Abstract
Kupffer’s vesicle (KV), a ciliated fluid-filled sphere in the zebrafish embryo with a critical role in laterality determination, is derived from a group of superficial cells in the organizer region of the gastrula named the dorsal forerunner cells (DFC). We have examined the role of the expression of sox17 and chordin (chd) in the DFC in KV formation and laterality determination. Whereas sox17 was known to be expressed in DFC, its function in these cells was not studied before. Further, expression of chd in these cells has not been reported previously. Targeted knockdown of Sox17 and Chd in DFC led to aberrant Left-Right (L-R) asymmetry establishment, as visualized by the expression of southpaw and lefty, and heart and pancreas placement in the embryo. These defects correlated with the formation of small KVs with apparently diminished cilia, consistent with the known requirement for ciliary function in the laterality organ for the establishment of L-R asymmetry.
Keywords: Sox17, Chordin, Kupffer’s Vesicle (KV), Left-Right asymmetry, Dorsal Forerunner Cells (DFC)
Introduction
Left–right (L-R) patterning in the developing vertebrate embryo has been extensively studied because of its intrinsic interest and because of the importance of L-R defects in human pathology. Initial symmetry breaking events may occur very early in embryogenesis and may involve ion flux and calcium transients (Levin and Palmer, 2007; Raya and Izpisua Belmonte, 2008; Schneider et al., 2008). While the evidence for such early events varies between different species, it is clear that laterality establishment requires the function of a laterality organ, identified as the node in mice, Hensen’s node in chick, the posterior notochord in rabbit, the gastrocoel roof plate (GRP) in frogs and Kupffer’s vesicle (KV) in zebrafish. Beating of cilia in the laterality organ to generate directional fluid flow is essential in symmetry breaking around the node (Essner et al., 2005; Hirokawa et al., 2006; Schweickert et al., 2007). Creation of leftward fluid flow instead of local vertices may be explained by the fact that cilia are tilted posteriorly, a feature that is conserved among vertebrates including mice, rabbits, and zebrafish (Nonaka et al., 2005; Raya and Izpisua Belmonte, 2008). It is still not fully resolved whether morphogen transport or sensing of directional flow is critical in laterality organ function (Bisgrove and Yost, 2006; Levin and Palmer, 2007; Raya and Izpisua Belmonte, 2008). The proximal consequence of ciliary flow is the asymmetric expression of different genes on the left and right side of the embryo, in particular of genes encoding agonists and antagonists of nodal signaling (Essner et al., 2005; Kramer-Zucker et al., 2005; Kreiling et al., 2007; Okabe et al., 2008). In zebrafish, the nodal family member Spaw, and the nodal antagonists Lefty1 and Lefty2 are expressed on the left side and inhibited on the right, and this asymmetric expression is required for subsequent visceral organ placement (Long et al., 2003; Shen, 2007).
In zebrafish, the lumen of the KV forms from a group of noninvoluting cells named the Dorsal Forerunner Cells (DFC) that are located at the leading edge of the shield during gastrulation (Kupffer, 1868; Cooper and D'Amico, 1996). KV formation involves the specification and movement of superficial cells under the influence of nodal signaling to form the DFC that constitute a distinct population of precursor cells (Oteiza et al., 2008). Nodal signaling appears to occupy a high position in the regulatory hierarchy of DFC specification, with T-box factors, known targets of nodal signaling, involved in the control of individual steps of KV differentiation (Amack et al., 2007; Oteiza et al., 2008). Molecules mediating cell communication, some of them known as targets of the regulatory factors mentioned above, have a role in the cell shape changes and cell movements that lead from the precursor population to the mature KV (Bisgrove et al., 2005; Hatler et al., 2009).
Beyond the regulatory factors whose role in DFC formation and differentiation has been described, expression of the sox17 gene has been described in these cells without having a functional role assigned to it. In addition, we noted that chordin is expressed in DFC, a fact not previously reported. Thus we asked whether these two factors are required for KV formation and for the establishment of L-R asymmetry.
Sox17, a transcription factor that functions downstream of casanova (cas) in endoderm formation, is strongly expressed in the DFC and KV (Reiter et al., 2001; Ober et al., 2003; Kobayashi et al., 2006; Oteiza et al., 2008; Schneider et al., 2008) (see also supplementary Fig. 4A,B). Cas mutants have fewer DFC and a defective KV, and exhibit L-R asymmetry defects (Alexander et al., 1999; Liang et al., 2000; Essner et al., 2005; Wang and Yost, 2008). This could be due to a direct effect of Cas on DFC development or might be mediated by Sox17. Chordin is a well-known organizer gene that is involved in dorsal-ventral patterning and neural induction in zebrafish and other vertebrate embryos (Miller-Bertoglio et al., 1997). A possible role for chd in KV formation or function is suggested by the fact that chordino (dino) mutant embryos show L-R asymmetry defects in zebrafish (Bisgrove et al., 2000; Tiso et al., 2002) and medaka (Takashima et al., 2007). As in the case of cas mutants, it is not known whether the effects of dino on L-R asymmetry are secondary to the ventralization in these embryos, which is known to affect the anterior-posterior patterning of endodermal organs (Tiso et al., 2002). Endodermal organs such as the pancreas and liver show L-R asymmetry by 2–3dpf, and ventralization might affect this process as well.
In the present work we assess whether expression of sox17 and chd in DFC is required for the determination of L-R asymmetry in zebrafish. We take advantage of the technique of targeting knockdown with morpholinos (MO) to the DFC by injection into the yolk at the midblastula stage, which excludes the MO from most of the embryonic cells, maintaining generally normal development (Amack and Yost, 2004; Bisgrove et al., 2005). Using this approach we obtained evidence that sox17 and chd have a function in KV formation and in the establishment of laterality in zebrafish.
