 and HR of Pet-1−/− neonates would be reduced relative to wild-type pups during mild cooling.
and HR of Pet-1−/− neonates would be reduced relative to wild-type pups during mild cooling.
Serotonergic neurones in adult animals contribute to the ventilatory response to increasing CO2/H+ within the brainstem parenchyma, either directly as CO2 chemosensors or through positive interactions with other chemosensitive sites in the brainstem (Dias et al. 2008; Hodges et al. 2008; Nattie & Li, 2010). Data related to the role of 5-HT neurones in the CO2 response of unanaesthetised neonatal animals is conflicting. Pet-1−/− neonates have a normal ventilatory response to CO2 at P4.5. Piglets with acute inhibition of 5-HT neurones and rat pups fed a tryptophan-deficient diet (resulting in a ∼50% decrease in medullary 5-HT content) show an increased response early in postnatal life then a decreased response with development (Messier et al. 2004; Penatti et al. 2010). The reduced response in older animals is consistent with the observation in vitro that CO2 sensitivity emerges with development (Wang & Richerson, 1999). We hypothesize that the hypercapnic ventilatory response will be reduced in Pet-1−/− neonates older than P12, the age at which rodent 5-HT neurones become sensitive to CO2in vitro (Wang & Richerson, 1999).
In this study, we first measured the degree of 5-HT deficiency resulting from a loss of 60–70% of 5-HT-positive neurones from the brainstem of Pet-1−/− animals, and then assessed the consequences of this deficiency on their thermogenic, ventilatory and HR responses to a mild and brief drop in TA. We also measured the hypercapnic ventilatory response in separate groups of Pet-1−/− and WT animals. We demonstrate an apparent requirement for brainstem 5-HT neurones for cooling-induced thermogenesis, with consequences for both respiratory and heart rate control.
Methods
Animals
We used five litters from five different Pet-1+/− breeding pairs. All animals were studied in the unanaesthetised state. Dams were provided food and water ad libitum and were housed with a 12 h light–dark cycle (lights on from 06.00 to 18.00 h) at a TA of 21–23°C. To assess monoamine content, high-pressure liquid chromatographic (HPLC) analysis was performed on the brainstems (medulla and pons) of Pet-1−/− animals and WT (n = 4 of each) at ∼1 week of age. Prior to tissue harvesting, animals were killed with a lethal intra-peritoneal dose of a ketamine–xylazine mixture. There is no difference between Pet-1+/+ and +/− animals with respect to 5-HT cell counts (Hendricks et al. 2003). We have not observed any difference between Pet-1+/+ and +/− animals with respect to weight or 5-HT content (including the current data), so these two genotypes are grouped together (WT) for comparison with Pet-1−/− animals. To examine thermoregulatory ability, Pet-1−/− animals (n = 5; 2 males, 3 females) and WT (n = 5; 3 males, 2 females) were studied at P12, at TA= 34°C and 29°C. We chose P12 animals because younger WT animals did not display a robust increase in 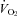 with cooling, and in our experience, older animals respond to cooling with excessive body movement which confounds the assessment of the autonomic thermoregulatory response. Average weights for WT and Pet-1−/− animals were 6.5 ± 0.3 g and 4.7 ± 0.2 g, respectively.
with cooling, and in our experience, older animals respond to cooling with excessive body movement which confounds the assessment of the autonomic thermoregulatory response. Average weights for WT and Pet-1−/− animals were 6.5 ± 0.3 g and 4.7 ± 0.2 g, respectively.
For examining the CO2 response, we studied two additional groups of animals: one at P5 (10 WT: 8 male, 2 female; 8 Pet-1−/−: 2 male, 6 female) and another at P15 (6 WT: 2 male, 4 female; 9 Pet-1−/−: 6 male, 3 female). Average weights for WT and Pet-1−/− animals at P5 were 2.8 ± 0.1 g and 2.1 ± 0.1 g, respectively, and at P12 were 8.2 ± 0.5 g and 5.4 ± 0.2 g, respectively. Heart rate data from these animals during normoxic, normocapnic conditions have appeared in a previous publication (Cummings et al. 2010). All experimental protocols were approved by the Institutional Animal Care and Use Committee of Dartmouth College.
Genotyping
Genotyping on isolated DNA was performed using primers: 5′-CGC ACT TGG GGG GTC ATT ATC AC-3′, 5′-CGG TGG ATG TGG AAT GTG TGC-3′ and 5′-GCC TGA TGT TCA AGG AAG ACC TCG G-3′ according to a previous study (Hendricks et al. 2003). PCR was performed using an initial 5 min denaturing step at 95°C, followed by 35 cycles of 94°C for 1 min, 62°C for 30 s and 72°C for 50 s. PCR products generated were a wild-type allele and knockout allele of 209 and 361 base pairs, respectively.
Experimental setup
Experiments were performed using a setup described previously (Cummings & Frappell, 2009). Briefly, the animal chamber (volume ∼40 ml) was constructed from a water-jacketed glass cylinder. TA was precisely controlled by changing the temperature of the water perfusing the glass chamber. Breathing was measured with a head-out system consisting of a mask and a pneumotach. This method provides accurate measurements of VT (and hence 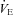 ) in neonatal animals (Mortola & Frappell, 1998). The head chamber was made by fitting a section of vinyl over the end of a syringe tube (volume ∼3 ml), held in place with another rubber gasket that fitted into the anterior end of the chamber. The snout of the animal was placed into a small hole in the vinyl and sealed with polyether material (Impregum F Polyether Impression material, 3M, St Paul, MN, USA).
