Abstract
To meet the continuous demand for energy, organisms use diverse signals to match food intake with energy needs. This paper reviews the effect of satiation signals and adiposity signals on food intake, including how they interact in the brain and how their influence changes with experience. Whereas meal initiation is influenced by external environmental factors, meal size is influenced by an array of signals that can be partitioned according to their reliability in indicating caloric content of food. It is argued that the malleability of satiation signals renders them poor candidates as pharmacological targets to control body weight.
Introduction
Rapid advances in understanding cell biology and in unraveling the complex neurocircuitry of the brain have led to a wealth of novel findings on the molecular and neural control of food intake and energy homeostasis, and one consequence is that important intersections have arisen among scholars interested in ingestive behavior, neuroscience, cell and molecular biology, and genetics. The accelerating pace at which this is occurring makes it imperative that occasional pauses be interspersed so that new information and its implications can be appropriately interpreted and assimilated. I herein review what is known of the factors that contribute to the control of food intake, emphasizing areas where interpretations based on molecular biology versus those based on behavioral science may lead to different conclusions, as well as the implications for therapy and where research should be directed.
Energy Homeostasis
The brain is a key player in the control of energy homeostasis (Elmquist et al., 2005; Flier, 2004; Schwartz et al., 2000). It receives a continuous stream of diverse signals regarding energy status throughout the body and consequently influences energy consumption as well as the entry of nutrients into the blood and their utilization by most tissues (Figure 1). The brain integrates incoming information in the form of hormonal and neural signals with data on energetic needs or anticipated needs, with environmental factors such as where and when food might be available, with aspects of the social situation, with memory for past experiences, with hedonic factors, and with many others, as well.
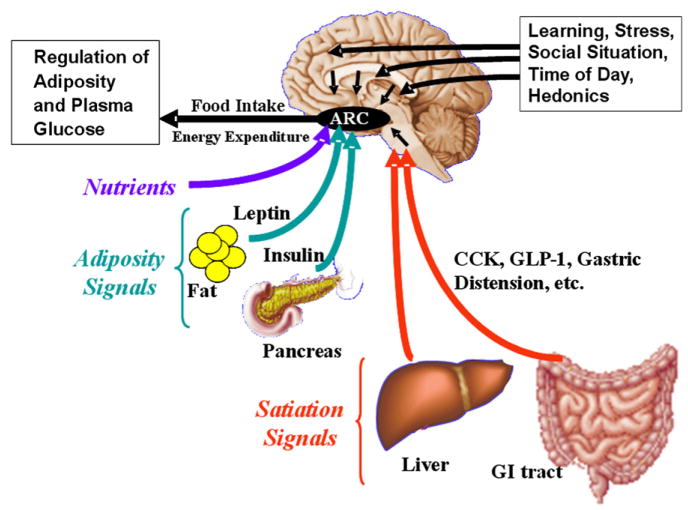
Figure 1. Physiology of Satiation.
Several categories of signals converge on the brain to influence energy homeostasis. Satiation signals such as CCK and GLP-1 arise from the gastrointestinal tract and related organs during meals and are conveyed to the hindbrain. Adiposity signals are hormones whose secretion is proportional to body fat and that stimulate receptors in several areas of the brain, including the hypothalamic arcuate nucleus (ARC). Energy-rich nutrients also provide a direct signal to the ARC. These sensory inputs are integrated with circuits from other brain areas related to cognitive, social, and emotional activities, and the output alters food intake, energy expenditure, and ultimately body adiposity.
An important role of the brain is to ensure adequate circulating energy for immediate tissue needs as well as adequate stored energy to weather intervals when external energy is scarce (Seeley and Woods, 2003). Finding and ingesting food are consequently tightly coordinated with the control of plasma glucose, fatty acids, and other nutrients. Under most circumstances, the levels of energy-rich fuels in the blood are relatively constant, with use by tissues matched well to secretion by liver and adipocytes. Meals constitute an exception as newly digested nutrients enter the blood, raising plasma glucose and other nutrients considerably above basal levels, and these in turn generate signals that are relayed to the brain (Friedman, 1998). An important consideration of the brain is to limit meal size, thus precluding especially large perturbations of plasma nutrients (Woods, 1991; Woods and Ramsay, 2007). This is accomplished in part by accurately anticipating meals and subsequently coordinating ongoing information about calories being consumed (via satiation signals), the levels of fuels already in the plasma (via direct sensing by specialized cells in the brain and elsewhere), and the amount of energy present in various storage depots (via adiposity signals). In sum, a key function of the brain is to coordinate diverse processes that allow optimal circulating and storage levels of energy-rich nutrients.
Satiation Signals
Satiation signals arise during meals and limit meal size (Moran, 2004; Strader and Woods, 2005). The best known is the duodenal peptide cholecystokinin (CCK), which is secreted in proportion to lipids and proteins in the meal and which stimulates receptors on nearby vagal axons (Raybould, 2007). The signal is relayed neurally to the hindbrain and onto diverse brain areas, including the hypothalamus and reward areas. Most satiation signals follow a similar pattern as CCK, either stimulating sensory nerves passing to the hindbrain or else stimulating the hindbrain directly. The signals are integrated in the hindbrain, and digestive reflexes influencing gastrointestinal activity are coordinated and initiated. Satiation signals are also relayed to other brain areas, where they are integrated with adiposity signals, with hedonic and social factors, and with local levels of nutrients (Berthoud, 2007). The net effect is that as satiation signals are generated during a meal, their impact gradually accumulates, ultimately activating circuits that cause individuals to stop eating. This is true even when ample food remains and more could be eaten, in part to prevent extreme postprandial elevations of plasma fuels (Woods, 1991).
Exogenous administration of compounds that stimulate the receptors for endogenous satiation factors cause people or animals to respond as if additional extra calories have been consumed; i.e., they cease eating prematurely and consequently eat less than otherwise. Analogously, when the activity of endogenous satiation factors is experimentally reduced, larger meals are consumed (Moran, 2004; Strader and Woods, 2005). The important point is that when the activity of CCK and/or other satiation signals is manipulated in either direction, the size of the ongoing meal is altered. This is an acute, within-meal phenomenon, and its relevance to more chronic situations and to body weight is discussed below. It is noteworthy that administering CCK prior to the start of a meal does not delay the onset of eating, but rather reduces the amount consumed once eating begins (Moran, 2004; Smith and Gibbs, 1992).