Results
Targeted knockdown of sox17 and chordin
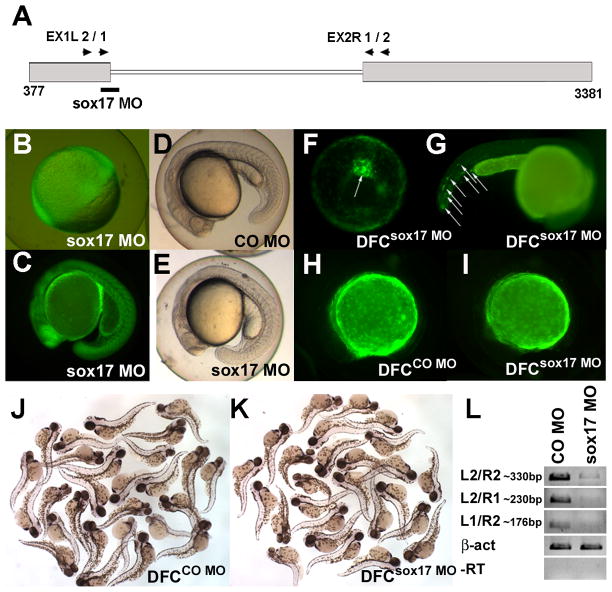
To ask whether the expression of sox17 in the DFC has a role in L-R asymmetry, we designed a splice MO (Fig. 1A) that reduced the level of mature sox17 mRNA (Fig. 1L). Injection of fluorescein-tagged sox17 MO into the yolk of one-cell stage embryos led to uptake of the MO by most cells (Fig. 1B and C), and caused global phenotypes including a slightly shortened axis, delay in somitogenesis, a thickened yolk extension, cell death, and heart edema after 1dpf (Fig. 1D,E and data not shown). In addition, these embryos show reduced or no blood flow, as reported in cas mutants and for Sox17-deficient mice (Alexander et al., 1999; Kikuchi et al., 2001; Jang and Sharkis, 2007; Kim et al., 2007; Liu et al., 2007). Injection of fluorescein-tagged sox17 MO into the yolk at the midblastula stage targeted the DFC (Cooper and D'Amico, 1996; Bisgrove et al., 2005; Amack et al., 2007), and also labeled a few cells in the tail (Fig. 1F–I); we will refer to these morphants as DFCsox17 MO embryos. The overall morphology of DFCsox17 MO embryos was similar to that of embryos injected with CO MO (DFCCO MO embryos) (Fig. 1H,I and J,K).
Fig. 1.
Global phenotypes arise in sox17 morphants, but not in DFCsox17 MO embryos. (A) Location of the sox17 MO. 3’-Fluorescein tagged sox17 MO was widely distributed in the embryos injected at the one-cell stage (B, shield; C, late somite stage). (D,E) 22hpf old embryos injected at the one-cell stage with 10ng control (CO) MO, and sox17 MO, respectively. (F–I) Injection into the yolk at the midblastula stage excludes the MO from most embryonic cells except the DFC (F, arrow. 80% epiboly,) and a few cells in the tail (G, arrows. 1dpf). (H–K) Embryos injected into the yolk at the midblastula stage with 20ng CO MO (H,J) or sox17 MO (I,K) were similar. Pictures taken under fluorescence illumination at about 8-somite stage (H,I), and in visible light at 2dpf (J,K). (L) The efficiency of sox17 MO, 10ng, injected at the one-cell stage, was assessed at 60–80% epiboly by RT-PCR with primers shown in (A); β-act was used as control. All pictures are lateral view, except vegetal view with dorsal on top in F. C–E and H–I anterior to the left, G anterior to the right.
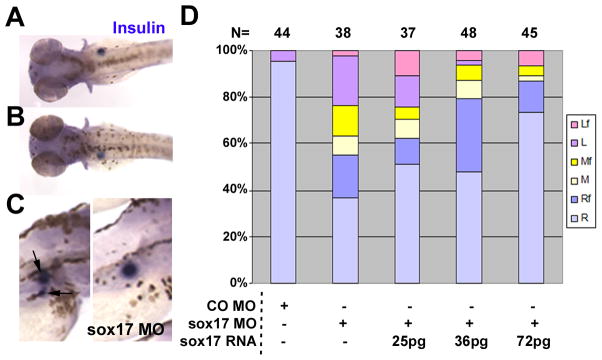
As sox17 has a role in endoderm formation we tested morphants for the expression of insulin and shh. At 2dpf, shh is expressed in the anterior endoderm and gut in addition to other regions (diIorio et al., 2007). Sox17 morphants showed reduction in shh expression in the anterior endoderm with no change in the floor plate (supplementary Fig. 1A–D). This reduction is consistent with the observation that cas mutants lack the gut (Alexander et al., 1999). In contrast, DFCsox17 MO embryos showed no effect on shh expression in endodermal derivatives (supplementary Fig. 1E–H), further validating the targeting approach. Formation of another endodermal tissue, the pancreas, was tested by insulin (ins) expression. Injection of sox17 MO at the one-cell stage altered the ins-expressing domain, with some morphants having fragmented pancreas (left panel of Fig. 2C). More interestingly, localization was altered, with normal right side localization of unfragmented ins-positive domains dropping from about 95% in controls to 37% in sox17 morphants (Fig. 2A,B,D). This defect was rescued in a dose dependent manner by coinjection of sox17 RNA, rising to 73% at the highest level of injected RNA (Fig. 2D). Similar rescue was seen when right-side localization of both intact and fragmented ins-positive domains was considered. These results indicate that the sox17 MO used in these experiments specifically affects the expression of this gene.
Fig. 2.
Injection of sox17 MO leads to abnormal pancreas placement, which is rescued by sox17 RNA co-injection. Embryos injected at the one-cell stage with 10ng CO MO, or 10ng sox17 MO alone or combined with sox17 RNA as indicated, were in situ hybridized with insulin at 3dpf. (A–C) Sox17 MO injected embryos showed normal expression on the right side (A), and abnormal opposite expression on the left (B); dorsal views, anterior to left. Some embryos showed fragmented ins-positive domains (C left panel, arrows), with an example of an intact domain in the right panel (lateral views, anterior to right). (D) Localization of ins expression after injection of MOs with or without RNA, as indicated. R, Right; M, Middle; L, Left; f in Rf, Mf or Lf refers to fragmented ins-positive domains. Number of embryos is shown at the top.
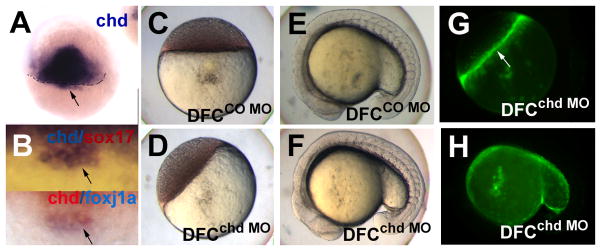
We noticed that chd is expressed in the DFC in mid to late epiboly stages (Fig. 3A,B), in addition to its major expression domain in dorsal mesoderm (Piccolo et al., 1996). In the DFC domain, chd expression overlapped with sox17 (Fig. 3B upper panel; arrow) and foxj1a (Fig. 3B lower panel; arrow) (Aamar and Dawid, 2008). Expression of chd in DFC was transient and could not be detected in the KV at early somite stages (supplementary Fig. 2C–H). To explore Chd function in the DFC we used targeted injection of a previously established MO (Nasevicius and Ekker, 2000). MO injection at the one-cell stage resulted in the expected ventralized embryos with expanded expression of bmp2b and bmp4, whereas injection of the MO at the midblastula stage did not affect dorsal-ventral polarity (Fig. 3C–F; supplementary Fig. 3). Again, fluorescent MO was excluded from the embryonic cells and enriched in the yolk syncytial layer (Fig. 3G, arrow, and H).