) in neonatal animals (Mortola & Frappell, 1998). The head chamber was made by fitting a section of vinyl over the end of a syringe tube (volume ∼3 ml), held in place with another rubber gasket that fitted into the anterior end of the chamber. The snout of the animal was placed into a small hole in the vinyl and sealed with polyether material (Impregum F Polyether Impression material, 3M, St Paul, MN, USA).
A downstream pump (AEI Technologies, Naperville, IL, USA) connected to the outlet port of the mask pulled air or 5% CO2 (balanced with air or hyperoxia) through the pneumotach and mask at a flow of 100 ml min−1. 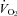 in air was determined by pulling the expired gas through an O2 analyser (AEI Technologies, Pittsburgh, PA, USA). CO2 was delivered directly from a tank to the surrounds of the pneumotach through the open end of a 50 cc syringe placed over the end of the pneumotach. In this way, the downstream pump pulled the gas through the mask with a very fast wash-in time. TB was continually monitored with a fine rectal thermocouple (Omega Engineering, Stamford, CT, USA). ECG was obtained with two surface electrodes embedded in a small vest worn by the animal. The electrodes rested on the ventral surface of the animal, displaced from each other ∼1 cm in the anterior–posterior and medial–lateral axes. Thermocouples and ECG leads exteriorized by way of a hole in a rubber gasket (Terumo Medical Corp., Japan) in the posterior end of the chamber.
in air was determined by pulling the expired gas through an O2 analyser (AEI Technologies, Pittsburgh, PA, USA). CO2 was delivered directly from a tank to the surrounds of the pneumotach through the open end of a 50 cc syringe placed over the end of the pneumotach. In this way, the downstream pump pulled the gas through the mask with a very fast wash-in time. TB was continually monitored with a fine rectal thermocouple (Omega Engineering, Stamford, CT, USA). ECG was obtained with two surface electrodes embedded in a small vest worn by the animal. The electrodes rested on the ventral surface of the animal, displaced from each other ∼1 cm in the anterior–posterior and medial–lateral axes. Thermocouples and ECG leads exteriorized by way of a hole in a rubber gasket (Terumo Medical Corp., Japan) in the posterior end of the chamber.
Inspiratory and expiratory airflows were detected by connecting both side-arms of the pneumotach to a differential pressure transducer (Validyne Engineering, Northridge, CA, USA). Integration of the flow trace provided respiratory volume, calibrated by injecting and withdrawing known volumes of air (0.025, 0.05 ml) at the end of each experiment. The pneumotach responded in a linear fashion to these volumes.
Experimental protocols
General
Experimentation was performed while blinded to genotype. Pups were removed from the litter and immediately weighed. Fur from the snout was removed as well as a small (0.2 cm × 0.5 cm) area on the dorsal surface of the tail, at approximately the midpoint for placement of a thermocouple. Animals were then instrumented with ECG leads contained in a small vest made from tensor bandage. A rectal thermocouple was inserted ∼1 cm and lightly glued to the base of the tail, and a separate thermocouple was fixed to the midpoint of the tail with a small drop of glue and then covered with a small bead of Impregum F. The snout of the animal was sealed into the mask. Animals rested comfortably in the chamber; no change in HR was observed in animals after being placed in the mask.
Protocol A: Thermoregulation (P12)
Each animal was allowed to equilibrate to a TA of 34 ± 0.2°C for 20 min. TA was then reduced from 34°C to 29°C (Fig. 2A). TA was measured with a thermocouple placed within the chamber, ∼1 cm off the upper surface of the chamber at the midpoint. Because of the hole in the rubber gasket at the rear of the chamber, a small temperature gradient existed within the chamber; TA directly over the tail was measured during each experiment and found to be ∼27.5°C when TA at the chamber midpoint was 29°C. Most of the decrease in TA during the cooling step occurred over the first 5 min after changing the temperature of the water perfusing the chamber. After 25 min at 29°C, TA was subsequently increased back to 34°C over the course of 15 min. We measured VT, f, 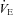 , the co-efficient of respiratory variation (CV%= (standard deviation of the respiratory period/average period) × 100),
, the co-efficient of respiratory variation (CV%= (standard deviation of the respiratory period/average period) × 100), 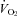 , TB, TT and HR in each animal during the last 5 min of each TA.
, TB, TT and HR in each animal during the last 5 min of each TA.
Figure 2. Reduced thermogenesis and heart rate (HR) in Pet-1−/− (KO) neonates during ambient cooling.
A, raw traces showing the changes in the fractional concentration of O2 leaving the mask (mask O2) and body temperature (TB) in Pet-1−/− animals (n = 5, grey lines) and littermates (WT) (n = 5, black lines) as ambient temperature (TA) is cooled from 34°C to 29°C over 25 min (shaded region). B, at 34°C, metabolic rate  is not significantly different in Pet-1−/− animals compared to WT. The disparity between genotypes becomes apparent as TA is reduced to 29°C (shaded region). C, at 34°C, TB is slightly but significantly lower in Pet-1−/− animals compared to WT. As is the case with
is not significantly different in Pet-1−/− animals compared to WT. The disparity between genotypes becomes apparent as TA is reduced to 29°C (shaded region). C, at 34°C, TB is slightly but significantly lower in Pet-1−/− animals compared to WT. As is the case with 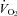 , the disparity becomes greater with cooling to 29°C. D, there were no differences in tail temperature (TT) between Pet-1−/− and WT animals at either TA. Note: TT is less than 29°C because of a TA gradient within the chamber (TA was 1.5°C lower at the tail end compared to the middle). E, there is a significantly greater reduction in HR with cooling in Pet-1−/− animals compared to WT. All data in B–E are means ± SE. *Significant genotype effect, P < 0.05; †genotype ×TA interaction, P < 0.05.
