Non-technical summary
Even when the bladder is full, emptying can be deferred voluntarily until individuals find themselves in a socially appropriate environment. This indicates that the brain is important in the control of bladder function. This study used drugs that either mimic or block the effects of the neurotransmitter chemicals involved in communication between nerve cells to investigate the brain nerve circuits that govern the control of the bladder. A small region in the midbrain was shown to be critical for normal bladder emptying to occur. Normally the excitability of the nerve circuits in this region was controlled by the action of an inhibitory neurotransmitter chemical called GABA. However, if GABA's effect was removed, bladder emptying became abnormally frequent. These results advance our understanding of the normal control of bladder function, and have implications for the development of new treatments for some forms of incontinence.
Abstract
Abstract
In urethane-anaesthetised rats continuous infusion of saline into the bladder (6 ml h−1) evoked periodic sharp rises in intravesicular pressure accompanied by rhythmic bursting of external urethral sphincter (EUS) EMG and expulsion of urine from the urethral meatus. Microinjection of the GABA agonist muscimol (250 pmol) into the caudal ventrolateral periaqueductal grey (PAG), but not at other sites in the PAG, either depressed reflex voiding frequency (−60%, n = 7) and tonic EUS EMG activity (−38%, n = 6) or completely inhibited voiding (four sites). Microinjection of the GABA antagonist bicuculline (BIC; 1 nmol) into the same region, to reduce ongoing GABA tone, increased reflex voiding frequency (+467%, n = 16) and tonic activity in the EUS (+56%, n = 7) whilst bursting activity in the EUS became desynchronised. Although muscimol failed to change reflex micturition when microinjected into the dorsal caudal PAG, microinjection of BIC at these sites evoked pronounced autonomic arousal and increased reflex voiding frequency (+237%, n = 34). The results demonstrate that the functional integrity of synapses in the caudal ventrolateral PAG is essential to permit micturition. Transmission through the region is normally regulated by a tonic GABAergic inhibitory influence. In contrast, the functional integrity of the dorsal caudal PAG is not essential for reflex micturition. However, micturition may be initiated from this region via projections to the caudal ventrolateral PAG, as part of the behavioural response to psychological threat or other stressful stimuli.
Introduction
In most socialised animals micturition is a reflex event that is under inhibitory control from the brain. Even when the bladder is full, voiding can be suppressed until the individual finds itself in a safe and socially acceptable environment. This implies that under normal circumstances, activity in the micturition reflex pathway is tonically inhibited. The micturition reflex pathway includes a supraspinal loop that relays in the midbrain. As the bladder fills, activity from stretch afferents in the bladder wall is relayed via the sacral cord to the periaqueductal grey matter (PAG). To complete the reflex loop, neurones in the PAG then project to the pontine micturition centre (PMC, also known as Barrington's nucleus), which in turn sends projections directly to preganglionic parasympathetic neurones in the sacral cord that regulate the activity of motor neurones innervating the detrusor and urethral sphincter muscles (Holstege, 2005, 2010; Fowler et al. 2008; Drake et al. 2010 for reviews). In some species, but not the rat, more laterally in the pons lies the pontine storage centre or ‘L-region’. Electrical stimulation of this region induces contraction of the external urethral sphincter (EUS) and relaxation of the bladder (Holstege et al. 1986). Anatomical connections between this region, the PMC and the PAG have yet to be demonstrated (Blok & Holstege, 1999).
Recent evidence points to the PAG as a site where the level of ongoing GABAergic tone may be important in determining whether micturition can occur. The PAG contains a dense population of GABAergic interneurones (Reichling & Basbaum, 1990; Lovick & Paul, 1999; Griffiths & Lovick, 2005), which exert a tonic inhibitory influence on output neurones (Behbehani et al. 1990; Ogawa et al. 1994; Brack & Lovick, 2007). Studies in conscious animals have shown that aspects of several behavioural responses controlled by the PAG such as defensive behaviour, flight, anti-nociception and tonic immobility are subject to ongoing inhibitory GABAergic control (Bandler et al. 1985; Carrive et al. 1986; Monassi et al. 1999; Morgan & Clayton, 2005). Furthermore, recent studies in conscious rats indicate that the act of voiding is associated with a decrease in the concentration of extracellular GABA in the PAG (Kitta et al. 2008) suggesting that in between voids, transmission in the micturition reflex pathway may normally be inhibited by ongoing GABAergic activity. However, the role of GABAergic systems in the PAG in the control of micturition has yet to be investigated systematically.
In the present study we examined the effect on micturition of manipulating the level of GABAergic inhibition in the PAG. Experiments were carried out to determine (a) the location of synaptic relays within the PAG that are essential components of the micturition reflex pathway, and (b) whether transmission through these regions is normally suppressed by a tonic GABAergic influence. Part of this work has been published in abstract form (Stone et al. 2009).
Methods
Ethical approval
These experiments were approved by the Joint Ethics and Research Governance Committee of The University of Birmingham and were carried out in accordance with the Animals (Scientific Procedures) Act 1986. All efforts were made to reduce the number of animals used and to minimise the risk of any suffering.
Experiments were performed on 79 urethane-anaesthetised (1.5 g kg−1i.p.) spontaneously breathing male Sprague–Dawley rats (Charles River, Kent, UK) 197–410 g body weight. In all rats blood pressure was monitored in a femoral artery and a cannula in a femoral vein was used for infusion of fluids. The trachea was cannulated and attached to a spirometer to monitor tracheal airflow. Rectal temperature was maintained at 37 ± 1°C by a thermostatically controlled heating blanket. The animal was mounted prone in a stereotaxic frame with the head in the attitude described by Paxinos & Watson (1986). The lower body was twisted to allow access to the abdomen. The bladder was exposed via a midline laparotomy and the dome pierced by a 23G stainless steel needle connected via polythene tubing to a T-piece. One arm was used for infusion of saline (6 ml h−1) (Van Asselt et al. 1995; Matsuura et al. 1998, 2000; Kitta et al. 2008) into the bladder and the other was connected to a pressure transducer to monitor intravesicular pressure. A drop counter was positioned to record the passage of drops of urine as they fell from the penis. Two 26G stainless steel needles, insulated except for the tips, were inserted bilaterally into the external urethral sphincter muscle (EUS) proximal to the symphysis pubis, to record EMG activity. A recovery period of 30 min was allowed following surgery before infusion of saline into the bladder was started.
