Abstract
Adults of freshwater ostracod Stenocypris major (Crustacea, Candonidae) were exposed for a four-day period in laboratory conditions to a range of copper (Cu), cadmium (Cd), zinc (Zn), lead (Pb), nickel (Ni), iron (Fe), aluminium (Al), and manganese (Mn) concentrations. Mortality was assessed, and median lethal times (LT50) and concentrations (LC50) were calculated. LT50 and LC50 increased with the decrease in mean exposure concentrations and times, respectively, for all metals. LC50s for 96 hours for Cu, Cd, Zn, Pb, Ni, Fe, Al, and Mn were 25.2, 13.1, 1189.8, 526.2, 19743.7, 278.9, 3101.9, and 510.2 μg/L, respectively. Metals bioconcentration in S. major increases with exposure to increasing concentrations, and Cd was the most toxic to S. major, followed by Cu, Fe, Mn, Pb, Zn, Al, and Ni (Cd>Cu>Fe>Mn>Pb>Zn>Al>Ni). Comparison of LC50 values for metals for this species with those for other freshwater crustacean reveals that S. major is equally or more sensitive to metals than most other tested crustacean.
1. Introduction
Widespread uses of metals, the legacies of past contamination and new technologies, continue to pose and important ecological risk in aquatic environment [1]. Metals such as Cu, Cd, Zn, and Pb are released from natural sources as well as human activity. Impact of these metals to the environment is an increasing problem worldwide. Malaysia, as a developed country, is no exception and faces metals pollution caused especially by anthropogenic activities such as manufacturing, agriculture, sewage, and motor vehicle emissions [2, 3]. Metals research in Malaysia, especially using organisms as bioindicator, is still scarce. Therefore, it is important to conduct studies with local organisms that can be used to gain data on metal toxicity, to determine the organism's sensitivity and to derive a permissible limit for Malaysian's water that can protect aquatic communities. Managing trace metal contamination requires understanding the concentration dependence of toxicity. Toxicity testing is an essential tool for assessing the effect and fate of toxicants in aquatic ecosystems and has been widely used as a tool to identify suitable organisms as a bioindicator and to derive water quality standards for chemicals. Toxicity testing provides the underpinning for traditional regulatory approaches for all chemicals and is an important part of many risk assessment [1, 4].
Ostracods are microscopic, bivalve crustaceans, with valve of low-Mg calcite. Ostracods are ubiquitous in fresh waters. They are mainly benthic, and fairly common in shallow water bodies. On area basis, small lentic systems such as pond and pools support more ostracods taxa than large lakes. They also thrive in temporary habitats including rice fields and containers like tins, discarded tires, tree holes, crab hole, and so forth. Benthic ostracods are mainly detritivores and also readily feed on dead animals. Ostracods form an important component in the food chain of some fish. Freshwater ostracods reproduce sexually and also by parthenogenesis. Females are more abundant and common than males. They have resistant eggs that can withstand adverse environmental conditions. Ostracods can also aestivate (dormant) or hibernate as resistant larval stages. Like other crustacean, ostracods moult, generally passing eight stages to reach adulthood, and life cycle may last a few months or more than 2 years. The most prevalent genus in Southeast Asia is Strandesia with about 30 species. Other genera that are common occurrence are Stenocypris, Hemicypris, and Cypretta [5, 6]. There are approximately 11 species of Stenocypris genus. Stenocypris major is widely distributed and has been reported from South America, Africa, and Asia. It is a nektobenthic species and is known to be eurytopic species, that is, taxa with a broad tolerance for ecological conditions and it prefers shallow and running water [7].
Ostracods have been used as one of freshwater invertebrates in ecotoxicological studies and as a test model organisms for environmental, paleoenvironmental, and toxic stress studies and also for toxicity monitoring of soil and river sediment [8–10]. Ruiz et al. [11] suggest that ostracods are highly sensitive to heavy metal pollution, oil-discharges, and anoxic conditions, and a study by Khangarot and Das [10] demonstrated the need to include crustacean ostracods in a battery of biotest to detect the presence of hazardous chemicals in soils, sewage sludge, sediments, and aquatic systems. Some metals toxicity studies have been conducted with freshwater ostracods such as Cypris subglobosa [10, 12], and toxicity to organic pollutants with Ilyocypris dentifera, Cypridopsis vidua, and Cypretta seurati [13]. However, no toxicity studies have been reported on S. major in the literature especially to metals. The purpose of this study was to determine the acute toxicity of Cu, Cd, Zn, Pb, Ni, Fe, Al, and Mn to freshwater ostracod, S. major, and to examine bioconcentration of these metals in the body after four days of exposure.