Fig. 3.
Chordin expression in the dorsal forerunner cells in zebrafish embryos and targeting of DFC by chd MO. (A,B) Whole mount in situ hybridization for chd at 50% epiboly (A), and double staining of chd (blue) and sox17 (red) (B, upper panel), and chd (red) and foxj1a (blue) (B, lower panel) at 60% epiboly; arrows point to DFC. Embryos were injected at the midblastula stage with 2.5ng CO MO (C,E) or chd MO (D,F), and photographed at the oblong (C,D) and 16–18 somite (E,F) stages. The embryos in (D,F) are also shown as fluorescent images (G,H respectively. Arrow in G, YSL). A,B, dorsal views; E,F,H, lateral views, with anterior to the left.
Sox17 and Chordin are required for left-right asymmetry determination in zebrafish
As DFC are precursors of the KV, the zebrafish laterality organ, we examined a possible role of the DFC-specific expression of sox17 and chd in establishing L-R asymmetry in the embryo. For this purpose we tested the expression of spaw in the lateral plate mesoderm (LPM), lefty2 in the heart region and, in some experiments, lefty1 in the brain of embryos with reduced sox17 and chd expression. For sox17, we compared the effect of general knockdown by injection at the one-cell stage with that of targeted knockdown in the DFC by injection at the midblastula stage. For chd, we tested only the effect of targeted knockdown because of the severe phenotype of broad inhibition of chd expression.
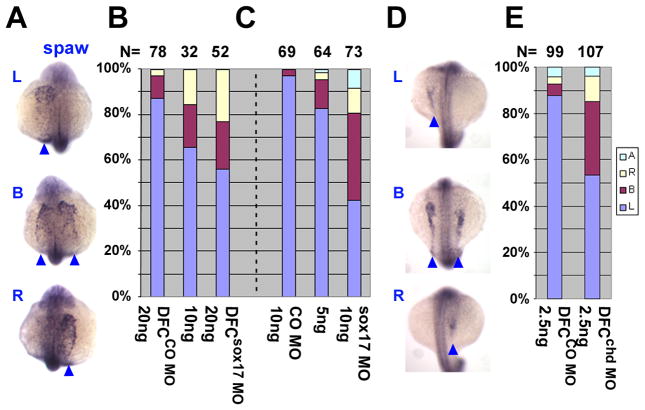
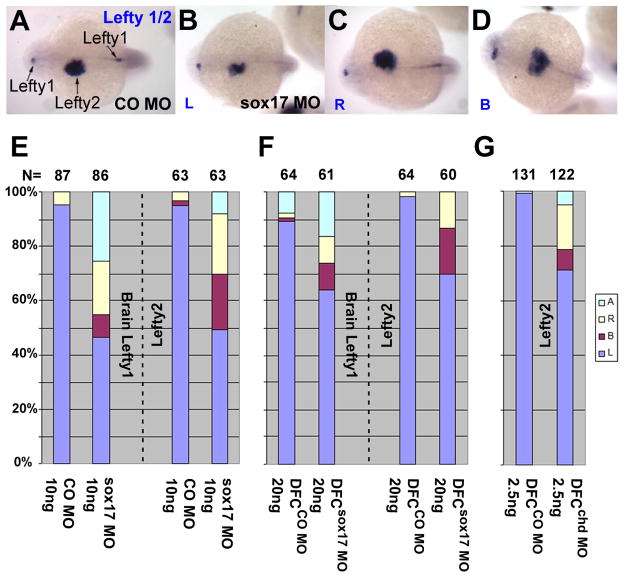
General knockdown in sox17 morphants led to abnormal laterality of spaw, lefty1 and lefty2 expression (Fig. 4C and Fig. 5E). Spaw expression was altered in a dose dependent manner by sox17 MO, with normal left-side expression reduced to as low as 42% (Fig. 4C). Changes in Lefty1 and 2 expression were also dose dependent, even though only one level is shown here; after injection of 10ng sox17 MO, normal left expression of both lefty1 and 2 dropped to 45–50% (Fig. 5A–E).
Fig. 4.
Sox17 and chd are required for left-right asymmetry determination in zebrafish. Embryos were injected into the yolk at one-cell (C) or midblastula (A,B,D,E) stages, with CO MO (B,C,E), sox17 MO (A and B,C), or chd MO (D and E) as indicated in the bar graphs. Whole mount in situ hybridization for spaw (A,D: arrow heads, 15–18 somite; dorsal view with anterior on top) showed aberrant expression when either sox17 MO or chd MO was injected, as summarized in B,C and E. L: Left, B: Bilateral, R: Right, A: absent. Number of embryos is shown at the top.
Fig. 5.
Lefty1,2 expression after suppression of sox17 and of chd in DFC. Embryos were injected into the yolk at one-cell (A–D,E) or midblastula (F,G) stages, with CO MO (A and E–G), sox17 MO (B–D and E–F), or chd MO (G) as indicated. Whole mount in situ hybridization for lefty1 and/or lefty2 at 22hpf (A–D: arrows; dorsal view with anterior to the left) showed aberrant expression when either sox17 MO or chd MO were injected, as summarized in E–G. Expression of lefty1 in the brain and lefty2 in the heart field was scored, as indicated (E, F) in sox17 MO injected embryos, but only lefty2 was scored after chd MO injection (G). L: Left, B: Bilateral, R: Right, A: absent. Number of embryos is shown at the top.
Targeted knockdown in DFCsox17 MO and DFCchd MO embryos resulted in defects in L-R expression of spaw and lefty. Normal left-side expression of spaw in the LPM was reduced to 55% and 52%, respectively, in DFCsox17 MO and DFCchd MO embryos compared to 87–88% in DFCCO MO embryos (Fig. 4B; A and D show examples and illustrate the scoring criteria). Likewise, the proportion of embryos showing normal left-side expression of lefty1/2 was reduced to between 63 and 72% in DFCsox17 MO and DFCchd MO embryos (Fig. 5F,G; A–D are examples). Consistent with the effects on early markers we observed that MO-mediated inhibition of sox17 or chd affected heart looping at one and two dpf. The dose dependence of heart looping defects in sox17 MO morphants are shown in supplementary Fig. 4A,C. Targeted inhibition of sox17 or chd by midblastula injection likewise led to aberrant heart looping, in the case of sox17 at a similar frequency as was achieved by global inhibition of its expression (supplementary Fig. 4B).