The reliability with which meal size can be acutely manipulated makes it tempting to consider whether satiation factors have therapeutic potential to control body weight; i.e., can formulations of drugs that act on receptors for CCK or any other satiation factor be developed so that the size of every meal is reduced? A first-order answer is that if individuals are coerced to eat smaller meals, they will compensate and defend their body weight by eating more often, and this has been observed when CCK is administered prior to every meal; i.e., animals eat smaller and more frequent meals while keeping body weight constant (West et al., 1984). That said, long-acting formulations of some compounds that reduce meal size when given acutely (e.g., long-acting GLP-1 [D’Alessio and Vahl, 2005]) do result in weight loss (Buse et al., 2007), but it is not clear that the chronic effect is due to continued hypophagia as opposed to other, nonbehavioral actions of the compounds (Woods and D’Alessio, 2008). Pertinent to this, genetically altered animals that uniquely lack CCK (Lo et al., 2008) have normal food intake and body weight, suggesting that CCK is not necessary for normal homeostatic regulation. Further, as discussed below, the ability of satiation factors to influence meal size is plastic and subject to change based on experience. To summarize, satiation signals inform the brain of the quantity and quality of food being eaten, and the brain incorporates this information into a complex interaction with other factors in deciding when to stop the meal.
Adiposity Signals
Adiposity signals, hormones secreted in proportion to body fat that influence the activity of the brain and other tissues, include insulin and leptin (Myers et al., 2007). A higher level of either in the blood is indicative of more stored fat. Each is transported through brain capillaries to gain access to receptors on neurons in the hypothalamus, the hindbrain, and elsewhere (Banks, 2006; Woods et al., 2003). When either insulin or leptin is delivered in a way that increases its activity in the brain, less food is consumed, and when the action of either hormone in the brain is reduced, food intake increases (Schwartz et al., 2000; Woods et al., 1998). Unlike what occurs with satiation signals, chronically changing insulin or leptin activity locally in the hypothalamus changes body weight as well. Animals respond to changes in the activity of adiposity signals as if the amount of fat in their bodies had changed; i.e., they alter food intake and consequently change body fat. The conundrum is that whereas administering adiposity signals to normal-weight individuals reduces food intake and body fat, obese individuals are relatively resistant; i.e., while the obese state is characterized by hyperinsulinemia and hyperleptinemia, it is also characterized by insulin and leptin resistance. One consequence is that chronically administering these compounds, at least systemically, causes little or no loss of weight in obese humans, and chronic insulin can actually increase body weight as intermittent hypoglycemia can elicit persistent overeating (Langhans, 1996).
It should not be inferred that insulin and leptin are either interchangeable or exhaustive as adiposity signals. Each has multiple unique actions throughout the body, and they also differ as adiposity signals. Leptin is disproportionately secreted from subcutaneous fat, whereas insulin secretion reflects mainly visceral fat (Bjorntorp, 1997). Females, whether rodents or humans, have proportionally more subcutaneous and less visceral fat than males (Dusserre et al., 2000), and females have higher plasma leptin and lower plasma insulin than comparably obese males (Clegg et al., 2006). This dichotomy is also manifest in the brain, females being relatively more sensitive to the anorexigenic action of leptin and males more sensitive to insulin (Clegg et al., 2003, 2006), the difference due in part to estrogen action locally in the hypothalamus (Musatov et al., 2007). In some hypothalamic areas, insulin and leptin activate overlapping enzymatic cascades (Air et al., 2002; Niswender and Schwartz, 2003), and the anorexigenic action of each can be blocked, for example, by inhibitors of PI3 kinase or melanocortin 3/4 receptor antagonists (Benoit et al., 2002; Seeley et al., 1997). Hence, while insulin and leptin have many differential actions, they also stimulate common pathways, resulting in reduced food intake and weight loss.
Other hormones are secreted in proportion to body fat and may serve as adiposity signals. Amylin is cosecreted with insulin, and its levels are directly proportional to visceral fat (Cooper, 1994). Amylin reduces food intake and body weight by acting at receptors in the hindbrain, and administration of antagonists that block amylin action increase food intake (Lutz, 2006). Amylin functions both as a satiation signal, reducing meal size, and as an adiposity signal, influencing body weight (Lutz, 2006). Adiponectin is secreted from fat cells in inverse proportion to fat mass (Wang and Scherer, 2008). Although adiponectin has not been reported to alter food intake, it does increase energy expenditure via a central mechanism (Qi et al., 2004). Thus, several peripherally originating hormones are proportional to adipose tissue stores, all gain access to receptors in the brain, and all exert a central influence over energy homeostasis. Insulin and leptin provide useful examples, since they have been most investigated in this regard.
Factors that Influence Meal Size
Determining when and how much to eat are key challenges of the brain. An organism living in a stable environment with ample available food has the luxury of establishing regular eating patterns and optimally integrating caloric intake with other behaviors (Strubbe and Woods, 2004). It can optimize the amount of energy to store as well as how much to eat in individual meals spread over the day. Because its food supply is consistent and reliable, it learns to make responses to take in and process the energy most efficiently (Woods, 1991). Temporal cues dictate the optimal times to eat in relation to other behaviors. Odors and tastes become reliable bellwethers of food quality and energy content, and these relatively distal cues acquire the ability to guide food taking behavior (i.e., how much to eat of a particular food). They are distal cues in the sense that while their presence has been associated with energy content in the past, they have no energy content themselves (Figure 2). In a less predictable environment, the association between distal cues and actual energy content can be tenuous, requiring the individual to rely to a greater extent upon more proximal cues: i.e., with cues more closely tied to actual caloric content. Signals such as CCK are intermediate cues, being secreted in response to a physicochemical analysis of ingested food by intestinal cells. In a stable environment, in which the food is constant, a certain level of CCK activity or of gastric distension accurately presages the number of calories that can be anticipated to enter the blood from the intestines over the next hour or so; nonetheless, activity of CCK or any other “satiation” signal is not necessarily hard-wired to caloric content. The ultimate proximal cues are glucose, fatty acids or other energy-rich molecules reaching a sensory cell, or else the consequences of their intracellular metabolism (i.e., metabolites, altered enzyme activity). The point is that there is an energy-reliability gradient that maps onto cues an individual can use to guide energy intake. In a highly predictable world, distal cues habitually dictate when meals should end.
