Abstract
A rapid, selective, and sensitive gradient HPLC method was developed for the analysis of dissolution samples of levothyroxine sodium tablets. Current USP methodology for levothyroxine (l-T4) was not adequate to resolve co-elutants from a variety of levothyroxine drug product formulations. The USP method for analyzing dissolution samples of the drug product has shown significant intra- and inter-day variability. The sources of method variability include chromatographic interferences introduced by the dissolution media and the formulation excipients. In the present work, chromatographic separation of levothyroxine was achieved on an Agilent 1100 Series HPLC with a Waters Nova-pak column (250mm × 3.9mm) using a 0.01 M phosphate buffer (pH 3.0)–methanol (55:45, v/v) in a gradient elution mobile phase at a flow rate of 1.0 mL/min and detection UV wavelength of 225 nm. The injection volume was 800 µL and the column temperature was maintained at 28 °C. The method was validated according to USP Category I requirements. The validation characteristics included accuracy, precision, specificity, linearity, and analytical range. The standard curve was found to have a linear relationship (r2 > 0.99) over the analytical range of 0.08–0.8 µg/mL. Accuracy ranged from 90 to 110% for low quality control (QC) standards and 95 to 105% for medium and high QC standards. Precision was <2% at all QC levels. The method was found to be accurate, precise, selective, and linear for l-T4 over the analytical range. The HPLC method was successfully applied to the analysis of dissolution samples of marketed levothyroxine sodium tablets.
Keywords: Levothyroxine, Dissolution, Drug product, HPLC, Validation
1. Introduction
Levothyroxine sodium (l-3,5,3′,5′-tetraiodothyronine sodium salt) pentahydrate (Fig. 1a) is the sodium salt of the levo-isomer of thyroxine (T4) (Fig. 1b), and is the primary hormone secreted by the thyroid gland to regulate metabolic processes and physical development [1]. Levothyroxine sodium serves as a replacement therapy for the inadequate secretion of T4 in the body and is commonly used to treat hypothyroidism, simple non-endemic goiters, and chronic lymphocytic thyroiditis, thyroxine, a prohormone and iodothyronine (T3) the more active form produced from T4, are solely responsible for the normal development of the central nervous system in infants, and the regulation of the normal functioning of multiple organ systems in adults [2].
Fig. 1.
Structures of (a) levothyroxine sodium pentahydrate and (b) thyroxine.
Levothyroxine sodium is a white to pale, odorless, tasteless, hygroscopic, crystalline powder. It is slightly soluble in water and alcohols, and insoluble in acetone, chloroform, and ether [3]. Levothyroxine has three ionizable groups: carboxyl group (pKa1 = 2.40), phenolic group (pKa2 = 6.87) and amino group (pKa3 = 9.96) and can exist as a cation, zwitterion, or anion [4]. The aqueous solubility of levothyroxine decreases as pH increases from 1 to 3 and begins to increase above pH 7 [5].
It has been suggested that the two main problems with achieving clinical efficacy of levothyroxine are: (1) compliance to long-term treatment and (2) inadequate bioequivalence, which may result from a lack of product quality of commercial formulations [6]. Levothyroxine has also been a major topic of discussion at FDA Advisory Committee meetings, where the clinical consequences of marketing products with approved specifications limits of 90–110% has been reported as an ongoing problem [7]. Therefore, formulation characterization and the assessment of product quality with in vitro methods, such as dissolution, are essential.
The accurate determination of levothyroxine in complex media such as dissolution requires selective and sensitive analytical methodologies. There are various analytical methods cited in the literature used for the quantitative determination for levothyroxine for pharmaceutical purposes. Examples include isotope dilution tandem mass spectroscopy [8], capillary electrophoresis [9], high performance liquid chromatography [10–15], spectrophotometry [16], inductively coupled plasma (ICP) mass spectrometry [17,18], liquid chromatography/tandem mass spectrometry [19], and liquid chromatography using electrochemical and MS detection [20]. None of the HPLC methodologies were applied to dissolution studies of multiple drug product formulations for product quality assessment. Additionally, past HPLC methods for the quantitative determination of T4 have suffered from an inherent lack of sensitivity resulting from the low molar absorptivity coefficient of T4. The issue of analytical sensitivity for the dissolution test has been further complicated by the low dose strength (30–300 µg) of levothyroxine formulations.