2. Materials and Methods
Ostracods were collected from filter system of fish pond in Bangi, Selangor, Malaysia. The filter system was consisting of several layers of filter mate and made from polyester wool and the water is continuously circulating using water pump from the fish pond to the filter, and back to the pond. Identification of species was based on Victor and Fernando [5] and Victor [6]. Prior to toxicity testing, the ostracods were acclimatized for one week under laboratory conditions (28–30°C with 12 h light : 12 h darkness) in 50-L stocking tanks using dechlorinated tap water (filtered by several layers of sand and activated carbon; T.C. Sediment Filter) aerated through an air stone. During acclimation, the ostracods were fed with commercial fish food Aquadene. The standard stock solution (100 mg/L) of Cu, Cd, Zn, Pb, Ni, Fe, Al, and Mn were prepared from CuSO4·5H2O, CdCl2·2.5H2O, ZnSO4·7H2O, Pb(NO3)2, NiSO4·6H2O, FeCl3, Al2(SO4)3·18H2O, and MnSO4·H2O, respectively. The stock solutions were prepared with deionized water in 1-L volumetric flasks. Acute Cu, Cd, Zn, Pb, Ni, Fe, Al, and Mn toxicity experiments were performed for a four-day period using adult ostracods (approximately 1.5 mm body length, mean wet weight 0.3 mg) obtained from stocking tanks. Following a range finding test, five Cu (32, 56, 100, 560, and 870 μg/L), Cd (56, 87, 320, 560, and 870 μg/L), Zn (560, 1000, 2400, 3200, and 5600 μg/L), Pb (560, 1000, 3200, 5600, and 10000 μg/L), Ni (1800, 3200, 5600, 8700, and 10000 μg/L), Fe (560, 750, 1000, 3200, and 5600 μg/L), Al (1000, 5600, 8700, 10000, and 18000 μg/L), and Mn (560, 870, 1000, 3200, and 5600 μg/L) concentrations were chosen. Metal solutions were prepared by dilution of a stock solution with dechlorinated tap water. A control with dechlorinated tap water only was also used. The tests were carried out under static conditions with renewal of the solution every two days. Control and metal-treated groups each consisted of five replicates of four randomly allocated ostracods in a 10 mL glass vial containing 8 mL of the appropriate solution. No stress was observed for the ostracods in the solution, indicated by 100% survival for the ostracods in the control water until the end of the study. A total of 20 animals per treatment/concentration were used in the experiment and a total of 820 animals were employed in the investigation [14, 15]. Samples of water for metal analysis taken before and immediately after each solution renewal were acidified to 1% with ARISTAR nitric acid (65%) before metal analysis by flame or furnace Atomic Absorption Spectrophotometer (AAS–Perkin Elmer model AAnalyst800) depending on the concentrations.
During the toxicity test, the ostracods were not fed. The experiments were performed at room temperature of 28–30°C with photoperiod 12 h light : 12 h darkness, using fluorescent lights (334–376 lux). Water quality parameters (pH, conductivity, and dissolved oxygen) were measured every two days using portable meters (model Hydrolab Quanta), and water hardness samples (0.45 μm filtered) were fixed with ARISTAR nitric acid and measured by flame atomic absorption spectrophotometer. Mortality was recorded every 3 to 4 hours for the first two days and then at 12 to 24 hour intervals throughout the rest of the test period. The criteria used to determine mortality were failure to respond to gentle physical stimulation. Any dead animals were removed immediately.
At the end of day four, the live ostracods were used to determine bioconcentration of the metals in whole body according to the concentrations used. The ostracods were rinsed with distilled water and each sample contained three replicates of three to five animals in a glass test tube (depending on how many live animals were left) and was oven dried (80°C) for at least 48 hours before being weighed. Each replicate was digested (whole organism) in 1.0 mL Aristar nitric acid (65%) in a block thermostat (80°C) for 2 hours. Upon cooling, 0.8 mL of hydrogen peroxide (30%) was added to the solutions. The test tubes were put back on the block thermostat for another 1 hour until the solutions became clear. The solutions were then made up to 25 mL with addition of deionized water in 25-mL volumetric flasks. Efficiency of the digestion method was evaluated using mussel and lobster tissue reference material (SRM 2976 and TORT-2, National Institute of Standard and Technology, Gaithersburg, USA and National Research Council Canada, Ottawa, Ontario, Canada, resp.). Efficiencies obtained were within 10% of the reference values. To avoid possible contamination, all glassware and equipment used were acid-washed (20% HNO3), and the accuracy of the analysis was checked against blanks. Procedural blanks and quality control samples made from standard solutions for Cu, Cd, Zn, Pb, Ni, Fe, Al, and Mn were analyzed in every ten samples in order to check for sample accuracy. Percentage recoveries for metals analyses were between 85–105%.