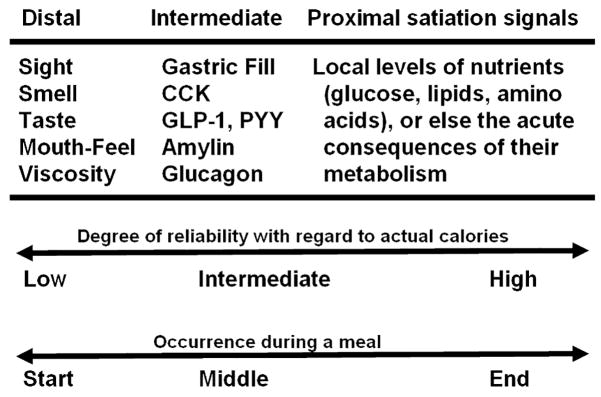
Figure 2. Satiation Signals.
Satiation signals can be partitioned in terms of their relationship to the caloric content of food being eaten. Distal signals such as the taste and smell of food occur relatively early during meals and have a low and variable degree of reliability to caloric content. Intermediate signals such as CCK and GLP-1 are secreted in response to the physicochemical properties of ingested food as it interacts with receptors in the gastrointestinal lumen. Proximal signals are energy-rich nutrients themselves and/or the consequences of their local metabolism in the brain, and they arise later in time than distal or intermediate signals.
This continuum also correlates with time until ingested nutrients enter the blood. An individual using taste or other oral cues can stop eating long before most nutrients are digested and absorbed with the assurance that sufficient but not excessive calories have been gained. As it shifts to using more proximal cues, the lag between ingestion and its consequences lessens. Controlling intake via distal cues bestows the advantage of being able to eat relatively large meals because appropriate anticipatory responses can be made sufficiently far in advance to lessen the meal’s metabolic impact. Anticipatory responses made prior to the actual ingestion of food, such as the secretion of cephalic insulin, reduce the prandial increase of glucose and other nutrients that would otherwise occur (Woods, 1991).
Having to rely upon more proximal cues comes at a cost of risking consuming too many calories at once or else adopting a pattern of eating smaller and more frequent meals, thus perhaps interfering with other behaviors (Woods, 1991; Woods and Ramsay, 2007). The point is that myriad cues may be available to assist in the decision as to when to stop a meal. Distal cues are preferred so long as they reliably predict nutrients, and individuals easily learn to rely upon them. However, in a variable world, individuals revert to cues more closely tied to actual nutrient content. As a common example, animals use taste to guide intake when food is consistent; however, when taste is experimentally dissociated from caloric content, animals abandon it as a cue in deciding when to end a meal (Sclafani, 2006). Analogously, when confronted with novel-tasting food (i.e., food with no previous gustatory associations), individuals are neophobic, eating very little until their more proximal nutrient sensors have a chance to experience and certify the consequences (Rozin, 1968). Similarly, when caloric content is experimentally dissociated from CCK activity, the ability of CCK to reduce meal size becomes attenuated (Duncan et al., 2005; Goodison and Siegel, 1995).
Numerous considerations enter into the determination as to when and how much to eat. When to eat is largely dictated by environmental factors, especially time of day. How much to eat once a meal is underway is governed by a potentially broad array of satiation signals. In a predictable world, individuals learn to utilize signals farther and farther from actual energy content, enabling the consumption of large meals with little metabolic perturbation. In more variable worlds, more proximal cues come into play at the expense of eating smaller meals, requiring the individual to eat more often to meet its energy requirements.
Models of Satiation
Satiation signals could work in many different ways. There may be a satiation threshold such that when an integrated signal (e.g., combinations of taste, gastric distension, CCK, etc.) reaches a certain level of intensity, eating stops (Figure 3). Such a model is implicitly assumed in many experiments, where an administered satiation signal is presumed to push the balance of already-present factors over the stop-eating threshold. While certainly possible, especially if the threshold increases or decreases with body fat, the model nonetheless seems maladaptive, as it could unduly constrain behavior. Thresholds imply a degree of certainty, yet as discussed below, the evidence suggests that satiation signals act in a more probabilistic manner.
Figure 3. Modeling the Satiating Effect of an Exogenous Compound.
Satiation is hypothesized to occur and the ongoing meal therefore hypothesized to end when a satiation threshold is reached. Early in a normal (control) meal (Time A), when not many calories have been consumed, mouth factors (MF) and gastric distension (GD) presumably combine with intermediate signals such as amylin (AMY), glucagon (GL), and CCK, providing an integrated satiation signal insufficient to cause the meal to end. Later during the meal (Time B), other signals such as GLP-1 (GLP) and PYY come online, increasing the total satiation signal. If an exogenous satiation factor such as CCK has been administered (test meal), the combined satiation signal (endogenous plus exogenous factors) is sufficient to reach threshold, and eating stops. Normally, however, more food is consumed (Time C), and other factors such as perhaps nutrients themselves (NUT) enter into the calculus, and the meal ends when the combined endogenous signals reach threshold.
A different model assumes that the sensory inputs that guide eating follow the same principles as those in other sensory systems. Intrinsic signals that influence the start or finish of meals are always present at some level (e.g., there are always, albeit low, basal levels of CCK; and stomach distension is always monitored by the brain), but these signals generally go unheeded unless other factors intervene. Just as occurs for most sensory systems (e.g., olfaction), adaptation occurs until other inputs cause the brain to attend and focus on already-present signals. With regard to satiation, the behavioral act of eating could sensitize brain circuits responsive to CCK and other signals generated during meals, thereby shifting attention to relevant circuits constantly active but normally off the radar screen. Likewise, when to initiate eating would also depend on extrinsic factors causing the brain to focus upon already-present signals. Time of day is one such extrinsic factor. Individuals eat at a particular time because they are accustomed to eat at that time and have learned to make anticipatory responses to cope with the caloric load at that time (Strubbe and Woods, 2004). One consequence is that if always-present signals such as blood glucose, stomach distension, or any other hypothesized “hunger” signal changes at that time as a result of making meal-anticipatory responses, the change can as easily be attributed to knowing a meal is imminent as to a biological need for energy. The point is that anticipating eating, even at a subconscious level, elicits a broad array of endocrine and gastrointestinal changes that are themselves detected and associated with eating. In this schema, rather than a need for energy causing a threshold to be crossed and triggering changes that are then perceived and interpreted as “hunger,” under most circumstances the perception of hunger arises secondarily to the reality that eating normally occurs in that situation.