A dorsal craniotomy was made to expose the midbrain and the dura was reflected. A stainless steel cannula (500 μm diameter), connected to a 1 μl microsyringe was inserted into the midbrain, inclined at an angle of 10 deg facing caudally. The cannula was filled with either 5 × 10−3m muscimol, 2 × 10−2m bicuculline methiodide (BIC, both from Sigma, Dorset, UK) or saline. Drug and saline solutions were injected in 50 nl volumes. Doses were based on those published in the literature and used in preliminary experiments. Between 1 and 6 microinjections (usually 2–4) were made in any one rat. To aid histological localisation of injection sites, a few milligrams of pontamine sky blue dye were added to the solutions of muscimol and saline (pH 7.4), whilst solutions of BIC contained 0.2% yellow-green fluorescent microbeads (FluoSphere, Invitrogen Molecular Probes, Eugene, OR, USA). Recordings of physiological variables were captured using a PowerLab data acquisition system (ADInstruments) running Chart V4.2.3 software.
Two experimental protocols were employed. In most experiments saline was infused continuously into the bladder. Once stable cycles of filling and voiding had been established (typically after 1–3 h), the effect on bladder pressure and EUS activity of microinjection of GABAergic drugs or saline into the PAG was tested. In order to determine whether the changes in EUS activity seen after injection of drugs into the PAG represented a primary response or were secondary to changes in intravesicular pressure, a further six experiments were carried out in which the bladder was first emptied by withdrawing the contents via the infusion cannula. The effects of microinjecting BIC into the PAG on EUS activity with the bladder empty and in response to stepped increases in bladder volume were then tested.
At the end of each experiment the animal was killed with an overdose of anaesthetic and the brain removed and fixed in 10% formol saline. To visualise blue dye marks serial frozen coronal sections 60 μm thick were cut and stained with Neutral Red. For visualisation of the fluorescent microbeads, alternate unstained sections were examined in a fluorescence microscope exciting at 450–500 nm and detecting at 475–700 nm, whilst the remaining sections were stained with Neutral Red to identify brain structures. The locations of injection sites were reconstructed from dye marks and cannula tracks present in the 60 μm thick histological sections. Subsequently the centre of each microinjection site was plotted onto the closest representative section of the PAG at levels P-5.6, P-6.2, P-8.0 and P-8.8, with reference to the atlas of Paxinos & Watson (1986).
The significance of differences following treatments was assessed using Student's paired t test with significance level set at P < 0.05. All values are represented as mean ± SEM. A bladder ‘contraction’ was defined as a change in the intravesicular pressure of more than 7 mmHg. For analysis of the frequency of contractions, voiding and non-voiding contractions were grouped together. The raw EMG signal from the EUS taken over a 25 s period in between bladder contractions was rectified and the area under the curve calculated by summing the activity in individual bins. Mean values for arterial blood pressure, heart rate and respiratory rate over 1 min in the pre-drug control period were compared to the maximum values reached in the post-drug period.
Results
Response to infusion of saline into the bladder
When saline was infused continuously into the bladder (6 ml h−1), intravesicular pressure began to rise. As the infusion continued repeated phasic increases in bladder pressure developed, each one accompanied by the expulsion of several drops of urine from the penis (Fig. 1). During each contraction, bladder pressure rose steeply from threshold to a peak (mean change 17.59 ± 0.62 mmHg, range 6.43–30.69 mmHg) before returning to baseline within 14–124 s. In 25 rats that had recording electrodes in the EUS, a low level of tonic EMG activity, which was eliminated after the animal had been killed, could be detected during the filling phase. As the bladder pressure rose sharply prior to voiding, EMG activity increased markedly, changing from a tonic activity to a bursting pattern (Fig. 1). In between bursts of EMG activity spurts of urine could be seen exiting the penis. The rhythmic bursting of the EUS activity was reflected as small phasic changes in vesicular pressure (Fig. 1). Rhythmic contractile activity remained stable over periods up to 7 h without any significant change in residual pressure. There was a considerable degree of individual variation in parameters of voiding, e.g. threshold, maximum pressure, duration of contraction and frequency. With the exception of frequency, the mean values were the same in all treatment groups. In terms of frequency of voiding, the range was similar in all treatment groups (0.13–1.65, 0.31–1.88 and 0.59–1.08 contractions min−1 for muscimol, bicuculline and saline groups, respectively), the mean value was significantly lower in the bicuculline group compared to the muscimol group (0.65 ± 0.04 (n = 65) vs. 0.83 ± 0.04 (n = 61) but there was no difference between either bicuculline or muscimol and the saline control group (0.80 ± 0.04 (n = 19)) (one-way ANOVA between the groups with Bonferroni post hoc test (P = 0.007)). Despite these variations in baseline voiding frequency, the effects of GABA agonists and antagonists were independent of voiding frequency (see below). A wide weight range of animals was used in the present study. However, there was no significant difference between the mean weight of different treatment groups nor was there any difference in the responsiveness between the lightest and the heaviest rats.
Figure 1. Effect of continuous infusion of saline (6 ml h−1) into the bladder of anaesthetised rats.

Lower trace, response evoked by continuous infusion of saline into the bladder (6 ml h−1). Each phasic increase in intravesicular pressure was accompanied by a burst of activity in the EUS and expulsion of urine from the penis. Upper trace, expanded trace shows bursting pattern of activity in the EUS and expulsion of several drops of urine in quick succession. Oscillation in the bladder pressure corresponds with bursting activity in the EUS.
Effect of microinjection of a GABAA agonist into the PAG on reflex contractions of the bladder
Muscimol (250 pmol in 50 nl) was injected at 61 sites along the entire rostrocaudal axis of the PAG on either side of the midline (Fig. 3A). At the majority of sites (82.25%) there was less than a 5% change in the mean frequency, threshold, maximum pressure or duration of reflex bladder contractions (0.79 ± 0.04 to 0.79 ± 0.04 contractions min−1, P = 0.79 (n = 50), 13.09 ± 0.46 to 13.23 ± 0.39 mmHg, P = 0.70, 30.52 ± 0.66 to 30.18 ± 0.61 mmHg, P = 0.27 and 24.92 ± 1.47 to 27.24 ± 1.46 s, P = 0.20, respectively) or the other cardiovascular, respiratory and autonomic variables monitored. However, after injection of muscimol into a restricted area within the caudal ventrolateral PAG (filled circles, Fig. 3A), the micturition response was disrupted. As there was no difference in the response evoked from right- or left-hand side injections, the data have been pooled. At seven different sites, microinjection of muscimol produced a reduction in the frequency of reflex contractions (Fig. 2A) (0.93 ± 0.1 to 0.37 ± 0.1 min−1; P < 0.005), with recovery to control values after 43.4 ± 23.4 min (range 3.3–198.5 min), without affecting the amplitude of contraction (16.60 ± 1.98 to 18.79 ± 1.72 mmHg). At a further four sites, muscimol completely suppressed rhythmic bladder contractions and voiding (Fig. 2B). As the infusion of saline into the bladder continued intravesicular pressure rose gradually. Eventually a state of overflow incontinence developed and urine dripped from the penis in a regular manner (Fig. 2B). The animals remained in this state of retention for in excess of 1 h, when the experiment was terminated. Activity in the EUS was measured in 6 of the 11 experiments in which muscimol had inhibitory effects on bladder contractions. Tonic activity was reduced from 0.29 ± 0.06 to 0.18 ± 0.05 μV s (P = 0.03), but the pattern of bursting activity remained unchanged. There was no effect on cardio-respiratory or autonomic variables recorded following injections of muscimol at any of the sites tested (data not shown).