Median lethal times (LT50) and concentrations (LC50) for the ostracods exposed to metals were calculated using measured metal concentrations. FORTRAN programs based on the methods of Litchfield [16] and Litchfield and Wilcoxon [17] were used to compute and compare the LT50 and LC50. Concentration factors (CFs) were calculated for whole animals as the ratio of the metals concentrations in the tissues to the metals concentration measured in the water.
3. Results and Discussion
In all data analyses, the actual, rather than nominal, Cu, Cd, Zn, Pb, Ni, Fe, Al, and Mn concentrations were used (Table 1). The mean water quality parameters measured during the test were pH 6.51 ± 0.01, conductivity 244.3 ± 0.6 μS/cm, dissolved oxygen 6.25 ± 0.06 mg/L, and total hardness (Mg2+ and Ca2+) 15.63 ± 2.74 mg/L as CaCO3.
Table 1.
Median lethal times (LT50) for S. major exposed to different concentrations for Cu, Cd, Zn, Pb, Ni, Fe, Al, and Mn.
(a)
| Measured Cu concentration (μg/L) | LT50 (h) | 95% Confidence limits | Measured Cd concentration (μg/L) | LT50 (h) | 95% Confidence limits | Measured Zn concentration (μg/L) | LT50 (h) | 95% Confidence limits |
|---|---|---|---|---|---|---|---|---|
| 37.78 | 76.69 | 27.15–216.57 | 49.41 | 29.89 | 14.44–61.9 | 779 | 260.33 | 743.38–1562.33 |
| 53.18 | 19.05 | 9.02–40.26 | 88.57 | 16.72 | 8.53–32.77 | 1010 | 100.63 | 63.30–159.99 |
| 126.96 | 11.86 | 6.06–23.24 | 282.91 | 10.87 | 5.87–20.12 | 2456 | 52.52 | 32.18–85.71 |
| 573.91 | 7.31 | 4.30–12.43 | 508.43 | 6.29 | 3.77–10.48 | 3213 | 17.59 | 11.88–26.05 |
| 842.65 | 4.68 | 2.82–7.77 | 893.52 | 3.04 | 2.12–4.28 | 4822 | 13.17 | 10.31–16.82 |
(b)
| Measured Pb concentration (μg/L) | LT50 (h) | 95% Confidence limits | Measured Ni concentration (μg/L) | LT50 (h) | 95% Confidence limits | Measured Fe concentration (μg/L) | LT50 (h) | 95% Confidence limits |
|---|---|---|---|---|---|---|---|---|
| 475 | 110.26 | 79.32–153.28 | 19045 | 105.13 | 71.40–154.79 | 548.93 | 41.36 | 18.84–90.81 |
| 1160 | 72.39 | 53.61–97.76 | 29115 | 80.76 | 58.88–110.77 | 771.18 | 22.66 | 11.64–44.10 |
| 3410 | 55.66 | 42.32–73.21 | 52045 | 48.61 | 32.46–72.78 | 1084.0 | 10.13 | 5.53–18.57 |
| 4829 | 43.55 | 29.86–63.51 | 66665 | 17.59 | 11.88–26.05 | 3370.02 | 6.93 | 4.02–11.93 |
| 8973 | 9.15 | 5.90–14.17 | 90290 | 13.17 | 10.31–16.82 | 5836.64 | 4.20 | 2.82–6.25 |
(c)
| Measured Al concentration (μg/L) | LT50 (h) | 95% Confidence limits | Measured Mn concentration (μg/L) | LT50 (h) | 95% Confidence limits |
|---|---|---|---|---|---|
| 991 | 303.15 | 59.21–1552.17 | 562.28 | 155.41 | 53.84–448.63 |
| 4907 | 103.22 | 57.48–185.34 | 861.37 | 63.31 | 24.24–165.33 |
| 7454 | 55.66 | 42.32–73.21 | 1106.36 | 33.23 | 13.65–80.88 |
| 10210 | 43.55 | 29.86–63.51 | 3351.91 | 19.36 | 9.51–39.41 |
| 16348 | 9.15 | 5.90–14.17 | 5519.08 | 13.08 | 6.66–25.71 |
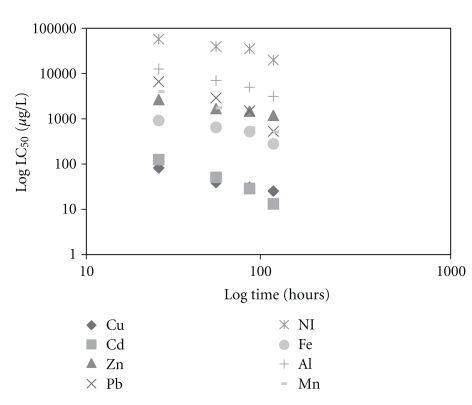
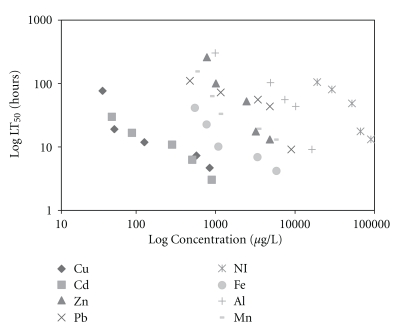
One hundred percent of control animals maintained in dechlorinated water survived throughout the experiment. The median lethal times (LT50) and concentrations (LC50) increased with a decrease in mean exposure concentrations and times, respectively, for all metals (Tables 1 and 2). However, the lethal threshold concentration could not be determined since the toxicity curves (Figures 1 and 2) did not become asymptotic to the time axis within the test period. Figures 1 and 2 also show that Cd was the most toxic to S. major, followed by Cu, Fe, Mn, Pb, Zn, Al, and Ni. Similar results were reported for the ostracod Cypris subglobosa [10]. Arambašić et al. [18] found that with Daphnia magna, the order of toxicity was Cu>Zn>Pb, and Bacher and O'Brien [19] showed that Cu was more toxic than Pb to Daphnia carinata.