, the disparity becomes greater with cooling to 29°C. D, there were no differences in tail temperature (TT) between Pet-1−/− and WT animals at either TA. Note: TT is less than 29°C because of a TA gradient within the chamber (TA was 1.5°C lower at the tail end compared to the middle). E, there is a significantly greater reduction in HR with cooling in Pet-1−/− animals compared to WT. All data in B–E are means ± SE. *Significant genotype effect, P < 0.05; †genotype ×TA interaction, P < 0.05.
Protocol B: Ventilatory CO2 responses (P5 and P15)
The TB of each animal was increased to 36.0 ± 0.2°C over the course of ∼20 min. Unlike Protocol A, these animals were held at a euthermic TB of 36.0°C for the entire protocol (requiring a higher TA in Pet-1−/− compared to WT animals). We chose to clamp TB rather than TA as TB has known effects on the ventilatory response to CO2 (Cummings & Frappell, 2009). Resting 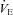 was measured for 10 min in normoxic, normocapnic conditions. The gas drawn through the mask was then changed to either normoxic hypercapnia (5% CO2, 21% O2, balance N2) or hyperoxic hypercapnia (5% CO2, 95% O2). Hypercapnia was maintained for 2 min. We measured VT, f and
was measured for 10 min in normoxic, normocapnic conditions. The gas drawn through the mask was then changed to either normoxic hypercapnia (5% CO2, 21% O2, balance N2) or hyperoxic hypercapnia (5% CO2, 95% O2). Hypercapnia was maintained for 2 min. We measured VT, f and 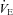 in the last minute of normocapnia and hypercapnia.
in the last minute of normocapnia and hypercapnia.
Data analysis
All analog signals were recorded and analysed in Labchart 6 (ADInstruments, Colorado Springs, USA) using Powerlab data acquisition system (ADInstruments). Heart rate and breathing were analysed using peak detection on the respiratory and R-wave traces. Heart rate was measured continually. Mass-specific 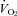 was determined using the formula (
was determined using the formula (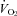 = (0.21 − fractional O2 exhausted from mask) × flow (ml min−1)/mass (kg)). Data are expressed as means ± SE.
= (0.21 − fractional O2 exhausted from mask) × flow (ml min−1)/mass (kg)). Data are expressed as means ± SE.
Statistical analysis
Pet-1−/− animals and many other 5-HT-deficient rodent models are invariably smaller than WT throughout neonatal life (Erickson et al. 2007; Alenina et al. 2009; Cummings et al. 2009, 2010), and this was the case with the current study. To assess the independent contribution of body mass to thermoregulation, we conducted two separate analyses of variance (ANOVA). In one, we performed a two-factor, repeated-measures ANOVA, with genotype as the between-subjects independent variable and TA as the within-subjects independent variable. In a second two-factor, repeated-measures ANOVA, we replaced genotype as the between-subjects variable with mass. Tukey's post hoc tests were performed when significant effects were found. Because TB responses to ambient cooling were not the same between genotypes, for each genotype we compared the observed 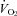 and HR after cooling with values predicted from a Q10 effect alone (assuming a Q10 co-efficient of 2). A two-factor, repeated-measures ANOVA was used to assess the effects of factor 1 (genotype) and factor 2 (observed or predicted response) on
and HR after cooling with values predicted from a Q10 effect alone (assuming a Q10 co-efficient of 2). A two-factor, repeated-measures ANOVA was used to assess the effects of factor 1 (genotype) and factor 2 (observed or predicted response) on 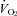 and HR.
and HR.
Results
Figure 1 shows that the brainstem 5-HT and 5-HIAA content of Pet-1−/− animals was reduced ∼90% compared to WT (P < 0.001). WT and Pet-1−/− animals had the same brainstem noradrenaline content. Figure 2A shows the raw metabolic and TB responses of each animal to the drop in TA. The change in the fractional concentration of O2 leaving the mask (mask O2) over the course of the cooling step reflects the change in 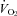 . From 34°C to 29°C, the
. From 34°C to 29°C, the 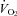 of all five WT animals increased, while there was no significant change in the
of all five WT animals increased, while there was no significant change in the  of any Pet-1−/− animals (Fig. 2A). An analysis of the mean data confirms a differential effect of cooling that depends on genotype: the
of any Pet-1−/− animals (Fig. 2A). An analysis of the mean data confirms a differential effect of cooling that depends on genotype: the 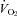 of WT animals increased 68 ± 19%, while the
of WT animals increased 68 ± 19%, while the 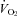 of Pet-1−/− animals declined 16 ± 8% (genotype ×TA interaction: P = 0.002; Fig. 2B). TB was slightly but significantly lower in Pet-1−/− animals compared to WT at TA of 34°C (P = 0.003; Fig. 2C). After ambient cooling, the TB of Pet-1−/− animals fell 4.2°C while that of WT fell 3.1°C (genotype ×TA interaction: P = 0.03, Fig. 2C). TT also dropped in all animals during cooling, but unlike TB, the fall in TT was the same in both genotypes (Fig. 2D). The HR of WT animals remained unchanged from 34°C to 29°C, while the HR of Pet-1−/− animals dropped 22 ± 2.3% (genotype ×TA interaction: P = 0.01, Fig. 2E).
of Pet-1−/− animals declined 16 ± 8% (genotype ×TA interaction: P = 0.002; Fig. 2B). TB was slightly but significantly lower in Pet-1−/− animals compared to WT at TA of 34°C (P = 0.003; Fig. 2C). After ambient cooling, the TB of Pet-1−/− animals fell 4.2°C while that of WT fell 3.1°C (genotype ×TA interaction: P = 0.03, Fig. 2C). TT also dropped in all animals during cooling, but unlike TB, the fall in TT was the same in both genotypes (Fig. 2D). The HR of WT animals remained unchanged from 34°C to 29°C, while the HR of Pet-1−/− animals dropped 22 ± 2.3% (genotype ×TA interaction: P = 0.01, Fig. 2E).
