Abstract
BACKGROUND
Cardiovascular diseases are among the leading causes of mortality in developed countries. It is widely recognized that troponin I (TnI) can be used for the assessment of a myocardial infarction.
METHODS
We investigated the use of the microwave-accelerated and metal-enhanced fluorescence (MA-MEF), a technique based on the combined use of low-power microwave heating, silver nanoparticle films (SNFs), and fluorescence spectroscopy for the detection of TnI from human whole blood samples. SNFs were deposited onto amine-modified glass microscope slides by use of Tollen’s reaction scheme and characterized by optical absorption spectroscopy and scanning electron microscopy. The detection of TnI from buffer solutions and human whole blood samples on SNFs was carried out by using fluorescence-based immunoassays at room temperature (control immunoassay, 2 h total assay time) or microwave heating (MA-MEF–based immunoassay, 1 min total assay time).
RESULTS
We found that the lower limits of detection for TnI from buffer solutions in the control immunoassay and MA-MEF–based immunoassay were 0.1 μg/L and 0.005 μg/L, respectively. However, we were unable to detect TnI in whole blood samples in the control immunoassays owing to the coagulation of whole blood within 5 min of the incubation step. The use of the MA-MEF technique allowed detection of TnI from whole blood samples in 1 min with a lower detection limit of 0.05 μg/L.
CONCLUSIONS
The MA-MEF–based immunoassay is one of the fastest reported quantitative detection methodos for detection of TnI in human whole blood and has low detection limits similar to those obtained with commercially available immunoassays.
Cardiovascular diseases are among the leading causes of mortality in developed countries. Of several conventional cardiac markers, including myoglobin, troponin I (TnI),2 troponin T (TnT), and the MB isoenzyme of creatine kinase (1–3), troponin is considered to be more sensitive and significantly more specific for the diagnosis of acute myocardial infarction than myoglobin or the MB isoenzyme of creatine kinase, and cTnI and TnT have been used as reliable markers for cardiac risk assessment for more than 10 years (4–6). For TnI, the accepted biological cutoff value is approximately 5 μg/L (7). TnI can be detected in blood 3–6 h after the onset of chest pain and reaches peak concentrations within 16–30 h. TnI is also useful for the late diagnosis of acute myocardial infarction, because increased concentrations can be detected in blood 5–8 days after chest-pain onset.
In a typical hospital setting, immunoassays for TnI are usually run on serum after a blood separation process (1–3). These immunoassays most commonly use antigen–antibody binding for analyte recognition and have several modes of readout (luminescence or absorption). The antigen–antibody recognition step can be very slow kinetically, often requiring incubation times in excess of 1 h (8–11). Semiautomated instruments for cardiac marker immunoassays that offer results from whole blood in approximately 15 min are commercially available, such as Abbott’s I-STAT System, Roche’s Cardiac Reader, Biosite’s Triage® Cardiac Panel, and Response Biomedical Corporation’s RAMP Cardiac Marker System. However, these systems measure 1 sample at a time and have high initial and maintenance costs. In this regard, there is still a need for the development of fast and sensitive immunoassays for the detection of TnI from whole blood that can be used to predict acute myocardial infarction accurately and inexpensively.
A new technique for the detection of proteins in whole blood samples, microwave-accelerated and metal-enhanced fluorescence (MA-MEF), was recently demonstrated (12). In the MA-MEF technique the benefits of MEF [a phenomenon that significantly increases the fluorescence signals of fluorophores in close proximity (<10 nm) to plasmonic nanostructures] is coupled with low-power microwave heating to accelerate biorecognition events (13, 14). It has also been shown that the antibody–antigen-binding steps in an immunoassay can be completed within seconds by using the MA-MEF technique (12, 15, 16). Furthermore, the enhanced fluorescence readout provided by MEF allows for the detection at a much lower antigen concentration (17). Therefore, MA-MEF technology has the potential to radically improve the speed and sensitivity of cardiac marker immunoassays. We report the application of the MA-MEF technique for rapid and sensitive detection of TnI.