Behaviors that serve other regulatory systems are analogous to eating. We become sleepy at times we usually go to sleep and wake up at around the same time most days. Certainly other factors intervene. The more chronically deprived one is (of food, sleep, or whatever), the more likely extrinsic signals are to trigger behavior, in part because activity in the appropriate circuits is more intense. When to urinate provides a common example. Neural signals constantly indicate bladder distension, increasing in magnitude gradually as urine accumulates. If they get especially high, the absolute intensity may be sufficient to appear on the conscious radar screen. Prior to that extreme, the slowly changing signals go unheeded unless an extrinsic factor focuses attention on them. For example, some people, when riding in a car and spying a service station, experience an urge to urinate. Rather than their bladder suddenly filling to threshold, an external cue focuses attention on already present signals. The feeling of urgency is real and can be alleviated either by reducing the signal (i.e., urinating) or else by focusing attention elsewhere. In an analogous fashion, some individuals, due to past associations, are especially sensitive to blood glucose, stomach distension, or any other parameter that varies systematically over time since the previous meal or with food deprivation, and some other factor is necessary to enable these always-present messages to be gated through to circuits controlling behavior. These individuals thus detect changes in internal parameters that occur as a consequence of the brain preparing for an impending meal and interpret those changes as “hunger.”
Control of Meal Onset and Meal Size
Meal size is more tightly controlled by homeostatic processes than meal onset because the specific time(s) at which humans initiate meals is/are largely dictated by factors outside those being considered here; i.e., we eat at times convenient for our schedules or because unexpected food becomes available (Strubbe and Woods, 2004). Opportunistic examples of eating, if they become habitual and predictable, fall under the same controls as those discussed above. They also highlight the importance of satiation signals in limiting individual meal size in order to keep daily caloric intake somewhat constant; i.e., since the times that meals occur as well as the number of meals per day may be variable, controlling meal size is an effective strategy for maintaining body weight.
An important goal of experiments evaluating factors that influence food intake is to minimize variance. To that end, subjects are first adapted to the laboratory environment in which light cycle, temperature, and food quality are standardized, consistent, and well controlled. Animals are housed individually to preclude social interactions. In this setting, time of day becomes a potent determinant of meal time, with the two largest meals occurring at the most predictable times; i.e., lights out and lights on (Kissileff, 1970). Laboratory rats and mice develop highly regular, individualized 24 hr eating patterns. Because the food (lab chow or some alternative specified diet) is the same at every meal, taste, mouth-feel, stomach distension, gastrointestinal secretions, and so on are all excellent correlates of ingested calories; i.e., distal cues become as reliable as intermediate or proximal cues in guiding behavior, for all have equivalent predictive power. What varies is the robustness of the link to caloric content.
The observation that one or another factor reliably reduces intake in an acute feeding test should not be taken as evidence that it would persist under chronic conditions. In fact, every time a compound is administered that mimics the action of an endogenous satiation signal and consequently reduces caloric intake, the established association between the signal and ultimate caloric gain is weakened. When repeated over many meals, the individual may simply learn to ignore the elevated signal, at least with regard to changing its behavior. For example, when food is constant from meal to meal, mouth cues become excellent correlates of caloric intake. If the customary food is diluted with nonnutritive filler, animals stop eating after the customary volume of food has passed through the mouth the first time this occurs, thereby failing to acquire the habitual calories. Over the course of a few meals, however, they abandon mouth cues and stomach volume and shift to more proximal signals, consuming enough bulk to get the customary calories (Adolph, 1947). Likewise, sham-eating, in which ingested food exits the body at the stomach, is associated with consuming huge volumes of food; but it takes several days of experience with the sham-feeding paradigm before animals completely abandon mouth cues (Davis and Smith, 1990).
There is an important consideration when evaluating pharmacological agents that act on receptors thought to be important in mediating satiation. Compounds such as CCK have numerous actions throughout the body. Because some of these actions are important in influencing digestion and other processes related to energy homeostasis, changes in the secretion and consequent levels of these compounds have historically been temporally associated with meals, and because the nervous system has receptors to detect the levels of these compounds, the compounds can acquire the ability to act as conditioned stimuli and influence eating behavior when conditions are stable and predictable. Once those criteria are no longer met, however, the ability of the compounds to influence behavior is lost (Duncan et al., 2005; Goodison and Siegel, 1995), although their ability to alter nonbehavioral metabolic processes presumably remains.
Interactions of Satiation Factors
Key questions relate to which signals take precedence when several are present simultaneously, how they interact, and whether some signals are hard-wired to drive consumption in one direction or the other. (Note that in most molecular biology/genetic experiments, environment is held constant, and there is an implicit assumption that what is being investigated is hard-wired.) Another consideration is whether repeatedly administering a compound that elicits premature satiation or that constantly implies elevated body adiposity will cause the compound to become ignored over time, limiting therapeutic potential. Normally the messages related to available nutrients in the blood, to a meal being consumed, and to adipose stores are congruent and the issue is moot, but this need not always hold. For example, what occurs when distal and proximal cues give mixed messages? One obvious possibility is that the more proximal cues should predominate, since they are more closely tied to caloric content. Although few experiments have directly assessed this, intermediate signals do trump distal signals such as taste and other oral factors, for when exogenous CCK is administered to bolster the total CCK signal, animals and people ignore what has passed through the mouth and eat less food. Studies in which nutrients are reduced locally in the hypothalamus at the same time that peripheral satiation signals are increased would be informative on these points.
Proximal Signals
Proximal signals that influence satiation as well as other metabolically relevant processes reflect actual usable energy and include energy-rich molecules (e.g., glucose, fatty acids) that interact with receptors on specialized cells and/or their ability to alter activity of intracellular enzymatic cascades or their metabolites. Receptor cells for proximal satiation signals are located in several regions of the hypothalamus, brainstem, and elsewhere. The enzymatic cascades in these cells that are influenced by nutrients are the subject of intense scientific inquiry in the search for novel therapeutic agents to treat obesity (Kahn and Myers, 2006; Woods et al., 2008). The hypothalamic arcuate nucleus (ARC) is an important site where proximal satiation signals interact with adiposity and other signals, and although the ARC serves as a convenient example in the following discussion, this is not intended to minimize the importance of other brain areas important in energy homeostasis.