These results indicate that the expression of sox17 and chd in the DFC is required for L-R asymmetry determination, as targeted knockdown of these genes in the DFC leads to laterality defects without causing gross morphological abnormalities in the affected embryos.
Sox17 and chd are required for the formation of Kupffer’s vesicle
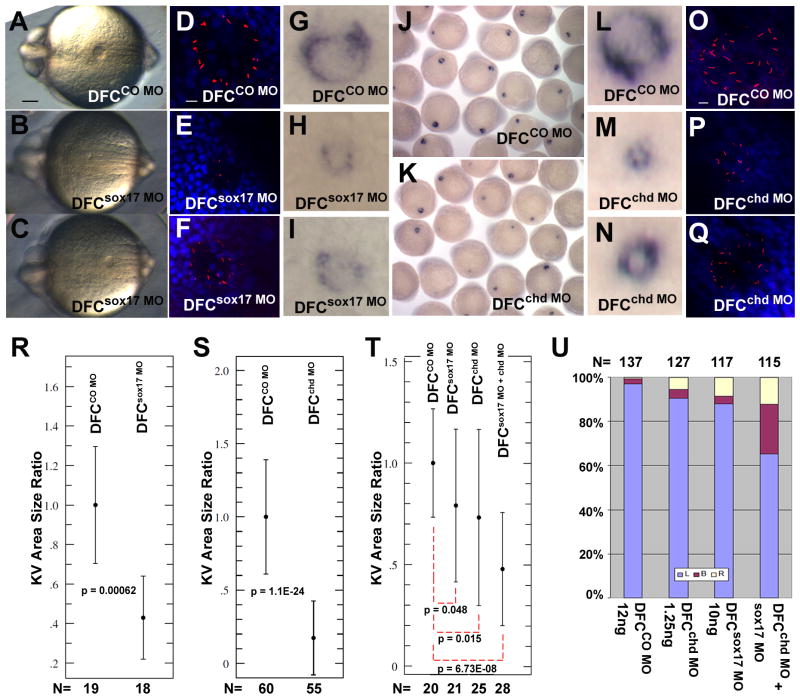
The KV, the laterality organ in zebrafish, is formed from descendents of the DFC (Kupffer, 1868; Cooper and D'Amico, 1996). Since our results show that sox17 and chd are expressed in the DFC and that their knockdown affects laterality, we asked whether function of these two genes is required for KV formation. Both sox17 and chd global morphants (not shown), and DFCsox17 MO and DFCchd MO embryos developed smaller and abnormal KVs when compared to controls, as seen in live embryos (Fig. 6A–C), or after in situ hybridization with charon (Fig. 6G–N; groups of embryos are shown in J,K). DFCsox17 MO embryos displayed a range of KV sizes: 68% of the embryos showed very small or missing KV (Fig. 6B,E,H), and 13% showed a moderate reduction in KV size (n=84) (Fig. 6C,F,I). KV in control embryos (DFCCO MO) were mostly unaffected, with 20% showing a slight reduction in size (n=64, Fig. 6A,D,G). Similarly, targeted injection of chd MO led to a strong reduction in KV size in 44% of embryos (Fig. 6M; K, group view) and a moderate reduction in 28% of embryos (n=61, Fig. 6N). Among the DFCCO MO embryos associated with this experiment, 28% showed a slight reduction in KV size (n=99, Fig. 6L; J, group view).
Fig. 6.
Sox17 and chd are required for proper formation of KV. CO MO (A,D,G: 20ng; R: 15ng; J,L,O,S: 2.5ng; T,U: 12ng), sox17 MO (B,C,E,F,H,I: 20ng; R: 15ng; T,U: 10ng) or chd MO (K,M,N,P,Q,S: 2.5ng; T,U: 1.25ng) were injected into the yolk at the midblastula stage, and embryos were examined at 6–8 somites. Sox17 MO and chd MO injected embryos developed small and abnormal KVs as seen in live embryos (A–C; posterior view), by whole mount in situ hybridization with charon (G–N), and by immunostaining for cilia using anti-acetylated tubulin antibody (red) and DAPI (blue) (D–F and O–Q). Scale bars in A, 0.1mm; in D and O, 10μm. (R,S) NIH ImageJ was used to measure the KV area in injected embryos, shown as ratio to the mean control area; error bars are standard deviations; p values are given in the panels. DFCsox17 MO (R) and DFCchd MO (S) embryos showed a significant reduction in KV size compared to controls. (T,U) Embryos injected at the midblastula stage with low levels of individual MO (12ng CO MO, 1.25ng chd MO, 10ng sox17 MO) or a combination of 1.25ng chd MO and 10ng sox17 MO. KV area was measured as above (T), or lefty2 expression was tested at the ~21 somite stage (U).
The results described above are based on visual classification of KV sizes. For a further evaluation of the data we used the NIH ImageJ program for quantification of KV size. In these measurements we calculated the average area in the control group, and express the sizes of individual KV as fractions of the control average; resulting values were subjected to the Student’s t-test. In the experiment shown in Fig. 6R, embryos were injected at the midblastula stage with 15ng of sox17 MO or CO MO. Setting the mean area in the control group as 1 (n=18; SD=0.29) resulted in a mean of 0.429 in the DFCsox17 MO embryos (n=19; SD=0.21), showing a highly significant difference (p=0.00062) (Fig. 6R). In an experiment in which 2.5ng chd or CO MO was injected, the mean area of the experimental group was 0.17 (n=60; SD=0.25), again setting the control group as 1 (n=55; SD=0.38). The difference in size was statistically significant with a p value of 1.1E-24 (Fig. 6S). These results confirm that reduction of sox17 or chd expression in DFC affects KV formation.
Laterality organs of all vertebrates tested are lined with cilia whose function is critical in the establishment of L-R asymmetry (Yost, 2003; Essner et al., 2005; Hirokawa et al., 2006; Basu and Brueckner, 2008). We tested for the presence of cilia in the defective KVs in DFCsox17 MO (Fig. 6D–F) and DFCchd MO embryos (Fig. 6O–Q). In either case cilia were present but appeared reduced, approximately in proportion to the size reduction of the KV. Thus depletion of Sox17 or Chd in the KV precursor cells led to abnormal KV, most likely impairing the function of the laterality organ in the affected embryos.