Materials and Methods
MATERIALS
Protein A, human whole blood (without anticoagulant), BSA, silver nitrate (99.9%), trisodium citrate, PBS (pH 7.4, 137 mmol/L NaCl, 8.1 mmol/L sodium phosphate, 2.7 mmol/L KCl, and 1.76 mmol/L KH2PO4), press-to-seal silicone isolators (8 well, depth × diameter: 1.0 mm × 9 mm), and silane-prep™ glass (amine-modified) slides were purchased from Sigma-Aldrich. Relevant antibodies (capture and FITC-labeled detection antibody) and standard solutions of TnI were purchased from Advanced Immunochemical. All chemicals were used as received.
METHODS
Coating of amine-modified glass slides with silver nanoparticle films (SNFs) was carried out by using a 6-step Tollen’s reaction scheme: (a) A solution of silver nitrate (0.5 g in 60 mL of deionized water) was placed in a clean 100-mL glass beaker with a magnetic stirrer. (b) While the silver nitrate solution was stirred at speed 10 (on a Fisher Heater/Stirrer), 200 μL of freshly prepared 5% (w/v) sodium hydroxide solution was added, resulting in the formation of dark-brown precipitates of silver particles in solution. (c) The precipitates were immediately redissolved by 2 mL ammonium hydroxide. (d) The resulting clear solution was cooled down to 5 °C by placing the beaker in an ice bath, then soaking the amine-modified glass slides in the solution. (e) After 2 min, a fresh solution of D-glucose (0.72 g in 15 mL water) was added. (f) Subsequently, the temperature of the mixture was warmed to 30 °C. As the color of the mixture turned from “yellow-green” to “yellow-brown” and the color of the amine-modified glass slides became green (in 2 min), the amine-modified glass slides with SNF deposits were removed from the mixture. SNFs were rinsed with deionized water and sonicated for 30 s at room temperature. SNFs were then rinsed with deionized water several times, then air-dried and kept in an air-tight vessel until further use.
The characterization of SNFs was carried out by optical absorption spectroscopy and scanning electron microscope (SEM). SEM images of SNFs were obtained at the Core Imaging Facility of the University of Maryland Dental School. Real-color photographs of SNFs and blank glass slides were taken by using a 5–mega-pixel digital camera.
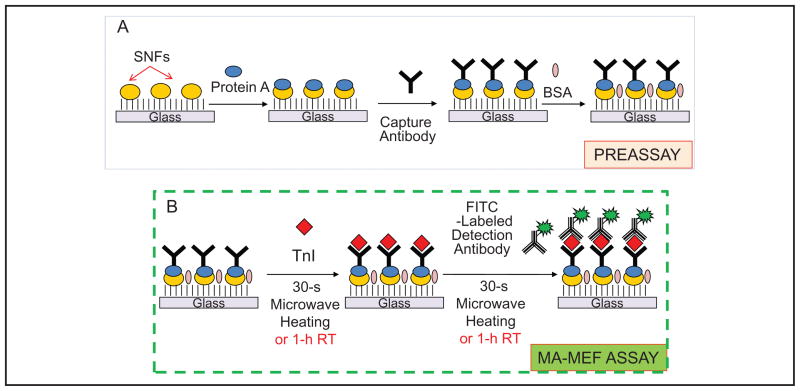
The construction of the MA-MEF–based immunoassay and control immunoassay for TnI is depicted in Fig. 1. The immunoassay procedure was divided into 2 parts:
Fig. 1. Schematic depiction of the MA-MEF–based immunoassay for TnI.
(A), Preassay: protein A, capture antibody, and BSA (in buffer) are attached to SNFs in sequence by incubation at room temperature (RT) for 30 min for each step. (B), MA-MEF assay: TnI (in buffer or whole blood) and detector antibody (in buffer) are incubated on SNFs with microwave heating for 30 s.
Preassay (Fig. 1A), in which protein A, capture antibody, and BSA (all in PBS buffer, pH 7.4) are attached to SNFs in sequence by incubation at room temperature for 30 min for each step, and
MA-MEF assay (Fig. 1B), in which TnI (in buffer or whole blood) and detector antibody (in buffer) are incubated on SNFs by use of microwave heating for 30 s. As a control immunoassay, these steps were also separately carried out at room temperature (60 min each step).