The current method employed by USP 32/NF 27 for dissolution samples is a direct injection procedure, however, the samples contain high amounts of surfactant present in the dissolution medium (0.2% sodium lauryl sulfate in 0.01% HCl) and can result in specificity issues that could impact the accurate determination of levothyroxine in certain levothyroxine drug products. Tzanavaras notes that a key determinant of the reliability of results for dissolution tests is the validity of the analytical methodology used to accurately determine the active pharmaceutical ingredient in test samples [21]. Therefore, valid analytical methodology for accurate dissolution testing is critical since sometimes in vitro measurements are an indirect measure of the in vivo activity, as well as quality control measures for a batch release.
Methodology issues are further highlighted by the fact that bioavailability and bioequivalence problems are still arising, which seem to be a result of formulation variability. Moreover, levothyroxine is a narrow therapeutic index drug, so products differing by as much as 10% can result in a large negative impact on patients who are at risk for over or under treatment when administering the medication. Therefore, a selective and sensitive HPLC method for the analysis of dissolution samples was developed. The method was used successfully to evaluate dissolution samples of five marketed levothyroxine drug products.
2. Materials and methods
2.1. Materials
l-Thyroxine sodium (l-T4) certified reference standard was purchased from the United States Pharmacopeia (Rockville, MD, USA). Levothyroxine sodium tablets were purchased from a local CVS Caremark pharmacy (Silver Spring, MD, USA); Sandoz, Mova Pharmaceutical Corporation (Caguas, Puerto Rico); Synthroid, Abbott Laboratories (North Chicago, IL, USA); Mylan, Mylan Pharmaceuticals, Inc. (Morgantown, WV, USA); Levothroid, Forest Pharmaceuticals, Inc. (Saint Louis, MO, USA); Levoxyl, King Pharmaceuticals (Bristol, TN, USA). Acrodisc CR 25mm syringe filters were purchased from the Pall Corp. (Ann Arbor, MI, USA). HPLC grade potassium phosphate monobasic, ACS grade phosphoric acid and ACS grade hydrochloric acid were purchased from Fisher Scientific (Fairlawn, NJ, USA). Sodium lauryl sulfate was purchased from Sigma (St. Louis, MO, USA). HPLC ready deionized 18MΩ water was obtained, in-house, from aMilli-Q Gradient A-10 water purification system, Millipore, (Bedford, MA, USA).
2.2. Dissolution
A calibrated dissolution apparatus (USP II) was used with paddles at 50 and 75rpm depending on the specification of the drug product tested. The bath temperature was maintained at 37 ± 1 °C. Five hundred milliliters of freshly prepared and degassed 0.01% HCl solution was prepared and 0.2% sodium lauryl sulfate added for the dissolution medium.
Five drug products (6 tablets per manufacturer) were evaluated and dissolution samples were collected at 15 and 45 min. At each time point, a 2 mL sample was removed from each vessel using a glass syringe and filtered through an acrodisc filter (0.45 µm, 25 mm) into labeled glass tubes. One milliliter is removed and transferred to the HPLC vial. Next, 1 mL of 0.01 M phosphate buffer (pH = 3.0):methanol solution (45:55) is added to the HPLC vial, vortexed, and analyzed by HPLC.
The amount of levothyroxine in the test samples was calculated, as quantity and percent dissolution, from the measured peak area response for the test samples (ru) and compared to peak area response (rs) for the standard levothyroxine solution using the following equations:
where C is the concentration in µg/mL of the USP levothyroxine reference standard and 798.85 and 776.87 are the molecular weights of levothyroxine sodium and levothyroxine.
2.3. Instrumentation and chromatographic conditions
An Agilent 1100 series HPLC (Wilmington, DE, USA) consisted of a quaternary pump, an automatic injector, variable wavelength detector, and a column oven. Data was collected using Agilent ChemStation software. Separation was achieved on a Waters Novapak C18 column (3.9mm × 150mm, 4 µm) fitted with a Waters Nova-pak guard column (3.9mm × 20 mm). The flow rate was 1.0 mL/min. The chromatographic conditions: 0.01 M phosphate buffer (pH = 3.0) (A) and methanol (B) from 45 to 20% A in 7 min, at 20% A from 7 to 12 min, from 20 to 45% A from 12 to 16 min, and at 45% A to 20 min as equilibration time. The column temperature was controlled at 28 °C and the injection volume was 800 µL. The UV detection wavelength was 225 nm.