The major output of the ARC is a pair of parallel neuronal circuits with functionally opposite actions, one providing an anabolic tone and the other providing a catabolic tone (Seeley and York, 2005). The balance between these two output systems is a major determinant as to whether more food is ingested and more fat laid down (i.e., anabolic activities) or else eating is suppressed and the body relies on stored fat for energy (i.e., catabolic activities). ARC catabolic neurons synthesize pro-opiomelanocortin (POMC), which is processed into α-melanocyte-stimulating hormone (αMSH). αMSH in turn acts at melanocortin (MC) receptors (especially MC4 receptors) in several brain areas to reduce food intake. MC4 agonists elicit hypophagia and weight loss, whereas MC4 antagonists cause hyperphagia and weight gain, and chronic reduction of αMSH activity results in extreme obesity (Naslund and Hellstrom, 2007). ARC anabolic neurons synthesize neuropeptide Y (NPY) and agouti-related protein (AgRP). Although NPY has diverse actions throughout the brain, ARC NPY acts on Y receptors to increase food intake, and if NPY is administered chronically, animals gain body weight (Beck, 2006). AgRP is an antagonist at MC4 receptors, and administration of AgRP or synthetic MC4 antagonists increases food intake and body weight (Flier, 2006).
Both the anabolic and the catabolic ARC circuits are normally active, such that manipulation of either the αMSH catabolic system or the NPY/AgRP anabolic system, in either direction, shifts the balance of control (Schwartz et al., 2000; Seeley and York, 2005). It is as if the ARC is simultaneously applying an accelerator and a brake to circuits controlling energy homeostasis, and that adjustments can be made to strengthen or lessen either side of the equation in the service of homeostasis. Both POMC and NPY/AgRP cells express both leptin and insulin receptors, and increased levels of either insulin or leptin locally in the ARC increase catabolic and decrease anabolic activity; the ability of leptin or insulin to reduce food intake is attenuated when the melanocortin system is blocked (Benoit et al., 2002; Seeley et al., 1997). Analogously, decreased leptin or insulin activity within the ARC increases food intake and weight gain (Munzberg and Myers, 2005; Obici et al., 2002).
Key for consideration of satiation, certain cells, including some ARC neurons, respond to changes in the activity of their own intracellular metabolic processes by generating messages that are passed to other cells and that influence the control of food intake and plasma glucose (Levin et al., 2004). For example, glucose-excited neurons in the ARC synthesize POMC and secrete αMSH (Ibrahim et al., 2003), whereas glucose-inhibited neurons secrete NPY (Muroya et al., 1999), and changes of glucose in the ARC elicit neural reflexes to the liver and the endocrine pancreas to modify plasma glucose. Local increases of oleic acid and some long-chain acetyl-CoAs (Lam et al., 2005) or the branched-chain amino acid leucine (Cota et al., 2006b) also initiate signals in ARC neurons. Hence, ARC neurons have several properties pertinent to the control of food intake. They directly sense and respond to local levels of energy-rich nutrients, they have receptors for adiposity signals, they receive information concerning satiation that is relayed from the hindbrain, and they are the origin of two major pathways influencing homeostatic balance. Thus, any of several possible inputs to the ARC can reduce feeding, including increased nutrients, increased satiation signals, or increased adiposity signals. These same signals also act in the ARC to influence glucose homeostasis.
In sum, numerous signals related to food intake converge on the hypothalamus, including the ARC. These hypothalamic areas comprise an important site of interaction of satiation and adiposity signals, and they modulate distinct anabolic and catabolic circuits to hindbrain areas directing meal size and plasma glucose. Administration of either insulin or leptin into the ARC area reduces food intake and body weight, and this is manifest as reduced meal size. Consistent with this, both insulin and leptin increase sensitivity to satiation factors such as CCK (Matson et al., 2000; Riedy et al., 1995).
Analogous to what occurs at the level of the whole animal, individual ARC neurons respond both to energy-rich proximal signals as well as to an array of distal cues. At the cellular level, distal cues are hormones and neurotransmitters generated in other, often remote, cells or tissues. Insulin is a distal cue, reaching receptors on ARC neurons in proportion to glucose reaching the pancreas in the recent past, and the ARC leptin signal reflects stored fat. One consequence of a change of ARC insulin or leptin signaling is altered food intake. The lag between changes of secretion and altered insulin or leptin signaling in the brain ranges from seconds to minutes or more. Neurotransmitters are distal signals but with a relatively short time constant. The target cell in turn integrates all of these signals and appropriately adjusts its neuronal output as well as its own energy intake, storage, and utilization. In a stable environment, the integrated message to ARC neurons is coordinated and consistent. Increased glucose interacting with receptors on the tongue, intestine, or liver elicits neuronal signals that arrive in the ARC prior to the local influx of new glucose molecules. ARC neurons are thus primed to be responsive to subsequent signals indicating increased available energy (i.e., via signaling from insulin, CCK, or increased local glucose).
Because energy-sensing cells in the ARC (and elsewhere) convert activity in fuel-sensitive enzymatic cascades into a signal that can be transmitted to other cells, intense research effort is aimed at determining precise receptor activity and/or intracellular enzyme activity that influence the output of cells to identify potential therapeutic targets. Hypothetically, the input to and hence output of these cells could be manipulated to provide a false message to brain areas controlling food intake and metabolism; i.e., if pivotal signaling cells could be pharmacologically tricked into responding as if there were more fat in the body than actually exists, or more glucose in the blood, reflexes could be activated to reduce food intake or glucose secretion from the liver.
Like most cells in the body (Woods et al., 2008), ARC energy-sensing neurons have dual intracellular kinase cascades that control their own metabolism, one activated when available energy is low (AMP-activated protein kinase, AMPK) and one when available energy is high (mammalian target of rapamycin, mTOR). Because increased AMPK activity elicits a coordinated pattern of cellular processes to reduce nonessential activities and acquire new energy (Hardie, 2007), AMPK is a proximal signal for insufficient energy. Conversely, mTOR is a proximal signal of increased intracellular energy. Neuronal AMPK is elevated by low glucose and by distal cues, such as AgRP or ghrelin, signifying nutrient need (Minokoshi et al., 2004). These distal signals increase during fasting and decrease upon refeeding, and they stimulate AMPK in ARC neurons. Consistent with this, administration of AgRP or ghrelin locally in the hypothalamus increases food intake. Conversely, leptin and insulin reduce ARC AMPK activity. Increasing AMPK activity in the ARC increases food intake and attenuates leptin anorexia (Minokoshi et al., 2004).