The most relevant steps in the MA-MEF–based immunoassay for TnI using microwave heating are the steps involving TnI and detector antibody incubation on SNFs. For this process, the preassay slides can be prepared at any time and stored for future use in the MA-MEF–based immunoassay. In both immunoassays (control and MA-MEF based), TnI was present in 2 forms: in buffer only or in human whole blood. In the immunoassays run for whole blood samples, a solution of TnI in phosphate buffer (Na2HPO4, pH 7) was mixed with human whole blood (50% v/v mixture; final volume: 50 μL; final concentration range for TnI: 0.001–100 μg/L). These mixtures or a buffer solution (control sample, no TnI) was placed inside the wells of silicon isolators, and the SNFs were heated for 30 s in a commercially available microwave oven (Emerson; maximum power 700 W microwave oven, model MW8784B; power setting 3 was used). The unbound material was removed by rinsing with phosphate buffer 3 times. Then, 50 μL of 10 μmol/L FITC-labeled detection antibody was subsequently added to the wells and heated for 30 s in the microwave cavity, followed by rinsing with buffer to remove the unbound material.
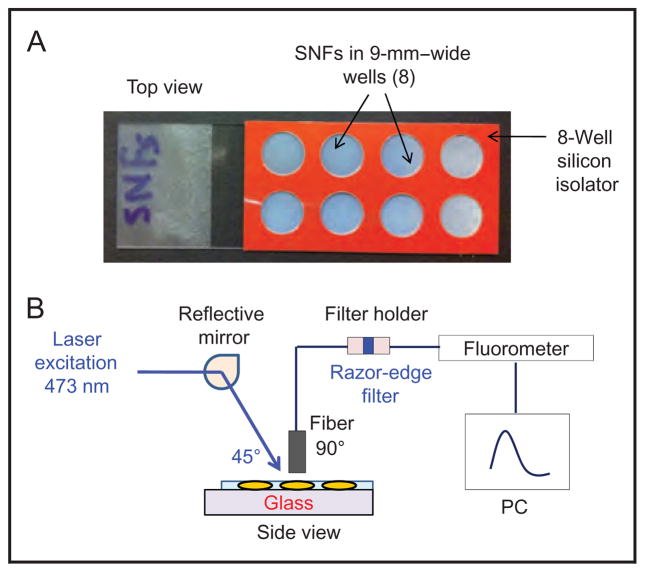
Fig. 2A shows the real-color image of SNFs with an 8-well silicon isolator that allows the processing of 8 different samples at once. Fig. 2B shows the experimental geometry used for the measurement of fluorescence emission from the immunoassays. SNFs were placed horizontally above a laser table on an optical post. The excitation source (473-nm continuous-wave laser; B&W Tek) is reflected at a 45° angle to the wells on SNFs (1 at a time). An optical fiber (1000-μm thick, Ocean Optics) was vertically placed over the samples to allow the fluorescence emission spectrum to be measured at a 45° angle to the excitation source. Excitation intensity was eliminated with a 473-nm razor-edge filter (Semrock) placed in a filter holder, which couples to the optical fibers from the sample side to a spectrofluorometer (Jaz; Ocean Optics). The fluorescence emission spectrum from each sample was collected by using the software provided by the vendor.
Fig. 2.
(A), Real-color image of SNFs with an 8-well silicon isolator that allows for the processing of 8 different samples at once.
(B), Experimental geometry used for the measurement of fluorescence emission from the immunoassays.
Results and Discussion
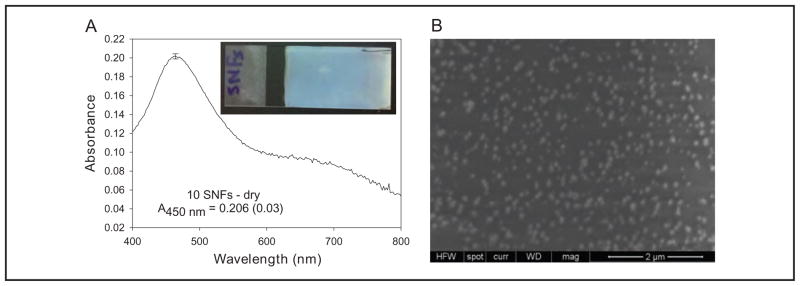
Because the MA-MEF–based immunoassay for TnI used in this study employs SNFs, it is important to substantiate the reproducibility of the deposition of SNFs from solution onto amine-modified glass slides. Ten different SNFs were prepared, and the absorption spectrum of each slide was measured as shown in Fig. 3A. The surface plasmon resonance peak for SNFs occurs at 450 nm and has an absorbance of 0.206. The SD for the surface plasmon resonance peak of 10 different samples was approximately %1.5, showing that deposition of silver nanoparticles onto an amine-modified glass slides was highly reproducible. The inset in Fig. 3A shows the real-color photograph of a typical SNF, which visually confirms that silver nanoparticles are deposited onto glass slides in a homogeneous fashion. An SEM image of SNFs (Fig. 3B) reveals that the size of the silver nanoparticles is approximately 100 nm. Previous studies on MEF-based applications using silver nanoparticles have shown that the optimum size of silver nanoparticles for maximum enhancement of fluorescence emission is approximately 100 nm (12). These results demonstrate that the deposition of silver nanoparticles onto amine-modified glass slides as demonstrated in this work yields highly reliable surfaces for quantitative detection of biomolecules and analytes based on the MA-MEF technique.