Figure 3. Location of sites where muscimol or saline was microinjected into the PAG plotted onto outlines of the PAG taken from the atlas of Paxinos & Watson (1986).

A, Black filled circles represent the centre of sites where microinjection of muscimol inhibited micturition. Grey filled circles are sites where microinjection evoked a decrease in the frequency of contractions. Open circles are sites where muscimol had no effect. Note that because the sites are plotted onto representative sections at 0.8 mm intervals, some points in close vicinity appear to overlap even though none were less than 200 μm apart and some lay up to 800 μm apart in the rostrocaudal axis. B, open triangles: sites where saline was microinjected into the PAG. Numbers indicate distance (in mm) caudal to Bregma.
Figure 2. Effect of microinjection of muscimol (250 pmol in 50 nl) into the PAG on phasic voiding evoked during continuous infusion of saline into the bladder (6 ml h−1).
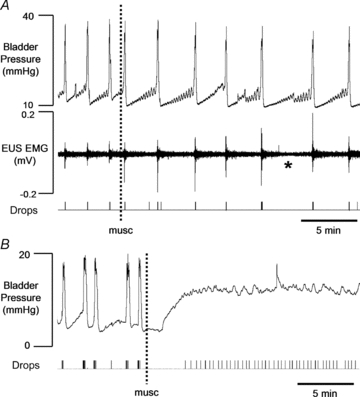
A, at most effective sites (n = 7) muscimol produced a reduction in the frequency of voiding accompanied by a decrease in the level of tonic activity in the EUS (asterisk). B, at 4 sites micturition was suppressed completely. As infusion of saline continued, intravesicular pressure rose and urine began to leak from the penis.
Sites at which the micturition reflex was completely inhibited by microinjection of muscimol were intermingled with the sites at which a transient response was seen. Thus the most effective sites were not the most caudal ones, closest to the PMC.
Microinjection of 50 nl of saline at sites throughout the PAG in a third group of rats (Fig. 3B) had no effect on micturition or on any of the cardiorespiratory and other autonomic variables measured (Table 1).
Table 1.
Effects of microinjection of saline (50 nl) throughout the PAG
| Pre-saline microinjection | Post-saline microinjection | P (paired t test) | |
|---|---|---|---|
| Frequency of contraction (contractions min−1) | 0.80 ± 0.04 | 0.77 ± 0.04 | 0.275 |
| MABP (mmHg) | 76.78 ± 3.89 | 84.20 ± 5.1 | 0.265 |
| Heart rate (beats min−1) | 487.95 ± 19.66 | 499.48 ± 19.67 | 0.683 |
| Respiratory rate (breaths min−1) | 156.22 ± 12.3 | 181.34 ± 15.62 | 0.223 |
Data for changes in cystometric (frequency of contractions of the bladder), cardiovascular (MABP and heart rate) and respiratory parameters recorded before and after microinjection of saline into the PAG. MABP, mean arterial blood pressure. Data are represented as mean ± SEM, and P values were calculated using Student's paired t test. n = 19.
Effect of microinjection of a GABAA antagonist on reflex contractions of the bladder
The inhibitory effect of muscimol injected into the caudal ventrolateral PAG suggested that relays in the micturition reflex pathway are present in this region. To determine whether the synaptic relays are normally under tonic GABAergic control, microinjections of the GABAA antagonist bicuculline (BIC, 1 nmol in 50 nl) were targeted at the region where muscimol had been effective. Microinjection of BIC at sites in the caudal ventrolateral PAG (cvlatPAG) shown by black filled circles in Fig. 5 enhanced reflex micturition. At most sites (16/26) this effect was characterised by an increase in the frequency of reflex voiding contractions combined with the appearance of low amplitude non-voiding contractions, so that the overall rate increased from 0.61 ± 0.06 min−1 to 3.46 ± 0.47 min−1 (P < 0.0001) but the amplitude of contraction was unchanged (18.96 ± 1.19 to 14.47 ± 2.28). This effect lasted for 21.4 ± 5.5 min. Tonic activity in the EUS also increased in response to BIC, from 0.25 ± 0.05 to 0.39 ± 0.06 μV s, P = 0.02 (n = 7), but the synchronised bursts of activity normally present during phasic increases in bladder pressure were disrupted (Fig. 4Ai).
Figure 5. Location of sites where bicuculline was microinjected into the periaqeductal grey (PAG), plotted onto outlines of the PAG taken from the atlas of Paxinos & Watson (1986).

Filled circles: centres of sites where microinjection of bicuculline facilitated micturition accompanied by only moderate autonomic changes. Grey circles: sites where microinjection of bicuculline produced intense autonomic activation accompanied by facilitation of micturition. Open circles: sites where microinjection of bicuculline had no effect. Because the points are plotted onto representative sections, some points in close vicinity appear to overlap although none were less than 200 μm apart and some lay up to 800 μm apart in the rostrocaudal axis. Numbers indicate distance (in mm) caudal to Bregma.
Figure 4. Effect of bicuculline (1 nmol in 50 nl) microinjected into the caudal ventrolateral PAG on micturition evoked in response to continuous infusion of saline into the bladder.

A, at most sites (n = 16) microinjection of bicuculline produced an increased frequency of voiding and increased activity in the EUS. Inset (Ai) shows the development of desynchronised activity in both detrusor and sphincter muscles (compare Fig. 1). B, at 10 sites micturition ceased after microinjection of bicuculline. Despite an increased level of activity in the EUS, overflow incontinence developed as the infusion of saline into the bladder continued.
At a further 10 sites, reflex micturition ceased after injection of BIC. In six cases the frequency of contractions showed an initial small non-significant increase (0.74 ± 0.16 to 1.68 ± 0.35 contractions min−1, P > 0.05) in the first 9.39 ± 2.24 min post injection, before ceasing completely. At the remaining four sites, micturition was suppressed immediately after injection of BIC. At all 10 sites, once bladder contractions had ceased, intravesicular pressure rose as the infusion of saline continued, before urine started to leak from the penis. In contrast to the regular pattern of drops seen when animals went into retention after microinjection of muscimol in the same region of the PAG, urine drops were produced in an irregular pattern. In addition, small oscillations became apparent on the pressure trace, suggesting that the detrusor might be in a state of active contraction (Fig. 4B). At the same time sustained activity was maintained in the EUS (Fig. 4B). In four experiments injections of BIC were made into the right cvlatPAG; these also facilitated micturition. However, injections made lateral or ventral to the cvlatPAG on either side or into the adjacent dorsal raphe nucleus, were without effect on reflex micturition (Fig. 5).
