Abstract
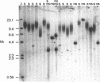
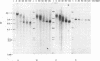
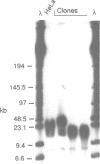
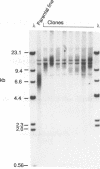
Cloned human telomeric DNA can integrate into mammalian chromosomes and seed the formation of new telomeres. This process occurs efficiently in three established human cell lines and in a mouse embryonic stem cell line. The newly seeded telomeres appear to be healed by telomerase. The seeding of new telomeres by cloned telomeric DNA is either undetectable or very inefficient in non-tumourigenic mouse or human somatic cell lines. The cytogenetic consequences of the seeding of new telomeres include large chromosome truncations but most of the telomere seeding events occur close to the pre-existing ends of natural chromosomes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolph S., Bartram C. R., Hameister H. Mapping of the oncogenes Myc, Sis, and int-1 to the distal part of mouse chromosome 15. Cytogenet Cell Genet. 1987;44(2-3):65–68. doi: 10.1159/000132345. [DOI] [PubMed] [Google Scholar]
- Brown W. R. A physical map of the human pseudoautosomal region. EMBO J. 1988 Aug;7(8):2377–2385. doi: 10.1002/j.1460-2075.1988.tb03082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. R., MacKinnon P. J., Villasanté A., Spurr N., Buckle V. J., Dobson M. J. Structure and polymorphism of human telomere-associated DNA. Cell. 1990 Oct 5;63(1):119–132. doi: 10.1016/0092-8674(90)90293-n. [DOI] [PubMed] [Google Scholar]
- Brown W. R. Mammalian artificial chromosomes. Curr Opin Genet Dev. 1992 Jun;2(3):479–486. doi: 10.1016/s0959-437x(05)80161-3. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr C., Fantes J., Goodfellow P., Cooke H. Functional reintroduction of human telomeres into mammalian cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7006–7010. doi: 10.1073/pnas.88.16.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Claus T. E., Szostak J. W. Characterization of two telomeric DNA processing reactions in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Nov;8(11):4642–4650. doi: 10.1128/mcb.8.11.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J., Evans E. P., Smith A. G. Establishment of germ-line-competent embryonic stem (ES) cells using differentiation inhibiting activity. Development. 1990 Dec;110(4):1341–1348. doi: 10.1242/dev.110.4.1341. [DOI] [PubMed] [Google Scholar]
- Pavan W. J., Hieter P., Reeves R. H. Generation of deletion derivatives by targeted transformation of human-derived yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1300–1304. doi: 10.1073/pnas.87.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera M. F., Cooper S., Mills J., Parrington J. M. Isolation and characterization of a multipotent clone of human embryonal carcinoma cells. Differentiation. 1989 Oct;42(1):10–23. doi: 10.1111/j.1432-0436.1989.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Rathjen P. D., Toth S., Willis A., Heath J. K., Smith A. G. Differentiation inhibiting activity is produced in matrix-associated and diffusible forms that are generated by alternate promoter usage. Cell. 1990 Sep 21;62(6):1105–1114. doi: 10.1016/0092-8674(90)90387-t. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- SCHERER W. F., SYVERTON J. T., GEY G. O. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J Exp Med. 1953 May;97(5):695–710. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surosky R. T., Tye B. K. Construction of telocentric chromosomes in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2106–2110. doi: 10.1073/pnas.82.7.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath D., Davis R. W., Connelly C., Hieter P. Physical mapping of large DNA by chromosome fragmentation. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6027–6031. doi: 10.1073/pnas.85.16.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T., Shiue L., Myers R. M., Cox D. R., Naylor S. L., Killery A. M., Varmus H. E. Structure and variability of human chromosome ends. Mol Cell Biol. 1990 Feb;10(2):518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]