To evaluate a possible relationship between the DFC-specific expression of sox17 and chd we tested whether these two genes cooperate in supporting the formation of the KV and the establishment of L-R asymmetry in the zebrafish embryo. When sox17 MO or chd MO was injected individually at half of their effective concentration at the midblastula stage, very little effect on KV formation could be detected. In contrast, coinjection of the MOs at these concentrations led to a clear reduction in KV size. We measured KV sizes with ImageJ as described above, again setting the control group mean value as 1 (n=20; SD=0.26). The ratios of KV area means after individual injection of sox17 MO or chd MO were 0.79 (n=21; SD=0.37; p=0.049) and 0.73 (n=25; SD=0.43; p=0.0174), respectively. The mean value dropped to 0.48 with much higher statistical significance when both MOs were co-injected (n=28; SD=0.27; p=6.78E-08) (Fig. 6T). The expression of lefty2 was also tested under these conditions, showing an enhancement of lefty2 mislocalization from about 10% in single DFCMO embryos to 35% in double DFCMO embryos (Fig. 6U). These results indicate that Sox17 and Chd cooperate in their function during KV formation and laterality establishment in the zebrafish embryo.
Finally, we also tested whether these defects in L-R asymmetry and KV formation are due to a defect in DFC size or proper formation and migration. To test that, we compared MO injected to control embryos at late epiboly stages and tested for DFC in situ markers. Both global sox17 morphants (data not shown) and DFCsox17MO embryos (Fig. 7) showed no significant reduction in expression of cas and foxj1a in DFC. In addition there was no significant effect on the overall formation and migration of the DFC (Fig. 7A–D). On the other hand, DFCchd MO embryos showed strong reduction in the size of the DFC domain as tested with cas, foxj1a, and sox17 in situ probes, and the expression intensity of sox17 appeared reduced (Fig. 7E–J). The reduction of the DFC domain in DFCchd MO but not in DFCsox17 MO embryos suggest that Chd and Sox17 affect KV formation in different ways or at different levels of the regulatory hierarchy. The reduction in sox17 expression after chd MO injection suggests that Chd may be upstream of sox17 in the regulatory network. This finding is consistent with the previous observations that BMP regulates endoderm formation by inhibiting endoderm markers in the ventral domain (Chan et al 2009).
Fig. 7.
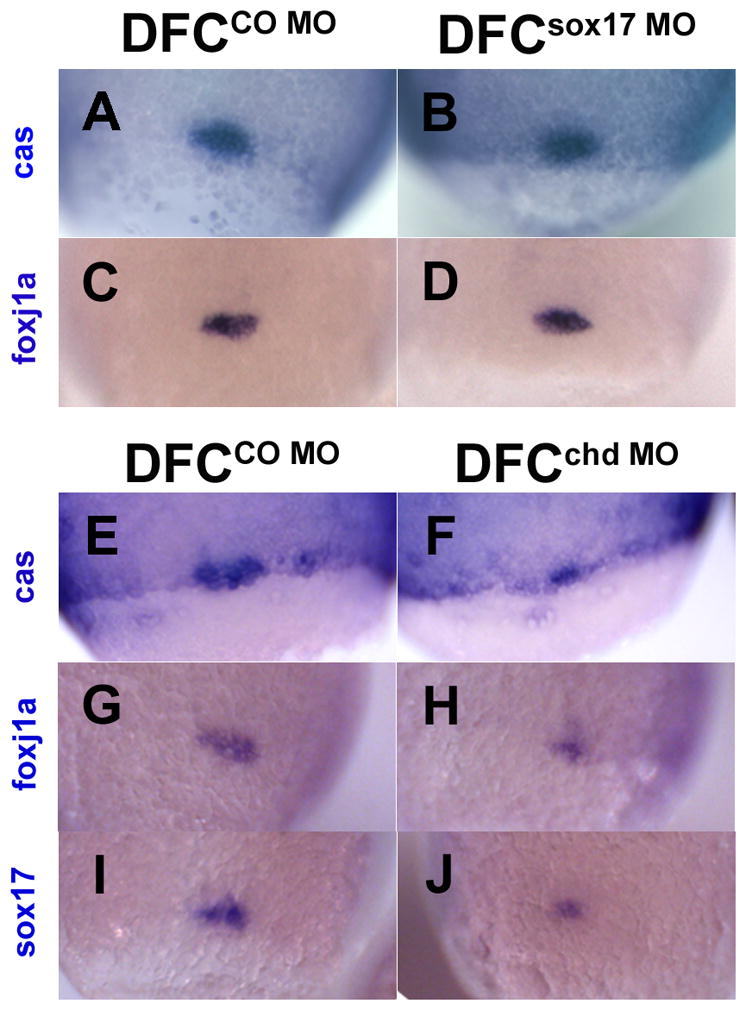
DFC formation in embryos after targeted injection of sox17 MO or chd MO. In situ staining of cas (A,B,E,F) foxj1a (C,D,G,H) and sox17 (I,J) at the 80–90% epiboly stage after midblastula injection of 20ng sox17 MO (B,D), 20ng CO MO (A,C), 2.5ng chd MO (F,H,J) or 2.5ng CO MO (E,G,I). B and D show no reduction in DFC size whereas F, H and J show size reduction; sox17 expression also appears reduced (J). All views are dorsal.
Discussion
The central role of the laterality organ in L-R establishment has been emphasized in studies on different vertebrates (Yost, 2003; Bisgrove and Yost, 2006; Hirokawa et al., 2006; Schweickert et al., 2007; Raya and Izpisua Belmonte, 2008). In zebrafish, the DFC constitute a group of precursor cells that form the KV, the laterality organ in this animal (Cooper and D'Amico, 1996; Essner et al., 2005; Oteiza et al., 2008). Multiple regulatory influences affect the formation and function of the KV, with special emphasis having been placed on the nodal pathway and also FGF, Wnt, Shh, Bmp and Notch pathways and several sets of transcription factors such as T-box and Fox family members (Amack et al., 2007; Levin and Palmer, 2007; Mine et al., 2008; Raya and Izpisua Belmonte, 2008; Blum et al., 2009; Hong and Dawid, 2009; Neugebauer et al., 2009). It seems important to obtain information on the entire range of components that can affect KV formation and laterality so as to move closer to a global understanding of the regulatory networks that are at play in this system. Starting from prior knowledge of sox17 expression in the DFC and our observations of chd expression in these cells we asked whether these two factors are functionally involved in KV formation and laterality establishment in the zebrafish. For this purpose we used injection of MOs into the yolk at the midblastula stage, which targets the MOs to the DFC, influencing KV formation without interference in general development (Amack and Yost, 2004).