Table 2.
Median lethal concentrations (LC50) for S. major at different exposure times for Cu, Cd, Zn, Pb, Ni, Fe, Al, and Mn.
(a)
| Time (hour) | LC50 (μg/L) for Cu | 95% Confidence limits | LC50 (μg/L) for Cd | 95% Confidence limits | LC50 (μg/L) for Zn | 95% Confidence limits | LC50 (μg/L) for Pb | 95% Confidence limits |
|---|---|---|---|---|---|---|---|---|
| 24 | 82.17 | 39.02–141.5 | 125.18 | 77.69–180.97 | 2655.27 | 2148.19–3282.04 | 6582.57 | 4920.11–8806.77 |
| 48 | 38.84 | 19.3–55.82 | 50.73 | 21.7–78.3 | 1682.70 | 1365.77–2073.17 | 2885.86 | 7919.06–4339.71 |
| 72 | 30.67 | 10.07–42.46 | 28.76 | 0.49–47.82 | 1475.74 | 1204.16–1808.58 | 1491.11 | 991.86–2241.66 |
| 96 | 25.2 | 4.51–35.75 | 13.15 | NA | 1189.83 | 955.19–1482.13 | 526.19 | 307.06–901.72 |
(b)
| Time (hour) | LC50 (μg/L) for Ni | 95% Confidence limits | LC50 (μg/L) for Fe | 95% Confidence limits | LC50 (μg/L) for Al | 95% Confidence limits | LC50 (μg/L) for Mn | 95% Confidence limits |
|---|---|---|---|---|---|---|---|---|
| 24 | 57280.92 | 49144.72–66764.11 | 911.44 | 654.2–1200.59 | 12530.33 | 10197.69–15396.55 | 3984.34 | 2341.04–16025.68 |
| 48 | 39177.81 | 33011.57–46495.85 | 644.39 | 450.21–770.2 | 6980.05 | 2360.23–20642.53 | 1733.27 | 1050.82–3095.45 |
| 72 | 35134.49 | 28902.18–42710.69 | 521.24 | 300.04–617.27 | 4964.23 | 3598.66–6848.01 | 636.03 | 278.65–943.71 |
| 96 | 19743.75 | 14774.15–26384.97 | 278.9 | NA | 3101.96 | 1281.61–7507.85 | 510.24 | 206.07–689.26 |
NA: not available: values could not be calculated from probit software.
Figure 1.
The relationship between median lethal concentration (LC50) and exposure times for S. major.
Figure 2.
The relationship between median lethal time (LT50) and exposure concentrations for S. major.
This study showed that LC50s for 96 hours for Cu, Cd, Zn, Pb, Ni, Fe, Al, and Mn were 25.2, 13.1, 1189.8, 526.2, 19743.7, 278.9, 3101.9, and 510.2 μg/L, respectively (Table 2). Few studies were reported on the toxicity of metals to ostracods. Khangarot and Ray [12] showed that toxicity of Cu to ostracod Cypris subglobosa increases as pH of the test medium decreases from 8.5 (EC50 = 5.1 mg/L) to 5.5 (EC50 = 0.35 mg/L) and vice versa. Khangarot and Das [10] showed that the 48 h EC50s (immobilization) for Cu, Cd, Zn, Pb, Ni, Fe, Al, and Mn for Cypris subglobosa were 0.55, 0.82, 85.04, 40.19, 75.78, 115.2, 100.90, and 11.77 mg/L, respectively. The toxicity baseline database on ostracod is still deficient; therefore, a comparison of LC50 values with other freshwater crustacean especially cladocerans, amphipods, and few ostracods is shown in Table 3. This study showed that for all metals tested, S. major showed highest sensitivity compared to other species such as ostracod Cypris subglobosa, cladoceran Daphnia carinata, and amphipod Hyalella azteca, except for Pb and Ni (Table 3). Present study showed that for Cu, Cd, Zn, Fe, Al, and Mn, 96h-LC50 values obtained were lower than for other ostracod, cladocerans, and amphipods (Table 3). This indicated that S. major is equally or more sensitive than most of the reported species for metals. Von Der Ohe and Liess [29] showed that 13 taxa belonging to Crustacea were among the most sensitive to metal compounds and concluded that taxa belonging to Crustacea are similar to one another and to Daphnia magna in terms of sensitivity to organics and metals.