Figure 1. Pet-1−/− animals (KO) have reduced brainstem 5-HT.
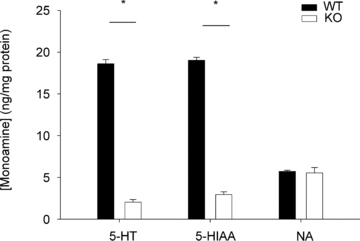
Shown is the 5-HT, 5-hydroxyindoleacetic acid (5-HIAA) and noradrenaline (NA) content from the medulla and pons of wild-type WT animals (n = 4) and Pet-1−/− animals (n = 4) at P7.
Mass also had a statistically significant effect on both TB and 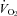 responses of WT and Pet-1−/− animals (TA× mass interaction effect: P < 0.05 for both TB and
responses of WT and Pet-1−/− animals (TA× mass interaction effect: P < 0.05 for both TB and 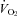 ; Fig. 3A and B). Rather than a reduced sympathetic response to the brown fat and heart, the reduced
; Fig. 3A and B). Rather than a reduced sympathetic response to the brown fat and heart, the reduced 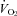 and HR responses of Pet-1−/− animals may have been due to their smaller size and larger drop in TB (i.e. Q10 effect). To address this, we compared the
and HR responses of Pet-1−/− animals may have been due to their smaller size and larger drop in TB (i.e. Q10 effect). To address this, we compared the 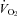 and HR responses of each animal with the responses expected from Q10 effects alone. The
and HR responses of each animal with the responses expected from Q10 effects alone. The 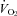 and HR of Pet-1−/− animals after cooling are not different from values that would result from the Q10 effect alone, while both
and HR of Pet-1−/− animals after cooling are not different from values that would result from the Q10 effect alone, while both 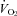 (Fig. 3C; P < 0.001) and HR (Fig. 3D; P = 0.02) responses of WT animals are greater than those that would result from Q10 alone.
(Fig. 3C; P < 0.001) and HR (Fig. 3D; P = 0.02) responses of WT animals are greater than those that would result from Q10 alone.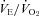 was significantly elevated in P12 Pet-1−/− animals compared to WT (genotype: P = 0.002; Fig. 4A). This was evident at both TA values, despite
was significantly elevated in P12 Pet-1−/− animals compared to WT (genotype: P = 0.002; Fig. 4A). This was evident at both TA values, despite 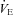 decreasing in Pet-1−/− and increasing in WT animals from 34°C to 29°C (genotype ×TA interaction: P = 0.002; Fig. 4B). The drop in
decreasing in Pet-1−/− and increasing in WT animals from 34°C to 29°C (genotype ×TA interaction: P = 0.002; Fig. 4B). The drop in 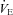 in Pet-1−/− animals after cooling resulted from reductions in both VT and f (genotype ×TA interaction: P = 0.04 and P = 0.02, respectively; Fig. 4C and D). Pet-1−/− animals had more respiratory variability than WT animals, irrespective of TA (genotype: P = 0.006; Fig. 4E).
in Pet-1−/− animals after cooling resulted from reductions in both VT and f (genotype ×TA interaction: P = 0.04 and P = 0.02, respectively; Fig. 4C and D). Pet-1−/− animals had more respiratory variability than WT animals, irrespective of TA (genotype: P = 0.006; Fig. 4E).
Figure 3. Effects of body size and Q10 on thermogenesis and HR during ambient cooling.
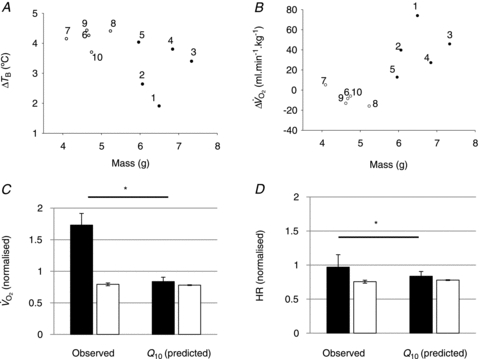
A and B, WT animals (filled circles, nos. 1–5) weigh more than Pet-1−/− animals (open circles, nos. 6–10), and mass has a significant relationship with TB and 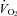 (2-factor repeated-measures ANOVA: P < 0.05 for each). C and D,
(2-factor repeated-measures ANOVA: P < 0.05 for each). C and D, 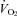 and HR after cooling (normalised to baseline values) for WT (filled bars), and Pet-1−/− (open bars). Shown are observed values and values predicted from the Q10 effect alone (assuming Q10 co-efficient of 2 and with a drop in TB of 4.2°C in Pet-1−/− and 3.1°C in WT). Data in C and D are means ± SE. *Significant difference exists between observed WT values for
and HR after cooling (normalised to baseline values) for WT (filled bars), and Pet-1−/− (open bars). Shown are observed values and values predicted from the Q10 effect alone (assuming Q10 co-efficient of 2 and with a drop in TB of 4.2°C in Pet-1−/− and 3.1°C in WT). Data in C and D are means ± SE. *Significant difference exists between observed WT values for 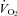 and HR and the values predicted from the Q10 effect alone. No such difference exists between the observed and predicted values for Pet-1−/− animals.
and HR and the values predicted from the Q10 effect alone. No such difference exists between the observed and predicted values for Pet-1−/− animals.