Sox17 is an endodermal factor known to be expressed in the DFC and KV in zebrafish and medaka (Kobayashi et al., 2006; Oteiza et al., 2008; Schneider et al., 2008), and in the area of the node in the mouse and chick (Chapman et al., 2007; Hassoun et al., 2009). Sox17 is downstream of cas, an endodermal gene also expressed in the DFC. Zebrafish cas mutants and Sox17 null mice exhibit defects that implicate these genes in laterality establishment (Alexander et al., 1999; Essner et al., 2005; Sakamoto et al., 2007). These results point to a role of sox17 in the DFC even though this gene is widely expressed and has broad functions in endoderm development and thus might have indirect effects (Hudson et al., 1997; Clements et al., 2003). Many genes are regulated by Sox17 in the Xenopus embryo (Sinner et al., 2004; Dickinson et al., 2006; Sinner et al., 2006), but the nature of these genes does not give specific clues on the possible function of this factor in L-R determination. An intriguing aspect is the interaction of Sox17 with β-catenin, the effector of the canonical Wnt signaling pathway, implicating different Sox factors in the regulation of Wnt signaling (Sinner et al., 2004; Sinner et al., 2006). While the Wnt pathway has been less extensively studied in the context of node/KV formation and laterality establishment than certain other signaling pathways, there is evidence implicating both the canonical and PCP arms of Wnt signaling in these processes (Oishi et al., 2006; Lin and Xu, 2009). Thus there may be a mechanistic link between the functions of Sox17 and the Wnt pathway in laterality establishment.
Similar issues as in the case of Sox17 apply to the question about the role of Chordin in L-R asymmetry. Chd is expressed in the node and the anterior part of the primitive streak of mice, and subsequently in the LPM (Bachiller et al., 2000; Monsoro-Burq and Le Douarin, 2001; Mine et al., 2008). Chd regulates Bmp4 expression in the node and LPM, derepressing Nodal expression possibly in concert with Notch signaling (Mine et al., 2008). Dino mutants in zebrafish and medaka, as well as Chd and Noggin double null mice, show defective body axes including aberrant L-R asymmetry and heart looping (Bachiller et al., 2000; Mine et al., 2008). Thus there is evidence for the view that Chd has a role in laterality establishment in different vertebrate embryos. However, as in the case of sox17, chd has a wide expression domain and a major function in dorsal-ventral patterning, and therefore targeted knockdown experiments are helpful in supporting the view that chd function is required within the DFC for normal KV development. This early requirement for chd function may be separate from the later requirement for Chordin and/or Noggin function in the left LPM of mouse embryos (Mine et al., 2008). Repeated functions of the same signaling component at successive steps of a developmental process are commonly observed.
We have obtained some evidence for synergism in the action of Sox17 and Chd in KV formation and laterality establishment, but the basis for this apparent synergism remains to be elucidated. Bmp can repress sox17 expression (Chan et al., 2009), suggesting that Chd and Sox17 are part of an epistatic relationship in which Chd is upstream of Sox17, but such a regulatory loop would not explain the observed synergism. Thus it seems more likely that Chd and Sox17 have parallel functions in the DFC that together promote their developmental progression in the formation of the KV.
Experimental Procedures
Embryos
Zebrafish (Danio rerio) were raised and maintained according to standard procedures (Westerfield, 2000) and staged as described (Kimmel et al., 1995).
RT-PCR
RNA isolation was performed using the RNeasy Mini Kit (Qiagen, http://www1.qiagen.com). Reverse transcription and PCR were performed as described in the SuperScript™ II Reverse Transcriptase manual (Invitrogen, http://www.invitrogen.com). The expression levels of sox17 mature RNA (NM_131287) were compared to those of beta-actin (β-act, BC154531). The primers used for β-act were: Forward, 5′-GAGGAGCACCCCGTCCTGC -3′ and Reverse, 5′-GATGGCTGGAACAGGGCC -3′ (58°C, 30 cycles). The primers used for sox17 were: EX1L1, 5′-CACAATGCGGAGCTGAGTAA-3′ and EX1L2, 5’-AGTCCGCTCTCAGACTCCAA-3’ for Forward primers; EX2R1, 5′-AATGGACGTTTGTCCACCAT-3′ and EX2R2, 5’-ATCGCTTGTTTCGTTTCACC-3’ for Reverse (58°C, 30 cycles, EX; Exon, L; Left/Forward primer, R; Right/Reverse primer).
Cloning and construction of expression plasmid
For Sox17 RNA, a full-length ORF cDNA (NM_131287) was subcloned into the EcorI-XhoI sites of the expression vector pCS2+ (Turner and Weintraub, 1994).
RNA and Morpholinos
Sox17 mRNA was prepared using clones linearized with NotI and transcribed using the mMESSAGE mMACHINE® SP6 Kit (Ambion). Morpholino antisense oligonucleotides (Gene Tools) were as follows: Sox17 splice MO, 3'-Fluorescein tagged, 5'-CTCATATTTCTGTACTCACCAAGCA-3' (bases complementary to the end of Exon1 are indicated in italics and underlined); this morpholino overlaps the first splice donor site/junction, including the Exon1 end and Intron1 start (Fig. 1A). We used the standard Chordin MO, 3'-Fluorescein tagged, available from Gene Tools; and Gene Tools standard control MO, both untagged and 3'-Fluorescein tagged. Morpholinos were injected into the yolk of one-cell stage embryos for whole knockdown in the embryonic cells (morphants), or into the yolk of midblastula stage (256–1000cells) embryos to target DFC (DFCMO embryos).
Whole-mount in situ hybridization
In situ hybridizations were performed as described by Thisse and Thisse (http://zfin.org/zf_info/zfbook/chapt9/9.82.html) (Westerfield, 2000). Antisense digoxigenin (or fluorescein in case of sox17) labeled probes were synthesized and used for chd, sox17, spaw (southpaw), lefty1, lefty2, insulin, shh, cas, foxj1a, bmp4, bmp2b, nma (BMP downstream target gene, data not shown), charon and cmcl2. The labeling kit from Roche Molecular Biochemicals was used as described (Westerfield, 2000).