Fig. 3.
(A), Absorbance (A) spectrum of SNFs; inset, a real-color photograph of a typical SNF.
(B), A typical SEM image of SNFs.
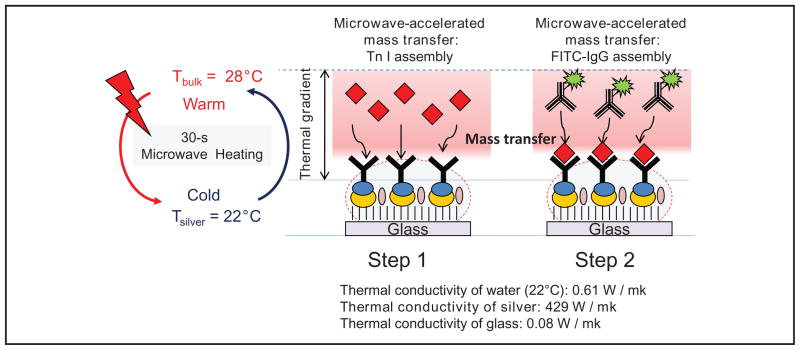
It is important to discuss the effect of combined use of microwave heating and SNFs in an MA-MEF immunoassay scheme that yields results in a matter of minutes (total assay time). Fig. 4 shows a schematic depiction of the proposed mechanism for the increased rapidity and sensitivity of the TnI immunoassay by use of low-power microwave heating and SNFs. On exposure of immunoassay medium placed on SNFs to microwave heating, a thermal gradient between water (or a mixture of whole blood and water) and SNFs is created because of the differences between their thermal conductivity (k) values. Because k values for water and glass slides are similar, a thermal gradient is not expected to occur on the unsilvered part of the SNFs. In the first step of the MA-MEF–based immunoassay, the thermal gradient between water and SNFs results in rapid microwave-accelerated mass transfer of TnI from the assay medium to the surface of SNFs. Subsequently, these proteins rapidly bind to capture antibodies on the surface of SNFs. It is important to note that whereas the Fab region of the capture antibodies is oriented toward the assay medium on SNFs, the Fc region is oriented toward SNFs because of binding of the Fc region with protein A. Although protein A is also present on the unsilvered part of the SNFs, because there is no thermal gradient between water and glass, it is thought that the binding of capture antibody with protein A predominantly occurs on SNFs. In the second step, the exposure of a fresh buffer solution containing FITC-labeled detection antibody to microwave heating results in rapid microwave-accelerated mass transfer of these molecules toward the SNF surface-presenting TnI proteins.
Fig. 4.
Schematic depiction of the proposed mechanism for the increased rapidity and sensitivity of TnI immunoassay by using low-power microwave heating and SNFs.
It was previously shown in experiments using fluorescence resonance energy transfer that low-power microwave heating of proteins in solution on SNFs does not denature the proteins (15). In that previously reported study, a model protein assay based on the interactions of biotinylated BSA–avidin was constructed on SNFs. After the attachment of biotinylated BSA on SNFs, avidins labeled with fluorescein (donor) and Alexa 532 (acceptor) were incubated on SNFs together at room temperature and with microwave heating. In these experiments, fluorescence emission spectra for these fluorophores were identical, which indicated that the structures of proteins and fluorophores were unchanged (15). In addition, the MA-MEF technique was also successfully demonstrated for sandwich-type immunoassays (16) and DNA-hybridization assays (13), in which no denaturation of proteins or DNA was observed. It is appropriate to assume that subsequently the antibodies used in this study, TnI and FITC, retained their structures upon exposure to low-power microwave heating.