Table 3.
Comparison of LC50 (or EC50) values of freshwater ostracod S. major with other freshwater crustacean (ostracod, cladoceran, and amphipod).
| Metal | Species | Live stage | Test duration | LC50 (μg/L) | Reference |
|---|---|---|---|---|---|
| Copper | Hyalella azteca | Adult | 96 h | 912 | [20] |
| Daphnia magna | 24 h | 48 h | 73.1 | [18] | |
| Gammarus fasciatus | Adult | 48 h | 190 | [21] | |
| Daphnia carinata | 6 hours | 96 h | 41 | [19] | |
| Cypris subglobosa | Adult | 48 h | 550* | [10] | |
| S. major | Adult | 96 h | 25.2 | This study | |
|
| |||||
| Cadmium | Hyalella azteca | Adult | 96 h | 17.5 | [20] |
| Echinogammarus meridionalis | Adult | 96 h | 36.17 | [22] | |
| Daphnia magna | 96 h | 12.7 | [23] | ||
| Gammarus pulex | Adult | 96 h | 82.1 | [24] | |
| Cypris subglobosa | Adult | 48 h | 821* | [10] | |
| S. major | Adult | 96 h | 13.1 | This study | |
|
| |||||
| Zinc | Hyalella azteca | Adult | 96 h | 1613 | [20] |
| Daphnia magna | 24h | 48 h | 752.8 | [18] | |
| Echinogammarus meridionalis | Adult | 96 h | 4610 | [22] | |
| Cypris subglobosa | Adult | 24 h | 3400* | [10] | |
| S. major | Adult | 96 h | 1189 | This study | |
|
| |||||
| Lead | Hyalella azteca | Adult | 96 h | 18 | [25] |
| Daphnia magna | 24 h | 48 h | 55641 | [18] | |
| Daphnia carinata | Neonate | 48 h | 170 | [19] | |
| Cypris subglobosa | Adult | 48 h | 40190* | [10] | |
| S. major | Adult | 96 h | 526 | This study | |
|
| |||||
| Nickel | Cypris subglobosa | Adult | 48h | 75780* | [10] |
| Daphnia magna | Adult | 48 h | 7290* | [26] | |
| S. major | Adult | 96 h | 19743 | This study | |
|
| |||||
| Iron | Cypris subglobosa | Adult | 48 h | 115200* | [10] |
| Daphnia magna | Adult | 48 h | 7200* | [26] | |
| Ceriodaphnia dubia | <24 h | 48 h | 36690 | [27] | |
| Daphnia pulex | neonate | 48 h | 12930 | [28] | |
| S. major | Adult | 96 h | 278 | This study | |
|
| |||||
| Aluminium | Cypris subglobosa | Adult | 48 h | 100900 | [10] |
| Daphnia magna | Adult | 48 h | 32000* | [26] | |
| S. major | Adult | 96 h | 3101 | This study | |
|
| |||||
| Manganese | Cypris subglobosa | Adult | 48 h | 11770 | [10] |
| Daphnia magna | Adult | 48 h | 8280* | [26] | |
| S. major | Adult | 96 h | 510 | This study | |
*EC50 value (immobilization).
In comparison with other freshwater ostracod (Cypris subglobosa) (Table 3), this study showed that LC50s for S. major were lower compared to EC50 (immobilization) of C. subglobosa for all the metals tested although our end point of study was higher (mortality) compared to C. subglobosa (immobilization) [10]. These differences are probably due to different species used, age, size of the organism, test methods, and water quality such as water hardness, as this can affect toxicity [30, 31]. In the present study, water hardness was considered low, and the water was categorized as soft water (<75 mg/L as CaCO3) compared to study by Khangarot and Das [10] where they used hard water (245 mg/L as CaCO3).