Figure 4. Hyperventilation in P12 Pet-1−/− animals.
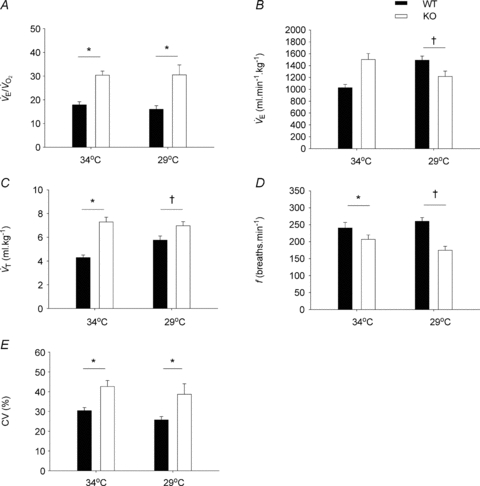
A, 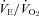 is elevated in P12 Pet-1−/− animals, irrespective of TA. B, from TA= 34°C to 29°C,
is elevated in P12 Pet-1−/− animals, irrespective of TA. B, from TA= 34°C to 29°C, 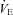 decreases in Pet-1−/− animals, but increases in WT animals. C and D,
decreases in Pet-1−/− animals, but increases in WT animals. C and D, 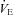 is selectively reduced in Pet-1−/− animals from a reduction in tidal volume (VT) and respiratory frequency (f) E, respiratory variability, as measured by the co-efficient of variation in the respiratory period (CV%) is higher in Pet-1−/− animals at both TA. All data are means ± SE. *Significant genotype effect, P < 0.05; †genotype ×TA interaction, P < 0.05.
is selectively reduced in Pet-1−/− animals from a reduction in tidal volume (VT) and respiratory frequency (f) E, respiratory variability, as measured by the co-efficient of variation in the respiratory period (CV%) is higher in Pet-1−/− animals at both TA. All data are means ± SE. *Significant genotype effect, P < 0.05; †genotype ×TA interaction, P < 0.05.
With TB held at 36.0°C, the room air 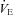 of P5 and P15 Pet-1−/− animals was the same as WT (Fig. 5), and there was no effect of genotype on
of P5 and P15 Pet-1−/− animals was the same as WT (Fig. 5), and there was no effect of genotype on 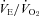 (not shown). At both ages, Pet-1−/− and WT animals had the same ventilatory response to both normoxic (Fig. 5A) and hyperoxic (Fig. 5B) hypercapnia. This is reflected in a similar percentage increase in
(not shown). At both ages, Pet-1−/− and WT animals had the same ventilatory response to both normoxic (Fig. 5A) and hyperoxic (Fig. 5B) hypercapnia. This is reflected in a similar percentage increase in 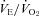 from room air to hypercapnia in both genotypes (Fig. 5C).
from room air to hypercapnia in both genotypes (Fig. 5C).
Figure 5. A loss of brainstem 5-HT neurones has no effect on the ventilatory response to CO2 at P5 or P15.
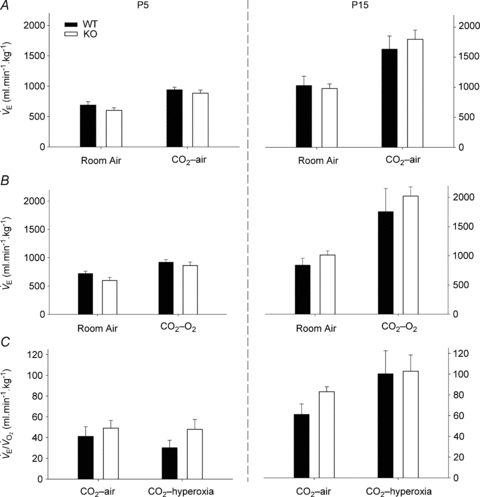
A, ventilation 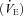 of WT and Pet-1−/− (KO) animals at P5 and P15, during room air and after 2 min of 5% CO2 balanced with air (CO2–air). B,
of WT and Pet-1−/− (KO) animals at P5 and P15, during room air and after 2 min of 5% CO2 balanced with air (CO2–air). B, 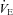 of WT and Pet-1−/− animals during room air and after 2 min of 5% CO2 balanced with O2 (CO2–O2). C, increase in
of WT and Pet-1−/− animals during room air and after 2 min of 5% CO2 balanced with O2 (CO2–O2). C, increase in 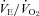 (% change) after 2 min of either CO2–air or CO2–O2 in each genotype at P5 and P15. All data are means ± SE.
(% change) after 2 min of either CO2–air or CO2–O2 in each genotype at P5 and P15. All data are means ± SE.