In contrast, ample intracellular energy increases mTOR activity, and the converse is true when energy is low. Like AMPK, mTOR activity is also sensitive to distal cues. Phosphorylation of some downstream products of mTOR occurs only in ARC NPY/AgRP and POMC neurons (Cota et al., 2006a). Thus, mTOR activity in ARC nutrient-sensing neurons varies with feeding status. Fasting decreases ARC mTOR, and refeeding increases it. Increased leptin locally near the ARC phosphorylates the mTOR enzymatic cascade, and the ability of leptin to reduce food intake is attenuated by mTOR inhibitors (Cota et al., 2006a). Thus, the reciprocal activities of ARC AMPK and mTOR are positioned to integrate adiposity signals with local energy availability, including glucose and some fatty acids and amino acids.
Implications
Eating, including both the initiation and termination of meals, is a complex behavior that interacts with the reflexive control of plasma glucose, with the maintenance of body fat, and with many other regulated parameters. Eating itself is not a regulated variable, but rather functions in the service of other parameters. To take body fat as an example, individuals readily abandon a preferred eating pattern when constraints are imposed; i.e., they eat at different times, or more often, or adopt whatever strategy allows them to acquire sufficient calories to maintain body fat (Collier and Johnson, 2004; Woods, 2002).
A broad array of signals influences both the onset and offset of eating, focusing attention on already-present inputs. An important principle is that it is advantageous to accurately know when to start eating and when to stop eating, and the sooner these behaviors can be pinpointed, the better the individual can prepare for the meal’s consequences (Woods, 1991). Hence, signals that herald a meal is imminent elicit cephalic responses, such as increased insulin and ghrelin, prior to the start of the meal (Drazen et al., 2006; Teff, 2000). Time of day is often a key determinant of meal time, and animals can also learn to make meal-anticipatory behaviors in response to arbitrary stimuli that reliably indicate food availability, and those same stimuli can elicit eating, even in sated animals (Sclafani, 1997). The point is that the most reliable indicators of food availability acquire the ability to control meal onset.
With regard to meal size, in most laboratory experiments, signals ranging from taste and smell to mouthfeel, to gastric distension, to CCK, and other gastrointestinal secretions are highly and equally correlated with ingested calories. Hence, the experimental manipulation of any of these signals elicits acute changes of meal size, but at the cost of dissociating the signal from its established link with subsequent metabolic changes. If such trials are interspersed only occasionally, the intervening normal meals serve to restore the signal’s potency. However, if a signal is repeatedly and reliably dissociated from metabolic consequences, animals learn to ignore it, focusing on other more reliable correlates of ingested calories. This is problematic for therapeutic strategies aimed at controlling food intake by mimicking one or another naturalistic satiation signal.
A number of questions follow from this analysis: which signals are hard-wired genetically and/or modifiable by experience? Can any signal, internal or external, be co-opted to influence food intake chronically? Can an animal learn to disregard one or another signal within the ARC: e.g., of glucose, insulin, or AMPK? The answers to such questions would go a long way toward identifying which factors take precedence in the energy homeostatic calculus and would also shed light on which type of signal should be developed if controlling eating is a therapeutic endpoint.
The model presented here has implications for obesity. For example, some believe that the increased burden of everyday stressors is a culprit in stimulating food intake and consequent weight gain (Adam and Epel, 2007). The concept of comfort foods is a case in point (Dallman et al., 2005). When consumed, comfort foods reduce the stress response such that some individuals, faced with unavoidable stressors, self-medicate by ingesting these foods. Comfort foods are often calorically dense and palatable such that their frequent consumption predisposes to obesity. It is worth pondering why comfort food is comforting; i.e., is it because there is a genetic basis for some foodstuffs to activate systems that create pleasant sensations and/or suppress stress? Or is it that individuals have learned what to expect when they eat these foods and that there is comfort in knowing how the metabolic sequellae will play out when they are eaten? It may be that “comfort” is a consequence of being able to rely on distal cues when eating; i.e., in a changing, stressful world, there is an advantage to knowing that nutrients will be handled appropriately in advance of the actual entry of nutrients into the blood, so that effort can be diverted elsewhere to confront life’s other stressors (Ulrich-Lai et al., 2007). The point is that knowing when and how much will be eaten and the metabolic consequences of the act bestows an advantage for coping with life events. Opting for foods or food-related cues with a high degree of predictability may be a logical and economically viable response; it eliminates metabolic surprises.
There are several perspectives from which to consider the act of eating. It can be opportunistic, taking advantage of windfalls and other unpredictable situations. More often eating is habitual, the precise timing and specific eating situations being idiosyncratic and predictable, depending upon an individual’s unique history. A key point is that food intake itself is not a regulated variable but rather assists in the homeostatic maintenance of other variables that are themselves regulated, including body fat and blood glucose. As a result, when the environment allows, individuals have the luxury of adopting regular eating patterns that reflect a balance among many factors, including food availability, the social situation, and others. However, if constraints are superimposed on meal taking, animals abandon their preferred eating pattern in order to protect critical regulated parameters. For example, rats with food freely available prefer to spread their intake out, consuming numerous relatively small meals each day (Collier and Johnson, 2004). When required to exert considerable effort to gain access to their food, but able to eat as much as desired once food is available, they change their strategy, consuming a few large meals each day such that total daily intake and body weight remain constant. Likewise, if limits are placed on meal size, animals respond by eating more meals each day, again defending body weight (West et al., 1984).
If blood glucose (or glucose utilization) is acutely and severely reduced, eating is elicited (Langhans, 1996). This is generally considered an emergency response, since normal meals occur at glucose levels considerably above those extremes. Nonetheless, if glucopenic eating is repeated on a chronic basis, the enhanced daily caloric intake is sufficient to cause weight gain (Langhans, 1996). Thus, although the maintenance of body weight and blood glucose both interface with eating behavior, protecting blood glucose takes priority over maintaining a desired body weight when the two are pitted against each other. The point is that the size and pattern of meals are flexible and often represent compromises among the regulation of several variables.