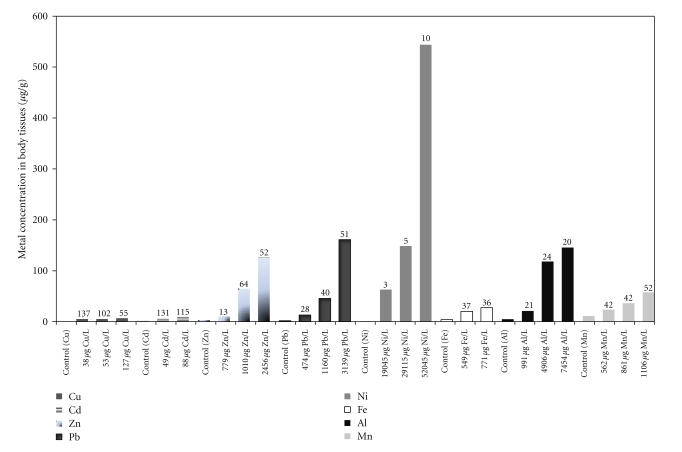
Bioconcentration of Cu, Cd, Zn, Pb, Ni, Fe, Al and Mn in surviving S. major are as shown in Figure 3. Bioconcentration data for live ostracods were obtained from three Cu (38, 53, and 127 μg/L), Zn (779, 1010, and 2456 μg/L), Pb (474, 1160, and 3139 μg/L), Ni (19045, 29115, and 52045 μg/L), Al (991, 4906, and 7454 μg/L) and Mn (562, 861, and 1106 μg/L) concentration exposures, and two Cd (49 and 88 μg/L) and Fe (549 and 771 μg/L) concentration exposures. In general, Cu, Cd, Pb, Zn, Ni, Fe, Al, and Mn bioconcentration in S. major increases with increasing concentration exposure. Luoma and Rainbow [1] reported that the uptake of trace metals from solution by an aquatic organism is primarily concentration dependent. The higher the dissolved concentration of the trace metal is, the higher will be the uptake of the metal from solution into the organism, until the uptake mechanism becomes saturated. Concentration factor (CFs) also showed some trend of increases with increasing concentration exposure (Figure 3) especially for Pb, Ni, and Mn. In general, the highest CF was noted for Cu (137) and Cd (131), and the lowest CF for Ni (3). Similar results were reported by Timmermans et al. [32] with Chironomus riparius, which showed that among four metals (Cu, Cd, Zn, and Pb), Cd had the highest bioconcentration factor (BCF). Shuhaimi-Othman and Pascoe [33] also showed that Cd had the highest BCF followed by Cu and Zn with amphipod Hyalella azteca.
Figure 3.
Bioconcentration of Cu, Cd, Zn, Pb, Ni, Fe, Al, and Mn (mean) in S. major after a four-day exposure to different concentrations of Cu, Cd, Zn, Pb, Ni, Fe, Al, and Mn. Concentration factor (CF) is indicated at the top of each bar.
Higher uptake of Cd in invertebrates probably due to availability of the uptake route through “major ion” channels as Cd can enter into the cell through Ca channels as both metals have very similar ionic radius [34]. Higher accumulation of Cd in S. major also seems to be associated with net accumulation of these metals. A study by Vijayram and Geraldine [35] with freshwater prawn, Macrobrachium malcolmsonii, showed that the prawn accumulated the nonessential metal Cd at all exposure levels (6.3–157 μg/L) without any regulation. However, the prawn regulated the essential metal Zn until it reached threshold level (373 μg/L), at which regulation collapsed and net accumulation began. Borgmann et al. [36] showed that the amphipod Hyalella azteca was capable of regulating Cu but unable to regulate Zn as effectively and did not regulate Hg, Cd, and Pb. Krantzberg and Stokes [37] reported that chironomid larvae were able to regulate or control the accumulation of Ni and Zn but could not regulate Pb and Cd. Comparison of uptake rate in aquatic organisms showed that in general the order of the uptake rate constant is Ag>Zn>Cd>Cu>Co>Cr>Se [1]. This discrepancy is probably due to short time of exposure (four days) to metals in this study.
4. Conclusions
This study showed that S. major was equally or more sensitive to metals compared to other freshwater crustaceans. Cd was the most toxic to S. major followed by Cu, Fe, Mn, Pb, Zn, Al, and Ni. A comparison of bioconcentration of metals in S. major showed that among the eight metals studied, Cu and Cd were the most accumulated and Ni was the least accumulated. This study indicates that S. major is a potential bioindicator organism of metals pollution and in toxicity testing.
Acknowledgment
This study was funded by the Ministry of Science and Technology, Malaysia (MOSTI) under e-Science fund code number 06-01-02-SF0217.
References
- 1.Luoma N, Rainbow PS. Metal Contamination in Aquatic Environment. Science and Lateral Management. New York, NY, USA: Cambridge University Press; 2008. [Google Scholar]
- 2.Shazili NAM, Yunus K, Ahmad AS, Abdullah N, Rashid MKA. Heavy metal pollution status in the Malaysian aquatic environment. Aquatic Ecosystem Health and Management. 2006;9(2):137–145. [Google Scholar]
- 3.DOE. Malaysia Environment Quality Report 2008. Department of Environment, Ministry of Natural Resources and Environment, Malaysia. ISSN 0127-6433, 2009.