Discussion
Given evidence from adult animals, we hypothesized that the thermogenic response of Pet-1−/− neonates – sustaining a 90% loss of brainstem 5-HT – would be reduced compared to WT. Not only was our hypothesis confirmed but surprisingly, Pet-1−/− animals at P12 were devoid of any measurable increase in 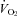 during a mild (5°C) drop in TA. Associated with this ablation of the
during a mild (5°C) drop in TA. Associated with this ablation of the 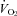 response during cooling in Pet-1−/− animals was a considerably larger drop in TB compared to WT. That TT was the same between WT and Pet-1−/− animals at reduced TA suggests that 5-HT loss does not compromise tail vasoconstriction, an important heat-conserving mechanism in rodents. The negative effect of brainstem 5-HT loss on the
response during cooling in Pet-1−/− animals was a considerably larger drop in TB compared to WT. That TT was the same between WT and Pet-1−/− animals at reduced TA suggests that 5-HT loss does not compromise tail vasoconstriction, an important heat-conserving mechanism in rodents. The negative effect of brainstem 5-HT loss on the 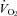 response to cooling is reflected in both the HR and
response to cooling is reflected in both the HR and 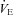 responses: both HR and
responses: both HR and 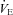 of Pet-1−/− animals fall with cooling while in WT HR is maintained and
of Pet-1−/− animals fall with cooling while in WT HR is maintained and 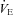 increases. Based on these data we propose that a loss of brainstem 5-HT in neonatal life eliminates the sympathetically mediated
increases. Based on these data we propose that a loss of brainstem 5-HT in neonatal life eliminates the sympathetically mediated 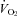 and HR responses to mild and brief cooling, with consequences for ventilatory control.
and HR responses to mild and brief cooling, with consequences for ventilatory control.
Brainstem 5-HT has the potential to influence both non-shivering and shivering thermogenesis. Bulbospinal 5-HT neurones innervate the intermediolateral cell column (Bowker et al. 1981), and activation of 5-HT receptors in this region enhances brown fat-mediated thermogenesis in adults (Madden & Morrison, 2010). Other data obtained from conscious piglets show that pharmacological inhibition of 5-HT neurones inhibits shivering thermogenesis (Hoffman et al. 2007; Brown et al. 2008). Adult Lmx-1b−/− mice (devoid of 5-HT neurones) do retain partial non-shivering and shivering thermogenesis in the severe cold (Hodges et al. 2008). Shivering thermogenesis, at least in neonatal rats, does not normally begin until the start of the third postnatal week (Jansky, 1973). Although we cannot completely discount an effect on shivering thermogenesis, it is likely that the effects we describe in Pet-1−/− animals at P12 are primarily the result of absent non-shivering thermogenesis. We cannot glean which 5-HT neurones are responsible for reduced thermogenesis in Pet-1−/− animals, or whether the effects are due to a developmental or physiological deficit; the lesion to the 5-HT system in Pet-1−/− animals is widespread throughout the brainstem raphe system (Hendricks et al. 2003). Bulbospinal 5-HT neurones originating in the raphe pallidus make an important physiological contribution to the sympathetic activation of the brown fat (Bowker et al. 1981; Allen & Cechetto, 1994; Nakamura & Morrison, 2007; Brown et al. 2008; Madden & Morrison, 2010), so a deficit in this neuronal population probably underpins the absence of thermogenesis in Pet-1−/− animals.
In addition to their compromised thermoregulation, the HR of Pet-1−/− animals fell considerably with ambient cooling, while that of WT remained unchanged. This observation is consistent with findings from anaesthetised adult rats in which both brown fat-mediated thermogenesis and HR are inhibited during acute skin cooling after application of muscimol (a GABAA receptor agonist) or 8-OH-DPAT (a 5-HT1A receptor agonist) to the raphe pallidus (Nakamura & Morrison, 2007). TB fell more in Pet-1−/− animals than WT with ambient cooling, leaving open the possibility that an effect of Q10 contributed to their reduced 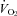 and HR responses to ambient cooling. That in WT, but not Pet-1−/− animals, there was a significant difference between the observed
and HR responses to ambient cooling. That in WT, but not Pet-1−/− animals, there was a significant difference between the observed 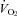 and HR after cooling and values predicted from Q10 alone suggests that 5-HT neurones are essential for the increase in sympathetic drive to the brown fat and heart with ambient cooling. Whether the sympathetic regulation of thermogenesis and heart rate during ambient cooling is served by the same set(s) of brainstem 5-HT neurones is another question we cannot answer with the current data, and is a matter of speculation even in adult animals (Ootsuka & Blessing, 2006). Brainstem 5-HT may be especially important during neonatal life for the appropriate sympathetic response to a variety of physiological stressors. An in-depth examination of the heart rate responses to other forms of physiological stress that may impact neonatal survival is warranted (e.g. hypoxia).
and HR after cooling and values predicted from Q10 alone suggests that 5-HT neurones are essential for the increase in sympathetic drive to the brown fat and heart with ambient cooling. Whether the sympathetic regulation of thermogenesis and heart rate during ambient cooling is served by the same set(s) of brainstem 5-HT neurones is another question we cannot answer with the current data, and is a matter of speculation even in adult animals (Ootsuka & Blessing, 2006). Brainstem 5-HT may be especially important during neonatal life for the appropriate sympathetic response to a variety of physiological stressors. An in-depth examination of the heart rate responses to other forms of physiological stress that may impact neonatal survival is warranted (e.g. hypoxia).