Thus, if designing pharmacotherapies to treat obesity is a goal, targeting meal size is not an optimal strategy, as other factors can intervene and interfere with the ultimate goal. A better strategy is to take advantage of the fact that food intake is reflexively reduced when the brain senses that the body is excessively fat. Signals such as insulin and leptin stimulate the brain in proportion to body fat, an increased signal activating catabolic circuits and a decreased signal activating anabolic circuits (Schwartz et al., 2000; Woods et al., 1998). This negative feedback aspect of body fat regulation is considered a major factor in the stability of weight over time and for the inevitable regain of body weight lost after dieting. The same process could presumably be harnessed to reduce body weight in obese individuals.
What is key regarding the weight-regulatory system is that its impact does not appear to dissipate over time; i.e., animals with a reduced leptin or insulin signal locally in the brain maintain a chronically elevated body weight, and elevating insulin or leptin locally in the brain causes weight loss as long as the treatment is maintained. What is pertinent is that food intake can be co-opted to achieve and maintain a particular level of weight. For example, animals typically eat less food when the insulin signal is increased in the brain (Schwartz et al., 2000; Woods et al., 1979, 1998). However, this does not mean that insulin causes an obligatory decrease of food intake in the brain. For whereas normal-weight controls eat less and lose weight when administered insulin into the brain, rats that have already lost significant weight actually increase their food intake when administered insulin into the brain, bringing their weight to the same level achieved by the controls receiving insulin (Chavez et al., 1995). The point is that the insulin seen by the brain dictated a certain level of body weight to be achieved, and changes of food intake, whether decreases or increases, were used to attain that weight.
The implication is that therapeutic strategies that mimic the action of adiposity signals in the brain are much more likely to accomplish desired endpoints than strategies targeting food intake per se. As discussed above, the factors that control individual meals are malleable, and meal patterns themselves are readily changed. Providing a signal to the brain that excess fat exists in the body allows homeostatic controls to rein in food intake in an optimal way.
Conclusions
In order to meet the continuous demand for energy, organisms utilize diverse signals at both the organismic and the cellular levels to optimize energy homeostasis. The coordinated regulation of these processes relies upon opposing effector systems at every level. Nutrient-sensing neurons in the ARC and elsewhere integrate distal and proximal signals to control their own metabolism as well as to generate signals transmitted to other cells, thereby changing the balance of anabolic and catabolic circuits directing behavioral, endocrine, and autonomic responses. The dual neuronal-circuitry response is mirrored by opposing intra-cellular enzymatic cascades (AMPK and mTOR), whose activity in turn affects food intake and body weight. An important point is that other factors, such as exposure to a high-fat diet, influence the sensitivity of these hypothalamic systems to hormonal and nutrient signals, predisposing to weight gain and/or dysregulated glucose. Considering the urgency that our society faces in curtailing the epidemics of obesity and type 2 diabetes, intense research is needed to understand how dietary constituents and energy content influence hypothalamic sensitivity to homeostatic signals.
Few behaviors of advanced organisms are simple knee-jerk reactions to sudden inputs or changes. Regulatory behaviors in particular must be plastic and adaptable in order to provide maximum flexibility for coping with a variable world. Eating is a behavior that serves many masters, including maintaining long-term energy stores with the least perturbation to circulating glucose and other fuels. Eating must be sufficiently opportunistic to take advantage of environmental windfalls and at the same time be integrated and coordinated with the myriad other behaviors in the individual’s repertoire. It would be maladaptive to constrain such an enterprise by making it a slave to specific stimuli relevant to one or another aspect of metabolism, and there is compelling evidence that this is not in fact how it works. Rather than being tied to specific stimuli, both the onset and termination of meals are able to exploit whichever signals guarantee the best outcomes, and these vary with past reliability and experience. Because of this, uninformed pharmacological approaches to control eating chronically may prove fruitless, as the chronically treated individual could easily switch to more reliable cues; i.e., a deeper understanding of the system will be necessary to provide insights as to how the system can be exploited to achieve metabolic if not behavioral endpoints.
Acknowledgments
Consideration of these topics benefited from discussion with many individuals, including Randy Seeley, Douglas Ramsay, Randall Sakai, Debbie Clegg, Stephen Benoit, Dan Porte, Michael Schwartz, Wolfgang Langhans, Nori Geary, Thomas Lutz, Gertjan van Dijk, Anton Scheurink, Mary Dallman, Elissa Epel, Daniela Cota, Matthias Tschöp, Silvana Obici, David D’Alessio, Patrick Tso, James Herman, Yve Ulrich-Lai, and Ed Stricker. This work was supported in part by DK 067550 and DK 017844.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Adolph EF. Urges to eat and drink in rats. Am J Physiol. 1947;151:110–125. doi: 10.1152/ajplegacy.1947.151.1.110. [DOI] [PubMed] [Google Scholar]
- Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology. 2002;143:2449–2452. doi: 10.1210/endo.143.6.8948. [DOI] [PubMed] [Google Scholar]
- Banks WA. The blood-brain barrier as a regulatory interface in the gut-brain axes. Physiol Behav. 2006;89:472–476. doi: 10.1016/j.physbeh.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Beck B. Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos Trans R Soc Lond B Biol Sci. 2006;361:1159–1185. doi: 10.1098/rstb.2006.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav. 2007;91:486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Hormonal control of regional fat distribution. Hum Reprod. 1997;12:21–25. doi: 10.1093/humrep/12.suppl_1.21. [DOI] [PubMed] [Google Scholar]
- Buse JB, Klonoff DC, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Maggs DG, Wintle ME. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther. 2007;29:139–153. doi: 10.1016/j.clinthera.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats. Behav Neurosci. 1995;109:528–531. doi: 10.1037//0735-7044.109.3.528. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Collier G, Johnson DF. The paradox of satiation. Physiol Behav. 2004;82:149–153. doi: 10.1016/j.physbeh.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Cooper GJ. Amylin compared with calcitonin gene-related peptide: structure, biology, and relevance to metabolic disease. Endocr Rev. 1994;15:163–201. doi: 10.1210/edrv-15-2-163. [DOI] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006a;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Cota D, Proulx K, Woods SC, Seeley RJ. Role of leucine in regulating food intake. Science. 2006b;313:1236–1238. doi: 10.1126/science.313.5791.1236b. [DOI] [PubMed] [Google Scholar]