- 4.Adams WJ, Rowland CD. Aquatic toxicology test methods. In: Hoffman DJ, Rattner BA, Burton GA Jr., Cairns J Jr., editors. Handbook of Ecotoxicology. 2nd edition. Boca Raton, Fla, USA: Lewis Publisher, CRC Press; 2003. [Google Scholar]
- 5.Victor R, Fernando CH. An Illustrated Key to the Freshwater Ostracod Genera of the Oriental Region. Vol. 23. University of Waterloo Biology Series; 1981. [Google Scholar]
- 6.Victor R. Crustacea: ostracoda. In: Yule CM, Sen YH, editors. Freshwater Invertebrates of the Malaysian Region. Kuala Lumpur, Malaysia: Academy of Science; 2004. pp. 225–253. [Google Scholar]
- 7.Pérez L, Lorenschat J, Brenner M, Scharf B, Schwalb A. Extant freshwater ostracodes (Crustacea: Ostracoda) from Lago Petén Itzá, Guatemala. Revista de Biologia Tropical. 2010;8(3):871–895. doi: 10.15517/rbt.v58i2.5252. [DOI] [PubMed] [Google Scholar]
- 8.Pascual A, Rodriguez-Lazaro J, Weber O, Jouanneau JM. Late Holocene pollution in the Gernika estuary (southern Bay of Biscay) evidenced by the study of Foraminifera and Ostracoda. Hydrobiologia. 2002;475-476:477–491. [Google Scholar]
- 9.Chial B, Persoone G. Cyst-based toxicity tests XIV—application of the ostracod solid-phase microbiotest for toxicity monitoring of river sediments in Flanders (Belgium) Environmental Toxicology. 2002;17(6):533–537. doi: 10.1002/tox.10087. [DOI] [PubMed] [Google Scholar]
- 10.Khangarot BS, Das S. Acute toxicity of metals and reference toxicants to a freshwater ostracod, Cypris subglobosa Sowerby, 1840 and correlation to EC50 values of other test models. Journal of Hazardous Materials. 2009;172(2-3):641–649. doi: 10.1016/j.jhazmat.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz F, González-Regalado ML, Borrego J, Abad M, Pendón JG. Ostracoda and foraminifera as short-term tracers of environmental changes in very polluted areas: the Odiel Estuary (SW Spain) Environmental Pollution. 2004;129(1):49–61. doi: 10.1016/j.envpol.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Khangarot BS, Ray PK. Response of a freshwater ostracod (Cypris subglobosa Sowerby) exposed to copper at different pH levels. Acta Hydrochimica et Hydrobiologica. 1987;15:553–558. [Google Scholar]
- 13.Sánchez-Bayo F, Goka K. Influence of light in acute toxicity bioassays of imidacloprid and zinc pyrithione to zooplankton crustaceans. Aquatic Toxicology. 2006;78(3):262–271. doi: 10.1016/j.aquatox.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 14.APHA (American Public Health Association) Standard Method for the Examination of Water and Wastewater. 18th edition. Washington, DC, USA: American Public Health Association; 1992. [Google Scholar]
- 15.Cooney JD. Freshwater test. In: Rand GM, editor. Fundamental of Aquatic Toxicology: Effects, Environmental fate and Risk Assessment. 2nd edition. Boca Raton, Fla, USA: Taylor & Francis; 1995. pp. 71–102. [Google Scholar]
- 16.Lichfield TJ. A method for the rapid graphic solution of time-percentage effect curves. Journal of Pharmacology and Experimental Therapeutics. 1949;97:399–408. [PubMed] [Google Scholar]
- 17.Lichfield TJ, Wilcoxon F. A simplified method of evaluating dose-effect experiments. Journal of Pharmacology and Experimental Therapeutics. 1949;96:99–113. [PubMed] [Google Scholar]
- 18.Arambašić MB, Bjelić S, Subakov G. Acute toxicity of heavy metals (Cu, Pb, Zn), phenol and sodium on Allium cepa, Lepidium sativum and Daphnia magna: comparative investigations and the practical applications. Water Research. 1995;29(2):497–503. [Google Scholar]
- 19.Bacher GJ, O’Brien TA. Scientific Series Report. 88/18. Victoria, Australia: Victorian Environmental Protection Authority; 1990. The sensitivity of Australian freshwater aquatic organisms to heavy metals. [Google Scholar]
- 20.Shuhaimi-Othman M, Pascoe D. Acute toxicity of copper, zinc and cadmium to the freshwater amphipod Hyalella azteca . Malaysian Applied Biology. 2001;30(1-2):1–8. [Google Scholar]