In order to determine whether the increase in activity in the EUS was a direct response to disinhibiting GABAergic tone in the cvlatPAG or secondary to raised bladder pressure evoked by the microinjection of BIC, experiments were performed on six rats in which the effect of BIC on EMG activity of the EUS was recorded after stepped increases in bladder pressure produced by infusing increasing volumes of saline into the bladder. In the absence of BIC, increasing bladder volume incrementally elicited a pressure-related increase in EMG activity (Fig. 6A). When the bladder was empty microinjection of BIC at four sites in the cvlatPAG produced a 139.44 ± 36.87% increase (range 40.1–277.7%) in the level of tonic activity recorded in the EUS, accompanied by a modest increase in bladder pressure (8.70 ± 2.64 mmHg) (Fig. 6B). In addition, the pressure–response relationship evoked by stepped increases in bladder volume was shifted upwards (Fig. 6A). Interestingly, at a further seven sites (open circles in Fig. 6C), where bicuculline failed to change resting EUS EMG activity, the response to stepped increases in bladder volume was not changed (data not shown).
Figure 6. Effect of microinjection of bicuculline into the caudal ventrolateral PAG on response of the external urethral sphincter to stepped infusion of saline into the bladder.

A, effect of microinjection of bicuculline (BIC) into the caudal ventrolateral PAG on response of external urethral sphincter (EUS) to stepped infusion of saline into the bladder before (open triangles) and after (filled circles) the microinjection. Note that due to the necessity of limiting the experiment to within the period that BIC was active after microinjection, it was not possible to test the response to the complete range of stepped increases in bladder volume in all experiments. *P < 0.05 compared to control for the same increase in bladder volume (Mann–Whitney U test). B, example of EUS EMG activity and increase in bladder pressure evoked after microinjection of BIC into the cvlatPAG in a rat in which the bladder had been previously emptied. C, location of injection sites in the caudal PAG where microinjection of BIC increased responses to stepped increases in bladder pressure (filled circles) and sites where microinjection of BIC had no effect (open circles). Because the points are plotted onto representative sections, some points in close vicinity appear to overlap although none were less than 200 μm apart and some lay up to 800 μm apart in the rostrocaudal axis.
In addition to its effect on micturition, microinjection of BIC into the cvlatPAG produced modest cardiovascular and respiratory changes (Fig. 7A). There was a comparatively small, transient (27.5 ± 2.9 min) pressor response (32.7 ± 4.9 mmHg), tachycardia (59.9 ± 9.6 beats min−1) and hyperpnoea (respiratory rate increased from 153.7 ± 4.3 to 278.5 ± 16.2 breaths min−1) often accompanied by pupillodilatation. We were concerned that this pattern of cardiorespiratory change was contradictory to that reported by others following activation of cells in the caudal ventrolateral PAG (Snowball et al. 1997) and when using the same anaesthetic as used in the present study (Lovick, 1992; Wang & Lovick, 1993). To determine whether the preparative surgery could be influencing responsiveness of the midbrain, in four experiments we microinjected BIC into the cvlatPAG before performing the laparotomy to expose and cannulate the bladder. In these rats microinjection of BIC prior to abdominal surgery produced a transient depressor response (mean arterial blood pressure depressed by 22.1 ± 5.9 mmHg for 17.9 ± 13.2 min). The bladder was then prepared for cystometry and the microinjection was repeated at the same site whilst infusing saline into the bladder. The second injection of BIC facilitated the bladder contractions, as described above, but the depressor response seen after the first microinjection was absent and replaced instead by a rise in blood pressure (27.0 ± 12.2 mmHg) (data not shown).
Figure 7. Cardiorespiratory changes evoked by microinjection of bicuculline into the periaqueductal grey (PAG).

A, ventral, and B, dorsal half of the caudal PAG. Abbreviations: BP, blood pressure; HR, heart rate; BPM: beats min−1; TA, tracheal airflow; Arb.U, arbitrary units; RR, respiratory rate; Br.PM, breaths min−1.
At the beginning of the study some injections were made into the dorsal half of the caudal PAG in the region where muscimol was without effect. These produced striking facilitatory effects, which we judged worthy of further investigation. Bicuculline was therefore microinjected at 34 sites into the dorsal half of the caudal PAG and found to potentiate reflex micturition (Fig. 5). At 21 sites the frequency of reflex voiding contractions increased and small amplitude, phasic changes in intravesicular pressure, which were not associated with voiding, also appeared. Thus the overall frequency increased from 0.73 ± 0.05 to 2.46 ± 0.39 contractions min−1 (P = 0.0001). The level of tonic activity present in the EUS in the intervals between phasic bladder contractions also increased markedly (from 0.14 ± 0.02 to 0.47 ± 0.04 μV s (n = 14) P≤ 0.0001) and the synchronous bursting pattern of activity that had been present during the phasic increases in bladder pressure became disrupted. At the further 13 sites, after an initial increase in frequency, the rhythmic high amplitude voiding contractions ceased and bladder pressure remained high, accompanied by sustained contraction of the EUS. Tonic activity increased from 0.11 ± 0.02 to 0.73 ± 0.23 μV s (n = 3) although the difference failed to reach significance (P = 0.12). As the infusion of saline continued the bladder appeared to go into a state of overflow incontinence whereby urine continually leaked from the penis. In these cases no recovery was seen even 2 h after the injection, when the experiment was terminated.
In addition to its effect on bladder activity, application of BIC at dorsal sites in the caudal PAG produced pronounced autonomic changes. There was a marked pressor response (57.17 ± 5.58 mmHg), tachycardia (122.6 ± 15.4 beats min−1) and tachypnoea (respiratory rate increased by 152.34 ± 15.74 breaths min−1) (Fig. 7B) accompanied by pronounced pupillary dilatation, exophthalmos and twitching of the vibrissae and hindlimb muscles. The latency to onset of these effects was similar to the effects evoked by injection of bicuculline into the ventral half of the PAG suggesting that they were separate events and not simply due to diffusion of the antagonist from dorsal to ventral sites (Table 2). Microinjection of bicuculline into the right-hand side of dorsal regions of the caudal PAG (n = 9) produced responses that were similar to those evoked by left-sided injections.
Table 2.