2.4. Preparation of standard solutions
Levothyroxine stock solutions I and II were prepared in 100% methanol and sonicated for 10 min to obtain stock solution concentrations of 100 µg/mL. From these individual stock solutions, 10 mL was transferred to 100 mL amber volumetric flasks and filled to volume with dissolution media for two separate working solutions of 10 µg/mL.
2.4.1. Preparation of levothyroxine calibration standards
Levothyroxine stock I and mixture of 0.01 M phosphate buffer:MeOH solution (45:55) (1:1, v/v) was used to prepare a working standard of 4 µg/mL. Calibration standard solutions were prepared daily at 5 concentrations by diluting the working standard to concentrations of 0.08, 0.2, 0.4, 0.5, and 0.8 µg/mL. Standard solutions were then transferred to the autosampler for HPLC analysis. Standard solutions were analyzed in duplicate.
2.4.2. Preparation of levothyroxine quality control standards
Levothyroxine stock II and mixture of 0.01 M phosphate buffer:MeOH solution (45:55) (1:1, v/v) was used to prepare a working standard of 4 µg/mL. Quality control (QC) standards solutions were prepared by diluting the working standard to final QC concentrations of 0.08, 0.4, and 0.8 µg/mL. QC solutions were then transferred to an automatic injector for HPLC analysis. Each QC solution was analyzed at a low and high concentration three times. The intermediate QC was analyzed six times.
2.5. Method validation
The method was validated according to the United States Pharmacopeia Category I requirements. The following validation characteristics were addressed: accuracy, precision, linearity, range, and specificity.
2.5.1. System suitability standard
System suitability standard solution was prepared daily using stock solution I and mixture of 0.01 M phosphate buffer:MeOH solution (45:55) (1:1, v/v) to prepare a 0.4 µg/mL solution. System suitability was determined from six replicate injections of the system suitability standard before sample analysis. The acceptance criteria were less than 2% relative standard deviation (RSD) for peak area, greater than 3000 column plates, USP tailing factor less than 1.5, and capacity factor (k′) greater than 3.0.
2.5.2. Linearity and range
Standard calibration curves were prepared with five calibrators over a concentration range of 0.08–0.8 µg/mL (0.08, 0.2, 0.4, 0.5, and 0.8 µg/mL) for levothyroxine. The data of peak area versus drug concentration were treated by linear least square regression analysis. The standard curves were evaluated for intra- and inter-day linearity. The analytical range was established by the highest and lowest concentrations of analyte where acceptable linearity, accuracy and precision were obtained.
2.5.3. Accuracy and precision
Accuracy and precision of the method were determined for levothyroxine by analyzing the QC standard samples at three concentrations of levothyroxine (low QC 0.08, intermediate QC 0.4, and high QC 0.8 µg/mL). The method precision was established by six injections of the standard QC sample at a 0.4 µg/mL concentration level for the intra-day precision and on 3 days for the intermediate precision. Precision was expressed as a coefficient of variation percentage (CV%) of the analyte peak. Accuracy was determined by the three QC standards and evaluated for 3 days as an average drug content percentage.
2.5.4. Specificity
Specificity of the method was determined by analyzing the system suitability standard plus drug products by various manufacturers. All chromatograms were examined to determine if the active compound had any co-elution with the surfactant peak from the dissolution medium.
2.5.5. Dissolution analysis of drug products
Analysis was performed on five marketed levothyroxine drug products using a Van Kel (VK 7000) dissolution apparatus. The dissolution procedure was performed using the USP 32/NF 27 method for levothyroxine sodium tablets. All products were run using the paddle method at 50 rpm except one product with a release specification of 75 rpm. The USP requires that only one dissolution time point be used for analysis; however, this method tested two time points for verification.