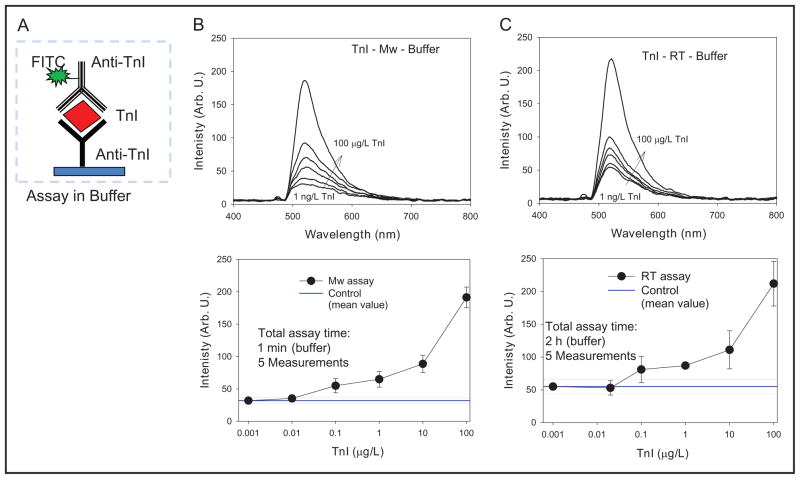
Fig. 5 shows the schematic depiction of the TnI immunoassay run on SNFs (Fig. 5A); TnI-concentration–dependent fluorescence emission spectra (Fig. 5, B and C, top); and TnI-concentration–dependent fluorescence emission intensity at 520 nm (Fig. 5, B and C, bottom) from the TnI immunoassays run in buffer at room temperature and using microwave heating. Fluorescence emission spectra (Fig. 5, B and C, top) revealed that both immunoassays yield similar spectra for the range of concentrations of TnI (1 ng/L to 100 μg/L). A better quantitative comparison of these immunoassays can be made by plotting the peak emission intensity (520 nm) of the spectra vs the concentration of TnI as shown in Fig. 5, B and C, bottom, which reveals that both immunoassays for TnI in buffer yielded nearly identical results. The lower detection limits for the immunoassays run at room temperature and using microwave heating were determined to be 0.1 μg/L and 0.005 μg/L (3 SD above the emission intensity for a 0-concentration sample), respectively. That is, one can detect lower amounts of TnI in buffer using SNFs and microwave heating within minutes than using SNFs and room temperature incubations that take in excess of 2 h to complete.
Fig. 5.
(A), Schematic depiction of the TnI immunoassay run on SNFs in buffer.
(B and C, top), TnI-concentration– dependent fluorescence emission spectra. (B and C, bottom), TnI-concentration– dependent fluorescence emission intensity at 520 nm from the TnI immunoassays run in buffer at room temperature (RT) and using microwave (Mw) heating. Arb. U., arbitrary units.
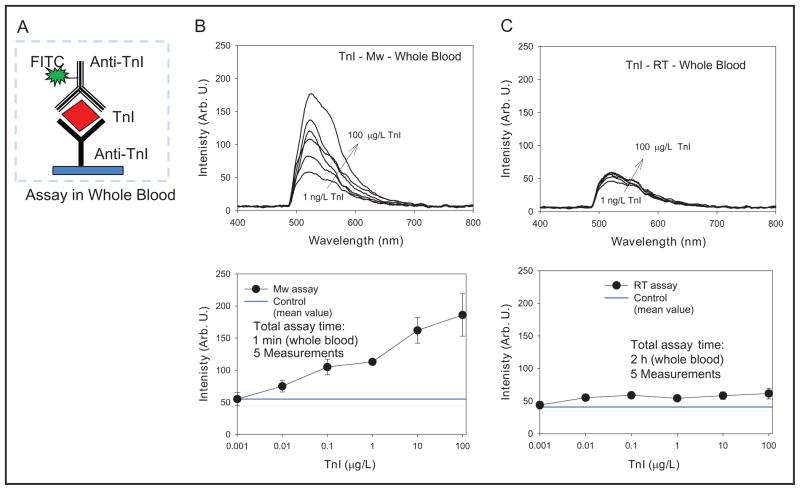
Subsequently, the detection of TnI in human whole blood at room temperature and with microwave heating (Fig. 6) was carried out on the basis of encouraging results obtained with the buffer samples (Fig. 5). It is important to note that the results shown in Figs. 5 and 6 for TnI immunoassay were identical with one exception: a solution of TnI in human whole blood (Fig. 6) was used instead of a solution in buffer (Fig. 5). It is clearly evident that the fluorescence emission spectra (Fig. 6, B and C, top) for the immunoassay run at room temperature and with microwave heating were not the same. Although there is a concentration-dependent increase in the emission spectrum for the immunoassay run using microwave heating, the emission spectra measured from the immunoassay at room temperature are virtually the same for all the concentrations of TnI. That is, TnI was detectable only with the use of the MA-MEF technique. It is important to note that TnI samples in whole blood coagulated within 5 min during the incubation at room temperature. It is thought that the coagulation of whole blood prevented TnI from reaching the capture antibodies on the surface of SNFs, which were washed away after the subsequent washing step. It is important to note that whole blood samples in this study did not contain an anticoagulant agent (e.g., EDTA) because the effects of these chemicals on SNFs are not known.