Others have shown that during the first two postnatal weeks, neonatal rodents with a genetically induced loss of brainstem 5-HT have reduced, unstable breathing (Erickson et al. 2007; Cummings & Frappell, 2009; Hodges et al. 2009; Cummings et al. 2010). The inhibitory effect of cooling on the 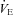 of Pet-1−/− animals is probably due to their reduced
of Pet-1−/− animals is probably due to their reduced 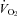 ;
; 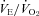 did not change in either genotype with cooling although it was higher in Pet-1−/− at both TA values. Lmx-1b−/− neonates lacking nearly all serotonergic neurones have reduced breathing, including apnoeas nearly 1 min long (Hodges et al. 2009). Although reduced
did not change in either genotype with cooling although it was higher in Pet-1−/− at both TA values. Lmx-1b−/− neonates lacking nearly all serotonergic neurones have reduced breathing, including apnoeas nearly 1 min long (Hodges et al. 2009). Although reduced 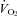 could contribute to the Lmx-1b−/− phenotype, the more severe respiratory dysfunction observed in Lmx1b−/− mice compared to Pet-1−/− mice may result from a loss of co-expressed factors (e.g. substance P, thyrotropin releasing factor) along with 5-HT. This may also explain why the CO2 response is reduced in Lmx1b−/− mice but not in Pet-1−/− neonates. Alternatively, 5-HT neurones may contribute more to the CO2 response at later points in development or at more extreme levels of hypercapnia (Hodges et al. 2008; Nattie & Li, 2010; Penatti et al. 2010).
could contribute to the Lmx-1b−/− phenotype, the more severe respiratory dysfunction observed in Lmx1b−/− mice compared to Pet-1−/− mice may result from a loss of co-expressed factors (e.g. substance P, thyrotropin releasing factor) along with 5-HT. This may also explain why the CO2 response is reduced in Lmx1b−/− mice but not in Pet-1−/− neonates. Alternatively, 5-HT neurones may contribute more to the CO2 response at later points in development or at more extreme levels of hypercapnia (Hodges et al. 2008; Nattie & Li, 2010; Penatti et al. 2010).
P12 Pet-1−/− animals hyperventilate (increased 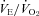 ) compared to WT. This implies that these animals are hypocapnic and potentially alkalotic relative to controls. We did not observe hyperventilation in P5 or P15 Pet-1−/− animals when TB was held at 36°C. TB is unlikely to be playing a role, as further hyperventilation did not occur in P12 Pet-1−/− animals when TB was reduced after ambient cooling. Alternatively, Pet-1−/− animals may only hyperventilate during a narrow window of postnatal life. Others have shown that a loss of brainstem 5-HT induces hyperventilation in rats and goats (Olson et al. 1979; Mitchell et al. 1983). The influence of brainstem 5-HT on the matching of
) compared to WT. This implies that these animals are hypocapnic and potentially alkalotic relative to controls. We did not observe hyperventilation in P5 or P15 Pet-1−/− animals when TB was held at 36°C. TB is unlikely to be playing a role, as further hyperventilation did not occur in P12 Pet-1−/− animals when TB was reduced after ambient cooling. Alternatively, Pet-1−/− animals may only hyperventilate during a narrow window of postnatal life. Others have shown that a loss of brainstem 5-HT induces hyperventilation in rats and goats (Olson et al. 1979; Mitchell et al. 1983). The influence of brainstem 5-HT on the matching of 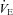 and
and 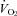 seems to depend on both species and stage of development. Our observations emphasize the need for accurate measurements of VT and
seems to depend on both species and stage of development. Our observations emphasize the need for accurate measurements of VT and 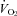 when assessing factors influencing respiratory control during the neonatal period. This is troublesome in small, developing mammals where the TB–TA difference is narrowed, and in which there exists a considerable amount of airway resistance that, when using whole-body arrangements, contaminates the respiratory signal (Mortola & Frappell, 1998).
when assessing factors influencing respiratory control during the neonatal period. This is troublesome in small, developing mammals where the TB–TA difference is narrowed, and in which there exists a considerable amount of airway resistance that, when using whole-body arrangements, contaminates the respiratory signal (Mortola & Frappell, 1998).
We have demonstrated that at a stage of development that may be close to the newborn period in humans (Clancy et al. 2001), brainstem 5-HT is essential for the thermogenic, HR and ventilatory responses to a mild and brief environmental cooling. In unanaesthetised neonatal animals, compromised non-shivering thermogenesis should be considered when assessing the effects of 5-HT deficiency on respiratory and HR control, especially at environmental temperatures below thermoneutrality. With previous findings in mind, we propose that brainstem 5-HT deficiency compromises, in a broad manner, the sympathetic responses to a variety of physiological stressors. Human infants with brainstem 5-HT deficiency may be prone to a variety of homeostatic deficits involving thermoregulatory, respiratory and cardiovascular control. Some of these deficits could manifest in the sudden infant death syndrome, recently linked to a deficiency in medullary 5-HT (Duncan et al. 2010).
Acknowledgments
Funding for this study was provided by an NIH Program Project grant HD36379 (NICHD, PI, to H.C. Kinney and PROJECT 2 PI, to E.E. Nattie) and NIH grant HL28066, (PI, to E.E. Nattie). We thank Dr James C. Leiter (Dartmouth) for help with statistical analyses. HPLC determinations were performed by the CMN/KC Neurochemistry Core Lab at Vanderbilt University. The CMN/KC Neurochemistry Core Lab is supported by Vanderbilt Kennedy Center for Research on Human Development, Vanderbilt Conte Center for Neuroscience Research and The Vanderbilt Center for Molecular Neuroscience. The authors also acknowledge the generous support of the Parker B. Francis Family and its Foundation (Fellowship to K.J. Cummings). K.J.C. thanks EPC for thoughtful discussion.