Whole-mount Immunohistochemistry
Whole-mount immunohistochemistry was performed as previously described (Essner et al., 2005). Briefly, zebrafish embryos were fixed in 4% parafomaldehyde, rinsed in PBST, dehydrated into methanol and stored at −20°C until used. After rehydration and blocking for 1 h in 10% goat serum (Sigma), 2% bovine serum albumin (BSA) (Sigma) in PBST (PBS and 0.1% TritonX-100), embryos were incubated overnight at 4°C in anti-acetylated tubulin antibody (1:1000, Sigma) in the blocking solution. After washing in 2% BSA in PBST, the embryos were incubated overnight at 4°C with Alexa Fluor 568 goat anti-mouse antibodies (1:500, Molecular Probes). The embryos then were washed with 2% BSA in PBST, transferred to PBS and mounted in low melt agarose for imaging.
Imaging
Live, fixed, or in situ stained embryos were observed and photographed using a Leica MZ APO dissecting microscope with a RETIGA 1300 digital camera (Quantitative imaging corporation) using the QCapture software. Fluorescence was observed in a Leica MZ FLIII fluorescence stereomicroscope and the Diagnostic Instruments corp. spot digital camera, RT slider system, and software version 4.5. Confocal micrographs were taken using the Zeiss LSM 510 Meta axioplan2 Laser Scanning Microscope.
Supplementary Material
Acknowledgments
We thank Dr. Wayne Lencer for support of this project. This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health.
References
- Aamar E, Dawid IB. Isolation and expression analysis of foxj1 and foxj1.2 in zebrafish embryos. Int J Dev Biol. 2008;52:985–991. doi: 10.1387/ijdb.072477ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J, Rothenberg M, Henry GL, Stainier DY. casanova plays an early and essential role in endoderm formation in zebrafish. Dev Biol. 1999;215:343–357. doi: 10.1006/dbio.1999.9441. [DOI] [PubMed] [Google Scholar]
- Amack JD, Wang X, Yost HJ. Two T-box genes play independent and cooperative roles to regulate morphogenesis of ciliated Kupffer's vesicle in zebrafish. Dev Biol. 2007;310:196–210. doi: 10.1016/j.ydbio.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Amack JD, Yost HJ. The T box transcription factor no tail in ciliated cells controls zebrafish left-right asymmetry. Curr Biol. 2004;14:685–690. doi: 10.1016/j.cub.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Basu B, Brueckner M. Cilia multifunctional organelles at the center of vertebrate left-right asymmetry. Curr Top Dev Biol. 2008;85:151–174. doi: 10.1016/S0070-2153(08)00806-5. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Essner JJ, Yost HJ. Multiple pathways in the midline regulate concordant brain, heart and gut left-right asymmetry. Development. 2000;127:3567–3579. doi: 10.1242/dev.127.16.3567. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Snarr BS, Emrazian A, Yost HJ. Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer's vesicle are required for specification of the zebrafish left-right axis. Dev Biol. 2005;287:274–288. doi: 10.1016/j.ydbio.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development. 2006;133:4131–4143. doi: 10.1242/dev.02595. [DOI] [PubMed] [Google Scholar]
- Blum M, Beyer T, Weber T, Vick P, Andre P, Bitzer E, Schweickert A. Xenopus, an ideal model system to study vertebrate left-right asymmetry. Dev Dyn. 2009;238:1215–1225. doi: 10.1002/dvdy.21855. [DOI] [PubMed] [Google Scholar]
- Chan TM, Chao CH, Wang HD, Yu YJ, Yuh CH. Functional analysis of the evolutionarily conserved cis-regulatory elements on the sox17 gene in zebrafish. Dev Biol. 2009;326:456–470. doi: 10.1016/j.ydbio.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Matsumoto K, Cai Q, Schoenwolf GC. Specification of germ layer identity in the chick gastrula. BMC Dev Biol. 2007;7:91. doi: 10.1186/1471-213X-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements D, Cameleyre I, Woodland HR. Redundant early and overlapping larval roles of Xsox17 subgroup genes in Xenopus endoderm development. Mech Dev. 2003;120:337–348. doi: 10.1016/s0925-4773(02)00450-1. [DOI] [PubMed] [Google Scholar]
- Cooper MS, D'Amico LA. A cluster of noninvoluting endocytic cells at the margin of the zebrafish blastoderm marks the site of embryonic shield formation. Dev Biol. 1996;180:184–198. doi: 10.1006/dbio.1996.0294. [DOI] [PubMed] [Google Scholar]
- Dickinson K, Leonard J, Baker JC. Genomic profiling of mixer and Sox17beta targets during Xenopus endoderm development. Dev Dyn. 2006;235:368–381. doi: 10.1002/dvdy.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- diIorio P, Alexa K, Choe SK, Etheridge L, Sagerstrom CG. TALE-family homeodomain proteins regulate endodermal sonic hedgehog expression and pattern the anterior endoderm. Dev Biol. 2007;304:221–231. doi: 10.1016/j.ydbio.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–1260. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- Hassoun R, Puschel B, Viebahn C. Sox17 Expression Patterns during Gastrulation and Early Neurulation in the Rabbit Suggest Two Sources of Endoderm Formation. Cells Tissues Organs. 2009 doi: 10.1159/000236044. [DOI] [PubMed] [Google Scholar]
- Hatler JM, Essner JJ, Johnson RG. A gap junction connexin is required in the vertebrate left-right organizer. Dev Biol. 2009;336:183–191. doi: 10.1016/j.ydbio.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Hong SK, Dawid IB. FGF-dependent left-right asymmetry patterning in zebrafish is mediated by Ier2 and Fibp1. Proc Natl Acad Sci U S A. 2009;106:2230–2235. doi: 10.1073/pnas.0812880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson C, Clements D, Friday RV, Stott D, Woodland HR. Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell. 1997;91:397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- Jang YY, Sharkis SJ. Fetal to adult stem cell transition: knocking Sox17 off. Cell. 2007;130:403–404. doi: 10.1016/j.cell.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Agathon A, Alexander J, Thisse C, Waldron S, Yelon D, Thisse B, Stainier DY. casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001;15:1493–1505. doi: 10.1101/gad.892301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Jindo T, Naruse K, Takeda H. Development of the endoderm and gut in medaka, Oryzias latipes. Dev Growth Differ. 2006;48:283–295. doi: 10.1111/j.1440-169X.2006.00870.x. [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- Kreiling JA, Williams G, Creton R. Analysis of Kupffer's vesicle in zebrafish embryos using a cave automated virtual environment. Dev Dyn. 2007;236:1963–1969. doi: 10.1002/dvdy.21191. [DOI] [PubMed] [Google Scholar]