- 21.Judy RD. The acute toxicity of copper to Gammarus fasciatus, a freshwater amphipod. Bulletin of Environmental Contamination and Toxicology. 1979;21(1-2):219–224. doi: 10.1007/BF01685414. [DOI] [PubMed] [Google Scholar]
- 22.Pestana JLT, Ré A, Nogueira AJA, Soares AMVM. Effects of Cadmium and Zinc on the feeding behaviour of two freshwater crustaceans: Atyaephyra desmarestii (Decapoda) and Echinogammarus meridionalis (Amphipoda) Chemosphere. 2007;68(8):1556–1562. doi: 10.1016/j.chemosphere.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 23.Suedel BC, Rodgers JH, Deaver E. Experimental factors that may affect toxicity of cadmium to freshwater organisms. Archives of Environmental Contamination and Toxicology. 1997;33(2):188–193. doi: 10.1007/s002449900241. [DOI] [PubMed] [Google Scholar]
- 24.Felten V, Charmantier G, Mons R, et al. Physiological and behavioural responses of Gammarus pulex (Crustacea: amphipoda) exposed to cadmium. Aquatic Toxicology. 2008;86(3):413–425. doi: 10.1016/j.aquatox.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Mackie GL. Tolerances of five benthic invertebrates to hydrogen ions and metals (Cd, Pb, Al) Archives of Environmental Contamination and Toxicology. 1989;18(1-2):215–223. [Google Scholar]
- 26.Khangarot BS, Ray PK. Investigation of correlation between physicochemical properties of metals and their toxicity to the water flea Daphnia magna Straus. Ecotoxicology and Environmental Safety. 1989;18(2):109–120. doi: 10.1016/0147-6513(89)90071-7. [DOI] [PubMed] [Google Scholar]
- 27.Fort DJ, Stover EL. Impact of toxicities and potential interactions of flocculants and coagulant aids on whole effluent toxicity testing. Water Environment Research. 1995;67(6):921–925. [Google Scholar]
- 28.Birge WJ, Black JA, Westerman AG, et al. Recommendations on Numerical Values for Regulating Iron and Chloride Concentrations for the Purpose of Protecting Warmwater Species of Aquatic Life in the Commonwealth of Kentucky Source. Lexington, KY, USA: University of Kentucky; 1985. [Google Scholar]
- 29.Von Der Ohe PC, Liess M. Relative sensitivity distribution of aquatic invertebrates to organic and metal compounds. Environmental Toxicology and Chemistry. 2004;23(1):150–156. doi: 10.1897/02-577. [DOI] [PubMed] [Google Scholar]
- 30.McCahon CP, Pascoe D. Use of Gammarus pulex (L.) in safety evaluation tests: culture and selection of a sensitive life stage. Ecotoxicology and Environmental Safety. 1988;15(3):245–252. doi: 10.1016/0147-6513(88)90078-4. [DOI] [PubMed] [Google Scholar]
- 31.Ebrahimpour M, Alipour H, Rakhshah S. Influence of water hardness on acute toxicity of copper and zinc on fish. Toxicology and Industrial Health. 2010;26(6):361–365. doi: 10.1177/0748233710369123. [DOI] [PubMed] [Google Scholar]
- 32.Timmermans KR, Peeters W, Tonkes M. Cadmium, zinc, lead and copper in Chironomus riparius (Meigen) larvae (Diptera, Chironomidae): uptake and effects. Hydrobiologia. 1992;241(2):119–134. [Google Scholar]
- 33.Shuhaimi-Othman M, Pascoe D. Bioconcentration and depuration of copper, cadmium, and zinc mixtures by the freshwater amphipod Hyalella azteca . Ecotoxicology and Environmental Safety. 2007;66(1):29–35. doi: 10.1016/j.ecoenv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Rainbow PS, Black WH. Cadmium, zinc and the uptake of calcium by two crabs, Carcinus maenas and Eriocheir sinensis . Aquatic Toxicology. 2005;72(1-2):45–65. doi: 10.1016/j.aquatox.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Vijayram K, Geraldine P. Are the heavy metals cadmium and zinc regulated in freshwater prawns? Ecotoxicology and Environmental Safety. 1996;34(2):180–183. doi: 10.1006/eesa.1996.0061. [DOI] [PubMed] [Google Scholar]
- 36.Borgmann U, Norwood WP, Clarke C. Accumulation, regulation and toxicity of copper, zinc, lead and mercury in Hyalella azteca . Hydrobiologia. 1993;259(2):79–89. [Google Scholar]
- 37.Krantzberg G, Stokes PM. Metal regulation, tolerance, and body burdens in the larvae of the genus Chironomus . Canadian Journal of Fisheries and Aquatic Sciences. 1989;46(3):389–398. [Google Scholar]