Glossary
Abbreviations
- 5-HT
5-hydroxytryptamine
- HR
heart rate
- TA
ambient temperature
- TB
body temperature
- TT
tail temperature
Author contributions
K.J.C., A.L. and E.E.N. contributed to the conception, design and analysis of experiments including interpretation of data, and drafting/revising/approving the final version of the manuscript for publication.
References
- Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GV, Cechetto DF. Serotoninergic and nonserotoninergic neurons in the medullary raphe system have axon collateral projections to autonomic and somatic cell groups in the medulla and spinal cord. J Comp Neurol. 1994;350:357–366. doi: 10.1002/cne.903500303. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Coulter JD. Serotonergic projections to the spinal cord from the midbrain in the rat: an immunocytochemical and retrograde transport study. Neurosci Lett. 1981;24:221–226. doi: 10.1016/0304-3940(81)90160-9. [DOI] [PubMed] [Google Scholar]
- Brown JW, Sirlin EA, Benoit AM, Hoffman JM, Darnall RA. Activation of 5-HT1A receptors in medullary raphe disrupts sleep and decreases shivering during cooling in the conscious piglet. Am J Physiol Regul Integr Comp Physiol. 2008;294:R884–R894. doi: 10.1152/ajpregu.00655.2007. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Commons KG, Fan KC, Li A, Nattie EE. Severe spontaneous bradycardia associated with respiratory disruptions in rat pups with fewer brain stem 5-HT neurons. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1783–R1796. doi: 10.1152/ajpregu.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Frappell PB. Breath-to-breath hypercapnic response in neonatal rats: temperature dependency of the chemoreflexes and potential implications for breathing stability. Am J Physiol Regul Integr Comp Physiol. 2009;297:R124–R134. doi: 10.1152/ajpregu.91011.2008. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Li A, Deneris ES, Nattie EE. Bradycardia in serotonin-deficient Pet-1−/− mice: influence of respiratory dysfunction and hyperthermia over the first 2 postnatal weeks. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1333–R1342. doi: 10.1152/ajpregu.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie E. Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J Appl Physiol. 2008;105:83–90. doi: 10.1152/japplphysiol.00120.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol. 2007;159:85–101. doi: 10.1016/j.resp.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frappell P, Lanthier C, Baudinette RV, Mortola JP. Metabolism and ventilation in acute hypoxia: a comparative analysis in small mammalian species. Am J Physiol Regul Integr Comp Physiol. 1992;262:R1040–R1046. doi: 10.1152/ajpregu.1992.262.6.R1040. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci. 2009;29:10341–10349. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JM, Brown JW, Sirlin EA, Benoit AM, Gill WH, Harris MB, Darnall RA. Activation of 5-HT1A receptors in the paragigantocellularis lateralis decreases shivering during cooling in the conscious piglet. Am J Physiol Regul Integr Comp Physiol. 2007;293:R518–R527. doi: 10.1152/ajpregu.00816.2006. [DOI] [PubMed] [Google Scholar]
- Jansky L. Non-shivering thermogenesis and its thermoregulatory significance. Biol Rev Camb Philos Soc. 1973;48:85–132. doi: 10.1111/j.1469-185x.1973.tb01115.x. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Endogenous activation of spinal 5-hydroxytryptamine (5-HT) receptors contributes to the thermoregulatory activation of brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2010;298:R776–R783. doi: 10.1152/ajpregu.00614.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience. 2000;98:301–309. doi: 10.1016/s0306-4522(00)00133-0. [DOI] [PubMed] [Google Scholar]
- Messier ML, Li A, Nattie EE. Inhibition of medullary raphe serotonergic neurons has age-dependent effects on the CO2 response in newborn piglets. J Appl Physiol. 2004;96:1909–1919. doi: 10.1152/japplphysiol.00805.2003. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Smith CA, Vidruk EH, Jameson LC, Dempsey JA. Effects of p-chlorophenylalanine on ventilatory control in goats. J Appl Physiol. 1983;54:277–283. doi: 10.1152/jappl.1983.54.1.277. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola JP, Frappell PB. On the barometric method for measurements of ventilation, and its use in small animals. Can J Physiol Pharmacol. 1998;76:937–944. doi: 10.1139/cjpp-76-10-11-937. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292:R127–R136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception in wakefulness and sleep: evidence for a distributed network and a role for orexin. J Appl Physiol. 2010;108:1417–1424. doi: 10.1152/japplphysiol.01261.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EB, Jr, Dempsey JA, McCrimmon DR. Serotonin and the control of ventilation in awake rats. J Clin Invest. 1979;64:689–693. doi: 10.1172/JCI109510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. Thermogenesis in brown adipose tissue: increase by 5-HT2A receptor activation and decrease by 5-HT1A receptor activation in conscious rats. Neurosci Lett. 2006;395:170–174. doi: 10.1016/j.neulet.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Penatti EM, Barina AE, Raju SC, Li A, Kinney HC, Commons KG, Nattie EE. Maternal dietary tryptophan deficiency alters cardiorespiratory control in rat pups. J Appl Physiol. 2010;110:318–328. doi: 10.1152/japplphysiol.00788.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathner JA, Owens NC, McAllen RM. Cold-activated raphe-spinal neurons in rats. J Physiol. 2001;535:841–854. doi: 10.1111/j.1469-7793.2001.t01-1-00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphe neurons. Neuroscience. 1999;90:1001–1011. doi: 10.1016/s0306-4522(98)00505-3. [DOI] [PubMed] [Google Scholar]