Latency to onset of cardiovascular/respiratory response evoked by microinjection of bicuculline into the caudal ventrolateral (cvlat) and dorsal periaqueductal grey (PAG)
| Latency to onset cvlatPAG (min) | Latency to onset dorsal PAG (min) | P value (unpaired t test) | |
|---|---|---|---|
| Pressor response | 2.68 ± 0.61 | 3.74 ± 0.69 | 0.25 |
| Tachycardia | 2.98 ± 0.63 | 3.84 ± 0.64 | 0.34 |
| Tachypnoea | 3.87 ± 0.88 | 3.84 ± 0.59 | 0.98 |
Data representing the latency to onset of the cardiovascular changes following microinjection of bicuculline into the cvlat (n = 26) and dorsal (n = 34) regions of the PAG. Data are represented as mean ± SEM, and P values were calculated using Student's unpaired t test.
The dorsal half of the caudal PAG was extremely sensitive to bicuculline. On 23 occasions insertion of the cannula alone (i.e. no injection made) evoked changes in both bladder and cardiovascular/respiratory system. These variables returned to baseline values within 31.8 ± 11.3 min once the cannula was withdrawn (Fig. 7B) and presumably reflect an effect produced by passive diffusion of bicuculline from the tip of the cannula.
Discussion
In the present study continuous infusion of saline into the bladder elicited cyclical increases in bladder pressure associated with the expulsion of fluid from the urethral meatus. During the filling phase whilst bladder pressure rose slowly, a low level of tonic activity was present in the EUS. As the bladder started to contract and intravesicular pressure rose more steeply, the level of tonic activity in the EUS showed a sharp increase before adopting a bursting pattern during the voiding phase at the peak of the contraction. Our data, together with similar findings by Peng et al. (2006, 2008) and discussion by de Groat (1990) indicate that a form of ‘guarding’ is present in the rat (de Groat & Steers, 1990). However, in the rat, unlike humans, a marked increase in tonic activity in the EUS accompanies the contraction of the detrusor. It is possible that this could facilitate voiding by initially producing an isovolumetric contraction, thus increasing the intravesicular pressure so that urine flows through the urethra with a greater velocity when activity in the EUS is silenced. The bursting activity which followed ‘guarding’ corresponded with the appearance of intraluminal pressure high frequency oscillations (IPHFOs) as described by others. It has been suggested that the bursting activity in the EUS seen in rats, also increases the velocity of flow in the urethra to allow spurting of urine for scent marking (Maggi et al. 1986; Conte et al. 1991; Kruse et al. 1993; Van Asselt et al. 1995; Matsuura et al. 1998, 2000; Chang & Havton, 2008; Peng et al. 2008).
Microinjection of a GABA agonist into the caudal ventrolateral region of the PAG, but not at other sites in the PAG, produced a significant decrease in the frequency of reflex bladder contractions, accompanied by reduced level of tonic activity in the EUS. At some sites despite the microinjection of muscimol being unilateral, micturition was suppressed completely. As the PAG projects bilaterally to the PMC (Taniguchi et al. 2002), it is possible that the inhibitory effects of microinjecting muscimol into one side of the cvlatPAG were sufficient to inhibit the PMC on the ipsilateral and contralateral sides. The failure of muscimol to act at rostral and dorsal sites was unlikely to be due to ineffective dosing as in preliminary experiments (not shown) double and triple doses (0.5 nmol in 100 nl and 0.75 nmol in 150 nl) were without effect.
On the map, active and inactive sites where muscimol was injected sometimes appeared to be intermingled (Figs 3 and 5). However, since the location of the centre of microinjection sites was collapsed on outline drawings of representative coronal sections at approximately 0.8 mm intervals, effective and ineffective microinjection sites plotted on the same level may actually have been up to 800 μm apart along the rostrocaudal axis of the brain. One injection that suppressed micturition totally was centred just lateral to the anatomically defined border of the PAG. However, this region is almost certainly within the reaches of the receptors on the dendrites of cells involved in transmission through the micturition reflex pathway at the level of the PAG.
Furthermore, it is also unlikely that microinjections of muscimol or bicuculline into the cvlatPAG imposed their effect directly on the PMC, despite their relatively close anatomical proximity, because dyes co-injected with the drugs never spread as far caudally as the PMC.
Although microinjection of muscimol into the cvlatPAG reduced or completely inhibited the frequency of reflex voiding contractions, there was no significant effect on the amplitude of the contraction, or other parameters such as the threshold and duration. This suggests that GABAergic influence in the region simply gates the pathway in an ‘on/off’ fashion, rather than contributing to the coordination of the void, which may be integrated downstream in the PMC. These findings are in accord with previous reports of suppression of micturition following microinjection of a μ opioid agonist or CoCl2 into a similar region within the PAG in rats (Matsuura et al. 1998, 2000; Matsumoto et al. 2004).
Interestingly, a recent study by Takasaki et al. (2010) in cats showed that micturition-related isovolumetric bladder contractions persisted after a transection through the caudal midbrain. However, their Fig. 2A clearly shows that the caudal PAG at the level where it opens into the fourth ventricle, was still intact. Importantly, following a lesion that extended into this region but spared part of the PMC (Fig. 2B of Takasaki), micturition-related contractions of the bladder were significantly reduced but not abolished totally. The authors of the study interpreted this to mean that the PAG was not involved in micturition in the cat. However, in our opinion, the data shown indicate that the transection spared the caudalmost tip of the PAG (see Fig. 2A in Takasaki et al. 2010). This suggests to us that there is a component of the response that is integrated via the PAG, in accord with the report of only sparse projections from the lumbosacral cord to the PMC in the cat (Blok et al. 1995). Our evidence shows that in contrast to Takasaki's interpretation in the cat, micturition (voiding in response to filling of the bladder) in the rat clearly requires participation of the caudal PAG.
Although the caudal ventrolateral PAG clearly contains a critical midbrain relay in the micturition reflex pathway, previous electrophysiological studies in the cat have reported that afferent information from the bladder relays through two areas within the PAG: the rostral dorsolateral as well as the caudal ventrolateral region (Duong et al. 1999). In the rat too, ascending pathways from the bladder and from the lumbosacral cord, where bladder afferents terminate, have been shown to terminate in both caudal ventrolateral and lateral/dorsolateral areas (Ding et al. 1997; Marson, 1997; Mitsui et al. 2003) although our data suggest that only the caudal relay is essential to perform reflex voiding in response to bladder filling.
It is likely that in the rat the cvlatPAG exerts tonic GABAergic inhibition via the PMC. This conclusion accords with the lack of evidence suggesting the presence of a pontine storage centre in the rat like that identified in cat and man (Holstege et al. 1986; Blok et al. 1997). Furthermore it is supported by there being strong bilateral connections between the PAG and the PMC (Taniguchi et al. 2002). Interestingly, in rodents there is a direct projection from the sacral cord to the PMC (Ding et al. 1997). If this projection is activated during bladder distension it could short-circuit the PAG. However, because transmission through the cvlatPAG is essential for micturition to occur this pathway does not appear to be functionally active, at least under urethane anaesthesia.