Fig. 6.
(A), Schematic depiction of the TnI immunoassay run on SNFs in whole blood.
(B and C, top), TnI-concentration– dependent fluorescence emission spectra. (B and C, bottom), TnI-concentration– dependent fluorescence emission intensity at 520 nm from the TnI immunoassays run in buffer at room temperature (RT) and using microwave (Mw) heating. Arb. U., arbitrary units.
The fluorescence emission intensity peak (520 nm) vs TnI concentration plot for the immunoassay run by using microwave heating (Fig. 6B, bottom) shows that the emission intensities for the range of TnI concentrations are well above those measured from control experiments. A signal-to-noise ratio of 3:1 or above is acceptable in fluorescence measurements (18). In contrast, the emission intensities for room temperature immunoassay are close to those values of control experiments (Fig. 6C, bottom). These results show that TnI in human whole blood was detectable with a lower detection level of 0.05 μg/L only with the MA-MEF technique, for which the total assay time was 1 min. In the study we report here we did not attempt to perform a comparison of the data generated for whole blood immunoassays by using the MA-MEF technique with a commercially available instrument for TnI immunoassays, as described in the Introduction, because such a system was not available to us.
The current limitations and the potential applicability of the MA-MEF–based immunoassays in a clinical setting are important considerations. As discussed in Materials and Methods, MA-MEF–based immunoassays use a widely available and inexpensive kitchen microwave (USA price: $40), SNFs, and fluorescence readers. The preparation of SNFs is carried out using a facile and well-established technique, in which single SNFs cost less than $10 in today’s estimate. Although fluorescence readers are also widely available, currently the cost of such a system, including the excitation source (a laser) described in this work, is more than $5000. On the other hand, with the fast-paced advancements in camera technology (e.g., charge-coupled devices) and inexpensive excitation sources (such as light-emitting diodes), one can predict that a significantly less expensive fluorescence reader/excitation system will be available in the near future.
The MA-MEF–based immunoassays can also be carried out with currently available charge-coupled–device cameras and light-emitting–diode excitation sources to convert the fluorescence readout automatically to images by use of digital camera software. Such digital images can also be automatically correlated to the quantitative values of TnI concentrations with the help of the same software. Consequently, it is the authors’ opinion that the potential application of the MA-MEF–based immunoassays for cardiac markers in a clinical setting is nearly a reality. However, additional work (i.e., instrumentation) will be needed to achieve the goal of implementing MA-MEF–based immunoassays in the emergency department, an application that is beyond the scope of current work.
Footnotes
Nonstandard abbreviations: TnI, troponin I; TnT, troponin T; SNF, silver nanoparticle film; SEM, scanning electron microscope.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bio-engineering or NIH.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: National Institute of Biomedical Imaging and Bioengineering, award number 5-K25EB007565-04; American Heart Association.