3. Results and discussion
3.1. Optimization of analytical method
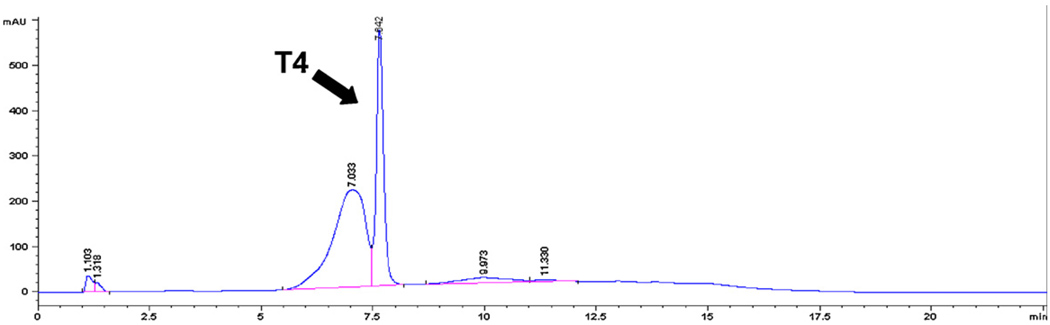
The purpose of this study was to address the chromatographic issues associated with resolving the levothyroxine peak from dissolution media constituents following direct injection. The initial chromatographic studies utilized the current USP method. It was observed that there were several confounding issues which included baseline stabilization difficulties, peak broadening, ionization, column chromatography selectivity, peak symmetry, injection volume, and co-elution of interferring peaks (Fig. 2). The current USP mobile phase consisted of an isocratic, filtered and degassed mixture of methanol and 0.1% phosphoric acid (60:40). With this method we found that peak broadening and co-elution were occurring between levothyroxine and surfactant from the dissolution media. After several attempts it was concluded that there is difficulty in consistently and effectively eluting the compound of interest utilizing the USP dissolution method for a variety of levothyroxine drug products.
Fig. 2.
Chromatogram of a drug product dissolution sample for current USP method.
Analytical considerations included a long equilibration (>3 h) which was required in order to establish a stable baseline for the USP method. This resulted in a lengthier run time overall, negatively impacting dissolution samples that are to be collected and analyzed. Baseline variability was a major issue that occurred due to the presence of the surfactant in the dissolution media. In order to try and resolve the baseline issues the isocratic method was converted to a gradient method, followed by modification of the gradient composition. Since levothyroxine is strongly retained using reverse-phase chromatography, an efficient method was needed to assure adequate baseline separation of the levothyroxine peak with no co-elution from components of dissolution media or formulation excipients from a variety of levothyroxine drug products. A gradient method was employed using 0.01 M phosphoric acid (pH = 3.0) (A) and methanol (B) from 55 to 80% B in 7 min, at 80% B from 7 to 12 min, from 80 to 55% B from 12 to 16 min, and at 55% B to 20 min as equilibration time was utilized. Converting to the gradient method immediately resolved the baseline stability issues as well as surfactant interference while establishing resolution and eluting levothyroxine efficiently.
Although resolution had been achieved under the gradient conditions, ionization issues were also observed in the chromatography using the USP phosphoric acid concentration of 0.01 M. To remedy this problem, a series of studies were conducted modifying the phosphoric acid concentration of the mobile phase. The experiment was conducted using 4 different phosphoric acid concentrations, individually, along with methanol using the newly developed gradient method: 0.1, 0.15, 0.2, and 0.25% with pH levels of 2.3, 2.1, 2.0, and 1.75, respectively. After evaluating all chromatography using the different acid concentrations 0.25% H3PO4 was found to be the most suitable, enhancing resolution between the levothyroxine and surfactant peak as well as improving peak symmetry. However, H3PO4 did not act as an effective buffer in the presence of pH modifiers that were present in the dissolution media and the formulations of drug products. Therefore, a buffer was needed that would not be so susceptible to changes in pH by these modifiers. A phosphate buffer (pH = 3.0) was utilized, which allowed for a more stable pH with minimal ionization and retention time shifting of the levothyroxine peak. The sample was diluted in the mobile phase buffer to reduce the negative affect of the surfactant on peak symmetry and column performance. It should be noted that plate counts decreased after a number of days resulting from the surfactant present in the dissolution.