- D’Alessio DA, Vahl TP. Utilizing the GLP-1 signaling system to treat diabetes: sorting through the pharmacologic approaches. Curr Diab Rep. 2005;5:346–352. doi: 10.1007/s11892-005-0092-2. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Learning to sham feed: behavioral adjustments to loss of physiological postingestional stimuli. Am J Physiol. 1990;259:R1228–R1235. doi: 10.1152/ajpregu.1990.259.6.R1228. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- Duncan EA, Davita G, Woods SC. Changes in the satiating effect of cholecystokinin over repeated trials. Physiol Behav. 2005;85:387–393. doi: 10.1016/j.physbeh.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Dusserre E, Moulin P, Vidal H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim Biophys Acta. 2000;1500:88–96. doi: 10.1016/s0925-4439(99)00091-5. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- Flier JS. AgRP in energy balance: will the real AgRP please stand up? Cell Metab. 2006;3:83–85. doi: 10.1016/j.cmet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Friedman MI. Fuel partitioning and food intake. Am J Clin Nutr. 1998;67(Suppl 3):513S–518S. doi: 10.1093/ajcn/67.3.513S. [DOI] [PubMed] [Google Scholar]
- Goodison T, Siegel S. Learning and tolerance to the intake suppressive effect of cholecystokinin in rats. Behav Neurosci. 1995;109:62–70. [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Myers MG., Jr mTOR tells the brain that the body is hungry. Nat Med. 2006;12:615–617. doi: 10.1038/nm0606-615. [DOI] [PubMed] [Google Scholar]
- Kissileff HR. Free feeding in normal and “recovered lateral” rats monitored by a pellet-detecting eatometer. Physiol Behav. 1970;5:163–173. doi: 10.1016/0031-9384(70)90060-0. [DOI] [PubMed] [Google Scholar]
- Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- Langhans W. Metabolic and glucostatic control of feeding. Proc Nutr Soc. 1996;55:497–515. doi: 10.1079/pns19960044. [DOI] [PubMed] [Google Scholar]
- Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53:2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- Lo CM, Samuelson LC, Chambers JB, King A, Heiman J, Jandacek RJ, Sakai RR, Benoit SC, Raybould HE, Woods SC, Tso P. Characterization of mice lacking the gene for cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2008;294:R803–R810. doi: 10.1152/ajpregu.00682.2007. [DOI] [PubMed] [Google Scholar]
- Lutz TA. Amylinergic control of food intake. Physiol Behav. 2006;89:465–471. doi: 10.1016/j.physbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Matson CA, Reid DF, Cannon TA, Ritter RC. Cholecystokinin and leptin act synergistically to reduce body weight. Am J Physiol. 2000;278:R882–R890. doi: 10.1152/ajpregu.2000.278.4.R882. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Moran TH. Gut peptides in the control of food intake: 30 years of ideas. Physiol Behav. 2004;82:175–180. doi: 10.1016/j.physbeh.2004.04.048. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- Muroya S, Yada T, Shioda S, Takigawa M. Glucose-sensitive neurons in the rat arcuate nucleus contain neuropeptide Y. Neurosci Lett. 1999;264:113–116. doi: 10.1016/s0304-3940(99)00185-8. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2007;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- Naslund E, Hellstrom PM. Appetite signaling: from gut peptides and enteric nerves to brain. Physiol Behav. 2007;92:256–262. doi: 10.1016/j.physbeh.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. 2003;24:1–10. doi: 10.1016/s0091-3022(02)00105-x. [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- Raybould HE. Mechanisms of CCK signaling from gut to brain. Curr Opin Pharmacol. 2007;7:570–574. doi: 10.1016/j.coph.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedy CA, Chavez M, Figlewicz DP, Woods SC. Central insulin enhances sensitivity to cholecystokinin. Physiol Behav. 1995;58:755–760. doi: 10.1016/0031-9384(95)00108-u. [DOI] [PubMed] [Google Scholar]
- Rozin P. Specific aversions and neophobia resulting from vitamin deficiency or poisoning in half-wild and domestic rats. J Comp Physiol Psychol. 1968;66:82–88. doi: 10.1037/h0025974. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Learned controls of ingestive behavior. Appetite. 1997;29:153–158. doi: 10.1006/appe.1997.0120. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Oral, post-oral and genetic interactions in sweet appetite. Physiol Behav. 2006;89:525–530. doi: 10.1016/j.physbeh.2006.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley RJ, Woods SC. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci. 2003;4:901–909. doi: 10.1038/nrn1245. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, York DA. Fuel sensing and the central nervous system (CNS): implications for the regulation of energy balance and the treatment for obesity. Obes Rev. 2005;6:259–265. doi: 10.1111/j.1467-789X.2005.00193.x. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW. Melanocortin receptors in leptin effects. Nature. 1997;390:349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- Smith GP, Gibbs J. The development and proof of the cholecystokinin hypothesis of satiety. In: Dourish CT, Cooper SJ, Iversen SD, Iversen LL, editors. Multiple Cholecystokinin Receptors in the CNS. Oxford: Oxford University Press; 1992. pp. 166–182. [Google Scholar]
- Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–191. doi: 10.1053/j.gastro.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Woods SC. The timing of meals. Psychol Rev. 2004;111:128–141. doi: 10.1037/0033-295X.111.1.128. [DOI] [PubMed] [Google Scholar]
- Teff K. Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite. 2000;34:206–213. doi: 10.1006/appe.1999.0282. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–1834. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51:8–14. doi: 10.1161/HYPERTENSIONAHA.107.099424. [DOI] [PubMed] [Google Scholar]
- West DB, Fey D, Woods SC. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am J Physiol. 1984;246:R776–R787. doi: 10.1152/ajpregu.1984.246.5.R776. [DOI] [PubMed] [Google Scholar]
- Woods SC. The eating paradox: how we tolerate food. Psychol Rev. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- Woods SC. The house economist and the eating paradox. Appetite. 2002;38:161–165. doi: 10.1006/appe.2001.0468. [DOI] [PubMed] [Google Scholar]
- Woods SC, Ramsay DS. Homeostasis: beyond Curt Richter. Appetite. 2007;49:388–398. doi: 10.1016/j.appet.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93:S37–S50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intra-cerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Cota D. Regulation of food intake through hypothalamic signaling networks involving mTOR. Annu Rev Nutr. 2008;28:295–311. doi: 10.1146/annurev.nutr.28.061807.155505. [DOI] [PubMed] [Google Scholar]