Expert Testimony: None declared.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
References
- 1.Ellenius J, Groth T, Lindahl B, Wallentin L. Early assessment of patients with suspected acute myocardial infarction by biochemical monitoring and neural network analysis. Clin Chem. 1997;43:919–25. [PubMed] [Google Scholar]
- 2.Panteghini M, Apple FS, Christenson RH, Dati F, Mair J, Wu AH. Use of biochemical markers in acute coronary syndromes. IFCC Scientific Division, Committee on Standardization of Markers of Cardiac Damage. International Federation of Clinical Chemistry. Clin Chem Lab Med. 1999;37:687–93. doi: 10.1515/CCLM.1999.107. [DOI] [PubMed] [Google Scholar]
- 3.Storrow AB, Gibler WB. The role of cardiac markers in the emergency department. Clin Chim Acta. 1999;284:187–96. doi: 10.1016/s0009-8981(99)00080-7. [DOI] [PubMed] [Google Scholar]
- 4.Gibler WB. Understanding true risk: evaluating troponins in the emergency setting. Am Heart J. 1999;137:985–6. doi: 10.1016/s0002-8703(99)70346-7. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien PJ. Blood cardiac troponin in toxic myocardial injury: archetype of a translational safety biomarker. Expert Rev Mol Design. 2006;6:685–702. doi: 10.1586/14737159.6.5.685. [DOI] [PubMed] [Google Scholar]
- 6.Buschmann IR, Bondke A, Elgeti T, Kühnle Y, Dietz R, Möckel M. Positive cardiac troponin I and T and chest pain in a patient with iatrogenic hypothyroidism and no coronary artery disease. Int J Cardiol. 2007;115:e83–5. doi: 10.1016/j.ijcard.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Eggers KM, Oldgren J, Nordenskjöld A, Lindahl B. Diagnostic value of serial measurement of cardiac markers in patients with chest pain: limited value of adding myoglobin to troponin I for exclusion of myocardial infarction. Am Heart J. 2004;148:574–81. doi: 10.1016/j.ahj.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Bange A, Halsall HB, Heineman WR. Microfluidic immunosensor systems. Biosens Bioelectron. 2005;20:2488–503. doi: 10.1016/j.bios.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Hemmila LA. Applications of fluorescence in immunoassays. New York: John Wiley and Sons; 1992. p. 360. [Google Scholar]
- 10.Ozinkas AJ. Principals of fluorescence immunoassay. In: Lakowicz JR, editor. Topics in fluorescence spectroscopy: volume 4: probe design and chemical sensing. New York: Plenum Press; 1994. pp. 449–496. [Google Scholar]
- 11.Van Dyke K, Van Dyke R, editors. Luminescence immunoassay and molecular applications. Boca Raton: CRC Press; 1990. p. 341. [Google Scholar]
- 12.Aslan K. Rapid whole blood bioassays using microwave-accelerated metal-enhanced fluorescence. Nano Biomed Eng. 2010;2:1–9. doi: 10.5101/nbe.v2i1.p1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aslan K, Malyn SN, Geddes CD. Fast and sensitive DNA hybridization assays using microwave-accelerated metal-enhanced fluorescence. Biochem Biophys Res Commun. 2006;348:612–7. doi: 10.1016/j.bbrc.2006.07.093. [DOI] [PubMed] [Google Scholar]
- 14.Aslan K, Geddes CD. Microwave-accelerated metal-enhanced fluorescence (MAMEF): application to ultra fast and sensitive clinical assays. J Fluoresc. 2006;16:3–8. doi: 10.1007/s10895-005-0026-z. [DOI] [PubMed] [Google Scholar]
- 15.Aslan K, Geddes CD. Microwave-accelerated metal-enhanced fluorescence: platform technology for ultrafast and ultrabright assays. Anal Chem. 2005;77:8057–67. doi: 10.1021/ac0516077. [DOI] [PubMed] [Google Scholar]
- 16.Aslan K, Geddes CD. Microwave accelerated and metal enhanced fluorescence myoglobin detection on silvered surfaces: potential application to myocardial infarction diagnosis. Plasmonics. 2006;1:53–9. doi: 10.1007/s11468-006-9006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aslan K, Gryczynski I, Malicka J, Matveeva E, Lakowicz JR, Geddes CD. Metal-enhanced fluorescence: an emerging tool in biotechnology. Curr Opin Biotechnol. 2005;16:55–62. doi: 10.1016/j.copbio.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakowicz JR. Principles of fluorescence spectroscopy. 2. New York: Kluwer Academic; 1999. p. 192. [Google Scholar]