To evaluate method sensitivity, injection volume tests were conducted at 400, 500, 600, 700, and 800 µL. Since levothyroxine has a relatively low extinction coefficient, it is necessary to optimize sensitivity while maintaining peak symmetry. It was determined that sample injection volumes of 800 µL provided the best results for dissolution sampling at both the early and later time points. Although levothyroxine sodium is present at very low concentrations in dissolution media, the detection and quantitation was successfully achieved due to the large on column injection volume and adequate peak symmetry by UV detection at 225 nm.
The optimization goal was to develop a simple chromatographic method to resolve the levothyroxine peak without co-elution with excipients incorporated in the various dosage forms.
3.2. Method validation
The following method validation characteristics were addressed for levothyroxine: accuracy, precision, specificity, linearity, and range. The validation characteristics met the acceptance criteria for USP Category I.
3.2.1. System suitability
The system suitability test ensures the validity of the analytical procedure as well as confirms the resolution between different peaks of interest. All critical parameters tested met the acceptance criteria on all days (Table 1). The system suitability assessment for the analytical HPLC method established instrument performance parameters such as retention time, peak area, capacity factor, and USP tailing factor for levothyroxine peak. All parameters maintained a %RSD of <1, respectively. All critical parameters tested met the acceptance criteria on all days.
Table 1.
System suitability test results.
| Parameters | Specifications | Day 1 | Day 2 | Day 3 |
|---|---|---|---|---|
| Retention time (% RSD) | ≤2.0 | 0.65 | 0.75 | 0.12 |
| Capacity factor (k′) | >3.0 | 5.64 | 5.70 | 5.63 |
| Area (% RSD) | ≤2.0 | 0.95 | 0.84 | 0.42 |
| Plates (column) | >3000 | 43,725 | 30,900 | 28,200 |
| USP tailing | <1.5 | 1.24 | 1.43 | 1.39 |
3.2.2. Linearity and range
Linearity of the method was confirmed by preparing levothyroxine standard curves for the analytical range of 0.08–0.8 µg/mL. A correlation between analyte peak area and concentration of the drug was observed with r2 ≥0.99 for all standard curves (Table 2). Range was set by establishing acceptable precision, accuracy, and linearity over the analytical range from 0.08 to 0.8 µg/mL.
Table 2.
Parameters and linearity data of levothyroxine calibration curves.
| Standard curve | Analytical range (µg/mL) |
Calibrators | Slope | y-Intercept | R2 value |
|---|---|---|---|---|---|
| Validation day 1 | 0.08–0.8 | 5 | 1260.3 | 11.315 | >0.998 |
| Validation day 2 | 0.08–0.8 | 5 | 1234.6 | 8.8371 | >0.998 |
| Validation day 3 | 0.08–0.8 | 5 | 1314.8 | 9.6029 | >0.999 |
3.2.3. Accuracy and precision
Accuracy and precision were established across the analytical range for levothyroxine. The accuracy and intra- and inter-day precision were calculated from the QC samples for levothyroxine. Results for the intra-day accuracy of levothyroxine are summarized in Table 3. Results for the intra- and inter-day precision are summarized in Table 4.
Table 3.
Accuracy: drug substance (% RSD, n = 3).
| Sample | 0.08 µg/mL | 0.4 µg/mL | 0.8 µg/mL |
|---|---|---|---|
| Validation day 1 (%) | 94.9 | 101.1 | 104.8 |
| Validation day 2 (%) | 102.8 | 102.5 | 100.1 |
| Validation day 3 (%) | 98.1 | 103.7 | 102.2 |
Table 4.
Precision: Drug Substance (% RSD, n = 3).