After microinjection of the GABAA antagonist bicuculline into the caudal ventrolateral PAG the frequency of reflexly evoked micturition was enhanced. Similarly to the responses seen after microinjection of muscimol, there was no overall change in amplitude of contractions following microinjection of BIC into the cvlatPAG, again suggesting that modulating the GABAergic tone does not affect the profile of a contraction, but it determines whether a void can or cannot take place. There was, however, a significant increase in the post-contraction pressure, suggesting that either the bladder was tonically contracting during the ‘filling period’ or that the post-void residual volume had increased as a result of less successful voids. This indicates that the excitability of synaptic relays in this region is normally subject to a tonic inhibitory GABAergic influence. Modulating the level of GABA tone in this region could be used to gate transmission through the reflex pathway to permit bladder emptying. The source of inhibitory tone has not been established. However, a number of factors suggest that it could originate in frontal cortical regions. In rats and primates the prefrontal cortex sends a dense projection to the ventrolateral PAG (An et al. 1998; Floyd et al. 2000). In man, the medial prefrontal cortex is recognised for its involvement in decision making in a social context, based on the social situation (Adolphs, 1999). Recent evidence indicates that this influence may extend to control of bladder function. Based on meta-analyses of imaging studies in human subjects, Griffiths and co-workers have proposed that during bladder filling afferent information on bladder status is relayed via the PAG to the anterior cingulate cortex and then via the insula, to the lateral and medial prefrontal cortices (Fowler et al. 2008; Griffiths & Tadic, 2008; Fowler & Griffiths, 2010). They propose that during the storage phase, the voiding reflex remains continuously suppressed due to activity in an inhibitory pathway to the PAG. When the voluntary decision to void is made in the medial prefrontal cortex, the inhibition is relaxed. In support of these ideas recent imaging studies using multilevel path modelling approaches have been able to show that social evaluative threat (SET) can influence functional connections between the frontal cortical regions, the PAG and autonomic output whereby mental appraisals are translated into adaptive physiological responses (Wager et al. 2009). In the context of micturition, SET might be experienced as the potential humiliating consequences of failure to maintain continence in a given social situation. In the urethane-anaesthetised preparation reported here it is likely that activity in the frontal cortices has been dampened. However, some activity in the motor cortex and higher centres may be preserved, even under urethane anaesthesia (Tai et al. 2009). These sites could be the source of descending signals to the PAG, which gate GABAergic control of the micturition reflex at the level of the cvlatPAG.
Unexpectedly the effects of BIC on blood pressure were reversed upon infusion of saline into the bladder. This reversal could have been due to sympathetic redistribution of blood flow during bladder stretch leading to an increase in blood pressure, which masked any depressor response caused by a decrease in GABAergic inhibition in the cvlatPAG during micturition. This pressor response during bladder stretch has been demonstrated in man as well as in cats and dogs (Guttmann & Whitteridge, 1947; Cunningham et al. 1953; Mukherjee, 1957; Weaver, 1985; Ward et al. 1995).
Whilst the cvlatPAG is clearly an essential component of the micturition reflex pathway, the role of the dorsal half of the PAG in the control of micturition is less clear. The failure of muscimol to influence reflex micturition indicates that the functional integrity of this region is not a prerequisite for voiding to occur. However, the neurones are clearly able to influence the micturition process, since microinjection of bicuculline into the dorsal PAG enhanced reflex micturition. Interestingly, previous studies have reported that electrical stimulation in the dorsal PAG elicited micturition in conscious rats (Bittencourt et al. 2004). The initiation of micturition when the bladder is not necessarily full suggests that in some circumstances, voiding may be an active process rather than a reflex response to a full bladder. In many animal species including rodents, volitional micturition, which is unrelated to the need to empty a full bladder, occurs in the form of scent marking to define territory. The pattern of marking is significantly affected by social status and by stressful encounters (Desjardins et al. 1973; Wood et al. 2009). In conscious animals the dorsal half of the PAG initiates defensive or aggressive behaviour, which is accompanied by autonomic activation and micturition (Schenberg et al. 2005). Such behaviour is believed to reflect an active coping strategy characterised by engagement with the environment (Keay & Bandler, 2001). To initiate this form of limited bladder voiding, the efferent limb of the micturition reflex pathway must be directly activated at PAG level, or even further downstream. Indeed, in the present study we were able to evoke contractile activity by stimulating the cvlatPAG when the bladder was empty. The intrinsic connections between dorsal and ventral regions of the PAG (Jansen et al. 1998) would appear to be strategically located to initiate volitional micturition under defined behavioural circumstances.
In summary, the present study has shown that the functional integrity of synapses in a discrete area within the caudal ventrolateral PAG is a prerequisite to enable reflex voiding in response to a full bladder. Transmission through this region is normally depressed by ongoing activity in GABAergic neurones. Changes in the level of GABA tone may be the mechanism by which micturition can be suppressed or permitted according to the individual's social situation. In contrast, the dorsal half of the PAG is not involved in reflex voiding, but can initiate micturition when the tonic GABAergic tone that is normally present in this region, is withdrawn. This active micturition is always accompanied by pronounced autonomic arousal suggesting that under certain conditions, such as defensive behaviour, micturition can become an active event rather than a reflex response.
Acknowledgments
This research was funded by BBSRC/Pfizer CASE Doctoral Training Grant BB/E528752/1 in favour of E.S.
Glossary
Abbreviations
- BIC
bicuculline
- cvlatPAG
caudal ventrolateral periaqueductal grey
- EUS
external urethral sphincter
- PAG
periaqueductal grey
- PMC
pontine micturition centre
Author contributions
The experimental work was carried out in the laboratory of T.A.L. in the College of Medical and Dental Sciences, University of Birmingham. The original conception and design of the experiments was made by T.A.L. and J.H.C. in consultation with J.A., with subsequent input from E.S. E.S. carried out the experiments and was responsible for the collection and analysis of the data, with input from T.A.L. and J.H.C. The original draft of the article was prepared by T.A.L., with substantial input from E.S. and critical input from J.H.C. and J.A. All of the authors approved the final version of the manuscript.