- Kupffer C. Beobachtungea uber die Entwicklung der Knochenfische. Arch Mikrob Anat. 1868;4:209–272. [Google Scholar]
- Levin M, Palmer AR. Left-right patterning from the inside out: widespread evidence for intracellular control. Bioessays. 2007;29:271–287. doi: 10.1002/bies.20545. [DOI] [PubMed] [Google Scholar]
- Liang JO, Etheridge A, Hantsoo L, Rubinstein AL, Nowak SJ, Izpisua Belmonte JC, Halpern ME. Asymmetric nodal signaling in the zebrafish diencephalon positions the pineal organ. Development. 2000;127:5101–5112. doi: 10.1242/dev.127.23.5101. [DOI] [PubMed] [Google Scholar]
- Lin X, Xu X. Distinct functions of Wnt/beta-catenin signaling in KV development and cardiac asymmetry. Development. 2009;136:207–217. doi: 10.1242/dev.029561. [DOI] [PubMed] [Google Scholar]
- Liu Y, Asakura M, Inoue H, Nakamura T, Sano M, Niu Z, Chen M, Schwartz RJ, Schneider MD. Sox17 is essential for the specification of cardiac mesoderm in embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:3859–3864. doi: 10.1073/pnas.0609100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S, Ahmad N, Rebagliati M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development. 2003;130:2303–2316. doi: 10.1242/dev.00436. [DOI] [PubMed] [Google Scholar]
- Miller-Bertoglio VE, Fisher S, Sanchez A, Mullins MC, Halpern ME. Differential regulation of chordin expression domains in mutant zebrafish. Dev Biol. 1997;192:537–550. doi: 10.1006/dbio.1997.8788. [DOI] [PubMed] [Google Scholar]
- Mine N, Anderson RM, Klingensmith J. BMP antagonism is required in both the node and lateral plate mesoderm for mammalian left-right axis establishment. Development. 2008;135:2425–2434. doi: 10.1242/dev.018986. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A, Le Douarin NM. BMP4 plays a key role in left-right patterning in chick embryos by maintaining Sonic Hedgehog asymmetry. Mol Cell. 2001;7:789–799. doi: 10.1016/s1097-2765(01)00223-4. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene 'knockdown' in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Neugebauer JM, Amack JD, Peterson AG, Bisgrove BW, Yost HJ. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature. 2009;458:651–654. doi: 10.1038/nature07753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. De novo formation of left-right asymmetry by posterior tilt of nodal cilia. PLoS Biol. 2005;3:e268. doi: 10.1371/journal.pbio.0030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober EA, Field HA, Stainier DY. From endoderm formation to liver and pancreas development in zebrafish. Mech Dev. 2003;120:5–18. doi: 10.1016/s0925-4773(02)00327-1. [DOI] [PubMed] [Google Scholar]
- Oishi I, Kawakami Y, Raya A, Callol-Massot C, Izpisua Belmonte JC. Regulation of primary cilia formation and left-right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nat Genet. 2006;38:1316–1322. doi: 10.1038/ng1892. [DOI] [PubMed] [Google Scholar]
- Okabe N, Xu B, Burdine RD. Fluid dynamics in zebrafish Kupffer's vesicle. Dev Dyn. 2008;237:3602–3612. doi: 10.1002/dvdy.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteiza P, Koppen M, Concha ML, Heisenberg CP. Origin and shaping of the laterality organ in zebrafish. Development. 2008;135:2807–2813. doi: 10.1242/dev.022228. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya A, Izpisua Belmonte JC. Insights into the establishment of left-right asymmetries in vertebrates. Birth Defects Res C Embryo Today. 2008;84:81–94. doi: 10.1002/bdrc.20122. [DOI] [PubMed] [Google Scholar]
- Reiter JF, Kikuchi Y, Stainier DY. Multiple roles for Gata5 in zebrafish endoderm formation. Development. 2001;128:125–135. doi: 10.1242/dev.128.1.125. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Hara K, Kanai-Azuma M, Matsui T, Miura Y, Tsunekawa N, Kurohmaru M, Saijoh Y, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochem Biophys Res Commun. 2007;360:539–544. doi: 10.1016/j.bbrc.2007.06.093. [DOI] [PubMed] [Google Scholar]
- Schneider I, Houston DW, Rebagliati MR, Slusarski DC. Calcium fluxes in dorsal forerunner cells antagonize beta-catenin and alter left-right patterning. Development. 2008;135:75–84. doi: 10.1242/dev.004713. [DOI] [PubMed] [Google Scholar]
- Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol. 2007;17:60–66. doi: 10.1016/j.cub.2006.10.067. [DOI] [PubMed] [Google Scholar]
- Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- Sinner D, Kirilenko P, Rankin S, Wei E, Howard L, Kofron M, Heasman J, Woodland HR, Zorn AM. Global analysis of the transcriptional network controlling Xenopus endoderm formation. Development. 2006;133:1955–1966. doi: 10.1242/dev.02358. [DOI] [PubMed] [Google Scholar]
- Sinner D, Rankin S, Lee M, Zorn AM. Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development. 2004;131:3069–3080. doi: 10.1242/dev.01176. [DOI] [PubMed] [Google Scholar]
- Takashima S, Shimada A, Kobayashi D, Yokoi H, Narita T, Jindo T, Kage T, Kitagawa T, Kimura T, Sekimizu K, Miyake A, Setiamarga DH, Murakami R, Tsuda S, Ooki S, Kakihara K, Hojo M, Naruse K, Mitani H, Shima A, Ishikawa Y, Araki K, Saga Y, Takeda H. Phenotypic analysis of a novel chordin mutant in medaka. Dev Dyn. 2007;236:2298–2310. doi: 10.1002/dvdy.21245. [DOI] [PubMed] [Google Scholar]
- Tiso N, Filippi A, Pauls S, Bortolussi M, Argenton F. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech Dev. 2002;118:29–37. doi: 10.1016/s0925-4773(02)00252-6. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Wang X, Yost HJ. Initiation and propagation of posterior to anterior (PA) waves in zebrafish left-right development. Dev Dyn. 2008;237:3640–3647. doi: 10.1002/dvdy.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. A guide for the laboratory use of zebrafish (Danio rerio) 4. Univ. of Oregon Press; Eugene: 2000. The zebrafish book. [Google Scholar]
- Yost HJ. Left-right asymmetry: nodal cilia make and catch a wave. Curr Biol. 2003;13:R808–809. doi: 10.1016/j.cub.2003.09.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.