| Sample | 0.08 µg/mL | 0.4 µg/mL | 0.8 µg/mL |
|---|---|---|---|
| Validation day 1 | 0.22 | 0.16 | 0.74 |
| Validation day 2 | 3.66 | 0.68 | 0.56 |
| Validation day 3 | 2.12 | 1.09 | 1.37 |
3.2.4. Specificity
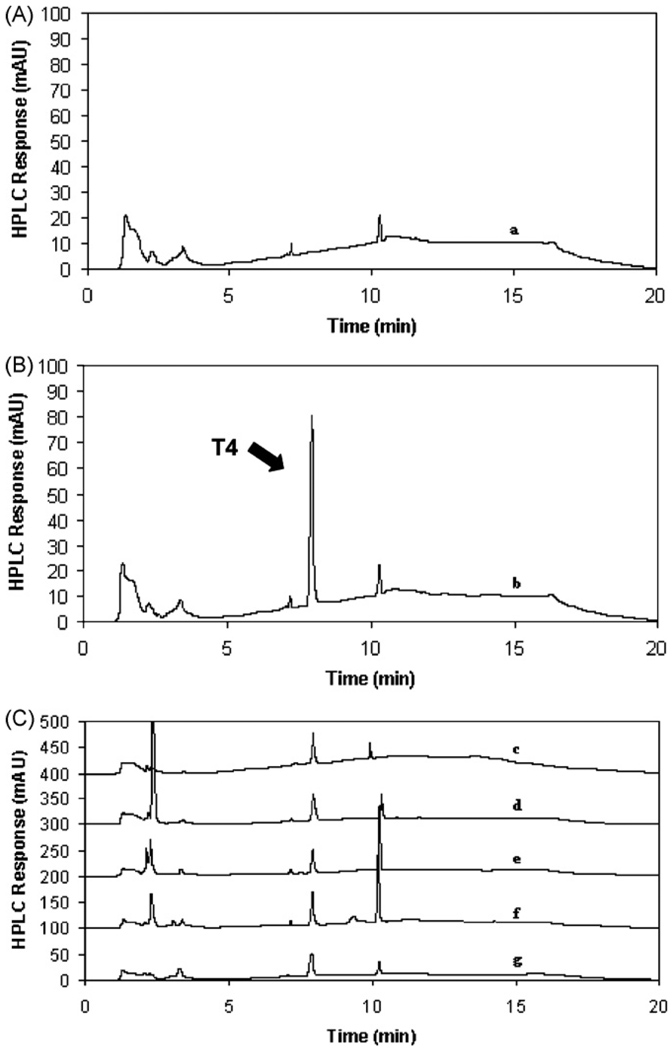
The analysis of the 0.01 M phosphate buffer:MeOH (45:55) (1:1, v/v) solution showed the absence of any major peaks beyond the void volume (Fig. 3), with the exception of the surfactant peak at 10.1 min. In addition, the resolution between levothyroxine and the surfactant peak was always greater than 5. Due to the absence of any co-eluting peaks we determined this method to be specific for levothyroxine.
Fig. 3.
Chromatography of (A) 0.01 M phosphate buffer:MeOH (a); (B) the system suitability standard (b) and (C) (c)–(g) levothyroxine sodium drug products.
3.2.5. Drug product evaluation
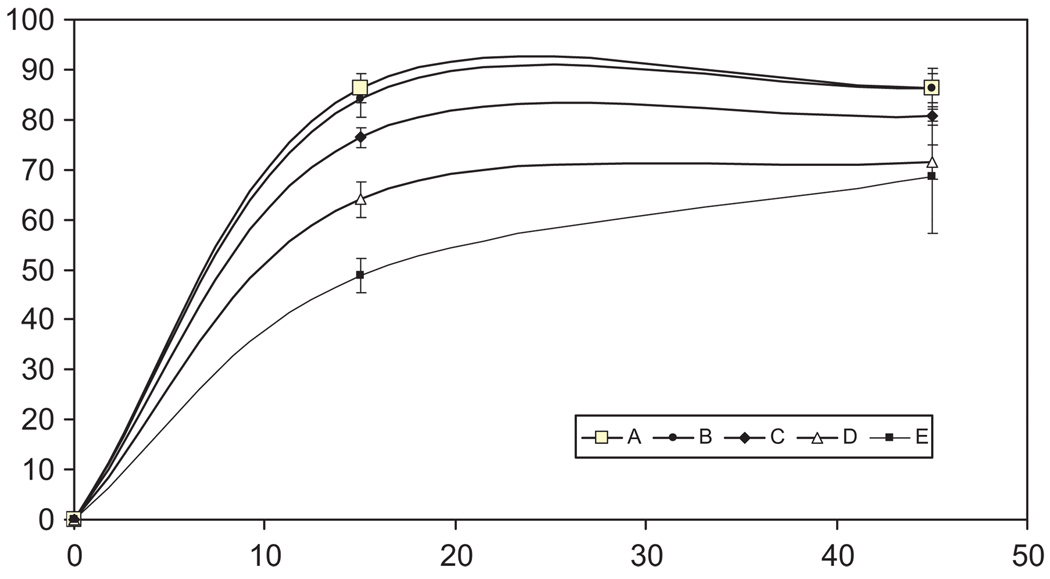
The validated method was successfully applied for the evaluation of five marketed levothyroxine sodium drug products from five different manufacturers. All products were an oral dosage form tablet with a 200-µg dosing strength. Dissolution profiles of each product are presented in Fig. 4. The dissolution profiles showed sig-nificant differences between the drug products. Both Product A and Product B maintained a dissolution rate of >80% at the 15 and 45 min time points. Product C reached a dissolution rate of 80% by the end of the 45 min time point. However, Product D displayed only a dissolution rate below 80% for both time points. Product E displayed the lowest dissolution performance of <70% at 45 min. Nonetheless, significant differences were observed between the dissolution profiles of all 5-drug products at 15 min. An independent t-test revealed a significant difference in release at 45 min between the highest percent dissolution (Product B) and lowest percent dissolution (Product E) at a 95% confidence level (p = 0.0038). In addition, a one-way ANOVA showed that the 5 drug products at 45 min were statistically different at a 95% confidence level (p < 0.0001). However, all the drug products met the USP specifications at 15 or 45 min time points, which was either Q> 70% in 15 or 45 min or Q> 80% in 45 min depending upon the drug product.
Fig. 4.
Dissolution profiles of levothyroxine sodium tablet products A–E at 15 and 45 min.
4. Conclusion
A simple and efficient HPLC method was developed and validated for levothyroxine. The method addressed each of the analytical validation characteristics such as accuracy, precision, specificity, linearity, and range, and met the USP acceptance criteria. The usefulness of this method is demonstrated by successful application for the analysis of dissolution samples from five levothyroxine drug products.
Footnotes