References
- Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- An X, Bandler R, Ongür D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- Bandler R, Depaulis A, Vergnes M. Identification of midbrain neurones mediating defensive behaviour in the rat by microinjections of excitatory amino acids. Behav Brain Res. 1985;15:107–119. doi: 10.1016/0166-4328(85)90058-0. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Jiang MR, Chandler SD, Ennis M. The effect of GABA and its antagonists on midbrain periaqueductal gray neurons in the rat. Pain. 1990;40:195–204. doi: 10.1016/0304-3959(90)90070-T. [DOI] [PubMed] [Google Scholar]
- Bittencourt AS, Carobrez AP, Zamprogno LP, Tufik S, Schenberg LC. Organization of single components of defensive behaviors within distinct columns of periaqueductal gray matter of the rat: role of N-methyl-d-aspartic acid glutamate receptors. Neuroscience. 2004;125:71–89. doi: 10.1016/j.neuroscience.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Blok BF, De Weerd H, Holstege G. Ultrastructural evidence for a paucity of projections from the lumbosacral cord to the pontine micturition center or M-region in the cat: A new concept for the organization of the micturition reflex with the periaqueductal gray as a central relay. J Comp Neurol. 1995;359:300–309. doi: 10.1002/cne.903590208. [DOI] [PubMed] [Google Scholar]
- Blok BF, Holstege G. Two pontine micturition centres in the cat are not interconnected directly: implications for the central organization of micturition. J Comp Neurol. 1999;403:209–218. doi: 10.1002/(sici)1096-9861(19990111)403:2<209::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Blok BF, Willemsen AT, Holstege G. A PET study on brain control of micturition in humans. Brain. 1997;120:111–121. doi: 10.1093/brain/120.1.111. [DOI] [PubMed] [Google Scholar]
- Brack KE, Lovick TA. Neuronal excitability in the periaqueductal grey matter during the oestrous cycle in female Wistar rats. Neuroscience. 2007;144:325–335. doi: 10.1016/j.neuroscience.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Carrive P, Schmitt P, Karli P. Flight induced by microinjection of D-tubocurarine or alpha-bungarotoxin into medial hypothalamus or periaqueductal gray matter: cholinergic or GABAergic mediation. Behav Brain Res. 1986;22:233–248. doi: 10.1016/0166-4328(86)90068-9. [DOI] [PubMed] [Google Scholar]
- Chang HY, Havton LA. Differential effects of urethane and isoflurane on external urethral sphincter electromyography and cystometry in rats. Am J Physiol Renal Physiol. 2008;295:F1248–F1253. doi: 10.1152/ajprenal.90259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte B, Maggi CA, Parlani M, Lopez G, Manzini S, Giachetti A. Simultaneous recording of vesical and urethral pressure in urethane-anesthetized rats: effect of neuromuscular blocking agents on the activity of the external urethral sphincter. J Pharmacol Methods. 1991;26:161–171. doi: 10.1016/0160-5402(91)90041-3. [DOI] [PubMed] [Google Scholar]
- Cunningham DJC, Guttmann L, Whitteridge D, Wyndham CH. Cardiovascular responses to bladder distension in paraplegic patients. J Physiol. 1953;121:581–592. doi: 10.1113/jphysiol.1953.sp004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC. Central neural control of the lower urinary tract. In: Bock G, Whelan J, editors. Neurobiology of Incontinence. Vol. 151. UK: John Wiley & Sons Ltd; 1990. pp. 27–56. Ciba Foundation Symposium. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Steers WD. Autonomic regulation of the urinary bladder and sexual organs. In: Loewy AD, Spyer KM, editors. In Central regulation of autonomic functions. USA: Oxford University Press; 1990. pp. 310–333. [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Zheng HX, Gong LW, Lu Y, Zhao H, Qin BZ. Direct projections from the lumbosacral spinal cord to Barrington's nucleus in the rat: a special reference to micturition reflex. J Comp Neurol. 1997;389:149–160. doi: 10.1002/(sici)1096-9861(19971208)389:1<149::aid-cne11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Drake MJ, Fowler CJ, Griffiths D, Mayer E, Paton JF, Birder L. Neural control of the lower urinary and gastrointestinal tracts: supraspinal CNS mechanisms. Neurourol Urodyn. 2010;29:119–127. doi: 10.1002/nau.20841. [DOI] [PubMed] [Google Scholar]
- Duong M, Downie JW, Du HJ. Transmission of afferent information from urinary bladder, urethra and perineum to periaqueductal gray of cat. Brain Res. 1999;819:108–119. doi: 10.1016/s0006-8993(98)01294-3. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol. 2000;422:556–578. doi: 10.1002/1096-9861(20000710)422:4<556::aid-cne6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths DJ. A decade of functional brain imaging applied to bladder control. Neurourol Urodyn. 2010;29:49–55. doi: 10.1002/nau.20740. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths D, Tadic SD. Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn. 2008;27:466–474. doi: 10.1002/nau.20549. [DOI] [PubMed] [Google Scholar]
- Griffiths JL, Lovick TA. GABAergic neurones in the rat periaqueductal grey matter express α4, β1 and δ GABAA receptor subunits: plasticity of expression during the estrous cycle. Neuroscience. 2005;136:457–466. doi: 10.1016/j.neuroscience.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Guttmann L, Whitteridge D. Effects of bladder distension on autonomic mechanisms after spinal cord injury. Brain. 1947;70:361–404. doi: 10.1093/brain/70.4.361. [DOI] [PubMed] [Google Scholar]
- Holstege G. Micturition and the soul. J Comp Neurol. 2005;493:15–20. doi: 10.1002/cne.20785. [DOI] [PubMed] [Google Scholar]
- Holstege G. The emotional motor system and micturition control. Neurourol Urodyn. 2010;29:42–48. doi: 10.1002/nau.20789. [DOI] [PubMed] [Google Scholar]
- Holstege G, Griffiths D, de Wall H, Dalm E. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol. 1986;250:449–461. doi: 10.1002/cne.902500404. [DOI] [PubMed] [Google Scholar]
- Jansen ASP, Farkas E, Mac Sams J, Loewy AD. Local connections between the columns of the periaqueductal gray matter: a case for intrinsic neuromodulation. Brain Res. 1998;784:329–336. doi: 10.1016/s0006-8993(98)00473-9. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Kitta T, Matsumoto M, Tanaka H, Mitsui T, Yoshioka M, Nonomura K. GABAergic mechanism mediated via D1 receptors in the rat periaqueductal gray participates in the micturition reflex: an in vivo microdialysis study. Eur J Neurosci. 2008;27:3216–3225. doi: 10.1111/j.1460-9568.2008.06276.x. [DOI] [PubMed] [Google Scholar]