This scientific contribution is intended to support regulatory policy development. The views presented in this article have not been adopted as regulatory policies by the Food and Drug Administration at this time.
References
- 1.Gika H, Samanidou V, Papadoyannis I. Development of a validated HPLC method for the determination of iodotyrosines and iodothyronines in pharmaceuticals and biological samples using solid phase extraction. J. Chromatogr. B. 2005;814:163–172. doi: 10.1016/j.jchromb.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Gika H, Lammerhofer M, Papadoyannis I, Lindner W. Direct separation and quantitative analysis of thyroxine and triiodothyronine enantiomers in pharmaceuticals by high-performance liquid chromatography. J. Chromatogr. B. 2004;800:193–201. doi: 10.1016/j.jchromb.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Post A, Warren R. Sodium levothyroxine. In: Florey K, editor. Analytical Profiles of Drug Substances. vol. 5. New York: Academic Press; 1976. p. 230. [Google Scholar]
- 4.Won C. Kinetics of degradation of levothyroxine in aqueous solution and in solid state. Pharm. Res. 1992;9:226–281. doi: 10.1023/a:1018952415732. [DOI] [PubMed] [Google Scholar]
- 5.Patel H, Stalcup A, Dansereau R, Sakr A. The effect of excipients on the stability of levothyroxine sodium pentahydrate tablets. Int. J. Pharm. 2003;264:35–43. doi: 10.1016/s0378-5173(03)00387-9. [DOI] [PubMed] [Google Scholar]
- 6.Volpato N, Silva R, Brito A, Goncalves J, Vaisman M, Noël F. Multiple level C in vitro/in vivo correlation of dissolution profiles of two l-thyroxine tablets with pharmacokinetics data obtained from patients treated for hypothyroidism. Eur. J. Pharm. 2004;21:655–660. doi: 10.1016/j.ejps.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Shah R, Bryant A, Collier J, Habib M, Khan M. Stability indicating validated HPLC method for quantification of levothyroxine with eight degradation peaks in the presence of excipients. Int. J. Pharm. 2008;360:77–82. doi: 10.1016/j.ijpharm.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Gu J, Soldin O, Soldin S. Simultaneous quantification of free triiodothyronine and free thyroxine by isotope dilution tandem mass spectrometry. Clin. Biochem. 2007;40:1386–1391. doi: 10.1016/j.clinbiochem.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Carducci C, Lucangioli S, Rodriguez V, Fernandez G. Application of extraction disks in dissolution tests of clenbuterol and levothyroxine tablets by capillary electrophoresis. J. Chromatogr. A. 1996;730:313–319. doi: 10.1016/0021-9673(95)00935-3. [DOI] [PubMed] [Google Scholar]
- 10.Richheimer S, Amer T. Stability-indicating assay, dissolution, and content uniformity of sodium levothyroxine in tablets. J. Pharm. Sci. 1983;72:1349–1351. doi: 10.1002/jps.2600721129. [DOI] [PubMed] [Google Scholar]