- Kruse MN, Belton AL, de Groat WC. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am J Physiol Regul Integr Comp Physiol. 1993;264:R1157–R1163. doi: 10.1152/ajpregu.1993.264.6.R1157. [DOI] [PubMed] [Google Scholar]
- Lovick TA. Midbrain influences on ventrolateral medullo-spinal neurones in the rat. Exp Brain Res. 1992;90:147–152. doi: 10.1007/BF00229266. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Paul NL. Co-localization of GABA with nicotinamide adenine dinucleotide phosphate-dependent diaphorase in neurones in the dorsolateral periaqueductal grey matter of the rat. Neurosci Lett. 1999;272:167–170. doi: 10.1016/s0304-3940(99)00607-2. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Giuliani S, Santicioli P, Meli A. Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetised rats. Am J Physiol Regul Integr Comp Physiol. 1986;251:R250–R257. doi: 10.1152/ajpregu.1986.251.2.R250. [DOI] [PubMed] [Google Scholar]
- Marson L. Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1997;389:584–602. [PubMed] [Google Scholar]
- Matsumoto S, Levendusky MC, Longhurst PA, Levin RM, Millington WR. Activation of mu opioid receptors in the ventrolateral periaqueductal gray inhibits reflex micturition in anesthetized rats. Neurosci Lett. 2004;363:116–119. doi: 10.1016/j.neulet.2004.03.055. [DOI] [PubMed] [Google Scholar]
- Matsuura S, Allen GV, Downie JW. Volume-evoked micturition reflex is mediated by the ventrolateral periaqueductal gray in anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 1998;275:R2049–R2055. doi: 10.1152/ajpregu.1998.275.6.R2049. [DOI] [PubMed] [Google Scholar]
- Matsuura S, Downie JW, Allen GV. Micturition evoked by glutamate microinjection in the ventrolateral periaqueductal gray is mediated through Barrington's nucleus in the rat. Neuroscience. 2000;101:1053–1061. doi: 10.1016/s0306-4522(00)00404-8. [DOI] [PubMed] [Google Scholar]
- Mitsui T, Kakizaki H, Matsuura S, Tanaka H, Yoshioka M, Koyanagi T. Chemical bladder irritation provokes c-fos expression in the midbrain periaqueductal gray matter of the rat. Brain Res. 2003;967:81–88. doi: 10.1016/s0006-8993(02)04226-9. [DOI] [PubMed] [Google Scholar]
- Monassi CR, Leite-Panissi CR, Menescal-de-Oliveira L. Ventrolateral periaqueductal gray matter and the control of tonic immobility. Brain Res Bull. 1999;50:201–218. doi: 10.1016/s0361-9230(99)00192-6. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Clayton CC. Defensive behaviors evoked from the ventrolateral periaqueductal gray of the rat: comparison of opioid and GABA disinhibition. Behav Brain Res. 2005;164:61–66. doi: 10.1016/j.bbr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Mukherjee SR. Effect of bladder distension on arterial blood pressure and renal circulation: Role of splanchnic and buffer nerves. J Physiol. 1957;138:307–325. doi: 10.1113/jphysiol.1957.sp005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Kow LM, Pfaff DW. In vitro electrophysiological characterization of midbrain periaqueductal gray neurons in female rats: responses to GABA- and Met-enkephalin-related agents. Brain Res. 1994;666:239–249. doi: 10.1016/0006-8993(94)90778-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain: In Stereotaxic Coordinates. 2nd edn. Sydney: Academic Press; 1986. [Google Scholar]
- Peng CW, Chen JJ, Chang HY, de Groat WC, Cheng CL. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourol Urodyn. 2006;25:388–396. doi: 10.1002/nau.20229. [DOI] [PubMed] [Google Scholar]
- Peng CW, Chen JJ, Cheng CL, Grill WM. Role of pudendal afferents in voiding efficiency in the rat. Am J Physiol Integr Comp Physiol. 2008;294:R660–R672. doi: 10.1152/ajpregu.00270.2007. [DOI] [PubMed] [Google Scholar]
- Reichling DB, Basbaum AI. Contribution of brainstem GABAergic circuitry to descending antinociceptive controls: I. GABA-immunoreactive projection neurons in the periaqueductal gray and nucleus raphe magnus. J Comp Neurol. 1990;302:370–377. doi: 10.1002/cne.903020213. [DOI] [PubMed] [Google Scholar]
- Schenberg LC, Póvoa RM, Costa AL, Caldellas AV, Tufik S, Bittencourt AS. Functional specializations within the tectum defense systems of the rat. Neurosci Biobehav Rev. 2005;29:1279–1298. doi: 10.1016/j.neubiorev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Snowball RK, Dampney RA, Lumb BM. Responses of neurones in the medullary raphe nuclei to inputs from visceral nociceptors and the ventrolateral periaqueductal grey in the rat. Exp Physiol. 1997;82:485–500. doi: 10.1113/expphysiol.1997.sp004041. [DOI] [PubMed] [Google Scholar]
- Stone E, Coote JH, Allard J, Lovick TA. Midbrain control of micturition in the rat. Proc Physiol Soc. 2009;15:C100. [Google Scholar]
- Tai C, Wang J, Jin T, Wang P, Kim S-G, Roppolo JR, de Groat WC. Brain switch for reflex micturition control detected by fMRI in rats. J Neurophysiol. 2009;102:2719–2730. doi: 10.1152/jn.00700.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki A, Meng Hui M, Sasaki M. Is the periaqueductal gray an essential relay center for the micturition reflex pathway in the cat? Brain Res. 2010;1317:108–115. doi: 10.1016/j.brainres.2009.12.057. [DOI] [PubMed] [Google Scholar]
- Taniguchi N, Miyata M, Yachiku S, Yamaguchi S, Numata A. A study of micturition inducing sites in the periaqeductal gray of the mesencephalon. J Urol. 2002;168:1626–1631. doi: 10.1016/S0022-5347(05)64532-6. [DOI] [PubMed] [Google Scholar]
- Van Asselt E, Groen J, Van Mastrigt R. A comparative study of voiding in rat and guinea pig: simultaneous measurement of flow rate and pressure. Am J Physiol Regul Integr Comp Physiol. 1995;269:R98–R103. doi: 10.1152/ajpregu.1995.269.1.R98. [DOI] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Lovick TA. The inhibitory effect of the ventrolateral periaqueductal grey matter on neurones in the rostral ventrolateral medulla involves a relay in the medullary raphe nuclei. Exp Brain Res. 1993;94:295–300. doi: 10.1007/BF00230299. [DOI] [PubMed] [Google Scholar]
- Ward J, de Burgh Daly M, Wood LM. Urinary bladder distension: its effects on carotid baroreceptor reflex left ventricular inotropic response in the dog. J Physiol. 1995;489:857–868. doi: 10.1113/jphysiol.1995.sp021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LC. Organization of sympathetic responses to distension of urinary bladder. Am J Physiol Regul Integr Comp Physiol. 1985;248:R236–R240. doi: 10.1152/ajpregu.1985.248.2.R236. [DOI] [PubMed] [Google Scholar]
- Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of corticotrophin-releasing factor. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1671–R1678. doi: 10.1152/ajpregu.91013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