- 11.Rapaka R, Knight P, Prasad V. Revered-phase high-performance liquid chromatographic analysis of liothyronine sodium and levothyroxine sodium in tablet formulations: preliminary studies on dissolution and content uniformity. J. Pharm. Sci. 1981;70:131–134. doi: 10.1002/jps.2600700205. [DOI] [PubMed] [Google Scholar]
- 12.Rapaka R, Roth J, Brine G, Prasad V. A simple HPLC method for the dissolution studies on levothyroxine sodium tablets. Int. J. Pharm. 1982;12:285–294. [Google Scholar]
- 13.Smith D, Biesemeyer M, Yaciw C. The separation and determination of liothyronine and levothyroxine in tablets by reversed-phase high performance liquid chromatography. J. Chromatogr. Sci. 1981;19:72–78. doi: 10.1093/chromsci/19.2.72. [DOI] [PubMed] [Google Scholar]
- 14.Brower J, Toler D, Reepmeyer J. Determination of sodium levothyroxine in bulk, tablet, and injection formulations by high-performance liquid chromatography. J. Pharm. Sci. 1984;73:1315–1317. doi: 10.1002/jps.2600730937. [DOI] [PubMed] [Google Scholar]
- 15.Garnick R, Burt G, Long D, Bastian J, Aldred J. High-performance liquid chromatographic assay for sodium levothyroxine in tablet formulations: content uniformity applications. J. Pharm. Sci. 1884;73:75–77. doi: 10.1002/jps.2600730120. [DOI] [PubMed] [Google Scholar]
- 16.Pastor F, Milovanovic G, Todorovic M. Kinetic method for the determination of traces of thyroxine by its catalytic effect on the Mn(III) metaphosphate-As(III) reaction. Talanta. 2008;74:1556–1561. doi: 10.1016/j.talanta.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 17.Pabla D, Akhalaghi F, Zia H. A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur. J. Pharm. Biopharm. 2009;72:105–110. doi: 10.1016/j.ejpb.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Pabla D, Akhalaghi F, Zia H. Development and validation of an inductively coupled plasma mass spectrometry method for quantification of levothyroxine in dissolution studies. Rapid Commun. Mass Spectrom. 2008;22:993–996. doi: 10.1002/rcm.3457. [DOI] [PubMed] [Google Scholar]
- 19.Piehl S, Heberer T, Balizs G, Scanlan T, Köhrle J. Development of a validated liquid chromatography/tandem mass spectrometry method for the distinction of thyronine and thyronamine constitutional isomers and for the identification of new deiodinase substrates. Rapid Commun. Mass Spectrom. 2008;22:3286–3296. doi: 10.1002/rcm.3732. [DOI] [PubMed] [Google Scholar]
- 20.Kazemifard A, Moore D, Aghazadeh A. Identification and quantitation of sodium-thyroxine and its degradation products by LC using electrochemical and MS detection. J. Pharm. Biomed. Anal. 2001;25:697–711. doi: 10.1016/s0731-7085(01)00370-3. [DOI] [PubMed] [Google Scholar]
- 21.Tzanavaras P, Themelis D, Zotou A, Stratis J, Karlberg B. Optimization and validation of a dissolution test for selegiline hydrochloride tablets by a novel rapid HPLC assay using a monolithic stationary phase. J. Pharm. Biomed. Anal. 2008;46:670–675. doi: 10.1016/j.jpba.2007.11.039. [DOI] [PubMed] [Google Scholar]